Abstract
Studies have determined the effects of joint immobilization on the articular cartilage of sedentary animals, but we are not aware of any studies reporting the effects of joint immobilization in previously trained animals. The objective of the present study was to determine whether exercise could prevent degeneration of the articular cartilage that accompanies joint immobilization. We used light microscopy to study the thickness, cell density, nuclear size, and collagen density of articular cartilage of the femoral condyle of Wistar rats subjected to aerobic physical activity on an adapted treadmill five times per week. Four groups of Wistar rats were used: a control group (C), an immobilized group (I), an exercised group (E), and an exercised and then immobilized group (EI). The right knee joints from rats in groups I and EI were immobilized at 90 °C of flexion using a plastic cast for 8 weeks. Cartilage thickness decreased significantly in group I (mean, 120.14 ± 15.6 μm, P < 0.05), but not in group EI (mean, 174 ± 2.25), and increased significantly in group E (mean, 289.49 ± 9.15) compared with group C (mean, 239.20 ± 6.25). The same results were obtained for cell density, nuclear size, and collagen density (in all cases, P < 0.05). We concluded that exercise can prevent degenerative changes in femoral articular cartilage caused by immobilization of the knee joint.
Keywords: articular cartilage, exercise, immobilization, knee joint
Introduction
The articular cartilage is composed of chondrocytes and an extracellular matrix (ECM) rich in macromolecules such as proteoglycans and collagen (Huber et al. 2000; Smith et al. 2000). In adult humans, chondrocytes account for approximately 1% of tissue volume (Stockwell, 1967). Under normal physiological conditions, chondrocytes are responsible for maintaining cartilage homeostasis by balancing the synthesis and degradation of ECM components (Angel et al. 2003; Alford & Cole, 2005), and nutritional intake of the tissue (Huber et al. 2000). There are four layers or zones in the articular cartilage based on the arrangement of the collagen fibers of the ECM and chondrocytic morphology, which include the superficial, intermediate, deep, and calcified cartilage zones. The organization of the zones is functionally important and the zone size varies among joints and between various species (Huber et al. 2000). Chondrocytes in various layers differ in size, shape, and metabolic activity (Aydelotte & Kuettner, 1988). In humans, chondrocytic density is highest at the superficial zone and decreases with increasing distance from the surface (Huber et al. 2000).
The superficial zone is the thinnest in articular cartilage, as the chondrocytes are small, flattened, positioned parallel to the articular surface (Oda et al. 2007), and contain the highest water concentration, a low proteoglycan content, and densely packed layers of collagen fibers (Wu & Herzog, 2002). The specific organization of the superficial zone allows it to act as a barrier to the passage of large molecules from the synovial fluid to the cartilage. The intermediate zone contains rounded chondrocytes surrounded by an ECM with its apparent organization due to collagen fibers. In the deep zone, the chondrocytes form columns and the collagen fibers are organized radially. The calcified zone is characterized by rounded chondrocytes with collagen fibers arranged perpendicular to the articular surface (Castano Oreja et al. 1995).
Joint immobilization is necessary for the treatment of fractures and dislocations and although immobilization is beneficial, it promotes atrophy of the articular cartilage both in humans (Buckwalter, 1995; Hudelmaier et al. 2006; Liphardt et al. 2009) and animal models (Haapala et al. 1999; Ando et al. 2008; Hagiwara et al. 2009). Following immobilization, there is a decrease in proteoglycan staining intensity (Kiviranta et al. 1987; Haapala et al. 1999; Hudelmaier et al. 2006), increased collagen synthesis, decreased thickness, and decreased volume of chondrocytic nuclei (Buckwalter, 1995; Haapala et al. 1999; Hagiwara et al. 2009). The decrease in chondrocytic nuclear volume induces a reduction in cellular activity that negatively affects cartilage structure (Oda et al. 2007).
Moderate exercise influences the thickness and structure of articular cartilage; however, the results of some studies on the effects of exercise on cartilage thickness are controversial. Some authors found that increased cartilage thickness (Palmoski & Brandt, 1981) resulted from chondrocytic stimulation and subsequent production of ECM components (Kiviranta et al. 1988; Haupt et al. 1989; Moriyama et al. 2012), whereas others have reported a decrease in cartilage thickness (O'Connor, 1997) or no change in thickness (Carvalho & Langfeldt, 1977; Kiviranta et al. 1984; O'Connor, 1997). Exercise also alters the composition and mechanical properties of articular cartilage and improves adaptation to mechanical loads. According to Kiviranta et al. (1988) and Otterness et al. (1998), moderate exercise maintains proteoglycan content in the ECM, whereas a lack of exercise can result in the reduction of these elements. Exercise is considered one of the most important treatments to combat the deleterious changes promoted by osteoarthritis (Thorstensson et al. 2005). There have been a number of experimental studies on the immobilized articular cartilage in sedentary individuals and animals; however, changes in articular cartilage following joint immobilization on exercised animals remain unknown. Herein, we analyzed the deleterious effects of immobilization on the femoral articular cartilage from sedentary and exercised rats to test our hypothesis that exercise increases the resistance of articular cartilage to atrophy that occurs during joint immobilization.
