Abstract
Introduction
The Roux-en-Y gastric bypass (RYGB) has been employed for more than 3 decades. However, there are no normative data to aid the bariatric surgeon in assessing the adequacy of weight loss at each postoperative visit.
Objective
To construct nomograms to aid in assessment of weight loss.
Setting
University Hospital, United States
Methods
We used data prospectively collected from 1216 patients who underwent RYGB at Duke University between April 2000 and September 2007. Percent excess weight loss (EWL) was determined for each follow-up visit (1, 3, 6, 12, and 36 months). EWL velocity was also determined using postoperative data collected at 1 and 3 month visits. Multivariate analysis was used to determine predictive factors that influence long term results.
Results
At 12 months follow up, the majority of patients, especially those in the 1st and 4th quartiles (p=0.01) continue to be in the same weight loss quartile where they were initially classified at 1 month postoperatively. The positive and negative predictive values for 1st quartile EWL at 1 month resulting in 1st quartile EWL at 12 months was 39% and 81%, respectively. The multivariate analysis indicated that sex, preoperative body mass index (BMI), EWL at 1 month, and EWL velocity were statistically significant predictors of EWL at 12 months.
Conclusions
We are the first group to determine that weight loss performance in the early period is a significant predictor of long term outcome. The clinical utility of the weight loss chart is to identify underperformers early postoperatively and potentially improve outcomes.
Keywords: roux-en-y gastric bypass, weight loss charts, outcomes, prediction model
Introduction
According to a study published in 2004 as a part of the National Health and Nutrition Examination Survey (NHANES), two-thirds of adults in the US are overweight or obese (1). The Centers for Disease Control reported a tremendous rate of increase in the incidence of obesity (body mass index (BMI) >=30) across the country from 1985 through 2010 (2).
Many of the chronic medical conditions and common cancers plaguing the US today are directly caused by or exacerbated by obesity (3, 4). It has also been shown that all cause mortality increases with increasing BMI over 30 (3, 4). Surgical options for weight loss were developed and refined because medical weight loss programs and dieting have a high recidivism rate, often with weight regain in excess of previous weight (5).
The Roux-en-y gastric bypass (RYGB) and adjustable gastric band are the two most commonly performed bariatric procedures in the U.S. Weight loss after RYGB is very rapid but tends to plateau after about 12-18 months following surgery. Percent excess weight loss (EWL) following RYGB is generally very good between 60-80% at one year and between 50-75% at 3 or more years post-operatively (6-11).
Some weight regain is generally seen overtime and may be secondary to maladaptive eating behaviors of the patient, pouch dilatation, large gastrojejunal stoma, gastrogastric fistula, or gut hormonal changes (12-15). There is still a lack of knowledge about factors that influence magnitude of postoperative weight loss and weight regain eventually.
Normative data for weight loss at each postoperative time point has not been specifically reported. As such, the limits of satisfactory and unsatisfactory weight loss for any given postoperative visit are also unknown. The first 12 to 18 months following RYGB is a golden opportunity to maximize weight loss and therefore monitoring weight loss during this period is of paramount importance. Like growth charts for children, identifying when patients have fallen below the normal curve for weight loss during the first year may provide an opportunity to intervene to maximize weight loss.
The purpose of this study was to determine the normal weight loss patterns for patients at each of the postoperative visits at 1, 3, 6, 12, and 36 months following RYGB. As a secondary aim, we tested the hypothesis that weight loss in the early postoperative period was predictive of long term weight loss.
Methods
The Duke University Center for Metabolic and Weight-loss Surgery database was queried for all patients undergoing RYGB from April 2000 through September 2007. Patients were included if they were >18 years of age and had follow-up at 4 weeks and 12 or 36 months postoperatively. Patients undergoing revisional bariatric surgery were excluded. Demographic data was collected including sex, age, preoperative weight (determined on the day of surgery), preoperative BMI, excess weight, and insulin or non-insulin dependent diabetes status.
At Duke University the vast majority of patient undergo a laparoscopic antecolic, antegastric lesser curve based roux-en-y gastrojejunostomy unless there would be tension on the roux limb necessitating a retrocolic, retrogastric approach. The gastrojejunostomy is performed via a linear stapled technique and hand-sewn closure of the common enterotomy. Postoperatively, all patients are placed on a “stage II” liquid protein diet for 3 weeks and are then advanced to “stage III” soft diet at 3 weeks, and later to “stage IV” small portion regular diet at 3 months.
