Abstract
Context
A previous cross-sectional study showed an association of migraine with a higher prevalence of magnetic resonance imaging (MRI)–measured ischemic lesions in the brain.
Objective
To determine whether women or men with migraine (with and without aura) have a higher incidence of brain lesions 9 years after initial MRI, whether migraine frequency was associated with progression of brain lesions, and whether progression of brain lesions was associated with cognitive decline.
Design, Setting, and Participants
In a follow-up of the 2000 Cerebral Abnormalities in Migraine, an Epidemiological Risk Analysis cohort, a prospective populationbased observational study of Dutch participants with migraine and an age- and sexmatched control group, 203 of the 295 baseline participants in the migraine group and 83 of 140 in the control group underwent MRI scan in 2009 to identify progression of MRI-measured brain lesions. Comparisons were adjusted for age, sex, hypertension, diabetes, and educational level. The participants in the migraine group were a mean 57 years (range, 43–72 years), and 71% were women. Those in the control group were a mean 55 years (range, 44–71 years), and 69% were women.
Main Outcome Measures
Progression of MRI-measured cerebral deep white matter hyperintensities, infratentorial hyperintensities, and posterior circulation territory infarctlike lesions. Change in cognition was also measured.
Results
Of the 145 women in the migraine group, 112 (77%) vs 33 of 55 women (60%) in the control group had progression of deep white matter hyperintensities (adjusted odds ratio [OR], 2.1; 95%CI, 1.0–4.1; P=.04). There were no significant associations of migraine with progression of infratentorial hyperintensities: 21 participants (15%) in themigraine group and 1 of 57 participants (2%) in the control group showed progression (adjusted OR, 7.7; 95% CI, 1.0–59.5; P=.05) or new posterior circulation territory infarctlike lesions: 10 of 203 participants (5%) in the migraine group but none of 83 in the control group (P=.07). There was no association of number or frequency of migraine headaches with progression of lesions. There was no significant association of high vs nonhigh deep white matter hyperintensity load with change in cognitive scores ( 3.7 in the migraine group vs 1.4 in the control group; 95% CI, 4.4 to 0.2; adjusted P=.07).
Conclusions
In a community-based cohort followed up after 9 years, women with migraine had a higher incidence of deep white matter hyperintensities but did not have significantly higher progression of other MRI-measured brain changes. There was no association of migraine with progression of any MRI-measured brain lesions in men.
Migraine affects up to 15% of the general population. 1–3 One-third of patients with migraine have associated symptoms of neurological aura.2,3 Previous work in the cross-sectional community-based Cerebral Abnormalities in Migraine, an Epidemiological Risk Analysis (CAMERA-1) study demonstrated a higher prevalence and greater volume of magnetic resonance imaging (MRI)–measured deep white matter hyperintensities, infratentorial hyperintensities, and posterior circulation territory infarctlike lesions in participants withmigraine.4–6 A higher volume of deep white matter hyperintensities7 and increased prevalence of posterior circulation territory infarctlike lesions has also been demonstrated in women with migraine with aura8 and the prevalence of deep white matter hyperintensities was increased among patients with migraine identified from neurology clinics.9
White matter hyperintensities, infratentorial hyperintensities, and posterior circulation territory infarctlike lesions are believed to be of ischemic origin. In particular, white matter hyperintensities are associated with atherosclerotic disease risk factors,9 increased risk of ischemic stroke,10–12 and cognitive decline.13 The associations of migraine with these MRI-measured lesions and clinical ischemic stroke7,14 are consistent with the hypothesis that recurring migraine headaches may be associated with cerebral ischemia and that migraine-associated cerebral ischemia may be attack related. In the current study, we report associations of migraine and migraine subtype with the progression of MRI-measured cerebral ischemic lesions at the 9-year follow-up of the original CAMERA study population. In exploratory analyses, we report associations of migraine frequency, total number of migraine attacks during follow-up, and presence of current migraine headache symptoms with progression of brain lesions. In additional exploratory analyses, we determined whether progression of brain lesions was associated with cognitive decline and whether the presence of migraine headache influenced any association of brain lesion progression with cognitive decline.
METHODS
Study Population and Procedures
The original participants of the CAMERA-1 study included 295 well characterized individuals with migraine3 and 140 age- and sex-matched controls who were randomly selected from a community-based study of the general population.1 The MRI scans were completed in 2000.4 All participants were invited to return for follow-up scan in 2009. In 2000, the mean age of the sample was 48 years (SD, 7.8 years) and 71% were women (eTable 1, available at http://www.jama.com). The CAMERA-2 study, conducted in 2009, included a structured computer guided telephone interview (programmed using Ishell software, World Health Organization), brain MRI, physical examination, and cognitive testing similar to the CAMERA-1 protocol. Participants were administered questionnaires to determine previous, current, and newly developed migraine attacks since 2000. The interview was structured so that participants could recount their history of migraine using personal benchmarks (eg, pregnancy) for when a different pattern started and stopped. These benchmarks were used to define periods. Information was collected on migraine prophylaxis and treatment. All nonimaging data were collected blinded to diagnosis and MRI findings. To avoid introduction of false-positive differences due to upgraded MRI techniques, we used the same scanners and protocols that were used for CAMERA-1.4 The protocol was approved by the local medical ethics committees. All participants gave written informed consent.
