Abstract
Superficial leiomyosarcomas are rare malignant smooth-muscle tumors accounting for 4-6.5% of all soft-tissue sarcomas, less than 2-3% of cutaneous soft-tissue neoplasms and 0.04% of all cancers. They are divided into cutaneous or dermal and subcutaneous leiomyosarcomas. Subcutaneous tumors have been reported to be associated with an increased risk of local recurrences and distant metastases, compared to their cutaneous counterparts. In this study, we describe a rare case of a recurrent subcutaneous trunk leiomyosarcoma in a 68-year-old male patient. Local recurrence developed two years after the complete surgical resection with wide margins and adjuvant postoperative radiotherapy. The management of the patient is discussed along with a review of the literature. We conclude that subcutaneous leiomyosarcoma is a rare clinical entity which may be associated with an atypical clinical presentation. Physicians should be aware of the misleading features of this tumor in order to avoid delay in diagnosis and treatment. Early complete surgical resection with wide margins of at least 2 cm is the cornerstone of treatment and has been reported to mostly influence the prognosis. However, the tumor has a high tendency to recur locally and metastasize. Recurrence may develop despite wide resection and radiotherapy. Long-term follow-up is mandatory.
Keywords: Leiomyosarcoma, recurrent, subcutaneous
INTRODUCTION
Superficial leiomyosarcomas are rare malignant smooth-muscle tumors accounting for 4-6.5% of all soft-tissue sarcomas, less than 2-3% of cutaneous soft-tissue neoplasms and 0.04% of all cancers.[1,2] They are divided into cutaneous or dermal and subcutaneous types depending on their histogenesis and different clinical and prognostic implications. Cutaneous or dermal tumors are thought to arise from the arrector pili or genital dartoic muscles whereas their subcutaneous counterparts arise from the smooth muscle wall of blood vessels.[3–5] Subcutaneous leiomyosarcomas account for 1-2% of all soft-tissue sarcomas.[6] Although they may arise anywhere in the body,[1,7] they most frequently occur in the lower extremities.[7–10] The thigh has been reported as the most frequently affected site.[8] Only 10-15% of subcutaneous leiomyosarcomas arise in the trunk.[1,6] Leiomyosarcomas arising in the extremities, the trunk wall and the superficial parts of the head and neck have not been characterized well and the experience is usually limited.[8] We present here a case of a recurrent trunk leiomyosarcoma in a 68-year-old male. The tumor recurred two years after wide surgical excision and administration of adjuvant radiotherapy. Diagnostic evaluation and management of the patient are discussed along with a review of the relevant literature.
CASE REPORT
A 68-year-old Caucasian male presented to our department with a two-month history of a small palpable nodule at the inferior angle of the right scapula. His medical history was remarkable for arterial hypertension.
Physical examination revealed a well-defined, non-tender and relatively mobile nodule at the inferior angle of the right scapula. There were no signs of infection and the overlying skin was normal. The remainder of the clinical examination was unremarkable. Ultrasonography demonstrated a well-circumscribed hypoechoic mass measuring 1.8 × 1.5 cm. Full blood count, biochemical tests and tumor markers were within the normal range.
A surgical resection with wide margins of at least 2 cm was performed. Histological examination revealed a tumor measuring 1.8 × 1.5 × 0.7 cm consisting of elongated malignant cells characterized by nuclear polymorphic figures combined with mitoses. The surgical margins were free of disease. On detailed immunohistochemical analysis the tumor cells stained positive for smooth muscle actin (SMA), a1-antitrypsin, and vimentin [Figure 1] and negative for desmin, S-100 protein, myocin and a1-antichymotrypsin. Based on immunohistochemical findings, the diagnosis of a subcutaneous leiomyosarcoma was established. Staging investigations including computed tomography scans of the chest and abdomen were all negative. Although a complete resection with wide surgical margins had been performed, adjuvant postoperative radiation therapy was administered due to the high mitotic rate of the tumor.
