Abstract
Objective:
We sought to evaluate the incidence of metabolic syndrome and non-metabolic syndrome among type 2 diabetic patients attending the diabetic outpatient clinic at tertiary care hospital, Warangal.
Materials and Methods:
A cross-sectional study was conducted in a period of 6 months from January 2011 to June 2011. The study group consisted of 75 type 2 diabetic patients. They were screened for hypertension, hyperlipidemia, obesity, and clinical characteristics, and other co-morbidities were recorded. Metabolic syndrome diagnosis was made as per ATP III guidelines.
Results:
The prevalence of metabolic syndrome was significant in men (54.8%) compared to women (45.2%). Incidence of metabolic syndrome was found to be more in normal weight patients (43.56%). Low high density lipoprotein (HDL) levels were observed in both rural (90.63%) and urban (95.65%) patients with metabolic syndrome, followed by increase in waist circumference. The mean HDL level was found to be 23.77 mg/dl. Patients in the age group 51-60 years were found to be more affected with metabolic syndrome. Sedentary household female patients (58.3%) and illiterates (41.8%) were suffering from metabolic syndrome. Patients with metabolic syndrome had been suffering with diabetes (duration of diabetes) from 1 to 5 years. In summary, this cross-sectional study characterizes the metabolic and non-metabolic syndromes of type 2 diabetes patients living in Telangana regions, using ATP III guidelines, and generates a biological resource that enables further investigation of numerous hypotheses related to genetic exposure of both in a population.
Conclusion:
These results suggest that higher prevalence of metabolic syndrome was observed in non-obese male patients and was significantly associated with aging. Nevertheless, further studies are required to confirm the metabolic syndrome in larger population.
Keywords: Body mass index, diabetes, high density lipoprotein, metabolic syndrome, mortality
INTRODUCTION
The metabolic syndrome (MetS), as defined by the National Cholesterol Education Program Adult Treatment Panel III (NCEP-ATP III) criteria, is a constellation of fasting lipids and lipoproteins, waist circumference, glucose, and blood pressure abnormalities that have been associated with increasing risk for developing cardiovascular disease (CVD) and diabetes.[1–3] These definitions are based on expert opinion and not on evidence resulting from prospective epidemiologic studies. Therefore, it remains unclear whether single component features of the MetS or the thresholds at which each component is defined as present or absent are informative and optimal for predicting the risk of cardiovascular disease or early death.[4] Weiss and colleagues reported that the prevalence of the MetS increased with the severity of obesity and reached a proportion of 50% in severely obese youngsters. The prevalence of the MetS increased significantly with increasing insulin resistance after adjustment for the ethnic group factors and degree of obesity.[5] Type 2 diabetes and the MetS have been regarded as a disease of adults for a long time,[6] but it has clearly become evident that the disease can begin at different ages in all ethnic groups and can be already present in childhood.[7–9] This observation also points toward the existence of a metabolic endocrine susceptibility factor determining the risk for clinically developing MetS already during childhood. An aggressive global approach to screening and management of the MetS is therefore recommended to slow the growth of the syndrome throughout the United States.[10] Therefore, our present study highlights the incidence of MetS and non-metabolic syndrome (NMetS) in type 2 diabetic patients.
MATERIALS AND METHODS
We performed a cross-sectional study from January 2011 to June 2011. This research was jointly conducted by Vaagdevi College of Pharmacy and Mahatma Gandhi Memorial (MGM) Hospital. Both are affiliated to Kakatiya University, Warangal, Andhra Pradesh, India.
Study participants
The inclusion criteria were: Age >18 years and diabetic. Exclusion criteria were: Endocrine diseases such as Cushing's disease, type 1 diabetes mellitus, acromegaly, hypothyroidism, hypogonadism, patient on prolong steroid use, and those who were on active drug treatment for obesity at the time of admission.
The source of the data was the outpatients who were attending the MGM Hospital, Warangal. The Performa included co-morbidities, characteristics, and details of physical and biochemical information and treatment, which were reviewed and recorded.
The physical and biochemical measurements were made at the first visit. The independent variables of interest were age, gender, ethnicity, smoking, alcohol consumption, and history of diabetic types. All patients were interviewed regarding duration of diabetes and anti-diabetic drugs.