Outcome Measures
Primary outcome measure of this study was change in number and volume of MRI-measured deep white matter hyperintensities in individuals with migraine vs controls during follow-up. In addition, progression of posterior circulation territory infarctlike lesions as well as infratentorial hyperintensities was evaluated. Results of automatic segmentation of white matter lesions (QBrain1.1software) were, if necessary, corrected manually in a conservative manner by 1 rater, in anonymized baseline and follow-up scans separately, blinded for scan order and diagnosis. Reproducibility data include (random, n=40 of participants reanalyzed): 1.0T-scanner: ρ, 0.999 (P<.001) and 1.5T-scanner: ρ, 0.963 (P<.001). Periventricular white matter hyperintensities were attached to the lateral ventricle; other supratentorial hyperintensities were deep white matter hyperintensities, which were calculated by number, total, and mean volume for each participant. Geographical location was evaluated by normalizing the individual MRI scans with segmented lesions to standard Montreal Neurological Institute–space, and projecting the lesions (weighted for group size) of all participants per diagnostic group in a transparent 3-dimensional map (glass brain). Infratentorial hyperintensities were hyperintense on T2- and proton-density weighted and not hypointense on fluid attenuated inversion recovery images. Presence and progression of lesions was assessed by one rater, who was blinded to diagnosis, by comparing baseline and follow-up scans side by side. Reproducibility data (random, n=40 [14%]; baseline, κ=0.908; P=.09 and follow-up, κ=1.000; P<.001). Lesion progression was defined as an increase in size, number, or both (FIGURE 1). Infarctlike lesions were nonmass parenchymal defects with a vascular distribution, isointense to cerebrospinal fluid signal on all sequences, and, when supratentorial, surrounded by a hyperintense rim on FLAIR images.4 Virchow-Robin spaces were excluded based on typical location, shape, and absence of a hyperintense rim. In the basal ganglia, only parenchymal defects larger than 3 mm in diameter were considered in order to exclude nonspecific lesions. Location and vascular territory of new and preexisting infarcts were read by 2 neuroradiologists, who were blinded to diagnosis (κ=0.87, P<.001). All sequences of baseline and follow-up scans were presented side by side (angulation corrected and position linked). A third senior neuroradiologist made the final diagnosis in the 9 cases in which the 2 raters disagreed. An exploratory outcome measure of this study was the changes in cognition related to white matter hyperintensities at baseline and at follow-up. Similarly, the change in cognition between baseline and follow-up was evaluated as function of baseline and follow-up lesion volumes well as lesion volume change. For each participant, normalized test scores (Z scores of separate tests in domains of memory, executive function, attention, visuospatial ability, and speed) were summed to achieve a total composite cognitive score for each time point. Change in raw test scores (follow-up minus baseline) was normalized by Z scores. The tests, evaluating cognitive performance in the domains of memory, concentration, and attention, executive functioning, psychomotor, and processing speed, organization, fine motor skills, fluid intelligence, and visuospatial skills, consisted of the 15-word Verbal Learning Test15; abbreviated Stroop test,16 consisting of 3 subtasks; verbal Fluency test17; Letter Digit Substitution Test,18 which is a modified version of the Symbol Digit Modalities Test; and the Purdue pegboard test.19 In follow-up investigation, the Block Design Test from the Wechsler Adult Intelligence Scale III test battery20 was added. Further details on cognition testing are provided in eTable 3 (available at http://www.jama.com).
Figure 1.
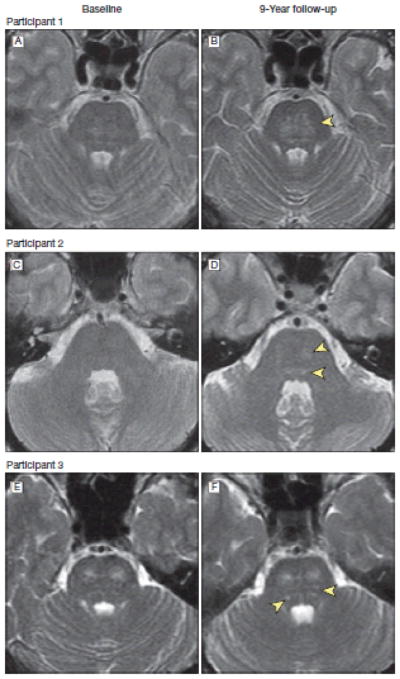
Brain Magnetic Resonance T2-Weighted Images at Baseline and Follow-up From three Representative Participants Showing Progression of Infratentorial Hyperintensities
Image B shows pontine hyperintensity (arrowhead) increased in size compared with baseline image (A). Image D shows new hyperintensities (arrowheads) compared with baseline image (C). Image F shows additional hyperintensities(arrowheads) compared with baseline image (E).
Covariates and Definitions
Sociodemographic and medical history characteristics were assessed by interview. Educational level was dichotomized into low, primary school or less than vocational education, and high, more than higher vocational or professional education, college, or university. A diagnosis of diabetes or hypertension was based on patient report of a physician’s diagnosis.
Statistical Analysis
Differences in the distributions and means of measured characteristics among the study groups were assessed with χ2, 2-tailed Fisher exact, unpaired t, and Mann-Whitney U tests and 1-way analyses of variance where appropriate. Using logistic regression, the risk for MRI outcome measures was examined by migraine diagnosis (yes/no) and subtype of migraine (with and without aura vs controls), controlling for age, sex, educational level, hypertension, and diabetes. Statistical interactions of hypertension and diabetes for associations of migraine and MRI measured outcomes were tested for by adding the interaction terms to the models. Analyses of deep white matter hyperintensity volumes were a priori stratified by sex, based on earlier findings of increased association of migraine with MRI lesions only among women.4 Likewise, infarct analyses were a priori stratified by anterior or posterior vascular territory. In logistic regression models, exploratory analyses were conducted on the effects of several migraine characteristics on measures of lesion progression. Associations between deep white matter hyperintensity load and normalized scores of the baseline and follow-up cognitive tests were assessed using linear regression models, adjusting for age, sex, and educational level (model 1) and additionally for migraine (model 2) to assess the effect of migraine diagnosis. Data were analyzed using the statistical software package for social sciences (SPSS, version 17.0. for Windows).