Body weight loss was measured at 1, 3, 6, 12, 36 months postoperatively and the percent EWL was calculated. We then established normative categories for percent EWL based on quartiles and patient's preoperative BMI < 50 or ≥ 50 (16). Ideal body weight was calculated by the Devine method (17). Weight loss velocity was calculated as the difference between the percent EWL at 3 and 1 months divided by 10 weeks and is reported as %EWL/week.
The data are presented as mean ± standard deviation for continuous variables, and as counts or proportions (%) for categorical variables. Continuous outcomes were evaluated by unpaired t-tests. Discrete variables were analyzed with Pearson's chi-square test or Fisher's exact test. Pearson correlation was used to examine the correlation coefficient for each patient between EWL at 1 month and EWL at 12-36 months. Multivariate logistic regression analysis was used to identify patients' variables that were predictive of long term outcome. A receiver operating characteristic (ROC) curve was plotted in order to choose the best cut-off point for the variable excess weight loss velocity. The optimal cut-off value was determined by maximizing the Youden's index, i.e. the true positive rate (sensitivity) minus the false positive rate (1-specificity). The SPSS statistical software program (version 15.0; SPSS, Chicago, IL) was used for all analyses. All tests were 2-tailed. P values of less than .05 were considered to indicate statistical significance.
Results
The database query revealed 1216 patients that met inclusion criteria. There were 1051 women (86%). The mean patient age was 46 (range 21-69), mean preoperative BMI was 49, and 76% of men (n=125) and 58% of women (n=606) had a preop BMI <50. There were 317 patients with diabetes, 21% were insulin dependent preoperatively.
The average EWL at 1 year for women, men, and all patients were 63%, 70%, and 64%, respectively and at 3 years was 64%, 74%, 64%, respectively. At 36 months follow up (compared with the 12 months follow up), 53% of patients experience a modest weight regain (mean EWL regain: 8.7%). Figure 1 illustrates that the quartile attribution for EWL at the 1 month visit was significantly conserved throughout the first year. For example, patients in the 1st quartile (lowest postoperative weight loss) at 1 month were more likely to remain in the lowest quartile at 12 months (PPV=39%, p<0.001). Patients in the fourth quartile (greatest EWL) at 1 month were more likely to remain in the 3rd and 4th quartiles at 1 year. Patients who were not in the 1st quartile at 1 month were unlikely to have poor weight loss at 1 year (NPV= 81%, p<0.001). The Pearson correlation for each patient between EWL at 1 month and EWL at both 12 and 36 months was significant (r=0.36, p=0.0001 for EWL at 12 months; r=0.2, p=0.001 for EWL at 36 months).
Figure 1.
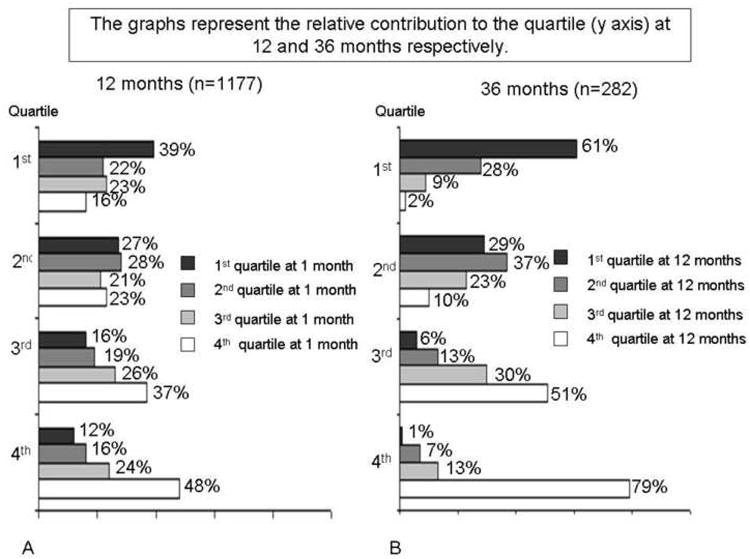
Relative contribution to the quartile at 12 and 36 months respectively.
Multivariate analysis of the variables sex, preoperative BMI, and 1st quartile EWL at 1 month were statistically significant predictors of quartile distribution for EWL at 12 months. The presence of insulin dependent or non-insulin dependent diabetes was not a significant predictor of poor weight loss. Age was also not a statistically significant variable. The relative contribution of each significant variable to the model revealed that 1st quartile EWL at 1 month carried the greatest influence, followed by preoperative BMI, and sex.
