Abstract
Vascularization and blood cell circulation are crucial steps during lung development. However, how blood vessels are generated and when lung circulation is initiated is still a matter of debate. A morpho-functional analysis of pulmonary vasculature was done using human lung samples between 31 and 56 days post-fertilization (pf). The immunolocalization and expression of CD31, CD34, FLT-1, KDR and the vascular growth factor (VEGF) were investigated. The results showed that at day 31 pf, a capillary plexus is already installed, and a few primitive erythroblasts were seen for the first time within the lumen of some blood vessels. Around day 45 pf, an increase in the amount of primitive erythroblasts was detected in the parenchyma surrounding the distal segment of the bronchial tree. The expression of FLT-1, KDR, CD31 and CD34 was observed in endothelial cells of the capillary plexus and the VEGF was detected in the endodermal epithelium. Our results support the hypothesis that the initial formation of the capillary plexus around the tip of the growing airway bud occurs by vasculogenesis, probably regulated by VEGF and KDR. We also showed a very early onset of blood circulation, starting from day 34 pf, concomitant with the generation of new lung buds. In addition, the increasing number of primitive erythroblasts from week 6 onward, associated with a change in the shape of the blood vessels, suggests a remodeling process and that the generation of new distal vessels at the tip of the lung bud occurs mainly by a process of angiogenesis.
Keywords: angiogenesis, blood circulation, embryogenesis, human embryo, lung development, vasculogenesis
Introduction
Human lung morphogenesis begins around day 26–27 post-fertilization (pf) during early embryonic development; the lungs emerge as two epithelial buds from the distal segment of the laryngotracheal primordium (Cardoso & Lu, 2006). These lung buds elongate and grow into the surrounding splanchnopleuric mesenchymal tissue. As the lungs mature, the endoderm gives rise to the epithelium lining, while the mesoderm turns into connective tissue. Reciprocal interactions between the endodermal epithelial cells of the lung buds and the surrounding mesenchyme lead to the formation of the respiratory tree by a process of branching morphogenesis (Metzger et al. 2008; Warburton, 2000). By the end of the embryonic period, the lung lobar and segmental organization is established. During this period, blood vessels are forming in the surrounding mesoderm, and the pulmonary vasculature essential for the metabolic homeostasis is already installed. Vascularization plays a central role in embryonic lung function because an inadequate or poor blood flow distribution may result in lung malformation (Joshi & Kotecha, 2007). Surprisingly, in humans the initial steps of vascular development as well as the determination when blood cells start to invade the vessels of the lungs are still unsolved questions.
In humans, there are very limited data on vascular ontogeny, and the starting points of this process are still a matter of controversy. Three different experimental models have been proposed to explain vascular development in the lung. The first model postulates that human blood vessels originate from continuous expansion and coalescence of the primary capillary plexus derived from the mesenchyme by vasculogenesis, with new blood vessels forming in situ from endothelial cell precursors (Hall et al. 2000). A second model proposes two mechanisms in the formation of the human pulmonary vasculature: on the one hand, angiogenesis from central vessels branching and, on the other hand, the generation of a distal capillary network formed by vasculogenesis (Demello & Reid, 2000). Parera et al. (2005) working on mice lung have questioned the mechanisms of distal pulmonary vessels formation by vasculogenesis, suggesting that the vasculature grows in the periphery of the lung by expansion of new capillaries from preexisting ones; they proposed the concept of distal angiogenesis as a new model for pulmonary vascular morphogenesis. This new concept is reinforced by the presence of primitive erythroblasts (PEs) in blood vessels as a proof of circulation. According to these authors, distal angiogenesis in mice starts in the embryonic phase of lung development, representing the major blood vessel-forming process.
During the early stages of organogenesis, blood circulation is essential for the transport of nutrients and other factors necessary for organ growth and development, inducing cell proliferation and differentiation. The relationship between human lung vasculature development and blood cell circulation has received little attention in the past. In the present study we investigate, using a morphological and immunohistochemical approach, the time interval between the vascularization of human lung primordium and the appearance of PEs in the network of blood vessels as an indicator that circulation has started. Our results confirm the thesis that growth and development of blood vessels from the end of the embryonic period is mainly by distal angiogenesis.
