Abstract
Aims
We determined the role of the Rho kinase (ROCK) isoforms in diabetes-induced vascular endothelial dysfunction and enhancement of arginase activity and expression.
Methods and results
Studies were performed in aortic tissues from haplo-insufficient (H-I) ROCK1 and ROCK2 mice and wild-type (WT) mice rendered diabetic with streptozotocin and in bovine aortic endothelial cells (BAECs) treated with high glucose (HG, 25 mM). Protein expression of both ROCK isoforms was substantially elevated in aortas of WT mice after 8 weeks of diabetes and in BAECs after 48 h in HG. Impairment of endothelium-dependent vasorelaxation of aortas was observed in diabetic WT mice. However, there was no impairment in aortas of diabetic ROCK1 H-I mice and less impairment in aortas of diabetic ROCK2 H-I mice, compared with non-diabetic mice. These vascular effects were associated with the prevention of diabetes-induced decrease in nitric oxide (NO) production and a rise in arginase activity/expression. Acute treatment with the arginase inhibitor, BEC, improved endothelium-dependent vasorelaxation of aortas of both diabetic WT and ROCK2, but not of ROCK1 mice.
Conclusion
Partial deletion of either ROCK isoform, but to a greater extent ROCK1, attenuates diabetes-induced vascular endothelial dysfunction by preventing increased arginase activity and expression and reduction in NO production in type 1 diabetes. Limiting ROCK and arginase activity improves vascular function in diabetes.
Keywords: Type 1 diabetes, Vascular function, Arginase, Rho kinase, Isoforms
1. Introduction
In diabetic patients, vascular disease is a major cause of morbidity and mortality.1 Endothelial dysfunction is considered a critical factor in the development of diabetic vascular disease.2,3 A hallmark of endothelial dysfunction is decreased nitric oxide (NO) bioavailability, which may be caused by the inactivation of NO by oxidative stress and/or reduced expression and function of endothelial NO synthase (eNOS). Studies in macrophages and vascular endothelial cells (ECs) have shown that arginase, an enzyme in the urea cycle, can be an important limiting factor in NO production by competing with NOS for their common substrate, l-arginine.4,5 Associated with this mechanism, increased arginase activity has been implicated in vascular dysfunction associated with diabetes, atherosclerosis, hypertension, asthma, inflammation, and ageing.5–10 Furthermore, our previous studies have shown that treatment of bovine coronary ECs with arginase I siRNA can prevent the high-glucose (HG)-induced impairment of NO production.5 Additionally, knockout of arginase I or II gene can partially prevent diabetes-induced endothelium-dependent vascular dysfunction (EDVD) in mouse aorta and cavernosal tissues.11,12 Therefore, regulatory mechanisms of arginase activity in diabetic blood vessels and ECs exposed to HG need to be understood and under extensive study.
Much evidence indicates that expression and activity of eNOS are regulated by the RhoA/ROCK pathway.13,14 Moreover, inhibition of ROCK improves diabetes and hypertension-induced endothelial dysfunction.15–18 Studies have demonstrated that the small GTPase, RhoA, and its downstream effector ROCK are involved in the activation of arginase in diabetes and upon exposure to HG.5,19 Elevation of arginase activity/expression can be blocked by inhibiting either RhoA or ROCK.5,19–21 These data indicate that activation of the RhoA/ROCK pathway is a critical step towards elevated arginase activity and expression in the vasculature.
Two isoforms of ROCK have been identified in mammalian tissues. ROCK1 is preferentially expressed in the lung, liver, spleen, kidney, and testis, whereas ROCK2 is most highly expressed in the brain and heart.22,23 Both ROCK1 and ROCK2 are expressed in vascular ECs and smooth muscle cells (SMCs). Regulation of ROCK isoform activity and expression is not well understood. However, angiogensin II and IL-1 β have been shown to up-regulate expression of both ROCK isoforms in human coronary vascular SMCs by a mechanism involving activation of protein kinase C and NF-kappa B.24 Additionally, lysophosphatidic acid-induced up-regulation of intercellular adhesion molecule 1 and vascular cell adhesion molecule-1 has been shown to involve activation of ROCK2 in human umbilical vein ECs.25 However, the specific role of ROCK isoforms in diabetes-induced vascular impairment is unknown. Therefore, our study was designed to evaluate the specific role of ROCK1 and ROCK2 in diabetes-induced vascular endothelial dysfunction.
2. Methods
Detailed methods are provided in Supplementary material online.
2.1. Diabetic mice
Our investigations conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication, 8th edition, 2011) and were conducted with the approval of the Georgia Health Sciences University's Animal Use for Research and Education Committee. Ten-week-old male C57BL/6J mice and heterozygous ROCK 1 and ROCK 2 knockout (KO) mice were rendered diabetic with streptozotocin (STZ, 50 mg/kg) injected ip every other day up to three times. Mice with blood glucose levels >350 mg/dL were considered diabetic. Body weight and blood glucose levels of each mouse were measured at the time of injections and 8 weeks after treatment, when experiments were performed.
2.2. Cell culture
Bovine aortic endothelial cells (BAECs) were used from passages 4 to 7. They were maintained, prior to treatments, in M-199 media (Invitrogen, Carlsbad, CA, USA) containing 0.2% FBS, 50 µM l-arginine, 100 U/mL penicillin, 100 µg/mL streptomycin, and l-glutamine.
