Abstract
The Syrian hamster Harderian gland (HG) has a marked sexual dimorphism and exhibits an extraordinary rate of porphyrinogenesis. The physiological oxidative stress, derived from constant porphyrin production, is so high that the HG needs additional survival autophagic mechanisms to fight against this chronic exposure, provoking the triggering of a holocrine secretion in female glands that forms two types of secretory masses: intra-tubular-syncytial and inter-tubular-syncytial masses. The aim of this work was to study the development of this inter-tubular holocrine secretion. To approach this task, we have considered that the steps developed during the formation of the so-called invasive masses consist of the growth of epithelial cells, cell detachment from the basal lamina and invasion of surrounding tissues. The presence of these masses, particularly in the female HG, are closely linked to sexual dimorphism in redox balance and to alterations in the expression of certain factors such as cytokeratins, P-cadherin, matrix metalloproteinases, cathepsin H, proliferating cell nuclear antigen, p53, CD-31 and vascular endothelial growth factor, which seem to be involved in tissue remodeling. The results document unusual mechanisms of secretion in Syrian hamster HG: an extraordinary system of massive secretion through the conjunctive tissue, disrupting the branched structure of the gland.
Keywords: cell detachment, extracellular matrix, Harderian gland, invasive secretion, oxidative stress
Introduction
The Syrian hamster Harderian gland (HG) is a large orbital lacrimal gland behind the eye, which at morphological and biochemical levels shows sexual differences. Morphologically, it is an exocrine gland composed of branching duct systems that combine and form unspecialized secretory ducts. The female HG presents epithelial cells with small lipid droplets (Type I cells), whereas male HG displays both small and large lipid droplets (Type I and Type II cells). In addition, the female HG contains huge amounts of porphyrins that are, under normal conditions, secreted into the acinar lumen to form porphyric accretions, whereas the male HG does not show these deposits. Both sexes have a porphyrin content that exceeds, by far, the levels observed in other porphyrinogenic tissues, such as the liver and kidney (Spike et al. 1986). In the liver, δ-aminolevulinic acid synthase (ALA-S), the first enzyme in the porphyrinogenic pathway, is negatively regulated by heme, the final product (Fujita, 1997). However, in the HG ALA-S is constitutively expressed and it is not under the influence of heme (Sassa & Nagai, 1996). Moreover, the activity of the enzyme ferrochelatase, which converts protoporphyrin IX into heme, is very low in the HG, so that the uncomplexed porphyrins can rise to unusually high levels (Vilchis et al. 2006).
Our group has analyzed, in recent years, the extraordinary conditions present in the Syrian hamster HG. Porphyrin metabolism modulates HG physiological responses with regard to antioxidant defense and protein damage (Tomas-Zapico et al. 2002a,b). Porphyrin formation and accumulation generate reactive oxygen species (ROS), which may have deleterious effects on gland integrity. Part of the porphyrin-derived deleterious ROS effects may be attributed to protoporphyrinyl cation radicals, superoxide anions (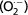 ) and singlet oxygen (1O2), all of which are generated by excited protoporphyrin IX. Additionally, hydroxyl radicals (·OH) derived from photoreactions of δ-aminolevulinic acid (ALA) and ·OH formed via Fenton-type reactions by copper-porphyrins found in the HG (Hardeland & Uria, 1995) contribute to the cellular destruction. Because of this, we have used HG as a physiological model to study oxidative stress (Coto-Montes et al. 2001a). The cumulative production of ROS induces oxidative stress, leading to a cellular redox imbalance, which is linked to altered redox regulation of cellular signaling pathways and is common in many cancer cells (Valko et al. 2006).
) and singlet oxygen (1O2), all of which are generated by excited protoporphyrin IX. Additionally, hydroxyl radicals (·OH) derived from photoreactions of δ-aminolevulinic acid (ALA) and ·OH formed via Fenton-type reactions by copper-porphyrins found in the HG (Hardeland & Uria, 1995) contribute to the cellular destruction. Because of this, we have used HG as a physiological model to study oxidative stress (Coto-Montes et al. 2001a). The cumulative production of ROS induces oxidative stress, leading to a cellular redox imbalance, which is linked to altered redox regulation of cellular signaling pathways and is common in many cancer cells (Valko et al. 2006).
In the liver, a porphyrinogenic organ par excellence, a similar situation of huge porphyrin production may cause dramatic effects, such as erythropoietic protoporphyria (Libbrecht et al. 2003) and porphyria cutanea tarda in the skin (Badminton & Elder, 2002). The porphyrin precursor, ALA, is considered a carcinogen and has been implicated in the development of primary carcinoma in symptomatic acute intermittent porphyria patients (Karbownik & Reiter, 2002).
Remarkably, the HG survives in a state of ‘permanent porphyria’, although at the expense of some unavoidable cellular alterations that are morphologically evident. Extensive cell damage has been described by several authors who observed a large number of degenerative signs in the gland (Antolin et al. 1994; Tolivia et al. 1996). In previous studies, we have established that autophagic processes in the Syrian hamster HG are an early result of an elevated porphyrin metabolism; this is observed in both sexes (Tomas-Zapico et al. 2005; Coto-Montes & Tomas-Zapico, 2006). However, the female Syrian hamster HG, which presents intraluminal porphyrins, frequently shows morphological features which culminate in cell-detachment-derived cell death, leading to massive glandular secretion (Vega-Naredo et al. 2009). This process plays a major role in the secretory activity of the gland, leading to two types of massive holocrine secretion: through the lumen (intra-tubular secretion) or through the conjunctive tissue (inter-tubular or invasive secretion). These secretory processes have no apparent effects on the rest of the organ and are controlled by oxidative stress sensed by redox-sensitive transcription factors (Vega-Naredo et al. 2012).
The mechanisms of the formation and development of inter-tubular secretions are not yet established. The aim of the present study was to analyze the process observed in the HG and, as it starts in epithelium and penetrates the conjunctive tissue, to approach this analysis, we have monitored the steps during the process of invasion and tissue remodeling to explain the concordance between the two secretory types.
Materials and methods
Animals
Twenty-four 1-month-old male and female Syrian hamsters (Mesocricetus auratus) (Harlan Interfauna Ibérica, Barcelona, Spain) were divided four per cage and housed in a 14 : 10 h light/dark cycle and temperature-controlled (22 ± 2 ºC) environment. Animals received tap water and a standard pelleted diet ad libitum.
The Oviedo University Local Animal Care and Use Committee approved the experimental protocol. All experiments were carried out according to the Spanish Government Guide and the European Community Guide for Animal Care (Council Directive 86/609/EEC).
After 1 month in the animal house, hamsters were sacrificed and HGs and livers were immediately removed, frozen in liquid nitrogen, and stored at −80 ºC until the experiments were performed.
If not otherwise indicated, HGs (0.1 g) and livers were homogenized with a Polytron homogenizer in 1 mL ice-cold lysis buffer (50 mm Tris/HCl, pH 7.4, 150 mm NaCl) with protease inhibitors (1 mm Na3VO4, 1 mm PMSF, 1 μg mL−1 aprotinin). The tissue homogenates were then centrifuged for 6 min at 3000 g at 4 °C, and supernatants were collected and centrifuged again using the same conditions. To assess p53 expression, nuclear extracts were prepared using the sucrose gradient method (Coto-Montes et al. 2003). The amount of protein in the supernatants was measured by the Bradford method (Bradford, 1976).