All subjects enrolled in the study underwent detailed clinical examinations, including measurement of height, weight, waist circumference, and blood pressure. Patients were considered hypertensive if these were already on antihypertensive therapy or with a systolic pressure ≥135 mm Hg and a diastolic pressure ≥85 mm Hg. Fasting venous blood was sampled from antecubital vein from all patients for the measurement of plasma glucose, total cholesterol, high density lipoprotein (HDL) cholesterol, and serum triglycerides.
Lifestyle habits
Patients were assessed for smoking habits, physical inactivity, and alcohol consumption. Briefly, patients were categorized based on their smoking habits into never smoker, former smoker, and current smoker
Data collection
In the first two weeks of January 2011, a workshop was conducted in cardiac unit at MGM Hospital, Warangal, which standardized the protocol and methodology (administering the structure questionnaires, drawing blood, and taking medications). Thus, the investigators (diabetologist, cardiologist) epidemiologist, PG students, Interns, students, training nurse, and Pharm. D students took part in the 2-day training course on using NCEP-ATP III guidelines.
Patients were instructed and encouraged not to eat after 7:00 p.m. before the laboratory measurement. Baseline overnight fasting blood was drawn from 7:00 to 9:00 a.m. Fasting serum and plasma samples [preserved with ethylene diamine tetraaceticacid (EDTA) and sodium fluoride (NaF)] were collected within 2 h of venous blood sampling and immediately frozen to −20°C until triglycerides, HDL, and cholesterol were analyzed and measured using standard methods.[11]
Ethical approval was obtained from the Medical Review Board of Kakatiya Medical College (KMC), Andhra Pradesh, India. Eligible participants were invited to attend the diabetic unit on a specified date. They were free to participate and gave written consent according to Helsinki declaration. Descriptive strategies were calculated for each variable. All continuous variables were expressed as a mean with standard deviation; median, intraquartile range, frequency, and percentage were obtained for categorized variables. Univariables, multivariables, binary logistics, and regression analysis were used to identify factors significantly associated with MetS and NMetS classified based on NCEP-ATP III criteria. All analyses were performed using the GraphPad Prism version 5.
