Abstract
Background and Purpose
Understanding the mechanisms underlying stroke can aid the development of therapies and improve the final outcome. The purposes of this study were to establish whether there are characteristic mechanistic differences in the frequency, severity, functional outcome, and mortality between left- and right-hemisphere ischemic stroke and, given the velocity differences in the carotid circulation and direct branching of the left common carotid artery from the aorta, whether large-vessel ischemia (including cardioembolism) is more common in the territory of the left middle cerebral artery.
Methods
Trial cohorts were combined into a data set of 476 samples. Using Trial of Org 10172 in Acute Stroke Treatment criteria, ischemic strokes in a total 317 patients were included in the analysis. Hemorrhagic stroke, stroke of undetermined etiology, cryptogenic stroke, and bilateral ischemic strokes were excluded. Laterality and vascular distribution were correlated with outcomes using a logistic regression model. The etiologies of the large-vessel strokes were atherosclerosis and cardioembolism.
Results
The overall event frequency, mortality, National Institutes of Health Stroke Scale (NIHSS) score, Glasgow Coma Scale score, and rate of mechanical thrombectomy interventions differed significantly between the hemispheres. Left-hemispheric strokes (54%) were more common than right-hemispheric strokes (46%; p=0.0073), and had higher admission NIHSS scores (p=0.011), increased mortality (p=0.0339), and higher endovascular intervention rates (p≤0.0001). ischemic strokes were more frequent in the distribution of the left middle cerebral artery (122 vs. 97; p=0.0003) due to the higher incidence of large-vessel ischemic stroke in this area (p=0.0011).
Conclusions
Left-hemispheric ischemic strokes appear to be more frequent and often have a worse outcome than their right-hemispheric counterparts. The incidence of large-vessel ischemic strokes is higher in the left middle cerebral artery distribution, contributing to these hemispheric differences. The hemispheric differences exhibit a nonsignificant trend when strokes in the middle cerebral artery distribution are excluded from the analysis.
Keywords: motor grade, collaterals, ischemic stroke, endovascular intervention, outcome after stroke, mortality after stroke, acute stroke, endovascular treatment, mechanical thrombectomy
Introduction
Lateralization is important in rehabilitation due to the compartmentalized functional differences between the hemispheres.1 Left cerebral hemispheric (LH) infarctions are more frequent than infarctions of the right hemisphere (RH) among young adults.2 Previous studies have found hemodynamic differences between the right and left carotid artery circulations. This is primarily attributable to differences in the intima-media complex and flow velocity in the left carotid artery, resulting in higher stress and intimal damage therein. This may induce atherosclerotic changes, leading to more severe LH ischemic events.3 These studies also show that large-vessel ischemic events and cardioembolism may be more common in the LH. Our unpublished clinical observation data from the transcranial Doppler (TCD) laboratory have revealed a higher predilection of the agitated saline bubbles to flow to the LH. Among 50 patients undergoing TCD for the detection of right-to-left shunt, a higher trend was seen for bubbles to travel to the LH (57% vs. 43%, p<0.001). The left common carotid artery is a direct branch off the aorta, and cardiogenic emboli may prefer a more direct path.
The main purpose of this study was to determine whether in adult patients there is a higher incidence of LH vs. RH ischemic strokes, and if such asymmetry is related to stroke in the middle cerebral artery territory. Since there are differences in the median stroke volume between the hemispheres for a given neurological examination,4-6 we also investigated whether there were asymmetries in clinical severity, functional outcome, and death.
Methods
Patients and techniques
All patient data were obtained from the University of Florida stroke database between January 2009 and December 2009 and combined into a single data set totaling 476 patients. Of those, 359 were ischemic strokes, 89 were hemorrhagic strokes, and 28 were transient ischemic attacks/previous chronic strokes. Among the 359 ischemic strokes, 30 patients with bilateral strokes and 12 patients with missing data were excluded from the laterality analysis. The final cohort comprised 317 patients.
