Abstract
Background:
Underreporting of spontaneous adverse drug reaction (ADR) is a threat to pharmacovigilance. Various factors related with the knowledge and attitudes are responsible for underreporting of ADRs.
Aims:
The study was aimed at investigating the knowledge and attitudes of doctors to ADR reporting.
Materials and Methods:
It was a questionnaire-based cross-sectional study. One hundred and eight questionnaires were administered to doctors working in a teaching hospital with an ADR monitoring center.
Statistical Analysis Used:
The descriptive statistics were used for responses to evaluate the knowledge and attitudes toward ADR reporting. Pearson's Chi-square test was used to observe the association of knowledge and attitude with experience and position.
Results:
The response rate was 62.9%. Spontaneous reporting rate was found to be 19.1%. The major factors found to be responsible for underreporting of ADR include inadequate risk perception about newly marketed drugs (77.9%), fear factor (73.5%), diffidence (67.7%), lack of clarity of information on ADR form about reporting (52.9%), lethargy (42.7%), insufficient training to identify ADRs (41.2%), lack of awareness about existence of pharmacovigilance program (30.9%) and ADR monitoring center in the institute (19.1%), and inadequate risk perception of over-the-counter (OTC) product (20.6%) and herbal medicines (13.2%). Experience and position did not influence the knowledge and attitudes of doctors.
Conclusion:
The deficiencies in knowledge and attitudes require urgent attention not only to improve the rate of spontaneous reporting, but also for enhanced safety of the patients and society at large.
Keywords: Attitude, doctors, knowledge, pharmacovigilance, spontaneous reporting
INTRODUCTION
Adverse drug reaction (ADR) is defined by World Health Organization (WHO) as “a response to a drug which is noxious and unintended, and which occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease or for the modification of physiological function.”[1] ADRs are one of the major health care problems occurring throughout the world. They affect the people with varying magnitudes, causing both morbidity and mortality.[2,3]
Pharmacovigilance is the science and activities related to the detection, assessment, understanding, and prevention of adverse effects or any other possible drug-related problems.[4] To transform the concept of pharmacovigilance into practice for enhancing the safety of patients, ADR monitoring centers (AMCs) are being set up across the country under Pharmacovigilance Programme of India (PvPI).[5]
Spontaneous reporting of ADRs has played a major role in the detection of unsuspected, serious, and unusual ADRs previously undetected during the clinical trial phases. This has led to the withdrawal of many drugs in the recent past, i.e., rofecoxib, cisapride, terfenadine.[6] The contribution of health professionals is enormous in this regard. However, underreporting still remains a major obstacle in the complete success of pharmacovigilance program.[7] It is found that only 6- 10% of all ADRs are reported.[8,9] This high rate of underreporting is a matter of great concern which can delay detection of serious ADRs and consequently have a major negative impact on the public health.
Various factors have been attributed for underreporting of ADRs among health professionals. These factors are based on knowledge and perception of health professionals to reporting. Inman has described them as “seven deadly sins.” These include: Financial incentives: Rewards for reporting; legal aspects: Fear of litigation or enquiry into prescribing costs and ambition to compile or publish a personal case series; complacency: The belief that very serious ADRs are well documented by the time a drug is marketed; diffidence: The belief that reporting an ADR would only be done if there was certainty that it was related to the use of a particular drug; indifference: The belief that the single case an individual doctor might observe could not contribute to medical knowledge; ignorance: The belief that it is only necessary to report serious or unexpected ADRs, and excuses made by professionals; and lethargy: The procrastination and disinterestedness in reporting or lack of time to find a report card, and other excuses.[10]
The factors responsible for underreporting have not been extensively studied in India. A previous study from India has found inadequate knowledge of resident doctors about ADRs.[11] However, the study excluded other cadres of doctors. Therefore, the present study was proposed to investigate the knowledge and attitudes of doctors to ADR reporting in a teaching hospital in India and to suggest possible ways of improving spontaneous reporting based on our findings. It would be also interesting to see the influence of work experience and position/level on the knowledge and attitudes of doctors.