Materials and methods
Animals
Twenty adult male Wistar rats (mean body mass = 390 ± 10 g) were used in this study that were 180 days old, the age at which the growth plates are closed (Simpson, 1950). The animals were kept in plastic cages at room temperature under a 12-h light/dark cycle (7:00–19:00 h) and provided with water and food ad libitum. The animals were divided into four groups (n = 5 animals each): C, the control group (not exercised and not immobilized); I, an immobilized group; E, an exercised group with no immobilization; and EI, an exercised and immobilized group.
Exercise protocol
The animals in the exercised groups performed a maximal exercise test (TEM) on an adapted motorized treadmill (Inbrasport, Rio Grande do Sul, Brazil) to determine a baseline rate to be used in the experiments. The TEM was then performed at the baseline speed of 0.3 km h−1 with increments of 0.3 km h−1 every 4 min until the animal showed signs of fatigue, i.e. when it lost the ability to maintain the speed increase on the treadmill (Silva et al. 1997). At the end of week 4, new TEMs were adopted and the training speed was adjusted for the 4 weeks if the animal performances varied. At the end of the experiment, new TEMs were conducted before sacrifice of the animals.
The protocol of physical training was as follows: during the first week, the animals in the exercise groups ran for 30 min with increasing speed up to 60% of that achieved in the TEM. From week 2 of training, the time was gradually increased by 10 min each week until reaching 60 min in week 4. At the end of week 4, a new TEM was adopted to adjust the exercise intensity for the next 4 weeks.
Animals in groups E and EI were submitted to long-term joint immobilization using plastic casts (Kiviranta et al. 1984). Briefly, the rats from these two groups were anesthetized with sodium pentobarbital (50 mg kg−1) and a plastic cast was wrapped around the right limb from the lower leg to abdomen to maintain the knee joint at 90 °C of flexion. After 8 weeks, the rats were sacrificed.
Tissue sample preparation
After week 8, rats from all groups were sacrificed by intraperitoneal injections of sodium pentobarbital (150 mg kg−1). The capsule of the knee was sectioned, the joint was opened, and the menisci isolated. The distal extremity of the femur containing cartilage was isolated and fixed with 4% paraformaldehyde in 0.1 m phosphate-buffered saline (PBS) at pH 7.4. The fixed specimens were decalcified in 10% ethylenediaminetetraacetic acid in 0.01 m PBS at pH 7.4 for 2 months at 4 °C (Kiviranta et al. 1984). The blocks were then washed in PBS and dehydrated, cleared, and paraffin-embedded (Paraplast) (Stockwell, 1967). Sections (6-μm-thick) were cut perpendicular to the articular surface of the femoral cartilage in contact with the tibia and dried overnight at 37 °C.
Quantitative analysis
Each tissue sample was cut into 50 sections. To minimize variability, all sections were taken from the center of the block containing the medial condyle of the femur. Every sixth section from each animal was then stained with hematoxylin and eosin (HE) and every seventh section was stained with Picrosirius red (a total of nine sections for each staining technique). These techniques are particularly useful for investigating quantitative data of the cartilage (Stockwell, 1967; Lothe et al. 1979; Oda et al. 2007).
Articular cartilage thickness
To determine the cartilage thickness, histological sections stained with HE were analyzed using a digital image analysis system for quantitative histomorphometry (KS-300; Carl Zeiss, Inc., Oberkochen, Germany). Each microscopic image was projected to a monitor and the thickness of the entire cartilage was measured at three equidistant points from the articular surface of femur based on the cartilage morphometry measurement methods of others (Castano Oreja et al. 1995; Oda et al. 2007; Hagiwara et al. 2009).
Cell density
To count the number of chondrocytes, we set a certain range of interest (a rectangle 100 μm wide and 400 μm long) and superimposed it over histological sections using a digital image analysis program (KS-300; Carl Zeiss, Inc.) (Hagiwara et al. 2009). The chondrocytes present in three non-consecutive sections were counted and the means calculated.
Nuclear volume of chondrocytes
The nuclear volume of chondrocytes from the intermediate zone of the articular cartilage was estimated under 1000× magnification from two randomly selected fields in each of five HE-stained sections using the following formula (Fig. 1):
Fig. 1.
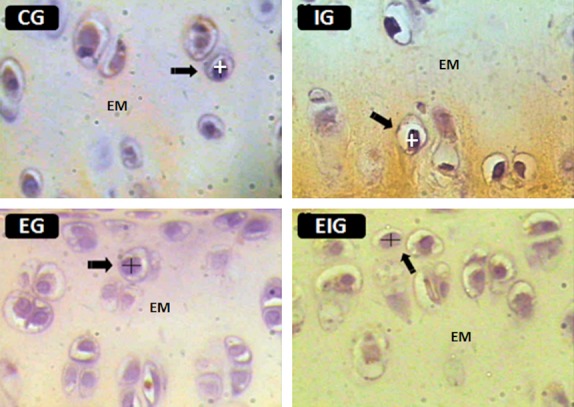
Histological sections of the femoral articular cartilage from each of the four groups of rats (C, I, E, and EI) were used to measure the major and minor nuclear diameters of chondrocytes (arrows). EM, ECM. Figures are from the intermediate zone of the cartilage. HE staining. Scale bar: 30 μm.
where V is the volume of the nucleus; a is the shortest nuclear diameter; b is the longest nuclear diameter; and 1.91 is a constant (Salvatore & Schreider, 1947).