Lipid peroxidation
Lipid peroxidation was measured by determining malondialdehyde (MDA) and 4-hydroxy-2(E)-nonenal (4-HNE) using a lipid peroxidation assay kit from Calbiochem (No. 437634) (EMD Biosciences, San Diego, CA, USA). Results were expressed as nmol MDA+4-HNE/mg protein.
Cathepsin H activity
The cysteine-proteinase cathepsin H (EC 3.4.22.16) was assayed fluorimetrically (CytofluorTM 2350; Millipore, Eschborn, Germany) according to Barrett (Barrett, 1980) with minor modifications (Schreurs et al. 1995), using Z-Phe-Arg-MCA (Sigma, Saint Quentin, France) as substrate. The cathepsin results were expressed as enzymatic milliunits per mg protein.
Immunoblotting
Seventy-five μg of protein were separated by 12% SDS-PAGE at 100 V and transferred to PVDF membrane at 350 mA. The membranes were blocked for 1 h at room temperature in 5% skimmed milk in phosphate-buffered saline (PBS) containing 0.05% Tween-20 (PBS-T) and then probed for 1 h at room temperature with antibodies against cytokeratins (C9687; Sigma), P-cadherin (sc-1501; Santa Cruz Biotechnology, Santa Cruz, CA, USA), cathepsin H (sc- 6496; Santa Cruz Biotechnology), PCNA (sc-56; Santa Cruz Biotechnology) and p53 (sc-6243; Santa Cruz Biotechnology), previously diluted 1 : 1000 in blocking buffer. After washing in PBS-T, the membranes were incubated with the corresponding horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. Development was carried out using the Western Blotting Luminol Reagent (sc-2048; Santa Cruz Biotechnology) according to the manufacturer's protocol. Ponceau S staining was used for loading the control due to the cytoskeletal disorganization found in female Syrian hamster HG (Vega-Naredo et al. 2009). The images were imported and incorporated into the electronic figure using corel draw x13 and/or Microsoft powerpoint, and are representative of at least three separate experiments.
Gelatin zymography
Gelatinase activity was assayed by zymography analysis as described by Xu et al. (2001). Briefly, 100 μg of protein was separated by SDS-PAGE on a 10% gel containing 1 mg mL−1 gelatin. The gel was washed twice in 2.5% Triton X-100 solution and incubated overnight at 37 °C in developing buffer (50 mm Tris-HCl pH 7.4, 10 mm CaCl2), stained with 0.5% Coomassie Blue and then destained in a 40% methanol/10% acetic acid solution.
Morphological studies
HG for structural and ultrastructural studies were pre-fixed by immersion in a solution containing 1.5% glutaraldehyde and 2.5% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). The fixation was prolonged overnight in fresh fixative at 4 °C. The tissues were postfixed in 1% OsO4 for 2 h. After dehydration in graded acetone, the pieces were embedded in Taab 812. Semithin sections (1 μm) were stained with toluidine blue, studied, and photographed using an Olympus CAST2 microscope (Olympus, Denmark).
Immunohistochemistry
Tissues for immunohistochemical analysis were fixed and embedded in paraffin using standard methods. The sections were rinsed three times for 10 min in 0.01 M PBS with 0.1% Triton X-100 and 0.25% bovine serum albumin (PBS-TB) and blocked in 30% serum in PBS-TB for 30 min. This was followed by incubation with antibodies against P-cadherin (sc-1501; Santa Cruz Biotechnology), PCNA (sc-56; Santa Cruz Biotechnology), VEGF165 (Neomarkers, Fremont, CA, USA) or CD31/PECAM-1 (RB-10333; LabVision, Fremont, CA, USA) for 24 h at 4 °C in a humidity chamber. After washing in PBS-TB, the sections were incubated with a horseradish peroxidase-conjugated secondary antibody and thereafter incubated in peroxidase anti-peroxidase complexes (Sigma), for 1 h at room temperature. Finally, the sections were washed and developed using 0.05% 3,3′-diaminobenzidine/HCl with 0.005% H2O2 in Tris/HCl. The stained sections were studied using a light microscope (CAST2 System, Olympus, Denmark). The images were imported and incorporated into the electronic figure using corel draw x13 and/or Microsoft powerpoint 2007.
Statistical analysis
Data were presented as mean values ± SD calculated from at least three separate experiments, each performed in triplicate. The normality of the data was analyzed by the Kolmogorov–Smirnov test. Statistical comparisons between sexes were carried out using Student's t test. The Mann–Whitney non-parametric test was applied in non-normal data. The level of significance accepted was P < 0.05.
Results
Oxidative damage
The comparison of the content in lipid peroxidation products (nmol 4-HNE+MDA per mg protein) reveals higher lipid oxidative damage in female glands (12.02 ± 1.26 in males vs. 17.56 ± 0.69 in females, P < 0.01).
Morphological analysis of tubulo-alveoli: intra-tubular masses
Figure 1 shows the glandular epithelial cell growth. Micrographs of Harderian gland from female hamster show protuberances from acinar cells towards the acinar lumen (Fig. 1A). Some areas of the female Harderian gland display cellular debris in the acinar lumen with nuclei inside and, simultaneously, acinar cells seem to grow and occupy all the luminal space. In addition, some cells protrude from the basal pole towards the connective tissue, forming wads (Fig. 1B).
Fig. 1.
Morphological analysis of tubulo-alveoli. (A) Micrograph of Harderian gland from female hamster showing protuberances from acinar cells towards acinar lumen (arrows). Scale bar: 50 μm. (B) Toluidine blue-stained semithin section of a Harderian gland from female hamster showing cellular content in the acinar lumen with nuclei inside (arrowheads). Acinar cells begin to grow and occupy all the luminal space (asterisks) with porphyrin deposits (p). Some cells protrude from the basal pole towards the connective tissue, forming wads (arrows). Scale bar: 100 μm. (C) Harderian gland from female hamster showing the initiation of acini coalescence; eventually the acini lose their tubulo-alveolar morphology (stars). Scale bar: 100 μm. (D) Image of female Harderian gland showing how epithelial cells lose their individuality; nuclei (arrowheads) in the cytoplasmic masses are clearly delimited by basal membrane. Note myoepithelial cells surrounding the acinus (arrow). Scale bar: 100 μm.
Figure 1C shows the initiation of acini coalescence and how these acini eventually lose their tubulo-alveolar morphology. Even some images of female Harderian gland shows epithelial cells losing their individuality and forming syncytial masses clearly delimited by basal membranes (Fig. 1D).
