Abstract
In the present study, the effects of oral administration of imidacloprid for 4 weeks on serum biochemical, oxidative stress, histopathological and ultrastructural alterations were assessed in the liver of male rats. This study also aimed to investigate whether vitamin C could protect against the imidacloprid-induced oxidative stress. Forty-eight male Sprague dawley rats were divided into four groups of 12 animals each. Group 1 served as the control, while groups 2 and 4 were administered with imidacloprid (80 mg/kg body weight) daily by oral gavage for 28 days. In addition to imidacloprid, group 4 also received vitamin C at 10 mg/kg daily by oral gavage for 28 days. Group 3 was maintained as the vitamin C control (dose as above). The serum biochemical assays revealed a significant (P < 0.05) increase in alanine transaminase and aspartate transaminase and decrease in total protein in group 2. The tissue biochemical profile revealed a significant (P < 0.05) reduction in reduced glutathione concentration in the liver of group 2 animals. Histologically, the liver showed marked dilation, congestion of central vein, portal vein and sinusoidal spaces, vacuolation/fatty change and degenerated hepatocytes. Ultra thin sections of the liver revealed swollen nuclei, varied size and shape of mitochondria, disrupted chromatin and rough endoplasmic reticulum. Co-treatment with vitamin C significantly (P < 0.05) reversed the imidacloprid-induced changes.
Keywords: Hepatotoxicity, imidacloprid, vitamin C
INTRODUCTION
Indiscriminate usage of pesticides in agriculture is leading to contamination of the environment and natural resources and, thereby, producing an adverse impact on animal and human health. Imidacloprid is a neonicotinoid insecticide first registered for agricultural usage in 1994 and classified under toxicity class II/III agents by the United States Environmental Protection Agency.[1] It is extensively used against various sucking insects, viz. aphids, leaf hoppers, thrips, white fleas, leaf miners and beetles,[2] and is also used as a foliar treatment for soil and seed dressing.[3] In veterinary medicine, it is used as a flea control agent on dogs and cats[4] and also to control houseflies on poultry farms. It is one of the fastest sold insecticides across the world because of its high selective toxicity in insects and apparent safety in humans. It acts on the nervous system by blocking postsynaptic acetylcholine receptors, which kills the insect.[5] Its selective toxicity results from its high affinity to the insect's nicotinic acetylcholine receptors compared with mammals.[6] A case of acute poisoning was reported in a human following ingestion of a pesticide formulation containing 10% imidacloprid,[7] and two fatal intoxication cases have been reported recently.[8]
The potential role of oxidative stress in imidacloprid exposure suggests that antioxidant supplementation may mitigate imidacloprid-induced toxicity. Vitamin C plays an important role in protection against insecticide-induced hepatic toxicity as an antioxidant agent and prevents the effect of free radicals on vital cells.[9]
Although imidacloprid is widely used across the world, there is still less work done related to its toxicity in male rats. Therefore, a study has been designed to evaluate the serum biochemical, tissue antioxidant, histopathological and ultrastructural alterations induced by imidacloprid in the liver of male rats, and the protective role of vitamin C against imidacloprid-induced toxicity was also assessed.
MATERIALS AND METHODS
Chemicals
Imidacloprid was procured from GSP Crop Science Pvt. Ltd., Gujarat, India and Vitamin C was obtained from Abbott Health Care Pvt. Ltd., Bhiwandi, India.
Animals
Forty-eight male Sprague dawley rats weighing 200-250 g were procured from the National Institute of Nutrition (NIN), Hyderabad, India. The experiment was conducted as per CPCSEA guidelines and approved by the Institutional Animal Ethics Committee (Approval No. I/3/2012). The rats were housed in solid bottom polypropylene cages at the lab animal house in the Department of Pharmacology and Toxicology, College of Veterinary Science, Hyderabad, and were maintained in a controlled environment (temperature 20-22°C) throughout the course of the experiment. Rice husk was used as the bedding material. All the rats were provided ad libitum with standard pellet diet (procured from NIN) and water throughout the experimental period.
Experimental design
Following an acclimatization period of 1 week, the animals were divided into four groups consisting of 12 animals in each group. Group 1 served as the control, group 2 was treated with imidacloprid at the rate of 80 mg/kg body weight, group 3 was treated with vitamin C at the rate of 10 mg/kg body weight and group 4 was treated with both imidacloprid and vitamin C. These drugs were administered by oral gavage every day consequently for 28 days. At the end of the 14th day, six rats from each group and the remaining at the end of the 28th day were sacrificed by cervical dislocation. A day before sacrifice, blood was collected from the retro-orbital plexus for studying the serum biochemical profile (alanine transaminase (ALT), aspartate transaminase (AST) and total protein).
