Abstract
Objective:
The study investigates the possible role of oxidative stress on renal tissues in association with petroleum hydrocarbon-induced nephrotoxicity.
Materials and Methods:
Rats of comparable weights were randomly distributed into 10 groups: Control and groups exposed to kerosene, petrol, and diesel via inhalation, contamination by food, and contamination by water. The exposure lasted for eight weeks.
Results:
Exposure to petroleum hydrocarbon led to significant rise in serum urea and creatinine, and renal tissue malondialdehyde. It also caused significant reduction in urinary urea and creatinine, and reduced glutathione, superoxide dismutase, and catalase activities of renal tissue homogenate. However, serum and urine concentrations of albumin and total protein were comparable in all groups.
Conclusion:
Results from this study shows that exposure to petroleum hydrocarbon led to renal dysfunction via oxidative stress, increasing lipid peroxidation and reducing the antioxidant defense mechanism.
Keywords: Diesel, kerosene, oxidative stress, petrol, renal function
INTRODUCTION
Crude petroleum is a mixture of different hydrocarbons and metals.[1] The chemical composition of crude petroleum varies between geologic formations.[2] It may be refined into fractions of kerosene, petrol, diesel, heavy gas oils, lubricating oils, as well as residual and heavy fuels among others; however, kerosene, petrol, and diesel are the most commonly used fractionated crude petroleum products. These fractions contain aliphatic, aromatic, and a variety of other branched saturated and unsaturated hydrocarbons at variable concentrations.[3,4]
Domestic and industrial use of petroleum, either in its crude or refined forms, has increased tremendously,[4] leading to increased exposure of users to its various constituent hydrocarbons. The common forms of exposure are inhalation, dermal contact, and ingestion of petroleum-contaminated food and water. Studies have documented the adverse environmental and health effects of petroleum hydrocarbons over the years. Previous studies reported the cardiotoxic,[5,6] hepatotoxic,[7,8] nephrotoxic,[4,9] and hematoxic[10] effects of hydrocarbons.
Exposure to various fractionated products of crude petroleum has been reported to cause impairment of renal function evident by the derangement of serum electrolytes.[4,7,9] However, to the best of our knowledge and as at the time of carrying out this study, no study has documented the effects of the common fractionated products of petroleum on renal function in association with oxidative stress. Therefore, this study investigates the possible role of oxidative stress on renal tissues in association with petroleum hydrocarbon-induced nephrotoxicity.
MATERIALS AND METHODS
Animal treatment
Sprague Dawley rats of comparable weights were obtained from the animal house of the Physiology Department, Ladoke Akintola University of Technology, Ogbomoso, Nigeria. They were housed in well-ventilated cages maintained at 25 ± 2°C, on a 12-hour light-dark cycle. The rats were on standard rat chow and tap water ad libitum. They were acclimatized for two weeks before the experimental period.[11,12] Procedures involving the animals and their care were performed in accordance with the guidelines of the Animal Ethics Committee of the institution and guidelines of the National Institutes of Health (NIH) for the care and use of animals.
Rats were assigned randomly to one of the ten experimental groups ( n=5) as follows: Control; Kerosene-inhalation (Ki); Petrol-inhalation (Pi); Diesel-inhalation (Di); Kerosene-food (Kf); Petrol-food (Pf); Diesel-food (Df); Kerosene-water (Kw); Petrol-water (Pw); Diesel-water (Dw).
Rats on inhalation were kept in the exposure chambers saturated with the appropriate vapors of the petroleum fractionated product. All treatments lasted for eight weeks.
Exposure to kerosene, petrol, and diesel vapors
A modified nose inhalation exposure method was used as previously described.[13,14] The cages housing the animals were placed in respective exposure chambers with calibrated beakers of 1000 cm3 containing 500 cm3 of kerosene, petrol, and diesel, respectively. The petroleum fractionated products were allowed to evaporate freely within the respective exposure chambers at ambient humidity and temperature, and the animals were exposed to vapors (0.5 ± 0.08 cm3/min/kg/m3/day) generated from direct evaporation of the petroleum products. The animals were exposed for five minutes daily. At the end of the exposure, the animals were transferred to a petroleum-free section of the animal house. The initial and final volumes of petroleum products in the beaker before and after exposure were respectively recorded. The differences in volume per day were used as an estimate of the relative concentrations of vapors used.