Weight loss charts were constructed for patients with BMI<50 and BMI>=50. Figures 2 and 3 show the percentile distribution for EWL at each of the postoperative time points for all patients and for patients stratified by preoperative BMI. Although sex was a significant predictive variable for long term outcome, only 14% of the patients were men (n=165) and thus the data was not stratified by sex.
Figure 2.
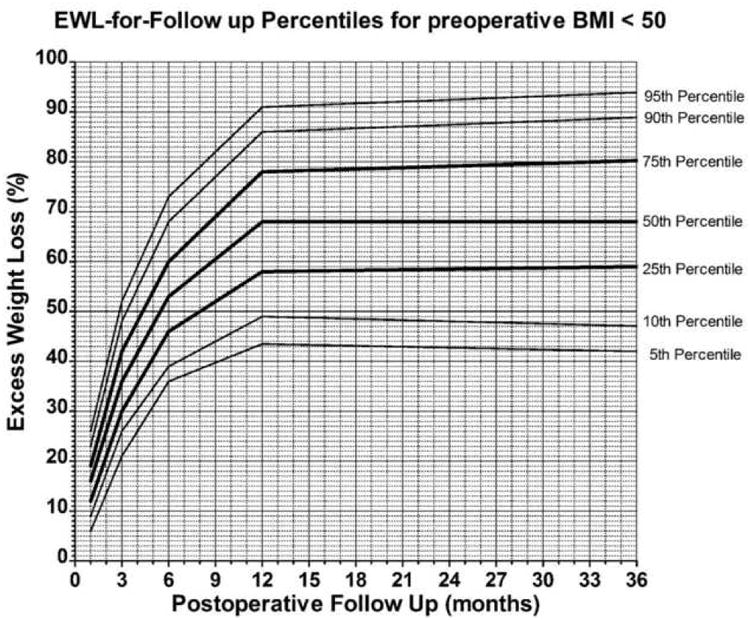
Weight loss chart for preoperative body mass index (BMI) < 50. Total number of patients at 1, 3, 6, 9, 12 and 36 months are respectively 731, 645, 690, 706, 168.
Figure 3.
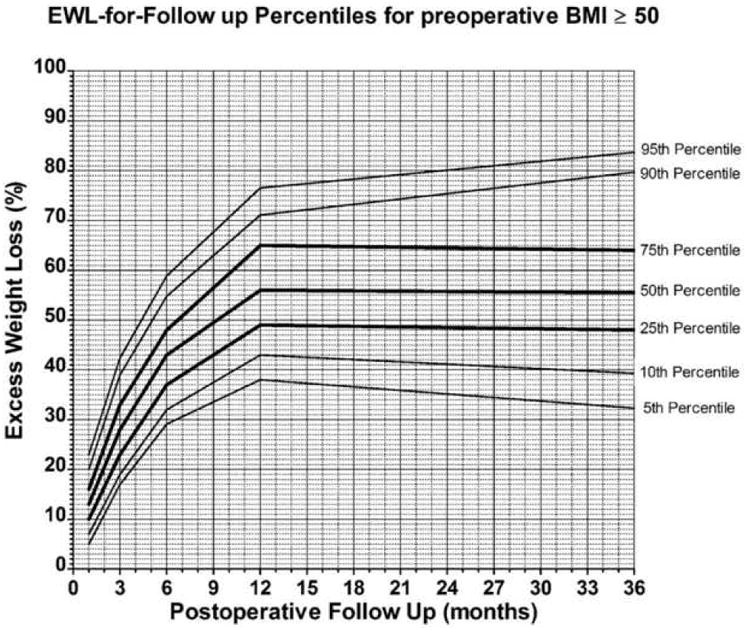
Weight loss chart for preoperative body mass index (BMI) ≥ 50. Total number of patients at 1, 3, 6, 9, 12 and 36 months are respectively 485, 434, 464, 471, 114.
The weight loss velocity in the early postoperative period was calculated as the difference between EWL at 3 and 1 months divided by 10 weeks (represented as %EWL/week). After adjusting for covariates, the multivariate analysis indicated EWL velocity as an independent statistically predictive variable for long term EWL (r=0.51, p=0.0001 for EWL at 12 months; r=0.35, p=0.0001 for EWL at 36 months). A Receiver Operator Curve (ROC) was constructed to determine the point at which the weight loss velocity predicted 2nd, 3rd, or 4th quartile EWL at 12 months. This inflection point corresponds to a weight loss velocity of 2% EWL / week (Sn=44%, Sp=90%). Ninety percent of patients with a weight loss velocity greater than 2%/week, remained in the 2nd, 3rd, or 4th quartile for EWL at 12 months. This cutoff value for weight loss velocity should be used only to monitor weight loss occurring during the initial 14 week period postoperatively.