Figure 1.
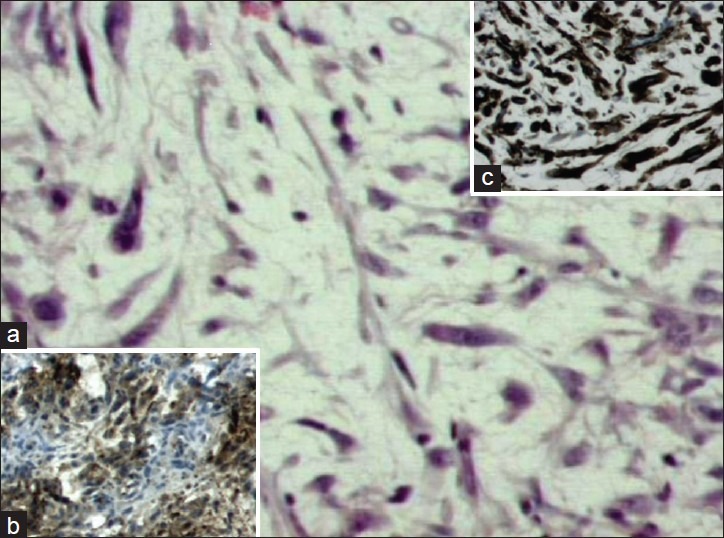
Histological features of subcutaneous leiomyosarcoma. (a) Note the elongated malignant cells characterized by nuclear polymorphic figures combined with mitoses (H and E stain, original magnification ×20). (b, c) Immunohistochemical analysis showed positive staining pattern for smooth muscle actin and a1-antitrypsin, respectively (original magnification ×20)
Two years after surgery, the patient was readmitted with a palpable small, non-tender nodule very close to the scar of the previous operation within the irradiated field. A tumor recurrence was suspected and a new surgical resection with wide margins was performed. Complete blood count and tumor markers were within the normal range.
Histological examination and immunohistochemical analysis revealed a recurrent leiomyosarcoma measuring 1 cm in diameter. Staging investigations were negative. Adjuvant radiation therapy was not considered at this point by the multidisciplinary oncology team because the patient had received the maximum dose after the primary tumor resection. The patient is being followed up and is well without signs of disease six years after the resection of the recurrent tumor [Figure 2].
Figure 2.
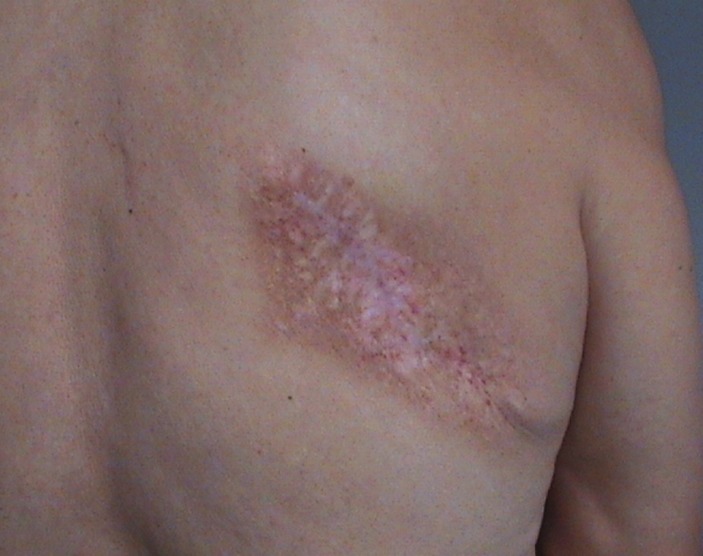
The area of the resected recurrent leiomyosarcoma
DISCUSSION
Superficial leiomyosarcomas affect men more frequently than women with a male to female ratio of 2:1 to 3:1, whereas patients typically present in the fifth to seven decades of life.[1,7,8,10–12] The tumor, however, may occur at any age. Stout and Hill[12] reported a case of leiomyosarcoma in a five-month-old infant girl.