Exposure to feed and water contaminated by kerosene, petrol, and diesel
All feeding and drinking troughs were cleaned and kept hygienically throughout the experimental period. The animals were randomly allocated into dietary groups as stated. Rats on petroleum-contaminated water were administered 2.5 mL/ KgBW/5 mL water per oral feed. For petroleum-contaminated food, 100 mL of the appropriate petroleum fractionated product was incorporated into 150 g of rat feed and concentrated by simple mixing and homogenization with a manual mixer. The animals were starved for 24 hours before being introduced to the contaminated feed and water. This conforms to the method used in the previous study.[7]
Biochemical assay
Blood sample was collected via cardiac puncture. The 24-hour urine sample was collected; the volume was determined and recorded for each rat. Urine and serum levels of creatinine and urea were determined using alkaline picrate method and diacetyl monoxime method as previously described.[15] Urine and serum levels of total protein and albumin were determined using standard assay kits.[16]
Renal tissue malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT), and reduced glutathione (GSH) were assayed using standard laboratory methods using kidney homogenate.[17,18]
Statistical analysis
All data are expressed as mean ± SEM (SEM: Standard error of mean). The results were analyzed by one-way analysis of variance (ANOVA), followed by pairwise comparison between tests and control groups using student's t-test. Duncan's multiple range test was used post hoc. Differences between the groups were considered significant at P < 0.05.
RESULTS
Exposure to petroleum hydrocarbon led to a significant increase in serum urea and creatinine, and significant reduction in urinary urea and creatinine. Ingestion of petroleum hydrocarbon-contaminated food and water caused a more significant rise in serum creatinine when compared with that seen in inhalation of petroleum hydrocarbon, whereas serum urea was comparable in all hydrocarbon-exposed groups. Interestingly, the significant reduction in urinary urea and creatinine seen in hydrocarbon-exposed animals was not dependent on the route of exposure [Figures 1 and 2].
Figure 1.
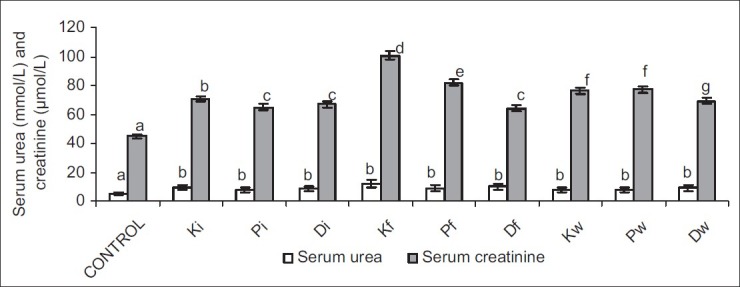
Effect of petroleum hydrocarbon exposure on serum urea and creatinine - Bars carrying different letters on the same parameter are significantly different at P < 0.05
Figure 2.
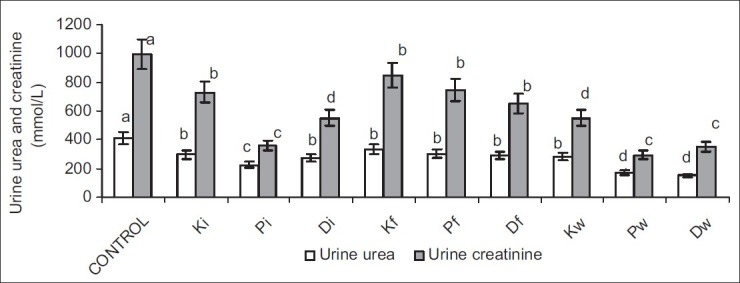
Effect of petroleum hydrocarbon exposure on urine urea and creatinine - Bars carrying different letters on the same parameter are significantly different at P < 0.05
Serum albumin and total protein were comparable in all groups. Similarly, urinary albumin and total protein were not significantly different across all groups [Figures 3 and 4].