Discussion
Our initial goal was to define normative data and develop weight loss charts for patients after RYGB. The purpose was to develop a tool to aid the bariatric surgeon gauge a patient's success during each postoperative visit. Once a patient has been identified as being at risk of poor weight loss, as determined by 1st quartile weight loss, then factors that affect weight loss can be identified and modified.
One unexpected finding of our study was to observe patients staying on the same weight loss trajectory overtime, i.e. there is a tendency for a patient to remain in or near their initial EWL quartile. This is particularly true with patients in the 1st (poor performers) and 4th quartiles (top performers) at the first postoperative visit. Patients in the 2nd and 3rd quartiles had great variability in their final EWL quartile at 12 months. However, if a patient was in the 2nd, 3rd, or 4th quartile for EWL at the 1 month follow-up, they were unlikely to end-up in the 1st quartile for EWL at 12 months (NPV=81%). The likelihood that a patient in the 1st quartile for EWL at 1 month would remain there was 39%.
Most authors have defined weight loss success after bariatric surgery as the loss of at least 50% of EBW (18). The upper value for 1st quartile EWL at one year was 53%, confirming inadequate weight loss in this group. The normative data allows the surgeon to identify patients early who are at risk of having the operation “fail” for them. Early identification allows early intervention and may allow improved outcomes long term.
Assessment of the therapeutic effect of any intervention is also important. Patients can be identified at the first postoperative visit as being at risk for poor weight loss long term. Interventions can then be selected such as indentifying the need for increased physical activity (19), assessing dietary infringements or maladaptive eating behaviors such as snacking or grazing (20), and then instituting changes. The weight loss velocity then allows the bariatric surgeon to reassess the efficacy of that intervention also early in the weight loss period (between weeks 4 and 14 postoperative). A weight loss velocity >2% EWL per week is indicative of a 90% likelihood of moving out of the 1st quartile for EWL at 12 months.
Of the variables analyzed, 1st quartile for EWL at 1 month carried the greatest impact on the model, followed by preoperative BMI and then gender. We then developed normative data for weight loss based on a patient's preoperative BMI <50 and >=50, as a patient with a higher BMI has a greater excess weight and thus likely a slower and ultimately lower EWL at 12 months. However, patients can still be identified within the preoperative BMI groups as being at risk for poor weight loss compared to the group itself.
Despite reports showing that the presence of diabetes results in less weight loss over time, our analysis did not show diabetes to be a statistically significant predictor of EWL quartile at 12 months (21-23). Additionally, age was also not found to be a significant predictor of weight loss, in concordance with previous reports (24-28).
Studies have shown that patients who follow-up have better long term weight loss (29, 30). Additionally, programs that incorporate dietary education and evaluation, exercise program, behavior modification and support groups have better outcomes following bariatric surgery. One way to perhaps rescue patients at risk for poor weight loss long term would be to have more intensive follow-up early to identify barriers to good exercise habits, identify eating habits or food choices that may be counterproductive, and provide continued peer support and assess adequacy of current treatment regimens of depression and anxiety (31-33). Of course, after any intervention there needs to be reassessment of the effects of the intervention and additional changes as needed.
There are several limitations of this study. The generalization of our normative data for monitoring weight loss in the general population of patients undergoing RYGB can be challenged considering that there is a wide range of reported outcomes in the literature (11). However, our large simple size reduces a potential generalization bias and is one of the strength of our study. Another approach to overcome this limitation will be to design a larger scale multi-institutional study where quartiles distribution could reflect more accurately the real population outcomes. These data are also only relevant for the RYGB and could not be applied to other procedures like adjustable gastric banding, sleeve gastrectomy or biliopancreatic diversion with or without duodenal switch. Another limitation was the number of male patients included in the study. This small sample did not allow us to develop weight loss charts based on sex despite the fact that this was a significant predictive variable for long term outcome. We also acknowledge that at 3 years follow up, our sample size was significantly smaller than the 12 month time point.
Conclusion
We have developed weight loss charts stratified by preoperative BMI based on normative data from over 1200 patients following RYGB. This data can be used to identify patients at risk of poor weight loss long term and allows an opportunity for early intervention to possibly change their weight loss trajectory. The weight loss velocity can be a quick reference to assess success of an intervention in the early postoperative period and allow for modification of any intervention before the golden year for weight loss following RYGB is completed.