MATERIALS AND METHODS
This was a questionnaire-based cross-sectional study. The study tool was a pre-designed questionnaire adapted from previous studies,[11–12,13] with some changes to suit local environment. The questionnaire was structured to observe the knowledge and attitudes of doctors toward reporting ADRs and the various factors that doctors perceived may influence reporting. It was a closed-ended questionnaire. The respondents were allowed to strike multiple options wherever applicable. Suggestions on possible ways to improve ADR reporting were also obtained. After explaining the purpose of the study, the questionnaire was administered to 108 doctors working in pre-clinical, para-clinical and clinical departments. To enhance the response rate, the doctors were requested to complete the questionnaire and hand it back immediately, and those who were busy at that moment were requested to return back the duly filled questionnaires within 1 week. The study was done in the period between July 2011 and September 2011.
Statistical analysis
The data were entered into the computer database and responses of frequencies were calculated and analyzed by using statistical software SPSS version 11.0. The descriptive statistics were used for responses among doctors to identify the knowledge and attitudes toward ADR reporting. Pearson's Chi-square test was used to observe the association of knowledge and attitude regarding ADR reporting and experience/position in medical field at P < 0.05 significant level.
RESULTS
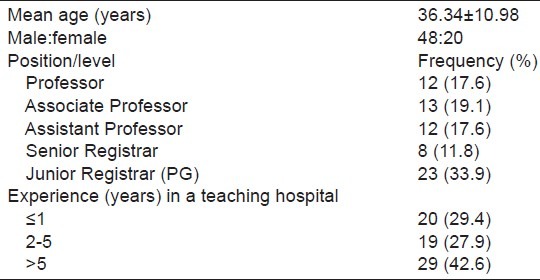
Of a total of 108 questionnaires administered to doctors, 68 were duly filled and returned, thus giving a response rate of 62.9%. The observed demographics and characteristics of the respondents are depicted in Table 1.
Table 1.
Demographics and experience (years)
Awareness of ADR reporting system and pharmacovigilance
Out of 68 respondent doctors, 48 (70.6%), 18 (26.5%), and 2 (2.9%) were from clinical, para-clinical, and pre-clinical departments, respectively.
Almost all doctors (97.06%) revealed that they were qualified to report adverse reactions to drugs, while pharmacists and physiotherapists were the least considered to report an ADR [Figure 1].
Figure 1.
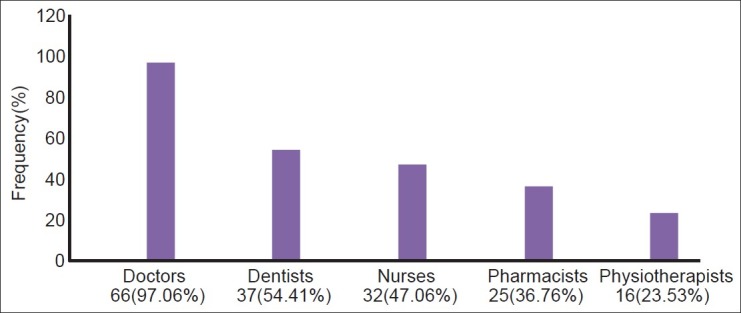
Health professionals qualified to report adverse drug reactions (N = 68)
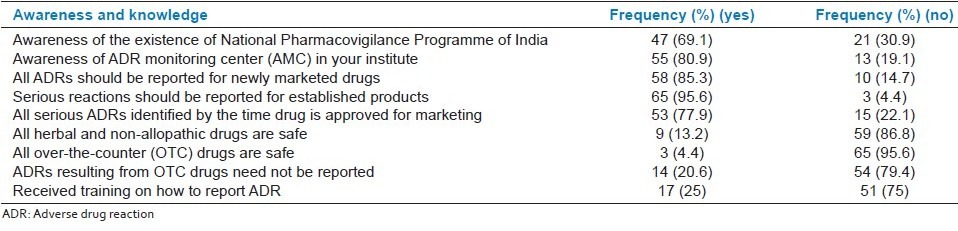
Forty-seven (69.1%) participants were aware of the existence of PvPI, while 55 (80.9%) doctors were aware of the AMC in the institute [Table 2a]. Major proportion (85.3%) of the doctors were aware that all ADRs should be reported to newly marketed drugs and almost all respondents (95.6%) knew that serious reactions should be reported for established products. Nearly all the respondents (95.6%) opined that all over-the-counter (OTC) drugs are not safe, whereas 54 (79.4%) agreed that ADRs resulting from OTC drugs need to be reported. Fifty-three (77.9%) doctors felt that all serious ADRs to a drug are identified by the time it is approved for marketing, while 59 (86.8%) considered herbal and non-allopathic drugs to be unsafe [Table 2a].
