Abstract
Objective:
The aim of this work was to investigate the effect of β-cyclodextrin complexation on the solubility and hydrolysis rate of icariin.
Material and Methods:
The inclusion complex of icariin at the molar ratio of 1:1 was obtained by the dropping method and was characterized by differential scanning calorimetry. The solubility of icariin complex in water at 37°C was 36 times greater than that of free icariin. Enzymatic hydrolysis conditions were tested for the bioconversion of icariin by mono-factor experimental design.
Methods:
The inclusion complex of icariin at the molar ratio of 1:1 was obtained by the dropping method and was characterized by differential scanning calorimetry. The solubility of icariin complex in water at 37°C was 36 times greater than that of free icariin. Enzymatic hydrolysis conditions were tested for the bioconversion of icariin by mono-factor experimental design.
Results:
The enzymatic hydrolysis experiment showed that icariin can be transformed into baohuoside I. The optimum conditions determined were as follows: pH 5.0, 50°C, the ratio of cellulase/substrate (0.6), the concentration of icariin 20 mg/ml, and reaction time 12 h. Under these enzymatic conditions, 98.2% transforming rate of baohuoside I from icariin in inclusion complexes was obtained.
Conclusion
The aqueous solubility and enzymatic hydrolysis rate of icariin were improved owing to the inclusion complexation.
Keywords: Baohuoside I, enzymolysis, icariin, inclusion complex, β-cyclodextrin
INTRODUCTION
Baohuoside I, one kind of metabolite from icariin, is present in the herb Epimedium (Berberidaceae). Its molecular formula is C27H30O10 and molecular weight is 514 Da. It has been confirmed to display many bioactivities in vivo or in vitro. It has been reported that baohuoside I can enhance the differentiation and proliferation of osteoblasts, and facilitate matrix calcification. At the same time, it inhibits osteoclastic differentiation in both osteoclast progenitor cell culture and osteoblast–preosteoclast coculture. It is also found to induce osteoclastic apoptosis and inhibit bone resorption in vivo.[1–5] It effectively inhibits the cell proliferation activated by mitogenic antigen, and suppresses the growth of several tumor cell lines.[6] It has also shown relatively apparent repression on the ischemia/reperfusion-induced protein tyrosine kinase activation to protect human umbilical vein endothelial cells.[7,8] However, its low level in natural hinders its further studies on its pharmacological action. Based on the close structural relationship with icariin and the advantage of a relatively high content of natural icariin, baohuoside I can be obtained by enzymatic hydrolysis of icariin.[9] The chemical structures of icariin and baohuoside I are depicted in [Figure 1].
Figure 1.
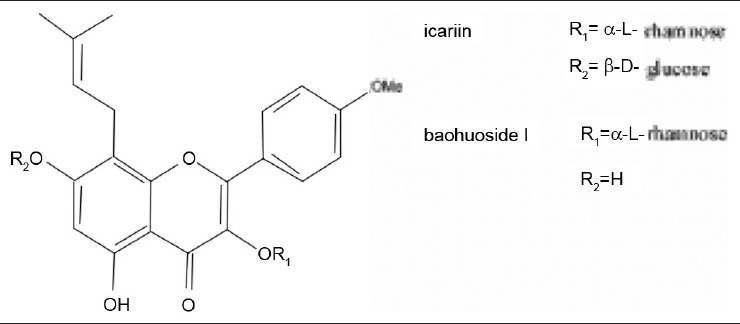
Chemical structures of icariin and baohuoside I
There has been no corresponding report of preparing baohuoside I from the inclusion complex by using cellulase. Cyclodextrins (CDs) are cyclic oligosaccharides containing six α-cyclodextrin, seven β-cyclodextrin (β-CD), and eight g-cyclodextrin units connected by α-(1,4) bonds [Figure 2]. Hydrophobic drug can be entrapped by CDs as guest in a cage-like meshwork, thereby enhancing its solubility.[10–12] On account of their structure and the relatively lipophilic surface of the internal cavity, which is in contrast with the hydrophilic feature of the external hydroxyl faces, CD molecules can easily form inclusion complexes with a wide variety of molecules and molecular ions.[13–16] β-CD might be the best natural β-CD due to its drug complexation and availability in pure form.[17] Meanwhile, its low cost and simple synthesis also have expanded its application.
Figure 2.