Acknowledgments
We would like to thank the following individuals for their contribution to the study: James Alexander; Aurora Pryor, MD; Eric DeMaria, MD; John Grant, MD; Ross McMahon, MD.
Dr. Torquati is supported by a National Institute of Health Grant: K23DK075907
Footnotes
Conflict of Interest Statement: All authors do not have any conflict of interest with an institution or product that is mentioned in the manuscript and/or is important to the outcome of the study presented.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999-2002. Jama. 2004;291:2847–50. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 2.CDC. [Accessed June 2012]; http://www.cdc.gov/nccdphp/dnpa/obesity/trend/maps/index.htm.
- 3.Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults--The Evidence Report. 2. Vol. 6. National Institutes of Health. Obesity research; 1998. pp. 51S–209S. [PubMed] [Google Scholar]
- 4.Overweight, obesity, and health risk. National Task Force on the Prevention and Treatment of Obesity. Arch Intern Med. 2000;160:898–904. doi: 10.1001/archinte.160.7.898. [DOI] [PubMed] [Google Scholar]
- 5.Robison JI, Hoerr SL, Strandmark J, Mavis B. Obesity, weight loss, and health. J Am Diet Assoc. 1993;93:445–9. doi: 10.1016/0002-8223(93)92293-7. [DOI] [PubMed] [Google Scholar]
- 6.Hickey MS, Carey JO, Azevedo JL, et al. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. The American journal of physiology. 1995;268:E453–7. doi: 10.1152/ajpendo.1995.268.3.E453. [DOI] [PubMed] [Google Scholar]
- 7.Wittgrove AC, Clark GW. Laparoscopic gastric bypass, Roux-en-Y- 500 patients: technique and results, with 3-60 month follow-up. Obesity surgery. 2000;10:233–9. doi: 10.1381/096089200321643511. [DOI] [PubMed] [Google Scholar]
- 8.Higa KD, Boone KB, Ho T. Complications of the laparoscopic Roux-en-Y gastric bypass: 1,040 patients--what have we learned? Obesity surgery. 2000;10:509–13. doi: 10.1381/096089200321593706. [DOI] [PubMed] [Google Scholar]
- 9.Higa KD, Ho T, Boone KB. Laparoscopic Roux-en-Y gastric bypass: technique and 3-year follow-up. J Laparoendosc Adv Surg Tech A. 2001;11:377–82. doi: 10.1089/10926420152761905. [DOI] [PubMed] [Google Scholar]
- 10.DeMaria EJ, Sugerman HJ, Kellum JM, Meador JG, Wolfe LG. Results of 281 consecutive total laparoscopic Roux-en-Y gastric bypasses to treat morbid obesity. Annals of surgery. 2002;235:640–5. doi: 10.1097/00000658-200205000-00005. discussion 5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. Jama. 2004;292:1724–37. doi: 10.1001/jama.292.14.1724. [DOI] [PubMed] [Google Scholar]
- 12.Faria SL, de Oliveira Kelly E, Lins RD, Faria OP. Nutritional Management of Weight Regain After Bariatric Surgery. Obesity surgery. 2008 doi: 10.1007/s11695-008-9610-z. [DOI] [PubMed] [Google Scholar]
- 13.Dapri G, Cadiere GB, Himpens J. Laparoscopic Placement of Non-Adjustable Silicone Ring for Weight Regain After Roux-en-Y Gastric Bypass. Obesity surgery. 2009 doi: 10.1007/s11695-009-9807-9. [DOI] [PubMed] [Google Scholar]
- 14.Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: a significant 20% complication related to PYY. Nutrition. 2008;24:832–42. doi: 10.1016/j.nut.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 15.Faria SL, Kelly E, Faria OP. Energy Expenditure and Weight Regain in Patients Submitted to Roux-en-Y Gastric Bypass. Obesity surgery. 2009 doi: 10.1007/s11695-009-9842-6. [DOI] [PubMed] [Google Scholar]
- 16.Pontiroli AE, Pizzocri P, Librenti MC, et al. Laparoscopic adjustable gastric banding for the treatment of morbid (grade 3) obesity and its metabolic complications: a three-year study. The Journal of clinical endocrinology and metabolism. 2002;87:3555–61. doi: 10.1210/jcem.87.8.8708. [DOI] [PubMed] [Google Scholar]
- 17.Shah B, Sucher K, Hollenbeck CB. Comparison of ideal body weight equations and published height-weight tables with body mass index tables for healthy adults in the United States. Nutr Clin Pract. 2006;21:312–9. doi: 10.1177/0115426506021003312. [DOI] [PubMed] [Google Scholar]
