Abstract
Goal:
To investigate the correlation between TSH and HbA1c in the treatment of L-thyroxine in the process of glycemic control in patients with subclinical hypothyroidism.
Patients and methods:
The sample consisted of 100 patients, mean age 51.75±3.23 years, BMI=27.97±4.52 kg/m2, with SH (TSH>4.2 mU/L and normal serum T3 and T4). Laboratory diagnosis included the determination of free T3, free T4, thyroid antibodies, Tg, insulin, C-peptide and glucose during the OGTT, HbA1c, CRP and lipid levels. 20 patients with SH had prediabetes and 38 patients had DM. All patients were treated with low doses of L-thyroxine (25-50ug) and all were physically active.
Results:
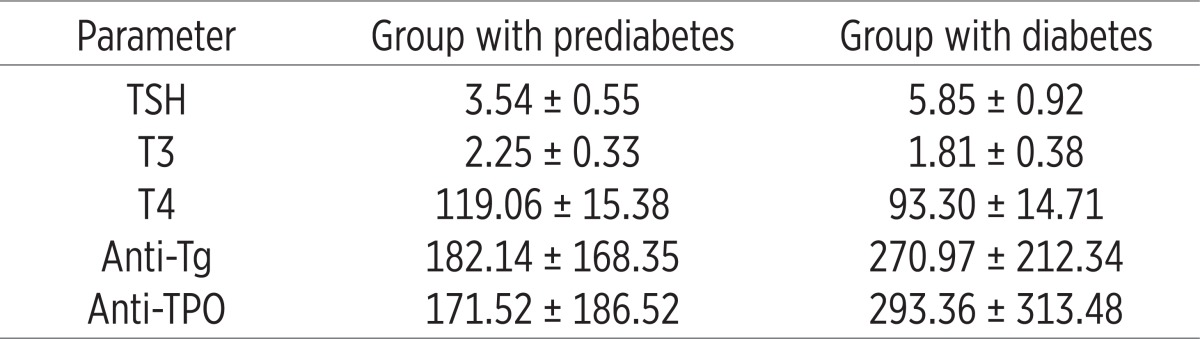
After 6 months of treatment with L-thyroxine, the patients had normal or decreased TSH (5.85±0.92 vs. 3.54±0.55 mU/L), insulin levels (114.64±24.11 vs. 96.44±17.26 pmol/L) significantly reduced HbA1c (6.74±1.01 vs. 6.26±1.12) is reduced.
Conclusion:
The correlation between TSH and HbA1c was positive and significant (r=0.46). This indicates a significant effect of treatment with L-thyroxine on glycemic control in patients with subclinical hypothyroidism.
Keywords: subclinical hypothyroidism, L-thyroxine, glycemic control.
1. INTRODUCTION
The thyroid gland (lat.glandula thyroidea) is located in the neck in front of the larynx (lat.larinx), and consists of two lobes (lat.lobus) connected by narrowing (lat.isthmus). The gland is usually asymmetric with stronger right lobe. Seen from the front has the shape of a letter H or a butterfly with its wings outstretched. The consistency is soft and red color. Average weight is 15-35 grams. Thyroid gland is odd endocrine gland that secretes two important hormones: thyroxine (T4) and triiodothyronine (T3) (1, 2, 3, 4, 5).
Their secretion is controlled by thyroid-stimulating hormone (TSH), secreted by the anterior lobe of the pituitary gland. As a prelude to the release of hormones from the thyroid gland into the blood and to convert them in circulation, it is necessary to form a proteolytic degradation of thyroglobulin. This process occurs through the effect of the enzyme (proteinase and peptidase), which are normally present in the thyroid. Thyroxin and triiodine-tironin thus separating the molecules of thyroglobulin and then as free hormones released into the blood (6, 7, 8, 9, 10).
Of the hormones that are secreted in the blood about 90% is the thyroxine, and 10% triiodine-tironin but triiodine-tironin is four times more potent than thyroxine. In contrast effect of thyroxine takes about four times longer than the active triiodine-tironin. Therefore, the effect of each of these hormones in the period in which it operates, expressed per unit mass of hormones, probably equal. Once it enters the peripheral cells, mainly thyroxine loses iodine and creates triiodine-tironin. Therefore it is considered that the true intracellular hormone mainly triiodine-tironin rather than thyroxine.
Released T3 and T4 crossing the blood bind to its specific protein carriers with firm but reversible bond. These carriers are: globulin (TBG), prealbumin, albumin and those obtained by bonding characteristics of macromolecules, which affects their metabolism and distribution in the body. Less than 1% of the released hormones are free hormones, that is not tied to carriers and only this fraction of free hormone is available to tissues and has metabolic effects (1, 3, 5).
1.1. FUNCTIONS OF THYROID HORMONES IN TISSUES
T3 and T4 are general metabolic stimulants which act on virtually all tissues of the body. Their most important effects are: a) strengthening the metabolism of lipids, proteins, and carbohydrates; b) reinforcement of growth and development; c) the regulation of water and electrolyte transport; d) stimulation of the cardiovascular system; e) stimulation of the central nervous system. Thyroid hormones increase the body’s sensitivity to catecholamines. There are two main modes of action of thyroid hormone in the body: a) increase in overall metabolism; b) promote growth in children.
Thyroid hormones increase the metabolic activity in virtually all tissues of the body (except the brain, retina, testicles and lungs). Basal metabolism may increase by as much as 60-100% above normal values if secrete large amounts of hormones. Enhances the utilization of nutrients to release energy.
Protein synthesis was once more in the same time and increased protein catabolism. Young people’s growth is greatly accelerated. Mental processes become more intense and the activity of most endocrine glands often becomes larger.
Some of the mechanisms of action of thyroid hormones are: a) increased protein synthesis; b) increasing the amount and activity of enzyme systems; c) increased volume and number of mitochondria and d) the effect on the active transport of ions (1, 3).
1.2. THYROID HORMONES EFFECT TO DIFFERENT PROCESSES IN THE BODY
Most likely, the main effect of thyroid hormones is their ability to activate transcription process in the cell nucleus, which leads to increased production of proteins. Thyroid hormones act on the following processes in the human body:
The metabolism of carbohydrates–promote metabolism;
The metabolism of fat–fat mobilization from adipose tissue, which leads to a greasy stock exhaustion, while increasing the concentration of free fatty acids in plasma;
The metabolism of vitamins–increase the need for vitamins, increasing the amount of many enzymes, which are essential ingredients and vitamins;
The basal metabolism–increase basal metabolism 60-100% above normal;
The weight–large quantities of the hormone leads to weight loss and vice versa, reducing the secretion of hormones will result in weight gain;
The cardiovascular system–increasing blood flow and an increase in cardiac output, increased heart rate, increases the strength of heart muscle (up to a certain limit), changes in blood pressure;
The respiratory system–increasing the frequency and depth of breathing;
The digestive tract–increase appetite and nutrient absorption, and intestinal motility;
CNS–speed up the brain;
On muscle function–the stimulation of muscle function;
On sleep–if the increased amount of the hormone occurs insomnia and vice versa if is reduced, there is a strong drowsiness;
The other endocrine glands–increases secretion of other glands, but the need tissues for these hormones increases;
1.3. REGULATION OF THYROID HORMONES SECRETION
The thyroid gland is controlled by the pituitary gland and its hormone TSH (thyroid stimulating hormone or thyrotropin), which depend on the production and release of thyroid hormone levels. In other words, when the level of T3 and T4 decreases, the pituitary gland is activated and begins to secrete TSH and its concentration in the blood increases. TSH stimulates the thyroid gland to produce and secrete T3 and T4, increasing their levels in the blood and leads to normalization of the situation.