Although the exact pathogenesis of leiomyosarcoma is unknown, several predisposing factors have been reported in the literature including precursor leiomyomas, history of trauma and radiation exposure.[2]
Pain is the most common symptom occurring in 80-95% of the patients[1] and can be spontaneous or induced by pressure.[7] Clinically, the tumor may appear as a solitary nodule with irregular or well-defined borders, pedunculation, umbilication and skin discoloration.[1] Subcutaneous leiomyosarcomas most often are nodular well-circumscribed lesions[10] associated with clinically normal overlying skin[3] and a hemispherical skin elevation ranging from 0.6 to 5 cm in diameter.[7] Dermal leiomyosarcomas usually present as small firm nodules measuring less than 2 cm in size but their subcutaneous counterparts are larger[4,11] with a median tumor size of 4 cm at presentation.[8] The median duration from onset to presentation is 12 months.[7] In rare cases the patient may present with multiple nodules. In these cases the possibility of metastasis from another soft-tissue sarcoma site, mainly the retroperitoneum, should be excluded.[3,10] Dahl et al.,[10] reported that 4 of 7 patients who were diagnosed with multiple superficial leiomyosarcomas had been previously operated for retroperitoneal leiomyosarcomas. In some cases, however, the clinical presentation is non-specific[13] and leiomyosarcomas may be misdiagnosed on clinical grounds.[14] Since some tumors are not firmly attached to the surrounding structures, they can be considered innocuous and excised or shelled out as if they were benign.[7,12] Awareness of the misleading features of this tumor is therefore necessary in order to avoid delay in diagnosis and treatment.
The imaging features of superficial leiomyosarcomas are not specific. Large tumors are usually heterogeneous due to the presence of necrosis, cystic changes and hemorrhage, whereas microcalcifications may be demonstrated in 10-15% of the cases on radiographs or computed tomography scans.[4] On ultrasonography, the tumor may appear as a hypoechoic solid mass with well-defined or ill-defined borders. Magnetic resonance imaging should be used for staging purposes in subcutaneous tumors larger than 3-5 cm.[4]
Early surgical excision with wide margins is the treatment of choice.[1,4,7,10,15,16] The guidelines, however, for the optimal width of the surgical margins have not been clearly defined.[2] Most authors suggest that margins of at least 2 cm are necessary.[2,17] However, McKee et al.,[18] reported that surgical margins >1 cm independently predicted longer local recurrence-free survival and are optimal in the resection of extremity soft-tissue sarcomas. Mohs micrographic surgery has been used in the management of superficial leiomyosarcomas with a reported recurrence rate of 0-14%.[19–21] In the largest series including 11 patients treated with Mohs surgery, Starling et al.,[20] reported that after a median follow-up of 4.47 years no tumor recurrences were detected. The average tumor size was 4.69 cm2 and the average size of the surgical defect was 14.95 cm2. Larger series of patients treated with Mohs surgery and longer follow-up are however needed before definite conclusions can be drawn.[3]
The initial surgical intervention is considered to be the most important factor influencing the prognosis in patients with superficial leiomyosarcoma.[10] Inadequate surgical margins place the patient at high risk for local recurrence or in a case of a subcutaneous leiomyosarcoma expose the patient to a potentially incurable and fatal metastatic disease.[1]
Histologically, the tumor is characterized by the presence of perpendicularly arranged fascicles of spindled cells, with abundant eosinophilic cytoplasm that is dense but vacuolated and elongated blunt-ended nuclei.[5] Immunochistochemical analysis plays a key role in the differentiation diagnosis of leiomyosarcomas from other spindle-cell tumors,[1,17] especially in cases of poorly differentiated and anaplastic tumors.[3] The a-Smooth Muscle Actin is the most sensitive marker indicating smooth-muscle differentiation[5] and has been reported to be positive in 100% of the cases in many series of leiomyosarcomas.[13,16] In most cases the histopathology report will reveal a high-grade tumor.[8,9,16] In cases of dermal leiomyosarcomas it is essential to mention in the histopathology report the depth of a possible subcutaneous extension because even a minimal subcutis invasion may be associated with late local recurrences and distant metastases.[22]