Materials and methods
Lung tissue
Human lung samples were taken from 10 normal human embryos ranging from 4 to 8 weeks pf (6–10 weeks of pregnancy). The embryos' ages were estimated by the last menstrual period, morphology of the limb, and body shape. Embryos aged 4 weeks (n = 2), 5 weeks (n = 2), 6 weeks (n = 2), 7 weeks (n = 2), and 8 weeks (n = 2) pf were obtained from salpingectomies performed due to tubal ectopic pregnancies and conserved in 10% formalin in buffer phosphate at the Human Embryo Collection, Universidad de Santiago de Chile. After fixation, lung samples were dehydrated and embedded in paraffin. Histological sections were made for immunohistochemical analysis. The study was approved by the University Ethics Committee of the Universidad de Santiago de Chile (authorization number C-1004).
Light microscopy (LM)
Lung samples, fixed in 10% neutral-buffered formalin, were dehydrated in graded ethanol (70–100%) and embedded in paraffin for routine histology. Serial sections of 7 μm thick were stained with Mayer's hematoxylin (Merck, Darmstadt, Germany) and hematoxylin/eosin.
Scanning electron microscopy (SEM)
For SEM, lung samples were post-fixed in 2% osmium tetroxide in 0.1 m phosphate buffer, pH 7.4 at 4 °C (PBS), dehydrated in graded acetone series, critical point dried from CO2, mounted on aluminum stubs, sputter-coated with gold palladium, and viewed in a Hitachi S-4000 field emission SEM (Department of Human Anatomy, Faculty of Medicine, Università di Roma ‘La Sapienza’).
Immunohistochemistry
The immunohistochemical analysis of lung tissue was performed according to Godoy-Guzmán et al. (2012). Briefly, sections were treated with 3% (v/v) H2O2 in phosphate-buffered saline (PBS) for 30 min to block endogenous peroxidase. Each of the succeeding steps was followed by a through rinse with PBS. Antigenic retrieval was made with citrate sodium solution (10 mm, pH 6.0) for 15 min at 95 °C. Non-specific staining was blocked for 10 min with Cas-Block solution (Zymed Laboratories, South San Francisco, CA, USA).
The primary antibodies VEGF (mouse monoclonal, 1 : 100, Abcam), FLT-1/R1-VEGF (rabbit polyclonal, 1 : 100, Abcam), KDR/R2-VEGF (rabbit polyclonal, 1 : 100, Abcam), CD31 (rabbit polyclonal, 1 : 100, Abcam), and CD34 (Mouse monoclonal, 1 : 25, Cell Signalling) diluted in PBS containing 0.3% (v/v) Tween-20 were incubated overnight at 4 °C. Specific secondary antibody (Vector) was incubated for 1 h at room temperature followed by incubation with Vectastain RTU kit (Vector) for 1 h at room temperature. The peroxidase reaction was visualized using the Nova RED kit (Vector, Burlingame, CA, USA). After immunostaining, sections were lightly stained with Mayer's hematoxylin.
For each immunohistochemical reaction, negative controls were run by omitting the primary antibody. Sections were examined in an IX81-Olympus microscope, and images were captured using a digital camera (Olympus DP-71) and the Olympus dp-bsw software.
Double immunofluorescence staining
Double immunofluorescence for CD34 (1 : 50) and CD31 (1 : 100) was carried out using secondary antibodies conjugated with the fluorescent agents Alexa 488 and 555 (1 : 500, Molecular Probes) for 60 min at room temperature. The nuclei were stained with TO-PRO-3-iodide (1 : 200, Invitrogen) for 40 min. Finally, slides were mounted with fluorescence mounting media (DAKO) and examined in a model IX81 confocal microscope with an Olympus FV1000 camera.
Results
Lung morphology during the embryonic period
In embryos at day 31 pf, the right and left main bronchi were formed and entirely surrounded by a thick layer of splanchnopleuric mesenchyme that includes the esophagus. Between days 31 and 34 pf, the lobar organization of the lung was identified and the intrapulmonary airway branches were installed (Fig. 1). A sheath of a very lax mesenchymal tissue surrounding the airways was detected at these ages, and abundant PEs were seen in the ventricle, auricle and blood vessels of the body wall (Fig. 1A). Meanwhile, single PEs were seen for the first time within the lumen of some blood vessels at the distal segment of the terminal lung bud (Fig. 2A).
Fig. 1.
