Abstract
Objective
To report the use of inspiratory muscle strength training (IMST) to treat repeated ventilatory insufficiency in a child with nemaline myopathy (NM) who underwent cardiac and renal transplantation.
Design
Case report.
Setting
Pediatric intensive care unit of a tertiary care university teaching hospital.
Intervention
IMST was provided five days weekly for two weeks, accompanied by progressive weaning from non-invasive ventilation.
Measurements and Main Results
Maximal inspiratory pressure (MIP) increased from −36.7 cm H2O to −77.8 cm H2O, accompanied by improved inspiratory flow, volume, pressure activation and power. During the training period, the patient weaned from continuous non-invasive ventilatory assist to her pre-operative level of ventilatory function.
Conclusions
Inspiratory muscle training may be a beneficial component of care for children with NM who experience acute ventilatory insufficiency.
Keywords: Myopathy, nemaline; Respiratory insufficiency; Muscle weakness; Respiratory muscles; Strength training; Organ transplantation
Introduction
Nemaline myopathy (NM) is a congenital muscular disease characterized by cytosolic accumulation of thin-filament protein aggregates, resulting in the appearance of electron-dense rods in affected sarcomeres (1). Thin filament myopathies such as NM have been associated with impaired myofiber regeneration, disrupted calcium homeostasis, and altered contraction speed (2, 3). Clinically, symptoms of NM include stable or slowly progressive weakness of the facial, respiratory and proximal appendicular muscles (3). The type of genetic variation does not reliably predict disease course (4). Disease presentation may vary from a severe congenital form, marked by lack of spontaneous movement, respiratory failure and early mortality, to adult-onset disease, with comparatively mild proximal and respiratory weakness (5).
In patients with NM, respiratory muscle weakness can lead to symptoms including impaired central respiratory drive leading to sleep apnea (6), pulmonary restriction due to chest wall defects, progressive scoliosis, and inspiratory muscle weakness, or a combination of deficits (5). Greater numbers of nemaline rods in the diaphragm and accessory respiratory muscles have been reported in patients with ventilatory insufficiency (4). Progressive ventilatory insufficiency has been associated with early mortality (5). Despite the presence of histological and functional adaptations of respiratory muscle in NM, specific inspiratory strength training strategies have not been previously reported.
The purpose of this case report is to illustrate the use of inspiratory muscle strength training (IMST) to increase inspiratory performance in an adolescent with NM affecting cardiorespiratory function, recent organ transplantation, and delayed post-operative ventilatory recovery. We present this case as evidence that children with NM may be able to improve inspiratory performance with moderate-intensity training.
Case Report
The patient was a 16 year-old female diagnosed with NM as a young child, with moderate impairment of respiratory, trunk and proximal extremity muscle strength. Prior to organ transplantation, the child was ambulatory throughout the community. In addition to NM, her medical history included end-stage dilated cardiomyopathy, severe restrictive lung disease (FEV1 33% predicted, FVC 31% predicted), a several-year history of sleep apnea requiring the nighttime use of bilevel positive airway pressure ventilation (BiPAP), hypertension, and end-stage renal disease, secondary to focal segmental glomerulosclerosis (FSGS). She required hemodialysis for two years prior to transplantation. The girl was admitted to the hospital for orthotopic heart transplantation and concurrent cadaveric renal transplantation. The post-operative clinical course was remarkable for acute tubular necrosis of the donor kidney necessitating temporary renal replacement therapy, and recurrent episodes of ventilatory failure. Although the patient was extubated on postoperative day (POD) #1, she required use of continuous BiPAP to maintain ventilatory homeostasis. Her initial physical therapy evaluation on POD #2 revealed moderate generalized weakness. The patient developed recurrent ventilatory failure and required intubation during the first two post-operative weeks. Arterial blood gases revealed repeated episodes of respiratory acidosis, and she required mechanical ventilation for eight of the first 17 post-operative days. Ventilator weaning was accomplished through gradual reduction of rate and pressure support. By POD #18, she was extubated, but needed continuous ICU monitoring and used BiPAP 24 hours per day. At this time, she was referred for inspiratory muscle strength training (IMST).