RESULTS
There were 275 patients who attended the diabetic OP clinic from January 2011 to June 2011. However, only 75 (27.2%) patients were eligible for the study. Based on the inclusion and exclusion criteria, diabetic patients had complete data and comprised the study population. Mean duration of diabetes was 6.52 years in MetS and 4.8 years in NMetS. The relative proportion of alcoholic patients with MetS was 41.81%, while 50% of patients were non-alcoholic with NMetS. Current smokers (20.8%) and past smokers (18.18%) with MetS were equally distributed. However, illiterate patients (41.8%) had higher incidence of MetS and reduced incidence (20%) of NMetS. The data are summarized in Table 1.
Table 1.
Prevalence (%) of the metabolic syndrome versus non-metabolic syndrome in Warangal adults according to socio-demographic details
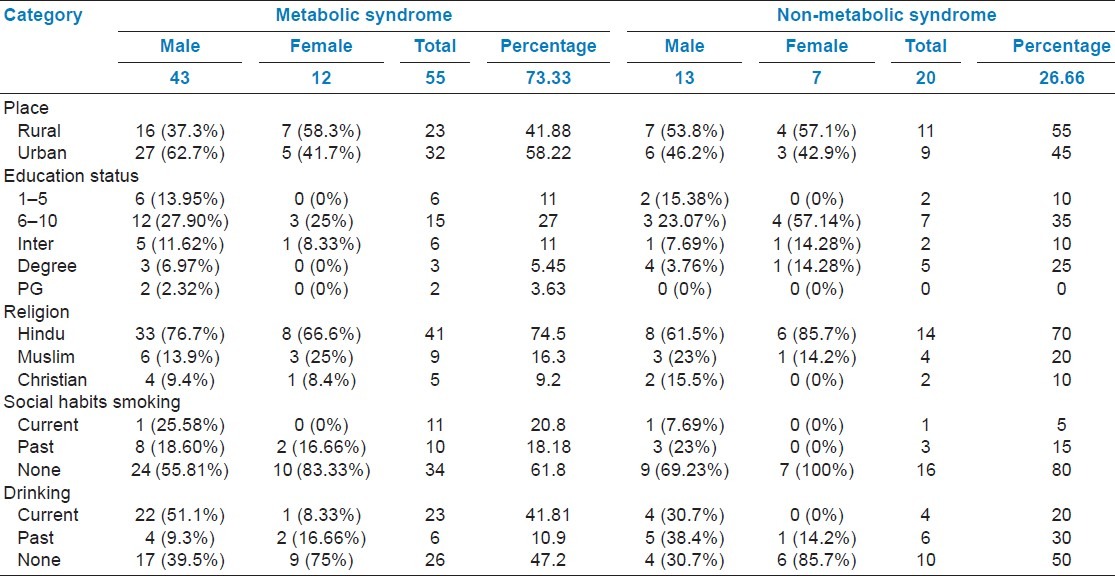
Table 2 presents prevalence of MetS and NMetS according to NCEP-ATP III guidelines. Sedentary lifestyle is more prone to MetS (64.18%) compared to NMetS (55%). Women's sedentary lifestyle (91.66%) resulted in higher incidence of MetS. Patients with high prevalence of MetS compared to those with NMetS were higher (34.88%) in 51-60 years age group, followed by 25.59% in 61-70 years age group [Table 3]. An individual component of the MetS and NMetS in gender and living area was also measured. Interestingly, urban people had higher incidence of developing MetS compared to rural people. Low HDL levels were consistently observed in rural and urban population (95.65% and 90.625%, respectively), followed by increase in waist circumference in rural and urban population (65.62% and 73.19%, respectively).
Table 2.
Prevalence of metabolic and non-metabolic syndrome according to NCEP-ATP III guidelines
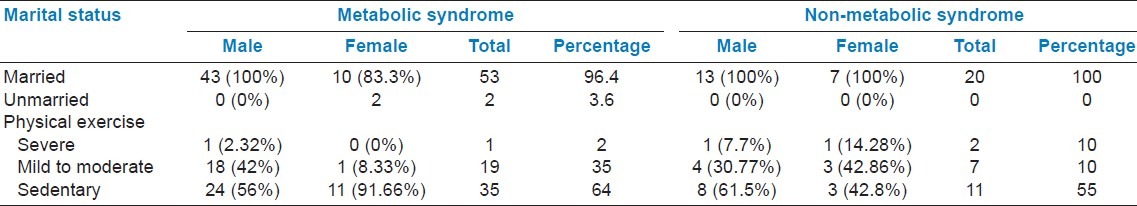
Table 3.
Prevalence of metabolic and non-metabolic syndrome in patients aged ≥18 years by gender, age, and group
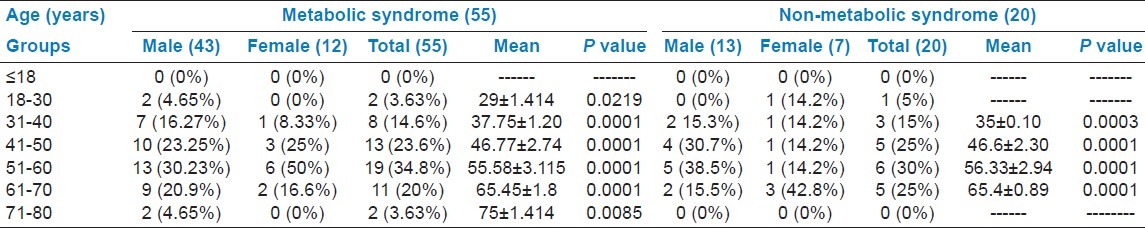
Table 4 presents the physical characteristics of South Indian participants (Warangal adults) with type 2 diabetes. Interestingly, high incidence of MetS was observed in normal weight patients (43.56%) compared to obese (10.9%), overweight (40%), and underweight (5.45%) patients.
Table 4.