RESULTS
Study Population
A total of 411 of 435 (95%) of baseline participants were successfully recontacted; 14 participants had moved, 4 were lost to civil registry information, and 6 had died (eTable 1). Two hundred eighty-six participants (66%) underwent follow-up MRI scan (114 migraine with aura, 89 migraine without aura, 83 controls). Mean follow-up was 8.5 years (range, 7.9–9.2; SD, 0.24 years). Reasons for nonparticipation were no interest (n=51), inability to visit the research center (n=30), claustrophobia (n=8), and nonneurological illness (n=36). There was no association between responder rate and diagnosis of migraine (response rate in both migraine groups was 203 of 296 (69%) vs 83 of 139 (60%) in the control group (P=.07). Compared with nonparticipants, participants were younger at baseline (48 vs 50 years; P=.01), more often reported high educational level (52% vs 40%; P=.01), smoked fewer packyears (8 vs 14 years; P<.001; eTable 1), had a similar prevalence of posterior circulation territory infarctlike lesions (4%), brain infarcts (6% vs 9%; P=.24), and a high load of deep white matter hyperintensities (based on semi quantitative measures at baseline; 19% vs 22%; P=.44). At follow-up, participants in the migraine group were slightly older than those in the control group (57 vs 55 years; P=.03) and had a higher prevalence of diabetes (9% vs 2%; P=.05; TABLE 1).
Table 1.
Follow-up Characteristics of Study Participants
| Characteristic | Total (n = 286) | Controls(n = 83) | Migraine (n = 203) | Migraine
|
|
|---|---|---|---|---|---|
| No Aura (n = 89) | Aura (n = 114) | ||||
| Age, mean (SD), y | 57 (7.7) | 55 (7.3) | 57 (7.8)f | 58 (7.5) | 67 (8.0) |
|
| |||||
| Women | 202 (71) | 57 (69) | 145 (71) | 64 (72) | 81 (71) |
|
| |||||
| Maastricht research center | 128 (45) | 38 (46) | 90 (44) | 35 (40) | 55 (48) |
|
| |||||
| Low educationa | 137 (48) | 38 (46) | 99 (49) | 46 (52) | 63 (47) |
|
| |||||
| BMI, mean (SD) | 26 (4.1) | 26 (38) | 26 (4.3) | 25 (4.3) | 26 (4.2) |
|
| |||||
| Hypertensionb | 97 (34) | 24 (29) | 73 (36) | 33 (36) | 41 (36) |
|
| |||||
| Use of antihypertensive medicationa | 79 (28) | 19 (23) | 60 (30) | 28 (32) | 32 (28) |
|
| |||||
| Blood pressure, mean (SD), mm Hgb | |||||
| Systolic | 151 (21) | 152 (19) | 151 (21) | 148 (20) | 154 (22) |
|
| |||||
| Diastolic | 94 (11) | 94 (12) | 94 (11) | 92 (10) | 96 (12) |
|
| |||||
| Diabetes (self-reported) | 20 (7) | 2 (2) | 10 (9)g | 9 (10) | 9 (8) |
|
| |||||
| History of stroked | 8 (3) | 0 (0) | 8 (4) | 2 (2) | 6 (5) |
|
| |||||
| History of transient ischemic attack | 12 (4) | 2 (2) | 10 (5) | 5 (6) | 6 (4) |
|
| |||||
| Smoking | |||||
| Ever | 193 (68) | 58 (70) | 135 (67) | 58 (65) | 77 (68) |
|
| |||||
| Current | 67 (35) | 19 (33) | 48 (36) | 22 (38) | 26 (34) |
|
| |||||
| Pack-years, mean (SD) | 11 (15) | 12 (15) | 11 (15) | 13 (18) | 10 (13) |
|
| |||||
| Alcohol use | |||||
| None during last 12 mo | 42 (15) | 10 (12) | 32 (16) | 18 (20) | 14 (12) |
|
| |||||
| ≥3 U/d | 29 (10) | 11 (13) | 18 (9) | 6 (7) | 12 (11) |
|
| |||||
| Current use of migraine medicatione | |||||
| Triptans | 25 (12.3) | 8 (9) | 17 (14.9) | ||
|
| |||||
| Ergotamines | 5 (2.5) | 1 (1.1) | 4 (3.5) | ||
|
| |||||
| Prophylactic drugs | 7 (3.4) | 1 (1.1) | 6 (5.3) | ||
|
| |||||
| Oral contraceptive use, women only | |||||
| Current | 16 (6) | 6 (12) | 10 (8) | 3 (6) | 7 (9) |
|
| |||||
| ≥15 y | 71 (25) | 71 (25) | 47 (38) | 21 (42) | 26 (35) |
Abbreviations: EMI body mass index calculated as weight in kilograms divided by height in matans squared.
Low education indicates primary school or lower vocational education.
Hypertension self-reported, physician diagnosed.
Use of antihypertensive medication by participants with hypertension; not used as migraine prophylaxis. Mean blood pressure indicates mean of 2 blood pressure measurements after transcranial Doppler examination with Vaiselva
ischemic or hemorrhage self-reported.
Current use of migraine medication defined as use in the year of investigation.
Compared with controls: P = .03. Unless indicated otherwise, differences were not significant (P > .06).
Compared with controls: P = .06.