It was determined that the patient would be a good candidate for IMST because she experienced repeated hypercapnic respiratory failure (resting arterial blood gases upon evaluation: pH: 7.37, PaCO2: 57 torr, PaO2: 85 torr, SaO2: 99%, HCO3: 33 mEq/L), accompanied by a decline from her baseline respiratory function. In addition, she took immunosuppressant medications that may alter striated muscle performance, including 20 mg of prednisone (7) and 6mg of tacrolimus daily. The patient and her parents consented to our assessment and intervention, and initial consultation occurred on POD #20. At this academic medical center, Institutional Review Board approval was not required for case reports.
The following measures were recorded: maximal inspiratory pressure (MIP), respiratory parameters at rest and with extrinsic loads, and rate of pressure development at standard inspiratory threshold loads (5, 10, and 15 cm H2O). Respiratory testing was conducted with a respiratory monitor (CO2SMO Plus, Novametrix, Murrysville, PA) connected to a laptop computer, and the data were integrated using AnalysisPlus software (Phillips-Respironics, Murrysville, PA).
The patient completed MIP maneuvers from a sitting position. MIP has been used extensively to estimate inspiratory strength in healthy adults and children, as well as people with neuromuscular impairments (8, 9). We assessed MIP upon initial exam and weekly thereafter, according to American Thoracic Society testing guidelines (10). The patient exhaled to residual volume, and then attempted a maximal inspiration against a one-way valve connected to a digital pressure manometer (Sper Scientific, Scottsdale, AZ). She rested for two minutes between attempts, and repeated the test three times. Testing occurred following inspiratory training sessions, to reduce variability and facilitate inspiratory drive.
In addition to MIP, we evaluated inspiratory performance during maximal-effort breathing against standardized inspiratory threshold loads (5, 10, and 15 cm H2O). At each threshold load, an average value was obtained for the following ventilatory parameters: inspiratory tidal volume (VT), peak inspiratory flow (PIF), inspiratory (TI) and expiratory (TE) times, and the peak inspiratory pressure achieved during the threshold breaths (PI). The imposed work (WOBi = VT x PI) and power (POBi = (VT x PI ) / TI) of breathing were calculated to quantify the work and power produced by the inspiratory pump in response to the loaded breaths. In addition, inspiratory pressure activation (Pact) was assessed during the loaded breaths, defined as the negative pressure generated 100 milliseconds into the inspiratory effort. Pact reflected the patient’s ability to generate pressure quickly against the graded threshold loads. The Pact was used to reflect the ability of the inspiratory muscles to dynamically generate pressure during a concentric contraction, and thus was not considered to be equivalent to the static occlusion pressure (P0.1) used to estimate neural drive. Finally, we recorded the number of hours of BiPAP used daily, arterial blood gases, when available, perceived exertion and vital signs during training.
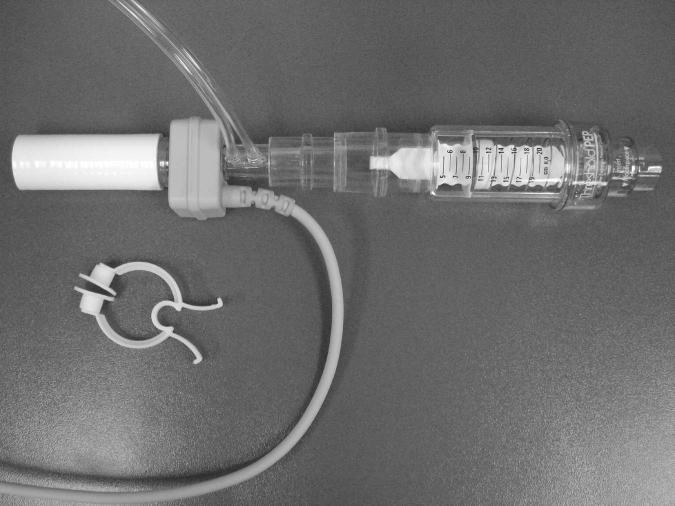
The patient completed IMST 5 days per week for 2 weeks. IMST was conducted using a Threshold PEP trainer, (HS735, Phillips-Respironics, Murrysville, PA). The opening of the Threshold PEP device contained a poppet valve occluded by a spring, and the spring tension was adjusted to modify the pressure required to open the valve. The PEP device was inverted to deliver inspiratory threshold training loads. (Fig. 1). The patient completed 4 sets of 10 threshold-loaded breaths per training session, and rested for 2 minutes between exercise sets. The intensity of the training device was set at the highest inspiratory load that the subject could tolerate and consistently open, according to the following guidelines: the patient could generate an inspiratory volume greater than 50% of her unloaded resting inspirations, vital signs were stable with perceived exertion less than 7 on the modified Borg 10-point scale (11), and valve opening occurred in 100% of threshold breaths.
