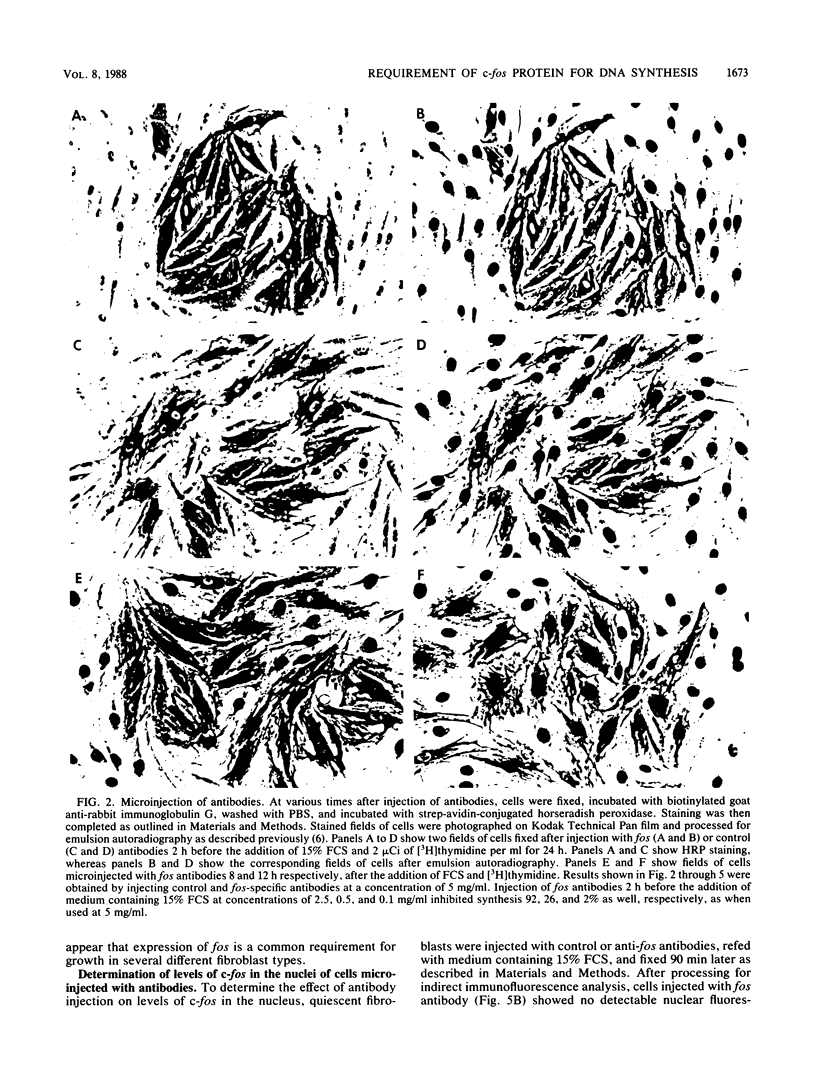
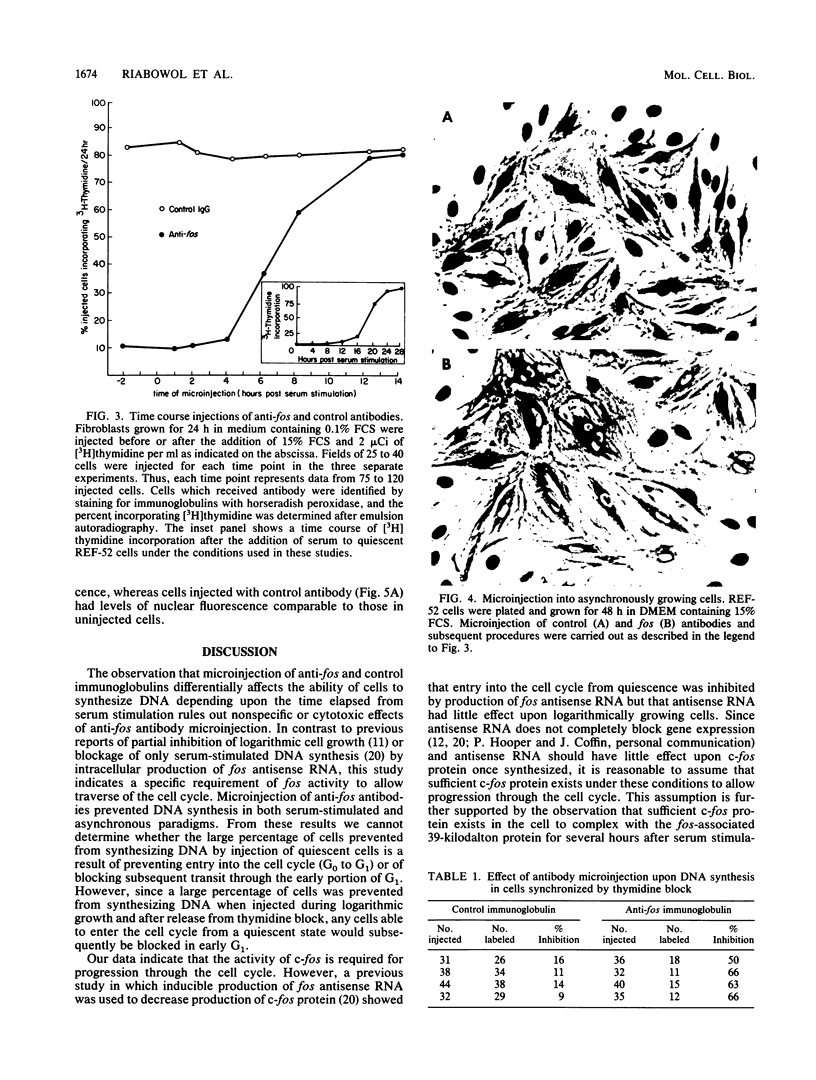
Abstract
Transcription of the protooncogene c-fos is increased greater than 10-fold within minutes of treatment of fibroblasts with serum or purified growth factors. Recent experiments with mouse 3T3 cell lines containing inducible fos antisense RNA constructs have shown that induced fos antisense RNA transcripts cause either a marked inhibition of growth in continuously proliferating cells or, conversely, a minimal effect except during the transition from a quiescent (G0) state into the cell cycle. Since intracellular production of large amounts of antisense RNA does not completely block gene expression, we microinjected affinity-purified antibodies raised against fos to determine whether and when during the cell cycle c-fos expression was required for cell proliferation. Using this independent method, we found that microinjected fos antibodies efficiently blocked serum-stimulated DNA synthesis when injected up to 6 to 8 h after serum stimulation of quiescent REF-52 fibroblasts. Furthermore, when fos antibodies were injected into asynchronously growing cells, a consistently greater number of cells was prevented from synthesizing DNA than when cells were injected with nonspecific immunoglobulins. Thus, whereas the activity of c-fos may be necessary for transition of fibroblasts from G0 to G1 of the cell cycle, its function is also required during the early G1 portion of the cell cycle to allow subsequent DNA synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber J. R., Verma I. M. Modification of fos proteins: phosphorylation of c-fos, but not v-fos, is stimulated by 12-tetradecanoyl-phorbol-13-acetate and serum. Mol Cell Biol. 1987 Jun;7(6):2201–2211. doi: 10.1128/mcb.7.6.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Burckhardt J., Curran T., Müller R. Expression of c-fos in NIH3T3 cells is very low but inducible throughout the cell cycle. EMBO J. 1986 Apr;5(4):695–700. doi: 10.1002/j.1460-2075.1986.tb04269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Curran T., Van Beveren C., Verma I. M. Viral and cellular fos proteins are complexed with a 39,000-dalton cellular protein. Mol Cell Biol. 1985 Jan;5(1):167–172. doi: 10.1128/mcb.5.