Epithelial plasticity: cytokeratins and P-cadherin expression
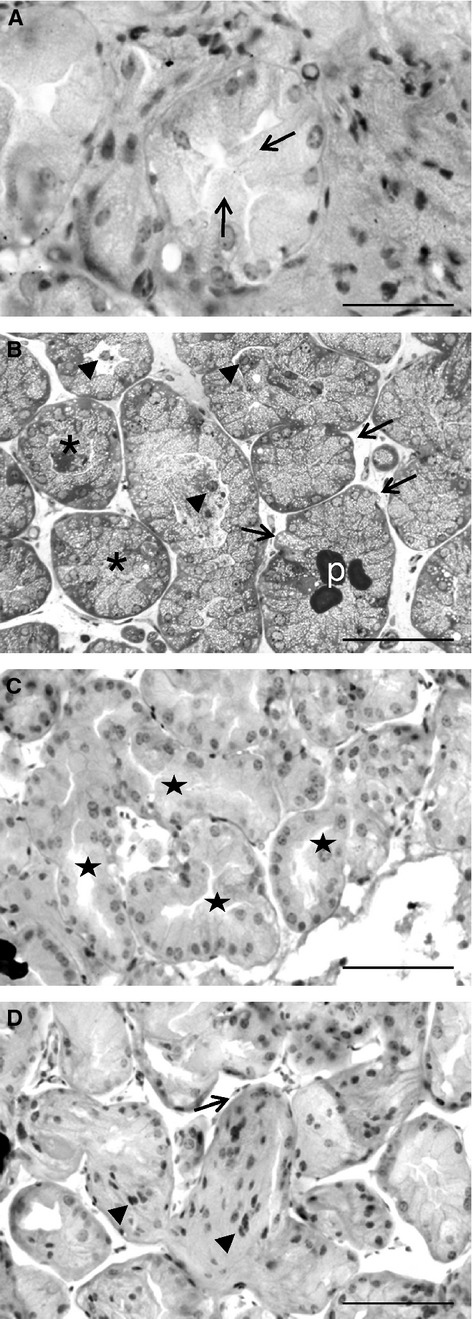
Figure 2A shows gender differences in cytokeratins and P-cadherin expression revealing changes in epithelial plasticity. The immunoblot analysis of cytokeratins from total HGs total extracts identified three bands corresponding to cytokeratins 5/6, 8/18 and 19 (Fig. 2A), indicating a loss of cytokeratins 8/18 and 19 in females compared with males. Cytokeratins 8/18, together with cytokeratin 19, are considered the most abundant cytokeratins in simple epithelia. Cytokeratin 6 is a ‘hyperproliferation’ cytokeratin expressed in tissues with a high natural or pathological turnover and was observed by us in both male and female HG. In addition, HGs presented an additional band of about 33 kDa, below the molecular weight expected for cytokeratin, showing higher amounts of this protein in male than in female hamsters. This additional band was also observed previously by us in the Flank organ (Coto-Montes et al. 2009) and by Bursch et al. (2000) in MCF-7 cells.
Fig. 2.
Epithelial plasticity. (A) Immunoblot analysis of cytokeratins and P-cadherin from total extracts of male (M) and female (F) Harderian glands. The anti-pan cytokeratin antibody shows a loss of high molecular weight cytokeratins in females. Both sexes presented a band at 33 kDa, below the molecular weight expected for cytokeratin. Immunoblot analysis of P-cadherin displays higher expression levels in males and the presence of a 50-kDa truncated form. Ponceau S staining was used for loading control and blot bands are representative of at least three separate experiments using the pair of glands from at least three different animals per gender. (B) Photographs of Harderian gland from male (♂) and female (♀) hamsters showing a gradual expression of P-cadherin from the apical pole (+) of the acinar cells to the basal zone (−). Note the presence of immunostaining around vesicles, especially apparent in Type II cells (asterisks). Scale bar: 50 μm.
Western blot analysis of P-cadherin displays higher expression levels in males and the presence of a 50-kDa truncated form (Fig. 2A). As shown in Fig. 2B, immunohistochemistry analysis of P-cadherin shows a gradual expression of P-cadherin from the apical zone of the acinar cells to the basal pole. The presence of immunostaining around vesicles is especially apparent in Type II cells.
Cell proliferation: PCNA and p53 expression
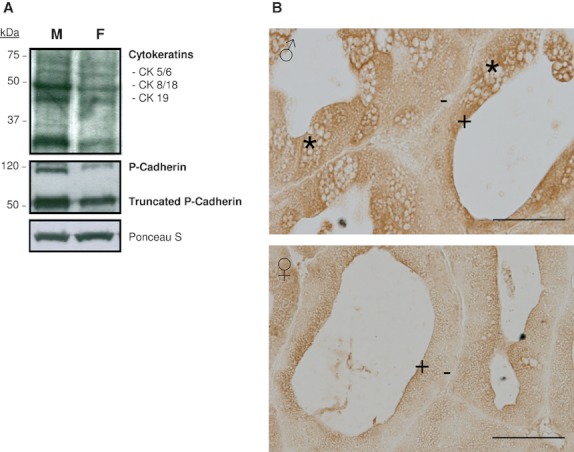
Figure 3(A,B) presents the immunohistochemical and immunoblot analyses for proliferating cell nuclear antigen (PCNA), which confirm high nuclear expression in Syrian hamster HG, especially in female glands (Fig. 3A,B). Furthermore, Western blotting was performed to detect the presence of p53 in Syrian hamster HG in nuclear extracts. The analysis of the bands obtained shows a higher expression in female than in male HGs (Fig. 3B).
Fig. 3.
Cell proliferation and adjacent tissue invasion. (A) Immunohistochemical demonstration of PCNA in nuclei of epithelial cells (arrows) from male (♂) and female (♀) Syrian hamster Harderian gland. Note also immunopositive nucleus in the acinar lumen (arrowhead) and the protuberances in the acinar cells (asterisk). Scale bar: 100 μm. (B) Western blot analysis for the detection of PCNA and nuclear p53 in Harderian gland extracts from male (M) and female (F) Syrian hamsters. Ponceau S staining was used for loading control and blot bands are representative of at least three separate experiments using the pair of glands from at least three different animals per gender. (C) Stained semithin section of Harderian gland from female hamster showing a syncytial mass (SM) with evaginations into the connective tissue (asterisk). Inter-tubular material with frayed appearance are observed near these evaginations (stars). Note the presence of two types of nuclei: heterochromatic nuclei present in some acinar cells and in the inter-tubular material (black arrowheads), and euchromatic nuclei in the intra-tubular mass (white arrowheads). Scale bar: 100 μm. Note the details of an evagination releasing into the connective tissue from the syncytial mass where it is possible to observe the irregular contour and the frayed material. Scale bar: 20 μm.
Morphological analysis of inter-tubular masses
Figure 3C shows evidence of adjacent tissue invasion: our morphological results from female HGs show syncytial masses with a frayed appearance with evaginations protruding into the connective tissue. The ultrastructural appearance of a baso-lateral region in normal glandular cells shows a defined and compact aspect. However, the zone with evaginations that releases their glandular material into the connective tissue shows a breakage of the basal layer (Fig. 4A). This syncytial material forms interstitial masses filling all available space between tubulo-alveoli and along the inter-tubular strands of connective tissue (Fig. 4B). Some tubules reached by this invasive material seem to lose their morphology and fuse with it (Fig. 4C).
Fig. 4.