Table 2a.
Evaluation of awareness and knowledge of doctors to adverse drug reaction reporting (N = 68)
Factors influencing ADR reporting
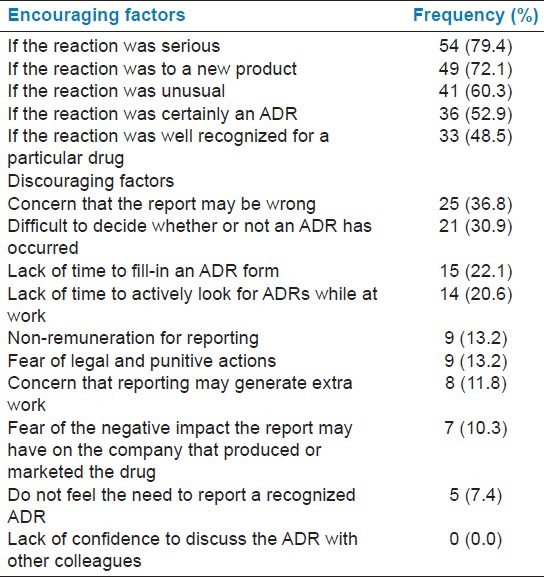
Most respondents were encouraged to report ADRs if the reaction was serious (79.4%), if the reaction was to a new product (72.1%), and was unusual (60.3%) in nature. Concern that the report may be wrong (36.8%), difficulty in deciding whether an ADR has occurred or not (30.9%), lack of time to fill-in ADR form (22.1%), and lack of time to actively look for ADRs while at work (20.6%) were the most discouraging factors [Table 3].
Table 3.
Study of factors influencing ADR reporting (N = 68)
Attitudes to reporting ADRs
Forty-five (66.2%) respondents considered ADR reporting to be professional obligation. 16.2% of the respondents opined that reporting of only one ADR makes no significant contribution to ADR database. Thirty-six (52.9%) doctors did not find the information on ADR form very clear about what to report. That ADR reporting should hide the identity of the prescriber was felt by 21 (30.9%) and that it should hide the identity of the reporter was expressed by 29 participants (42.6%) [Table 2b].
Table 2b.
Evaluation of attitudes and practice of doctors to adverse drug reaction reporting (N = 68)
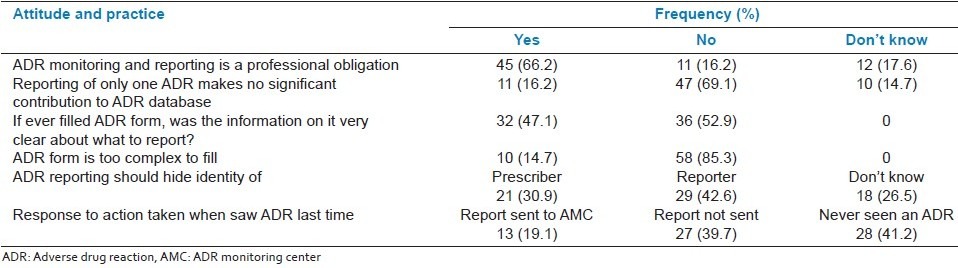
The response to action taken when the ADR was seen last time, only 13 (19.1%) respondents stated that ADR report was sent to AMC. Twenty-eight (41.2%) doctors disclosed that they had never seen an ADR [Table 2b].
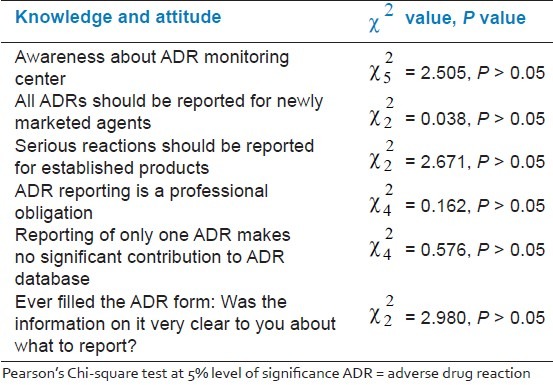
No significant association was observed when experience was compared with the following: Awareness of AMC, reporting ADRs to newly marketed drugs, serious reactions to established products, ADR reporting is a professional obligation, reporting of only one ADR makes no significant contribution to the ADR database, and ever filled the ADR form: Was the information on it very clear about what to report? [Table 4]. Similarly, knowledge and attitude was not significantly influenced when compared with the position/level of the doctors.