The pituitary gland is controlled by the hypothalamus. It is a part of the brain that produces TRH (thyroid releasing hormone). TRH stimulates the pituitary gland to secrete TSH (14, 15, 16).
1.4. DISEASES OF THYROID GLAND
General classification of diseases of the thyroid gland looks like this:
Hyperthyroidism–hyperactivity of the thyroid gland (thyrotoxicosis);
Hypothyroidism–hypo function of the thyroid gland (cretinism–myxedema innate–acquired disease);
Thyroiditis–inf lammation of the thyroid gland (Hashimoto, subacute–de Quervain, and chronic– silent);
Goiter–enlargement (diffuse–simple and multinodular–toxic or non-toxic),
The nodes–nodes (functional, non-functional, solitary and multiple);
Tumors of the thyroid gland (benign–adenomas, malignant–cancer: papillary, follicular, medullary, and anaplastic).
1.5. HYPOTHYROIDISM
Hypothyroidism is a condition which occurs due to decreased production, distribution disruption or lack of action of thyroid hormones. Disruption of production caused by disease or disorder of the thyroid gland controls the pituitary or hypothalamus function. Resistance to peripheral effects of thyroid hormone is a rare condition with an image of hypothyroidism and increased levels of circulating iodine tironine. Hypothyroidism is traditionally divided into primary, caused by insufficiency of thyroid function, secondary, due to the absence of pituitary stimulation of thyrotropin, tertiary, due to insufficient secretion tiroliberina and quaternary because peripheral resistance to thyroid hormones.
Hypothyroidism, therefore, can arise due to disturbances in glandular disorders or monitoring mechanisms in higher brain structures. Causes of primary hypothyroidism may include: a) the reduction of thyroid tissue; b) normal or hyperplastic glands; c) decreased stimulation of the thyroid gland and d) peripheral resistance to thyroid hormones.
Iodine deficiency is a major cause of hypothyroidism in the world. In countries with sufficient iodine in food, autoimmune disease dominated in 90% of cases (70% Hashimoto and Basedow 20%) as a cause of hypothyroidism, thyroid surgery followed. Application tirosupressants during pregnancy (propylthiouracil, metamizol), antithyroid drugs (tionamidi) and other agents such as lithium, amiodarone recombined cytokines in tumor treatment (IFN-α, IL-2), p-aminosalicylic acid, aminoglutethimide, beta-carotene, contrast agents are the main causes transient iatrogenic hypothyroidism.
1.6. SUBCLINICAL HYPOTHYROIDISM
Subclinical hypothyroidism (SH) is a disorder that is defined as a condition with elevated serum levels of thyroid stimulating hormone TSH and normal serum concentrations of thyroid hormones by the absence of clinical signs and symptoms (Canaris GJ). By compensatory TSH elevation, thyroid gland temporarily stimulates the production of approximately adequate amount of thyroid hormones. SH or mild thyroid failure is a common problem for the population, as indicated by the prevalence of 4-10%. The prevalence increases with age, with females compared to male up to 5 times, as evidenced by data on the prevalence of 20% among women older than 60 years. The incidence of overt hypothyroidism is about 1-2% in women and 0.1% in men.
Progression from subclinical to overt hypothyroidism is expected in 5-18% of cases (Canaris GJ). If TSH> 10 mIU/L, the risk of crossing the SH to MH will be higher. Antithyroid antibodies were detected in 80% of the SH, and 80% of these patients had serum TSH <10 mIU/L. It is believed that the measurement of anti-TPO antibodies ensures proper evaluation of patients with SH as an excellent indicator of the transition to overt hypothyroidism. Expected clinical progression to overt hypothyroidism in TPOAb was negative 2.6% of patients, whereas patients with positive TPOAb 4.3% per year. The significance of antibodies increases due conspicuous association of the disease with other autoimmune diseases such as diabetes mellitus, Addison’s disease, myasthenia gravis, lupus erythematosus, pernicious anemia, rheumatoid arthritis and idiopathic thrombocytopenia, which also represents confirmation that the disease is a secondary result of an autoimmune reaction (Dfez JJ, Seinfeld).
In the background of subclinical hypothyroidism is autoimmune etiology. Autoimmune thyroid disease (Hashimoto, Basedow) leads to hypothyroidism in two ways. One is the destruction of thyroid tissue in the chronic autoimmune inflammation, and the creation of antibodies that bind to the TSH receptor. Hashimoto’s thyroiditis tends to occur in the group of 5 HLADR antigens in the white race, and HLADR 53 in Japanese (Farid). Chronic lymphocytic (Hashimoto’s) thyroiditis occurs as a result of humoral and cellular autoimmune thyroid dysfunction and destruction. Hashimoto’s thyroiditis and other organ specific endocrinopathy is the result of a specific genetic defect in immunoregulation. This defect is expressed in qualitative and quantitative organ dysfunction clones specific suppressor (CD8 +) T lymphocytes, and it allows you to spontaneously mutated clone of helper T lymphocytes (CD4 +) directed by thyroid tissue survives (Okita). Self-reacting lymphocytes react with complementary antigens on thyroid membrane and establish local and localized cellular immune response. The reaction does not require any change in the structure of antigens, but only the presence of antigens and the expression of HLA DR antigens, either on the cell surface or through antigen senting cells. As a result of reaction with complementary antigens, included the activation of B cells, which produce the appropriate antibodies that will get into your blood by the thyroid gland and induce autoimmune lesions (Feldt-Rasmussen, 1996).
Among the largest share autoantibodies antibodies tiroid peroxidase (anti-TPO) and thyroid microsomal antigen (McAb). Autoantibodies are rarely present antithyreoglobulin antibody (Anti-Tg) antibodies directed against T4 and T3, also autoantibodies against the TSH receptor (TRAb).
TPOAb–antibodies to thyroid peroxidase (TPOAb) are features of HT, which are present in 95% of patients with HT. Are also seen in patients with idiopathic mixed edema, Graves’s disease, and in patients with some tumors, but lower titers. Lesions of the thyroid gland occur as a result of the role of these antibodies with the complement fixation and induction of cytotoxic changes. Histological lesions and the titer of these antibodies show good correlation. TPOAb were immune globulin G class with a different distribution of subclasses IgG1-IgG4.
As each sub class has a different biological activity, and the effect of HT on the clinical status of the thyroid gland is from euthyreodism through subclinical hypothyroidism to overt hypothyroidism, it is expected that in each variant to exist under the domination of different classes of IgG. If the class is dominated by IgG2 and IgG4 in these patients, he will carry with them a higher risk for the development of overt hypothyroidism (L.–D. Xie).