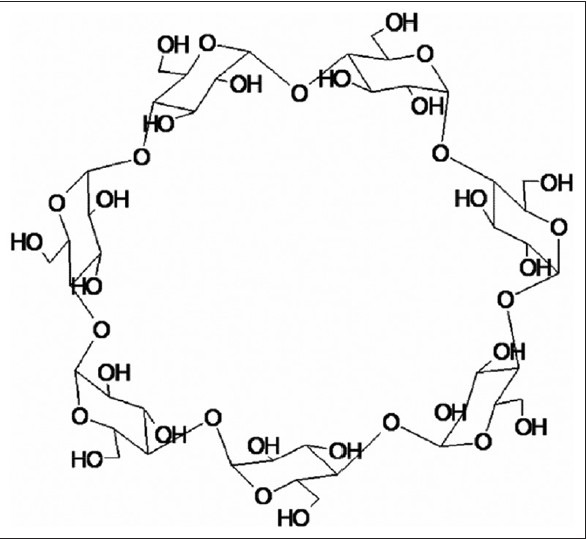
Chemical structure of cyclodextrin
Cellulases, enzymes which hydrolyze the β-1,4-glucosidic linkages of cellulose, are present in 13 of the 82 glycoside hydrolase families identified by sequence analysis.[18] The complete cellulase system comprises endoglucanase (EG), cellobiohydrolase (CBH), and β-glucosidase (BGL) components. Cellulases are currently the third largest industrial enzyme worldwide because of their wide applications in cotton processing, paper recycling, in juice extraction, as detergent enzymes, and animal feed additives.[19,20]
Based on these, the main objectives of the present study were to evaluate the effect of β-CD on the aqueous solubility and hydrolysis rate of icariin, and identify the key parameters (pH value, temperature, ratio of substrate/enzyme, concentration of the substrate, and reaction time) for the enzymatic hydrolysis by mono-factor experimental design. By preparing the inclusion complex and selecting the optimum enzymatic parameters, the higher enzymatic hydrolysis rate of icariin can be obtained.
MATERIALS AND METHODS
Materials and equipment
Standard icariin (purity > 98%) and baohuoside I (purity > 98%) were provided by the Laboratory of Pharmaceutical Preparation (Jiangsu Provincial Academy of Chinese Medicine, Nanjing, China). The cellulase, which required maintenance temperature of 0°C, was purchased from Baier Di Biotechnology Co., Ltd (Beijing, China). ββ-CD (average MW = 1135) was purchased from Shanghai Chemical Reagent Company of China (Shanghai, China). Pharmaceutical Group. Glacial acetic acid, anhydrous sodium acetate, and absolute ethanol were purchased from Nanjing Chemical Reagent Co., Ltd (Nanjing, China). All the other reagents were of analytical grade and were purchased from different companies. The ultra-pure water was purified by the Milli-Q water purification system (Millipore, Bedford, MA, USA).
All quantitative analyses were carried out by a high-performance liquid chromatographic system, the high-pressure liquid chromatography (HPLC) Waters 2690 Separation Module (comprising in-line degasser, quaternary solvent delivery pumps, automatic injector, and a column oven) with a Photodiode Array Detector (Model Waters 2996) and Phenomenex® C18 column (250 × 4.60 mm, Phenomen Tech Co, Ltd, Tianjin, China). The Waters software was used to handle the data. The enzymatic hydrolysis was carried out by a digital constant temperature water bath HH-4 (Guohua Electric Appliance Co., Ltd, Changzhou, China). A TGL-16H high-speed centrifuge (Shanghai Precision Instrument Factory, Anping, Shanghai, China.) was employed to treat the samples. DSC204F1 differential scanning calorimeter (Germany) was used to verify whether icariin was complexed.
Preparation and characterization of the inclusion complex
Preparation of inclusion complex
The inclusion complex of icariin with the β-CD at 1:1 molar ratio was prepared using saturated solution method. Accurately weighed β-CD was dissolved in distilled water to get a saturated solution. Then, the icariin solution in absolute ethanol was added drop by drop. The formed suspension was agitated for 4 h at 60°C. Subsequently, it was placed at 4°C for 24 h to precipitate the complex. The suspension was filtered and the free icariin was washed from the residue using ethanol. Finally, the precipitation was dried at 100°C for 1 h on the water bath.
Preparation of the physical mixture of icariin and β-CD
A physical mixture of icariin and β-CD in the same weight ratio as the complex was prepared. Physical mixture was previously sieved into individual components through a 315-μm mesh in a mortar and pestle for 5 min.
