Abstract
Over three decades ago, it was found that intermittent electrical stimulation from the vagus nerve produces inhibition of neural processes, which can alter brain activity and terminate seizures. This paved way for the concept of vagal nerve stimulator (VNS). We describe the evolution of the VNS and its use in different fields of medicine. We also review the literature focusing on the mechanism of action of VNS producing desired effects in different conditions. PUBMED and EMBASE search was performed for ‘VNS’ and its use in refractory seizure management, depression, obesity, memory, and neurogenesis. VNS has been in vogue over for the past three decades and has proven to reduce the intensity and frequency of seizure by 50% in the management of refractory seizures. Apart from this, VNS has been shown to promote neurogenesis in the dentate gyrus of rat hippocampus after 48 hours of stimulation of the vagus nerve. Improvement has also been observed in non-psychotic major depression from a randomized trial conducted 7 years ago. The same concept has been utilized to alter behavior and cognition in rodents, and good improvement has been observed. Recent studies have proven that VNS is effective in obesity management in patients with depression. Several hypotheses have been postulated for the mechanism of action of VNS contributing to its success. VNS has gained significant popularity with promising results in epilepsy surgery and treatment-resistant depression. The spectrum of its use has also extended to other fields of medicine including obesity, memory, and neurogenesis, and there is still a viable scope for its utility in the future.
Keywords: Depression, neurogenesis, obesity, seizure, vagus nerve stimulator
INTRODUCTION
The vagus nerve, also known as the pneumogastric nerve or cranial nerve X, has the most extensive course and distribution of all the cranial nerves.
It originates from four nuclei in the medulla oblongata:
Dorsal Nucleus: Axons from this nucleus gives rise to the pre-ganglionic parasympathetic visceromotor fibers of the vagus nerve.
Nucleus Ambiguus: Cells from here contain motor neurons associated with three cranial nerves- IX, X, XI. Sends parasympathetic outputs to the heart.
Nucleus of Tractus Solitarius: This receives viscerosensory information from the gastrointestinal tract, and the respiratory system, as well as afferent taste information. The vagus nerve is composed of 80% afferent sensory fibers, and relay information to the nucleus tractus solitarius (NTS). Via the NTS, the vagus nerve has extensive projections to different important aspects of the brain (including the locus ceruleus, and dorsal raphe nuclei), which form the foundation for most of the functions considered in this review.
Spinal Nucleus of Trigeminal nerve: Receives general somatic sensory afferent information from the external auditory meatus and back of the ear.
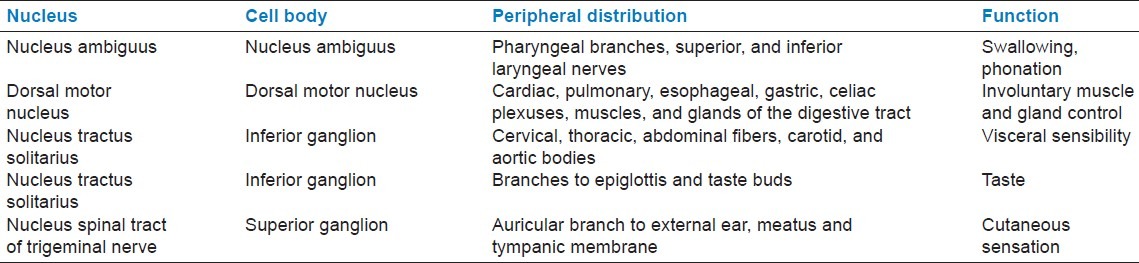
Eight to Ten rootlets extend from the nuclei forming the fibers of the vagus nerve. The nerve exits the cranium via the jugular foramen, lies in the carotid sheath at the neck level (between the common carotid artery and the internal jugular vein). [Table 1][1] summarizes the origin, peripheral distribution, and functions of the vagus nerve [Table 1].
Table 1.
Summary of nuclei, distribution and function of the vagus nerve[1]
History of vagus nerve stimulation (VNS)
In 1934, Soma Weiss[2] proposed that compression of the carotid sinus produced a direct cerebral response, causing syncope in human beings that is different from the effects of this stimulation on blood pressure or heart rate.
In 1938, Bailey and Bremer[3] reported that vagal stimulation caused electro-encephalogram changes. Dell and Olson,[4] in 1951, showed that stimulation of the cut cervical vagus nerve evoked responses in the ventroposterior complex and intralaminar regions of the thalamus. Since then, various experimental studies have established the effects of vagus nerve stimulation on the brain.