Physical characteristics of South Indian participants in the Warangal adults of type 2 diabetic patients
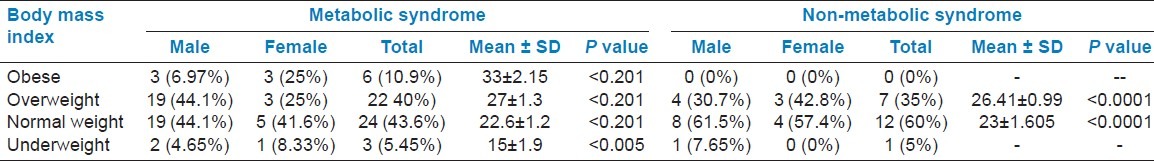
Table 5 shows the gender wise Mean ± SD of the MeS versus NMetS patients . Sixty percent of patients with NMetS were normal weight and 35% were overweight. Mean body mass index (BMI) was 25.01 kg/m2 in MetS and 24.165 in NMetS patients. The waist circumference mean was 92.7 ± 10.3 cm in MetS and 84.9 ± 8.25 cm in NMetS patients. However, triglycerides were 186.12 ± 76.5 mg/dl in MetS and 156.82 ± 75.43 mg/dl in NMets patients (P < 0.001). HDL levels were 23.77 ± 10.1 mg/dl in MetS, compared to 33.47 ± 16 mg/dl in NMetS patients. The overall systolic blood pressure was 130 ± 13.7 mm Hg in MetS and 118.17 ± 7.95 mm Hg in NMetS, and diastolic blood pressure was 84.36 ± 7.65 mm Hg in MetS and 77.85 ± 5.53 in NMetS patients. Fasting blood glucose level was 116.12 ± 55.4 mg/dl in MetS and 111.58 ± 34.6 mm Hg in NMetS. Table 5 also depicts the prevalence of MetS and NMetS according to gender and age. Men and women aged between 51 and 60 years suffered more frjom MetS, followed by those from 41 to 50 years of age. 26.66% of total diabetic patients were not suffering from MetS. 73.33% of total diabetic patients were suffering from MetS. Mean age was 52.8 years in MetS and 51.3 years in NMetS.
Table 5.
Mean ± SD of the components of the metabolic syndrome versus non-metabolic syndrome according to gender
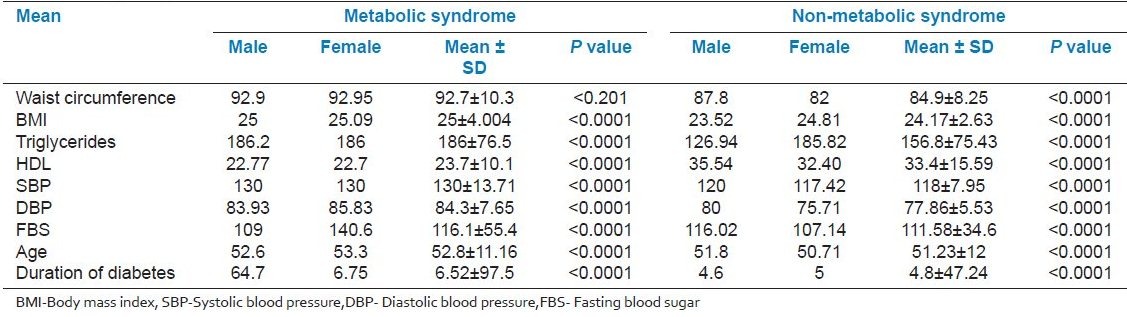
Table 6 presents the top 12 drugs utilized concomitantly in MetS patients with type 2 diabetes; anti-diabetics were found to be highly used (33.2%), followed by vitamins (20.7%), anti-ulcer drugs (17.84%), antihypertensives (11.61%), analgesics and antipyretics (4.9%), anti-inflammatory drugs (4.14%), antibiotics (2.5%), anti-allergic drugs (1.7%), hyperlipidemic drugs (1.7%), anti-angina drugs (0.8%), anti-diarrheal (0.4%), and anti-platelet drugs (0.4%).
Table 6.