Differential histological diagnoses of leiomyosarcoma include fibrosarcoma, malignant fibrous histiocytoma, rhabdomyosarcoma, malignant schwannoma, dermatofibroma, nodular fasciitis, and neurofibroma.[1]
A cumulative five-year survival of 65% has been reported for patients with superficial leiomyosarcomas.[11,17] Svarvar et al.,[8] reported that in 225 patients from the Scandinavian Sarcoma Group the 5-and 10-year overall survival for patients without metastases at presentation was 69% and 49% respectively. However, the five-year survival for patients with metastases at presentation dropped significantly to 16%. In this study 8.4% of the patients had metastases at presentation.[8]
Local recurrence has been reported in 50-60% of the patients with subcutaneous leiomyosarcomas.[12] Recurrences have been reported following the removal of very small primary tumors measuring 0.5 cm, whereas 50% of the tumors recur within 4-18 months after initial diagnosis.[7] On the contrary, patients with cutaneous tumors usually run an indolent clinical course and recurrences are uncommon.[1,9]
Metastases have been reported in 30-60% of the patients with subcutaneous leiomyosarcomas[7,12,17] and in 5% of the patients with cutaneous tumors.[17] Metastases are usually hematogeneous whereas the lung is the most frequently affected organ.[7,9,10,12] Although in 80% of the cases metastases occur within the first two years after diagnosis,[17] they may also develop many years later.[3] Lymph node metastases are uncommon and have been reported in 25% of the cases.[12] The presence of metastases at presentation has been reported as the strongest risk factor related to death.[8]
Factors that have correlated with adverse prognosis in patients with subcutaneous leiomyosarcoma include tumor size, high mitotic rate, presence of necrosis, deep-seated tumors with fascia involvement and intratumoral vascular invasion.[8,9,11,15,23] Survival for tumors smaller than 2 cm and larger than 5 cm has been reported to be 95% and 30% respectively.[17] Gustafson et al.,[11] suggested that advanced age, vascular invasion and DNA aneuploidy could be used to identify prognostic subgroups. They found that 16 of 30 patients with two or three risk factors died of the disease, but only one patient died of 15 patients who had one or no risk factors.[11]
The role of adjuvant chemotherapy and radiation therapy in patients with superficial leiomyosarcoma is controversial and seems that the tumor is resistant in these modalities.[1,6,7] However, rare cases of patients successfully treated with chemotherapy have been reported. Haffner et al.,[24] reported a case of complete remission of an advanced cutaneous leiomyosarcoma following isolated limb perfusion with high-dose tumor necrosis factor-alpha and melphalan. Chemotherapy has been used in the management of widespread metastatic disease but the response rate was 15-30% and the median survival was one year.[17]
Adjuvant radiotherapy may be reserved for tumors larger than 5 cm, for high-grade tumors in order to reduce the likelihood of recurrence and in combination with surgery in cases of recurrent tumors.[17]
The long-term prognosis, however, of patients with subcutaneous leiomyosarcoma remains poor despite adequate local control with or without adjuvant radiation therapy.[8] The tumor may run a more aggressive clinical course in the presence of immunosuppression.[19]
In our case, although an initial wide surgical excision with margins of 2 cm was performed and postoperative adjuvant radiotherapy was administered, the tumor recurred within the irradiated field two years later. Long-term follow-up of the patients is therefore essential.[22]
In conclusion, subcutaneous leiomyosarcoma is a rare clinical entity. Clinical presentation may be non-specific. Physicians should be aware of the misleading features of the tumor in order to avoid delay in diagnosis and treatment. Early surgical resection with wide margins of at least 2 cm is the treatment of choice and has been reported to mostly influence the prognosis. However, the tumor has a high tendency to recur locally and metastasize. Recurrences may develop despite the initial wide surgical resection and administration of adjuvant radiation therapy. Long-term follow-up of the patients is mandatory.