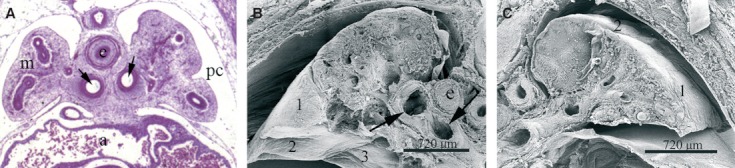
Histological and SEM images illustrating the lobar organization of the embryonic lung. (A) A transverse section of the lung stained with H/E at day 34 pf. Two bronchi (arrows) on both sides of the esophagus (e) are seen. Note that the esophagus and the main bronchi are entirely surrounded by splanchnopleuric mesenchyme (m). Abundant blood cells are seen in the auricle (a). Pleural cavity (pc). Magnification, 20×. (B,C) SEM images of the lung at day 50 pf. Three pulmonary lobes can be seen in the right lung (B), and only two (numbered) in the left lung (C).
Fig. 2.
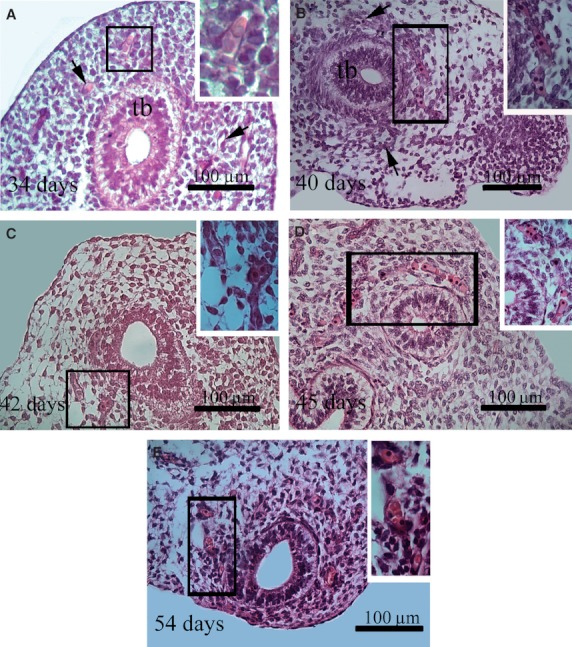
Histological sections of human lung stained with H/E. The insets correspond to higher magnification of the area enclosed in the rectangles. (A,B,C) The capillary plexus (arrows) around a lung terminal bud (tb) of embryos aged 34, 40 and 42 days pf. Primitive erythroblasts are seen within the lumen of some blood vessels (inset). (D) Primitive erythroblasts arranged in a single line within the capillary network of the lung (inset) at day 45 pf. From this embryonic age on, an increase of the amount of blood cells in the distal vascular plexus as well of their diameter was observed. Note that some definitive erythrocytes can be seen within the capillaries (inset) (E).
Serial histological sections show that at embryonic age between 34 and 42 days pf each airway terminal bud expanded and the number of airway generations increased. In the mesenchyme surrounding the terminal airway buds, an irregularly shaped capillary network was identified (Fig. 2A–C). Between 40 and 45 days pf, a continuous primitive-like vascular network filled with a single line of PEs was identified in the distal lung terminal bud mesenchyme (Fig. 2B–D). At week 6 pf, the vascular lumen was evident, showing a diameter between 13 and 14 μm, which is very close to the PE size. In contrast, from 45 days pf on, an increase in the amount of blood cells in the distal vascular plexus was seen (Fig. 2D–E).
By SEM, at embryonic age between 40 and 49 days pf, a new generation of airways was observed. The segmental organization of the lung was clearly defined and three lobes were seen on the right side and two on the left (Fig. 1B,C). Each lung lobe showed consecutive branching in a dichotomous and axial pattern. We noted that the airways increased in length and in the number of generations progressing from the center to the periphery (Fig. 3A). The tip of each lung bud was covered by a basal membrane and no mesenchymal cells were seen at this location (Fig. 3B). However, mesenchymal cells were seen at the base of the bud and some migrating cells were detected moving toward the tip (Fig. 3B,C). By LM we observed that the network of thin-walled peripheral vessels formed a ring in the parenchyma; the ring was always some distance from the endodermal epithelial basement membrane. A normal feature was the observation of mesenchymal cells arranged in a circumferential pattern just beneath the epithelial tubules. However, these cell layers were not observed around blood vessels.
Fig. 3.