In 1985, Zabara et al.[5] reported that electrical stimulation from the vagus nerve produces inhibition of the neural processes, which can alter brain electrical activity and terminate seizures in dogs. Since this research, vagus nerve stimulation has been used for patient benefit in various clinical conditions.
Vagus nerve stimulator implantation[6–9]
Vagus nerve stimulator implantation is usually done on the left side so as to avoid cardiac complications (the right vagus nerve supplies the sinoatrial node, while the left innervates the atrioventricular node). This, thus, prevents the untoward effect of cardiac dysrhythmia. Performed under general anesthesia. Aseptic measures must be ensured to minimize infection. Single induction dose of antibiotics is administered. Patients head is positioned on a donut head support and slightly extended. A 3-4 cm incision is made about the cricothyroid interval on the left side, on the anterior border of the sternocleidomastoid muscle. The platysma muscle is divided in the direction of its fibers, and the deep cervical fascia opened to identify the sternocleidomastoid muscle. This muscle is mobilized and retracted to expose the neuromuscular bundle. This bundle is incised to expose the common carotid and the internal jugular vein. The vagus nerve lies in between the two vessels. Approximately 3-4 cm of the nerve should be mobilized from the adventitia. The left chest incision is made along the anterior axillary line (another option could be a single neck incision) with a #10 scapel. A subcutaneous pocket is made over the pectoralis fascia. A tunneler is passed between the neck and chest incision, and the electrode passed. The electrodes (three spiral coils- a tethering coil, a cathode, and an anode) are implanted on the Vagus nerve. The distal wire is connected to the generator. The whole circuit is tested- a hand-held wand is placed over the generator, and a Computer programmer is used to test the lead impedance and to assess the integrity of the system. The generator internalized, and surgical wound closed. The system is turned on 14 days post-surgery.
Potential side effects of VNS[10] could be related to the implantation procedure or during actual stimulation of the vagus nerve. They include but not limited to – infection, scaring, voice alteration, dysphagia, coughing, neck pain, cardiac arrhythmia.
Method
The authors performed extensive PUBMED and EMBASE search for VNS and its current application in different areas of medicine including epilepsy, depression, obesity, memory and cognition as well as neurogenesis.
DISCUSSION
Epilepsy
In 1988 Penry et al.[11] performed the first human implant of a vagal stimulating device into a human. In 1997, FDA approved the use of VNS as an adjunctive treatment for medically refractory epilepsy.
Two pilot studies (E01, E02) on a total of 14 patients in whom programmable device was implanted with 14, and 35-month follow-ups showed 47% reduction in the frequency of seizures. Subsequent E03 study was approved, and carried out on 54 patient patients, with similar outcome.[12]
Muller et al.,[13] in 2010, reported a 50% reduction in the frequency of seizures within the first year. Englot et al.,[14] in 2011, in a meta-analysis of 74 clinical studies with 3321 patients, reported >50% reduction of seizure. Bao et al.,[15] in 2011, reported 64% response in a retrospective analysis of 45 cases.
Mechanism of action of vagus nerve stimulator for epilepsy
The precise mode of action of VNS for reduction of seizure frequency and intensity in not clearly understood. The possible hypothesis on the mechanism of action is described below.
Synchronization theory
Through its projections to the amygdala, the nucleus tractus solitarius gains access to the amygdala-hippocampus-entorhinal cortex loop of the limbic system, which are sites that most often generate complex partial seizures. It has been demonstrated that cortical and thalamo-cortical neuronal interactions become hyper synchronized during seizures in animal models. Early neurophysiologic studies had shown that cervical VNS can induce EEG desynchronization in cats. Zabara et al.[16] then postulated that desynchronization of these overly synchronized neuronal activities would confer the anti-seizure effects of VNS.
Neurotransmitter theory
The locus ceruleus (receives neuronal projections from the NTS) is the site for many norepinephrine-containing neurons. Krahl et al.[17] showed that lesioning the locus ceruleus in rats eliminates the ability of VNS to suppress seizures. It was also demonstrated that after chronically depleting the norepinephrine in the locus ceruleus by infusing 6-hydroxydopamine into the structure bilaterally, the acute anti-seizure effects of VNS was reversed. Ben-Menachem et al.[18] reported increase in cerebrospinal fluid (CSF) concentration of Gamma-amino-butyric acid (GABA), and 5-hydroxy-indole-acetic acid, as well as simultaneous decrease in the concentrations of glutamate and aspartate in 16 patients following VNS. These seem to advocate a neurotransmitter mechanism of the ability of VNS to suppress seizures.