Volume density (Vv) of collagen
Every seventh section of the cartilage was stained for 1 h in a 0.1% solution of Picrosirius (Sirius Red F3B; color index 35782; Sigma-Aldrich, St. Louis, MO, USA) dissolved in aqueous saturated picric acid. The sections were then rapidly washed in running tap water, counterstained with fresh Harris hematoxylin for 6 min (Junqueira et al. 1979; Kiernan, #b500), and analyzed using Polaroid filters under conventional light microscopy. The best results were obtained when very high light intensity was used. When analyzed by this method, the cartilage containing type one collagen appears red in color and that containing type three appears green (Junqueira et al. 1978; Whittaker et al. 1994).
The collagen volume density (Vv) was obtained using two fields from each section, which were generated by the digital image analysis program. Two frames (5 cm2 each), containing 38 equidistant points each, were superimposed on the corresponding sectional fields of view (Fig. 2). Vv was estimated according to the methods of Gundersen et al. (1988). Briefly, the number of points corresponding to the area of interest (Pcollagen) was divided by the number of points corresponding to the reference area (Pref) using the formula: Vv = Pcollagen/Pref. The volume fractions of collagen are expressed in percentages.
Fig. 2.
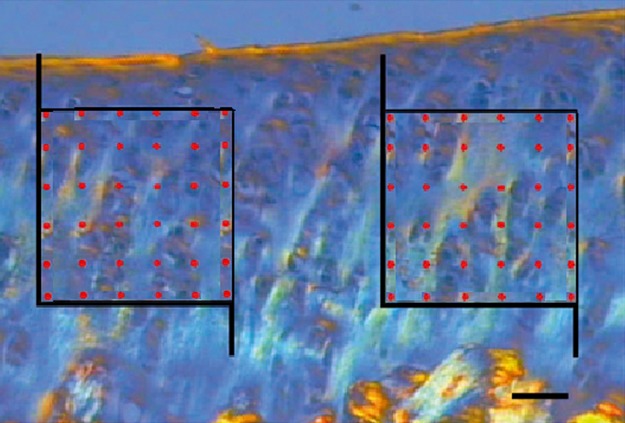
A photomicrograph showing unbiased counting using two square frames containing 36 points each and superimposed on the intermediate zone of the femoral articular cartilage. Collagen appears as orange, white, and green vertical fiber bundles on a blue background inside of the frames. Scale bar: 20 μm.
Statistical analysis
Quantitative data were analyzed using analysis of variance and the Kruskal–Wallis test and expressed as mean ± standard error (SE). A P-value < 0.05 was considered statistically significant.
Results
Morphological data
The changes observed in the articular cartilage of immobilized rats at 8 weeks were as follows: the zones of cells were irregular, the cells of the superficial zone had practically disappeared, and the mean cell concentration in the intermediate zone of the articular cartilage was reduced compared with groups C, E, and EI, which did not contain these alterations (Fig. 3). The collagen concentration seemed to have been reduced in group I in relation to the other groups (Fig. 4).
Fig. 3.
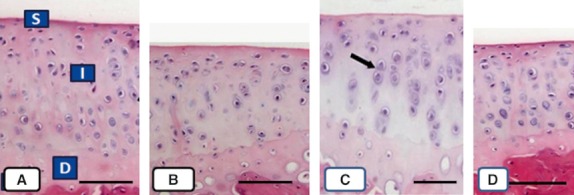
Microphotographs of sections of cartilage sectioned perpendicular to the articular surface from rats in groups C (A), I (B), E (C), and EI (D). Observations of the superficial (S), intermediate (I), and deep zones (D) were clearly differentiated in (A), (C), and (D), but not in (B). Two chondrocytes are indicated in (C) with arrows. Scale bars: 100 μm.
Fig. 4.
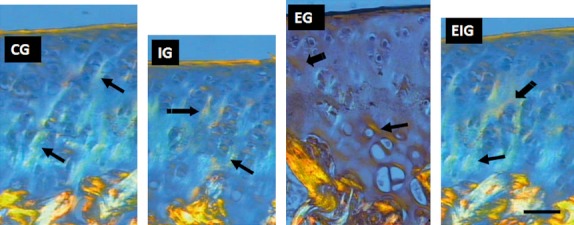
Light-field photomicrographs via polarization microscopy of the femoral cartilage sections from groups C, I, E, and EI stained with Picrosirius to observe the changes in collagen (arrows) in the immobilization (I) and exercise (E and EI) groups. Collagen appears as orange, white, and green vertical fiber bundles on a blue background above the cancellous bone. Collagen concentration seemed to have been reduced in group I and augmented in group E in relation to the other groups. Scale bar: 30 μm.