Figure 3.
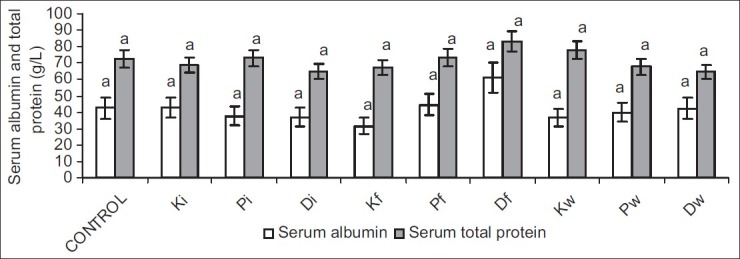
Effect of petroleum hydrocarbon on serum albumin and total protein - Bars carrying different letters on the same parameter are significantly different at P < 0.05
Figure 4.
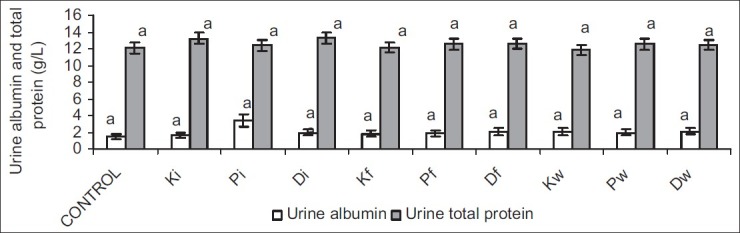
Effect of petroleum hydrocarbon exposure on urine albumin and total protein - Bars carrying different letters on the same parameter are significantly different at P < 0.05
Petroleum hydrocarbon led to a significant rise in MDA, and significant reduction in GSH, SOD, and CAT activities in the rat kidney. Petroleum hydrocarbon-induced rise in MDA was not route dependent; however, the rats exposed to diesel showed the most significant increase in MDA in each route of exposure followed by those exposed to petrol. However, in the inhalation groups, kerosene and petrol caused a comparable rise in renal MDA.
Ingestion of hydrocarbon-contaminated food caused a significantly higher reduction in SOD and CAT activities when compared with other exposed groups. Nevertheless, alterations in GSH following petroleum hydrocarbon exposure were not route related [Figures 5–8].
Figure 5.
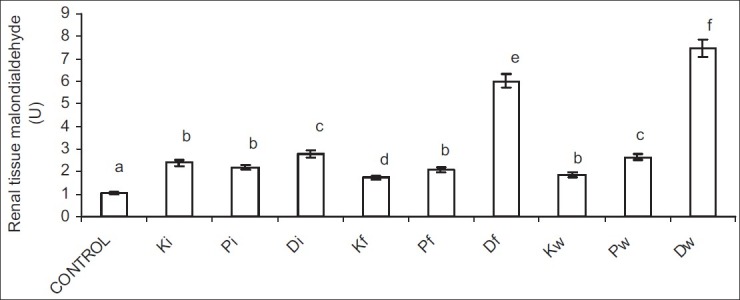
Effect of petroleum hydrocarbon on renal tissue homogenate malondialdehyde - Bars carrying different letters on the same parameter are significantly different at P < 0.05
Figure 8.
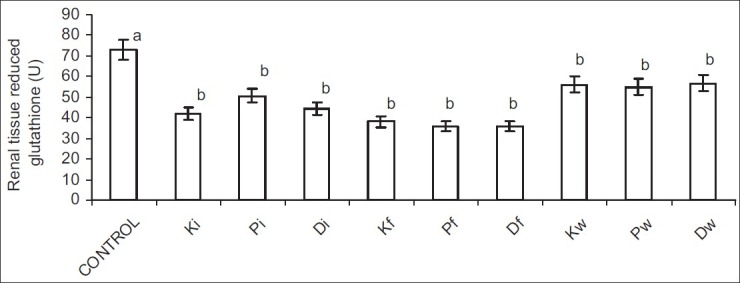
Effect of petroleum hydrocarbon on renal tissue homogenate reduced glutathione -Bars carrying different letters on the same parameter are significantly different at P < 0.05
Figure 6.