Differential scanning calorimetry
To obtain the differential scanning calorimetry (DSC) curves of icariin, β-CD, equimolecular physical mixture of icariin, and inclusion complex, a Photo DSC204 F1 differential scanning calorimeter calibrated with indium was utilized. Accurately weighed samples (1-5 mg) were placed in a 40-μl aluminum pan, which was sealed and pierced. Alumina was used as a reference material and the scanning rate was 10.0°C/min with a scanning temperature range of 0-450°C. Measurements were made in duplicate for each sample.
Solubility studies of inclusion complex
Quantification of icariin in the inclusion complex
Icariin (98%, 26.10 mg) was accurately weighted and dissolved in 50 ml purified water to get store solutions. Solutions with volumes of 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 ml were taken out and ethanol was added to each to obtain a volume of 25 ml. The samples were analyzed by the HPLC system using an isocratic elution of CH3OH:H2O (75:25, v/v) as the mobile phase at a flow rate 1.0 ml/min, and detected at 270 nm wavelength. The regression equation was derived from the values between the peak area (Y) and the concentrations (X) of icariin. The content of icariin was calculated with the regression equations.
Sample processing
Solubility measurements were carried out as follows: Excess amounts of the inclusion complex or free icariin were added to 10 ml of aqueous solutions. The suspensions were shaken for 72 h at 37°C at a constant speed of 150 r/min and protected from light to achieve equilibration. After equilibration, the suspensions were filtered through 0.45-μm membranes. The filtrate thus obtained was collected. All procedures were conducted at the specified test temperature to avoid any precipitation of the drug. The filtrate was appropriately diluted with ethanol and the concentration of the substrate (the term “substrate” in the whole article refers to icariin) was determined by the HPLC system.
Enzymatic hydrolysis experiment
Enzymatic technology for inclusion complex
The inclusion complex of icariin with the β-CD at 1:1 molar ratio was prepared using saturated solution method. Accurately weighed β-CD was dissolved in distilled water to get a saturated solution. Then, the icariin solution in absolute ethanol was added drop by drop and a suspension was formed. The suspension was agitated for 4 h at 60°C and the ethanol was removed by rotary evaporation. Subsequently, the pH of the reaction system was adjusted and cellulase was added. Finally, the enzymatic hydrolysis experiment could be carried out.
Mono-factor experiment for inclusion complex and free icariin
The pH value, temperature, ratio of enzyme/substrate in the reaction mixture, concentration of the substrate, and reaction time were fixed at 4.5, 50°C, 1:1, 10 mg/ml, and 24 h, respectively. To evaluate the effect of each factor on enzymatic hydrolysis, the other four factors were fixed and all the reactions were tested in triplicate. The pH value was investigated, including the values 4.0, 4.5, 5.0, 5.5, 6.0, and 6.5. The temperature was studied, including 25°C, 37°C, 50°C, 60°C, and 70°C. The ratio of enzyme/substrate in reaction mixture was inspected, including 1:10, 1:5, 2:5, 3:5, 4:5, and 1:1. The substrate concentration was investigated, including 1, 5, 10, 20 and 30 mg/ml. Finally, the reaction time was studied, including 2, 4, 6, 12, 24, and 48 h.
The final reaction product was dried by the N 2 blow and dissolved in a specific volume of ethanol. Subsequently, 10 μl filtered solution was injected into the HPLC system. The content of baohuoside I was analyzed using the single-point external standard method. The transforming rate of icariin in each reaction was used to reflect the enzymatic hydrolysis efficiency.
Preparation of baohuoside I under optimum condition
The inclusion complex and free icariin were transformed into baohuoside I according to the optimized screening conditions. Consequently, the maximum product of baohuoside I was obtained individually for further purification. The produced baohuoside I was extracted from the reaction mixture with ethyl acetate. Subsequently, baohuoside I was dissolved in methanol and subjected to silica gel column using CHCl3–MeOH for elution. The eluent that only contained baohuoside I was merged. Finally, baohuoside I was obtained through reduced pressure recovery. The identification of produced baohuoside I was carried out by 1H NMR and 13C NMR.