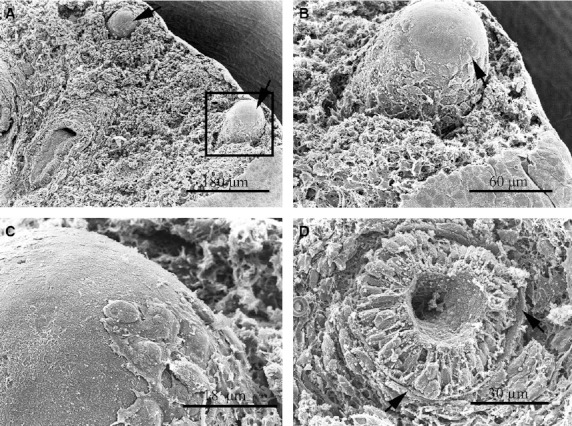
SEM images of the human lung at day 50 pf. (A) Two lung buds at the periphery of the lung (arrows). (B) Higher magnification of the tip of the blind projection of the lung bud enclosed in (A). The tip of the lung bud appears covered by a basal membrane and no mesenchymal cells were observed at this location. A higher magnification of the tip image is shown in (C). Some mesenchymal cells that look like migratory cells are attached to the basal lamina. (D) A transverse section through a terminal bud, showing a very regular-shaped endodermal epithelium surrounded by a strong basal lamina (arrows). Note that the mesenchymal tissue forms a sheath around the bud.
Each terminal bud was formed by cylindrical endodermal cells of regular shape and size (Fig. 3D). At the epithelial-mesenchymal interface the basal lamina was thick and consistent in all cases (secondary and tertiary bronchi), separating the endodermal epithelium from the surrounding mesenchyme (Fig. 3D). In general, the extracellular matrix material was very scanty along the distal outgrowths.
At 54 days pf, PEs and some definitive erythrocytes were present in the lumen of blood vessels in the distal segment of the terminal lung bud (Fig. 2E).
VEGF expression
Between 34 and 56 days pf, VEGF was expressed in the airway epithelial cells (Fig. 4A,B). At the same time, VEGF was expressed in some blood vessels around the terminal buds.
Fig. 4.
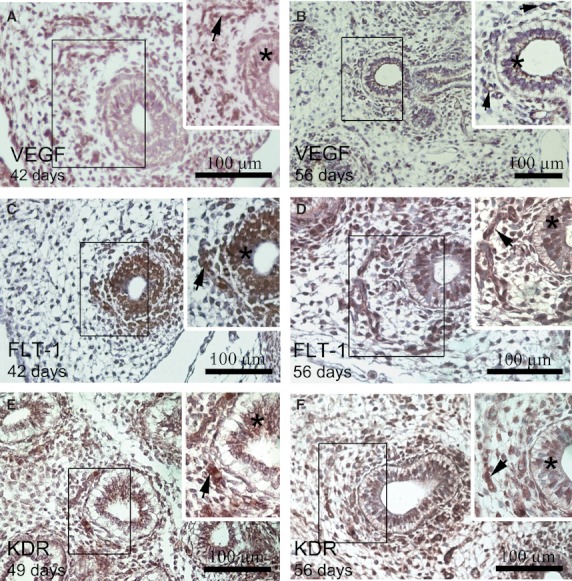
Immunostaining of VEGF, FLT-1 and KDR in embryonic lung sections. The insets correspond to a higher magnification of the area enclosed in the rectangles. In lungs from 42 (A) to 56 days pf (B), VEGF was expressed in the airway epithelial cells (*) and in some blood vessels (arrow). (C) FLT-1+ group endothelial cells (arrows) around the terminal buds, forming a vascular plexus at 42 days pf. (D) An increased number of FLT-1+ endothelial cells (arrow) are seen in the distal mesenchyma surrounding the developing airway at 56 days pf. Insets (C,D) show FLT-1+ endodermal cells (*). (E) KDR+ group endothelial cells (arrow) around the terminal buds, forming a vascular plexus at 49 days pf. (F) An increased number of KDR+ endothelial cells (arrow) is seen in the distal mesenchyma surrounding the developing airway at 56 days pf. Insets (E,F) shows KDR+ endodermal cells (*).
FLT-1 expression
At 34 days pf, the FLT-1+ endothelial cells were restricted to the mesenchyme area surrounding the distal lung buds. A group of FLT-1+ endothelial cells was seen around the terminal bud at 42 days pf (Fig. 4C). From 49 to 56 days pf, an increase in the number of FLT-1+ endothelial cells was detected in the distal mesenchymal cells surrounding the developing airway, maintaining their association with the developing airway (Fig. 4D). At the same time, FLT-1 was expressed in the endodermic epithelial cells between 34 and 56 days pf (Fig. 4C,D).