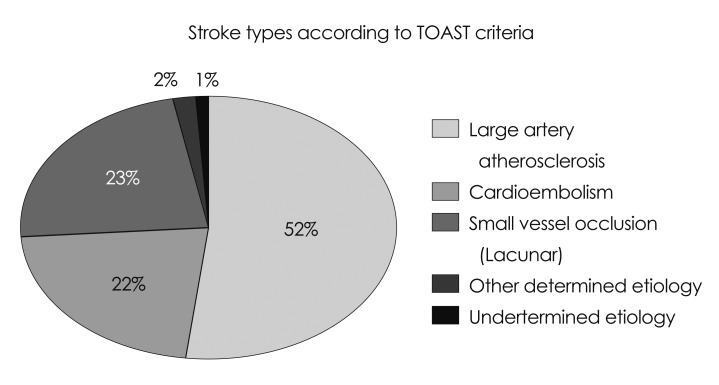
Based on the Trial of Org 10172 for Acute Ischemic Strokes (TOAST) criteria,7 strokes were categorized into five major infarction groups (Fig. 1): small-vessel (lacunar), large-vessel atherosclerotic (AS; including both extracranial and intracranial disease), cardioembolic (CE), cryptogenic, and stroke of undetermined causes. Among 317 patients, the stroke etiology was AS in 165 (52%), CE in 68 (22%), small vessel in 74 (23%), and cryptogenic and stroke of undetermined origin in 10 (3%). Large-vessel stroke included infarctions caused by AS and CE etiology. The inclusion criteria for this study included 1) cerebral infarctions, 2) availability of a well-documented on-site evaluation of examination, and 3) availability of brain-imaging studies comprising computed tomography angiography (CTA) or magnetic resonance angiography (MRA) obtained as part of the stroke alert and that were of sufficient quality to aid unequivocal TOAST criteria employed in this investigation. All patients included in the study either had a CTA or MRA of the head and neck as part of their stroke evaluation. The follow-up period was from admission due to the current stroke until their discharge from the hospital.
Fig. 1.
Distribution of strokes according to TOAST criteria in study population (n=317). Large vessel strokes constitute the largest category in ischemic stroke distribution. TOAST: Trial of Org 10172 for Acute Ischemic Strokes.
The retrospective analysis of admission data for these participants included the following parameters: demographics, the results of the neurological examination performed by a neurologist, and brain imaging with and without contrast and perfusion. Laterality was correlated to outcomes using a logistic regression model. This study was approved by the University of Florida Institutional Review Board.
Clinical variables and measurement of outcome
The following items were included in the analysis: age, TOAST classification, event frequency, troponin elevation, admission National Institutes of Health Stroke Scale (NIHSS) score, admission Glasgow Coma Scale (GCS) score, outcome including mortality, disposition, endovascular mechanical thrombectomy (MT) data, and discharge modified Rankin Scale (m-RS) score. The etiologies of large-vessel strokes comprised AS and CE.
Statistical analysis
The database was analyzed using a chi-square test with Bonferroni adjustment. Few factors were dichotomized at clinically relevant cutoffs. All analyses were performed by a biostatistician with the aid of JMP software (Version 10.0). Models were built using forward/backward stepwise logistic regression with variables entered into the model at the 0.05 significance level.
Results
Overall stroke distribution (Table 1, Fig. 1)
Table 1.
Overall stroke distribution
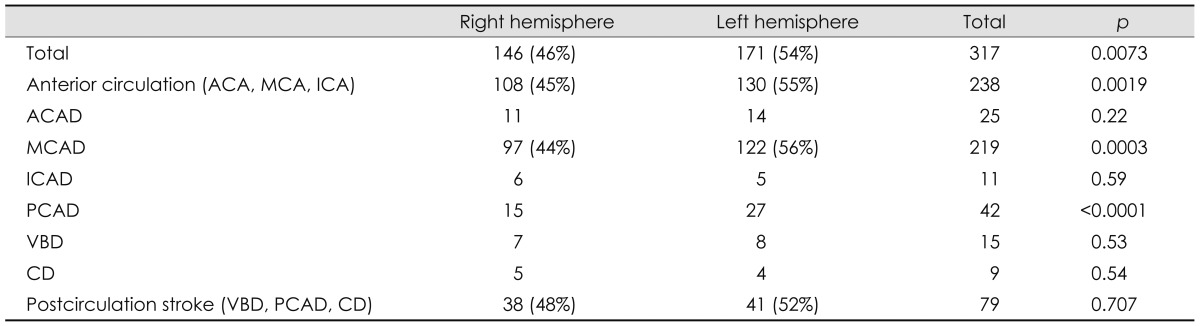
Data are presented as either n or n (%) values.