Sero-biochemical markers
ALT, AST and total protein were estimated in serum by using standard diagnostic kits.
Antioxidant markers
Glutathione (GSH) was estimated based on a reaction of reduced GSH with 5-5 ditiobis-2-nitrobenzoic acid.[10]
Histopathology
For histopathological examination, the formalin-fixed tissues were dehydrated through ascending grades of alcohol, cleared in three changes of xylene and embedded in paraffin. 5-micron thickness sections were cut and stained with hematoxylin and eosin as per standard protocols.[11]
Ultrastructure pathological examination
Soon after sacrifice, thin slices of liver tissue were dissected into 2.5% gluteraldehyde in 0.1M phosphate buffer (pH 7.3, stored at 4°C), washed in buffer, post-fixed in 1% osmium tetraoxide in 0.1M phosphate buffer, dehydrated with ascending grades of acetone, embedded in Spurr's resin and incubated overnight at 60°C for complete polymerization of the tissue. Semi thin (1000-1500 nm thickness) sections were made with ultra microtome and stained with 1% toludine blue to locate the exact area to be sectioned for TEM. Then, ultrathin sections were made (500-700 nm thickness), mounted on hexagonal copper grids, allowed to air dry overnight and stained with saturated urenyl acetate and 1% Reynolds's lead citrate as per the protocol of.[12] All grids were dried at room temperature and observed under a transmission electron microscope.
Statistical analysis
The data were subjected to statistical analysis by applying one-way Analysis of Variance. Differences between means were tested using Duncan's multiple comparison test and significance was set at P < 0.05.
RESULTS