Deep White Matter Hyperintensities
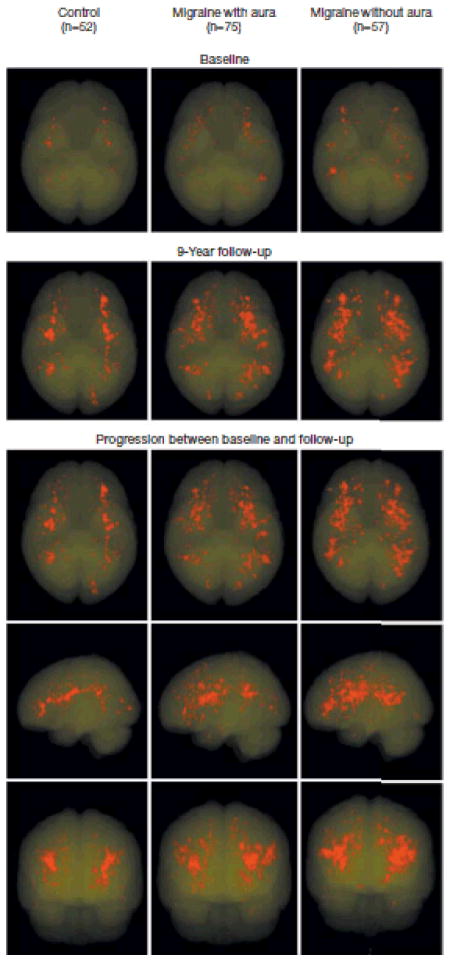
There were no differences in baseline and follow-up white matter hyperintensities between men in the migraine group and those in the control group (TABLE 2). However, among women, both at baseline and follow up, deep white matter hyperintensity volume was higher in the migraine group than in the control group (baseline: 0.02mLvs 0.00 mL; P=.009; follow-up: 0.09mL vs 0.04 mL; P=.04). Women in the migraine group also had a higher median increase in volume of deep white matter hyperintensities(mL), as well as a higher incidence of progression (defined as >0.01 mL) than women in the control group (yes/no,≥0.01 mL)(77% vs 60%; P=.02). The incidence of deep white matter hyperintensity progression was highest among women with migraine without aura (83%; Table 2). In multivariate logistic regression analyses involving only women, migraine was independently associated with deep white matter hyperintensity progression (adjusted odds ratio [OR], 2.1; 95%CI, 1.0–4.1; P=.04; TABLE 3). Similarly, women in the migraine group had a higher incidence of high progression than women in the control group (23%vs 9%; P=.03; Table 2). Hypertension was not associated with a higher incidence of white matter hyperintensity progression (P=.06). Interaction terms for hypertension (P=.90) and diabetes (P=.60) were not significant. Further exploratory analyses showed no association of the number of migraine attacks, migraine attack duration, migraine frequency, type of attack, or migraine therapy with lesion progression (eTable 2). The increase in total deep white matter hyperintensity volume among women with migraine was related to an increased number of new lesions rather than an increase in the size of preexisting lesions. The mean size of individual hyperintensities at follow-up did not differ between groups (P=.97). Participants in the migraine group had a higher incidence of 10 or more new lesions among 43 of 145 participants (30%) vs 5 of 57 in the control group (9%) (adjusted OR, 3.5; 95% CI, 1.3–9.6; P=.01).Among women with migraine, deep white matter hyperintensities were more diffusely distributed in the deep white matter than among controls (FIGURE 2).
Table 2.
Prevalence and Progression of Infarct and Deep White Matter and Infratentorial Hyperintensities
| Controls (n = 83) | Migraine Headache (n = 203) | P Valuea | Migraine Headache
|
P Valuea | ||
|---|---|---|---|---|---|---|
| Without Aura (n = 89) | With Aura (n = 114) | |||||
| Deep white matter hyperintensities | ||||||
| Men, No. (%) | 26 (31) | 58 (29) | .67 | 25 (28) | 33 (29) | >.99 |
|
| ||||||
| Lesion volume, median (IQR), mL | ||||||
| Baseline | 0.04 (0.00–0.20) | 0.02 (0.00–0.07) | .18 | 0.01 (0.00–0.08) | 0.02 (0.00–0.09) | .76 |
|
| ||||||
| 9-y Follow up | 0.14 (0.01–0.67) | 0.06 (0.08–0.34) | .36 | 0.05 (0.00–0.15) | 0.11 (0.01–0.42) | .47 |
|
| ||||||
| Difference | 0.08 (0.01–0.43) | 0.04 (0.00–0.29) | .31 | 0.04 (0.00–0.10) | 0.08 (0.01–0.31) | .47 |
|
| ||||||
| Lesion progression, No. (%)b | 21 (81) | 40 (69) | .30 | 15 (60) | 25 (76) | .26 |
|
| ||||||
| High progression, No. (%)c | 6 (23) | 12 (21) | .78 | 5 (20) | 7 (21) | >.99 |
|
| ||||||
| New lesions | ||||||
| Median (IQR) | 3 (1–11) | 3 (0–8) | .64 | 3 (0–5) | 4 (0–10) | .50 |
|
| ||||||
| ≥10, No. (%) | 8 (31) | 12 (21) | .41 | 4 (16) | 8 (24) | .53 |
|
| ||||||
| Mean volume, median (IQR) | 0.09 (0.01–0.05) | 0.02 (0.01–0.04) | .23 | 0.02 (0.01–0.07) | 0.02 (0.01–0.04) | .65 |
|
| ||||||
| Women, No. (%) | 57 (69)d | 145 (71) | .67 | 64 (72) | 81 (71) | >.99 |
|
| ||||||
| Lesion volume, median (IQR), mL | ||||||
| Baseline | 0.00 (0.00–0.04) | 0.02 (0.00–0.09) | .009 | 0.03 (0.00–0.12) | 0.0l (0.00–0.06) | .08 |
|
| ||||||
| 9-y Follow-up | 0.04 (0.00–0.19) | 0.09 (0.02–0.34) | .04 | 0.16 (0.02–0.43) | 0.05 (0.01–0.28) | .03 |
|
| ||||||
| Difference | 0.02 (0.00–0.14) | 0.05 (0.01–0.27) | .04 | 0.11 (0.01–036) | 0.04 (0.00–0.15) | .04 |
|
| ||||||
| Lesion progression, No. (%)b | 33 (60) | 112 (77) | .02 | 53 (83) | 59 (73) | .17 |
|
| ||||||
| High progression, No. (%) | 5 (9) | 33 (23) | .03 | 19 (30) | 14 (17) | .11 |
|
| ||||||
| New lesions | ||||||
| Median (IQR) | 1 (0–6) | 3 (0–11) | .04 | 1 (0–9) | 5 (0–16) | .10 |
|
| ||||||
| >10, No. (%) | 5 (9) | 43 (30) | .009 | 25 (39) | 18 (22) | .03 |
|
| ||||||
| Mean volume, median (IQR) | 0.02 (0.01–0.04) | 0.02 (0.01–0.03) | .97 | 0.02 (0.01–0.03) | 0.02 (0.01–0.04) | .59 |
|
| ||||||
| Infratentorial hyperintensities, No. (%) | ||||||
| Men | ||||||
|
| ||||||
| Prevalence | 3 (12) | 9 (16) | .75 | 4 (16) | 5 (15) | >.99 |
|
| ||||||
| Progressionb | 1 (4) | 5 (9) | .66 | 2 (8) | 3 (9) | >.99 |
|
| ||||||
| Women | ||||||