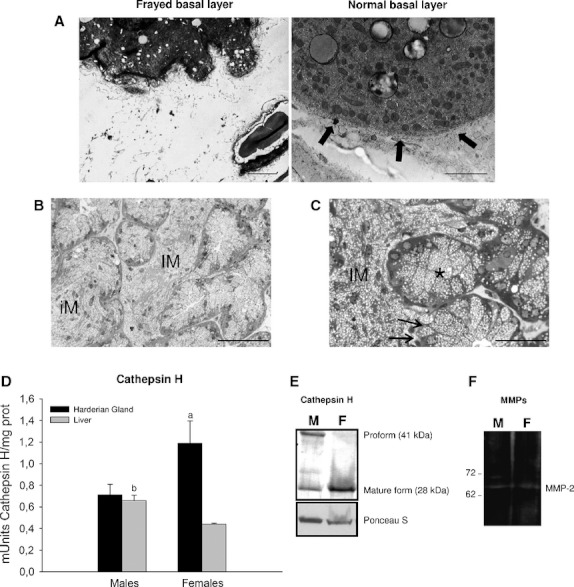
Matrix remodeling and inter-tubular masses. (A) Ultrastructural appearance of a normal baso-lateral region from the Harderian gland, showing a defined and compact aspect, compared with a zone with evaginations that release their glandular material into the connective tissue, showing breakage of the basal layer. Scale bar: 1 μm (B) Toluidine blue-stained semithin sections of Harderian gland from female hamster showing an interstitial mass (IM) filling all available space between the tubulo-alveoli next to an intra-tubular mass (iM) where it is still possible to distinguish the acinar border. Scale bar: 100 μm. (C) Some tubules reached by this invasive material (IM) fuse with it (arrows) and others with secretory cells coexist with the mass, which maintains its individuality (asterisk). Scale bar: 50 μm. (D) Cathepsin H activity determined using Z-Phe-Arg-MCA as substrate, in liver  and Harderian gland (HG)
and Harderian gland (HG)  from male and female Syrian hamster. Values are means ± SD. (a) P < 0.05, HG female vs. (HG male, Liver female); (b) P < 0.05, Liver male vs. Liver female. (E) Immunoblot analysis of cathepsins H in male (M) and female (F) Harderian gland showed different expression between sexes, with females showing higher levels of mature cathepsin H (28 kDa). Ponceau S staining was used for loading control, and blot bands are representative of at least three separate experiments using the pair of glands from at least three different animals per gender. (F) Gelatin zymography of Harderian gland homogenates from male (M) and female (F) hamster showing MMP-2 activity, especially in males who also possess proMMP-2.
from male and female Syrian hamster. Values are means ± SD. (a) P < 0.05, HG female vs. (HG male, Liver female); (b) P < 0.05, Liver male vs. Liver female. (E) Immunoblot analysis of cathepsins H in male (M) and female (F) Harderian gland showed different expression between sexes, with females showing higher levels of mature cathepsin H (28 kDa). Ponceau S staining was used for loading control, and blot bands are representative of at least three separate experiments using the pair of glands from at least three different animals per gender. (F) Gelatin zymography of Harderian gland homogenates from male (M) and female (F) hamster showing MMP-2 activity, especially in males who also possess proMMP-2.
Matrix remodeling: cathepsin H and collagenase activities
In Fig. 4D, we compare cathepsin H activity from Syrian hamster HG and liver. The results show that this activity is higher in the HG, particularly in female glands (Fig. 4D). Immunoblot analysis of cathepsins H in male and female HG demonstrate different expression patterns between sexes, with females showing higher levels of mature 28-kDa cathepsin H (Fig. 4E).
Analysis of type IV collagenase activity (MMP-2 and MMP-9) by gel zymography revealed the presence of active gelatinases in the HG. We observed MMP-2 but not MMP-9, in male and female glands with a similar overall activity (Fig. 4F).
Interaction of secretory material and the vascular system
Figure 5A shows vascular endothelial growth factor (VEGF) immunoreactivity in female HGs, mainly localized in the basal area of the epithelial cells next to the connective tissue and the vessels. In addition, morphological studies with toluidine blue-stained semithin sections revealed thin-walled blood vessels reached by the interstitial material and even vessels almost totally filled with cellular debris (Fig. 5B). Interestingly, immunohistochemical demonstration of CD31 (platelet/endothelial cell adhesion molecule-1, PECAM-1) was observed only in female Harderian gland and was restricted to the invasive masses (Fig. 5C).
Fig. 5.
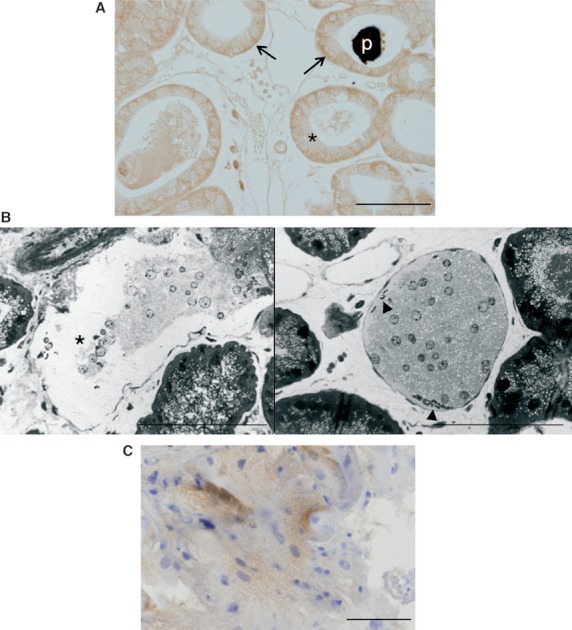
Interaction between secretory material and vascular system. (A) Harderian gland from female hamster showing VEGF immunoreactivity, mainly localized in the basal area of the epithelial cells (arrows) next to vessels. A slight reaction in apical areas was observed (asterisks). Porphyrin deposit in the lumen (p). Scale bar: 100 μm. (B) Toluidine blue-stained semithin sections showing thin-walled blood vessels (asterisk) reached by the interstitial material and vessels almost completely filled with cellular debris. Arrowhead indicates erythrocytes. Scale bar: 100 μm. (C) Immunohistochemical demonstration of CD31 in female Harderian gland in an inter-tubular mass. No positive reaction was observed in males. Scale bars: 50 μm.
Discussion
The main factor that contributes to the HG physiology and morphology is their chronic exposure to high oxidative stress levels, which determines the development of mechanisms that alter the normal structure, leading to a tubulo-alveolar disorganization (see Payne et al. 1985; Spike et al. 1988). In those articles, the clumps of interstitial cells were considered to be foreign-body giant cells. However, the existence of invasive inter-tubular masses was described in 1996. The masses are composed of cells and cellular debris from the walls of the tubules (Tolivia et al. 1996). A decade later, this phenomenon was studied again and linked to a survival system for removing cells damaged by oxidative stress (Tomas-Zapico et al. 2005). More recently, we have shown that the Syrian hamster HG, especially in the females, exhibits cell death processes with features intermediate between apoptosis and autophagy: autophagic morphology, microtubule-associated protein 1 light chain 3 (LC3) lipidation, absence of DNA fragmentation and caspase-3 independence (Vega-Naredo et al. 2009). The detachment of the dead cells plays a central role in the secretory activity of the gland, which can be modified by changes in oxidative stress and by treatment with melatonin, an antioxidant (Vega-Naredo et al. 2012). The HG secretion consists of cytoplasmic lipid droplets with nuclei that are generally heterochromatic, pyknotic and have an irregular profile. This material seems to flow and expands to completely fill the tubules (intra-tubular secretion). On occasion, these masses seem to penetrate the tube walls, forming the inter-tubular secretion that occupies the available space between tubulo-alveoli.