- 18.Livhits M, Mercado C, Yermilov I, et al. Behavioral factors associated with successful weight loss after gastric bypass. Am Surg. 2010;76:1139–42. [PubMed] [Google Scholar]
- 19.Hatoum IJ, Stein HK, Merrifield BF, Kaplan LM. Capacity for physical activity predicts weight loss after Roux-en-Y gastric bypass. Obesity (Silver Spring) 2009;17:92–9. doi: 10.1038/oby.2008.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colles SL, Dixon JB, O'Brien PE. Grazing and loss of control related to eating: two high-risk factors following bariatric surgery. Obesity (Silver Spring) 2008;16:615–22. doi: 10.1038/oby.2007.101. [DOI] [PubMed] [Google Scholar]
- 21.Carbonell AM, Wolfe LG, Meador JG, Sugerman HJ, Kellum JM, Maher JW. Does diabetes affect weight loss after gastric bypass? Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2008;4:441–4. doi: 10.1016/j.soard.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Ma Y, Pagoto SL, Olendzki BC, et al. Predictors of weight status following laparoscopic gastric bypass. Obesity surgery. 2006;16:1227–31. doi: 10.1381/096089206778392284. [DOI] [PubMed] [Google Scholar]
- 23.Perugini RA, Mason R, Czerniach DR, et al. Predictors of complication and suboptimal weight loss after laparoscopic Roux-en-Y gastric bypass: a series of 188 patients. Arch Surg. 2003;138:541–5. doi: 10.1001/archsurg.138.5.541. discussion 5-6. [DOI] [PubMed] [Google Scholar]
- 24.Papasavas PK, Gagne DJ, Kelly J, Caushaj PF. Laparoscopic Roux-En-Y gastric bypass is a safe and effective operation for the treatment of morbid obesity in patients older than 55 years. Obesity surgery. 2004;14:1056–61. doi: 10.1381/0960892041975541. [DOI] [PubMed] [Google Scholar]
- 25.Dunkle-Blatter SE, St Jean MR, Whitehead C, et al. Outcomes among elderly bariatric patients at a high-volume center. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2007;3:163–9. doi: 10.1016/j.soard.2006.12.004. discussion 9-70. [DOI] [PubMed] [Google Scholar]
- 26.Gomez V, Riall TS, Gomez GA. Outcomes in bariatric surgery in the older patient population in Texas. J Surg Res. 2008;147:270–5. doi: 10.1016/j.jss.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Trieu HT, Gonzalvo JP, Szomstein S, Rosenthal R. Safety and outcomes of laparoscopic gastric bypass surgery in patients 60 years of age and older. Surgery for obesity and related diseases: official journal of the American Society for Bariatric Surgery. 2007;3:383–6. doi: 10.1016/j.soard.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Alkoraishi AS, Saltzman E, Rand W, Shikora SA. Does age affect outcome of gastric bypass surgery? Curr Surg. 2000;57:502. doi: 10.1016/s0149-7944(00)00334-2. [DOI] [PubMed] [Google Scholar]
- 29.Yanovski SZ, Yanovski JA. Obesity. N Engl J Med. 2002;346:591–602. doi: 10.1056/NEJMra012586. [DOI] [PubMed] [Google Scholar]
- 30.Barakat HA, McLendon VD, Marks R, Pories W, Heath J, Carpenter JW. Influence of morbid obesity and non-insulin-dependent diabetes mellitus on high-density lipoprotein composition and subpopulation distribution. Metabolism: clinical and experimental. 1992;41:37–41. doi: 10.1016/0026-0495(92)90188-g. [DOI] [PubMed] [Google Scholar]
- 31.Livhits M, Mercado C, Yermilov I, et al. Exercise following bariatric surgery: systematic review. Obesity surgery. 2010;20:657–65. doi: 10.1007/s11695-010-0096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook CM, Edwards C. Success habits of long-term gastric bypass patients. Obesity surgery. 1999;9:80–2. doi: 10.1381/096089299765553872. [DOI] [PubMed] [Google Scholar]
- 33.Herpertz S, Kielmann R, Wolf AM, Hebebrand J, Senf W. Do psychosocial variables predict weight loss or mental health after obesity surgery? A systematic review Obesity research. 2004;12:1554–69. doi: 10.1038/oby.2004.195. [DOI] [PubMed] [Google Scholar]