Table 4.
Comparison of knowledge and attitudes with experience
DISCUSSION
Underreporting of ADRs is a major threat to the success of pharmacovigilance program. Various factors have been found to be responsible for underreporting of ADRs by doctors. These factors are mainly related with the knowledge and attitudes.[10] Very few studies have been conducted to find out these factors in Indian doctors. Therefore, the present study was performed to investigate the knowledge and attitudes of doctors to ADR reporting in a tertiary care teaching hospital with an AMC.
Spontaneous ADR reporting by other health professionals is being recommended by national pharmacovigilance program[14] but not recognized by the participants, as is reflected from the above results [Figure 1]. Similar results were obtained in another study.[11] Involvement of other health care professionals and paramedical staff will go a long way in improving spontaneous reporting of ADRs.
The results reflect upon the lack of awareness of participants about the existence of ADR reporting system [Table 2a], which would ultimately affect the reporting. Similar observations were also reported in other studies.[15–18] The deficits in the spontaneous reporting can be significantly reduced if the medical professionals are aware of the importance of reporting ADRs and the reporting system. Therefore, increasing awareness about pharmacovigilance program and AMC through personal communication and advertisement appears necessary to enhance reporting.
Although majority of the doctors felt that ADR reporting is a professional obligation, they would be encouraged to report if the reaction is serious (79.4%), to a new product (72.1%), and is unusual (60.3%), which is similar to the results obtained in other studies.[15,18] However, 17.6% doctors were unaware of professional obligation to report ADRs and 16.2% declined to accept ADR reporting as a professional obligation [Table 2b]. Personal discussions and awareness programs will help to remove misconceptions and modify the attitudes of doctors, whereby ADR reporting is perceived as an integral part of clinical practice. Furthermore, attitude (16.2%) that reporting of only one ADR makes no significant difference also needs to be changed. This may translate into enhanced spontaneous reporting in the long term.
ADRs resulting from OTC product need not be reported was felt by 20.6% participants. To foster reporting culture, pharmacovigilance programme recommends reporting of all suspected, even non-serious and common ADRs related with the drugs, including OTC products.[14] This needs to be communicated to the doctors to improve ADR reporting. ADRs of herbal and traditional medicine is well known and reported in literature,[19] but still 13.2% doctors considered all herbal and non-allopathic drugs to be safe. Marketing approval is given to a product after phase III clinical trial. Many a time, serious and unusual ADRs are not identified during phase III trial, but are detected later on when the drug is available for use to general population. Inability of the respondents (77.9%) to identify the serious risk of newly marketed drug is an alarming situation and needs to be addressed urgently. As the Indian market is flooded with the arrival of newer and newer drugs, delayed detection of serious ADR may prove to be disastrous to the patients and society at large.
The findings of our study are indicative of the inadequate risk perceptions of participants about newly marketed drugs as well as OTC and herbal medicines. Probably, the training in medicine risk perceptions is insufficient to prepare the doctors for the task of ADR monitoring and reporting.
The present study found that diffidence (67.7%) (concern that the ADR may be wrong and difficult to decide whether an ADR has occurred or not) and lethargy (42.7%) (lack of time to fill-in ADR form and lack of time to actively look for ADRs while at work) would significantly affect the ADR reporting among the doctors working in a teaching hospital. Ignorance, fear, and financial incentives had a little influence on the respondents [Table 3]. These findings suggest underreporting of ADRs to be associated with gaps in the knowledge and perception, which is also pointed out in other studies.[13,20–22]
Interestingly, fear is not a discouraging factor for majority of the doctors to report an ADR Table 3. But fear factor is evident from high proportion of doctors suggesting to hide the identity of prescriber (30.9%) or reporter (42.6%). Similar results were also found in another study.[11] Fear or apprehension has a potential to undermine the spontaneous reporting of ADRs. Therefore, this needs to be allayed urgently through informing doctors that ADRs are natural accompaniments of therapy and can be prevented through diligent and rational use of drugs. Doctor cannot be held responsible for any such reaction provided he/she adheres to the principles of rational prescription writing.[23]
Majority of the doctors (85.3%) opined that the ADR form was not too complex to fill. However, the information on filling of the ADR form about what to report were identified by only 47.1% respondents [Table 2b]. Therefore, there appears a need to further simplify the ADR form to make it more objective and clear about what to report.
