Summary
Neuroimmune processes contribute to hypoxic-ischemic damage in the immature brain and may play a role in the progression of particular variants of neonatal encephalopathy. The present study was designed to elucidate molecular mediators of interactions between astrocytes, neurons and infiltrating peripheral immune cells after experimental neonatal hypoxia-ischemia (HI). Splenectomy was performed on postnatal day-7 Sprague-Dawley rats 3 days prior to HI surgery; in which the right common carotid artery was permanently ligated followed by 2 hours of hypoxia (8% O2). Quantitative analysis showed that natural killer (NK) and T cell expression was reduced in spleen but increased in the brain following HI. Elevations in cyclooxygenase-2 (COX-2) expression after HI by immune cells promoted interleukin-15 expression in astrocytes and infiltration of inflammatory cells to site of injury; additionally, down-regulated the pro-survival protein, phosphoinositide-3-kinase, resulting in caspase-3 mediated neuronal death. The removal of the largest pool of peripheral immune cells in the body by splenectomy, COX-2 inhibitors, as well as rendering NK cells inactive by CD161 knockdown, significantly ameliorated cerebral infarct volume at 72 hours, diminished body weight loss and brain and systemic organ atrophy, and reduced neurobehavioral deficits at 3 weeks. Herein we demonstrate with the use of surgical approach (splenectomy), with pharmacological loss-gain function approach using COX-2 inhibitors/agonists, as well as with NK cell-type specific siRNA that after neonatal HI, the infiltrating peripheral immune cells may modulate downstream targets of cell death and neuroinflammation by COX-2 regulated signals.
Keywords: NK cells, neuroinflammation, hypoxia-ischemia, neonatal, cyclooxygenase-2
Introduction
The inflammatory response, which is characterized by recruitment of circulating immune cells after their deployment from peripheral lymphatic organs, has been implicated as a core component of damage to the immature brain following neonatal hypoxia-ischemia (HI) [1]. Although many therapeutic interventions have been explored to prevent and/or mitigate the inflammatory sequelae of perinatal HI, few such interventions have proven clinically viable. One explanation has been that the immunoinflammatory response is multifaceted, in that activation of immune cells may have both detrimental and neuroprotective effects [2]. Increasing evidence suggests that a more integrative approach to therapy may resolve this paradox [3, 4]. In theory, re-directing our attention from neuron-driven outcomes toward the molecular mediators believed to orchestrate interactions between brain and immune cells may prove a more fruitful investigative approach in neonatal HI [5].
Cyclooxygenase-2 (COX-2), a well-established contributor to ischemic brain injury, might serve as a prime candidate for such a molecular-mediated investigation [6, 7]. In particular, COX-2 may mediate the mechanism by which activated immune cells induce pro-inflammatory cytokine production by astrocytes [8]. Recent data suggests that amongst these cytokines the enhanced interleukin-15 (IL-15) expression in astrocytes is a major propagator of inflammatory responses after central nervous system injury [9]. Yet it still remains to be determined whether astrocytes respond to COX-2 effectors from infiltrating immune cells by producing IL-15, which then further orchestrates the inflammatory response and/or cell death in the immature brain.
Additionally, the degree of involvement from the innate immune system correlates with the extent of neuronal damage in the post-ischemic tissue [2]. Studies suggest dysfunction of the phosphoinositide-3-kinase (PI3K)/Akt survival pathway in triggering apoptotic cascades by inflammatory cells in the brain [10]. However, it is unknown whether down-regulation of the PI3K pathway and subsequent neuronal death in HI-injured rats occurs in response to COX-2 activation in infiltrating immune cells.
From the ischemic stroke model comes an indication that progression of brain injury can be mediated by immune cells originating in the spleen [11]. We hypothesized that neurological outcomes in stroked neonatal rats can be ameliorated by targeting splenic immune cells and their modulatory functions mediated by COX-2. To elucidate the possible impact of the peripheral immune cells on astroglia-neuron interactions, we removed the spleen, the largest pool of peripheral immune cells prior to HI and studied neuronal and astrocytic inflammatory pathways followed by the evaluation of short- and long-term outcomes in the neonatal rats. We also used a gain and loss of function approach (pharmacological activation or inhibition, respectively) for COX-2, a neutralizing antibody for IL-15, and a gene silencer for natural killer (NK) cells in both splenectomized and non-splenectomized rats to verify the role of COX-2 in splenic immune cell responses following HI. Here, we identify infiltrating splenic immune cells as a major source of enhanced COX-2 expression in the ischemic brain, and implicate COX-2 for causation of signaling pathways in astrocytes and neurons that lead to a worsened outcome.
Methods
Surgical Procedures
The protocol detailing this study was approved by the Institutional Animal Care and Use Committee at Loma Linda University. Splenectomy, on postnatal day-7 pups, entailed a skin incision at the upper left quadrant of the abdomen, exteriorization of the spleen through the incision, and cauterization of the blood vessels. Un-splenectomized groups had the abdominal cavity opened, the spleen isolated, and then re-closed. Postnatal day-10 pups were placed on a surgical table maintained at 37°C and anesthetized by inhalation with 3% isoflurane. Briefly, HI surgery entailed permanent ligation of the right common carotid artery, followed by 1.5 hours (h) of recovery, then placement in a glass jar perfused for 2 h with 8% oxygen [7]. Rats were sacrificed under general anesthesia by decapitation at 3 h, 3 days (d) or 3 weeks (w) post-HI.
Pharmacological Manipulation
Some postnatal day-9 pups were treated intraperitoneally with 30mg/kg COX-2 inhibitor (NS398; Cayman Chemical), or intraventricularly with 0.01mg/kg COX-2 agonist, 4-hydroxynonenal (4-HNE; Cayman Chemical) or with 1µl NK cell (CD161) siRNA. Others were treated intraventricularly with 1.5µl IL-15 neutralizing antibody (Santa Cruz Biotech) 1 h pre-HI.
Measurement of Infarct Size
Brain tissue was collected after trans-cardial perfusion with 0.1 mol/L phosphate buffered saline; and cut at 2-mm intervals into 5 coronal sections and incubated in 2% 2,3,5-triphenyltetrazolium chloride (TTC) for infarct volume measurement [12].
Evaluation of Organ Damage
Cerebral hemispheres (separated by a midline incision) of animals and spleen of un-splenectomized animals was isolated then weighed on a high-precision balance (sensitivity ± 0.001 g).
Neurological Assessment
Rats were placed onto an elevated wire grid floor (36 × 13 in) for 2 minutes; foot-faults were noted when a complete paw fell through the bars for assessment of motor coordination [13]. Rats were placed in the stem of the T-maze and allowed to explore until an arm of the maze was chosen, as an assessment of short-term or working memory [14]. The sequence of left and right arm choices over 10 trials was expressed as the rate of spontaneous alternation. For the following tests, rats were given a score of 100 for immediate and correct placement, 50 for delayed and/or incomplete placement, or 0 for no placement: Postural Reflex, Proprioceptive Limb Placing, Lateral Pressure Towards Edge, and Lateral Limb Placing [7].
Immunofluorescence Analysis
The following antibodies were used: CD161 (1:100; Serotec Co.), CD3 (1:100; Serotec Co.), COX-2 (1:100; Santa Cruz Biotech), IL-15 (1:100; Santa Cruz Biotech.), GFAP (1:100; Millipore), cleaved caspase-3 (1:100; Cell Signaling), NeuN (1:100; Millipore), Iba1 (1:100; Wako Chemicals), CD68 (1:100; Millipore), or myeloperoxidase - MPO (1:100; Dako) on 10 um frozen sections of tissue collected 3 d post-HI. The absolute number of positive cells per square millimeter was counted in three peri-infarct regions of the ipsilateral cerebral cortex or in three marginal zone areas immediately adjacent to the white pulp of the spleen [7].