Top 12 drugs utilized concomitantly with metabolic syndrome in diabetes mellitus
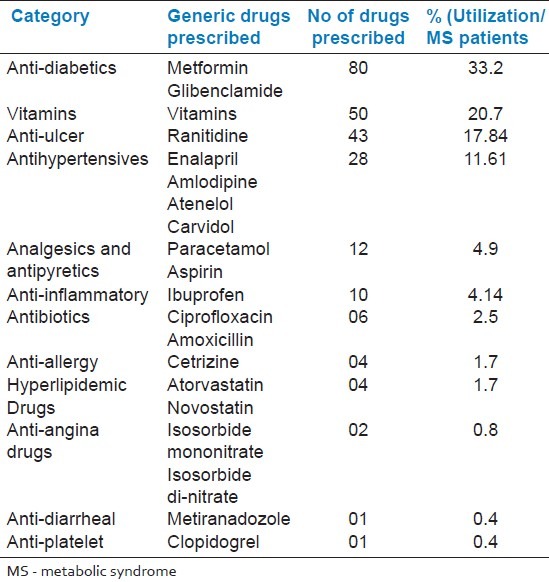
Table 7 shows the Indian Diabetes Risk Score (IDRS) which was developed based on multiple logistic regression analysis. In 2007, Mohan et al. reported that IDRS was calculated using four simple variables, namely age, family history, regular exercise, and waist circumference. The individuals were classified as being at high risk (score > 60), moderate risk (score 30–50), and low risk (score < 30) out of total score. 55% of MetS patients had high score, followed by 43.63% of patients with moderate score and 1.8% with low score. High score leads to the development of cardiovascular disease. Score was calculated on the basis of Figure 1. Even patients with NMetS had a high score (45%), followed by moderate 35% and low (20%) scores.
Table 7.
Indian diabetes risk score (IDRS) developed based on multiple logistic regression analysis
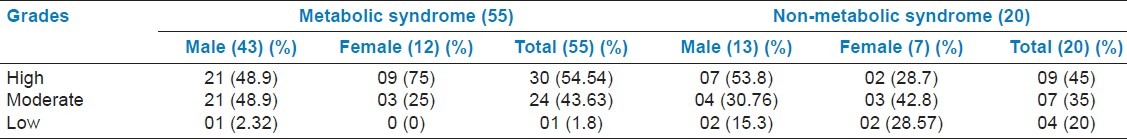
Figure 1.
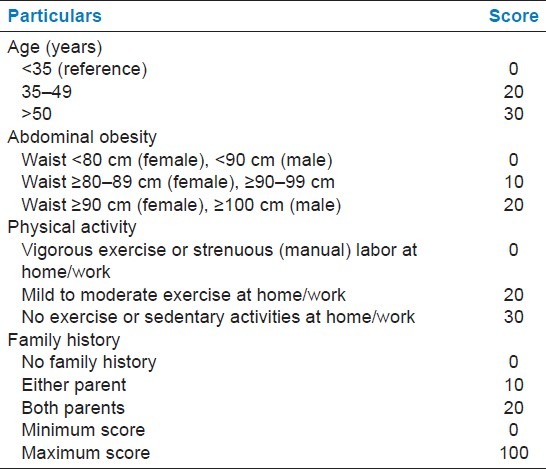
Indian diabetes risk score (IDRS)
DISCUSSION
This study analyzed the incidence of the MetS and NMetS among type 2 diabetic patients attending diabetic outpatient clinic at a tertiary care hospital, using the recently introduced definition of the MetS by the NCEP-ATP III guidelines. The findings of the present study show that the incidence of MetS was significantly higher in males than in females. Contrary to this finding, a study in Iran found that MetS was significantly higher in women than in men. Nizal et al. highlighted the very low levels of physical activity in women than in men. Physical activity is a complex behavior which consists of different components, including leisure time transportation, hours of work, and household activities.[12]
In our study, the MetS was more prevalent in urban areas. This finding is similar to the findings of recent surveys conducted in China and Greece, showing a higher prevalence of the MetS in urban compared to rural residents.[13,14]
We found energy expenditure to be lower and the MetS to be considerably higher in women compared to men, in urban residents compared to rural residents, and in sedentary individuals compared to active individuals of both genders in urban and rural areas. This finding was similar to the previous reports.[15,16]
Our findings highlight the very low levels of physical activity in women than in men. Physical activity is a complex behavior which consists of different components, including leisure time transportation, hours of work, and household activities. Physical inactivity could well be a major contributor for women being more prone to obesity problems than men.