Work experience in a medical college/hospital does not influence the knowledge and attitudes of doctors toward reporting of ADRs [Table 4]. Perhaps, the undergraduate and post-graduate training lacks in preparing the doctors for the task of performing pharmacovigilance work in their future endeavor.
The present study found spontaneous reporting rate of only 19.1% [Table 2b]. Previous studies have also found low rate of spontaneous reporting.[18,20] ADR related hospital admission has been found to be as high as 6.5%,[2] but still more than one-third (41.2%) doctors revealed that they had never seen an ADR [Table 2b]. These findings are of great concern and suggest that there is a serious and urgent need of education and training of doctors on ADRs from identification to reporting, which would ultimately improve the rate of spontaneous reporting.
Only 17 (25.0%) doctors had received training on how to report an ADR [Table 2a]. Educational intervention has been found to improve spontaneous reporting of ADRs.[24] Therefore, there is a need to conduct training program to provide training to all the doctors for improving spontaneous reporting of ADRs.
The doctors included in the present study suggested various methods to improve ADR reporting like Continuous Medical Education (CMEs) and refresher courses. Other suggested measures to improve spontaneous reporting included regular meeting on ADRs and establishing AMC in each hospital. This is indicative of willingness of the doctors to improve their knowledge of ADR reporting and participation in the spontaneous reporting if education and awareness program is held in the hospital. Last but not least, the importance of including pharmacovigilance related activity in undergraduate and post-graduate training program could not be overemphasized.
Based on the findings of the present study, the authors suggest a number of short-term and long-term measures to improve spontaneous reporting of ADRs in the hospital. The short-term measures include the following:
Increase awareness of the pharmacovigilance program and existence of AMC through personal communications and advertisements.
Encourage doctors to report all suspected ADRs irrespective of the level of association with the interventions.
Encourage doctors to report all suspected ADRs whether known, unknown, common, uncommon, and serious or nonserious, even with established drugs and OTC products.
Allay fear through informing doctors that intervention is responsible for ADRs, not the rational prescriber and reporter.
Training in pharmacovigilance with special emphasis on the risk perceptions of drugs including newly marketed drugs, OTC, and herbal medicines.
Long-term measures include the following:
ADR forms to be made more clear and objective about what to report.
Attitudinal changes through personal discussion and training whereby ADR reporting is perceived as an integral part of medical practice.
Involvement of other health professionals and paramedical staff in ADR reporting.
Inclusion of pharmacovigilance activity during undergraduate and post-graduate training program.
This was a single-center study involving limited number of doctors; therefore, results of the study could not be directly extrapolated to other teaching hospitals or institutes. A large multicentric study involving different teaching hospitals across India may provide greater insight into the factors responsible for underreporting of ADRs among doctors and their subsequent remedial measures. The undersigned authors are looking forward for a large multicentric study for the same in near future.
CONCLUSION
The results of the study strongly suggest that underreporting of ADRs is associated with gaps in the knowledge and attitudes. Important factors responsible for underreporting of ADRs among doctors working in a teaching hospital include inadequate risk perception about newly marketed drugs, fear factor, diffidence, lack of clarity of information on ADR form about reporting, lethargy, insufficient training to identify ADRs, lack of awareness about existence of pharmacovigilance program and AMC in the institute, and inadequate risk perception of OTC product and herbal medicines. Special attention should be given to these factors while conducting training programs and CMEs in order to enhance spontaneous reporting rate and safety of the patients at large.
ACKNOWLEDGMENT
We are thankful to all the doctors who participated in the study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.International drug monitoring: The role of National Centres. Report No: 498. Geneva, Switzerland: World Health Organization; 1972. World Health Organization. [PubMed] [Google Scholar]
- 2.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, et al. Adverse drug reactions as cause of admission to hospital: Prospective analysis of 18820 patients. Br Med J. 2004;329:15–9. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279:1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 4.The importance of pharmacovigilance. Safety monitoring of medicinal products. Geneva: World Health Organization; 2002. World Health Organization Collaborating Centre for International Drug Monitoring. [Google Scholar]
- 5.Pharmacovigilance programme of India 2010. CDSCO, Ministry of Health and Family Welfare, Government of India. 2010. [Last accessed on 2012 March 21]. Available from: http://cdsco.nic.in/pharmacovigilance.htm .