ACKNOWLEDGMENT
The author would like to thank Dr Tsiambas E. from the Pathology Department for providing the histology slide.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Wascher RA, Lee MY. Recurrent cutaneous leiomyosarcoma. Cancer. 1992;70:490–2. doi: 10.1002/1097-0142(19920715)70:2<490::aid-cncr2820700218>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Tsutsumida A, Yoshida T, Yamamoto Y, Itoh T, Minakawa H, Sugihara T. Management of superficial leiomyosarcoma: A retrospective study of 10 cases. Plast Reconstr Surg. 2005;116:8–12. doi: 10.1097/01.prs.0000169711.70525.10. [DOI] [PubMed] [Google Scholar]
- 3.Jimenez-Acosta F, Poblet E. Dermal and subcutaneous tumors. In: Kerbel FA, Jimenez-Acosta F, editors. Dermatology just the facts. New york: The McGraw-Hill; 2003. pp. 293–308. [Google Scholar]
- 4.Morel M, Taieb S, Penel N, Mortier L, Vanseymortier L, Robin YM, et al. Imaging of the most frequent soft-tissue sarcomas. Skeletal Radiol. 2011;40:271–84. doi: 10.1007/s00256-009-0855-y. [DOI] [PubMed] [Google Scholar]
- 5.Guillén DR, Cockerell CJ. Cutaneous and subcutaneous sarcomas. Clin Dermatol. 2001;19:262–8. doi: 10.1016/s0738-081x(01)00177-8. [DOI] [PubMed] [Google Scholar]
- 6.Yajima K, Shirai Y, Fujita N, Sato D, Umezu H, Hatakeyama K. A giant subcutaneous leiomyosarcoma arising in the inguinal region. World J Surg Oncol. 2005;3:14. doi: 10.1186/1477-7819-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields JP, Helwig EB. Leiomyosarcoma of the skin and subcutaneous tissue. Cancer. 1981;47:156–69. doi: 10.1002/1097-0142(19810101)47:1<156::aid-cncr2820470127>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Svarvar C, Böhling T, Berlin O, Gustafson P, Folleras G, Bjerkehagen B, et al. Scandinavian Sarcoma Group Leiomyosarcoma Working Group.Clinical course of nonvisceral soft tissue leiomyosarcoma in 225 patients from the Scandinavian Sarcoma Group. Cancer. 2007;109:282–91. doi: 10.1002/cncr.22395. [DOI] [PubMed] [Google Scholar]
- 9.Jensen ML, Jensen OM, Michalski W, Nielsen OS, Keller J. Intradermal and subcutaneous leiomyosarcoma: A clinicopathological and immunohistochemical study of 41 cases. J Cutan Pathol. 1996;23:458–63. doi: 10.1111/j.1600-0560.1996.tb01435.x. [DOI] [PubMed] [Google Scholar]
- 10.Dahl I, Angervall L. Cutaneous and subcutaneous leiomyosarcoma.A clinicopathologic study of 47 patients. Pathol Eur. 1974;9:307–15. [PubMed] [Google Scholar]
- 11.Gustafson P, Willén H, Baldetorp B, Fernö M, Akerman M, Rydholm A. Soft tissue leiomyosarcoma.A population-based epidemiologic and prognostic study of 48 patients, including cellular DNA content. Cancer. 1992;70:114–9. doi: 10.1002/1097-0142(19920701)70:1<114::aid-cncr2820700119>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 12.Stout AP, Hill WT. Leiomyosarcoma of the superficial soft tissues. Cancer. 1958;11:844–54. doi: 10.1002/1097-0142(195807/08)11:4<844::aid-cncr2820110425>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 13.Fauth CT, Bruecks AK, Temple W, Arlette JP, DiFrancesco LM. Superficial leiomyosarcoma: A clinicopathologic review and update. J Cutan Pathol. 2010;37:269–76. doi: 10.1111/j.1600-0560.2009.01405.x. [DOI] [PubMed] [Google Scholar]