Figure 1.
Image of the IMST apparatus used to administer training and test muscle performance during standardized breathing loads.
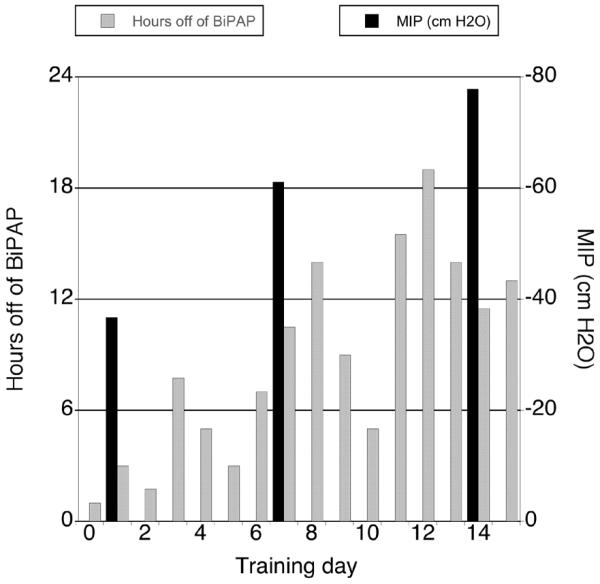
During the course of IMST, the load on the training device increased from 7cm H2O on the first training day (19% of MIP) to 18cm H2O on day 15 (23% of MIP). The patient demonstrated consistent weekly gains in MIP. After one week, MIP increased from −36.7 cm H2O to −61.1 cm H2O (66% increase). By the end of week two, MIP improved to −77.8 cm H2O (112% increase from baseline). Functionally, the patient progressed from 1 hour of unassisted ventilation prior to training, to 11-13 hours daily, and used BiPAP only when asleep (Fig. 2).
Figure 2.
Hours off of BiPAP increased progressively during the two-week training period as maximal inspiratory pressure improved (pressure scale inverted for visual clarity). Prior to the first training session, the patient tolerated 1 hour off of BiPAP daily. Following inspiratory training, she achieved her baseline ventilatory function, and used Bi-PAP only while sleeping.
Ventilatory parameters improved during maximal-effort, threshold-loaded breathing (Table 1). Initially, the patient was able to complete the inspiratory performance tests at each load. However, PIF, VT, and Pact progressively decreased at the higher loads, accompanied by a prolonged TI and shortened TE. After training, both TI and TE decreased during threshold-loaded breaths, accompanied by increased VT and PIF. The largest training-induced gains occurred in measures of muscle power: PIF, POBi, and Pact. Because threshold devices provide consistent pressure loads that are independent of inspiratory flow under most conditions (12), it was not surprising that the PI measured during the loaded breaths did not increase appreciably after training.
Table 1.