We have hypothesized that the secretion processes are a consequence of a chronic exposure to high oxidative stress levels, and are responsible for the main changes which characterize non-neoplastic tissue remodeling. In fact, far from being a pathological response, these masses account for the abundant release of glandular secretion, especially in female HG. We consider that the invasive secretion observed mainly in female HG is generated according to the steps outlined below.
Moderate oxidative stress environment
The porphyrinogenic activity of the Syrian hamster HG indicates that the level of oxidative stress and damage is greater in females than in males, as revealed by the higher lipid peroxidation activity found in females. There is a link between increased levels of ROS and disturbed activities of enzymatic antioxidants. Data obtained before (Vega-Naredo et al. 2009) showed highly elevated superoxide dismutase and catalase activities in female HG but lipid peroxidation remained high. We hypothesized that an imbalance between 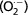 formation and H2O2 degradation, occurring in cells that overexpress superoxide dismutase, might activate cell death and differentiation. In fact, oxidation in the cellular environment increases progressively during organ remodeling (Menon & Rozman, 2007; Piantoni et al. 2010), cell growth and proliferation (Dragin et al. 2006).
formation and H2O2 degradation, occurring in cells that overexpress superoxide dismutase, might activate cell death and differentiation. In fact, oxidation in the cellular environment increases progressively during organ remodeling (Menon & Rozman, 2007; Piantoni et al. 2010), cell growth and proliferation (Dragin et al. 2006).
Cell growth in epithelium
In the Syrian hamster HG, at the microscopic level, a large number of unusual features are apparent. These signs exhibit a temporal pattern, which starts with protuberances from acinar cells towards the lumen. These protuberances grow until they are discharged into the lumen. Thus, some tubules in the gland appear filled with a mass of cellular debris. Thereafter, tubulo-alveoli begin to coalesce until they lose their normal morphology. Additionally, the glandular cells gradually lose their individuality, becoming a mass of cytoplasm containing several nuclei.
To develop this morphological transformation, changes in the epithelial features and in the interactions between the acinar epithelial cells and the components of the basal lamina are necessary. Cytokeratin expression has been closely linked to the differentiation state of epithelial cells. Alterations in cytokeratin expression have been reported during cell transformation (Franzen et al. 1996). In the case of HG, it is not possible to compare our results with a control condition since our animals are in an activated physiological state, but significant changes in cytokeratin expression between males and females were observed. Likewise, the loss of high molecular weight cytokeratins CK 8/18 and CK 19 is unusual in glandular cells and could reflect decreased differentiation of female HG epithelial cells, which lose some characteristics of simple epithelia.
It also seems likely that the acinar disorganization may involve a change in the interactions between the acinar epithelial cells and the components of the basal lamina. To investigate this, we analyzed a member of the cadherin family. Cadherins are transmembranal calcium-dependent proteins with important roles in cell adhesion. Placental, or P-, cadherin is a classical cadherin whose expression is restricted to basal or lower layers of the epithelia; this suggests that in addition to maintaining cellular adhesion, P-cadherin may have another function in differentiation and cell growth (Shimoyama et al. 1989). Reduction in expression has been associated with poor survival and a number of recent publications have noted striking alterations in the expression of P-cadherin in a range of disease conditions, where aberrant P-cadherin expression involves neoplastic transformation, cell migration and proliferation (Bauer et al. 2006). P-cadherin could be one of the proteins responsible for the loss of cell polarity, as it mediates weaker and unstable cell–cell contacts that can be easily broken and reconstituted; thus, the cells are unable to maintain the differentiated epithelial structure (Pyo et al. 2007).
In the current study, P-cadherin expression was higher in male HGs than in female HGs, which suggests that, in the former, there is some change in the cell–cell interaction with regard to the latter. These changes precede a series of alterations of cell structure that eventually leads to cell detachment. However, more important than these differential expressions was the finding of a 50-kDa band with a high level of expression in both sexes; this has been identified as a truncated form of P-cadherin. Bauer et al. (2005) found in malignant melanoma, a 50-kDa form of P-cadherin secreted by the cells was associated with a mobile phenotype, playing a role in migration. Thus, P-cadherin may have an additional role in Syrian hamster HG, acting as a secretory product. Accordingly, our immunohistochemical results for P-cadherin displayed a defined transmembrane staining around vesicles, especially in male Type II cells, and a gradual loss of expression towards basal layers. In addition, we can associate the reduction in P-cadherin expression in female glands with the lack of Type II cells found in this gender, further supporting its participation in the secretory activity of the gland.
Cell proliferation
We also studied the proliferative processes within the gland. PCNA is a nuclear protein essential for cell replication and is a useful marker for cell proliferation (Tachibana et al. 2005). The higher expression of PCNA in the female HG is correlated to the levels of oxidative stress observed in this sex (Coto-Montes et al. 2001b; Tomas-Zapico et al. 2005). It is possible that in the Syrian hamster HG, due to its high oxidative stress levels, proliferation is detrimental to gland integrity, as was seen at the morphological level. However, cells possess mechanisms to counteract the possible deleterious effects of such a phenomenon. In addition to proliferation, PCNA also exhibits anti-apoptotic functions and promotes tumor cell growth (Vairapandi et al. 2000).
p53 is considered a ‘guardian of the genome’, as it prevents tumor formation. This protein plays important roles in cell cycle control and apoptosis. In normal cells, p53 levels are low. However, DNA damage and other stress signals increase its expression within the cell. Herein, we analyzed p53 levels to determine whether this protein is elevated to counteract the high proliferative levels and thus the risk of mutations in the gland. In fact, the higher levels of p53 observed by us in female HG may activate genes that prevent cell growth.
In addition to the p53 observations, we previously determined caspase-3 activity within the gland, with negative results (Tomas-Zapico et al. 2005). Hence, Syrian hamster HG exhibits a high cell proliferation index, especially in females, which exhibit elevated levels of oxidative stress and, more frequently, inter-tubular secretion, by inhibiting apoptosis, as revealed by the upregulation of PCNA and undetectable caspase-3 activity. The situation in the glands requires the elimination of huge amounts of secretory material and the presence of glandular tubules filled with a mass of cellular debris is evidence of such a process. Our group has described, in Syrian hamster HG, how autophagic-related cell death processes play a role in the holocrine secretion which is controlled by redox status through p53 and NF-κB cross-regulation (Vega-Naredo et al. 2012). Thus, as a side effect, p53 in female HG would counteract tumor formation.
Adjacent tissue invasion
The described masses, consisting of cellular debris, are also frequently observed protruding towards the connective tissue, which forms wads of several sizes. These wads have a frayed appearance, which differs markedly from the well-organized appearance of the baso-lateral region of these glandular cells. These evaginations gradually grow and eventually protrude into the connective tissue, producing the invasive material. The wads that enter into the connective tissue seem to be syncytia, as it was not possible to distinguish individual cells and only cytoplasm remains were seen together with nuclei. This material seems to move along the inter-tubular strands of connective tissue, expanding and progressively filling the available space between the tubulo-alveoli. Some tubules reached by these invasive wads lose their morphology and fuse with it, increasing the volume of the extruded material.