| Prevalence | 2 (4) | 30 (21) | .002 | 18 (28) | 12 (15) | .06 |
|
| ||||||
| Progressionb | 1 (2) | 21 (15) | .01 | 13 (20) | 8 (10) | .10 |
|
| ||||||
| Posterior circulation territory infarctlike lesions, No. (%)e | ||||||
| Baseline | 3 (4) | 11 (5) | .76 | 2 (2) | 9 (8) | .12 |
|
| ||||||
| 9-y Follow-up | 3 (4) | 18 (9) | .14 | 6 (7) | 12 (11) | .46 |
|
| ||||||
| New lesion | 0 | 10 (5) | .07 | 5 (6) | 5 (4) | .75 |
|
| ||||||
| Anterior circulation or basal ganglia infarctlike lesions (nonposterior circulation territory), No. (%) | ||||||
| Baseline | 8 (10) | 15 (7) | .63 | 6 (7) | 9 (8) | .79 |
|
| ||||||
| 9-y Follow-up | 11 (13) | 20 (10) | .41 | 12 (11) | 8 (9) | .82 |
|
| ||||||
| New lesions | 3 (4) | 5 (3) | .69 | 2 (2) | 3(3) | >.99 |
Abbbreviation: IQR, interquartile range,
P values are for difference between the control group and the migraine group and between those in the migraine group with and without aura.
Progression of deep white matter hyperintensities is defined as an increase in volume after 0 years (Δ between follow-up and baseline ≥0.01 mL); progression of intratentorial hyperintensities is defined as an increase in size, number, or both.
High progression of deep white mater hyperntensities defined as the upper 20th percentile of progression ditribution.
For analyses of deep white matter hyperintensity progression, 2 women in the control group were excluded (leaving n = 56), because of missing baseline volumes due to software failures during lesion segmentations. Visual comparison revealed no progression between baseline and follow-up for these 2 women.
The number of participants with 1 or more infarctlike lesions. Three of 10 participants who already had a posterior circulation territory infarctlike lesion at baseline developed additional lesions between scans.
Table 3.
Risk of Deep White Matter and Infratentorial Hyperintensities in Women by Migraine Statusa
| Controls (n = 57) | Migraine (n = 145) | P Value | Migraine Without Aura (n = 64) | Migraine With Aura (n = 81) | P value | |
|---|---|---|---|---|---|---|
| Deep white matter hyperintensities | ||||||
| Progression, No. (%)b | 33 (60)e | 112 (77) | 53 (83) | 59 (73) | ||
|
| ||||||
| OR (95% CI) | 1 [Reference] | 2.1 (1.0–4.1)f | .04 | 2.9 (1.2–6.7)f | 1.7 (0.8–3.5) | .23 |
|
| ||||||
| High progression, No. (%)c | 5 (9)e | 33 (23) | 19 (30) | 14 (17) | ||
|
| ||||||
| OR (95% CI) | 1 [Reference] | 2.3 (0.8–6.4) | .12 | 3.3 (1.1–9.9)f | 1.6 (0.5–5.0) | .12 |
|
| ||||||
| High increase in number, No. (%)d | 5 (9)e | 43 (30) | 25 (39) | 18 (22) | ||
|
| ||||||
| OR (95% CI) | 1 [Reference] | 3.5 (1.3–9.6)f | .01 | 5.3 (1.8–15.4)f | 2.4 (0.8–7.0) | .04 |
|
| ||||||
| Infratentorial hyperintensities | ||||||
| Prevalence, No. (%) | 2 (4) | 30 (21) | 18 (28) | 12 (15) | ||
|
| ||||||
| OR (95% CI) | 1 [Reference] | 6.5 (1.5–28.3) | .01 | 9.6 (2.1–44.1) | 4.4 (0.9–20.5) | .07 |
|
| ||||||
| Progression, No. (%)b | 1 (2) | 21 (15) | 13 (20) | 8 (10) | ||
|
| ||||||
| OR (95% CI) | 1 [Reference] | 7.7 (1.0–59.5) | .05 | 11.5 (1.4–92.9)f | 5.0 (0.6–41.7) | .10 |
Abbreviation: OR, odds ratio.
OR (95% CI) are adjusted for age, education, hypertension, and diabetes.
Progression is defined as an increase in volume after 0 years (Δ between follow up and baseline ≥ mL); progression of intratentorial hyperintensities is defined as an increase in size, number, or both.
Progression is defined as an upper 20th percentile of progression
High increase in number of lesions is defined as 10 or more new lesions, which reflects the upper 20th percentile of the distributions of lesions count.
For analyses of deep white matter hyperintensity progression, 2 women in the control group were excluded (leaving n = 56), because of missing baseline volumes due to software failures during lesion segmentations. Visual comparison revealed no progression between baseline and follow-up for these 2 women.
Compared with controls: P <.05
Figure 2.
Geographical Location of All Individual Deep White Matter Hyperintensities Projected on Transparent 3-Dimensional Maps After Normalization of the Individual Magnetic Resonance Scans With Segmented Lesions to Standard Montreal Neurological Institute Space
The upper 2 rows display hyperintensities per study group at baseline and follow-up separately; the lower rows show the difference (ie, progression) between baseline and follow-up in 3 directions. For visualization purposes, lesions are displayed after correction for group size, by adjusting their transparency level with a factor 0.69 for women in the migraine group with migraine with aura (n=52/n=75) and 0.91 for female participants with migraine without aura (n=52/n=57), using women in the control group as a reference.