KDR expression
KDR was always detected in endothelial cells in all the lung samples studied. At 34 days pf, a few KDR+ endothelial cells were seen in the mesenchymal area surrounding the distal lung bud. A group of KDR+ endothelial cells was seen around the terminal bud at 42 days pf. From 49 to 56 days pf, KDR+ endothelial cells increased in number in the distal mesenchymal area surrounding the developing airway (Fig. 4 E,F). At the same time, KDR was expressed in the endodermal epithelial cells between 34 and 56 days pf (Fig. 4E,F).
Double immunofluorescence for CD31/CD34
We examined the colocalization of CD31/CD34 from day 35 to day 42 pf using double immunofluorescence. We observed colocalization of these molecules in some endothelial cells, forming a vascular plexus around the distal pulmonary buds (Fig. 5A–C).
Fig. 5.
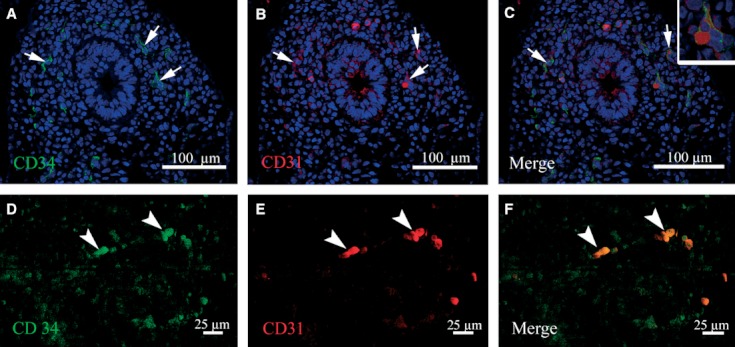
Confocal images of double immunofluorescence for CD34 and CD31 in human embryonic lungs at 35 days pf. (A) CD34+ cells (green) in the vascular plexus around the terminal bud. (B) Immunoreactivity for CD31 (red) in some endothelial cells. (C) The merged image of CD34 and CD31 in endothelial cells of the capillary plexus. A higher magnification of the endothelial cell is shown in the inset. (D,E) Single primitive CD34+ (green) and CD31+ (red) erythroblasts (arrow head). (F) Merged image of (D) and (E) showing CD34 and CD31 colocalization (arrowhead). Nuclei staining with TO-PRO-3-iodide.
On the other hand, single primitive CD34/CD31-positive erythroblasts were detected throughout the distal capillary plexus of the lung from day 35 pf on (Fig. 5D–F).
Discussion
Nutrient supply is an essential process during human organogenesis; therefore the installation of a network of blood vessels for nutrient distribution is mandatory during the embryonic period. According to its developmental timing, each emerging organ generated a vascular network to carry out such a nutritional task once the circulation starts. The timing of this process has not been followed in the human lung during the embryonic period. Our morphological and immunohistochemical results reveal that the timing between the generation of a vascular plexus at the terminal bronchial bud, and its physiological connection with the circulation of the embryo are processes that occur at almost the same time. This functional link is produced as early as day 31 pf, when PEs are seen for the first time within the capillary network of the embryonic lung.
This paper reports for the first time, a possible role of the VEGF signaling pathway in early human embryo lung development. Our results also suggest that the vascular plexus installed around the terminal buds is formed by vasculogenesis before week 5 pf. However, the increasing number of primitive erythroblasts from week 6 on, associated with a change in the shape of the blood vessels, suggests a vascular remodeling process and that the generation of new distal vessels occurs mainly by a process of distal angiogenesis. Finally, we showed a very early onset of blood circulation starting days 34 pf, concomitant with the generation of a new lung bud.
The increasing numbers of PEs observed from week 6 onward, associated with changes in the shape of blood vessels, are morphological indicators that new distal vessels are generated in the lung primordium, mainly by a process of distal angiogenesis. This assumption is in agreement with previous observations made by Parera et al. (2005) in mice, showing that vascular development in the distal segment of the lung occurs by the process of angiogenesis.
The morphofunctional events leading to segmental organization of the lung are early processes that occur in a very short period of time. This situation makes it difficult to establish whether vascularization and circulation are synchronous processes that run in parallel from the beginning of lung formation or are asynchronous phenomena, with an interval of time between both process. The only clear indication of a connection between extra- and intrapulmonary circulation in the current work was the morphological identification of PEs within the lumen of some blood vessels in the distal segment of the bronchial bud at day 31 pf. The scarcity of blood cells within the vessels before week 5 pf, leads us to consider that in the lung, vessel formation takes place initially by vasculogenesis.