Western Blot Analysis
Homogenates of ipsilateral cerebral hemispheres were processed (Fathali et al., 2010) for analysis using antibodies against: COX-2 (1:300), CD161 (1:200), CD3 (1:200), PI3K (1:300; Santa Cruz Biotech), pro caspase-3 (1:1000; Cell Signaling), cleaved caspase-3 (1:1000), or beta actin (1:1000, Santa Cruz Biotech). Optical density was determined using NIH Image J software and expressed relative to beta actin.
RT-PCR Analysis of NK Cells
Total RNA was isolated from the ipsilateral hemisphere with TRIZOL reagent and cDNA prepared using SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) [15]. PCR amplification used primers from Invitrogen (see below for sequence) and was done by means of thermocycler (iCycler; BIO-RAD). PCR products were electrophoresed in 2% agarose gel in Tris-borate-EDTA buffer. Densities of bands were determined by Bio-Rad Quantity One software, and were expressed relative to GAPDH bands.
Klrb1b (CD161) [16]:
Forward: 5'-GTTCTAGACTCGGCTGTGCTTGCCT-3'
Reverse: 5'-CTGAATTCTGGTAAAGTAATCGAGGTACG-3'
GAPDH [15]:
Forward: 5’-ACCACAGTCCATGCCATCAC-3’
Reverse: 5’-TCCACCACCCTGTTGCTGTA-3’
Assessment of Cell Death
Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) method was done with a kit (Roche). Sections were mounted with anti-fade mounting medium (Molecular Probes) under glass cover-slips.
Data Analysis
Observers were blind to the actual animal groupings. Results were expressed as mean ± SEM. One-way ANOVA Holm-Sidak correction or the unpaired Student’s t-test (when only two groups were available for comparison) were used to determine significance in differences between means. Kruskal-Wallis ANOVA followed by Dunn’s test was used for neurobehavioral analysis. P < 0.05 was taken as significant.
Results
Splenectomy Attenuates HI-Induced Cerebral Infarction
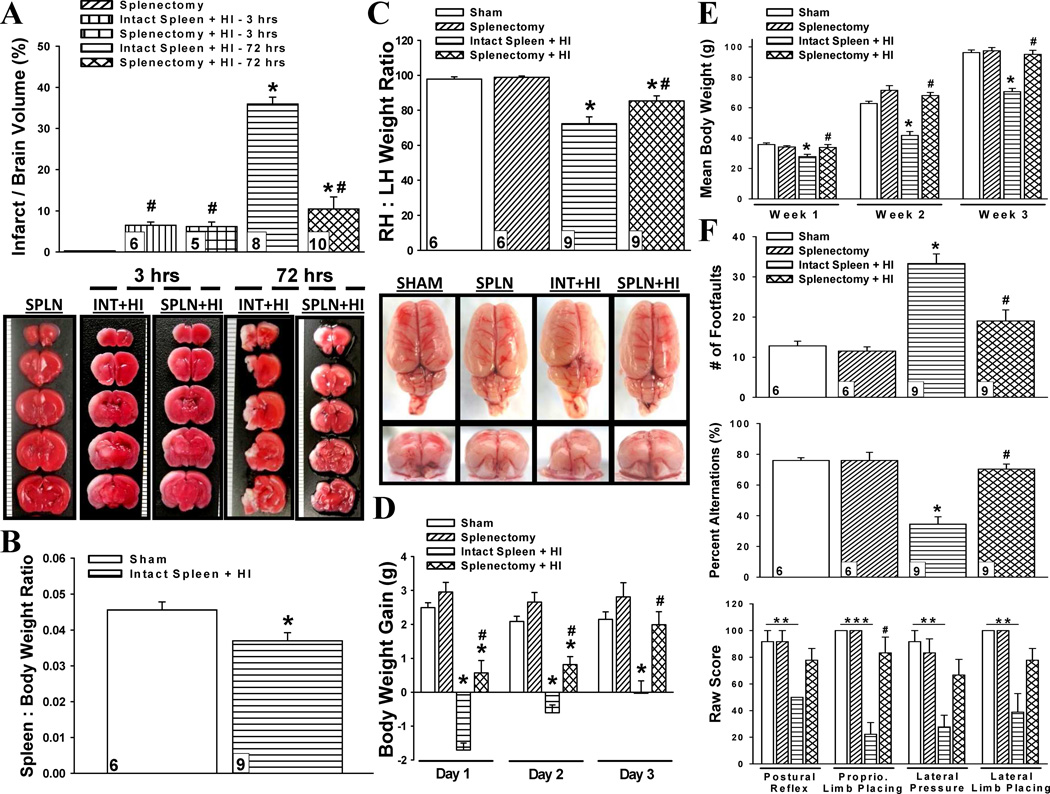
To detect whether spleen-derived immune cells contribute to cerebral infarction, we quantified primary (3 h post-HI) and secondary (3 d post-HI) infarct volume of splenectomized and un-splenectomized rats (Fig. 1a). Removal of the spleen prior to HI had no effect on primary infarct (6.19 ± 1.11 vs. 6.50 ± 0.77), but significantly reduced secondary infarct (10.45 ± 2.94 vs. 35.93 ± 1.69). These findings suggest that splenic cells mediate the progression of cerebral infarction having no effect on the initial extent of brain injury.
Fig. 1.
Effect of spleen on hypoxic-ischemic brain damage. (a) Infarct volumes at 3 hours and 72 hours in splenectomy (SPLN), intact spleen with hypoxia-ischemia (INT+HI), and splenectomy with HI (SPLN+HI) rats. *P < 0.001 versus splenectomy, #P < 0.001 versus intact spleen + HI − 72hrs. (b) Spleen to body weight ratio of sham and intact spleen with HI rats at 3 weeks. *P < 0.05 versus sham (c) Right to left hemispheric (RH:LH) weight ratio representing brain atrophy at 3 weeks. *P < 0.001 versus sham, #P < 0.001 versus intact spleen + HI. (d) Daily body weight gain of animals during the first 3 days after HI insult. *P < 0.001 versus sham, #P < 0.001 versus intact spleen + HI. (e) Weekly mean body weight of animals over 3 weeks. Week 1: *P < 0.01 versus sham, #P < 0.01 versus intact spleen + HI; Week 2 and 3: *P < 0.001 versus sham, #P < 0.001 versus intact spleen + HI. (f) Neurological scores at 3 weeks. *P < 0.05 versus sham, **P < 0.01, ***P < 0.001, #P < 0.05 versus intact spleen + HI. Data represent mean ± SEM. One-way ANOVA with Holm-Sidak correction (a,c,d,e); unpaired Student’s t-test (b); Kruskal-Wallis ANOVA with Dunn’s correction (f). Numbers in bars indicate animals/group.
Splenectomy Attenuates HI-Induced Extended Brain Damage
In order to determine the role of the spleen in long-term HI-related brain damage, we used reductions in spleen size and hemispheric weight as parameters [17, 18]. HI-injured rats had significant spleen (Fig. 1b) and brain (Fig. 1c) atrophy compared to sham animals 3 w post-HI. However, there was a significant attenuation of HI-induced brain loss by prior splenectomy (0.85 ± .03 vs. 0.72 ± .04).