The loss of epithelial features and the acquisition of a migratory phenotype, called the epithelial to mesenchymal transition, is considered a crucial event in tissue remodeling (Gregory et al. 2008). The invasion of subjacent tissue therefore requires another step, i.e. the breakdown of the extracellular matrix components. This step is crucial for the spread wads and requires proteases to degrade the extracellular matrix. Among these, lysosome proteases and matrix metalloproteinases stand out.
We have previously studied the activity of the cysteine cathepsin B and the aspartate cathepsin D in the Syrian hamster HG (Vega-Naredo et al. 2009). In the present study, we compared cathepsin H activity from HG and liver and showed that this activity is much higher in the HG. Data obtained from Western blots revealed that the female HG display greater cathepsin H processing than male glands, giving rise to the abnormally high levels of activity found in this gender. Cathepsin H has been used as a cancer prognostic marker as it plays an important role in tumorigenesis (Gocheva & Joyce, 2007; Gocheva et al. 2010), where it participates in extracellular matrix degradation.
Matrix metalloproteinases (MMPs) form a family of proteolytic zinc-containing enzymes that are implicated in the breakdown of the extracellular matrix, both in pathological and physiological conditions, changing cell–cell and cell–extracellular matrix interactions (Stallings-Mann & Radisky, 2007). These proteinases are widely expressed during tissue remodeling, as mediators of change and physical adaptation in tissues, whether developmentally regulated, environmentally induced or disease-associated (Page-McCaw et al. 2007). A subgroup within the MMPs is the gelatinase subfamily (MMP-2 and MMP-9), whose presence in actively remodeling tissue is indicative of basement membrane and extracellular matrix degradation (Weng et al. 2008). Analysis of MMP-2 and MMP-9 revealed the presence of active gelatinases in the HG, more specifically MMP-2. The outcome was unexpected since we anticipated more active MMPs in the female HG as invasive masses are more frequent in the gland of this sex. In any case, the presence of active MMPs in a normal organ is unusual and corroborates the special conditions encountered in this gland (Tomas-Zapico et al. 2005).
Collectively, our results identify the migratory phenotype of the epithelial cells that eventually form the inter-tubular wads.
Endocrine secretion
Although the majority of rodent HG-related functions are exocrine in nature (lubrication of the cornea, the secretion of pheromones, etc.) the blood supply of Syrian hamster HG is similar to that found in endocrine glands (Menendez--Pelaez et al. 1990). Also, it is known that MMPs are major contributors to angiogenesis (Sounni et al. 2011). To test whether HG cells secrete modulators that initiate an angiogenic process which enhances the characteristic endocrine blood supply of the gland, we chose to investigate the presence of VEGF by immunohistochemistry. VEGF is a potent mitogen for endothelial cells and it is critical for angiogenesis (Helotera & Alitalo, 2007). Its expression is usually restricted to endocrine glands, particularly, steroid-secreting cells (Raica et al. 2010). Accordingly, VEGF-positive cells were mainly found in the basal acinar area, next to the connective tissue, probably related to its paracrine role in the formation and maintenance of an endocrine vascular irrigation in Syrian hamster HG.
At this point, images of secretory material entering blood vessels are of particular interest. In the HG, particularly in female HGs, it is not unusual to observe inter-tubular secretions protruding into and surrounding blood vessels, which presumably exert an increasing pressure on the endothelial wall. Finally, these intravascular formations appear on occasion to almost completely fill the blood vessels. However, this process requires a relaxation of the endothelial cell junctions. Therefore, we tested for some proteins implicated in this phenomenon. Related to this, and to the obtaining of an endothelial phenotype, CD31 expression is considered a good marker. CD31 is a single chain type-1 transmembrane protein that plays a role in adhesive interactions between adjacent endothelial cells and it is its relaxation during infection which provokes leukocyte extravasation through endothelial cell junctions (Parums et al. 1990). Our results showed CD31 immunoreactivity in invasive masses from female HGs but not male HGs. The apparently random immunoreactivity found may be related to an attempt to transdifferentiate, rather than a specifically committed functional acquisition.
Our results show the interaction between HG and thyroid and pituitary glands. Earlier reports (Menendez-Pelaez et al. 1990; Tolivia et al. 1991) suggest a possible endocrine function for the HG. Thus, the Syrian hamster HG may be a mixed gland with exocrine and endocrine secretions.
Concluding remarks
The HG is located within the orbit adjacent to the third eyelid. Due to its retro-orbital localization, porphyrins are accessible to light and are therefore subjected to photoreactions. Indeed, a role for HG has been predicted in photoreception, with the HG acting as an extra-retinal photoreceptor (Wetterberg et al. 1970). Thus, changes in photoperiod cause critical variations in the production of porphyrins and in the secretory cell types (Coto-Montes et al. 1994, 2001c; Tomas-Zapico et al. 2003).
On the other hand, it is known that the porphyrinogenic activity of the HG fluctuates throughout the estrous cycle, pregnancy and lactation, suggesting that there is a link between HG activity and reproductive function (Vilchis et al. 2006). Consequently, the HG may be involved in a light-pineal-gonadal axis. Hence, we suggest that porphyrins may play an active role in the modulation of HG secretions by activating the autophagic cell death through a redox-mediated action in response to seasonal light variations.
The inter-tubular debris frequently observed in female HG was not present throughout all glands. Areas without these masses may be prone to develop future masses. These changes seem to follow a sequence, beginning with a degeneration of the tubule walls, which become very thin, and finishing with the formation of large protruding masses. The presence of glandular tubules filled with debris and empty tubules with thin walls is evidence that the HG requires a process of cell elimination and that this is carried out in cycles.
The hallmark of normal glands is the presence of a patent lumen, but in Syrian hamster HG, some secretory cells grow towards the lumen until being discharged. Thereby, some tubules appear filled with a mass of cellular debris (intra-tubular masses), leading to their collapse. Thereafter, the alterations in cell structure, the disruption of cell–cell anchorage, matrix remodeling and the absence of a morphologically specialized duct system with the gland, lead to the break-up of the collapsed tubules and the detachment of released material towards the connective tissue (inter-tubular masses).
We show in this article, a normal healthy gland, exposed to chronic oxidative stress, using different mechanisms of secretion for different purposes: apocrine, holocrine intra-tubular, inter-tubular and endocrine types. What determines the processes that the HG acini will undergo? Is the inter-tubular process a mechanism to rid the acini of extra cells? Could this situation be more common than once thought? Could this mechanism be a part of the response to a stressful situation? All these questions are still unanswered but it is evident, based on data presented in this article, that the Syrian hamster HG shows a pre-pathological state for the detrimental effect of oxidative stress during the multistage carcinogenesis process. The existence of this particular equilibrium among oxidative stress, detachment-derived cell death and secretory activity introduces a great number of questions about their interaction.