Quantitative results
Cartilage thickness
The thickness of the femoral articular cartilage in the contact area was significantly lower at 8 weeks in the femurs of group I rats (mean, 120.14 ± 15.6 μm) and significantly higher in the femurs of group E rats (mean, 289.49 ± 9.15 μm) compared with those of groups C (mean, 239.20 ± 6.25 μm) and EI (mean, 174 ± 2.25 μm) (in all cases P < 0.05) (Fig. 5). No significant difference was observed between groups C and EI (P > 0.05), i.e. the immobilization had no effect on the thickness of the cartilage of animals that had been exercised previously (group EI).
Fig. 5.
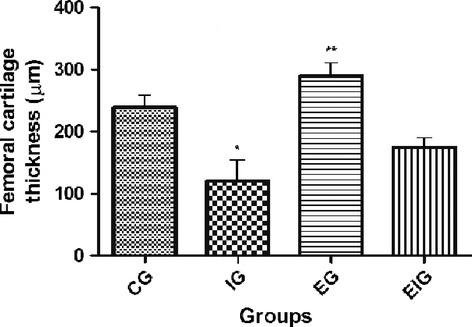
Articular cartilage thickness in the contact area from the four study groups (C, I, E, and EI). *Significance vs. groups C, E, and EI (P < 0.05); **Significance vs. groups C and EI (P < 0.05). No significant difference was observed between groups C and EI (P > 0.05), suggesting that exercise had no effect on cartilage thickness in immobilized animals. Five rats per group and nine tissue sections per animal.
Cell density
Cell density estimates for the four groups are presented in Fig. 6. The number of chondrocytes was significantly lower at 8 weeks in group I than in groups C, E, and EI. No significant difference was observed among groups C, E, and EI (P > 0.05). Exercise had no effect on the cell density of cartilage from animals that had not been immobilized (group E), but did affect the cartilage cell density of immobilized animals (group EI) and prevented the loss of chondrocytes induced by immobilization.
Fig. 6.
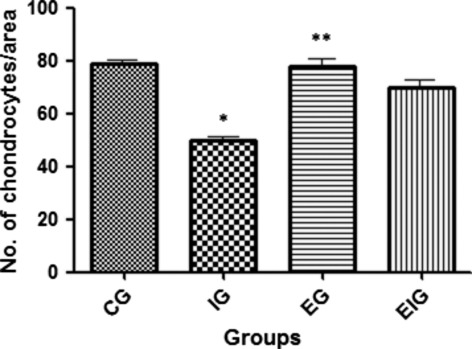
Cellular density (number per area) in femoral articular cartilage of the four study groups. *Significance vs. groups C, E, and EI (P < 0.05). No significant difference was observed among groups C, E, and EI (P > 0.05), suggesting that exercise had no effect on chondrocyte number from non-immobilized animals (group E), but prevented the effects of immobilization on the number of chondrocytes in immobilized animals (group EI). Five rats per group and nine tissue sections per animal.
Volume of the nucleus of chondrocytes
Statistical analysis showed that the mean nuclear volume from group I chondrocytes was significantly lower than those from all other groups (C, E, and EI) and the mean nuclear volume from group E samples was significantly higher than those from groups C and EI (Fig. 7). There was no significant difference between groups EI and C (P > 0.05). Apparently, exercise did have an effect on the nuclear volume of chondrocytes from animals that had been immobilized (group EI).
Fig. 7.
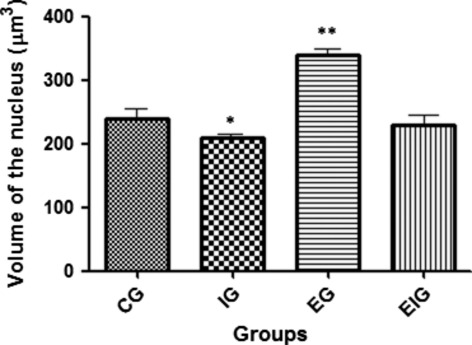
The volume of nuclear chondrocytes in articular cartilage of the femur in the four study groups of rats (C, I, E, and EI). *Significance vs. groups C, E, and EI (P < 0.05) and **significance vs. groups C and EI (P < 0.05). No significant difference was observed between groups C and EI (P > 0.05), suggesting that exercise did affect the nuclear volume of chondrocytes from immobilized animals (group EI) and prevented the effects of immobilization on nuclear volume. Five rats per group and nine sections per animal.
Collagen tissue Vv
The statistical analysis showed that Vv of the collagen fibers in group I was significantly lower (P < 0.05) than that of groups C, E, and EI and that Vv of collagen fibers in group E was significantly higher than that of groups C and EI (P < 0.05) (Fig. 8). No significant difference was observed between groups C and EI (P > 0.05). Apparently, exercise did not affect collagen tissue Vv in immobilized animals (group EI).