Splenectomy Improves Short- and Long-Term Body Weight Gain after HI
Body weight gain post-HI is an indicator of general well-being [19]. Accordingly, we found HI-injured rats with intact spleen gained less weight during the acute phase as compared to their splenectomized counterparts (Fig. 1d). Next, weekly body weights were taken to determine if these somatic differences had lasting effects (Fig. 1e). HI rats with intact spleen were unable to catch up to the weight of sham rats for the entirety of the study; while prior splenectomy maintained the weight of the animals within control limits at all weekly time-intervals.
Splenectomy Improves HI-Induced Neurological Behavior Deficits
Lifetime consequences of perinatal HI may include motor, cognitive, and behavioral deficits [20]. Several functional tests were used to evaluate the spleen’s role on long-term neurological behavior (Fig. 1f). When the spleen was intact, an ischemic event resulted in a significant decline in motor coordination (Foot-fault: 33.33 ± 2.39), memory (T-maze: 34.57% ± 4.70), and sensory function (Proprioceptive Limb Placing: 22.22 ± 8.78); while, prior removal of the spleen improved these outcomes (19.99 ± 2.77, 70.37% ± 3.21, and 83.33 ± 11.79, respectively). Additionally, HI rats with intact spleen demonstrated significant asymmetry in posture and extension of forelimbs (Postural Reflex: 50.00), and reduced ability for correct placement of limbs (Lateral Pressure: 22.78 ± 8.78; Lateral Limb Placing: 38.89 ± 13.89). Although splenectomy prior to HI appeared to improve these deficits (77.78 ± 8.78, 66.67 ± 11.79, 77.78 ± 8.78, respectively), significance was not reached.
Cerebral Ischemia Alters Expression of Immune Cells in Spleen and Brain
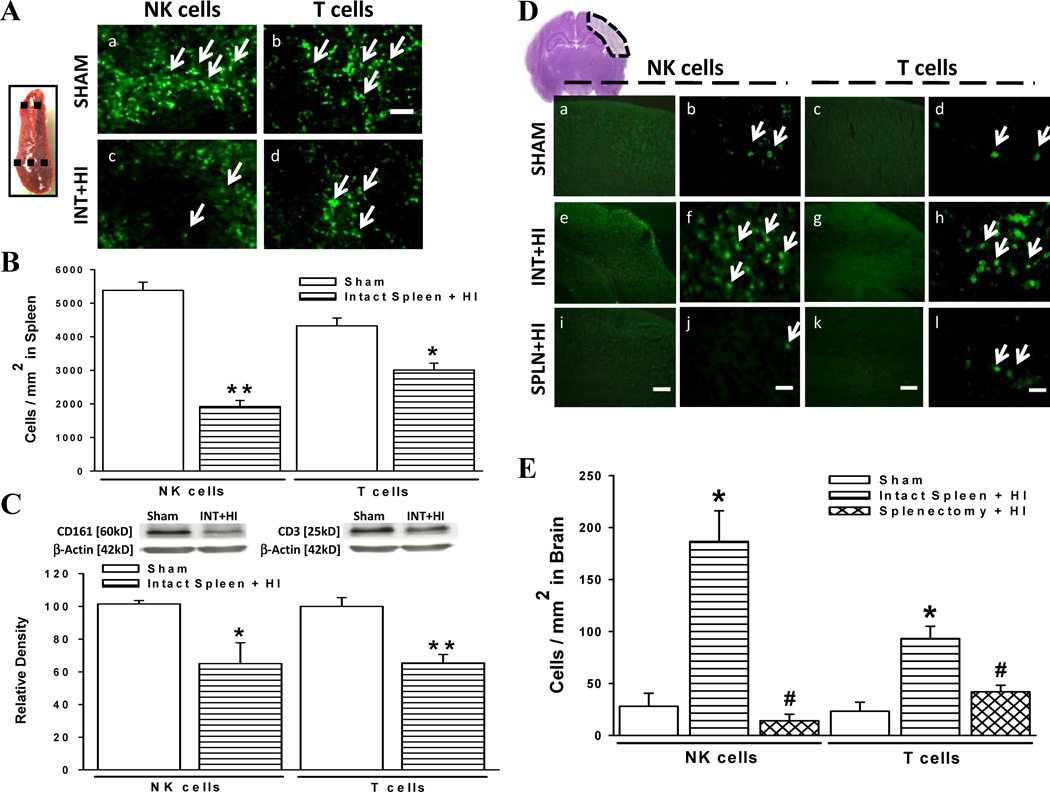
Lymphocytes are major inflammatory effectors of damage after cerebral ischemia [21, 22]. However, the contributions of peripheral immune cells to the ischemic neonatal brain are unclear; and due to vast differences in immune response between neonates and adults, much of the insight obtained from adult studies cannot be inferred to be the same in the immature brain [23, 24]. Accordingly, we sought to determine the leukocyte subsets possibly released by the spleen (Fig. 2a–c) and found a significant reduction in spleen immunoreactive-NK (1923 ± 175 cells/mm2 vs. 5381 ± 244 cells/mm2) and T (3010 ± 202 cells/mm2 vs. 4326 ± 233 cells/mm2) cells 3 d after HI. The same trends were also observed after quantitative Western blot analysis of spleen CD161 (NK cell marker): 65.02 ± 12.78 vs. 101.50 ± 2.10; CD3 (T cells): 65.43 ± 5.24 vs. 100.00 ± 5.31). Next, we verified whether the spleen is a major source of invading lymphocytes in the post-ischemic brain (Fig. 2d, e). Results indicated rats with intact spleen had increased NK (186.67 ± 29.52 cells/mm2 vs. 28.00 ± 12.52 cells/mm2) and T (93.33 ± 11.81 cells/mm2 vs. 23.33 ± 8.61 cells/mm2) cells in the brain 3 d after HI compared to sham; prior splenectomy significantly reduced these cell populations (NK cells: 14.00 ± 6.26 cells/mm2; T cell: 42.00 ± 6.26 cells/mm2). The reduced numbers of immune cells in the spleen after hypoxia-ischemia together with increased counts in brain parenchyma suggests their deployment from spleen to sites of brain injury.
Fig. 2.
Natural killer (NK) and T cell populations in spleen and brain tissues. (a) NK and T immunoreactive cells from sectioned spleen tissue between dotted lines of sham and intact spleen with HI (INT+HI) rats. Scale bar, 60 µm. (b) Absolute number of NK and T cells per square millimeter region in three marginal zone areas of the spleen immediately adjacent to the white pulp (n = 6/group). NK: **P < 0.001 versus sham, T: *P < 0.01 versus sham. (c) Splenic tissue probed for NK (CD161) and T (CD3) cell expression, relative to beta actin, on western blots. NK (n = 4/group): *P < 0.05 versus sham, T (n = 5/group): **P < 0.01 versus sham. (d) Immunofluorescent evaluation of NK and T cell populations in ipsilateral cerebral cortex (within dotted region) of sham, INT+HI, and splenectomy with HI (SPLN+HI) rats. Scale bar, 300 µm (panels i,k) or 30 µm (panels j,l). (e) Absolute number of NK and T cells per square millimeter (n = 6/group). *P < 0.001 versus sham, #P < 0.001 versus intact spleen + HI. Data represent mean ± SEM; Unpaired Student’s t-test (b,c); one-way ANOVA with Holm-Sidak correction (e).