- 6.Wysowsky DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969-2002: The importance of reporting suspected reactions. Arch Intern Med. 2005;165:1363–9. doi: 10.1001/archinte.165.12.1363. [DOI] [PubMed] [Google Scholar]
- 7.Lopez-Gonzalez E, Herdeiro MT, Figueiras A. Determinants of under-reporting of adverse drug reactions: A systematic review. Drug Saf. 2009;32:19–31. doi: 10.2165/00002018-200932010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Smith CC, Bennett PM, Pearce HM, Harrison PI, Reynolds DJ, Aronson JK, et al. Adverse drug reaction in a hospital general medical unit meriting notification to the Committee on Safety of Medicines. Br J Clin Pharmacol. 1996;42:423–42. doi: 10.1046/j.1365-2125.1996.04376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feely J, Moriarty S, O’Connor P. Stimulating reporting of adverse drug reaction by using a fee. Br Med J. 1990;300:22–3. doi: 10.1136/bmj.300.6716.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inman WH. Attitudes to adverse drug-reaction reporting. Br J Clin Pharmacol. 1996;41:433–5. [PubMed] [Google Scholar]
- 11.Gupta P, Udupa A. Adverse drug reaction reporting and pharmacovigilance: Knowledge, attitudes and perceptions amongst resident doctors. J Pharm Sci Res. 2011;3:1064–9. [Google Scholar]
- 12.Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9:14. doi: 10.1186/1472-6904-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belton KJ, Lewis SC, Payne S, Rawlins MD, Wood S. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol. 1995;39:223–6. doi: 10.1111/j.1365-2125.1995.tb04440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bavdekar SB, Karande S. National Pharmacovigilance programme: An over view. Indian Pediatr. 2006;43:27–32. [PubMed] [Google Scholar]
- 15.Belton K. The European Pharmacovigilance Research Group: Attitude survey of adverse drug-reaction reporting by health care professionals across the European Union. Eur J Clin Pharmacol. 1997;52:423–7. doi: 10.1007/s002280050314. [DOI] [PubMed] [Google Scholar]
- 16.Eland A, Belton KJ, van Grootheest AC, Meiners AP, Rawlins MD, Stricker BH. Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol. 1999;48:623–7. doi: 10.1046/j.1365-2125.1999.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Backstrom M, Mjorndal T, Dahlqvist R, Nordkvist-Olsson T. Attitudes to reporting ADR in northern Sweden. Eur J Clin Pharmacol. 2000;56:729–32. doi: 10.1007/s002280000202. [DOI] [PubMed] [Google Scholar]
- 18.Li Q, Zhang SM, Chen HT, Fang SP, Yu X, Liu D, et al. Awareness and attitudes of healthcare professionals in Wuhan, China to the reporting of adverse drug reactions. Chin Med J. 2004;117:856–61. [PubMed] [Google Scholar]
- 19.Edzard E. The risk- benefit profile of commonly used herbal therapies: Ginkgo, st.johns wort, ginseng, echinacea, saw palmetto, and kava. Ann Intern Med. 2002;136:42–53. doi: 10.7326/0003-4819-136-1-200201010-00010. [DOI] [PubMed] [Google Scholar]
- 20.McGettigan P, Feely J. ADR reporting: Opinion and attitudes of medical practitioners in Ireland. Pharmacoepidemiol Drug Saf. 1995;4:355–8. [Google Scholar]
- 21.Vallano A, Cereza G, Pedros C, Agusti A, Danes I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60:653–8. doi: 10.1111/j.1365-2125.2005.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosford J, Goettler M, Munter KH, Muller-Oerlinghausen B. Physician's knowledge and attitudes regarding the spontaneous reporting system for ADR. J Clin Epidemiol. 2002;55:945–50. doi: 10.1016/s0895-4356(02)00450-x. [DOI] [PubMed] [Google Scholar]
- 23.Dhikav V, Singh S, Anand KS. Adverse drug reaction monitoring in India. J Indian Acad Clin Med. 2004;5:27–33. [Google Scholar]
- 24.Tabali M, Jeschke E, Bockelbrink A, Witt CM, Willich SN, Ostermann T, et al. Educational intervention to improve physician reporting of adverse drug reactions (ADRs) in a primary care setting in complementary and alternative medicine. BMC Public Health. 2009;9:274. doi: 10.1186/1471-2458-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]