Average ventilatory parameters achieved during maximal-effort threshold-loaded breathing at standardized loads, before and after inspiratory muscle strength training. After 2 weeks of training, the child generated large gains in tidal volume, peak inspiratory flow, imposed work, imposed power, and inspiratory pressure activation.
| Threshold breathing parameters |
5 cmH2O load- baseline |
5 cmH2O load- Week 2 |
% change |
10 cmH2O load- baseline |
10 cmH2O load- Week 2 |
% change |
15 cmH2O load- baseline |
15 cmH2O load- Week 2 |
% change |
|---|---|---|---|---|---|---|---|---|---|
| PIF (L/min) | 46.83 | 93.77 | 100% | 40.90 | 82.45 | 102% | 22.68 | 60.83 | 168% |
| TI (sec) | 0.88 | 0.73 | −17% | 1.03 | 0.67 | −35% | 1.48 | 0.63 | −57% |
| TE (sec) | 0.98 | 0.65 | −34% | 0.68 | 0.52 | −24% | 0.58 | 0.35 | −40% |
| VT (mL) | 356 | 582 | 63% | 288 | 484 | 68% | 213 | 325 | 53% |
| PI (cm H2O) | −5.62 | −7.53 | 34% | −10.78 | −13.87 | 29% | −15.75 | −17.18 | 9% |
| Pact (cm H2O) | −2.50 | −4.63 | 85% | −2.25 | −8.45 | 276% | −1.28 | −13.56 | 959% |
| WOBi (J) | 0.20 | 0.43 | 115% | 0.30 | 0.66 | 120% | 0.33 | 0.55 | 67% |
| POBi (W) | 0.22 | 0.59 | 168% | 0.30 | 1.00 | 233% | 0.23 | 0.87 | 278% |
In addition to IMST, the patient received routine physical therapy three days per week while hospitalized, with an emphasis on upright mobilization and walking endurance. The patient’s functional mobility improved during the hospitalization. On POD #20 (IMT day #1), she was able to transfer to a chair with minimal assist and sit upright for one hour. On POD #28 (IMT #9), she could walk approximately 1000 feet at a self-selected pace. The strength of 12 extremity muscle groups was scored bi-monthly by clinical therapists, using the Medical Research Council (MRC, Table 3) 6-point ordinal scale (13). The MRC sumscore can be completed at the bedside, and benefits include rapidity of testing and low within-subject variance (14). Prior reports suggest that a score <48/60 indicates clinically significant weakness (13, 15). The patient’s MRC sumscore was 46/60 at POD #2 and the score remained unchanged upon the monthly therapy reassessment (POD #32).
Table 3.
The Medical Research Council (MRC) sumscore provides a non-invasive estimate of generalized extremity muscle strength that can be used in bed-bound, critically ill patients. Testers rate the strength of shoulder abduction, elbow flexion, wrist extension, hip flexion, knee extension, and ankle dorsiflexion in all extremities, using standard testing positions. Each plane of movement receives a 0 to 5-point ordinal score, for a total possible sumscore of 60.
| Grade | Muscle State |
|---|---|
|
| |
| 0 | No contraction |
| 1 | Flicker or trace contraction |
| 2 | Active movement with gravity eliminated |
| 3 | Active movement against gravity |
| 4 | Active movement against gravity and some resistance |
| 5 | Normal muscle power |
The girl’s medical condition was upgraded on POD #31 (IMST day #12), and she was transferred from intensive care to a regular pediatric nursing unit. By POD #34, (IMST day #15), the patient reached her pre-operative ventilatory function and demonstrated MIP within an expected age and gender-predicted range (10). IMST was discontinued at that time. Six days later, she was discharged to home.
Discussion
This report describes the effect of IMST on inspiratory strength and function in a 16-year-old girl with NM and persistent post-operative ventilatory insufficiency. After two weeks of IMST, the patient made significant gains in inspiratory performance, accompanied by improvements in resting ventilation. Gains in strength appeared to be most evident in the inspiratory muscles. A robust recovery of gender- and age-predicted MIP indicated that IMST increased the performance of the inspiratory muscles. Functionally, the patient was able to meet her ventilatory requirements with a reduced perception of respiratory effort. During our initial exam, her perceived exertion was 5/10 after one hour of unassisted ventilation. Upon completion of IMST, she reported no detectable exertion with tidal breathing on room air. Consequently, she regained her pre-operative baseline function, and used BiPAP only when asleep.