Splenic Immune Cells May Be a Major Source of COX-2 in HI Brain
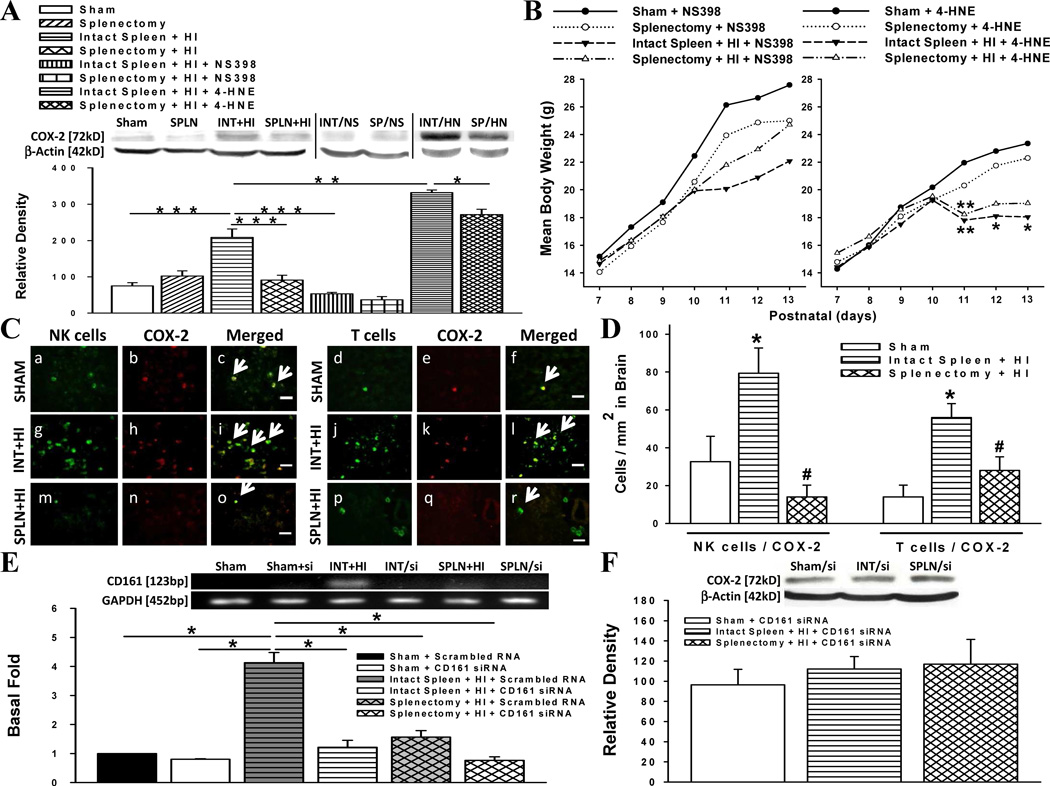
COX-2 is a major effecter of ischemic cerebral injury [6, 7]. Therefore, we sought to determine the contribution of the spleen on COX-2 expression in the HI-injured brain 3 d post-insult (Fig. 3a). Results showed that COX-2 expression was elevated in HI rats with intact spleen, but significantly suppressed by prior splenectomy. To verify the efficacy of the inhibitor (NS398) and agonist (4-HNE) used in our experimental studies for suppressing and upregulating COX-2, respectively; the pharmacological agents were administered to HI-induced rats with or without a spleen. NS398 given to HI rats with intact spleen significantly reduced COX-2 density compared to their untreated counterpart; but, there was no significant difference in COX-2 density between NS398-treated or NS398-untreated HI rats with prior splenectomy. 4-HNE significantly enhanced COX-2 expression in HI-injured groups, although less so in splenectomized rats.
Fig. 3.
Cyclooxygenase-2 (COX-2)-producing cells in the hypoxic-ischemic brain. (a) COX-2 protein levels relative to beta actin in the ipsilateral hemisphere of rats. Comparisons made after administration of COX-2 inhibitor (NS398) or agonist (4-HNE) (n = 5/group; interventions: n = 4/group). *P = 0.01, **P < 0.01, ***P < 0.001. (b) Daily mean body weight of rats administered NS398 (left-side of figure) or 4-HNE (right-side of figure) on postnatal day 9 followed by HI insult on day 10. Individual body weights were compared for statistical analysis. *P < 0.05, **P < 0.01. (c) Immunohistochemical analysis of ipsilateral cerebral cortex double-stained with antibodies against NK cells and COX-2 or T cells and COX-2. Scale bar, 30 µm. (d) Absolute number of NK and T cells co-expressing COX-2 per square millimeter (n = 6/group). *P < 0.01 versus sham, #P < 0.01 versus intact spleen + HI. (e) NK cell mRNA expression levels in rats pre-treated with scrambled RNA or NK (CD161) silencing (si) RNA (n = 6/group). *P < 0.001 (f) COX-2 protein levels relative to beta actin in the ipsilateral hemisphere of rats pre-treated with CD161 siRNA (n = 4/group). Data represent mean ± SEM; One-way ANOVA with Holm-Sidak procedure (a,b,d,e,f).
COX-2 May Contribute to HI-Induced Body Weight Reduction
To elucidate whether the low body weights of HI rats with intact spleen were COX-2-mediated, rats were administered NS398 or 4-HNE on postnatal day-9 followed one day later by sham- or HI-surgery. We found NS398 maintained body weights after HI (Fig. 3b, left-side); while 4-HNE groups showed a trend for lower body weights (Fig. 3b, right-side).
COX-2 Co-Localizes with Immune Cell Subsets after HI
To determine whether the immune cell subsets infiltrating the ischemic brain 3 d post-insult expressed COX-2, double-immunofluorescence was performed (Fig. 3c, d). Results showed elevated COX-2-expressing NK (CD161+) and T (CD3+) cells in brains of HI rats with intact spleen (79.33 ± 13.36 and 56.00 ± 7.23 cells/mm2, respectively) compared to sham (32.67 ± 13.36 and 14.00 ± 6.26 cells/mm2, respectively). Splenectomy prior to HI significantly reduced CD161+/COX-2+ (14.00 ± 6.26 cells/mm2) and CD3+/COX-2 (28.00 ± 7.23 cells/mm2) populations. Since NK cells appeared to be the majority in the ischemic tissue, CD161 siRNA was administered to verify its role on COX-2 expression. The efficacy of the siRNA was provided by RT-PCR (Fig. 3e) and further Western blot analysis demonstrated equalization of COX-2 levels between non-ischemic groups and ischemic group with CD161 knockdown (Fig. 3f).