Serum analysis revealed a significant (P < 0.05) decrease in mean values of total protein (5.82 ± 0.03 g/dL) on Day 14, and a similar trend was observed on Day 28 in group 2. A significant (P < 0.05) increase in ALT and AST activity (61.51 ± 0.28 IU/L and 101.81 ± 0.27 IU/L, respectively) was seen on Day 14, and a similar trend was observed on Day 28 following imidacloprid administration in group 2. Tissue antioxidant profile revealed a significant (P < 0.05) reduction of reduced GSH concentration (109.33 ± 0.25 μM/mg protein) on Day 14, and a similar trend was observed on Day 28 in the imidacloprid toxic group when compared with the control group. Co-administration of vitamin C significantly (P < 0.05) reversed the above values [Table 1].
Table 1.
Effect of vitamin C on serum biochemical and tissue antioxidant (glutathione) parameters against imidacloprid-induced toxicity in male rats
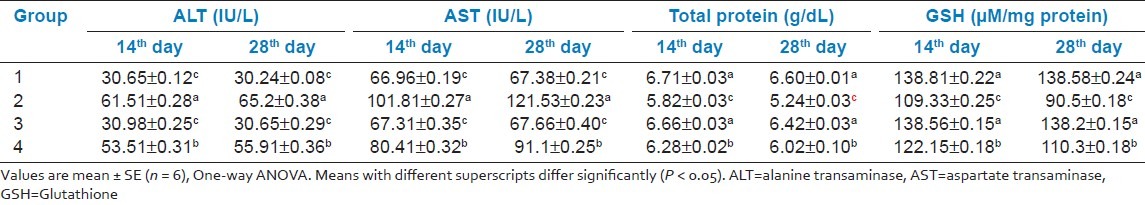
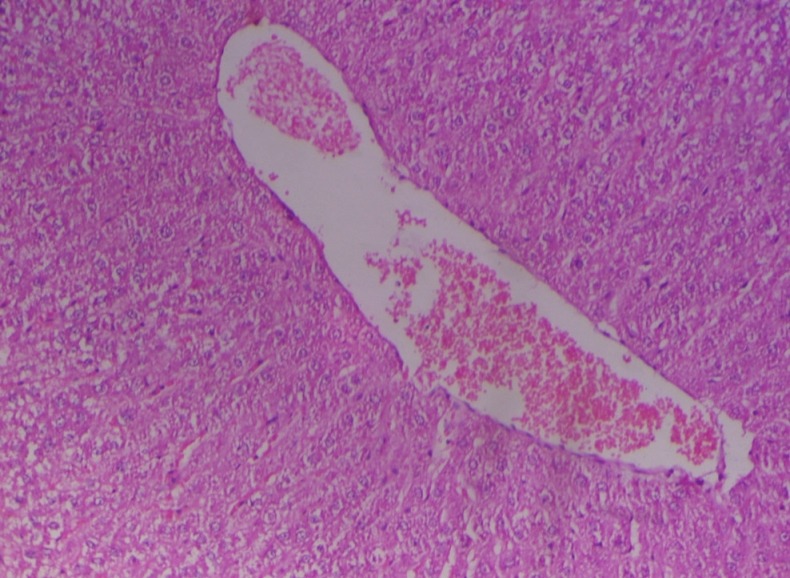
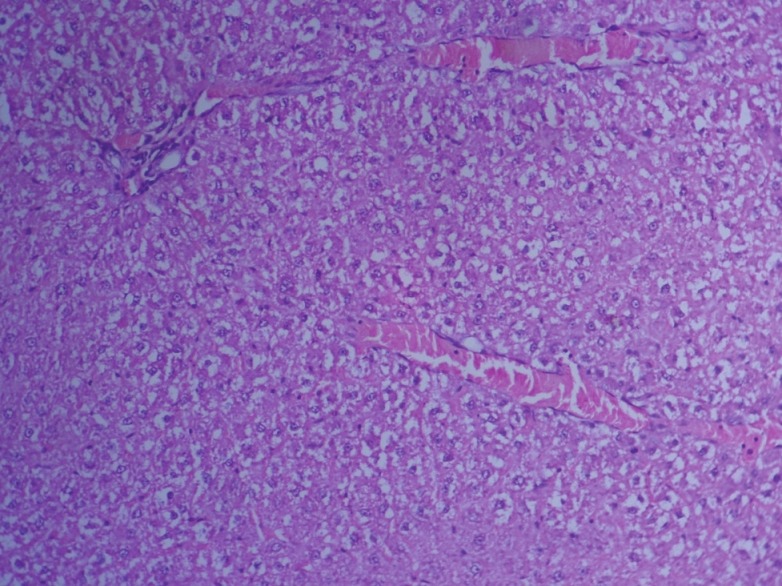
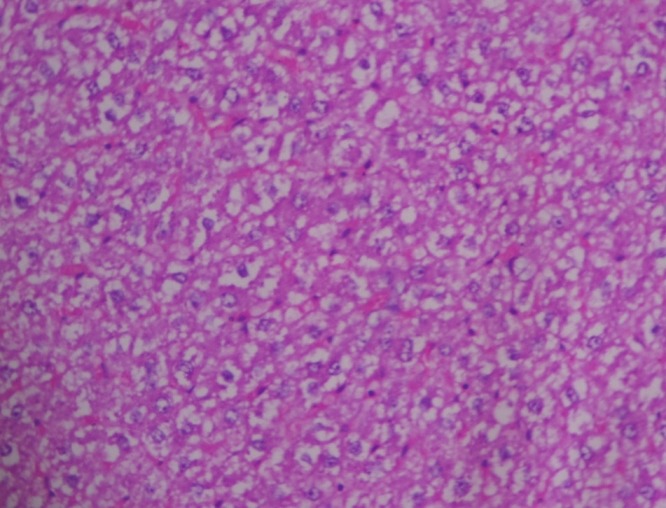
In group 2, hematoxylin and eosin sections of the liver revealed marked dilation and congestion of central vein [Figure 1] on Day 14, whereas on the 28th day, moderate congestion, dilation of sinusoids [Figure 2] and vacuolation of cytoplasm of hepatocytes/fatty change [Figure 3] were observed. Further, severe congestion of the portal vein and degeneration of hepatocytes [Figure 4] were noticed. In group 3, no lesions of pathological significance [Figure 5] were observed and liver sections of group 4 revealed marked dilation of the central vein and congestion of the portal vein on the 28th day of the experiment [Figure 6].
Figure 1.
Photomicrograph of liver showing marked dilation and congestion of central vein (Group 2, day 14): H and E ×100
Figure 2.
Photomicrograph of liver showing moderate dilation and congestion of sinusoidal spaces (Group 2, day 28): H and E ×200
Figure 3.
Photomicrograph of liver showing vacuolation/ fatty change in hepatocytes (Group 2, day 28): H and E ×200
Figure 4.