ACA: anterior cerebral artery, ACAD: anterior cerebral artery distribution, CD: cerebellar distribution, ICA: internal carotid artery, ICAD: internal carotid artery distribution, MCA: middle cerebral artery, MCAD: middle cerebral artery distribution, PCAD: posterior cerebral artery distribution, VBD: vertebral-basilar distribution.
The total number of participants in the study was 476. The final tally of participants after implementation of the inclusion and exclusion criteria was 317 (171 males, 146 females). The median age of the participants was 70 years [interquartile range (IQR), 58.0-78.5 years]. The ethnicity and racial distribution data were not used in the analysis due to poor documentation.
In the anterior circulation (n=255), 11 (5%) patients had strokes in the internal carotid artery distribution, 219 (85%) had them in the middle cerebral artery distribution (MCAD), and 25 (10%) had them in the anterior cerebral artery distribution. Bilateral infarcts were seen in 17 subjects and were not included in the analysis.
In the posterior circulation (n=79), 13 subjects had bilateral strokes and were excluded, and of the remaining subjects, 42 (53%) had strokes in the posterior cerebral artery distribution, 15 (19%) had them in the vertebral-basilar distribution, and 9 (12%) had them in the cerebellar distribution.
Hemispheric differences (Table 2 and 3, Fig. 2)
Table 2.
Hemispheric differences in the various vascular distributions

*Large-vessel strokes comprise atherosclerotic (AS) and cardioembolic (CE) etiologies.
MCAD: middle cerebral artery distribution.
Table 3.
Comparison of stroke outcome between hemispheres
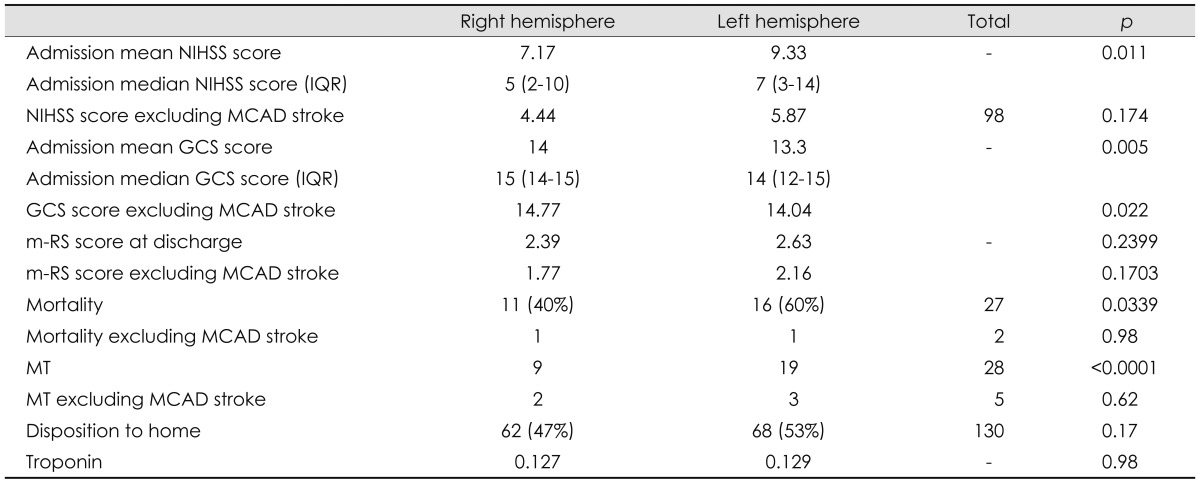
GCS: Glasgow Coma Scale, IQR: interquartile range, m-RS: modified Rankin Scale, MCAD: middle cerebral artery distribution, MT: mechanical thrombectomy, NIHSS: National Institutes of Health Stroke Scale.