Cerebral blood flow theory
Henry et al.,[19] in 1998, showed that cervical vagus nerve stimulation caused bilateral alteration in blood flow to the cortex, thalamus, hippocampus, amygdala, and posterior cingulate gyri, and may activate inhibitory structures in the brain.
Depression
It is predicted that by the year 2030, depression would become one of the major disease burdens to humanity, second only to human immunodeficiency virus (HIV).[20] The major challenge here is that the etiopathogenesis of depression is not clearly understood; being multi-factorial and as such developing effective treatment has been a challenge to both the research and pharmacology community.
Interestingly, it has been shown that depressed patient, who received vagus nerve stimulation for epilepsy, showed clinical improvement with depression unrelated to the effect of the VNS on their seizures. Rush et al.,[21] in a multi center trial, reported 40-50% improvement in patients with non-psychotic major depression. VNS has been approved by the Food and Drug Administration, USA for treatment-resistant depression July 2005.
Bajbouj et al.,[22] 2010, in a two-year outcome review involving 74 patients, reported a 53.1% response and 38.9% remission with VNS treatment in major depression. In an Editorial by Ashton A.K. 2010,[23] it was duly pointed out that patients relapsed into clinical depression after explantation of vagus nerve stimulator. Hence, it is evident that VNS for treatment-resistant depression has been proven to be beneficial as an adjunct therapy.
Mechanism of action of vagus nerve stimulator for depression
Alterations in several neurotransmitters have been noted in depression, particularly serotonin, norepinephrine, and dopamine. However, there is inadequate understanding of the exact pathophysiology of clinical depression. In the same light, the exact mode of action of VNS for depression is not clearly understood. The possible hypotheses are explained.
The anti-convulsant effect
It's a known fact that electroconvulsive therapy and other anti-convulsants like carbamazepine and lamotrigine have anti-depressant effects; therefore, since VNS have anti-convulsant effects, it is thought that VNS improves depression by manipulating the anti-convulsant system in the brain.[24]
Change in the regional anatomy
Via the nucleus tractus solitarius, the vagus nerve has projections to important areas of the brain involved in mood regulation. It is postulated that VNS exerts its anti-depressant effect by gradually changing the dynamics of this system over time.[25]
Stress sensitization theory
Chronic stress has been established as a major risk factor in the development of depression. It is thought by this theory that VNS might be blunting an otherwise amplified pathological reaction. Patient on selective serotonin re-uptake inhibitor report improved tolerance to stress. A study at the Medical university of South Carolina observed that patients on VNS for depression trials tolerated stressful events more than before.[26]
Neurotransmitter theory
It is a known fact that drugs like reserpine, which depletes neuronal stores of noradrenalin (NA), 5-hydroxytryptamine (5HT), and alpha-methyltyroxine, which inhibits NA synthesis, cause depression. As such, available anti-depressants (e.g. tricyclic anti-depressant and monoamine oxidase inhibitors) in the market today exert their function by increasing synaptic concentration of NA and 5HT. Given this background, Manta et al.,[27] using rat model of VNS, proved that VNS increased the activity norepinephrine neurons and 5-HT neurons. Therefore, the belief is that VNS functions to improve depression by up-regulating the activity of NE and 5-HT.
Memory and cognition
The nucleus tractus solitarius (NTS) is the main relay station for afferent vagus nerve fibers. Parts of the brain responsible for learning and memory formation (amygdala, hippocampus) receive projections from the NTS. Stimulation of the vagus nerve is believed to induce electrophysiological and metabolic changes in these structures.
Clarke et al.,[28] on working with animal models, reported that rats that received VNS showed improved retention performance compared to rats that underwent sham stimulation. Same author carried out experiments on human subject with similar results.[29] Ghacibeh et al.[30] found that VNS had no effect on learning but enhanced consolidation, which led to improved retention. Hallbook et al.[31] reported that there were no early changes in cognitive functioning following VNS; however, there seemed to be a gradual improvement over time. It, therefore, seems that longer study period is needed to detect significant cognitive changes.