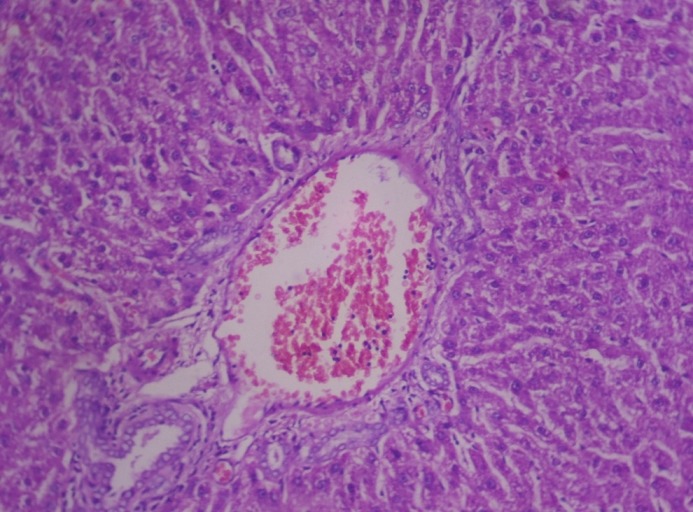
Photomicrograph of liver showing severe congestion of portal vein and degeneration of hepatocytes (Group 2, day 28): H and E ×200
Figure 5.
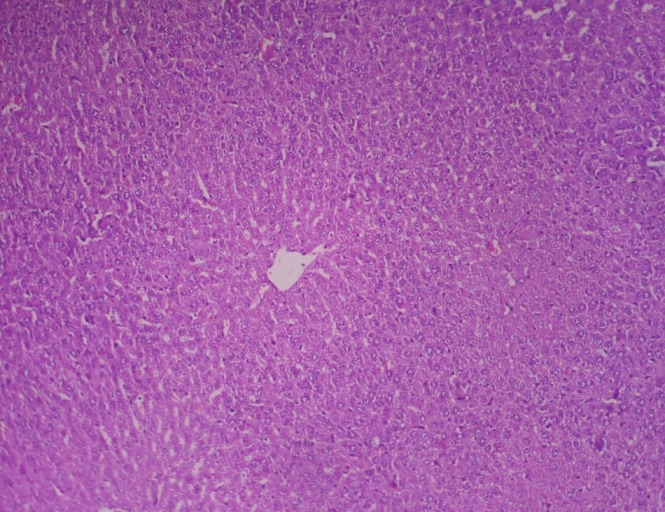
Photomicrograph of liver showing no pathological changes of significance (Group 3, day 28): H and E ×200
Figure 6.
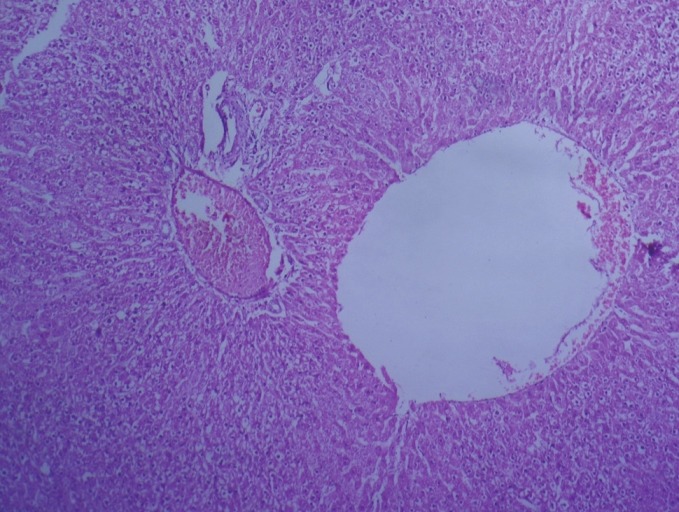
Photomicrograph of liver showing marked dilation of central vein and congestion of portal vein (Group 4, day 28): H and E ×100
Ultrathin sections of the liver in group 2 animals revealed swollen nuclei, sinusoidal congestion, disrupted chromatin, varied size and shape of mitochondria and disrupted rough endoplasmic reticulum [Figure 7] on Day 28. Group 4 sections showed swollen nuclei of hepatocytes [Figure 8] on the 28th day of the experiment.
Figure 7.

TEM of liver showing swollen nucleus (N), disrupted chromatin (arrow), disrupted endoplasmic reticulum (dER), varied size and shape of mitochondria (M)and normal kupffer cell (KC): UA and LC ×5580 (Group 2, day 28)
Figure 8.