COX-2 May Promote IL-15 Expression by Astrocytes
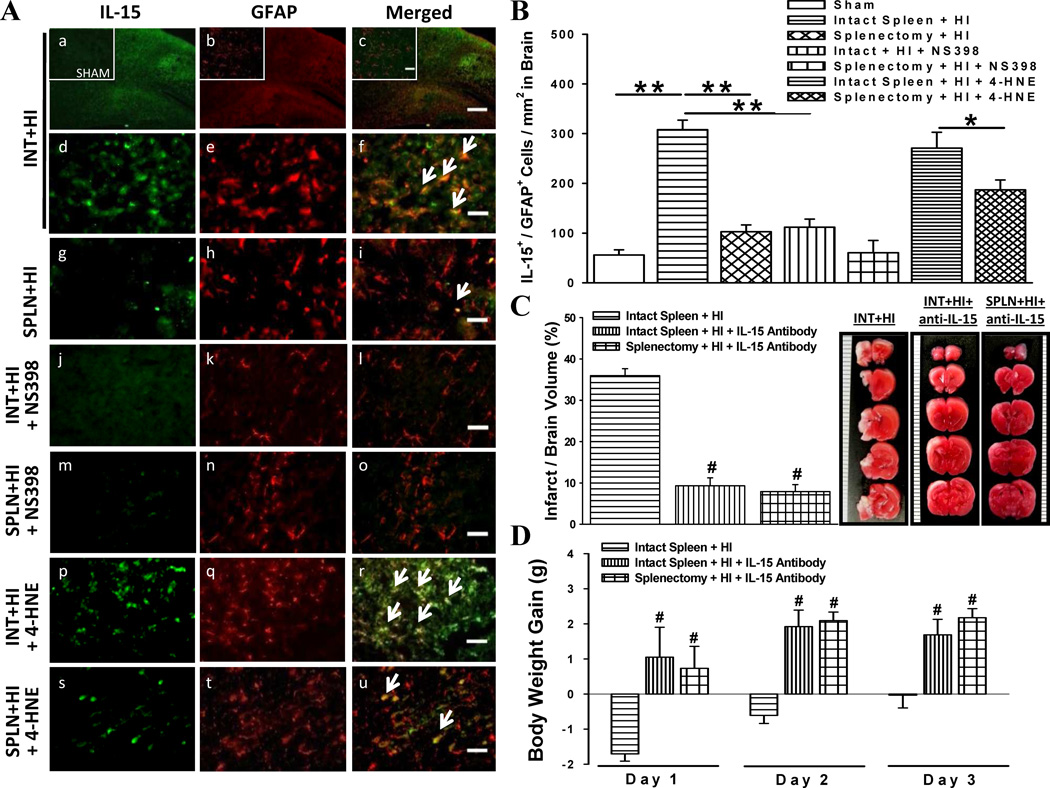
By-products of reactive astrocytes play a key role in regulating the extent of the immune response [25]. Therefore, we sought to elucidate whether peripheral immune cells propagate the neuroinflammatory response by inducing expression of the pro-inflammatory cytokine IL-15 in astrocytes 3 d post-HI (Fig. 4a, b). Ischemic cortical areas of rats with intact spleen revealed elevated IL-15-immunoreactive astrocytes, but a marked reduction in splenectomized animals. Next we verified whether these differences were COX-2-mediated, and found suppressed IL-15 immunoreactivity in NS398-treated HI animals. While 4-HNE enhanced IL-15-expressing astrocytes, co-localization was substantially less in HI rats with prior splenectomy. To determine the role of IL-15 in HI-induced damage, we evaluated infarct volume 3 d post-HI and body weights of rats after administration of IL-15 neutralizing antibody and found significant improvements in both parameters (Fig. 4c, d).
Fig. 4.
Interleukin-15 (IL-15) expression by astrocytes and its role in brain damage. (a) Immunofluorescent evaluation of IL-15 expression by astrocytes (GFAP). Comparisons made after administration of COX-2 inhibitor (NS398) or agonist (4-HNE). Scale bar, 300 µm (panel c) or 30 µm (subset panel c and others). (b) Absolute number of astrocytes co-expressing IL-15 per square millimeter (n = 6/group). *P < 0.05, **P < 0.001. (c) Infarct volumes at 3 days in IL-15 neutralizing antibody-administered groups: intact spleen with hypoxia-ischemia (INT+HI+anti-IL-15), and splenectomy with HI (SPLN+HI+anti-IL-15) (n = 8/intact spleen + HI group; n = 6/antibody groups). (d) Daily body weight gain, during first 3 days post-HI, of rats administered IL-15 neutralizing antibody. Data represent mean ± SEM; One-way ANOVA with Holm-Sidak test (b,c,d). #P < 0.001 versus intact spleen + HI.
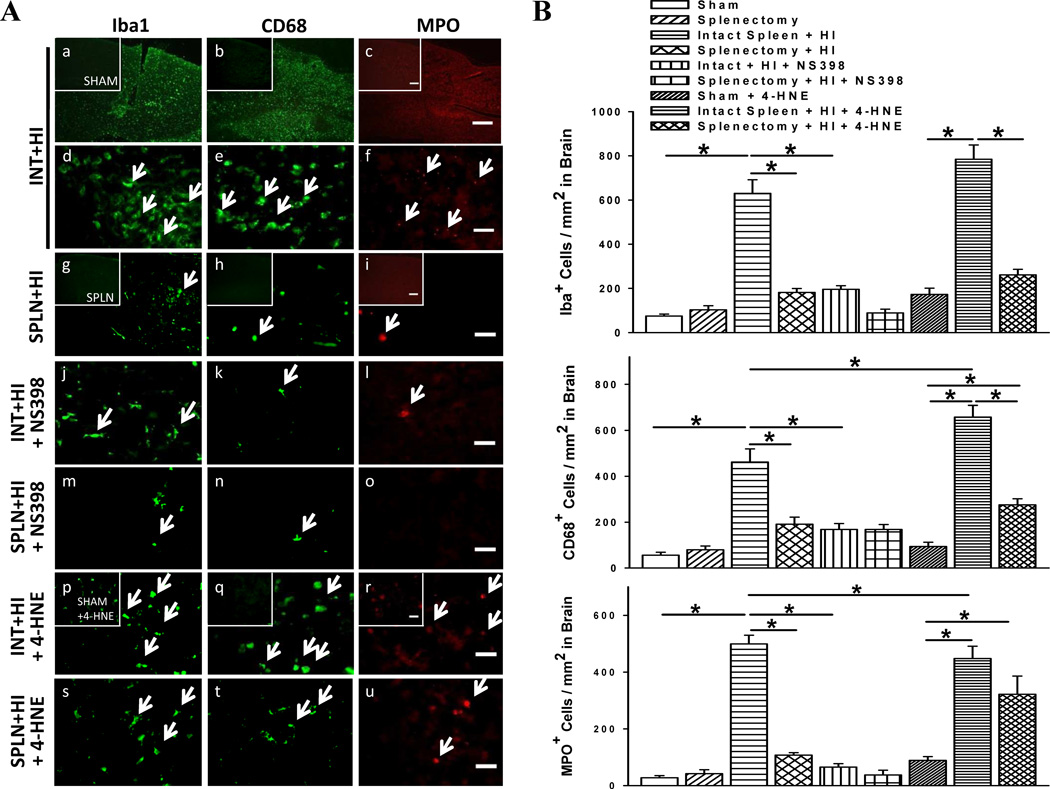
COX-2 May Mediate the Local Inflammatory Response
Microglia, macrophage, and neutrophil numbers in the ischemic hemisphere are greatest 3 d post-stroke [2]. We found HI-induced rats with intact spleen showed elevated expression of these inflammatory cells 3 d post-HI, while prior splenectomy suppressed this outcome (Fig. 5a, b). NS398 to HI-injured rats with intact spleen also reduced inflammatory cell abundance. On the other hand, 4-HNE significantly increased neuroinflammation although in splenectomized rats there were still less activated microglia and macrophages.
Fig. 5.
COX-2-mediated changes in inflammatory cell abundance. (a) Immunofluorescent evaluation of microglia (Iba1) activation, and expression of macrophages (CD68) and neutrophils (MPO) in cerebral cortex of rats. Comparisons made after administration of COX-2 inhibitor (NS398) or agonist (4-HNE). Scale bar, 300 µm (panel c) or 30 µm (subset panel c and others). (b) Absolute number of microglia, macrophage, and neutrophil abundance per square millimeter (n = 6/group). Data represent mean ± SEM; One-way ANOVA with Holm-Sidak correction (b). *P < 0.001.