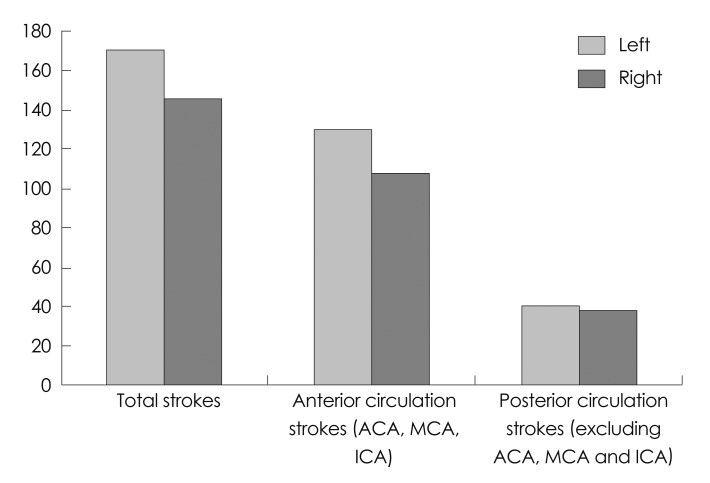
Fig. 2.
Distribution of strokes on left vs. right side in anterior and posterior circulation. In our cohort, overall event frequency was higher for left side ischemic strokes mainly in the anterior distribution. ACA: anterior cerebral artery, ICA: internal carotid artery, MCA: middle cerebral artery.
The total number of ischemic strokes was 317. LH stroke (LHS) in all vascular distributions was more common than RH stroke (RHS; 54% vs. 46%, p=0.0073). The incidence of anterior circulation strokes was higher in the LH (55% vs. 45%, p=0.0019). Large-vessel events were more common in the LH (57%) than in the RH (43%, p<0.0001). However, exclusion of the MCAD from the analysis resulted in a nonsignificant difference in the total frequency between the hemispheres or in the incidence of large-vessel ischemic events (RH 43% vs. LH 57%, p=0.0737).
Compared to RHSs, LHSs were more severe at presentation, with a higher admission mean NIHSS score (LHS, 9.33 vs. RHS, 7.17; p=0.011), median NIHSS score (IQR) [LHS, 7 (3-14) vs. RHS, 5 (2-10)], lower admission mean GCS score (LHS, 13.3 vs. RHS, 14; p=0.005), median GCS score (IQR) [LHS, 14 (12-15) vs. RHS, 15 (14-15)], higher mortality (LHS, 60% vs. RHS, 40%; p=0.0339), and increased occurrence of MT (LHS, 19 vs. RHS, 9; p<0.0001). Again, exclusion of the MCAD data from the analysis resulted in a nonsignificant difference in these parameters between the hemispheres. There were no significant differences in the small-vessel ischemic events (lacunar stroke) or posterior circulation events between the hemispheres. The total number of deaths was 33; patients discharged to a hospice were not included. There was no significant difference in the occurrence of troponin elevation (p=0.98) and disposition to home (p=0.17) between patients with RHS vs. LHS.
Characteristics of stroke in the MCAD (Table 4, Fig. 3)
Table 4.
MCAD stroke distribution and characteristics
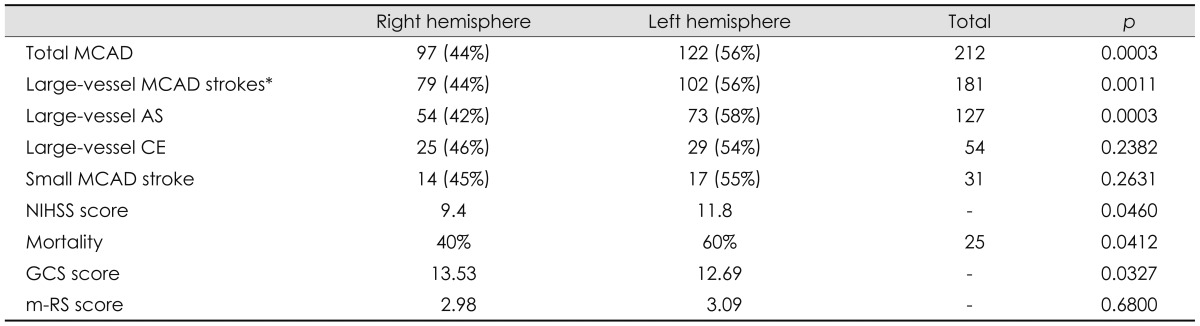
*Large-vessel strokes comprise AS and CE etiologies.