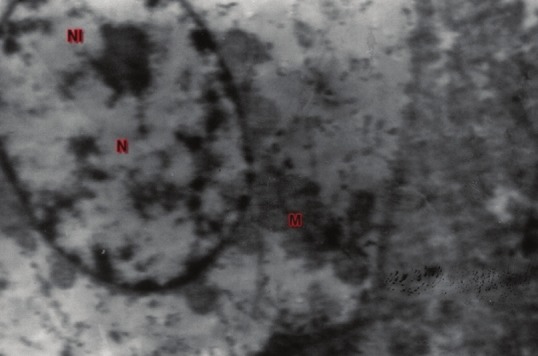
TEM of liver showing hepatocyte with swollen nucleus (N), normal nucleolus (NL) and mitochondria (M): UA and LC ×7440 (Group 4, day 28)
DISCUSSION
A significant increase in serum ALT and AST levels in group 2 rats was observed when compared with the control group. These results are inconsistent with the earlier reports.[13–15] It may be due to degeneration and necrosis of hepatocytes, which attributes an increased permeability of cell membrane that results in release of transaminases into the blood stream. These findings were correlated with the histopathological and ultrastructural changes observed in the liver of group 2 rats. Hepatotoxicity observed in the present study may be attributed to reactive oxygen species (ROS) induced by imidacloprid, as evident from significantly reduced concentration of GSH in toxic control group 2.
The total protein levels were significantly decreased in group 2 rats when compared with the control group. These findings are in accordance with the findings of Siddiqui et al.[16] The reduction in total protein may be due to hepatotoxicity induced by imidacloprid, which was evident in group 2 from elevated serum ALT and AST activities, reduced GSH and histological and ultrastructural alterations.
A significant reduction in GSH concentration in hepatic tissue was noticed in group 2. This observation is in accordance with the earlier report by Duzguner and Erdogen.[17] This signifies the generation of free radicals that induced oxidative stress following imidacloprid treatment, and this might be attributed to direct utilization of GSH as an antioxidant in terminating the free radical reaction resulting in exhaustion of the GSH during oxidative stress, which was evident from significant alterations in hepatic biomarkers and corresponding histological and ultrastructural changes in the liver sections of group 2.
In group 2 rats, liver sections revealed severe histological changes like marked dilation, congestion of central vein, sinusoidal spaces, portal vein and vacuolation/fatty change in hepatocytes. These results are in agreement with earlier reports.[14,18–20] Ultrathin liver sections in group 2 revealed swollen nuclei, sinusoidal congestion, disrupted chromatin, disrupted rough endoplasmic reticulum and altered size and shape of mitochondria. These changes might be due to the accumulation of imidacloprid metabolites in the liver as it is the principal target organ for any detoxification mechanism.
Vitamin C plays a primary role in neutralizing free radicals; it can work both inside and outside the cells to combat free radical damage. The free radicals will seek out an electron to regain their stability; vitamin C is an excellent source of electrons and, therefore, it can donate electrons to free radicals such as hydroxyl and superoxide radicals and quench their reactivity.[21] In the present study, supplementation of vitamin C brought moderate protection in all the above parameters. The results of the present study were in agreement with previous studies, where supplementation of vitamin C brought improvement in the histoarchitechture of the liver in Japanese quail[18] and restored GSH levels to normal in mice administered with imidacloprid.[22]
CONCLUSION
The study revealed that exposure to imidacloprid (80 mg/kg) affected hepatic function in rats, which was evident from biochemical, tissue antioxidant, histological and ultrastructural alterations in the liver. However, vitamin C supplementation along with imidacloprid to rats manifested significant protective effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Pesticide fact sheet: Imidacloprid. Washington, D.C: Office of Pesticide Programs; 1994. USEPA. [Google Scholar]
- 2.Abbink J. The biochemistry of imidacloprid. Planzenschutz Nachrichten- Bayer. 1991;44:183–95. [Google Scholar]
- 3.Felsot A. S. Admiring risk reduction. Does imidacloprid have what it takes? Agrichem. EnViron. News. 2001;186:1–12. [Google Scholar]