Fig. 8.
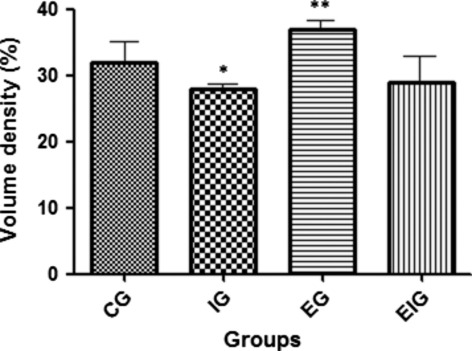
Vv of collagen fibers in femoral articular cartilage in the four study groups of rats (C, I, E, and EI). *Significance vs. groups C, E, and EI (P < 0.05) and **significance vs. groups C and EI (P < 0.05). No significant difference was observed between groups C and EI (P > 0.05), suggesting that exercise did affect collagen Vv in articular cartilage by immobilization (groups I and E). Five rats per group and nine sections per animal.
Discussion
In the present study, we used a joint immobilization model to explore whether changes in the morphology of femoral articular cartilage in exercised rats were similar to that observed in sedentary animals. Two major findings emerged from this study. First, under immobilization, the cartilage thinning that occurred in group I rats was not observed in group EI rats, showing that exercise protects cartilage thickness from the deleterious effects of immobilization. Secondly, the number of chondrocytes, the sizes of their nuclei, and the collagen Vv after immobilization was significantly decreased in the contact area in group I, but not in group EI. These results showed that exercise attenuated the effect of immobilization on chondrocytes and collagen. Furthermore, exercise increased cartilage thickness, collagen Vv, and nuclear volume of chondrocytes.
Immobilized animals exhibited a significant decrease in cartilage thickness compared with sedentary controls (P < 0.05) (Fig. 5). The results of previous studies on this subject are controversial, as some authors found increased cartilage thickness (Palmoski & Brandt, 1981), whereas others reported a decrease in cartilage thickness (O'Connor, 1997) or no change in thickness (Carvalho & Langfeldt, 1977; Kiviranta et al. 1984; O'Connor, 1997). Our results correspond well with the results of Haupt et al. (1989). According to some authors (Gyarmati et al. 1987; Haapala et al. 1999), these contradictory results may be explained by a lack of standard measurement sites, the use of young or old animals, or the use of contralateral knees as controls. The differences between the reports could also be due to the value of the immobilized angle, leading to major or minor joint laxity and different compressive force between the femur and tibia (Carvalho & Langfeldt, 1977).
The thinning of the cartilage and its layers in immobilized rats may be due to the decline in chondrocytic activity during immobilization (Palmoski & Brandt, 1981), which influenced the structure of the ECM and led to a gradual decrease in cartilage thickness (Palmoski & Brandt, 1981; O'Connor, 1997). Changes in ECM synthesis by cells, which is programmed and beneficial, occur throughout life to adapt the articular cartilage to mechanical and chemical requirements (Karvonen et al. 1994). It is also possible that the reduction in cartilage thickness occurs by decreasing synovial fluid production and the nutrient supply to the cartilage, as determined by a lack of motion and load, and thus produces a deficit in the diffusion of liquids and for pumping these elements into the cartilage (O'Connor, 1997).
The present study demonstrated a significant decrease in chondrocyte density in the femoral cartilage from rats in group I. The decrease in chondrocytic density in the cartilage of the immobilized rats was consistent with previous reports (Carvalho & Langfeldt, 1977; Hudelmaier et al. 2001), which was probably due to cell death; however, the specific mechanism remains unclear.
Further, the volume of chondrocytic nuclei in rats from group I was significantly lower than that in rats from the other groups (C, E, and EI) (Fig. 7). There was no significant difference observed among groups C, E, and EI (P > 0.05), suggesting that exercise affected the nuclear volume of chondrocytes from non-immobilized animals (group E) but prevented the effects of immobilization on nuclear volume in immobilized animals (group EI). Several studies have shown a direct relationship between nuclear volume and metabolic activity of cells (Harris, 1967; Elias et al. 2006; Pedrazzoli & Benedito, 2009; Gomes et al. 2011). The decrease in nuclear volume of chondrocytes is a clear indication of the decreased metabolic activity of these cells (Karvonen et al. 1994; Hudelmaier et al. 2001) with repercussions on the formation of ECM components.
As chondrocytes regulate the metabolism of the ECM and maintain cartilage structure, a decrease in chondrocyte number and nuclear volume could be responsible for the decrease in the turnover and subsequent collagen Vv and thinning in the immobilized group. Immobilization likely leads to a decline in cellular activity in all articular cartilage layers, which can be associated with a decrease in growth factors (Karvonen et al. 1994; Roughley, 2001) and subsequent decreases in collagen and other ECM components. These results differed from those of Akeson et al. (1987), who affirmed that articular immobilization did not cause a decrease in collagen fibers.