While IMST conferred specific respiratory performance benefits, we cannot state whether it facilitated improvements in generalized strength or mobility. We report that walking distance increased during the hospitalization without an apparent change in lower extremity strength. Although the patient’s extremity strength scores did not change appreciably, the sensitivity of the MRC scale decreases at higher scores (13). Thus, the MRC sumscore may not have detected clinically meaningful limb strength changes. On the other hand, walking tolerance remained below normal, suggesting persistent lower extremity deconditioning. Due to vigorous increases in respiratory performance, it is doubtful that inspiratory weakness impeded the reported gains in walking distance.
The function of related organ systems has been shown to affect respiratory muscle strength, namely cardiac(16) and renal (17) function. On POD #2, an echocardiogram revealed that the girl’s ejection fraction was 62%. Cardiac biopsy on POD #28 (IMST day #9) confirmed an absence of organ rejection. The child received temporary renal replacement therapy when a biopsy on POD #3 indicated acute tubular necrosis. A repeat biopsy on POD #15 showed improvement of renal function and no evidence of rejection. The patient did not undergo daily weights, but daily fluid intake and output was recorded. During week 1 of IMST, her average daily fluid balance was +175 mL, and the daily fluid balance averaged +204 mL during week 2 of IMST. Moreover, her blood urea nitrogen (BUN) and creatinine were stable. Daily BUN was 28 mg/dL on IMST day #1 and 32 mg/dL on IMST day #15; creatinine was 1.46 mg/dL on IMST day #1and 0.92 mg/dL on IMST day #15. Therefore, we concluded that changes in the patient’s cardiac or renal function were unlikely to have significantly influenced the respiratory performance gains observed during the training period. In addition, liver function tests did not change appreciably from pre-operative values, and remained largely within normal limits during the IMST training period. At a routine follow-up, the patient’s restrictive pulmonary disease had increased marginally two months after transplant (FEV1 36% predicted, FVC 33% predicted).
The child achieved the greatest performance improvements in POBi and Pact during maximal ventilatory efforts with high extrinsic loads. POBi and Pact specify the velocity-dependent characteristics of ventilatory muscle performance. The Pact estimated the maximal pressure activation the patient could generate in a dynamic fashion during threshold breathing challenges. In people with respiratory weakness, estimates of inspiratory drive (P0.1) may be normal or slightly elevated, but they do not sufficiently increase with added extrinsic demands (10). After IMST, the dynamic rate of pressure development (Pact) was increased during loaded inspirations. As a result, the patient quickly overcame the threshold pressure settings of the device, opened the poppet valve, and generated rapid inspiratory flow. Power is necessary to produce pressure quickly throughout the inspiration. Because POBi reflects pressure-time performance, it may describe dynamic inspiratory function to a greater degree than static performance measures such as MIP. Recovery of POBi and Pact indicate that IMST enhanced the patient’s respiratory pump reserve, and thereby increased her capacity to compensate rapidly to increased ventilatory loads.
The present case is unique in two aspects. To our knowledge, this is the first clinical report that describes a respiratory strengthening benefit for NM. The advantages of inspiratory training exercises have been described in children with other neuromuscular diseases, namely Duchenne muscular dystrophy (18-20). These investigations conclude that respiratory muscle performance can increase in children with mild to moderate disability (18), but children with severe dystrophic changes may experience no benefit (19). Animal research reveals that training adaptations may differ between dystrophies and myopathies. In contrast to progressive degeneration associated with many dystrophies, muscle loading does not appear to worsen disease activity in less severe NM gene variants (2, 21). Thus, it is feasible that some patients with childhood- or adult-onset NM may benefit from IMST.
This account illustrates that some children who meet IMST criteria (Table 2) may be able to achieve inspiratory strength gains in a monitored, critical care environment. Most published inspiratory training reports utilized low pressure loads over a sustained duration, designed to increase endurance (18). In contrast, our training regime consisted of higher loads delivered for a limited number of repetitions, more closely resembling strength training. However, it should be noted that targeted IMST exertion levels were achieved using relatively modest threshold loads (19-23% of MIP). This observation may reflect underlying excitation-contraction coupling impairments associated with NM.