- 4.Hutchinson MJ, Jacobs DE, Mencke N. Establishment of the cat flea (Ctenocephalides felis) on the ferret (Mustela putorius furo) and its control with imidacloprid. Med. Vet Entomol. 2001;15:212–4. doi: 10.1046/j.0269-283x.2001.00299.x. [DOI] [PubMed] [Google Scholar]
- 5.Tomizawa M, Lee DL, Casida JE. Neonicotinoid insecticide toxicology: Mechanisms of selective action. Annu Rev Pharmacol Toxicol. 2005;45:247–68. doi: 10.1146/annurev.pharmtox.45.120403.095930. [DOI] [PubMed] [Google Scholar]
- 6.Tomizawa M, Casida JE. Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors. Annu Revised Entomol. 2003;48:339–64. doi: 10.1146/annurev.ento.48.091801.112731. [DOI] [PubMed] [Google Scholar]
- 7.Wu IW, Lin JL, Cheng ET. Acute poisoning with nicotinoid insecticide imidacloprid in N-methyl pyrrlidone. Clin Toxicol. 2001;39:617–21. doi: 10.1081/clt-100108494. [DOI] [PubMed] [Google Scholar]
- 8.Proenca P, Teixeira H, Castanheira F, Pinheiro J, Monsanto PV. Two fatal intoxication cases with imidacloprid. Forensic Sci Int. 2005;153:75–80. doi: 10.1016/j.forsciint.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Abd-El-Ghaney A. Study the effect of imidacloprid insecticide on some physiological parameters in Japanese quail. Thesis for M.Sc. Faculty of science, Al-Azhar University. 2002 [Google Scholar]
- 10.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S transferase in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 11.Luna GL. Manual of histological and special staining techniques. 2nd ed. New York, Toronto, London: The Blakistone Divison McGraw-Hill Book Company, Inc; 1968. pp. 1–9. (9-34). [Google Scholar]
- 12.Bozzala JJ, Russel LD. Electron Microscopy Principles Techniques for Biologists. 2nd ed. Sudbury, Massachusetts: Jones and Barlett Publishers; 1998. pp. 19–45. (72-144). [Google Scholar]
- 13.Barinderjit K, Sandhu HS, Rajdeep K. Toxic effect of sub acute oral exposure of imidacloprid on biochemical parameters in cross bred cow calves. Toxicol Int. 2006;13:43–7. [Google Scholar]
- 14.Bhardwaj S, Srivastava MK, Upasana K, Srivastava LP. A 90 days oral toxicity of imidacloprid in female rats morphological, biochemicaland histopathological evaluation. Food Chem Toxicol. 2010;48:1185–90. doi: 10.1016/j.fct.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Balani T, Agarwal S, Thaker AM. Haematological and biochemical changes due to short-term oral administration of imidacloprid. Toxicol Int. 2011;18:2–4. doi: 10.4103/0971-6580.75843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siddiqui A A, Choudhary M, Goriya HV, Bhavsar SK, Thaker AM. Evaluation of immunotoxic effect of short-term administration of quinalphos and imidacloprid in white leghorn cockerels. Toxicol Int. 2007;14:15–9. [Google Scholar]
- 17.Duzguner V, Erdogan S. Acute oxidant and inflammatory effects of imidacloprid on the mammalian central nervous system and liver in rats. Pest Biochem Physiol. 2010;97:13–8. [Google Scholar]
- 18.Omiama SE. Protective effect of vitamin C and glutathione against the histopathological changes induced by imidacloprid in the liver and testis of Japanese quail. Egypt J Hosp Med. 2004;16:39–54. [Google Scholar]
- 19.Kammon AM, Brar RS, Banga HS, Sodhi S. Pato biochemical studies on hepatotoxicity and nephrotoxicity on exposure to imidacloprid in layer chickens. Veterinarski Arhiv. 2010;80:663–72. [Google Scholar]
- 20.Mohany M, Badr G, Refaat I, Garraud O, EI-feki M. Thymoquinone ameliorates immunological and histological changes induced by exposure to imidacloprid insecticide. Toxicol Sci. 2012;37:1–11. doi: 10.2131/jts.37.1. [DOI] [PubMed] [Google Scholar]
- 21.Bindhumol V, Chitra KC, Mathur PP. A induces reactive oxygen species generation in the liver of male rats. Toxicology. 2003;188:117–24. doi: 10.1016/s0300-483x(03)00056-8. [DOI] [PubMed] [Google Scholar]
- 22.El-Gendy KS, Aly NM, Mahmoud FH, Kenawy A, El-Sebae AK. The role of vitamin C as antioxidant in protection of oxidative stress induced by imidacloprid. Food Chem Toxicol. 2010;48:215–21. doi: 10.1016/j.fct.2009.10.003. [DOI] [PubMed] [Google Scholar]