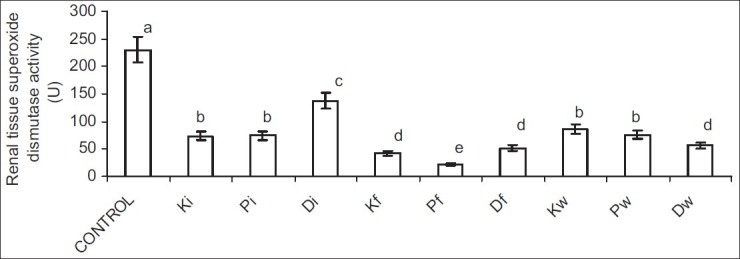
Effect of petroleum hydrocarbon on renal tissue homogenate superoxide dismutase activity - Bars carrying different letters on the same parameter are significantly different at P < 0.05
Figure 7.
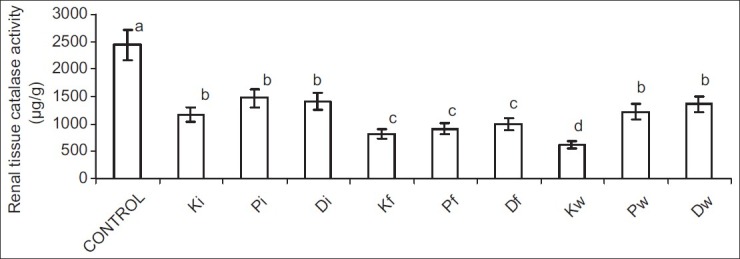
Effect of petroleum hydrocarbon on renal tissue homogenate catalase activity - Bars carrying different letters on the same parameter are significantly different at P < 0.05
DISCUSSION
Petroleum products have become an essential constituent of human life due to their industrial and domestic values. However, clinical and experimental studies point to the exposure to petroleum hydrocarbon as a risk factor for renal diseases.[7,19–22] This study thus investigated the effect of exposure to petroleum hydrocarbon on renal functions and the role of oxidative stress in hydrocarbon-induced alteration in renal function.
Plasma and urinary concentrations of urea and creatinine are indices of renal function. Low urinary clearance of urea and/or creatinine evident by their low urinary concentrations and consequent rise in their serum levels are pointers to impaired renal function.[23] This study revealed that exposure to petroleum hydrocarbon via various routes (inhalation and oral) caused a significant increase in serum urea and creatinine concentrations, and decrease in urinary urea and creatinine concentrations. This is in accord with previous studies[7,8,9] that reported similar results. This observation suggests an impaired glomerular filtration rate (GFR), a common manifestation of nephritic damage, possibly due to the damaged proximal tubule monolayer resulting in gaps in the epithelial lining leading to a back leak of filtrate.[24]
Albumin and total protein are substances with very low glomerular filterability due to their high molecular weights and negative electric charges.[25] The results from this study showed that serum and urinary concentrations of albumin and total protein were comparable across all groups. This could imply that though exposure to petroleum hydrocarbon via inhalation and ingestion of both contaminated food and water caused impaired renal function, it did not lead to proteinuria or albuminuria, suggesting a preservation of the negative charges of the basement membrane and podocytes.
Oxidative stress is the presence of reactive oxygen species (ROS) in excess of the available antioxidant-buffering capacity.[26] ROS can damage molecular targets—proteins, lipids and DNA, thus altering the structure and function of the cell, tissue, organ, or system.[27] This study seems to be the first to document the oxidant effect of petroleum hydrocarbon on renal tissue. Observations from this study revealed that exposure to petroleum hydrocarbon led to oxidative damage of the kidney evident by a rise in renal MDA, and reduction in glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) activities. This agrees with a previous study[28] that reported the prooxidant effect of hydrocarbons. The observed oxidative stress on the kidney could be associated with the hydrocarbon-induced renal dysfunction seen in this study. The results of this study thus suggest that oxidative stress is a primary mode of action of petroleum hydrocarbon-induced renal dysfunction.