AS: atherosclerotic, CE: cardioembolic, GCS: Glasgow Coma Scale, m-RS: modified Rankin Scale, MCAD: middle cerebral artery distribution, NIHSS: National Institutes of Health Stroke Scale.
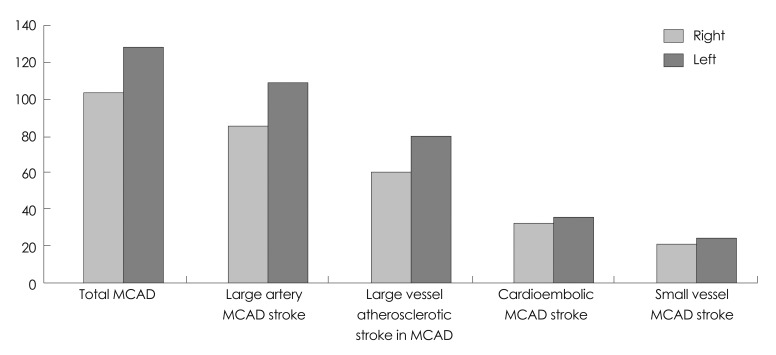
Fig. 3.
Middle cerebral artery stroke distribution (MCAD) according to TOAST criteria. In our cohort, there was higher incidence of large vessel stroke specifically atherosclerotic stroke in the left MCAD. There was no significant difference in the small vessel and cardioembolic ischemic stroke event rate. TOAST: Trial of Org 10172 for Acute Ischemic Strokes.
There was a total of 219 patients in the MCAD. Seven patients were excluded since they had cryptogenic stroke and stroke of unexplained etiology; the final cohort thus comprised 212 patients, of whom 97 (44%) had right MCAD and 122 (56%) had left MCAD. The overall MCAD stroke frequency differed significantly between the LH (n=122, 56%) and RH (n=97, 44%; p=0.0003). The incidence of large-vessel ischemic events was higher in the left MCAD (n=102, 56%) than in the right (n=79, 44%; p=0.0011). There were significantly more large-vessel AS events in the LH (n=73, 58%) than in the RH (p=0.0003). The incidence rates of CE and small-vessel events did not differ significantly between the hemispheres. In addition, left MCAD strokes were associated with a higher mortality (p=0.0412), higher admission NIHSS score (p=0.046), and lower GCS score (p=0.0327). There were no significant differences in the discharge m-RS score between the hemispheres.
Correlation between age, and stroke severity and mortality (Table 5)
Table 5.
Correlation of age with stroke severity and mortality
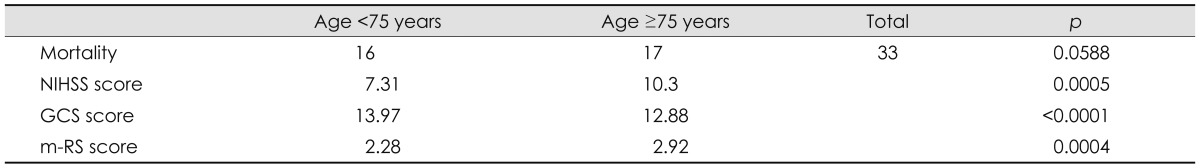
GCS: Glasgow Coma Scale, m-RS: modified Rankin Scale, NIHSS: National Institutes of Health Stroke Scale.
Age was not correlated with mortality (p=0.13); nevertheless, a mortality rate was higher in patients older than 75 years, which trended toward statistical significance independent of laterality (OD, 1.98; CI 95%, 0.96-4.07; p=0.0588). Patients aged ≥75 years upon admission were more impaired, with higher NIHSS score (p=0.0005), lower GCS score (p≤0.0001), and higher m-RS score (p=0.004). Independent of the age group, there was a greater admission stroke severity (NIHSS score) in the mortality group (p<0.01). The mortality rate was highest (60%) among patients with strokes in the left MCAD.