- 14.Schadendorf D, Haas N, Ostmeier H, Czarnetzki BM. Primary leiomyosarcoma of the skin. A histological and immunohistochemical analysis. Acta Derm Venereol. 1993;73:143–5. doi: 10.2340/0001555573143145. [DOI] [PubMed] [Google Scholar]
- 15.Massi D, Beltrami G, Mela MM, Pertici M, Capanna R, Franchi A. Prognostic factors in soft tissue leiomyosarcoma of the extremities: A retrospective analysis of 42 cases. Eur J Surg Oncol. 2004;30:565–72. doi: 10.1016/j.ejso.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 16.Auroy S, Contesso G, Spatz A, Genin J, Margulis A, Lecesne A, et al. Primary cutaneous leiomyosarcoma: 32 cases. Ann Dermatol Venereol. 1999;126:235–42. [PubMed] [Google Scholar]
- 17.Lang RG., Jr . Textbook of Dermatologic Surgery. Padova: Piccin Nuova Libraria; 2008. Malignant tumors of the dermis and subcutaneous tissue; pp. 435–70. [Google Scholar]
- 18.McKee MD, Liu DF, Brooks JJ, Gibbs JF, Driscoll DL, Kraybill WG. The prognostic significance of margin width for extremity and trunk sarcoma. J Surg Oncol. 2004;85:68–76. doi: 10.1002/jso.20009. [DOI] [PubMed] [Google Scholar]
- 19.Humphreys TR, Finkelstein DH, Lee JB. Superficial leiomyosarcoma treated with Mohs micrographic surgery. Dermatol Surg. 2004;30:108–12. doi: 10.1111/j.1524-4725.2004.30018.x. [DOI] [PubMed] [Google Scholar]
- 20.Starling J, 3rd, Coldiron BM. Mohs micrographic surgery for the treatment of cutaneous leiomyosarcoma. J Am Acad Dermatol. 2011;64:1119–22. doi: 10.1016/j.jaad.2010.11.051. [DOI] [PubMed] [Google Scholar]
- 21.Huether MJ, Zitelli JA, Brodland DG. Mohs micrographic surgery for the treatment of spindle cell tumors of the skin. J Am Acad Dermatol. 2001;44:656–9. doi: 10.1067/mjd.2001.112381. [DOI] [PubMed] [Google Scholar]
- 22.Massi D, Franchi A, Alos L, Cook M, Di Palma S, Enguita AB, et al. Primary cutaneous leiomyosarcoma: Clinicopathological analysis of 36 cases. Histopathology. 2010;56:251–62. doi: 10.1111/j.1365-2559.2009.03471.x. [DOI] [PubMed] [Google Scholar]
- 23.Miyajima K, Oda Y, Oshiro Y, Tamiya S, Kinukawa N, Masuda K, et al. Clinicopathological prognostic factors in soft tissue leiomyosarcoma: A multivariate analysis. Histopathology. 2002;40:353–9. doi: 10.1046/j.1365-2559.2002.01361.x. [DOI] [PubMed] [Google Scholar]
- 24.Häffner AC, Zepter K, Fritz T, Dummer R, Lejeune FJ, Burg G. Complete remission of advanced cutaneous leiomyosarcoma following isolated limb perfusion with high-dose tumour necrosis factor-alpha and melphalan. Br J Dermatol. 1999;141:935–6. doi: 10.1046/j.1365-2133.1999.03179.x. [DOI] [PubMed] [Google Scholar]