Hypertension is the most frequently occurring component of MetS when compared to NMetS in subjects with type 2 diabetes, and was found in 72.09% of men and 58.33% of women. This finding is in accordance with a previous report.[17]
Abdominal obesity was common in South Asians and evident in non-obese people. They have high percentage of body weight, thick subcutaneous adipose tissue, low muscular mass, insulin resistance, and hyperinsulinemia. This body composition is conducive to the development of MetS. According to a recent study on South Asians, the prevalence of MetS was estimated to be 25.8% in the general population as per international diabetes federation (IDF) definitions.[18] A very recent study has reported the prevalence of MetS in Pakistan, according to a different definition, to be from 18 to 46%, comparable to the data from other South Asian nations.[19] Our finding was similar to that previously reported.[20]
Moreover, the triglycerides were elevated and HDL levels reduced in patients with MetS when compared to NMetS patients. Hence, our results emphasize on prescription of hyperlipidemic drug to MetS patients to prevent further complications.
Our data support the notion that obesity and central obesity in particular is a strong risk factor for type 2 diabetes, a finding that is consistent with the previous reports on various racial/ethnic populations.[21,22] Globally, it has been estimated that approximately 58% of type 2 diabetes is attributable to overweight and obesity, and 90% of type 2 diabetes in western countries is attributed to weight gain.[23] These findings also support that preventive lifestyle interventions should be targeted at lowering both BMI and central obesity in Indian adults.
We found that high prevalence of MetS was primarily indicated by central obesity combined with dyslipidemia (low HDL, Hypertension and triglycerides). The high prevalence of hypertriglyceridemia, notably in men, might be due to the sedentary lifestyle. This finding was contrary to the previous report.[11]
CONCLUSION
Our results suggest that prevalence of MetS was observed in non-obese patients and was higher in male patients and positively correlated with aging. Nevertheless, further studies are required to validate the MetS in larger population.
ACKNOWLEDGMENTS
We thank the diabetic patients of MGM hospital who participated in this study. We also thank Dr. M. Satyadev, Superintendent, Mahatma Gandhi Memorial Hospital, for his valuable suggestion and guidance to complete this pilot study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Grundy S. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP). Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 2.Manson JE, Skerrett PJ, Greenland P, Vanitallie TB. The escalating pandemics of obesity and sedentary life style. A Call to action for clinicians. Arch Intern Med. 2004;164:249–58. doi: 10.1001/archinte.164.3.249. [DOI] [PubMed] [Google Scholar]
- 3.Alwan A, King H. Definition, diagnosis and Classification of Diabetes Mellitus and its Complications. Part I: Diagnosis and Classification of Diabetes Mellitus. Report of a WHO consultation. 1999 [Google Scholar]
- 4.Franks PW, Olsson T. Metabolic syndrome and early death: Getting to the heart of the problem. Hypertension. 2007;49:10–2. doi: 10.1161/01.HYP.0000251934.55488.ae. [DOI] [PubMed] [Google Scholar]
- 5.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 6.Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414:782–7. doi: 10.1038/414782a. [DOI] [PubMed] [Google Scholar]
- 7.Sinha R, Fisch G, Teague B, Tamborlane WV, Banyas B, Allen K, et al. Prevalence of impaired glucose tolerance among children and adolescents with marked obesity. N Engl J Med. 2002;346:802–10. doi: 10.1056/NEJMoa012578. [DOI] [PubMed] [Google Scholar]