Discussion
Few studies have addressed the hemispheric differences in stroke outcome.2-5,8,9 This retrospective study addressed a few key issues in this regard, and the findings indicate that LHSs are more common, more severe, and result in poorer outcomes than RHSs. These hemispheric differences in frequency and outcomes are due mainly to the higher incidence of LH large-vessel strokes in the MCAD. When MCAD data were excluded from the analysis and the data were adjusted, variables including frequency, severity, and mortality did not differ significantly between the hemispheres. The importance of isolating MCAD strokes from infarctions in other vascular territories when assessing outcome is highlighted by these findings. It will be interesting to learn whether the results of previous studies describing hemispheric differences would still be statistically significant if the MCAD strokes were analyzed independently. Theories such as intima-media complex variation and velocity differences in the left carotid artery accounting for greater stroke incidence in the LH need further validation.
While some studies have found a higher incidence of cardioembolism in the LH, the present study found no such difference. Our TCD data verified a trend for bubbles to travel to the LH. Prospective validity will be required to determine whether this translates to a higher rate of CE strokes in the LH.
One small retrospective imaging study of lacunar strokes revealed a higher event rate in the RH,10 even though that was not the primary intention of that particular study. Our results do not support this conclusion.
Studies of infants and children suggest that the LH has greater metabolic demands than the right.11 This hemispheric difference may place the LH at greater risk for functional decrement with a reduction of blood flow, and these asymmetrical hemispheric metabolic demands may influence neuroplasticity during the early and later stages of poststroke recovery, even in adults. There is also evidence that cerebral blood flow and neuroplasticity change with aging,12,13 and it is possible that these age-related changes also contribute to stroke risk and prognosis for recovery.
One retrospective study9 showed that LHS was associated with greater neurological deficits, based on the admission NIHSS score, but a better chance of recovery. However, our study contradicts these findings, in that LHS was associated with more severe strokes based on a higher NIHSS score on admission, but also a higher mortality.
Multiple studies have shown age to be an independent predictor of death in ischemic stroke.15-17 Few studies have investigated the effects of age on outcome in patients with left vs. right MCAD strokes, but animal studies have revealed that the animal's age is an important factor in stroke mortality in the MCAD.18 In the present small cohort of ischemic stroke patients, age ≥75 years conferred additional stroke mortality risk. The overall stroke mortality can be up to 17%14; in our cohort, it was slightly lower at 10% and 8% in the overall distribution and hemispheric distribution categories, respectively. This lower incidence was probably related to the exclusion from the analysis of hemorrhagic and bilateral strokes, and the shorter follow-up period. Our findings are in agreement with previous studies showing higher mortality in the group of patients aged ≥75 years of age, irrespective of the gender.19 The incidences of atrial fibrillation and other comorbidities increase significantly in patients older than 75 years. Further clarification is required to establish whether this higher incidence of mortality in the elderly relates to large-vessel strokes secondary to atrial fibrillation or a higher incidence of comorbidities. Future studies determining the cause of death and its prevention are needed.