Periventricular White Matter Hyperintensities
Progression of periventricular white matter hyperintensities did not differ between participants with migraine and controls. There was no association of sex, aura status, or migraine frequency with progression.
Infratentorial Hyperintensities
The prevalence of infratentorial hyperintensities at follow-up was 21% among women with migraine and 4% among controls (adjusted OR, 6.5; 95% CI, 1.5–28.3; P=.01; Table 3). Progression of infratentorial hyperintensities was not significantly higher among women with migraine (15%) than women in the control group (2%; adjusted OR, 7.7; 95% CI, 1.0–59.5; P=.05; Table 3). There was no relationship between migraine aura and number or frequency of migraine attacks with progression of infratentorial hyperintensities. Among men there were no differences in infratentorial hyperintensity prevalence or progression.
Infarcts and Infarctlike Lesions
None of the infarctlike lesions present at baseline had disappeared. No significant association of migraine with new posterior circulation territory infarctlike lesions existed between groups (migraine group, 5% vs control group, 0%; P=.07; Table 2). Among participants in the migraine group, 18 (8.9%) with posterior circulation territory infarctlike lesions had a less favorable cardiovascular risk profile than the 185 participants (91.1%) without it. Those with infarctlike lesions were older (mean age, 62 vs 57 years; P = .006); had higher prevalences of clinically diagnosed stroke (22% vs 3%; P<.001) or hypertension (67% vs 33%; P=.005), and were more likely taking statins (39% vs 17%; P=.03) or platelet inhibitors (33% vs 6%; P<.001). There was no difference between groups for new non–posterior circulation territory infarctlike lesions (migraine group, 2.5% vs control group, 3.5%; P=.69; Table 2). Of those with infarcts, 21% of those in the control group vs none in the control group reported a history of clinical stroke (P=.10).
Cognitive Changes
There were no differences in cognitive functioning between groups at follow-up (mean composite Z score, migraine group, 1.2 vs control group, 0; adjusted P=.90; 95% CI, −2.0 to 2.0). At follow-up, deep white matter hyperintensity load was not associated with cognitive performance (mean composite Z score high load, −3.7 vs low load, 1.4; adjusted P=.07; 95% CI, −4.4 to 0.2; men and women were analyzed together, see also eTable 3 for original clinical scores of the separate subtest domains). Presence of migraine did not influence this association (adjusted P=.30; 95% CI, −2.0 to 2.1). Individuals with a high deep white matter hyperintensity load at baseline did not experience greater change in cognitive function at the 9-year follow-up than those without a high load at baseline (mean composite Z score, −0.5 vs 0.2; adjusted P=.4; 95% CI, −1.7 to 0.7). Similarly, there were no significant differences between groups with respect to tests of individual cognitive domains (eTable 3).
COMMENT
We prospectively evaluated associations of migraine with structure and function of the brain at the 9-year follow-up. Among men, we found no association of migraine with progression of MRI-measured brain lesions. Women in the migraine group had a higher prevalence and a greater increase of deep white matter hyperintensities than women in the control group. Although migraine was associated with a higher prevalence of infratentorial hyperintensities at follow up, there were no significant associations of migraine with progression of infratentorial hyperintensities or posterior circulation territory infarctlike lesions among women. In addition, the number of migraines, frequency of migraines, migraine severity, type of migraine, and migraine therapy were not associated with lesion progression. Increase in deep white matter hyperintensity volume was not significantly associated with poorer cognitive performance at follow-up. This study has several strengths, including the longitudinal study design, length of follow-up, the relatively well characterized cohort, use of standardized International Headache Society criteria–based diagnosis of migraine by headache experts, and sensitive and reproducible methods of MRI reading. The sensitive MRI techniques used allowed for a more detailed analysis of the brain, in particular the cerebellum. Approximately one-third of the original baseline population could not be reinvestigated. This may have introduced selection bias. However, there were no differences in baseline MRI parameters between participants and nonparticipants and there was no imbalance between the proportions and demographic and clinical characteristics of nonparticipating individuals with migraine and controls. Because of differences between the semi quantitative baseline reading of deep white matter hyperintensities and the current quantitative volume measurements that were not available for the nonresponders, additional imputation analyses to support the sensitivity of the current results could not be performed. An additional study limitation is that confidence intervals are wide (Table 3). The number of migraine attacks, frequency of migraines, migraine severity, type of migraine headaches, and migraine therapy were not associated with lesion progression. In contrast, our baseline data showed that more frequent migraine headaches were associated with a higher prevalence of MRI findings.4 However, our findings at baseline regarding frequency-related difference in MRI findings was most pronounced among those in the migraine group who were 50 years or younger and less so in older patients. Thus, with increasing age of the study population, when attacks generally diminish,1 other migraine disease-related conditions leading to white matter hyperintensities are possibly increasing, complicating the detection of migraine attack-related associations. A similar, age-dependent mechanism is also seen for the risk of stroke in participants with migraine, which is increased in young patients only.14,21 At older age, other risk factors such as hypertension may obscure or overcome any potential role of migraine. In the present case, we hypothesize there are at least 2 different types of vascular mechanisms that may cause structural brain changes in migraine: one, which is primarily related to attacks and mainly present at younger age, and another, which is probably ongoing as part of having the disease migraine. The observation of migrainous stroke, with stroke occurring during a migraine attack, would support the hypothesis that ischemia may occur during attacks.22 However, our finding that migraine was not significantly associated with progression of all evaluated types of brain lesions at the 9-year follow-up raises questions about the role of cerebral ischemia over time in people with migraine.21,23 Possible explanations for an association of migraine headache with structural brain changes include a chronic procoagulatory or proinflammatory state due to endothelial dysfunction24,25 or elevated homocysteine levels,26,27 or recurrent paradoxical (micro-) emboli due to right-to-left shunts.28 Increased incidence of brain lesions among people with migraine headaches and atherosclerotic risk factors such as hypertension, diabetes, or other cardiovascular risk factors is also possible, but we did not identify any significant interactions for hypertension or diabetes. A relation with headache in general7 cannot be excluded. Finally, sex differences seem to play an important role because progression of deep white matter hyperintensities was only found in women. This finding is in line with results from another study8 and consistent with the higher risk of brain infarcts in women with migraine.14 Our sample size was too small for a proper analysis of sex-related differential interaction between migraine and cardiovascular risk factors. Participants in the migraine group with posterior circulation territory infarctlike lesions, however, did have a less favorable cardiovascular risk profile than those without posterior circulation territory infarctlike lesions. Further research is needed to unravel the pathogenesis and relevance of migraine-related structural brain changes and their possible relation with ischemic events. White matter hyperintensities have been associated with cognitive deficits in the elderly29,30 and some studies found evidence for worse cognitive performance in individuals with migraine. 31–34 We tested memory, speed, and attention35 in all participants at baseline and follow-up and found no significant association between deep white matter hyperintensity volume and cognitive dysfunction. Most prior studies were conducted in older participants with larger deep white matter intensity volumes; this cohort is rather young with relatively little volume.7 In summary, in a community-based cohort followed up for 9 years, migraine was associated only with a higher incidence of deep white matter brain changes among women. There were no significant associations of migraine with progression of other brain lesions among women, and there were no associations of migraine headache with progression of any brain lesions among men. These findings raise questions about the role of migraine headaches with progression of cerebral vascular changes. The functional implications of MRI brain lesions in women with migraine and their possible relation with ischemia and ischemic stroke warrant further research.