Acknowledgments
This work was partially performed with funding from grants MICINN-10-BFU2010-20919 from Ministerio de Economia y Competitividad (Spain) and AGL2010-21578-C03-02 from Ministerio de Economia y Competitividad (Spain). I.V.-N. thanks the Seventh Framework Program of the European Union (PIEF-GA-2009-251850) for a Marie Curie Intra-European Fellowship (IEF). B.C. thanks FICYT (Gobierno del Principado de Asturias, Spain) for a post-doctoral fellowship (Programa Clarin). M.G.-M. thanks ISCIII, Ministerio de Economia y Competitividad (Spain) for a PFIS fellowship.
Author contributions
A.C.-M. and I.V.-N carried out the design and execution of the study and wrote the manuscript. M.G.-M. and B.C. carried out the immunohistochemical analysis. V.S. contributed to data acquisition and analysis. M.J.R.-C. and R.J.R. participated in the writing of the manuscript with critical revisions. All authors have participated in the elaboration of this work and have approval for this submission.
References
- Antolin I, Uria H, Tolivia D, et al. Porphyrin accumulation in the harderian glands of female Syrian hamster results in mitochondrial damage and cell death. Anat Rec. 1994;239:349–359. doi: 10.1002/ar.1092390402. [DOI] [PubMed] [Google Scholar]
- Badminton MN, Elder GH. Management of acute and cutaneous porphyrias. Int J Clin Pract. 2002;56:272–278. [PubMed] [Google Scholar]
- Barrett AJ. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980;187:909–912. doi: 10.1042/bj1870909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer R, Hein R, Bosserhoff AK. A secreted form of P-cadherin is expressed in malignant melanoma. Exp Cell Res. 2005;305:418–426. doi: 10.1016/j.yexcr.2005.01.024. [DOI] [PubMed] [Google Scholar]
- Bauer R, Wild PJ, Meyer S, et al. Prognostic relevance of P-cadherin expression in melanocytic skin tumours analysed by high-throughput tissue microarrays. J Clin Pathol. 2006;59:699–705. doi: 10.1136/jcp.2005.034538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bursch W, Hochegger K, Torok L, et al. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J Cell Sci. 2000;113:1189–1198. doi: 10.1242/jcs.113.7.1189. [DOI] [PubMed] [Google Scholar]
- Coto-Montes A, Tomas-Zapico C. Could melatonin unbalance the equilibrium between autophagy and invasive processes? Autophagy. 2006;2:126–128. doi: 10.4161/auto.2.2.2351. [DOI] [PubMed] [Google Scholar]
- Coto-Montes AM, Rodriguez-Colunga MJ, Uria H, et al. Photoperiod and the pineal gland regulate the male phenotype of the Harderian glands of male Syrian hamsters after androgen withdrawal. J Pineal Res. 1994;17:48–54. doi: 10.1111/j.1600-079x.1994.tb00113.x. [DOI] [PubMed] [Google Scholar]
- Coto-Montes A, Boga JA, Tomas-Zapico C, et al. Physiological oxidative stress model: Syrian hamster Harderian gland-sex differences in antioxidant enzymes. Free Radic Biol Med. 2001a;30:785–792. doi: 10.1016/s0891-5849(01)00468-3. [DOI] [PubMed] [Google Scholar]
- Coto-Montes A, Boga JA, Tomas-Zapico C, et al. Porphyric enzymes in hamster Harderian gland, a model of damage by porphyrins and their precursors. A chronobiological study on the role of sex differences. Chem Biol Interact. 2001b;134:135–149. doi: 10.1016/s0009-2797(00)00320-3. [DOI] [PubMed] [Google Scholar]
- Coto-Montes A, Tomas-Zapico C, Rodriguez-Colunga MJ, et al. Effects of the circadian mutation ‘tau’ on the Harderian glands of Syrian hamsters. J Cell Biochem. 2001c;83:426–434. doi: 10.1002/jcb.1240. [DOI] [PubMed] [Google Scholar]
- Coto-Montes A, Tomas-Zapico C, Escames G, et al. Characterization of melatonin high-affinity binding sites in purified cell nuclei of the hamster (Mesocricetus auratus) harderian gland. J Pineal Res. 2003;34:202–207. doi: 10.1034/j.1600-079x.2003.00029.x. [DOI] [PubMed] [Google Scholar]
- Coto-Montes A, Tomas-Zapico C, Martinez-Fraga J, et al. Sexual autophagic differences in the androgen-dependent flank organ of Syrian hamsters. J Androl. 2009;30:113–121. doi: 10.2164/jandrol.108.005355. [DOI] [PubMed] [Google Scholar]
- Dragin N, Smani M, Arnaud-Dabernat S, et al. Acute oxidative stress is associated with cell proliferation in the mouse liver. FEBS Lett. 2006;580:3845–3852. doi: 10.1016/j.febslet.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Franzen B, Linder S, Alaiya AA, et al. Analysis of polypeptide expression in benign and malignant human breast lesions: down-regulation of cytokeratins. Br J Cancer. 1996;74:1632–1638. doi: 10.1038/bjc.1996.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita H. Molecular mechanism of heme biosynthesis. Tohoku J Exp Med. 1997;183:83–99. doi: 10.1620/tjem.183.83. [DOI] [PubMed] [Google Scholar]
- Gocheva V, Joyce JA. Cysteine cathepsins and the cutting edge of cancer invasion. Cell Cycle. 2007;6:60–64. doi: 10.4161/cc.6.1.3669. [DOI] [PubMed] [Google Scholar]
- Gocheva V, Chen X, Peters C, et al. Deletion of cathepsin H perturbs angiogenic switching, vascularization and growth of tumors in a mouse model of pancreatic islet cell cancer. Biol Chem. 2010;391:937–945. doi: 10.1515/BC.2010.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Hardeland R, Uria H. On the role of melatonin in mammalian Harderian glands: Does melatonin protect from free radicals generated by protoporphyrin-catalysed photooxidation? In: Hardeland R, editor. Cellular Rhythms and Indoleamines. Goettingen: University of Goettingen; 1995. pp. 145–151. [Google Scholar]
- Helotera H, Alitalo K. The VEGF family, the inside story. Cell. 2007;130:591–592. doi: 10.1016/j.cell.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Karbownik M, Reiter RJ. Melatonin protects against oxidative stress caused by delta-aminolevulinic acid: implications for cancer reduction. Cancer Invest. 2002;20:276–286. doi: 10.1081/cnv-120001154. [DOI] [PubMed] [Google Scholar]
- Libbrecht L, Meerman L, Kuipers F, et al. Liver pathology and hepatocarcinogenesis in a long-term mouse model of erythropoietic protoporphyria. J Pathol. 2003;199:191–200. doi: 10.1002/path.1257. [DOI] [PubMed] [Google Scholar]
- Menendez-Pelaez A, Tolivia D, Rodriguez-Colunga MJ, et al. Ultrastructure of the blood vessels in the Harderian gland of the hamster (Mesocricetus auratus): existence of sinusoids. J Morphol. 1990;204:257–263. doi: 10.1002/jmor.1052040304. [DOI] [PubMed] [Google Scholar]