In conclusion, LHSs are more common than RHSs, are more severe, and more often have a poorer outcome, principally due to the higher incidence of large-vessel ischemic strokes in the territory of the left middle cerebral artery.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Cassvan A, Ross PL, Dyer PR, Zane L. Lateralization in stroke syndromes as a factor in ambulation. Arch Phys Med Rehabil. 1976;57:583–587. [PubMed] [Google Scholar]
- 2.Naess H, Waje-Andreassen U, Thomassen L, Myhr KM. High incidence of infarction in the left cerebral hemisphere among young adults. J Stroke Cerebrovasc Dis. 2006;15:241–244. doi: 10.1016/j.jstrokecerebrovasdis.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 3.Rodríguez Hernández SA, Kroon AA, van Boxtel MP, Mess WH, Lodder J, Jolles J, et al. Is there a side predilection for cerebrovascular disease? Hypertension. 2003;42:56–60. doi: 10.1161/01.HYP.0000077983.66161.6F. [DOI] [PubMed] [Google Scholar]
- 4.Woo D, Broderick JP, Kothari RU, Lu M, Brott T, Lyden PD, et al. NINDS t-PA Stroke Study Group. Does the National Institutes of Health Stroke Scale favor left hemisphere strokes? Stroke. 1999;30:2355–2359. doi: 10.1161/01.str.30.11.2355. [DOI] [PubMed] [Google Scholar]
- 5.Fink JN, Selim MH, Kumar S, Silver B, Linfante I, Caplan LR, et al. Is the association of National Institutes of Health Stroke Scale scores and acute magnetic resonance imaging stroke volume equal for patients with right- and left-hemisphere ischemic stroke? Stroke. 2002;33:954–958. doi: 10.1161/01.str.0000013069.24300.1d. [DOI] [PubMed] [Google Scholar]
- 6.Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke. 2001;32:2559–2566. doi: 10.1161/hs1101.098524. [DOI] [PubMed] [Google Scholar]
- 7.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 8.Fink JN, Frampton CM, Lyden P, Lees KR Virtual International Stroke Trials Archive Investigators. Does hemispheric lateralization influence functional and cardiovascular outcomes after stroke?: an analysis of placebo-treated patients from prospective acute stroke trials. Stroke. 2008;39:3335–3340. doi: 10.1161/STROKEAHA.108.523365. [DOI] [PubMed] [Google Scholar]
- 9.Di Legge S, Saposnik G, Nilanont Y, Hachinski V. Neglecting the difference: does right or left matter in stroke outcome after thrombolysis? Stroke. 2006;37:2066–2069. doi: 10.1161/01.STR.0000229899.66019.62. [DOI] [PubMed] [Google Scholar]
- 10.Rothrock JF, Lyden PD, Hesselink JR, Brown JJ, Healy ME. Brain magnetic resonance imaging in the evaluation of lacunar stroke. Stroke. 1987;18:781–786. doi: 10.1161/01.str.18.4.781. [DOI] [PubMed] [Google Scholar]
- 11.Arditi H, Feldman R, Hammerman C, Eidelman AI. Cerebral blood flow velocity asymmetry, neurobehavioral maturation, and the cognitive development of premature infants across the first two years. J Dev Behav Pediatr. 2007;28:362–368. doi: 10.1097/DBP.0b013e318114315d. [DOI] [PubMed] [Google Scholar]
- 12.DeCarli C, Kawas C, Morrison JH, Reuter-Lorenz PA, Sperling RA, Wright CB. Session II: Mechanisms of age-related cognitive change and targets for intervention: neural circuits, networks, and plasticity. J Gerontol A Biol Sci Med Sci. 2012;67:747–753. doi: 10.1093/gerona/gls111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, et al. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Hum Brain Mapp. 2009;30:1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, et al. Executive summary: heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:188–197. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 15.Tsivgoulis G, Saqqur M, Sharma VK, Lao AY, Hill MD, Alexandrov AV, et al. Association of pretreatment blood pressure with tissue plasminogen activator-induced arterial recanalization in acute ischemic stroke. Stroke. 2007;38:961–966. doi: 10.1161/01.STR.0000257314.74853.2b. [DOI] [PubMed] [Google Scholar]
- 16.Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA Stroke Trial. Stroke. 1997;28:2119–2125. doi: 10.1161/01.str.28.11.2119. [DOI] [PubMed] [Google Scholar]
- 17.Nogueira RG, Liebeskind DS, Sung G, Duckwiler G, Smith WS, et al. MERCI. Predictors of good clinical outcomes, mortality, and successful revascularization in patients with acute ischemic stroke undergoing thrombectomy: pooled analysis of the Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi MERCI Trials. Stroke. 2009;40:3777–3783. doi: 10.1161/STROKEAHA.109.561431. [DOI] [PubMed] [Google Scholar]
- 18.Wang RY, Wang PS, Yang YR. Effect of age in rats following middle cerebral artery occlusion. Gerontology. 2003;49:27–32. doi: 10.1159/000066505. [DOI] [PubMed] [Google Scholar]
- 19.Khaw KT, Barrett-Connor E, Suarez L, Criqui MH. Predictors of stroke-associated mortality in the elderly. Stroke. 1984;15:244–248. doi: 10.1161/01.str.15.2.244. [DOI] [PubMed] [Google Scholar]