Table 2.
Clinical indications and contraindications for Inspiratory Muscle Strength Training.
| Clinical Indications for Inspiratory Muscle Strength Training | Contraindications for Inspiratory Muscle Strength Training |
|---|---|
|
| |
| Inspiratory strength below age and gender-predicted normal values |
Hemodynamic instability (systolic BP <90 mm Hg, or resting HR > 110 bpm) or requirement of continuous vasopressor medications |
| Decline from pre-morbid level of ventilatory function – acute requirement for assisted ventilation |
Evidence of uncontrolled infection (temperature >36.0 °C or > 38.5 °C, WBC > 19/mm2) |
| Failure to wean with routine clinical methods (i.e. reduced IMV, pressure support, lengthening spontaneous breathing trials) |
Acute pulmonary instability: untreated hemothorax or pneumothorax, unstable fractures |
| Gas exchange maintained with minimal ventilatory support (ie. FIO2 < .6, IMV <8, PS <15, PEEP <8) |
Presence of seizure activity, ventriculostomy, or evolving neurological injury |
| Current or previously prescribed medication known to impair skeletal muscle excitation-contraction coupling (such as corticosteroids, beta-blockers, neuromuscular blockade, aminoglycoside antibiotics, immunosuppressants) |
Inability to follow commands |
Conclusions
This report describes an IMST performance benefit in a child with NM and post-operative ventilatory insufficiency, and it corroborates the respiratory training effects identified in other neuromuscular diseases (18). Respiratory complications of NM have been associated with a more negative clinical prognosis (5, 6). Although other clinical reports illustrate that acute ventilatory insufficiency in NM can cause life-threatening medical complications (6, 22, 23), ours is the first to document specific respiratory performance and functional gains with IMST. IMST may be beneficial for some patients with NM who experience symptomatic respiratory muscle weakness and a decline from baseline ventilatory function.
Acknowledgments
Financial Support: Supported by NIH T32 HD043730A to BKS
Supported by Foundation for Physical Therapy
Footnotes
The authors do not have any potential conflicts of interest to disclose.
References
- 1.Laing NG. Congenital myopathies. Curr Opin Neurol. 2007;20(5):583–589. doi: 10.1097/WCO.0b013e3282ef6e69. [DOI] [PubMed] [Google Scholar]
- 2.Joya JE, Kee AJ, Nair-Shalliker V, et al. Muscle weakness in a mouse model of nemaline myopathy can be reversed with exercise and reveals a novel myofiber repair mechanism. Hum Mol Genet. 2004;13(21):2633–2645. doi: 10.1093/hmg/ddh285. [DOI] [PubMed] [Google Scholar]
- 3.Ochala J. Thin filament proteins mutations associated with skeletal myopathies: Defective regulation of muscle contraction. J Mol Med. 2008 doi: 10.1007/s00109-008-0380-9. [DOI] [PubMed] [Google Scholar]
- 4.Ryan MM, Ilkovski B, Strickland CD, et al. Clinical course correlates poorly with muscle pathology in nemaline myopathy. Neurology. 2003;60(4):665–673. doi: 10.1212/01.wnl.0000046585.81304.bc. [DOI] [PubMed] [Google Scholar]
- 5.Wallgren-Pettersson C, Laing NG. Report of the 70th ENMC International Workshop: nemaline myopathy, 11-13 June 1999, Naarden, The Netherlands. Neuromuscul Disord. 2000;10(4-5):299–306. doi: 10.1016/s0960-8966(99)00129-7. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki M, Takeda M, Kobayashi K, et al. Respiratory failure in nemaline myopathy. Pediatr Neurol. 1997;16(4):344–346. doi: 10.1016/s0887-8994(97)00032-5. [DOI] [PubMed] [Google Scholar]