TgAb–titer of these antibodies was significantly elevated in 65-90% of patients with Hashimoto’s thyroiditis, in 60-90% of patients with Graves’s hyperthyroidism, transient and diminish the degree in 50-75% of patients with subacute thyroiditis, a relatively modest increase is in other states of thyroid diseases (goiter other origin, malignancy), but in 16% of the population over 65 years, without any thyroid disorder. Because of less specificity, the significance of TgAb is less than the findings of antibodies to thyroid microsomes (1:92).
TSH receptor antibodies–antibodies to the TSH-receptor (TRAb) instead TSH bind and inhibit its effect on tiroicit (Thyroidstimulationblockingantibody, TSH RBAb; TBAb-Thyroidblockingantibody), or cause uncontrolled stimulation instead of the normal thyroid stimulating TSH (TSH Thyroidstimulatingantibody -RSBA). TRAb may be blocking and activating, and depending on their preponderance we have a picture of hypothyroidism or hyperthyroidism. Graves disease is dominated by TSI, in the predominant Hashimoto TGI (Thyreoidgrowh–stimulatingimmunoglobulins) to achieve growth of the thyroid gland. Antibody titers can vary in the course of disease, but the main feature of Hashimoto’s thyroiditis present TGI leading to the increase glands, while TSI produced in sufficient quantity or suppressed blocking antibody, which is probably conditioned by the progressive development of hypothyroidism (DeGroot).
The influence of the functional state of the thyroid gland on expression autoimmune phenomena–Influence of thyroid hormones on the expression of TSH and thyroid autoimmunity remains insufficiently understood. TRAb titer decreases with the introduction of L-thyroxine and increases again after the lifting of L-thyroxine (Trbojevic). Also the TPOAb titer, behaves in a similar manner. Simultaneously there is a positive correlation between level of TSH and TRAb and TPOAb titer. Thyroxine T3 can act directly on B cells, which produce antibodies to the TSH receptor. Evidence of such action not definitively collected. Another possibility is that thyroxine modifies the activity of enzymes that stimulate the formation of phospholipid membranes and thus reduces the production of antigen. Thyroid gland contains a thyroid receptor, which means it is itself a target organ for your hormones, which explains the modification of the production of antigens on the plasma membrane itirocites. After more thyroid hormone therapy, cases of complete recovery of patients with Hashimoto’s thyroiditis, in which there was a complete recovery of thyroid function (1:360).
In typical cases, the gland is moderately enlarged, symmetrical, firm, rubbery and sharply limited (the edges are jagged but the general contour of the gland preserved), pyramidal lobe can be highlighted. If there is a significant connective converting, it may be normal in size or even reduced. Typical HT manifested as symmetric or nearly symmetric increase of the thyroid gland in women after menopause, without significantly expressed irregular nodes, what can be expected in those cases in which exists in cancer. Patients with HT are reported to a doctor because of symptoms due to the increase pressure gland or signs of hypothyroidism. Due to the increase in gland disorders are often a feeling of fullness in the throat, dysphasic interference, hoarseness and dysphonia.
At an early stage the patient has a normal metabolism, but even then decreased thyroid reserve is often manifested in increased serum TSH. Progression of the disease can suddenly develop thyroid insufficiency (first subclinical) because of progressive replacement of thyroid parenchyma cells with fibrous tissue. Thyroid insufficiency is first evident increase in serum TSH. With time, the concentration of T4 in serum decreases, while T3 remains normal. Finally, serum T3 concentrations decrease below normal values and suddenly appear hypothyroidism.
In many patients with mild hypothyroidism disease remains not recognized, because disturbances, in which patients complain–drowsiness, fatigue, sensitivity to cold and general exhaustion are too vague. Slow and progressive development of the disease is a major problem in the rapid diagnosis. Already in the stage of mild thyroid failure are at risk for metabolic syndrome, which includes signs of central obesity, elevated triglycerides, LDL, the presence of insulin resistance and the risk of atherosclerosis, hypertension. Risk for atherosclerosis, further explains the discovery that SH causes elevated levels of factor X, which causes hyper coagulation condition.
Hypothyroidism can affect the gonadotropic axis at different levels and caused changes in the hypothalamic-pituitary unit, gonadal function and peripheral metabolism of sex hormones. The biological consequences of these effects on menstrual and ovulatory cycles can exacerbate women’s health. The prevalence of SH in women with infertility (inability to conceive after 1 year) ranks in a wide range of 1-40%. Examination of the patient with suspected hypothyroidism is in two directions: first assesses the state of thyroid function in order to determine whether it is really about hypothyroidism. The diagnosis is confirmed by finding of HT antithyroid antibodies in serum are usually in high titer. Antimicrosomial (TPOAb) peroxidase antibody was detected more frequently and in higher titers than TgAb.
Thyroid hormone levels and TSH depends on the stage of the disease. In the early stages of the disease there is an elevated TSH and normal levels of T4 and T3, when the radio iodine fixation test is usually elevated. In the beginning, when the goiter is higher, commonly found growing volume of fixation in the early period, up to three hours after the starting marker. Because thyroid epithelial injury, taking iodine is not followed by further metabolic stages so that it leaves the gland faster than normal. Scope fixation after 24h is usually lower than the normal value. In later stages of the disease (atrophic stage) the extent of fixation decreases, so that hypothyroid stage shows values as in hypothyroidism of any origin. In the initial stages of the disease the patient is eumetabolic, indicating that the response gland to TSH adequately compensated and abnormalities in the biosynthesis of thyroid hormones caused by disease. Over time, the ability to respond to the thyroid TSH level decreases and T4 uptake and fixation progressively falling. In the phase of reduced thyroid reserve or subclinical hypothyroidism is very elevated TSH and normal T3. Finally, the T3 is reduced below normal values and there are signs of hypothyroidism (13, 17, 18, 19, 20, 21).
1.7. DIAGNOSTIC TECHNOLOGY IN DETECTING HYPOTHYROIDISM
Scintigraphic findings initially show magnification of the gland with relatively uniform binding. In later stages observed characteristic uneven distribution of markers in the parenchyma, which gives the appearance of blotchy, with fields of normal and weakened binding. Once developed parenchymal atrophy, binding marker becomes weaker.
Ultrasound findings reveal the existence of goiter with no special features in the bloodstream glands. The striking feature is nodularity HT and if found such a finding should be considered in the degeneration of simple goiter or other reasons for the occurrence of nodes. Aspiration biopsy was no longer of great importance in the diagnosis, except in cases of HT in adolescents in whom autoantibody titers may be low. Open biopsy of the gland is rarely applied, except in cases of suspected malignancy. If taken as a criterion for elevated TPOAb titers, HT can be established in more than 30% of patients with nodal changes in the thyroid gland. If during fine needle aspiration biopsy finds large amount Hurthle’s cell, differential diagnosis to neoplasm is becoming a serious problem and must be solved biopsy findings (22, 23, 24, 25).