RESULTS AND DISCUSSION
Differential scanning calorimetry
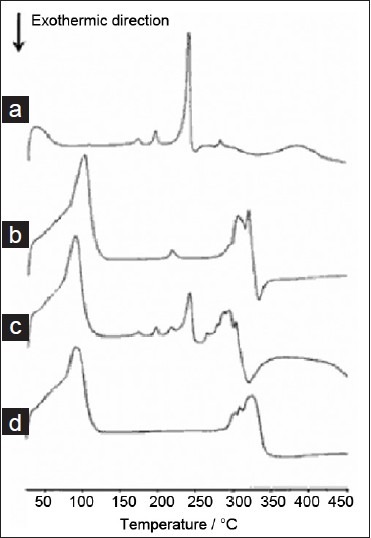
DSC analysis was generally performed to characterize inclusion compounds by comparing the thermal behaviors of the individual components as well as their physical mixtures and inclusion compounds.[21–23] The DSC curves of icariin, β-CD, icariin/β-CD physical mixture, and inclusion complex are shown in Figure 3. Icariin is indicated as a crystalline compound, in which an exothermic peak appears at its melting temperature (approximately 240°C). The dehydration and decomposition of β-CD occurred at approximately 100°C and 320°C, respectively. The curves of physical mixture obtained the same exothermic peak as that of icariin. However, this characteristic peak of icariin melt was not observed for the icariin–β?CD complex, indicating that the interaction of icariin molecules to form a crystal structure was destroyed by -CD complex, indicating that the interaction of icariin molecules to form a crystal structure was destroyed by β-CD in the icariin–β-CD complex. These results confirm that icariin in complexes exists in the amorphous state.[22] The result is in accordance with the referenced studies.[24]
Figure 3.
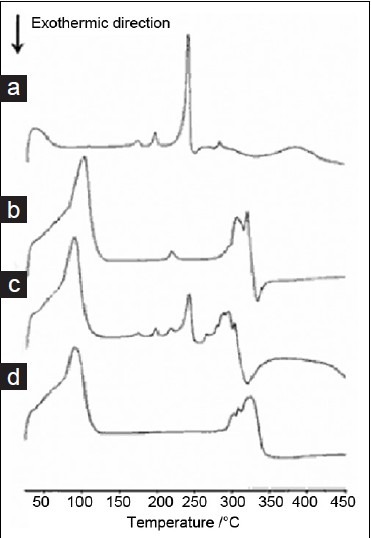
DSC curves (a) icariin, (b) CD, (c) equimolecular physical mixture of icariin and CD, (d) inclusion complex formed between CD and icariin
Solubility studies of inclusion complex
The results of HPLC analysis showed that the concentrations (X) and the peak area (Y) of icariin attained good linear relationship when the concentration of icariin was between 10.44 and 167.04 μg/ml. The regression equation was as follows:
![]()
According to Equation (1), the solubility of icariin in inclusion complex was determined as 525.6 μg/ml, whereas the value of the free icariin was only 14.6 μg/ml. β-CD significantly increased the solubility of icariin, which provided convenient condition for its hydrolysis.
Mono-factor experiments
The inclusion complex and free icariin can be transformed into baohuoside I by cellulase. For the inclusion complex and free icariin, the main parameters that affected enzymatic hydrolysis were pH value, temperature, ratio of enzyme/substrate, concentration of the substrate, and reaction time. The results are shown in Figures 4–8
Figure 4.
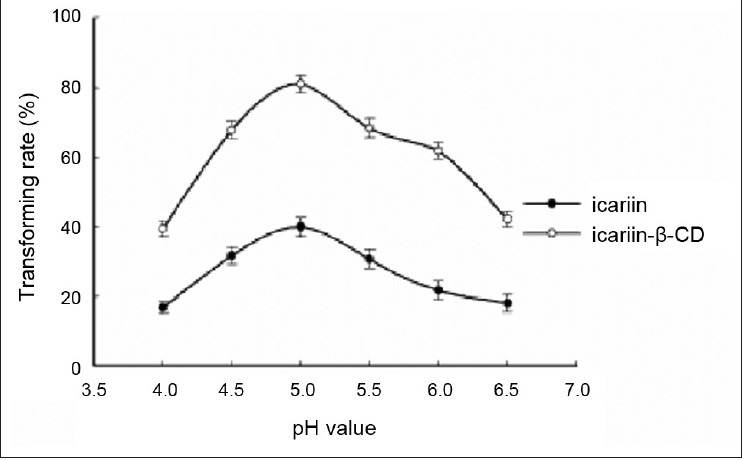
Effect of pH value of acetate buffer. Reaction was performed by adding 10 mg substrate, 10 mg cellulase, and 1 ml glacial acetic acid and sodium acetate anhydrous buffer (pH 4.0, 4.5, 5.0, 5.5, 6.0, and 6.5) at 50°C for 24 h. Each point represents the mean ± SD (n = 3)
Figure 8.