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel R. J., Ro H. S., Rosen B. S., Groves D. L., Spiegelman B. M. Nucleoprotein complexes that regulate gene expression in adipocyte differentiation: direct participation of c-fos. Cell. 1987 Jun 19;49(6):835–844. doi: 10.1016/0092-8674(87)90621-0. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., Gilman M. Z., Maruyama M., Weinberg R. A. c-myc and c-fos expression in differentiating mouse primary keratinocytes. EMBO J. 1986 Nov;5(11):2853–2857. doi: 10.1002/j.1460-2075.1986.tb04579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feramisco J. R., Gross M., Kamata T., Rosenberg M., Sweet R. W. Microinjection of the oncogene form of the human H-ras (T-24) protein results in rapid proliferation of quiescent cells. Cell. 1984 Aug;38(1):109–117. doi: 10.1016/0092-8674(84)90531-2. [DOI] [PubMed] [Google Scholar]
- Finkel M. P., Biskis B. O., Jinkins P. B. Virus induction of osteosarcomas in mice. Science. 1966 Feb 11;151(3711):698–701. doi: 10.1126/science.151.3711.698. [DOI] [PubMed] [Google Scholar]
- Franza B. R., Jr, Sambucetti L. C., Cohen D. R., Curran T. Analysis of Fos protein complexes and Fos-related antigens by high-resolution two-dimensional gel electrophoresis. Oncogene. 1987 May;1(2):213–221. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hayes T. E., Kitchen A. M., Cochran B. H. Inducible binding of a factor to the c-fos regulatory region. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1272–1276. doi: 10.1073/pnas.84.5.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht D. A., Loomis W. F. Antisense RNA inactivation of myosin heavy chain gene expression in Dictyostelium discoideum. Science. 1987 May 29;236(4805):1081–1086. doi: 10.1126/science.3576221. [DOI] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Mercer W. E., Avignolo C., Baserga R. Role of the p53 protein in cell proliferation as studied by microinjection of monoclonal antibodies. Mol Cell Biol. 1984 Feb;4(2):276–281. doi: 10.1128/mcb.4.2.