1.8. TREATMENT OF SUBCLINICAL HYPOTHYROIDISM
Levothyroxine (L-thyroxin)–is the thyroid hormone that is used as a replacement therapy in the treatment of hypothyroidism, to suppressed secretion of tire-stimulating hormone (TSH), and prevents an increase of the thyroid gland. Is contraindicated in thyrotoxicosis. T4 is converted to T3 intracellularly, so that giving T4 hormone produced by both hormones.
Prognosis is excellent if replacement therapy is taken regularly at a dose prescribed by a doctor and if the proper replacement therapy makes no complications. Th ey may occur in the case of HIV treatment or in case of overdose with thyroxine. In the case of almost all causes of hypothyroidism are no real preventive measures and its occurrence is virtually impossible to prevent. It is important to recognize it and prevent complications (25).
2. GOALS
The goal of this study was to determine whether there is between TSH and HbA1c significant correlation in patients with subclinical hypothyroidism treated with L-thyroxine therapy for glycemic control.
3. MATERIAL AND METHODS
The study involved 50 subjects from Clinic for Endocrinology, Diabetes and Metabolic Diseases, Clinical Center of Sarajevo University who were hospitalized or treated on outpatient basis between January 1st 2007 and December 31st 2009 with subclinical hypothyroidism (TSH>4.2 mIU/L and normal levels of T3 and T4) and who were treated with low doses of L-thyroxine. The average dose of L-thyroxine therapy of was 25-50 μg. All the tests were repeated 6 months aft er introduction of L-thyroxine therapy. The control group consisted of 50 patients with subclinical hypothyroidism that was not treated with L-thyroxine. Excluded from the study were subjects with subclinical hypothyroidism of iatrogenic origin (all states after surgical intervention on the thyroid gland or after treatment with radioactive iodine).
4. RESULTS
For each patient, a detailed history was taken and analyzed clinical and laboratory parameters. Statistical analysis was performed using the computer soft ware specific to this type of problem. Basic tables with calculated percentages are done in Winword 2007 and testing of significance between mean differences of quantitative variables was done using the Student t-test in the statistical package SPSS.
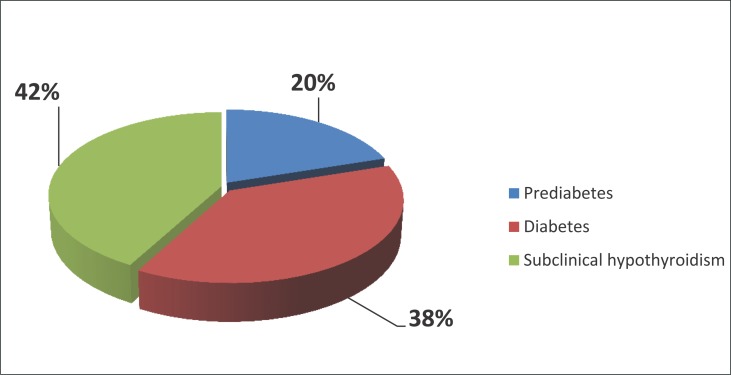
The results shown in Figure 1 indicate that the disorder of glycemic control was present in 58 (58%) of patients with subclinical hypothyroidism. In our sample, there were 20 patients with prediabetes (20%) and 38 with diabetes (38%).
Figure 1.
Analysis disorder glycemic control in patients with subclinical hypothyroidism.
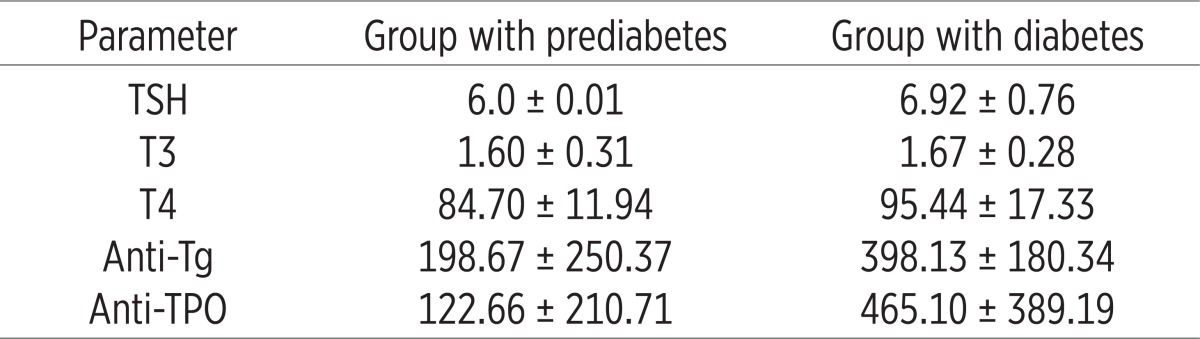
Table 1 shows mean values of thyroid hormones in patients with prediabetes and diabetes prior treatment with L-thyroxine. It is evident that the patients with diabetes had significantly higher mean values of all thyroid hormones in relation to the group of patients with prediabetes.
Table 1.
Analysis of thyroid hormones in the two groups before treatment with L-thyroxine
 |
The results presented in Table 2 indicate that the patients after 6 months of treatment with L-thyroxine had normal TSH (3.54±0.55 vs. 5.85±0.92, p=0.009) and normal values for total thyroid hormones.
Table 2.
Analysis of thyroid hormones in all patients before and after L-thyroxine therapy
 |
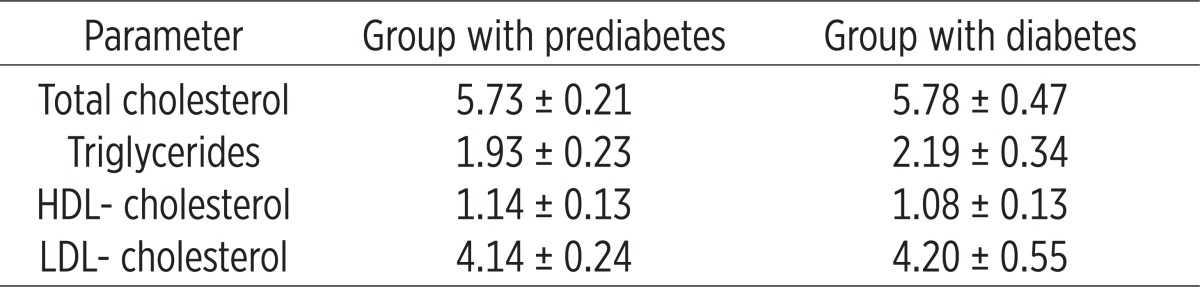
Tables 3 and 4 show the values of lipid profile in the two groups before and after treatment with L-thyroxine. It is obvious that in the group with diabetes had increased values of all lipid fractions or the values of total cholesterol, triglycerides, LDL-cholesterol while reducing HDL-cholesterol compared to the group of patients with prediabetes.
Table 3.