Here, we demonstrated that the effects of joint immobilization on articular cartilage of the femur were attenuated by exercise, as no significant difference was observed among exercised and then immobilized animals and controls. In other words, exercise prevented the deleterious effects of immobilization on cartilage, which was in accordance with studies demonstrating that movement and weight bearing ensure the functionality of the cartilage and maintain its cellular properties and mechanical behavior (Gyarmati et al. 1987; O'Connor, 1997). Other studies have shown that the application of constant compressive loading maintained normal structure of articular cartilage (Karvonen et al. 1994; Hudelmaier et al. 2001; Roughley, 2001), as the intensity of mechanical stimuli modulates chondrocytic responses (Beaupré et al. 2000; Loeser, 2000; Trudel et al. 2005). However, the reduced effects on exercised animals by immobilization remain to be elucidated. Nonetheless, constant moderate physical activity increases the synthesis and concentration of proteoglycans and glycosaminoglycans (Lothe et al. 1979; Kiviranta et al. 1988; Kim et al. 1994; Buckwalter, 1995) and other components of the cartilage matrix (Trattnig, 1997; Matyas et al. 2002), which are chondroprotective and lead to improvements in the biomechanical and biological properties of articular cartilage (Poole et al. 2001). As a consequence, reducing mechanical stimulus, as occurs in joint immobilization, may have a less deleterious effect in more resistant cartilage.
In the present study, it was shown that exercise increased cartilage thickness, collagen fiber Vv, and nuclear volume of chondrocytes. These results were similar to those obtained by Palmoski & Brandt (1981), Kiviranta et al. (1988), Haupt et al. (1989), and Moriyama et al. (2012), but differed from those of O'Connor (1997), who found a decrease in cartilage thickness with exercise and from that of Carvalho & Langfeldt (1977), Kiviranta et al. (1984), and O'Connor (1997), who reported no change in cartilage thickness with exercise.
A positive response of articular cartilage to exercise was mainly associated with the intensity and frequency of compressive stimuli that induced an increase in chondrocytic activity, which is identified by increased nuclear volume and cartilage thickness, and leads to greater resistance to compression. It is possible that another consequence of exercise is ECM hydration, resulting in greater mechanical resistance and elasticity and a consequent increase in the number of collagen fibrils, which generates greater resistance to deformation and, hence, less rigidity. These factors could reduce the risk of cartilage breakdown when it is subjected to less mechanical demand, as in the case of joint immobilization.
Author contributions
Diogo Correa Maldonado conceived and designed the study, acquired and interpreted the data, and drafted the manuscript. Romeu Rodrigues de Souza conceived and designed the study and analyzed and interpreted the data for the final version of the manuscript. Semaan El-Razi Neto acquired and prepared the tissue samples (fixation, embedding, and sectioning). Marcelo Cavenaghi Pereira da Silva and Mônica Rodrigues de Souza conceived and designed the study.
Acknowledgments
The authors thank Dr. Anselmo Moriscot (São Paulo University, São Paulo, Brazil) for his generous time and attention, as his expertise was invaluable to this project.
References
- Akeson WH, Amiel D, Abel MF, et al. Effects of immobilization on joints. Clin Orthop Relat Res. 1987;219:28–37. [PubMed] [Google Scholar]
- Alford JW, Cole BJ. Cartilage restoration, part 1: basic science, historical perspective, patient evaluation, and treatment options. Am J Sports Med. 2005;33:295–306. doi: 10.1177/0363546504273510. [DOI] [PubMed] [Google Scholar]
- Ando A, Hagiwara Y, Chimoto E, et al. Intra-articular injection of hyaluronan diminishes loss of chondrocytes in a rat immobilized-knee model. Tohoku J Exp Med. 2008;215:321–331. doi: 10.1620/tjem.215.321. [DOI] [PubMed] [Google Scholar]
- Angel J, Razzano P, Grande D. Defining the challenge: the basic science of articular cartilage repair and response to injury. Sports Med Arthrosc Rev. 2003;11:168–198. [Google Scholar]
- Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect Tissue Res. 1988;18:205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- Beaupré GS, Stevens SS, Carter DR. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000;37:145–151. [PubMed] [Google Scholar]
- Buckwalter JA. Osteoarthritis and articular cartilage use, disuse, and abuse: experimental studies. J Rheumatol Suppl. 1995;22:13–15. [PubMed] [Google Scholar]