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan J. I., Cohen D. R., Hempstead J. L., Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987 Jul 10;237(4811):192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- Mulcahy L. S., Smith M. R., Stacey D. W. Requirement for ras proto-oncogene function during serum-stimulated growth of NIH 3T3 cells. Nature. 1985 Jan 17;313(5999):241–243. doi: 10.1038/313241a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quade K. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology. 1979 Oct 30;98(2):461–465. doi: 10.1016/0042-6822(79)90569-5. [DOI] [PubMed] [Google Scholar]
- Sambucetti L. C., Curran T. The Fos protein complex is associated with DNA in isolated nuclei and binds to DNA cellulose. Science. 1986 Dec 12;234(4782):1417–1419. doi: 10.1126/science.3491427. [DOI] [PubMed] [Google Scholar]
- Setoyama C., Frunzio R., Liau G., Mudryj M., de Crombrugghe B. Transcriptional activation encoded by the v-fos gene. Proc Natl Acad Sci U S A. 1986 May;83(10):3213–3217. doi: 10.1073/pnas.83.10.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Watson T., Kung H. F., Curran T. Microinjection of transforming ras protein induces c-fos expression. Mol Cell Biol. 1987 Jan;7(1):523–527. doi: 10.1128/mcb.7.1.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Verrier B., Müller D., Bravo R., Müller R. Wounding a fibroblast monolayer results in the rapid induction of the c-fos proto-oncogene. EMBO J. 1986 May;5(5):913–917. doi: 10.1002/j.1460-2075.1986.tb04303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Nuclear and nucleolar localization of the 72,000-dalton heat shock protein in heat-shocked mammalian cells. J Biol Chem. 1984 Apr 10;259(7):4501–4513. [PubMed] [Google Scholar]
- Zullo J., Stiles C. D., Garcea R. L. Regulation of c-myc and c-fos mRNA levels by polyomavirus: distinct roles for the capsid protein VP1 and the viral early proteins. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1210–1214. doi: 10.1073/pnas.84.5.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]