Mechanism of action of vagus nerve stimulator for memory and cognition
The medial reticular formations of the medulla, which have projections to the nucleus reticularis thalami and to the intralaminar nuclei of the thalamus, receive afferents from the vagus nerve. Owing to these connections, patients tend to experience increased awareness with VNS. Could this improved alertness be responsible for improved learning and memory formation? This is still debatable.
Obesity
Obesity is fast becoming a major health issue, particularly in industrialized nations. It is defined as having excess adiposity or fat tissue. The body-mass-index (BMI) is the most accurate numerical assessment. A BMI OF 25-30 is considered overweight, 30-35 has class 1 obesity, 35-40 has class 2 obesity, while >40 has morbid obesity.[32] Known treatment options for obesity include- low-calorie regime, pharmaceutical agents, counseling, exercise programs, and surgery. Currently, surgical procedures that restrict the size of the stomach and/or bypass parts of the intestine are the only remedies that provide lasting results. Though most of these procedures are done laparoscopically and considered minimally invasive, they are still major surgery and have the potential for short-term complications and long-term nutritional problems. An optimal, ideal treatment for obesity is not yet at hand. It is thought that an ideal long-term treatment would need to target gut-brain interaction.
Bodenlos et al.,[33] through their studies, showed that VNS-treated patients had reduced craving for food. Pardo et al.[34] reported significant weight loss unrelated to depression improvement scores in 14 patients treated with VNS for major depression. They found that the degree of weight loss was directly proportional to the severity of obesity. A phase I study at Lenox Hill Hospital, New York, and University of Texas, Houston showed weight loss in 4 out of 6 patients that received VNS.[35]
Given that the prevalence of obesity continues to increase iatrogenically through the use of atypical neuroleptics, the fact the VNS, in addition to being an effective treatment for epilepsy and depression, also helps with weight reduction, is a more than welcome development.
Mechanism of action of vagus nerve stimulator for obesity
Gut-Brain feedback mechanism
While there is feedback to the brain from all areas of the gastrointestinal tract, distension of the stomach is the single greatest factor in satiety. This information reaches the brain via the vagus nerve. It has been shown that gastric distension, either by food or mechanically, increases vagus nerve activity.[36,37] Cholecystokinin (CCK) is released after meal consumption. Administration of CCK to animal models has been shown to reduce food intake. Interestingly, vagotomy attenuates this response. Capsaicin, a chemical that selectively destroys the vagal afferents, also significantly reduces the effects of CCK. This data demonstrates that afferent vagus fibers are responsible for satiating effect of CCK.[38,39] Thus, VNS accentuates the satiation information reaching the hypothalamic appetite center, making the individual to eat less, thereby losing weight.
Neurogenesis
Until in the recent pass, the general view was the adult brain did not undergo birth of new neurons, however, emerging scientific evidence point towards the ability of certain parts of the brain to undergo proliferation, even in adulthood through a process known as neurogenesis. Jacob et al.[40] explained that neurogenesis continues, even in adulthood and is particularly prominent in the dentate gyrus of the hippocampus. D. Revesz et al.[41] observed that there was increased uptake of bromodeoxyuridine (BRDU), a marker of cell proliferation, in the dentate gyrus of rat hippocampus that had VNS inserted for 48 hours. Gebhardt N. et al.[42] showed that stimulation of the vagus nerve ameliorated the expected olfactory lobectomy decrease in hippocampal neurogenesis. This consequently prevented the behavioral changes associated with bilateral olfactory lobectomy. Chronic VNS induced long-lasting increases in the number new cells formed in the hippocampus of rats as the newly formed cells remained at 3 weeks and persisted even after stimulation was discontinued.[43]
Mechanism of action of vagus nerve stimulator on neurogenesis
Neurotransmitter effect
It has been shown that selective noradrenergic depletion using neurotoxins caused a decrease in the number of proliferating progenitor cells in the dentate gyrus of adult rats.[44] Malberg and co[45] reported that increased synaptic levels of noradrenalin induced by anti-depressants enhanced hippocampal progenitor proliferation. Serotonin depletion has also been shown to inhibit neurogenesis both prenatally and in adult rat brain. It is, thereby, established that hippocampal neurogenesis is enhanced by these monoamine neurotransmitters (serotonin and noradrenalin). This does not come as a surprise as Sheline et al.[46] had reported neuroimaging confirmation of hippocampal atrophy in depressed patients. This effect is known to be reversed by anti-depressants, most of which function to enhance the availability of these neurotransmitters (the SSRI and tricyclics) as well as vagus nerve stimulation. Earlier, we had established that the vagus nerve has connection via the nucleus tractus solitarius in the medulla to the locus coerulus (noradrenergic) and dorsal raphe nuclei (serotonergic). It is, therefore, postulated that vagus nerve stimulation promotes neurogenesis by ramping up the activity in these neurotransmitter-producing sites.