- 8.Sung RY, Tong PC, Yu CW, Lau PW, Mok GT, Yam MC, et al. High prevalence of insulin resistance and metabolic syndrome in overweight/ obese preadolescent Hong Kong Chinese children aged 9-12 years. Diabetes Care. 2003;26:250–1. doi: 10.2337/diacare.26.1.250. [DOI] [PubMed] [Google Scholar]
- 9.Wei JN, Sung FC, Lin CC, Lin RS, Chiang CC, Chuang LM. National surveillance for type 2 diabetes mellitus in Taiwanese children. JAMA. 2003;290:1345–50. doi: 10.1001/jama.290.10.1345. [DOI] [PubMed] [Google Scholar]
- 10.Bestermann W, Houston MC, Basile J, Egan B, Ferrario CM, Lackland D, et al. Addressing the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome in the southeastern United States, part II: Treatment recommendations for management of the global cardiovascular risk of hypertension, dyslipidemia, diabetes mellitus, and the metabolic syndrome. Am J Med Sci. 2005;329:292–305. doi: 10.1097/00000441-200506000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Faeh D, Bopp M. Proposed obesity body mass index correction for self-reported data may not be appropriate. J Epidemiol Community Health. 2009;63:863–4. doi: 10.1136/jech.2009.089060. [DOI] [PubMed] [Google Scholar]
- 12.Sarrafzadegan N, Kelishadi R, Baghaei A, Hussein Sadri G, Malekafzali H, Mohammadifard N, et al. Metabolic Syndrome: An emerging public health problem in Iranian women: Isfahan healthy Heart Program. Int J Cardiol. 2008;13:90–6. doi: 10.1016/j.ijcard.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 13.Gu D, Reynolds K, WU X, Chen J, Duan X, Reynolds RF, et al. for the inter ASIA collaborate group prevalence of the MetS and overweight among adults in China. Lancet. 2005;365:1398–405. doi: 10.1016/S0140-6736(05)66375-1. [DOI] [PubMed] [Google Scholar]
- 14.Athyros VG, Bouloukos VI, Pehlivanidis AN, Papageorgiou AA, Dionysopoulou SG, Symeonidis AN, et al. The prevalence of the MetS in Greece. The MetS Greece multicentre study. Diabetes Obes Metab. 2005;7:397–405. doi: 10.1111/j.1463-1326.2004.00409.x. [DOI] [PubMed] [Google Scholar]
- 15.Laaksonen DE, Lakka HM, Salomen JT, Niskanen LK. Low levels of leisure-time physical activity and cardio-respiratory fitness predict development of the metabolic syndrome. Diabetes Care. 2002;25:1612–8. doi: 10.2337/diacare.25.9.1612. [DOI] [PubMed] [Google Scholar]
- 16.Ekelund U, Brag S, Franka PW, Hennings S, Emms S. Physical activity energy expenditure predicts progression towards the metabolic syndrome independently of aerobic fitness in middle aged healthy Caucasians; the medical research council Ely Study. Diabetes Care. 2005;28:1195–2000. doi: 10.2337/diacare.28.5.1195. [DOI] [PubMed] [Google Scholar]
- 17.Wokoma FS. Diabetes and hypertension in Africa-an overview. Diab int. 2002;12:36–40. [Google Scholar]
- 18.Deepa M, Farruq S, Datta M, Deepa R, Mohan V. Prevalence of metabolic syndrome using WHO, ATP III and IDF definitions in Asia Indians; the Chennai Urban Rural Epidemiology Study. Diabetes Metab Res Rev. 2007;23:127–34. doi: 10.1002/dmrr.658. [DOI] [PubMed] [Google Scholar]
- 19.Basit A, Shera AS. Prevalence of metabolic syndrome in Pakistan. Metab Syndr Relat Disord. 2008;6:171–5. doi: 10.1089/met.2008.0005. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Rimm EB, Stampfer MJ, Willett WC, Hu FB. Comparison of abdominal adiposity and overall obesity in predicting risk of type 2 diabetes among men. Am J Clin Nutr. 2005;81:555–63. doi: 10.1093/ajcn/81.3.555. [DOI] [PubMed] [Google Scholar]
- 21.Hu D, Xie J, FU P, Zhou J, Yu D, Whelton PK, et al. Central rather than overall obesity is related to diabetes in the Chinese population: The interASIA study. Obesity (Silver Spring) 2007;15:2809–16. doi: 10.1038/oby.2007.333. [DOI] [PubMed] [Google Scholar]
- 22.Visscher TL, Seidell JC. The public health impact of obesity. Annu Rev Public Health. 2001;22:355–75. doi: 10.1146/annurev.publhealth.22.1.355. [DOI] [PubMed] [Google Scholar]
- 23.Association CM‑aGoCOTFoILS. The recommendations of the body mass index categories for adult Chinese. Clin J Prev Med. 2001;35:349–50. [Google Scholar]