Effect of reaction time. Ten milligram substrate, 10 mg cellulase, and 1 ml glacial acetic acid and sodium acetate anhydrous buffer (pH 4.5) were mixed in a 1-ml Eppendorf tube. Subsequently, the buffer was incubated in an HH-4 digital constant temperature water bath at 50°C for 2, 4, 8, 12, 24, and 48 h. Each point represents the mean ± SD (n = 3)
Figure 5.
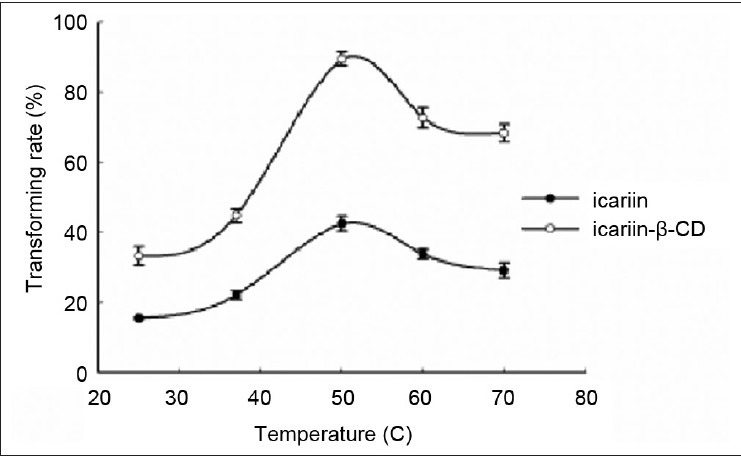
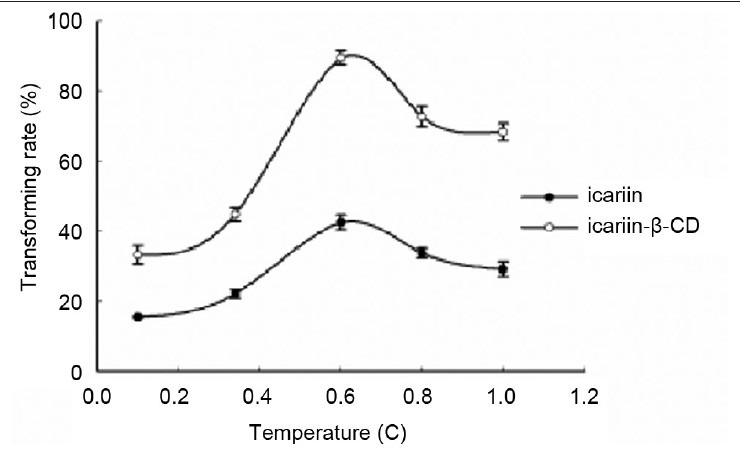
Effect of reaction temperature. Ten milligram substrate, 10 mg cellulase, and 1 ml glacial acetic acid and anhydrous sodium acetate buffer (pH 4.5) were mixed in a 1-ml Eppendorf tube. Subsequently, the buffer was incubated in an HH-4 digital constant temperature water bath at 25°C, 37°C, 50°C, 60°C, and 70°C for 24 h. Each point represents the mean ± SD (n = 3)
Figure 6.
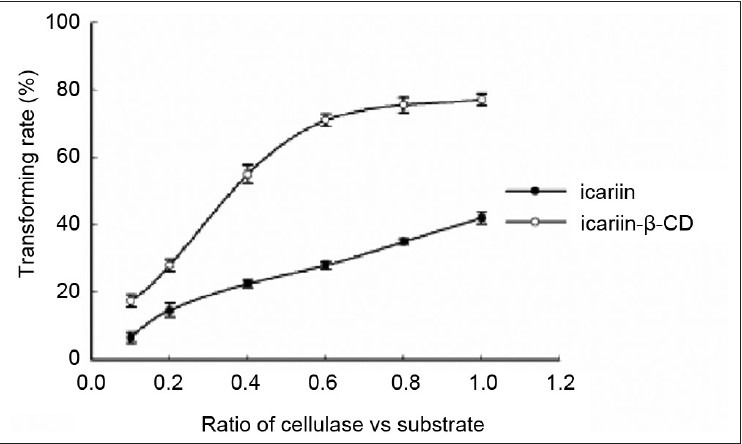
Effect of the ratio of cellulase/substrate. Ten milligram substrate was mixed with 1, 2, 4, 6, 8, 10 mg cellulase (cellulase/substrate = 0.1, 0.2, 0.4, 0.6, 0.8, and 1). Glacial acetic acid and sodium acetate anhydrous buffer (pH 4.5) were added to the mixture at a final volume of 1 ml. Subsequently, the buffer was incubated in an HH-4 digital constant temperature water bath at 50°C for 24 h. Each point represents the mean ± SD (n = 3)
Effect of pH on enzymatic hydrolysis of the inclusion complex and free icariin
The percent hydrolysis of the inclusion complex and free icariin increased with the increasing buffer pH, reached a peak value at 5.0, and then decreased slightly. Therefore, the optimal pH of the reaction for the inclusion complex and free icariin was 5.0.