CONCLUSION
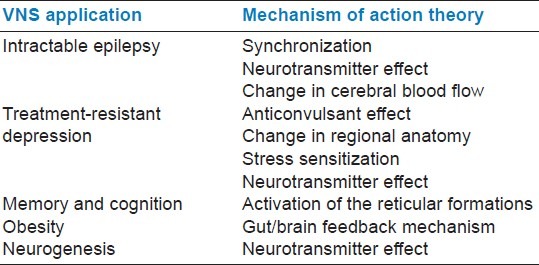
VNS has gained significant popularity in the recent years, yielding promising results in epilepsy surgery and treatment-resistant depression. The spectrum of its use has also extended to other fields of medicine, and there is still a viable scope for more research on its utility in the future [Table 2].
Table 2.
Summarizes vagus nerve stimulator application and mechanism of action theories
The nucleus tractus solitarius being one of the nuclei of the vagus nerve, with extensive network of connections to other regions of the brains, seems to play a central role in the current multiple applications of vagus nerve stimulator.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Agur AM, Dalley AE. The cranial nerves: Grants Atlas of Anatomy. Philadelphia: Wilkins and Williams; 2009. pp. 814–5. [Google Scholar]
- 2.Ferris EB, Capps RB, Weiss S. Carotid sinus syncope and its bearing on the mechanism of unconscious state and convulsions. Medicine. 1934;14:377–453. [Google Scholar]
- 3.Bailey P, Bremer FA. Sensory cortical representation of the Vagus nerve. J Neurophysiol. 1938;1:405–12. [Google Scholar]
- 4.Dell P, Olson R. Thalamic, cortical and cerebella projections of vagal visceral afferents. C R Seances Soc Biol Fil. 1951;145:1084–8. [PubMed] [Google Scholar]
- 5.Zabara J. Peripheral control of hypersynchronous discharge in epilepsy. Electroencephalography. 1985;61:S162. [Google Scholar]
- 6.Amar AP, DeGiorgio CM, Tarver WB, Apuzzo ML. Long term multicenter experience with Vagus nerve stimulation for intractable partial seizures. Stereotact Funct Neurosurg. 1999;73:104–8. doi: 10.1159/000029764. [DOI] [PubMed] [Google Scholar]
- 7.Amar AP, Heck CN, Levy ML, Smith T, De Giorgio CM, Oviedo S, et al. An institutional experience with cervical Vagus nerve trunk stimulation for medically intractable epilepsy: Rationale, technique, and outcome. Neurosurgery. 1998;43:1265–80. doi: 10.1097/00006123-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Farooqui S, Boswell W, Hemphill JM, Pearlman E. Vagus nerve stimulation in pediatric patients with intractable epilepsy: Case series and operative technique. Am Surg. 2001;67:119–21. [PubMed] [Google Scholar]
- 9.Rutecki P. Anatomical, physiological, and theoretical basis for the antiepileptic effect of Vagus nerve stimulation. Epilepsia. 1990;31:S1–6. doi: 10.1111/j.1528-1157.1990.tb05843.x. [DOI] [PubMed] [Google Scholar]
- 10.Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, et al. Vagus Nerve Stimulation for treatment resistant depression: Efficacy, side effects, and predictors of outcome. Neuropsychopharmacology. 2001;25:713–28. doi: 10.1016/S0893-133X(01)00271-8. [DOI] [PubMed] [Google Scholar]
- 11.Penry JK, Dean JC. Prevention of intractable partial seizures by intermittent vagal stimulation in humans: Preliminary results. Epilepsia. 1990;31(Suppl 2):S40–3. doi: 10.1111/j.1528-1157.1990.tb05848.x. [DOI] [PubMed] [Google Scholar]
- 12.Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:616–26. doi: 10.1111/j.1528-1157.1994.tb02482.x. [DOI] [PubMed] [Google Scholar]
- 13.Müller K, Fabó D, Entz L, Kelemen A, Halász P, Rásonyi G, et al. Outcome of Vagus nerve stimulation for epilepsy in Budapest. Epilepsia. 2010;51(Suppl S3):98–101. doi: 10.1111/j.1528-1167.2010.02620.x. [DOI] [PubMed] [Google Scholar]
- 14.Englot DJ, Chang EF, Auguste KI. Vagus nerve stimulation for epilepsy: A meta-analysis of efficacy, and predictors of response. J Neurosurg. 2011;115:1248–55. doi: 10.3171/2011.7.JNS11977. [DOI] [PubMed] [Google Scholar]