Acknowledgments
Funding/Support: This work was supported by grants 1R01NS061382-01 from the National Institutes of Health, 2007B016 from the Netherlands Heart Foundation, and 903-52-291, VICI 918-56-602, and Spinoza 2009 from the Netherlands Organisation for Scientific Research, and 907-00-217 and Vidi 917-11-319 from the Intramural Research Program at the National Institute on Aging.
Role of the Sponsor: None of the funding bodies had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Study supervision: Palm-Meinders, Koppen, Launer, Terwindt, van Buchem, Ferrari, Kruit.
Disclaimer: The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health or the Netherlands Heart Foundation.
Online-Only Material: Author Video Interview and eTables 1 through 3 are available at http://www.jama.com. Additional Contributions: We thank the research students and MRtechnicians for their assistance in screening and care of participants in this study.
Author Contributions: Drs Palm-Meinders and Kruit had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Drs Palm-Meinders and Koppen contributed equally. Drs Ferrari and Kruit share last authorship. Study concept and design: Palm-Meinders, Koppen, Launer, van Buchem, Ferrari, Kruit. Acquisition of data: Palm-Meinders, Koppen, Bakkers, Hofman, Middelkoop, van Buchem, Kruit. Analysis and interpretation of data: Palm-Meinders, Koppen, Terwindt, Launer, Konishi, Moonen, van Lew, Middelkoop, van Buchem, Ferrari, Kruit. Drafting of the manuscript: Palm-Meinders, Koppen, Moonen, Middelkoop, van Buchem, Kruit. Critical revision of the manuscript for important intellectual content: Palm-Meinders, Koppen, Terwindt, Launer, Konishi, Bakkers, Hofman, van Lew, Middelkoop, van Buchem, Ferrari, Kruit. Statistical analysis: Palm-Meinders, Koppen, Launer, Moonen, Middelkoop. Obtained funding: Palm-Meinders, Koppen, van Buchem, Ferrari, Kruit. Administrative, technical, or material support: Palm-Meinders, Koppen, Bakkers, Hofman, van Lew, Middelkoop, van Buchem.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Ferrari reported receiving grants and consultancy or industry support from Almirall, Coherex, Colucid, Eisai, GlaxoSmithKline, Linde, MAP, Medtronic, Menarini, Merck, Minster, Pfizer, and St Jude, and independent support from the Netherlands Organisation for Scientific Research (NOW). Dr Terwindt reported receiving consultancy support from Merck, Janssen-Cilag, Almirall, and Menarini. Dr Koppen reported consultancy or industry support from Allergan, Benecke congres, Pfizer, and In circulation website. The other authors reported no financial disclosures.