Mechanism involved in the development of the lung vascular plexus
In humans there is contradicting information about the mechanisms involved in the development of the lung vasculature. Based on morphological studies, Demello & Reid (2000) proposed two mechanisms of blood vessel formation, with central vessels being formed by angiogenesis and peripheral vessels by vasculogenesis. Hall et al. (2000) suggested from immunohistochemical studies of the endothelial marker CD31 that the main mechanism of blood vessel formation in the lung is distal vasculogenesis. Parera et al. (2005), studying the expression of PECAM-1, Flk-1, Fli-1 and CD31 in mice, proposed that lung vasculature arises by distal angiogenesis. In that model the capillary networks surrounding the terminal buds expand by the formation of new capillaries from preexisting vessels as the lung bud grows.
The recent observation of the expression of collagens (I, III, VI) and biglycan, and its relation with blood vessel development during the human pseudoglandular period, has raised new questions regarding the mechanism involved in vascular network formation (Godoy-Guzmán et al. 2012). It has been shown that the vascular endothelial growth factor (VEGF) plays a significant role in vasculogenesis and angiogenesis, regulating vascular endothelial cell proliferation and differentiation (Neufeld et al. 1999; Ferrara et al. 2003). In the human fetal lung between 16 and 22 weeks of gestation, VEGF was localized primarily in epithelial cells and myocytes, including the smooth muscle cells lining blood vessels (Shifren et al. 1994). These results suggest a paracrine mechanism whereby VEGF secreted by non-endothelial cells modulates physiological processes in the adjacent vascular endothelium. We found VEGF expression in the airway epithelial cells and in some blood vessels around the terminal buds between 34 and 56 days pf, suggesting that the distribution and expression of VEGF in the endodermal epithelial cells and in some blood vessels are associated with the development of the vascular pulmonary plexus.
VEGF and its high-affinity binding tyrosine kinase receptors FLT-1 and KDR are essential for the development of embryonic vasculature (Neufeld et al. 1999). Interestingly, high expression levels of KDR are detected in organs where vasculogenesis is the leading mechanism of vascularization, whereas lower levels are found in organs that receive their vascular supply by angiogenesis (Miquerol et al. 1999). Whereas the KDR receptor is dominant during early vasculogenesis, FLT-1 is prominent during remodeling of the primary vascular plexus and subsequent angiogenesis (Patan, 2000). In vivo, KDR null embryos lack endothelial and hematopoietic cells and thus fail in vasculogenesis and blood island formation (Shalaby et al. 1995). These observations are in line with our results showing that KDR+ and FLT-1+ endothelial cells may be related to the processes of vasculogenesis and angiogenesis, respectively.
In vivo and in vitro experiments suggest that KDR is the earliest angioblast marker and a suitable indicator of embryo vascularization and developing organs such as the lungs (Yamaguchi et al. 1993; Shalaby et al. 1995; Kabrun et al. 1997). We showed that between 34 and 56 days pf, KDR+ endothelial cells were detected in the mesenchyme around the distal lung bud. Therefore, the presence of precursor KDR+ cells in the mesenchyme of the more distal segments of the pulmonary airway suggests the occurrence of vasculogenesis. Our results agree with those reported by Gebb & Shannon (2000) in mice lung development concerning the spatial distribution of KDR in the mesenchyme of the lung periphery.
The colocalization of CD34/CD31 detected by inmmunofluorescence in some segments of blood vessel endothelium that form a vascular plexus around the terminal bud are consistent with those described by Han et al. (2003), where progenitor cells identified as angioblasts co-express CD31/CD34. An interesting finding was the observation of primitive CD31+/CD34+ erythroblasts in the distal capillary plexus that may contribute to the development of blood vessels by vasculogenesis. Rafii (2000) described the presence of circulating angioblast precursor cells in adults; these CD31+/CD34+ cells are recruited from the circulation, contributing to the endothelialization process by vasculogenesis.
The second tyrosine kinase receptor for VEGF, FLT-1, is also expressed during early embryonic development (Breier et al. 1995) and modulates vascular growth negatively, reducing endothelial cell proliferation (Fong et al. 1995). Despite the different functions described for KDR and FLT-1, we found a similar distribution of both receptors in the distal segments of the airway, suggesting an interaction of both molecules during the development of the vascular plexus. It has been suggested that both VEGF receptors regulate pulmonary vascular development, modulating VEGF signaling and forming a highly organized network in the lung (Yamamoto et al. 2007).