In conclusion, this study shows that exposure to petroleum hydrocarbon is a risk factor for impairment of renal function via a mechanism dependent on oxidative stress, increasing lipid peroxidation and reducing enzymatic antioxidant defense mechanism. We therefore suggest that caution should be taken to reduce exposure to hydrocarbons, and exposed subjects should regularly monitor their renal functions.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Edwards CW. Toxicology of oil field waste hazards to livestock associated with the petroleum industry. Vet Clin North Am. 1989;5:363–74. [PubMed] [Google Scholar]
- 2.Coppock RW, Mostrom MS, Khan A, Semalula SS. Toxicology of oil field pollutants in cattle: A review. Vet Hum Toxicol. 1995;37:369–576. [PubMed] [Google Scholar]
- 3.Kato M, Rocha ML, Carvallio AB, Chares ME, Rana MC, Oliverira FC. Occupational exposure to neurotoxicants; preliminary survey in five industries of Camacari petrochemical complex, Brazil. Environ Res. 1996;136:49–56. doi: 10.1006/enrs.1993.1057. [DOI] [PubMed] [Google Scholar]
- 4.Uboh FE, Akpanabiatu MI, Ndem JI, Alozie Y, Ebong PE. Comparative nephrotoxic effect associated with exposure to diesel and gasoline vapours in rats. J Toxicol Environ Health Sci. 2009;1:68–74. [Google Scholar]
- 5.Aboutabl ME, Zordoky BN, Hammock BD, El-Kadi AO. Inhibition of soluble epoxide hydrolase confers cardioprotection and prevents cardiac cytochrome P450 induction by benzo (a) pyrene. J Cardiovasc Pharmacol. 2011;57:273–81. doi: 10.1097/FJC.0b013e3182055baf. [DOI] [PubMed] [Google Scholar]
- 6.Barath S, Mills NL, Lundbäck M, Törnqvist H, Lucking AJ, Langrish JP, et al. Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions. Part Fibre Toxicol. 2010;7:19. doi: 10.1186/1743-8977-7-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovuru SS, Berepubo NA, Nodu MB. Biochemical blood parameters in semi-adult rabbits experimentally fed crude oil contaminated diets. Afr J Biotechnol. 2004;3:343–5. [Google Scholar]
- 8.George MI, Adegoke OA. Effect of vitamin E on biochemical parameters in albino rats treated with gasoline. J Sci Res. 2011;3:641–9. [Google Scholar]
- 9.Orisakwe E, Njan AA, Afonne OJ, Akumka DD, Orish VN, Udemezue OO. Investigation into the nephrotoxicity of Nigerian bonny light crude oil in albino rats. Int J Environ Res Public Health. 2004;1:106–10. doi: 10.3390/ijerph2004020106. [DOI] [PubMed] [Google Scholar]
- 10.Adebayo AH, Zeng GZ, Zhang YM, Ji CJ, Akindahunsi AA, Tan1 NH. Toxicological evaluation of precocene II isolated from Ageratum conyzoides L.(Asteraceae) in Sprague Dawley rats. Afr J Biotechnol. 2010;9:2938–44. [Google Scholar]
- 11.Akhigbe RE, Ige SF, Afolabi AO, Oyeyipo PI, Ajao FO, Ajayi FA. Water balance and serum levels of some electrolytes in oral contraceptive-treated female Wistar rats. J Med Sci. 2008;8:591–4. [Google Scholar]
- 12.Akhigbe RE, Olatunji LA, Soladoye AO, Oyeyipo IP. Effect of angiotensin 1-converting enzyme inhibitor, captopril, on body weight, and food and water consumption in oral contraceptive-treated rats. Am J Biochem Mol Biol. 2011;1:95–100. [Google Scholar]