- Carvalho A, Langfeldt B. Running practice and arthrosis deformans. A radiological assessment. Ugeskr Laeger. 1977;139:2621–2622. [PubMed] [Google Scholar]
- Castano Oreja MT, Quintáns Rodriguez M, Crespo Abelleira A, et al. Variation in articular cartilage in rabbits between weeks six and eight. Anat Rec. 1995;241:34–38. doi: 10.1002/ar.1092410106. [DOI] [PubMed] [Google Scholar]
- Elias S, Dardes RCM, Kemp C, et al. A pilot study of the effects of hormone therapy on normal breast tissue of postmenopausal women. Rev Bras Ginecol Obstet. 2006;28:658–663. [Google Scholar]
- Gomes RC, Verna C, Simões RS, et al. Effects of metoclopramide on the mouse anterior pituitary during the estrous cycle. Clinics (Sao Paulo) 2011;66:1101–1104. doi: 10.1590/S1807-59322011000600031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Bagger P, Bendtsen TF, et al. The stereological tools: dissector, fractionator and point sampled intercepts and their use in pathological research. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gyarmati J, Földes I, Kern M, et al. Morphological studies on the articular cartilage of old rats. Acta Morphol Hung. 1987;35:111–124. [PubMed] [Google Scholar]
- Haapala J, Arokoski JP, Hyttinen MM, et al. Remobilization does not fully restore immobilization induced articular cartilage atrophy. Clin Orthop Relat Res. 1999;362:218–229. [PubMed] [Google Scholar]
- Hagiwara Y, Ando A, Chimoto E, et al. Changes of articular cartilage after immobilization in a rat knee contracture model. J Orthop Res. 2009;27:236–242. doi: 10.1002/jor.20724. [DOI] [PubMed] [Google Scholar]
- Harris H. The reactivation of the red cell nucleus. J Cell Sci. 1967;2:23–32. doi: 10.1242/jcs.2.1.23. [DOI] [PubMed] [Google Scholar]
- Haupt R, Pieper KS, Heilmann HH, et al. The influence of mechanical loading on the knee-joint. Animal-experimental examinations. Beitr Orthop Traumatol. 1989;36:185–193. [PubMed] [Google Scholar]
- Huber M, Trattnig S, Lintner F. Anatomy, biochemistry and physiology of articular cartilage. Invest Radiol. 2000;35:573–580. doi: 10.1097/00004424-200010000-00003. [DOI] [PubMed] [Google Scholar]
- Hudelmaier M, Glaser C, Hohe J, et al. Age-related changes in the morphology on deformational behavior for knee joint cartilage. Arthritis Rheum. 2001;44:2556–2561. doi: 10.1002/1529-0131(200111)44:11<2556::aid-art436>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Hudelmaier M, Glaser C, Hausschild A, et al. Effects of joint unloading and reloading on human cartilage morphology and function, muscle cross-sectional areas, and bone density – a quantitative case report. J Musculoskelet Neuronal Interact. 2006;6:284–290. [PubMed] [Google Scholar]
- Junqueira LC, Cossermelli W, Brentani R. Differential staining of collagens type I, II and III by Sirius red polarization microscopy. Arch Histol Jpn. 1978;41:267–274. doi: 10.1679/aohc1950.41.267. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani R. Picrosirius red staining plus polarization microscopy, a specific method for collagen detection in tissue section. Histochem J. 1979;11:447–455. doi: 10.1007/BF01002772. [DOI] [PubMed] [Google Scholar]
- Karvonen RL, Negendank WG, Teitge RA, et al. Factors affecting articular cartilage thickness in osteoarthritis and aging. J Rheumatol. 1994;21:1310–1318. [PubMed] [Google Scholar]
- Kiernan JA. Oxford: Butterworth-Heinemann; 1999. Histological and histochemical methods : theory and practice. [Google Scholar]
- Kim YJ, Sah RL, Grodzinsky AJ, et al. Mechanical regulation of cartilage on byosunthetic behavior: physical stimuli. Arch Biochem Biophys. 1994;311:1–12. doi: 10.1006/abbi.1994.1201. [DOI] [PubMed] [Google Scholar]
- Kiviranta I, Tammi M, Jurvelin J, et al. Fixation, descalcification, and tissue processing effects on articular cartilage proteoglycans. Histochemistry. 1984;80:569–573. [PubMed] [Google Scholar]
- Kiviranta I, Jurvelin J, Tammi M, et al. Weight bearing controls glycosaminoglycan concentration and articular cartilage thickness in the knee joints of young beagle dogs. Arthritis Rheum. 1987;30:801–809. doi: 10.1002/art.1780300710. [DOI] [PubMed] [Google Scholar]
- Kiviranta I, Tammi M, Jurvelin J, et al. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the joint of young beagle dogs. J Orthop Res. 1988;6:188–195. doi: 10.1002/jor.1100060205. [DOI] [PubMed] [Google Scholar]
- Liphardt AM, Mündermann A, Koo S, et al. Vibration training intervention to maintain cartilage thickness and serum concentrations of cartilage oligometric matrix protein (COMP) during immobilization. Osteoarthritis Cartilage. 2009;17:1598–1603. doi: 10.1016/j.joca.2009.07.007. [DOI] [PubMed] [Google Scholar]