Analysis of the lipid profile in the two groups (before treatment)
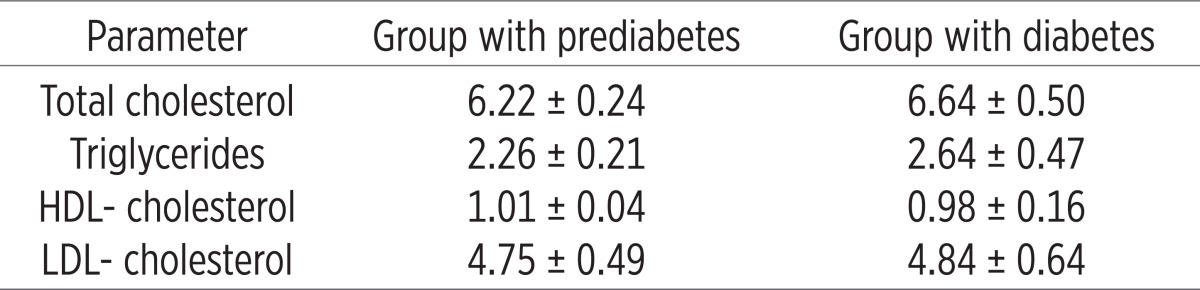 |
Table 4.
Analysis of the lipid profile in the two groups (after treatment)
 |
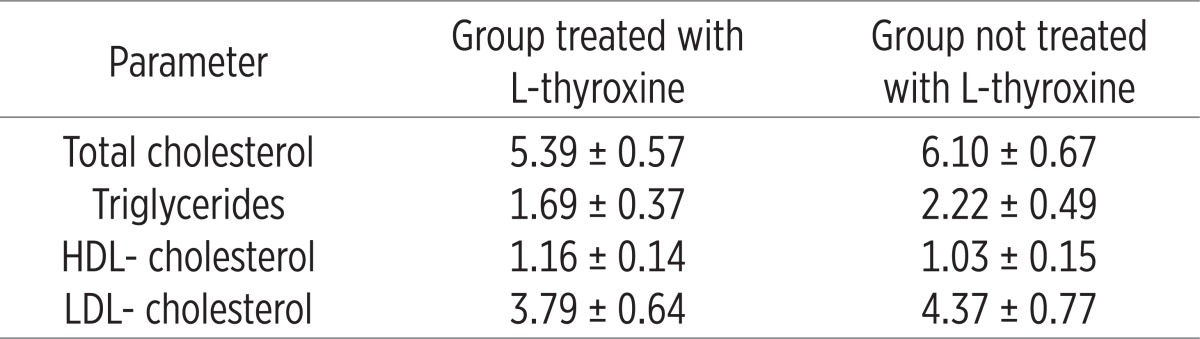
Patients treated with L-thyroxine had decreased basal insulin values (114.64±24.14 vs. 96.44±17.26, p=0.01) and the value of basal C-peptide (1120.58±299.17 vs. 883.58±213.14, p=0.0005) compared to the group that was not treated with L-thyroxine. The results of correlation between TSH and HbA1c were obtained through statistical analysis of coefficients of these parameters of our patients and are presented graphically as follows. (Table 5)
Table 5.
Analysis of lipid profile in all patients before and after treatment with L-thyroxine
 |
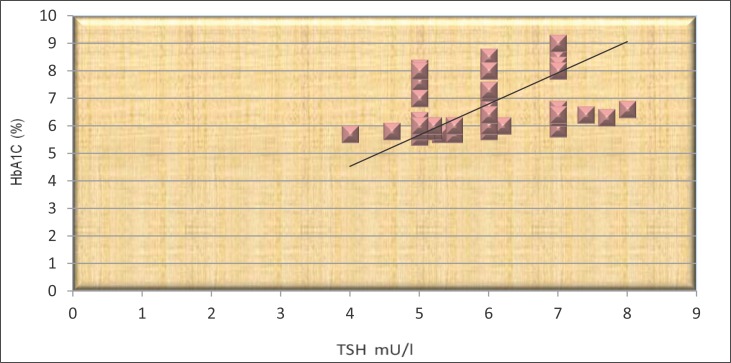
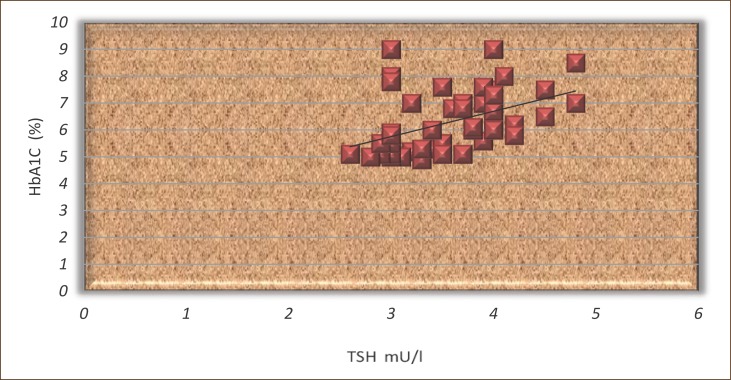
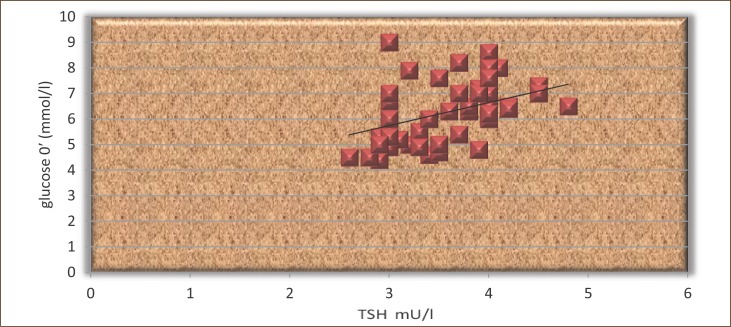
The results shown in Figures 2 and 3 show that the TSH and HbA1c signifi cantly correlated in both groups (r =0.46 and r=0.29, p <0.05).
Figure 2.
The correlation between TSH and HbA1c in patients not treated with L-thyroxine.
Figure 3.
The correlation between TSH and HbA1c in patients treated with L-thyroxine.
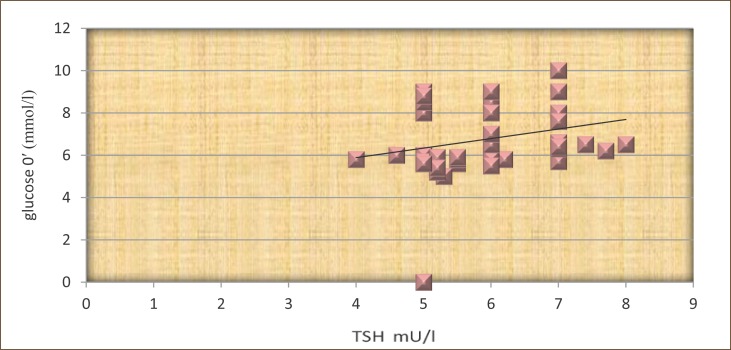
TSH and FPG in the group of patients who did not received L-thyroxine, was not statistically significantly correlated (r=0.21, p>0.05 (Figure 4).
Figure 4.