- 15.Bao M, Zhou J, Luan GM. Treatment of drug-resistant epilepsy with Vagus nerve stimulation-review of 45 cases. Chin Med J. 2011;124:4184–8. [PubMed] [Google Scholar]
- 16.Zabara J. Time course of seizure control to brief repetitive stimuli. Epilepsia. 1985;26:518. [Google Scholar]
- 17.Krahl SE, Clark KB, Smith DC, Browning RA. Locus coeruleus lesions suppress the seizure-attenuating effects of Vagus nerve stimulation. Epilepsia. 1998;39:709–14. doi: 10.1111/j.1528-1157.1998.tb01155.x. [DOI] [PubMed] [Google Scholar]
- 18.Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, et al. Effects of Vagus nerve stimulation on amino acids, and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995;20:221–7. doi: 10.1016/0920-1211(94)00083-9. [DOI] [PubMed] [Google Scholar]
- 19.Henry TR, Bakay RA, Votaw JR, Pennell PB, Epstein CM, Faber TL, et al. Brain blood flow alterations induced by therapeutic Vagus nerve stimulation in partial epilepsy. Epilepsia. 1998;39:983–90. doi: 10.1111/j.1528-1157.1998.tb01448.x. [DOI] [PubMed] [Google Scholar]
- 20.Mathers CD, Loncar D. Projections of global mortality, and burden of disease from 2002 to 2030. PloS Med. 2006;11:442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, et al. Vagus Nerve stimulation for treatment resistant depressions: A multicenter study. Biol Psychiatry. 2000;47:276–86. doi: 10.1016/s0006-3223(99)00304-2. [DOI] [PubMed] [Google Scholar]
- 22.Bajbouj M, Merkl A, Schlaepfer TE, Frick C, Zobel A, Maier W, et al. Two-year outcome of Vagus nerve stimulation in treatment-resistant depression. Clin Psychopharmacol. 2010;30:273–81. doi: 10.1097/JCP.0b013e3181db8831. [DOI] [PubMed] [Google Scholar]
- 23.Ashton AK. Depressive Relapse after Vagus nerve stimulator Explantation. Am J Psychiatry. 2010;167:719–20. doi: 10.1176/appi.ajp.2010.10010020. [DOI] [PubMed] [Google Scholar]
- 24.Sackeim HA, Decina P, Prohovnik I, Malitz S, Resor SR. Anticonvulsant, and antidepressant properties of electroconvulsive therapy: A proposed mechanism of action. Biol Psychiatry. 1983;18:1301–10. [PubMed] [Google Scholar]
- 25.Conway CR, Sheline YI, Chibnall JT, George MS, Fletcher JW, Mintun MA. Cerebral blood flow changes during Vagus nerve stimulation for depression. Psychiatry Res. 2006;146:179–84. doi: 10.1016/j.pscychresns.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Schacther SC, Schmidt D. Vagus Nerve Stimulation. Martin Dunitz. 2003;4:1755–84. [Google Scholar]
- 27.Manta S, Dong J, Debonnel G, Blier P. Enhancement of the function of rat serotonin, and norepinephrine neurons by sustained Vagus nerve stimulation. J Psychiatry Neurosci. 2009;34:272–80. [PMC free article] [PubMed] [Google Scholar]
- 28.Clark KB, Smith DC, Hassert DL, Browning RA, Naritoku DK, Jensen RA. Post-training electrical stimulation of vagal afferents with concomitant Vagal efferent inactivation enhances memory storage process in rat. Neurobiol Learn Mem. 1998;70:364–73. doi: 10.1006/nlme.1998.3863. [DOI] [PubMed] [Google Scholar]
- 29.Clark KB, Naritoku DK, Smith DC, Browning RA, Jensen RA. Enhanced recognition memory following Vagus nerve stimulation in human subjects. Natural Neurosci. 1999;2:94–8. doi: 10.1038/4600. [DOI] [PubMed] [Google Scholar]
- 30.Ghacibeh GA, Shenker JI, Shenal B, Uthman BM, Heilman KM. The influence of Vagus nerve stimulation on Memory. Cogn Behav Neurol. 2006;19:119–22. doi: 10.1097/01.wnn.0000213908.34278.7d. [DOI] [PubMed] [Google Scholar]