Effect of temperature on enzymatic hydrolysis of the inclusion complex and free icariin
The influence of temperature on the conversion rate in the present study was measured within a range of 25-70°C. The percent hydrolysis of the inclusion complex and free icariin increased remarkably from 25°C, reached its maximum at 50°C, and decreased with increasing temperature. These results indicate that the activity of cellulase increased with the increasing temperature and subsequently reached the highest point at 50°C. Thus, the temperature used in the succeeding experiments was chosen at 50°C for both the inclusion complex and free icariin.
Temperature is an important operational parameter in industrial large-scale production. Therefore, appropriate temperature is needed. In addition, numerous studies described temperature as a parameter with an optimal value in enzymatic processes.[25,26] The optimal temperature for the inclusion complex and free icariin was the same.
Effect of the ratio of cellulase/substrate on enzymatic hydrolysis of the inclusion complex and free icariin
For the ratio of cellulase/substrate, the percent hydrolysis of the inclusion complex and the free icariin increased with the incremental ratio of cellulase/substrate. The percent hydrolysis of the inclusion complex was almost constant from 0.6 to 1, whereas the percent hydrolysis of the free icariin increased linearly with increase in the ratio of cellulase/substrate. Based on these data, 0.6 and 1 were identified as the optimal ratios of cellulase/substrate in the reaction for the inclusion complex and free icariin, respectively. Thus, preparing a water-insoluble drug in the inclusion could reduce the amount of enzyme.
Effect of the substrate concentration on enzymatic hydrolysis of the inclusion complex and free icariin
The influence of the substrate concentration was evaluated at pH 4.5 and 50°C for 24 h. Curves that properly described the experiments over the range of the substrate concentration from 1 to 30 mg/ml were obtained. The percent hydrolysis of the inclusion complex or the free icariin increased with the incremental concentration and subsequently dropped. They reached their maximum at 20 mg/ml and 5 mg/ml, respectively.
Figure 7 shows the comparison of the improvement observed between the inclusion complex in concentration and that of free icariin. A visible improvement was observed in the former because the inclusion complex enhanced the solubility of poorly soluble icariin.
Figure 7.
Effect of concentration of the substrate. The substrate and cellulase were weighed according to the weight proportion of 1:1. Glacial acetic acid and sodium acetate anhydrous buffer (pH 4.5) were added and the following solution concentrations were obtained: 1, 5, 10, 20, and 30 mg/ml. Subsequently, the buffer was incubated in an HH-4 digital constant temperature water bath at 50°C for 24 h. Each point represents the mean ± SD (n = 3)
Effect of the reaction time on enzymatic hydrolysis of the inclusion complex and free icariin
The percent hydrolysis of both inclusion complex and free icariin increased with the ongoing reaction time. A maximum value was obtained at 12 h for the inclusion complex and at 48 h for icariin. A gradual increase in speed was observed. At the 12-h point, the hydrolysis percentage of the inclusion complex was greater than 90%, whereas percentage of the free icariin was only approximately 30%. This provides further evidence of the rationality of inclusion in enzymatic experiments.
Under the best reaction conditions, the hydrolysis percentage of the inclusion complex was 98.2% and of that free icariin was 80.4%.
This result laid the theoretical foundations for large-scale industrial production, owing to the higher substrate concentration of inclusion complex.