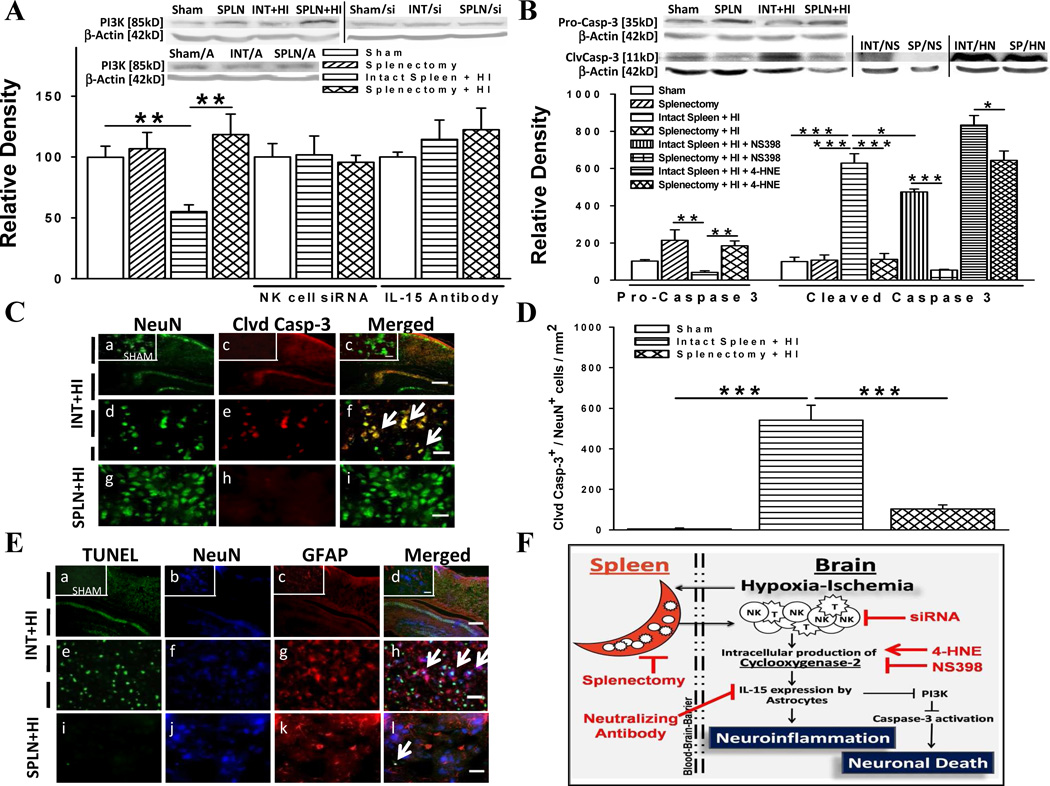
COX-2 May Promote Cell Death Pathway
To determine whether infiltrating immune cells exert detrimental effects by down-regulating specific pro-survival cellular signals, the expression of PI3K was measured at the 3 d time-point and found to be significantly reduced in HI-injured animals with intact spleen compared to those with prior splenectomy (Fig. 6a). NK cells may be responsible for these differences as PI3K levels were equal between groups after CD161 siRNA administration. Next, we determined whether IL-15 regulates PI3K expression and found that IL-15 inhibition normalized PI3K levels for HI rats with intact spleen. To then investigate the molecular mechanism by which decreased PI3K may have an apoptotic effect, we quantified cleaved- and pro-caspase-3 levels at 3 d post-HI. We found HI-induced rats with splenectomy had lower levels of the cleaved caspase-3 and higher levels of inactive pro-caspase-3 compared to those with intact spleen (Fig. 6b). Moreover, we found that NS398 to HI rats with intact spleen decreased expression of activated caspase-3 while the reverse was shown with 4-HNE.
Fig. 6.
Mechanism of neuronal cell death. (a) Phosphoinositide-3-kinase (PI3K) protein levels relative to beta actin in the ipsilateral hemisphere of rats. Comparisons made after administration of NK cell siRNA or IL-15 neutralizing antibody [A] (n = 5/group; interventions: n = 4/group). (b) Pro-caspase 3 and cleaved caspase 3 protein levels relative to beta actin in the ipsilateral hemisphere of rats. Comparisons made after administration of COX-2 inhibitor (NS398; NS) or agonist (4-HNE; HN) (n = 5/group; interventions: n = 4/group). (c) Immunofluorescence stain for cleaved caspase-3 (clvd casp-3) in neurons (NeuN) of the ipsilateral cerebral cortex. Scale bar, 300 µm (panel c) or 30 µm (subset panel c and others). (d) Absolute number of neurons co-expressing cleaved caspase-3 per square millimeter (n = 6/group). (e) Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end labeling (TUNEL) with markers for neurons and astrocytes (GFAP) (n = 6/group). Scale bar, 300 µm (panel d) or 30 µm (subset panel d and others). (f) Proposed mechanism with sites of intervention. Data represent mean ± SEM; One-way ANOVA with Holm-Sidak correction (a,b,d). *P < 0.05, **P < 0.01, ***P < 0.001.
Splenectomy Attenuates Caspase-Mediated Neuronal Death
An immunofluorescent study was done to examine whether neuronal cells were involved in caspase-mediated pathways induced by infiltrating immune cells. We found elevated cleaved caspase-3-expressing neurons in HI rats with intact spleen compared to splenectomized rats at 3 d post-HI (Fig. 6c, d). TUNEL analysis was done to determine whether the effects on cell death were limited to neurons (Fig. 6e). Results showed that HI rats with intact spleen abundantly expressed TUNEL-positive cells localized in the nucleus of neuronal cells and not astrocytes, thereby further supporting our proposed mechanism for astrocyte-neuron dynamics in response to immune cells after HI (Fig. 6f).
Discussion
All components of the neurovascular unit respond to inflammation; therefore, interventions aimed at preserving merely neurons, have proven not to be clinically effective. Therefore, it is crucial to understand how cell components within the unit interact with each other during inflammatory responses. In this study we characterize the mechanistic interaction between cell of the glial and neuronal compartments in response to immigrating peripheral immune cells after a hypoxic-ischemic insult in the neonatal rat. Our major new finding is that the magnitude of inflammatory response in the ischemic immature brain is proportional to the extent of peripheral immune cell invasion and that these cells employ COX-2 to modulate astrocyte-neuron signaling and the extent of injury including infarct growth, long-term brain atrophy, somatic restriction, and functional deterioration.
We evidenced marked reductions in infarct volume in HI-injured animals with prior splenectomy as compared to animals with an intact spleen. Since the operative variable was splenectomy, our data in a compelling way links progression of brain injury post-HI to splenic immune cell populations. Previous reports indicated that inflammatory responses are a core component in the sequelae of neonatal HI, comprising a significant portion of secondary brain injury [26].
More specifically, we have found that the influx of COX-2 expressing immune cells enhances astrocytic IL-15 production and caspase-3-mediated neuronal death, which in sum may propagate brain damage. Our study has first revealed that splenic cells appear to be a crucial source of COX-2 as splenectomy dramatically reduced COX-2 expression in the ischemic brain. Of the many spleen-derived immune cells that may be involved in COX-2 production, we focused on NK cells which are a component of the innate immune system. Our findings suggest a substantial involvement of NK cells in the production of COX-2 after HI; however to further verify this, a gene silencer against NK cells was administered. Results demonstrated that knocking down CD161 of NK cells restored baseline levels of COX-2 in brain injured animals with an intact spleen, further suggesting NK cells may be a key spleen-derived cell type involved in COX-2 production.