- Loeser RF., Jr Aging and the etiopathogenesis and treatment of osteoarthritis. Rheum Dis Clin North Am. 2000;26:547–567. doi: 10.1016/s0889-857x(05)70156-3. [DOI] [PubMed] [Google Scholar]
- Lothe K, Spycher MA, Rüttner JR. Human articular cartilage in relation to age. A morphometric study. Exp Cell Biol. 1979;47:22–28. doi: 10.1159/000162919. [DOI] [PubMed] [Google Scholar]
- Matyas JR, Huang D, Chung M, et al. Regional quantification of cartilage type II collagen and aggrecan messenger RNA in joints with early experimental osteoarthritis. Arthritis Rheum. 2002;46:1536–1543. doi: 10.1002/art.10331. [DOI] [PubMed] [Google Scholar]
- Moriyama H, Kanemura N, Brouns I, et al. Effects of aging and exercise training on the histological and mechanical properties of articular structures in knee joints of male rat. Biogerontology. 2012;13:369–381. doi: 10.1007/s10522-012-9381-8. [DOI] [PubMed] [Google Scholar]
- O'Connor KM. Unweighting accelerates tidemark advancement in articular cartilage at the knee joint of rats. J Bone Miner Res. 1997;12:580–589. doi: 10.1359/jbmr.1997.12.4.580. [DOI] [PubMed] [Google Scholar]
- Oda JY, Liberti EA, Maifrino LB, et al. Variation in articular cartilage in rats between 3 and 32 months old. A histomorphometric and scanning electron microscopy study. Biogerontology. 2007;8:345–352. doi: 10.1007/s10522-006-9076-0. [DOI] [PubMed] [Google Scholar]
- Otterness IG, Eskra JD, Bliven ML, et al. Exercise protects against articular cartilage degeneration in the hamster. Arthritis Rheum. 1998;41:2068–2076. doi: 10.1002/1529-0131(199811)41:11<2068::AID-ART23>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Palmoski MJ, Brandt KD. Running inhibits the reversal of atrophic changes in canine knee cartilage after removal of a leg cast. Arthritis Rheum. 1981;24:1329–1337. doi: 10.1002/art.1780241101. [DOI] [PubMed] [Google Scholar]
- Pedrazzoli M, Benedito MAC. Rapid eye movement sleep deprivation induces a decrease in neuronal nuclear volume in the locus coeruleus, hippocampus and cingulated cortex of the rat. Sleep Sci. 2009;2:130–134. [Google Scholar]
- Poole AR, Kojima T, Yasuda T, et al. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001;(Suppl 391):S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- Roughley PJ. Age-associated changes in cartilage matrix: implications for tissue repair. Clin Orthop Relat Res. 2001;(Suppl 391):S153–S160. doi: 10.1097/00003086-200110001-00015. [DOI] [PubMed] [Google Scholar]
- Salvatore CA, Schreider G. Pesquisas cariométricas no ciclo estral gravídico. Mem Inst Butantan. 1947;20:39–78. [Google Scholar]
- Silva GJJ, Brum PC, Negrâo CE, et al. Acute and chronic effects of exercise on baroreflexes in spontaneously hypertensive rats. Hypertension. 1997;30(pt 2):714–726. doi: 10.1161/01.hyp.30.3.714. [DOI] [PubMed] [Google Scholar]
- Simpson SL. The physiology of growth. Postgrad Med J. 1950;26:417–424. doi: 10.1136/pgmj.26.298.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RL, Lin J, Trindade MC, et al. Time-dependent effects of intermittent hydrostatic pressure on articular chondrocyte type II collagen and aggrecan mRNA expression. J Rehabil Res Dev. 2000;37:153–161. [PubMed] [Google Scholar]
- Stockwell RA. The lipid and glycogen content of rabbit articular hyaline and non-articular hyaline cartilage. J Anat. 1967;102(pt 1):87–94. [PMC free article] [PubMed] [Google Scholar]
- Thorstensson CA, Roos EM, Petersson IF, et al. Six-week high-intensity exercise program for middle-aged patients with knee osteoarthritis: a randomized controlled trial [ISRCTN20244858] BMC Musculoskelet Disord. 2005;30:6–27. doi: 10.1186/1471-2474-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trattnig S. Overuse of hyaline cartilage and imaging. Eur J Radiol. 1997;25:188–198. doi: 10.1016/s0720-048x(97)01173-x. [DOI] [PubMed] [Google Scholar]
- Trudel G, Himori K, Uhthoff HK. Contrasting alterations of apposed and unapposed articular cartilage during joint contracture formation. Arch Phys Med Rehabil. 2005;86:90–97. doi: 10.1016/j.apmr.2004.01.029. [DOI] [PubMed] [Google Scholar]
- Whittaker P, Kloner RA, Boughner DR, et al. Quantitative assessment of myocardial collagen with picrosirius red staining and circularly polarized light. Basic Res Cardiol. 1994;89:397–410. doi: 10.1007/BF00788278. [DOI] [PubMed] [Google Scholar]
- Wu JZ, Herzog W. Elastic anisotropy of articular cartilage is associated with the microstructures of collagen fibers and chondrocytes. J Biomech. 2002;35:931–942. doi: 10.1016/s0021-9290(02)00050-7. [DOI] [PubMed] [Google Scholar]