The correlation between TSH and fasting plasma glucose in patients not treated with L-thyroxine.
The results shown in Figure 5 show that the statistically significant positive correlation between TSH and fasting glucose in subjects treated with L-thyroxine (r=0.39, p<0.01).
Figure 5.
The correlation between TSH and fasting glucose in subjects treated with L-thyroxine.
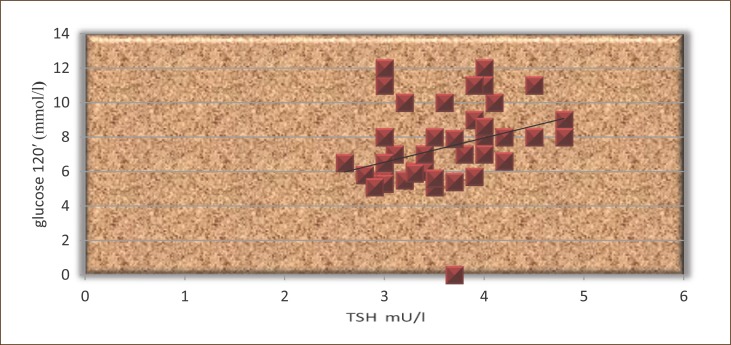
The results presented in Figure 6 show that TSH significantly correlated with postprandial glucose in patients not treated with L-thyroxine (r=0.34, p<0.05).
Figure 6.
The correlation between TSH and postprandial glucose in subjects not treated with L-thyroxine.
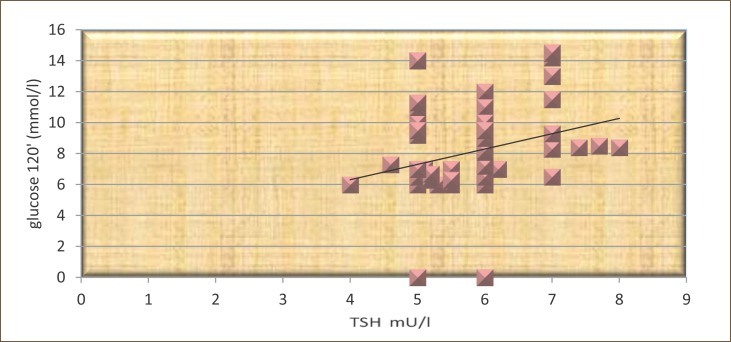
The results presented in Figure 7 shows that the TSH highly significantly correlated with postprandial glucose in patients treated with L-thyroxine (r=0.41, p<0.01).
Figure 7.
The correlation between TSH and postprandial glucose in patients treated with L-thyroxine.
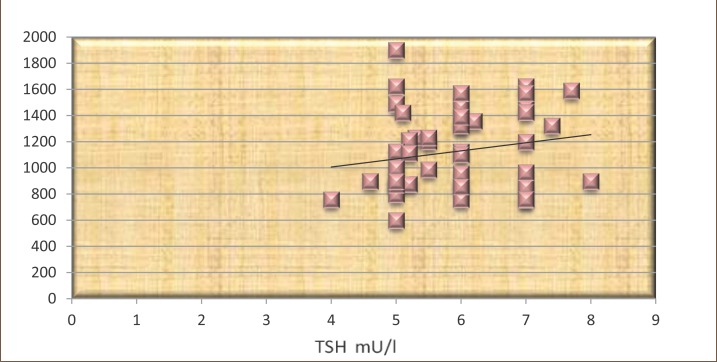
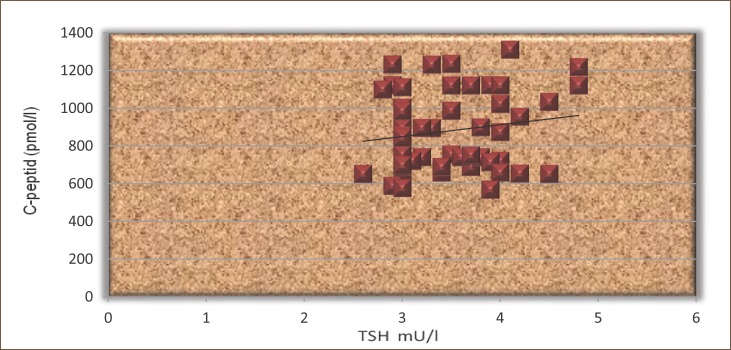
The results presented in Figures 8 and 9 show that TSH positively correlated with C-peptide, but not statistically significant in both groups (r=0.16 and r=0.19, p<0.05).
Figure 8.
The correlation between TSH and C-peptide in patients not treated with L-thyroxine.
Figure 9.
The correlation between TSH and C-peptide in patients treated with L-thyroxine.
5. DISCUSSION
Hypothyroidism is one of the most common diseases of the endocrine system. Most are subclinical hypothyroidism. The prevalence of subclinical hypothyroidism is 8-10%, even 15% of women and 3% of men. Thyroid hormones regulate metabolic processes throughout the body and affect the supply of sugar in the blood. In hypothyroidism there is the slow absorption of carbohydrates from the digestive system, the slower emptying of the stomach, but increased sensitivity to insulin. The prevalence of thyroid disease in patients with diabetes mellitus is approximately 10-15% (1, 3).
Already slight subclinical hypothyroidism can cause outbursts of sensitive functions, such as left ventricular diastolic dysfunction, lack of ovulation, expression of LDL receptors increase LDL and decrease HDL-cholesterol while the total cholesterol is still normal. The slowdown of all processes leading to the loss of both their mutual balance of thyroid hormones regulate metabolic processes throughout the body and affect the supply of sugar in the blood. In hypothyroidism slowed reabsorption carbohydrates from the digestive system, the slower emptying of the stomach, but increased sensitivity to insulin.
The absence of symptoms in patients with subclinical hypothyroidism and the serious health consequences, including cognitive disorders, stress the importance of timely diagnosis of subclinical hypothyroidism and adequate treatment of patients with small doses of L-thyroxine. Subclinical hypothyroidism can progress to manifest, especially in patients with circulating antibody thyroid gland. Determining the level of TSH is accurate, accessible, safe and inexpensive test to diagnose subclinical hypothyroidism. Determining the level of TSH can be used to define the risk of the occurrence of various complications (osteoporosis, cardiovascular disease, depression) for different intervals between TSH. As a result, the decision on the introduction of replacement therapy will be made not only on the level of TSH, but based on additional factors, such as gender, age, smoking, hypertension, cholesterol levels, diabetes.
Modern research has shown a link between type 1 diabetes and autoimmune thyroid disease. Type 1 diabetes is more common in young people, especially in puberty. Even at the beginning of diabetes, 15 to 30% of children were positive anti TPO antibodies and antithyreoglobulin. Antibodies were present more often in girls than boys. In half of these children will develop clinically manifested autoimmune thyroid disease. In children with diabetes there is rapidly increasing level of antibodies leading to subclinical hypothyroidism. Then the child does not have any problems. The laboratory is detected elevated TSH, and thyroid hormones (T3 and T4) are normal. Disease progression is towards development of manifest hypothyroidism, except when reduced thyroid hormones, there are problems. This disease usually develops gradually and insidiously. Patients usually have fatigue, malaise, lethargy, cold intolerance, and get fat if you often have increased appetite, then I have stomach bloating and constipation, sweating and weak often feel pain in your chest.