In line with evidence implicating astrocyte-derived IL-15 in microglial activation, we observed an increase in IL-15 expression by astrocytes, as well as increases in neutrophil and macrophage abundance and microglia activation in brain injured animals [9, 27, 28]. On the other hand, splenic removal prior to HI-insult markedly attenuated these associated changes. Moreover, pharmacological blockade of COX-2 afforded a tremendous reduction in IL-15 mediated inflammatory propagation, as did splenectomy in the HI-injured rats. This led us to believe that down-stream COX-2 effectors from splenic immune cells may be potent stimulators of IL-15 production by binding to E-prostanoid receptors, which are present on astrocytes [29]. These COX-2 regulated signaling patterns were further supported when opposite effects (enhanced IL-15 and inflammatory propagation) were seen in HI-injured rats with administration of 4-HNE, a COX-2 agonist [30, 31].
Although, other lymphocyte-exclusive by-products, such as IL-2, have also been shown to stimulate IL-15 production, experimental studies using IL-2-deficient mice have reported no changes in immune function [32, 33]. Previous studies also showed that IL-15 plays a major role in inflammatory cell chemo-attraction and stimulation of killer activity in NK cells [34, 35]. Concordantly, our study shows that COX-2 blockade, which results in reduced IL-15 brain expression, decreases infiltration of inflammatory cells. However, COX-2-dependent expression of IL-15 is important not only for regulation of neuroinflammation, but when chronically elevated, may lead to neuronal death through the downregulation of PI3K/Akt pathway [9]. Consequently, we found that inhibition of IL-15 in the ischemic brain led to increased levels of PI3K. Likewise, a reduction in COX-2-expressing NK cells correlated to increased PI3K levels and decreased cleaved caspase-3 expression in neurons. Furthermore, the HI-induced elevations in cell death appear to be neuron-limited and not include astrocytes. Studies by earlier authors have shown that co-culture studies incubated with COX inhibitors suppress glial-mediated paracrine damage to neurons [29]. Collectively, this supports our notion that astrocytes are actively participating in production of down-stream effectors that induce neurodegeneration. However, future studies are needed to determine the exact mechanism of COX-2-mediated increases in cell death proteins after HI, involving IL-15 binding to the IL-15/PI3K neuronal complex [32].
In the present study we reveal that the HI-induced neuronal apoptosis [36] and activation of local inflammation [37] may be triggered by spleen-derived NK cells. Depletion of splenic immune cells through splenectomy led to neuroprotection and a lasting and robust improvement across most neurobehavioral tasks. However, it cannot be ruled out that the splenectomy-induced neuroprotection afforded 3 weeks post-HI may, in fact, not be a direct effect of there being less COX-2 producing immune cells to infiltrate the ischemic brain in the acute stage of HI. At later stages, studies have shown that adaptive immune responses promote production of neurotrophic factors involved in remodeling the post-ischemic brain [38]. Therefore, a possible explanation for the long-term benefits in our study could be that immunologic cells involved in enhancing brain plasticity within weeks after HI may have migrated from other lymphoid organs in the absence of the spleen [39]. Most importantly, this amelioration of long-term outcomes provides the basis for the clinical relevance of this study. Additionally, despite an inclusion of sham-operated controls, the surgery by itself was more extensive in splenectomized rats in this present study as removal of the spleen was not replicated in the sham-operated rats. Thus, in theory, surgical intervention on spleen tissue/vasculature might alter inflammatory responses and produce effects unrelated to splenic cell ablation thereby affecting outcomes measured at 3 days after surgery. However it may appear that only cutting through body envelopes and muscles can produce a severe inflammatory reaction while surgical trauma due to spleen dissection alone may not be significant. Laparoscopic surgery studies have shown a reduced inflammatory response as compared to conventional open surgery [40]. Moreover our earlier study with splenic irradiation that eliminated splenic cells without splenectomy showed robust benefits in terms of reducing brain invasion by immune cells after cerebral ischemia [41]. Therefore, present experiments, by including sham-operated controls that underwent opening of abdominal wall similar to splenectomized animals, appear adequately guarded against factors confounding inflammatory response in the treatment group.
In summary, we have identified a previously unrecognized COX-2-dependent link between splenic immune cells and signaling pathways controlling cellular inflammatory targets and neuronal death after neonatal HI. We also confirmed that the spleen appears to be a major source of peripheral immunologic cells that infiltrate and exacerbate brain damage through their interactions with astrocytes and neurons. Consequently, a depletion of splenic cells offers lasting protection against HI-induced neonatal brain injury. There is conformity between our present study and the previous work showing that both splenectomy and ACE inhibitor can reduce monocyte infiltration in myocardium and improve healing process after ischemia [42]. Therefore we believe that new modalities and pharmacological agents capable of reducing deployment of immunological cells from spleen offer therapeutic potential for the treatment of cerebrovascular and cardiovascular injuries.
Acknowledgements
The authors thank W. Tong and K. Cordero for their technical assistance on this project. This work was supported by a grant from the National Institutes of Health [NS054685 to J.H.Z.].