Preparation of baohuoside I under optimum conditions
33.5 mg inclusion complex of icariin containing 20 mg icariin was mixed with 12 mg cellulase in 1 ml glacial acetic acid and anhydrous sodium acetate buffer (pH 5.0). The buffer was subsequently incubated in an HH-4 digital constant temperature water bath at 50°C for 12 h. The final reaction mixture was extracted with ethyl acetate three times. The combined ethyl acetate layer was evaporated to dryness under reduced pressure. HPLC analysis showed that 98.2% of the inclusion complex of icariin was transformed into baohuoside I. To isolate baohuoside I, the residue was dissolved in 10 ml methanol and subjected to silica gel column chromatography using CHCl3–MeOH system for elution. Finally, 19.64 mg baohuoside I was obtained with a purity of 99.3%. The identification of baohuoside I produced was carried out by the 1H NMR and 13C NMR. The obtained results proved that the formed product was baohuoside I.
CONCLUSIONS
The solubility of the icariin complex in water at 37°C was 36 times greater than that of the free drug. The inclusion complex of icariin was observed to be ideal for increasing the hydrolysis rate at the optimum condition compared with that of free icariin. The optimal enzymatic hydrolysis conditions for the inclusion complex were as follows: Acetate buffer with pH 5.0, 50°C, the ratio of cellulase/substrate (0.6), 20 mg/ml substrate, and reaction time 12 h. On the other hand, acetate buffer with pH 5.0, 50°C, cellulase to substrate ratio of 1:1, 5 mg/ml substrate, and reaction time 48 h were the optimum conditions for free icariin. The β-CD inclusion complex showed positive effect on shortening of the reaction time as well as on increasing the concentration of the substrate and reducing the amount of enzyme. Under the optimum conditions, the percent hydrolysis of the inclusion complex was 98.2%, whereas that of free icariin was 80.4%. Therefore, the method can significantly improve the conversion efficiency of icariin to baohuoside I.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Huang J, Yuan L, Wang X, Zhang TL, Wang K. Icaritin and its glycosides enhance osteoblastic, but suppress osteoclastic, differentiation and activity in vitro. Life Sci. 2007;81:832–40. doi: 10.1016/j.lfs.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 2.Xie F, Wu CF, Lai WP, Yang XJ, Cheung PY, Yao XS, et al. The osteoprotective effect of Herba epimedii extract in vivo and in vitro. Evid Based Complement Alternat Med. 2005;2:353–61. doi: 10.1093/ecam/neh101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun-Hee K, Kwang SA, Soo-Jin J, Tae-Rin K. Janus activated kinase 2/signal transducer and activator of transcription 3 pathway mediates icariside II-induced apoptosis in U266 multiple myeloma cells. Eur J Pharmacol. 2011;654:10–6. doi: 10.1016/j.ejphar.2010.11.032. [DOI] [PubMed] [Google Scholar]
- 4.Xu W, Zhang YP, Yang M, Shen ZY. Effect of Icariside II on the expression of OPG in mouse osteoblasts. Bone. 2010;47:S404. [Google Scholar]
- 5.Hwa JC, Jae-Soon E, Dae KK, Li RH. Icariside II from Epimedium koreanum inhibits hypoxia-inducible factor-1a in human osteosarcoma cells. Eur J Pharmacol. 2008;579:58–5. doi: 10.1016/j.ejphar.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Ma AL, Qi SJ, Xu DS, Pierre D, Chen HF. Baohuoside-1 inhibits activated T cell proliferation at G1-S phase transition. Transpl Immunol. 2005;15:55–62. doi: 10.1016/j.trim.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YW, Morita I, Shao G, Yao XS, Murota S. Screening of antihypoxia/reoxygenation agents by an in vitro model. Part 1: Natural inhibitors for protein tyrosine kinase activated by hypoxia/reoxygenation in cultured human umbilical vein endothelial cells. Planta Med. 2000;66:114–8. doi: 10.1055/s-2000-11128. [DOI] [PubMed] [Google Scholar]
- 8.Zhang YW, Morita I, Zhang L, Shao G, Yao XS, Murota S. Screening of anti-hypoxia/reoxygenation agents by an in vitro method. Part 2: Inhibition of tyrosine kinase activation prevented hypoxia/reoxygenation-induced injury in endothelial gap junctional intercellular communication. Planta Med. 2000;66:119–23. doi: 10.1055/s-2000-11126. [DOI] [PubMed] [Google Scholar]