- Menon J, Rozman R. Oxidative stress, tissue remodeling and regression during amphibian metamorphosis. Comp Biochem Physiol C Toxicol Pharmacol. 2007;145:625–631. doi: 10.1016/j.cbpc.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol. 2007;8:221–233. doi: 10.1038/nrm2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parums DV, Cordell JL, Micklem K, et al. JC70: a new monoclonal antibody that detects vascular endothelium associated antigen on routinely processed tissue sections. J Clin Pathol. 1990;43:752–757. doi: 10.1136/jcp.43.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne AP, McGadey J, Johnston HS. Interstitial porphyrins and tubule degeneration in the hamster Harderian gland. J Anat. 1985;140:25–36. [PMC free article] [PubMed] [Google Scholar]
- Piantoni P, Wang P, Drackley JK, et al. Expression of metabolic, tissue remodeling, oxidative stress, and inflammatory pathways in mammary tissue during involution in lactating dairy cows. Bioinform Biol Insights. 2010;4:85–97. doi: 10.4137/bbi.s5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyo SW, Hashimoto M, Kim YS, et al. Expression of E-cadherin, P-cadherin and N-cadherin in oral squamous cell carcinoma: correlation with the clinicopathologic features and patient outcome. J Craniomaxillofac Surg. 2007;35:1–9. doi: 10.1016/j.jcms.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Raica M, Coculescu M, Cimpean AM, et al. Endocrine gland derived-VEGF is down-regulated in human pituitary adenoma. Anticancer Res. 2010;30:3981–3986. [PubMed] [Google Scholar]
- Sassa S, Nagai T. The role of heme in gene expression. Int J Hematol. 1996;63:167–178. doi: 10.1016/0925-5710(96)00449-5. [DOI] [PubMed] [Google Scholar]
- Schreurs FJ, van der Heide D, Leenstra FR, et al. Endogenous proteolytic enzymes in chicken muscles. Differences among strains with different growth rates and protein efficiencies. Poult Sci. 1995;74:523–537. doi: 10.3382/ps.0740523. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Hirohashi S, Hirano S, et al. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989;49:2128–2133. [PubMed] [Google Scholar]
- Sounni NE, Paye A, Host L, et al. MT-MMPS as regulators of vessel stability associated with angiogenesis. Front Pharmacol. 2011;2:111. doi: 10.3389/fphar.2011.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spike RC, Johnston HS, McGadey J, et al. Quantitative studies on the effects of hormones on structure and porphyrin biosynthesis in the harderian gland of the female golden hamster. II. The time course of changes after ovariectomy. J Anat. 1986;145:67–77. [PMC free article] [PubMed] [Google Scholar]
- Spike RC, Payne AP, Moore MR. The effects of age on the structure and porphyrin synthesis of the harderian gland of the female golden hamster. J Anat. 1988;160:157–166. [PMC free article] [PubMed] [Google Scholar]
- Stallings-Mann M, Radisky D. Matrix metalloproteinase-induced malignancy in mammary epithelial cells. Cells Tissues Organs. 2007;185:104–110. doi: 10.1159/000101310. [DOI] [PubMed] [Google Scholar]
- Tachibana KE, Gonzalez MA, Coleman N. Cell-cycle-dependent regulation of DNA replication and its relevance to cancer pathology. J Pathol. 2005;205:123–129. doi: 10.1002/path.1708. [DOI] [PubMed] [Google Scholar]
- Tolivia D, Rodriguez-Colunga MJ, Menendez-Pelaez A. The basal pole of the acinar cells in the Harderian glands of Syrian hamsters: evidence of a secretory mechanism. Endocrinología. 1991;38:282–284. [Google Scholar]
- Tolivia D, Uria H, Mayo JC, et al. Invasive processes in the normal Harderian gland of Syrian hamster. Microsc Res Tech. 1996;34:55–64. doi: 10.1002/(SICI)1097-0029(19960501)34:1<55::AID-JEMT8>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Tomas-Zapico C, Coto-Montes A, Martinez-Fraga J, et al. Effects of delta-aminolevulinic acid and melatonin in the harderian gland of female Syrian hamsters. Free Radic Biol Med. 2002a;32:1197–1204. doi: 10.1016/s0891-5849(02)00812-2. [DOI] [PubMed] [Google Scholar]
- Tomas-Zapico C, Martinez-Fraga J, Rodriguez-Colunga MJ, et al. Melatonin protects against delta-aminolevulinic acid-induced oxidative damage in male Syrian hamster Harderian glands. Int J Biochem Cell Biol. 2002b;34:544–553. doi: 10.1016/s1357-2725(01)00149-2. [DOI] [PubMed] [Google Scholar]
- Tomas-Zapico C, Coto-Montes A, Martinez-Fraga J, et al. Effects of continuous light exposure on antioxidant enzymes, porphyric enzymes and cellular damage in the Harderian gland of the Syrian hamster. J Pineal Res. 2003;34:60–68. doi: 10.1034/j.1600-079x.2003.02951.x. [DOI] [PubMed] [Google Scholar]
- Tomas-Zapico C, Caballero B, Sierra V, et al. Survival mechanisms in a physiological oxidative stress model. FASEB J. 2005;19:2066–2068. doi: 10.1096/fj.04-3595fje. [DOI] [PubMed] [Google Scholar]
- Vairapandi M, Azam N, Balliet AG, et al. Characterization of MyD118, Gadd45, and proliferating cell nuclear antigen (PCNA) interacting domains. PCNA impedes MyD118 AND Gadd45-mediated negative growth control. J Biol Chem. 2000;275:16810–16819. doi: 10.1074/jbc.275.22.16810. [DOI] [PubMed] [Google Scholar]
- Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Vega-Naredo I, Caballero B, Sierra V, et al. Sexual dimorphism of autophagy in Syrian hamster Harderian gland culminates in a holocrine secretion in female glands. Autophagy. 2009;5:1004–1017. doi: 10.4161/auto.5.7.9610. [DOI] [PubMed] [Google Scholar]
- Vega-Naredo I, Caballero B, Sierra V, et al. Melatonin modulates autophagy through a redox-mediated action in female Syrian hamster Harderian gland controlling cell types and gland activity. J Pineal Res. 2012;52:80–92. doi: 10.1111/j.1600-079X.2011.00922.x. [DOI] [PubMed] [Google Scholar]
- Vilchis F, Ramos L, Timossi C, et al. The influence of sex steroid hormones on ferrochelatase gene expression in Harderian gland of hamster (Mesocricetus auratus. J Endocrinol. 2006;189:103–112. doi: 10.1677/joe.1.06300. [DOI] [PubMed] [Google Scholar]
- Weng MH, Yu TC, Chen SE, et al. Regional accretion of gelatinase B in mammary gland during gradual and acute involution of dairy animals. J Dairy Res. 2008;75:202–210. doi: 10.1017/S0022029908003130. [DOI] [PubMed] [Google Scholar]
- Wetterberg L, Geller E, Yuwiler A. Harderian gland: an extraretinal photoreceptor influencing the pineal gland in neonatal rats? Science. 1970;167:884–885. doi: 10.1126/science.167.3919.884. [DOI] [PubMed] [Google Scholar]
- Xu P, Wang Y, Piao Y, et al. Effects of matrix proteins on the expression of matrix metalloproteinase-2, -9, and -14 and tissue inhibitors of metalloproteinases in human cytotrophoblast cells during the first trimester. Biol Reprod. 2001;65:240–246. doi: 10.1095/biolreprod65.1.240. [DOI] [PubMed] [Google Scholar]