Subclinical hypothyroidism independently increases the risk for decreased insulin sensitivity, especially in the adipose tissue and muscle. Post receptors disorder of insulin signal seems to be the main reason for hyperinsulinemia. Most people with Excessive weight have insulin resistance. The concept of insulin resistance in patients with subclinical hypothyroidism has been further complicated by selective tissue sensitivity or order selective activity within the tissue that is resistant to its effect. There is an apparent correlation between subclinical hypothyroidism and hyperinsulinemia and insulin resistance. Subclinical hypothyroidism and insulin resistance via its numerous mechanisms involved in the disruption of glycemic control. Most professionals who deal with these clinical entities believed to be of great importance to keep in mind what kind and what is the impact of treatment on L-thyroxine glycemic control in patients with subclinical hypothyroidism.
Thyroid hormones affect the cardiovascular system of direct action on the heart and blood vessels, as well as the influence on lipid profile and atherogenesis. In overt hypothyroidism, cardiovascular function is characterized by increased vascular resistance and heart rate. In subclinical hypothyroidism, there is a disorder of systolic and diastolic function of the left and right ventricles. The latest research in the world speak of the existence of a positive correlation between serum TSH and total and LDL-cholesterol. Specifically, subclinical hypothyroidism is a risk indicator for atherosclerosis and coronary heart disease which was written by several authors.
Chronically elevated CRP levels also indicates the existence of long-term subclinical inflammation that exposes an individual’s to risk of developing hypertension and other cardiovascular damage, leading to loss of elasticity of the arterial wall. Some authors believe that CRP is not only a marker of risk for hypertension, but it induces formation of hypertension. Also, demonstrated a correlation between CRP and markers of endothelial dysfunction in patients with diabetes mellitus, which suggests a link between the activation of the endothelium and chronic inflammation in diabetic patients. Chronic subclinical inflammation is considered to be important for the initiation and / or progression of atherosclerosis in patients with diabetes mellitus.
The results of our study showed decrease in total cholesterol (5.39±0.57 vs. 6.10±0.67), a reduction in triglycerides (1.69±0.37 vs. 2.22±0.49), HDL cholesterol (1.16±0.14 vs. 1.03±0.15) and LDL cholesterol (3.79±0.64 vs. 4.37±0.77). Further, the results clearly showed that the concentration of CRP in the serum of patients who were not treated with Lthyroxine increased compared to the group of patients who were treated with L-thyroxine (2.27±0.8 vs. 3.32±1.1). Also, changes were found in the level of postprandial glucose (7.45±2.0 vs. 8.48±2.35) in basal insulin levels (96.44±17.26 vs. 114.64±24.11) as well as the level of the basal C-peptide (883.58±213.14 vs. 1.120±299.17).
The results we obtained in our study agree with the results reached by other authors. But it is important to note that this is a problem that is increasingly attracting the attention of world endocrinologist to keep it alive in their research. Unfortunately in South East Europe are still no extensive studies on this topic.
Statistically significant correlation was obtained for TSH and HbA1c values in patients treated with L-thyroxine (r=0.46, p<0.05), TSH and fasting glucose (r=0.39, p<0.05), TSH and postprandial glucose (r=0.41, p<0.05).
Statistically Significant correlation was obtained for TSH and HbA1c values in patients treated with L-thyroxine (r=0.46, p<0.05), TSH and fasting glucose (r=0.39, p<0.05), and TSH with postprandial glucose (r=0.41, p<0.05).
6. CONCLUSIONS
Hypothyroidism is one of the most common diseases of the endocrine system and is mostly of subclinical character. In most cases affects women at age of 40-60 years.
Normalization of TSH levels leads to a reduction in postprandial glucose levels, CRP, HbA1c and lipids. This indicates a significant effect of treatment with L-thyroxine on glycemic control in patients with subclinical hypothyroidism.
In our study, glycemic control disorder was present in 58% of patients with subclinical hypothyroidism. The correlation between TSH and HbA1c is positive and significant.
Patients with subclinical hypothyroidism exhibited elevated levels of atherogenic parameters (hyperinsulinemia, total cholesterol, LDL-cholesterol).
Determination of TSH is accurate, accessible, safe and inexpensive test to diagnose subclinical hypothyroidism. Determining the level of TSH can be used to define the risk of the occurrence of various complications (osteoporosis, cardiovascular disease, depression) for different intervals between TSH.
Subclinical hypothyroidism is quite hard to diagnose. In practice this is often overlooked. Adequate diagnosis requires: conducting extensive laboratory tests other than routine as the TSH test. Monitoring of body temperature and careful monitoring of clinical signs, then well taken case history helps to faster and easier detection of this disease in medical practice.
Conflict of interest
None declared.
REFERENCES
- 1.Trbojević B. Tiroidna žlezda: Patofiziološke osnove i klinički pristup, Beograd. 1998. pp. 305–310.
- 2.Manojlović D, Bogić M, Bošković D, Bulajić M, Čolović M, Ðorđević P. i ost. Bolesti žlezda sa unutrašnjim lučenjem. In: Bjeletić D, editor. U: Interna medicina. Beograd: Zavod za udžbenike i nastavna sredstva Beograd; 1998. pp. 1140–1158. [Google Scholar]
- 3.Diez JJ, Iglesias P, Burman KD. Spontaneous normalization of thyrotropin concentations in patients with subclinical hypothyreoidism. J Clin Endocrinol Metab. 2005;90:4124–4127. doi: 10.1210/jc.2005-0375. [DOI] [PubMed] [Google Scholar]
- 4.Milošević DP, Ðurica S, Davidović M, Stević R, Rajić M, Marković N. Subclinical and manifested hypothyroidism as a consequence of thyroid autoimmune disease. Serbian archives of medicine, Journal of the Serbian medical society. 2005;(Suppl 1):34–39. doi: 10.2298/sarh05s1034m. [DOI] [PubMed] [Google Scholar]
- 5.Bakker SJL, Ter Matten JC, Popp-Snijders C, Slaets JPJ, Heine RJ, Gans ROB. The relationship between thyrotropin and low density lipoprotein cholesterol is modified by insulin sensitivity in healthy euthyroid subjects. J Clin Endocrinol Metab. 2001;86:1206–1211. doi: 10.1210/jcem.86.3.7324. [DOI] [PubMed] [Google Scholar]
- 6.Komarica-Bilic E, Beciragic A. Effects of Treatment with L-thyroxin on Glucose Regulation in Patients with Subclinical Hypothyroidism. Med Arh. 2012 Dec;66(6):240–242. doi: 10.5455/medarh.2012.66.364-368. doi: 10.5455/medarh.2012.66.240-242. [DOI] [PubMed] [Google Scholar]
- 7.Fazio S, Palmieri EA, Lombardi G, Biondi B. Effects of thyroid hormone on the cardiovascular system. Recent Prog Horm. 2004;59:31–50. doi: 10.1210/rp.59.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 9.Radaideh A, El-Khateeb M, Batieha AM, Nasser AS, Ajlouni KM. Thyroid function and thyroid autoimmunityin patients with type 1 diabetes mellitus. Saudi Med J. 2003;24(4):352–355. [PubMed] [Google Scholar]
- 10.Schroner Z, Lazurova I. Diabetes mellitus and subclinical thyroid dysfunctions. Diabetologie Metabolismus Endokrinologie Vyziva. 2006;9(1):1–12. [Google Scholar]
- 11.Amino N, Tachi J. Wheeler MH, Lazarus JH. Diseases of the thyroid. Chapman & Hall; 1994. Hypothyroidism: Etiology and menagment. [Google Scholar]
- 12.Phelix P, Baxter J, Frohman LA. The thyroid. In: Graw MC, editor. Endocrinology and Metabolism. 1995. pp. 435–553. [Google Scholar]
- 13.Kovačević T, Lepšanović L. Endokrinologija. Beograd-Zagreb: Medicinska knjiga; 1988. [Google Scholar]
- 14.Heljić B. Heljić B, Dilić M, Čengić M, Čengić F, Lončarević N. U: Diabetes mellitus-klinički aspekti. Sarajevo: Jež; 2002. Diabetes mellitus; pp. 16–26. [Google Scholar]
- 15.IDF Diabetes Atlas. 2009.