References
- 1.Launer LJ, Terwindt GM, Ferrari MD. The prevalence and characteristics of migraine in a populationbased cohort: the GEM study. Neurology. 1999;53(3):537–542. doi: 10.1212/wnl.53.3.537. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari MD. Migraine. Lancet. 1998;351(9108):1043–1051. doi: 10.1016/S0140-6736(97)11370-8. [DOI] [PubMed] [Google Scholar]
- 3.Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(suppl 7):1–96. [PubMed] [Google Scholar]
- 4.Kruit MC, van Buchem MA, Hofman PA, et al. Migraine as a risk factor for subclinical brain lesions. JAMA. 2004;291(4):427–434. doi: 10.1001/jama.291.4.427. [DOI] [PubMed] [Google Scholar]
- 5.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Infarcts in the posterior circulation territory in migraine: the population-based MRI CAMERA study. Brain. 2005;128(Pt 9):2068–2077. doi: 10.1093/brain/awh542. [DOI] [PubMed] [Google Scholar]
- 6.Kruit MC, Launer LJ, Ferrari MD, van Buchem MA. Brain stem and cerebellar hyperintense lesions in migraine. Stroke. 2006;37(4):1109–1112. doi: 10.1161/01.STR.0000206446.26702.e9. [DOI] [PubMed] [Google Scholar]
- 7.Kurth T, Mohamed S, Maillard P, et al. Headache, migraine, and structural brain lesions and function: population based Epidemiology of Vascular Ageing-MRI study. BMJ. 2011;342:c7357. doi: 10.1136/bmj.c7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher AI, Gudmundsson LS, Sigurdsson S, et al. Migraine headache in middle age and late-life brain infarcts. JAMA. 2009;301(24):2563–2570. doi: 10.1001/jama.2009.932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dufouil C, de Kersaint-Gilly A, Besancçon V, et al. Longitudinal study of blood pressure and white matter hyperintensities: the EVA MRI Cohort. Neurology. 2001;56(7):921–926. doi: 10.1212/wnl.56.7.921. [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2010;341:c3666. doi: 10.1136/bmj.c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buyck JF, Dufouil C, Mazoyer B, et al. Cerebral white matter lesions are associated with the risk of stroke but not with other vascular events: the 3-City Dijon Study. Stroke. 2009;40(7):2327–2331. doi: 10.1161/STROKEAHA.109.548222. [DOI] [PubMed] [Google Scholar]
- 12.Vermeer SE, Hollander M, van Dijk EJ, Hofman A, Koudstaal PJ, Breteler MM Rotterdam Scan Study. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34(5):1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 13.van der Flier WM, van Straaten EC, Barkhof F, et al. Small vessel disease and general cognitive function in nondisabled elderly: the LADIS study. Stroke. 2005;36(10):2116–2120. doi: 10.1161/01.STR.0000179092.59909.42. [DOI] [PubMed] [Google Scholar]
- 14.Schürks M, Rist PM, Bigal ME, Buring JE, Lipton RB, Kurth T. Migraine and cardiovascular disease: systematic review and meta-analysis. BMJ. 2009;339:b3914. doi: 10.1136/bmj.b3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rey A. L’examin Clinique en Psychologie. Paris, France: Presses Universitaires de France; 1985. [Google Scholar]
- 16.Stroop J. Studies of interference in serial verbal reactions. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 17.Miller E. Verbal fluency as a function of a measure of verbal intelligence and in relation to different types of cerebral pathology. Br J Clin Psychol. 1984;23(pt 1):53–57. doi: 10.1111/j.2044-8260.1984.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 18.Van der Elst W, Van Boxtel MP, Van Breukelen GJ, Jolles J. Normative data for the Animal, Profession and LetterMNaming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. J Int Neuropsychol Soc. 2006;12(1):80–89. doi: 10.1017/S1355617706060115. [DOI] [PubMed] [Google Scholar]
- 19.Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32(3):234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- 20.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. New York, NY: Psychological Corp; 1981. [Google Scholar]
- 21.Kurth T, Diener HC. Current views of the risk of stroke for migraine with and migraine without aura. Curr Pain Headache Rep. 2006;10(3):214–220. doi: 10.1007/s11916-006-0048-5. [DOI] [PubMed] [Google Scholar]
- 22.Wolf ME, Szabo K, Griebe M, et al. Clinical and MRI characteristics of acute migrainous infarction. Neurology. 2011;76(22):1911–1917. doi: 10.1212/WNL.0b013e31821d74d5. [DOI] [PubMed] [Google Scholar]
- 23.Bigal ME, Kurth T, Santanello N, et al. Migraine and cardiovascular disease: a population-based study. Neurology. 2010;74(8):628–635. doi: 10.1212/WNL.0b013e3181d0cc8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee ST, Chu K, Jung KH, et al. Decreased number and function of endothelial progenitor cells in patients with migraine. Neurology. 2008;70(17):1510–1517. doi: 10.1212/01.wnl.0000294329.93565.94. [DOI] [PubMed] [Google Scholar]
- 25.Tietjen GE, Herial NA, White L, Utley C, Kosmyna JM, Khuder SA. Migraine and biomarkers of endothelial activation in young women. Stroke. 2009;40(9):2977–2982. doi: 10.1161/STROKEAHA.109.547901. [DOI] [PubMed] [Google Scholar]
- 26.Schurks M, Rist PM, Kurth T. MTHFR 677C_T and ACE D/I polymorphisms in migraine: a systematic review and meta-analysis. Headache. 2010;50(4):588–599. doi: 10.1111/j.1526-4610.2009.01570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scher AI, Terwindt GM, Verschuren WM, et al. Migraine and MTHFR C677T genotype in a populationbased sample. Ann Neurol. 2006;59(2):372–375. doi: 10.1002/ana.20755. [DOI] [PubMed] [Google Scholar]
- 28.Schwedt TJ, Demaerschalk BM, Dodick DW. Patent foramen ovale and migraine: a quantitative systematic review. Cephalalgia. 2008;28(5):531–540. doi: 10.1111/j.1468-2982.2008.01554.x. [DOI] [PubMed] [Google Scholar]
- 29.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27(8):1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 30.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128(Pt 9):2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 31.Le Pira F, Lanaia F, Zappalà G, et al. Relationship between clinical variables and cognitive performances in migraineurs with and without aura. Funct Neurol. 2004;19(2):101–105. [PubMed] [Google Scholar]
- 32.Meyer JS, Thornby J, Crawford K, Rauch GM. Reversible cognitive decline accompanies migraine and cluster headaches. Headache. 2000;40(8):638–646. doi: 10.1046/j.1526-4610.2000.040008638.x. [DOI] [PubMed] [Google Scholar]
- 33.Mulder EJ, Linssen WH, Passchier J, Orlebeke JF, de Geus EJ. Interictal and postictal cognitive changes in migraine. Cephalalgia. 1999;19(6):557–565. doi: 10.1046/j.1468-2982.1999.019006557.x. discussion 541. [DOI] [PubMed] [Google Scholar]
- 34.Waldie KE, Hausmann M, Milne BJ, Poulton R. Migraine and cognitive function: a life-course study. Neurology. 2002;59(6):904–908. doi: 10.1212/wnl.59.6.904. [DOI] [PubMed] [Google Scholar]
- 35.O’Bryant SE, Marcus DA, Rains JC, Penzien DB. The neuropsychology of recurrent headache. Headache. 2006;46(9):1364–1376. doi: 10.1111/j.1526-4610.2006.00579.x. [DOI] [PubMed] [Google Scholar]