- 13.Uboh FE, Akpanabiatu MI, Ekaidem IS, Ebong PE, Umoh IB. Effect of inhalation exposure to gasoline fumes on sex hormonesprofile in Wistar albino rats. Acta Endocrinol (Buc) 2007;3:23–30. [Google Scholar]
- 14.Uboh FE, Akpanabiatu MI, Eteng MU, Ebong PE, Umoh IB. Toxicological effects of exposure to gasoline vapours in male and female rats. Internet J Toxicol. 2008;4:59–63. [Google Scholar]
- 15.Ceriotti G, Spandro LA. A spectrophotometric method for determmation of urea. Clin Chim Acta. 1963;8:295–9. doi: 10.1016/0009-8981(63)90171-2. [DOI] [PubMed] [Google Scholar]
- 16.Akhigbe RE, Azeez OM, Ige SF, Oyeyipo IP, Ajao FO, Soladoye AO. Hemorheological effect of long-term administration of oral contraceptive in rats. Int J Pharm. 2008;4:403–6. [Google Scholar]
- 17.Ige SF, Akhigbe RE, Edeogho O, Ajao FO, Owolabi OQ, Oyekunle OS, et al. Hepatoprotective activities of Allium cepa in cadmium-treated rats. Int J Pharm Pharm Sci. 2011;3:60–3. [Google Scholar]
- 18.Adewole SO, Ojewole JA. Protective effects of Annona muricata Linn. (Annonaceae) leaf aqueous extract on serum lipid profiles and oxidative stress in hepatocytes of streptozotocin-treated diabetic rats. Afr J Tradit Complement Altern Med. 2009;6:30–41. doi: 10.4314/ajtcam.v6i1.57071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravnskov U. Hydrocarbon exposure may cause glomerulonephritis and worsen renal function: Evidence based on Hill's criteria for causality. QJM. 2000;93:551–6. doi: 10.1093/qjmed/93.8.551. [DOI] [PubMed] [Google Scholar]
- 20.Poole C, Dreyer NA, Satterfield MH, Levin L, Rothman KJ. Kidney cancer and hydrocarbon exposures among petroleum refinery workers. Environ Health Perspect. 1993;101(Suppl 6):53–62. doi: 10.1289/ehp.93101s653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaqoob M, Bell GM, Stevenson A, Mason H, Percy DF. Renal impairment with chronic hydrocarbon exposure. Q J Med. 1993;86:165–74. [PubMed] [Google Scholar]
- 22.Yaqoob M, Stevenson A, Mason H, Bell GM. Hydrocarbon exposure and tubular damage: Additional factors in the progression of renal failure in primary glomerulonephritis. Q J Med. 1993;86:661–7. doi: 10.1093/qjmed/86.10.661. [DOI] [PubMed] [Google Scholar]
- 23.Henry JB. Clinical diagnosis and management by laboratory methods. 20th ed. Philadelphia, PA: W. B. Saunders Company; 2001. [Google Scholar]
- 24.Counts RS, Nowak G, Wyatte RD, Schnellman RG. Nephrotoxicant inhibition of renal proximal tubule cell regeneration. Am J Physiol. 1995;169F:274–81. doi: 10.1152/ajprenal.1995.269.2.F274. [DOI] [PubMed] [Google Scholar]
- 25.Guyton AC, Hall JE. Textbook of medical physiology. 10th ed. New Delhi, India: Elsevier; 2001. pp. 309–10. [Google Scholar]
- 26.Adly AA. Oxidative stress and disease: An updated review. Res J Immunol. 2010;3:129–45. [Google Scholar]
- 27.Roberts JM, Hubel CA. Oxidative stress in preeclampsia. Am J Obstet Gynecol. 2004;190:1177–8. doi: 10.1016/j.ajog.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Alisi CS, Ojiako AO, Osuagwu CG, Onyeze GO. Response pattern of antioxidants to lipid peroxide concentration in carbon tetrachloride-induced hepato-toxicity is tightly logistic in rabbits. Eur J Med Plants. 2011;1:118–29. [Google Scholar]