The KDR and FLT-1 immunostaining seen in the endodermal epithelial cells suggests their participation in the branching morphogenesis processes. Studies in relation to the VEGF/VEGF-receptor system point at a linkage between the processes of branching morphogenesis, development of the vascular system, and morphogenesis of the epithelial system (Lassus et al. 2001; Del Moral et al. 2006; Ahlbrecht et al. 2008).
Early onset of blood circulation in the lungs
In humans, there are few data available concerning the beginning of blood circulation in the lungs. Morphological studies conducted by Demello & Reid (2000) suggest that it is not until week 10–11 of gestational age that a communication between small muscular vessels and nonmuscular ones can be observed. According to those authors, at that a breakthrough from an upstream pulmonary arterial bed to the peripheral microcirculation may be occurring. Maeda et al. (2002), using immunohistochemistry and 3D reconstruction, studied the distribution of CD34 and alpha smooth muscle actin between 8 and 16 weeks of gestation in the human fetal lung; they suggested a connection between the proximal and the distal blood network in the late pseudoglandular phase (16 weeks' gestation). In contrast, Hall et al. (2002) concluded from a three-dimensional reconstruction of a 34-day human embryo that the proximal and distal blood networks are already interconnected at that time. Parera et al. (2005) provided evidence in mice of the presence of a lung connection with the heart, liver and yolk sac starting from the very early signs of lung differentiation (9.5 days, equivalent to 22 days of human development). Based on the presence of primitive erythroblasts, our results suggest a very early onset of blood circulation starting from 34 days pf. Our observation indicates the existence of a connection between macrocirculation and pulmonary vascular microcirculation as early as 31 days pf. The increasing amount of blood cells in blood vessels might have an important role in the remodeling process of the lung vascular plexus. It has been shown that changes in viscosity caused by blood cells when they enter the circulation could result in a physical stimulus that triggers remodeling (Suri et al. 1996; Patan, 1998).
Acknowledgments
This work was supported by grants from DICYT (Universidad de Santiago de Chile, Santiago, Chile; grant 021001GG and 021001PT). S. San Martin is supported by DIPUV (Universidad de Valparaíso, Valparaíso, Chile; grant CI 05/2006) and Programa de Investigación Interdisciplinario (PIA) from the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) (Anillos ACT-73), Chile. We state that no author in this work had any conflict of interest.
References
- Ahlbrecht K, Schmitz J, Seay U, et al. Spatiotemporal expression of flk-1 in pulmonary epithelial cells during lung development. Am J Respir Cell Mol Biol. 2008;39:163–170. doi: 10.1165/rcmb.2007-0231OC. [DOI] [PubMed] [Google Scholar]
- Breier G, Clauss M, Risau W. Coordinate expression of vascular endothelial growth factor receptor-1 (flt-1) and its ligand suggests a paracrine regulation of murine vascular development. Dev Dyn. 1995;204:228–239. doi: 10.1002/aja.1002040303. [DOI] [PubMed] [Google Scholar]
- Cardoso WV, Lu J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–1624. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- Del Moral PM, Sala FG, Tefft D, et al. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 2006;290:177–188. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- Demello DE, Reid LM. Embryonic and early fetal development of human lung vasculature and its functional implications. Pediatr Dev Pathol. 2000;3:439–449. doi: 10.1007/s100240010090. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of vegf and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fong GH, Rossant J, Gertsenstein M, et al. Role of the flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature. 1995;376:66–70. doi: 10.1038/376066a0. [DOI] [PubMed] [Google Scholar]