- 31.Hallböök T, Lundgren J, Stjernqvist K, Blennow G, Strömblad LG, Rosén I. Vagus nerve stimulation in 15 children with therapy resistant epilepsy; its impact on cognition, quality of life, behavior and mood. Seizure. 2005;14:504–13. doi: 10.1016/j.seizure.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Global Database on Body Mass Index [Google Scholar]
- 33.Bodenlos JS, Kose S, Borckardt JJ, Nahas Z, Shaw D, O’Neil PM, et al. Vagus nerve stimulation acutely alters food craving in adults with depression. Appetite. 2007;48:145–53. doi: 10.1016/j.appet.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 34.Pardo JV, Sheikh SA, Kuskowski MA, Surerus-Johnson C, Hagen MC, Lee JT, et al. Weight loss during chronic, cervical Vagus nerve stimulation in depressed patients with obesity. Int J Obes. 2007;31:1756–9. doi: 10.1038/sj.ijo.0803666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schacther SC, Schmidt D. Vagus Nerve Stimulation. Martin Dunitz. 2003;6:2701–27. [Google Scholar]
- 36.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal Vagotomy blocks the satiety effect of cholecystokinin in rats. Science. 1981;213:1036–7. doi: 10.1126/science.7268408. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez MF, Deutsch JA. Vagotomy abolishes cues of satiety produced by gastric distension. Science. 1981;212:1283–4. doi: 10.1126/science.7233218. [DOI] [PubMed] [Google Scholar]
- 38.Ritter RC, Ritter S, Ewart WR, Wingate DL. Capsaicin attenuates hindbrain neuron responses to circulating cholecystokinin. Am J Physiol. 1989;257:1162–8. doi: 10.1152/ajpregu.1989.257.5.R1162. [DOI] [PubMed] [Google Scholar]
- 39.Bray GA. Afferent signals regulating food intake. Proc Nutr Soc. 2000;59:373–4. doi: 10.1017/s0029665100000422. [DOI] [PubMed] [Google Scholar]
- 40.Jacobs BL, Praag H, Gage FH. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol Psychiatry. 2000;5:262–9. doi: 10.1038/sj.mp.4000712. [DOI] [PubMed] [Google Scholar]
- 41.Revesz D, Tjernstrom M, Ben-Menachem E, Thorlin T. Effect of Vagus nerve stimulation on rat hippocampal progenitor proliferation. Exp Neurol. 2008;214:259–65. doi: 10.1016/j.expneurol.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Gebhardt N, Bär KJ, Boettger MK, Grecksch G, Keilhoff G, Reichart R, et al. Vagus nerve stimulation ameliorated deficits in one-way active avoidance learning, and stimulated hippocampal neurogenesis in bulbectomized rats. Brain Stimul. 2012 doi: 10.1016/j.brs.2012.01.009. In Press 42. [DOI] [PubMed] [Google Scholar]
- 43.Biggio F, Gorini G, Utzeri C, Olla P, Marrosu F, Mocchetti I, et al. Chronic Vagus nerve stimulation induces neuronal plasticity in the rat hippocampus. Neuropsychopharmacology. 2009;12:1209–21. doi: 10.1017/S1461145709000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulkarni VA, Jha S, Vaidya VA. Depletion of norepinephrine decreases the proliferation, but does not influence the survival and differentiation of cell progenitors in adult rat hippocampus. Eur J Neurosci. 2002;16:2008–12. doi: 10.1046/j.1460-9568.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- 45.Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in Adult rat Hippocampus. J Neurosci. 2000;20:9104–10. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheline YI, Wang PW, Gado MH, Csernansky JG, Vannier MW. Hippocampal Atrophy in recurrent major depression. Proc Natl Acad Sci USA. 1996;93:3908–13. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]