- 7.Menconi M, Fareed M, O’Neal P, et al. Role of glucocorticoids in the molecular regulation of muscle wasting. Crit Care Med. 2007;35(9 Suppl):S602–608. doi: 10.1097/01.CCM.0000279194.11328.77. [DOI] [PubMed] [Google Scholar]
- 8.Iandelli I, Gorini M, Misuri G, et al. Assessing inspiratory muscle strength in patients with neurologic and neuromuscular diseases : comparative evaluation of two noninvasive techniques. Chest. 2001;119(4):1108–1113. doi: 10.1378/chest.119.4.1108. [DOI] [PubMed] [Google Scholar]
- 9.Fauroux B. Respiratory muscle testing in children. Paediatr Respir Rev. 2003;4(3):243–249. doi: 10.1016/s1526-0542(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 10.ATS/ERS ATS/ERS Statement on respiratory muscle testing. Am J Respir Crit Care Med. 2002;166(4):518–624. doi: 10.1164/rccm.166.4.518. [DOI] [PubMed] [Google Scholar]
- 11.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14(5):377–381. [PubMed] [Google Scholar]
- 12.Gosselink R, Wagenaar RC, Decramer M. Reliability of a commercially available threshold loading device in healthy subjects and in patients with chronic obstructive pulmonary disease. Thorax. 1996;51(6):601–605. doi: 10.1136/thx.51.6.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kleyweg RP, van der Meche FG, Schmitz PI. Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve. 1991;14(11):1103–1109. doi: 10.1002/mus.880141111. [DOI] [PubMed] [Google Scholar]
- 14.Merkies IS, Schmitz PI, Van Der Meche FG, et al. Comparison between impairment and disability scales in immune-mediated polyneuropathies. Muscle Nerve. 2003;28(1):93–100. doi: 10.1002/mus.10410. [DOI] [PubMed] [Google Scholar]
- 15.De Jonghe B, Bastuji-Garin S, Sharshar T, et al. Does ICU-acquired paresis lengthen weaning from mechanical ventilation? Intensive Care Med. 2004;30(6):1117–1121. doi: 10.1007/s00134-004-2174-z. [DOI] [PubMed] [Google Scholar]
- 16.Ribeiro JP, Chiappa GR, Neder A, et al. Respiratory muscle function and exercise intolerance in heart failure. Curr Heart Fail Rep. 2009;6(2):95–101. doi: 10.1007/s11897-009-0015-7. [DOI] [PubMed] [Google Scholar]
- 17.Bark H, Heimer D, Chaimovitz C, et al. Effect of chronic renal failure on respiratory muscle strength. Respiration. 1988;54(3):153–161. doi: 10.1159/000195516. [DOI] [PubMed] [Google Scholar]
- 18.Koessler W, Wanke T, Winkler G, et al. 2 Years’ experience with inspiratory muscle training in patients with neuromuscular disorders. Chest. 2001;120(3):765–769. doi: 10.1378/chest.120.3.765. [DOI] [PubMed] [Google Scholar]
- 19.Wanke T, Toifl K, Merkle M, et al. Inspiratory muscle training in patients with Duchenne muscular dystrophy. Chest. 1994;105(2):475–482. doi: 10.1378/chest.105.2.475. [DOI] [PubMed] [Google Scholar]
- 20.Winkler G, Zifko U, Nader A, et al. Dose-dependent effects of inspiratory muscle training in neuromuscular disorders. Muscle Nerve. 2000;23(8):1257–1260. doi: 10.1002/1097-4598(200008)23:8<1257::aid-mus15>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 21.Nair-Shalliker V, Kee AJ, Joya JE, et al. Myofiber adaptational response to exercise in a mouse model of nemaline myopathy. Muscle Nerve. 2004;30(4):470–480. doi: 10.1002/mus.20138. [DOI] [PubMed] [Google Scholar]
- 22.Whitaker J, Love S, Williams AP, et al. Idiopathic adult-onset nemaline myopathy presenting with isolated respiratory failure. Muscle Nerve. 2009;39(3):406–408. doi: 10.1002/mus.21234. [DOI] [PubMed] [Google Scholar]
- 23.Kelly E, Farrell MA, McElvaney NG. Adult-onset nemaline myopathy presenting as respiratory failure. Respir Care. 2008;53(11):1490–1494. [PubMed] [Google Scholar]