- Gebb SA, Shannon JM. Tissue interactions mediate early events in pulmonary vasculogenesis. Dev Dyn. 2000;217:159–169. doi: 10.1002/(SICI)1097-0177(200002)217:2<159::AID-DVDY3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Godoy-Guzmán C, San Martin S, Pereda J. Proteoglycan and collagen expression during human air conducting system development. Eur J Histochem. 2012;56:e29. doi: 10.4081/ejh.2012.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Hislop AA, Pierce CM, et al. Prenatal origins of human intrapulmonary arteries: formation and smooth muscle maturation. Am J Respir Cell Mol Biol. 2000;23:194–203. doi: 10.1165/ajrcmb.23.2.3975. [DOI] [PubMed] [Google Scholar]
- Hall SM, Hislop AA, Haworth SG. Origin, differentiation, and maturation of human pulmonary veins. Am J Respir Cell Mol Biol. 2002;26:333–340. doi: 10.1165/ajrcmb.26.3.4698. [DOI] [PubMed] [Google Scholar]
- Han RN, Post M, Tanswell AK, et al. Insulin-like growth factor-i receptor-mediated vasculogenesis/angiogenesis in human lung development. Am J Respir Cell Mol Biol. 2003;28:159–169. doi: 10.1165/rcmb.4764. [DOI] [PubMed] [Google Scholar]
- Joshi S, Kotecha S. Lung growth and development. Early Hum Dev. 2007;83:789–794. doi: 10.1016/j.earlhumdev.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Kabrun N, Buhring HJ, Choi K, et al. Flk-1 expression defines a population of early embryonic hematopoietic precursors. Development. 1997;124:2039–2048. doi: 10.1242/dev.124.10.2039. [DOI] [PubMed] [Google Scholar]
- Lassus P, Turanlahti M, Heikkilä P, et al. Pulmonary vascular endothelial growth factor and Flt-1 in fetuses, in acute and chronic lung disease, and in persistent pulmonary hypertension of the newborn. Am J Respir Crit Care Med. 2001;164:1981–1987. doi: 10.1164/ajrccm.164.10.2012036. [DOI] [PubMed] [Google Scholar]
- Maeda S, Suzuki S, Suzuki T, et al. Analysis of intrapulmonary vessels and epithelial-endothelial interactions in the human developing lung. Lab Invest. 2002;82:293–301. doi: 10.1038/labinvest.3780423. [DOI] [PubMed] [Google Scholar]
- Metzger RJ, Klein OD, Martin GR, et al. The branching programme of mouse lung development. Nature. 2008;453:745–750. doi: 10.1038/nature07005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Gertsenstein M, Harpal K, et al. Multiple developmental roles of vegf suggested by a lacz-tagged allele. Dev Biol. 1999;212:307–322. doi: 10.1006/dbio.1999.9355. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Cohen T, Gengrinovitch S, et al. Vascular endothelial growth factor (vegf) and its receptors. FASEB J. 1999;13:9–22. [PubMed] [Google Scholar]
- Parera MC, van Dooren M, van Kempen M, et al. Distal angiogenesis: a new concept for lung vascular morphogenesis. Am J Physiol Lung Cell Mol Physiol. 2005;288:L141–149. doi: 10.1152/ajplung.00148.2004. [DOI] [PubMed] [Google Scholar]
- Patan S. Tie1 and tie2 receptor tyrosine kinases inversely regulate embryonic angiogenesis by the mechanism of intussusceptive microvascular growth. Microvasc Res. 1998;56:1–21. doi: 10.1006/mvre.1998.2081. [DOI] [PubMed] [Google Scholar]
- Patan S. Vasculogenesis and angiogenesis as mechanisms of vascular network formation, growth and remodeling. J Neurooncol. 2000;50:1–15. doi: 10.1023/a:1006493130855. [DOI] [PubMed] [Google Scholar]
- Rafii S. Circulating endothelial precursors: mystery, reality, and promise. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, et al. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Shifren JL, Doldi N, Ferrara N, et al. In the human fetus, vascular endothelial growth factor is expressed in epithelial cells and myocytes, but not vascular endothelium: implications for mode of action. J Clin Endocrinol Metab. 1994;79:316–322. doi: 10.1210/jcem.79.1.8027247. [DOI] [PubMed] [Google Scholar]
- Suri C, Jones PF, Patan S, et al. Requisite role of angiopoietin-1, a ligand for the tie2 receptor, during embryonic angiogenesis. Cell. 1996;87:1171–1180. doi: 10.1016/s0092-8674(00)81813-9. [DOI] [PubMed] [Google Scholar]
- Warburton D, Schwarz M, Tefft D, et al. The molecular basis of lung morphogenesis. Mech Dev. 2000;92:55–81. doi: 10.1016/s0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, et al. Flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Shiraishi I, Dai P, et al. Regulation of embryonic lung vascular development by vascular endothelial growth factor receptors, flk-1 and flt-1. Anat Rec. 2007;290:958–973. doi: 10.1002/ar.20564. [DOI] [PubMed] [Google Scholar]