- 9.Xia Q, Xu DJ, Huang ZG, Liu JJ, Wang XQ, Wang X, et al. Preparation of icariside II from icariin by enzymatic hydrolysis method. Fitoterapia. 2010;81:437–42. doi: 10.1016/j.fitote.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Blanco J, Jato JL, Otero F, Aguian S. Influence of method of preparation on inclusion complexes of naproxen with different cyclodextrin. Drug Dev Ind Pharm. 1991;17:943–57. [Google Scholar]
- 11.Palmieri GF, Angeli DG, Giovannucci G, Martelli S. Inclusion of methoxybutropate in β-and hydroxypropyl-β-cyclodextrins: Comparison of preparation methods. Drug Dev Ind Pharm. 1997;23:27–37. [Google Scholar]
- 12.Castillo JA, Canales JP, Garcia JJ, Lastres JL, Bolas F, Torrado JJ. Preparation and characterization of albendazole-β-cyclodextrin complexes. Drug Dev Ind Pharm. 1999;25:1241–8. doi: 10.1081/ddc-100102294. [DOI] [PubMed] [Google Scholar]
- 13.Rebecca LC, Lee AM, Imran A. The utility of cyclodextrins for enhancing oral bioavailability. J Control Release. 2007;123:78–99. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 14.Martin Del Valle EM. Cyclodextrins and their uses: A review. Process Biochem. 2004;39:1033–46. [Google Scholar]
- 15.Mamata S, Rohit S, Banerjee UC. Biotechnological applications of cyclodextrins. Biotechnol Adv. 2002;20:341–59. doi: 10.1016/s0734-9750(02)00020-4. [DOI] [PubMed] [Google Scholar]
- 16.Corti G, Capasso G, Maestrelli F, Cirri M, Mura P. Physical-chemical characterization of binary systems of metformin hydrochloride with triacetyl-beta-cyclodextrin. J Pharm Biomed Anal. 2007;45:480–6. doi: 10.1016/j.jpba.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 17.Marco B, Elisabetta R, Paolo F, Francesco T. Preparation and in vitro evaluation of the antiviral activity of the Acyclovir complex of a cyclodextrin/poly(amidoamine) copolymer. J Control Release. 2008;126:17–25. doi: 10.1016/j.jconrel.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 18.Schulein M. Protein engineering of cellulases. Biochim Biophys Acta. 2000;1543:239–52. doi: 10.1016/s0167-4838(00)00247-8. [DOI] [PubMed] [Google Scholar]
- 19.Li CZ, Makoto Y, Kimitoshi F, Katsumi N. Characterization and immobilization of liposome-bound cellulase for hydrolysis of insoluble cellulose. Bioresour Technol. 2007;98:1366–72. doi: 10.1016/j.biortech.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 20.Reeta RS, Rajeev KS, Anil KP, Christian L, Ashok P. Advancement and comparative profiles in the production technologies using solid-state and submerged fermentation for microbial cellulases. Enzyme Microb Technol. 2010;46:541–5. [Google Scholar]
- 21.Bilensoy E, Cırpanlı Y, Sen M, Doğan AL, Calı S. Thermosensitive mucoadhesive gel formulation loaded with 5-Fu: Cyclodextrin complex for HPV-induced cervical cancer. J Incl Phenom Macrocycl Chem. 2007;57:363–70. [Google Scholar]
- 22.Calderini, Pessine FB. Synthesis and characterization of inclusion complex of the vasodilator drug minoxidil with β-cyclodextrin. J Incl Phenom Macrocycl Chem. 2008;60:369–77. [Google Scholar]
- 23.Cevher E, Sensoy D, Zloh M, Mulazimoglu L. Preparation and characterisation of natamycin: R-cyclodextrin inclusion complex and its evaluation in vaginal mucoadhesive formulations. J Pharm Sci. 2008;97:4319–35. doi: 10.1002/jps.21312. [DOI] [PubMed] [Google Scholar]
- 24.Sansone F, Picerno P, Mencherini T. Flavonoid microparticles by spray-drying: Influence of enhancers of the dissolution rate on properties and stability. J Food Eng. 2011;103:188–96. [Google Scholar]
- 25.Liaset B, Julshamn K, Espe M. Chemical composition and theoretical nutritional evaluation of the produced fractions from enzymic hydrolysis of salmon frames with Protamex™. Process Biochem. 2003;38:1747–59. [Google Scholar]
- 26.Rosenthal A, Pyle D, Niranjan K, Gilmour S, Trinca L. Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzyme Microb Technol. 2001;28:499–509. doi: 10.1016/s0141-0229(00)00351-3. [DOI] [PubMed] [Google Scholar]