References
- 1.Alvarez-Diaz A, Hilario E, de Cerio FG, Valls-i-Soler A, Alvarez-Diaz FJ. Hypoxic-ischemic injury in the immature brain - key vascular and cellular players. Neonatology. 2007;92:227–235. doi: 10.1159/000103741. [DOI] [PubMed] [Google Scholar]
- 2.Gelderblom M, Leypoldt F, Steinbach K, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 3.Curin Y, Ritz MF, Andriantsitohaina R. Cellular mechanisms of the protective effect of polyphenols on the neurovascular unit in strokes. Cardiovasc Hematol Agents Med Chem. 2006;4:277–288. doi: 10.2174/187152506778520691. [DOI] [PubMed] [Google Scholar]
- 4.Lo EH. Experimental models, neurovascular mechanisms and translational issues in stroke research. Br J Pharmacol. 2008;153:S396–S405. doi: 10.1038/sj.bjp.0707626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.del Zoppo GJ. Inflammation and the neurovascular unit in the setting of focal cerebral ischemia. Neuroscience. 2009;158:972–982. doi: 10.1016/j.neuroscience.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou P, Qian L, Chou T, Iadecola C. Neuroprotection by PGE2 receptor EP1 inhibition involves the PTEN/AKT pathway. Neurobiol Dis. 2008;29:543–551. doi: 10.1016/j.nbd.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fathali N, Ostrowski RP, Lekic T, et al. Cyclooxygenase-2 inhibition provides lasting protection against neonatal hypoxic-ischemic brain injury. Crit Care Med. 2010;38:572–578. doi: 10.1097/CCM.0b013e3181cb1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21:570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gomez-Nicola D, Valle-Argos B, Pita-Thomas DW, Nieto-Sampedro M. Interleukin 15 expression in the CNS: blockade of its activity prevents glial activation after an inflammatory injury. Glia. 2008;56:494–505. doi: 10.1002/glia.20628. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34:249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]
- 11.Ajmo CT, Vernon DO, Collier L, et al. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–2234. doi: 10.1002/jnr.21661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hasegawa Y, Suzuki H, Sozen T, Rolland W, Zhang JH. Activation of sphingosine 1-phosphate receptor-1 by FTY720 is neuroprotective after ischemic stroke in rats. Stroke. 2010;41:368–374. doi: 10.1161/STROKEAHA.109.568899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yager JY, Wright S, Armstrong EA, Jahraus CM, Saucier DM. The influence of aging on recovery following ischemic brain damage. Behav Brain Res. 2006;173:171–180. doi: 10.1016/j.bbr.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 14.Hughes RN. The value of spontaneous alternation behavior (SAB) as a test of retention in pharmacological investigations of memory. Neurosci Biobehav Rev. 2004;28:497–505. doi: 10.1016/j.neubiorev.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Kusaka I, Kusaka G, Zhou C, et al. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- 16.Kveberg L, Dai KZ, Westgaard IH, Fossum MS, Naper C, Vaage JT. Two major groups of rat NKR-P1 receptors can be distinguished based on chromosomal localization, phylogenetic analysis and Clr ligand binding. Eur J Immunol. 2009;39:541–551. doi: 10.1002/eji.200838891. [DOI] [PubMed] [Google Scholar]
- 17.Vendrame M, Gemma C, Pennypacker KR, et al. Cord blood rescues stroke-induced changes in splenocyte phenotype and function. Exp Neurol. 2006;199:191–200. doi: 10.1016/j.expneurol.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Andine P, Thordstein M, Kjellmer I, et al. Evaluation of brain damage in a rat model of neonatal hypoxic-ischemia. J Neurosci Methods. 1990;35:253–260. doi: 10.1016/0165-0270(90)90131-x. [DOI] [PubMed] [Google Scholar]
- 19.Carty ML, Wixey JA, Colditz PB, Buller KM. Post-insult minocycline treatment attenuates hypoxia-ischemia-induced neuroinflammation and white matter injury in the neonatal rat: a comparison of two different dose regimens. Int J Dev Neurosci. 2008;26:477–485. doi: 10.1016/j.ijdevneu.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Balduini W, De Angelis V, Mazzoni E, Cimino M. Simvastatin protects against long-lasting behavioral and morphological consequences of neonatal hypoxic/ischemic brain injury. Stroke. 2001;32:2185–2191. doi: 10.1161/hs0901.094287. [DOI] [PubMed] [Google Scholar]
- 21.Gee JM, Kalil A, Shea C, Becker KJ. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38:783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- 22.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leonardo CC, Hall AA, Collier LA, Gottschall PE, Pennypacker KR. Inhibition of gelatinase activity reduces neural injury in an ex-vivo model of hypoxia-ischemia. Neuroscience. 2009;160:755–766. doi: 10.1016/j.neuroscience.2009.02.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strunk T, Hartel C, Temming P, Matzke N, Zimmer J, Schultz C. Erythropoietin inhibits cytokine production of neonatal and adult leukocytes. Acta Paediatr. 2008;97:16–20. doi: 10.1111/j.1651-2227.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 25.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 26.Hedtjarn M, Mallard C, Hagberg H. Inflammatory gene profiling in the developing mouse brain after hypoxia-ischemia. J Cereb Blood Flow Metab. 2004;24:1333–1351. doi: 10.1097/01.WCB.0000141559.17620.36. [DOI] [PubMed] [Google Scholar]
- 27.Bauer J, Rauschka H, Lassmann H. Inflammation in the nervous system: the human perspective. Glia. 2001;36:235–243. doi: 10.1002/glia.1112. [DOI] [PubMed] [Google Scholar]
- 28.Schroeter M, Jander S. T-cell cytokines in injury-induced neural damage and repair. Neuromolecular Med. 2005;7:183–195. doi: 10.1385/NMM:7:3:183. [DOI] [PubMed] [Google Scholar]
- 29.Shie FS, Montine KS, Breyer RM, Montine TJ. Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia. 2005;52:70–77. doi: 10.1002/glia.20220. [DOI] [PubMed] [Google Scholar]
- 30.Kakishita H, Hattori Y. Vascular smooth muscle cell activation and growth by 4-hydroxynonenal. Life Sci. 2001;69:689–697. doi: 10.1016/s0024-3205(01)01166-3. [DOI] [PubMed] [Google Scholar]
- 31.Kumagai T, Matsukawa N, Kaneko Y, Kusumi Y, Mitsumata M, Uchida K. A lipid peroxidation-derived inflammatory mediator: identification of 4-hydroxy-2-nonenal as a potential inducer of cyclooxygenase-2 in macrophages. J Biol Chem. 2004;279:48389–48396. doi: 10.1074/jbc.M409935200. [DOI] [PubMed] [Google Scholar]
- 32.Hanisch UK, Quirion R. Interleukin-2 as a neuroregulatory cytokine. Brain Res Rev. 1995;21:246–284. doi: 10.1016/0165-0173(95)00015-1. [DOI] [PubMed] [Google Scholar]
- 33.Kundig TM, Schorle H, Bachmann MF, Hengartner H, Zinkernagel RM, Horak I. Immune responses in interleukin-2-deficient mice. Science. 1993;262:1059–1061. doi: 10.1126/science.8235625. [DOI] [PubMed] [Google Scholar]
- 34.Carson WE, Giri JG, Lindemann MJ, et al. Interleukin (IL) 15 is a novel cytokine that activates human natural killer cells via components of the IL-2 receptor. J Exp Med. 1994;180:1395–1403. doi: 10.1084/jem.180.4.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diab A, Cohen AD, Alpdogan O, Perales MA. IL-15: targeting CD8+ T cells for immunotherapy. Cytotherapy. 2005;7:23–35. doi: 10.1080/14653240510018037. [DOI] [PubMed] [Google Scholar]
- 36.Beilharz EJ, Williams CE, Dragunow M, Sirimanne ES, Gluckman PD. Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Brain Res Mol Brain Res. 1995;29:1–14. doi: 10.1016/0169-328x(94)00217-3. [DOI] [PubMed] [Google Scholar]
- 37.Inder TE, Volpe JJ. Mechanisms of perinatal brain injury. Semin Neonatol. 2000;5:3–16. doi: 10.1053/siny.1999.0112. [DOI] [PubMed] [Google Scholar]
- 38.Linker R, Gold R, Luhder F. Function of neurotrophic factors beyond the nervous system: inflammation and autoimmune demyelination. Crit Rev Immunol. 2009;29:43–68. doi: 10.1615/critrevimmunol.v29.i1.20. [DOI] [PubMed] [Google Scholar]
- 39.Davis IA, Knight KA, Rouse BT. The spleen and organized lymph nodes are not essential for the development of gut-induced mucosal immune responses in lymphotoxin-alpha deficient mice. Clin Immunol Immunopathol. 1998;89:150–159. doi: 10.1006/clin.1998.4601. [DOI] [PubMed] [Google Scholar]
- 40.Novitsky YW, Litwin DE, Callery MP. The net immunologic advantage of laparoscopic surgery. Surgical endoscopy. 2004;18:1411–1419. doi: 10.1007/s00464-003-8275-x. [DOI] [PubMed] [Google Scholar]
- 41.Ostrowski RP, Schulte R, Ling T, Nie Y, Lee T, Manaenko A, Zhang JH. The acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Translational Stroke Res. 2012;3(4):473–481. doi: 10.1007/s12975-012-0206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leuschner F, Panizzi P, Chico-Calero I, et al. Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ Res. 2010;107:1364–73. doi: 10.1161/CIRCRESAHA.110.227454. [DOI] [PMC free article] [PubMed] [Google Scholar]