- 16.Müller MJ, Mitchinson PE, Paschen U, Seitz HJ. Glucoregulatory function of glucagon in hypo-, eu- and hyperthyroid miniature pigs. Diabetologia. 1998;31:368–374. doi: 10.1007/BF02341505. [DOI] [PubMed] [Google Scholar]
- 17.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 18.Fatourechi V. Subclinical Hypothyroidism: An Update for Primary Care Physicians. Mayo Clin Proc. 2009;84(l):65–71. doi: 10.4065/84.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Glonti S, Jasshi L. Prevalence of subclinical hypothyreosis and subclinical Thyreotoxicosis among females of average and elderlyages who hadntbeen examined before. Georgian Medical News. 2005;118:32–35. [PubMed] [Google Scholar]
- 20.De Groot LJ, Hoye K, Refetoff S. Serum antigenes and antybodies in the diagnosis of thyroid cancer. J Clin Endocrin Metab. 1980;45:1220. doi: 10.1210/jcem-45-6-1220. [DOI] [PubMed] [Google Scholar]
- 21.Poppe K, et al. Thyroid disease and female reproduction. Clin Endocrinol. 2007;66:309–321. doi: 10.1111/j.1365-2265.2007.02752.x. [DOI] [PubMed] [Google Scholar]
- 22.Flynn SD, Hishiyama RH, Bigos ST. Autoimmune thyroid disease: immunological, pathological an clinical aspects. Crit Rev Clin Lab Sci. 1988;26:43. doi: 10.3109/10408368809105889. [DOI] [PubMed] [Google Scholar]
- 23.Hamburger JI. The various presentations of thyroiditis: Diagnostic considerations. Ann Intern Med. 1986;104:219. doi: 10.7326/0003-4819-104-2-219. [DOI] [PubMed] [Google Scholar]
- 24.De Groot LJ, Quintans J. The causes of autoimune thyroid disease. Endocrin Rev. 1989;10:537. doi: 10.1210/edrv-10-4-537. [DOI] [PubMed] [Google Scholar]
- 25.Meier C, Staub JJ, Roth CB, Guglielmetti M, Kunz M, Miserez AR, Drewe J, Huber P, Herzog R, Muller B. TSH-controlled L-thyroxine therapy reduces cholesterol levels and clinical symptoms in subclinical hypothyroidism: A double blind, placebo-controlled trial (Basel Thyroid Study) J Clin Endocrinol Metab. 2001;86(10):4860. doi: 10.1210/jcem.86.10.7973. [DOI] [PubMed] [Google Scholar]
- 26.Jameson JL, Weetman PA. Kasper LD, Braunwald E, Fauci SA, Hauser LS, Longo LD, Jameson Lj. Harrison’s principles of internal medicine. 16th. McGraw-Hill: Medical Publishing Division; Disorders of the thyroid gland; pp. 2104–2127. [Google Scholar]
- 27.Arinc H, Gunduz H, Tamer A, Seyfeli E, Kanat M, Ozhan H, Akdemir R, Celebi H, Uyan C. Evaluation of right ventricular function in patients with thyroid dysfunction. Cardiology. 2006;105(2):89–94. doi: 10.1159/000089855. [DOI] [PubMed] [Google Scholar]
- 28.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 2004;291(2):228–238. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 29.Kvetny J, Heldgaard PE, Bladbjerg EM, Gram J. Subclinical hypothyroidismis associated with alow-grade inflammation, increased triglyceride elevel sand predicts cardiovascular disease inmales below 50 years. Clin Endocrinol. 2004;61:232–238. doi: 10.1111/j.1365-2265.2004.02088.x. [DOI] [PubMed] [Google Scholar]
- 30.Imaizumi M, Akahoshi M, Ichimaru S, et al. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab. 2004;89:3365–3370. doi: 10.1210/jc.2003-031089. [DOI] [PubMed] [Google Scholar]
- 31.Tuzcua Bahceci M, Gokalp D, Tuzun Y, Gunes K. Subclinical hypothyroidism may beassociated with elevated high-sensitive c-reactive protein (lowgradeinflammation) and fasting hyperinsulinemia. Endocr J. 2005;52:89–94. doi: 10.1507/endocrj.52.89. [DOI] [PubMed] [Google Scholar]; Radaideh A, El-Khateeb M, Batieha AM, Nasser AS, Ajlouni KM. Thyroid function and thyroid autoimmunityin patients with type 1 diabetes mellitus. Saudi Med J. 2003;24(4):352–355. [PubMed] [Google Scholar]
- 32.Heljić B. Heljić B, Dilić M, Čengić M, Čengić F, Lončarević N. U: Diabetes mellitus-klinički aspekti. Sarajevo: Jež; 2002. Diabetes mellitus; pp. 16–26. IDF Diabetes Atlas, 2009. [Google Scholar]
- 33.Müller MJ, Mitchinson PE, Paschen U, Seitz HJ. Glucoregulatory function of glucagon in hypo-, eu- and hyperthyroid miniature pigs. Diabetologia. 1998;31:368–374. doi: 10.1007/BF02341505. [DOI] [PubMed] [Google Scholar]
- 34.Canaris GJ, Manowitz NR, Mayor G, Ridgway EC. Frequency of thyroid dysfunction in diabetic patients: value of annual screening. Arch Intern Med. 2000;160:526–534. doi: 10.1001/archinte.160.4.526. [DOI] [PubMed] [Google Scholar]
- 35.Schroner Z, Lazurova I. Diabetes mellitus and subclinical thyroid dysfunctions. Diabetologie Metabolismus Endokrinologie Vyziva. 2006;9(1):1–12. [Google Scholar]