2.3. Aortic tissue preparation
Mice were anaesthetized with a mixture of ketamine/xylazine (100:10 mg/kg, ip) and supplemented as necessary to obtain a surgical level of anaesthesia as assessed by pinching of toes with a haemostat. The excised aorta was rapidly placed in chilled Krebs's solution. Half of each aorta was denuded of endothelium by gently rubbing the intimal surface with a needle. The absence of endothelium was determined by observing no relaxation response to acetylcholine (ACh, 10−6 M). The endothelium was left intact in the other half of the aorta. In this portion of the aorta, robust relaxation was observed in response to ACh (10−6 M).
2.4. Vascular function studies
The intact (E+) and endothelium-denuded (E−) aortas were cut into 2 mm rings. Rings were mounted in 5 mL myograph chambers (DMT). After 1 h equilibration, the ability of the preparation to develop contraction was assessed by applying 80 mM KCl solution. Cumulative concentration–response curves to ACh (endothelium-dependent vasodilator) and sodium nitroprusside (SNP, NO donor) were obtained in rings pre-contracted with α1-adrenergic receptor agonist, phenylephrine (PE, 10−7 to 10−6 M), to produce matching contractions in the study groups.11
2.5. Assessment of NO production by 4,5-diaminofluorscein
NO production was determined by using the fluorescent NO indicator 4,5-diaminofluorescein diacetate (DAF-2 DA).26 Serial cross-section rings (10 µm) from the OCT-frozen aorta with or without arginase inhibitor BEC treatment (10−4 M) were incubated in the dark at 37°C in HEPES buffer containing DAF-2 DA (10−7 M) for 15 min. The negative control was incubated with L-NAME (10−6 M). After fixation in 4% paraformaldehyde, aortic sections were examined under a microscope at ×100 magnification. Green fluorescence intensity was quantified using the MetaMorph image analysis software (version 6.3r7).
2.6. Blood pressure measurement
Systolic arterial pressure was determined by non-invasive tail-cuff plethysmography.27 The tail of each mouse was inserted in a tail-cuff attached to a pressure monitoring system. Ten measurement cycles were recorded. The mean of last four recordings, among which there was <10 mmHg difference, was accepted as systolic blood pressure (BP).
2.7. Arginase activity assay
Arginase activity was assayed by measuring urea produced from l-arginine as previously described.28 The supernatant from the pulverized mouse aorta or cell lysate (25 µL) was heated with MnCl2 (10−3 M) for 10 min at 56°C to activate arginase. The mixture was then incubated with 50 µL l-arginine (0.5 M, 1 h, 37°C) to hydrolyse the l-arginine. The hydrolysis reaction was stopped with acid and the mixture was heated at 100°C with 25 µL α-isonitrosopropiophenone (9% α-ISPF in EtOH) for 45 min for the colorimetric reaction. Samples were kept in dark at room temperature for 10 min and absorbance was measured at 540 nm.
2.8. Western blot analysis
Equal amounts of protein from homogenized aortas or BAECs were separated by electrophoresis using a 10% SDS–polyacrylamide pre-cast gel and transferred to nitrocellulose membrane. Protein expression levels for RhoA, arginase I, ROCK1, and ROCK2 were assessed and normalized to total actin.
2.9. Measurement of active RhoA
RhoA activation was determined by the detection of membrane-bound Rho.21 Homogenized mice aortas were centrifuged at 100 000g for 20 min at 4°C. Precipitate was solubilized in 1% Triton X-100 extraction buffer to obtain membrane fraction. Equal amounts of protein were loaded for western blot.
2.10. Statistical analysis
Data are shown as mean ± SEM of n experiments. Statistical comparisons were made using one-way and two-way analysis of variance (ANOVA) with Tukey's post hoc test. P < 0.05 was considered significant.
3. Results
3.1. Blood glucose, body weight, and BP
In our model, diabetes caused a significant increase in blood glucose levels and a reduction in the growth of body weight in wild-type (WT), ROCK1, and ROCK2 KO mice. Furthermore, diabetes significantly increased BP in WT mice compared with WT non-diabetic controls, but had no effect on BP in ROCK1 and ROCK2 KO mice. ROCK1 and ROCK2 KO control mice also had lower basal BP level than WT control mice (Table 1).
Table 1.
Blood glucose, body weight, and BP of WT, WT-D, ROCK1, ROCK1-D, ROCK2, ROCK2-D mice
| WT | WT-D | ROCK1 | ROCK1-D | ROCK2 | ROCK2-D | |
|---|---|---|---|---|---|---|
| Blood glucose (mg/dL) | 162.2 ± 15.1 | 377.9 ± 22.3** | 167.3 ± 17.2 | 411.2 ± 32.9** | 162.2 ± 9.3 | 390.4 ± 26.0** |
| Body weight (g) | 25.9 ± 0.5 | 20.4 ± 0.7** | 26.9 ± 0.9 | 18.8 ± 0.7** | 26.0 ± 1.2 | 19.1 ± 0.6** |
| BP/systolic (mmHg) | 108.3 ± 4.3 | 130.2 ± 6.1* | 92.1 ± 1.7* | 92.5 ± 1.9* | 88.5 ± 2.0* | 94.7 ± 2.6* |
Values are means ± S.E.M.
WT-D, wild-type diabetic; ROCK1, ROCK1+/−; ROCK1-D, diabetic ROCK1; ROCK2, ROCK2+/−; ROCK2-D, diabetic ROCK2.
*P < 0.05, vs. WT control group; **P < 0.05, diabetic vs. the time-matched control group. n = 8–10 mice/group.
3.2. Hyperglycaemia-induced activation of RhoA and induction of ROCK1 and ROCK2 expression
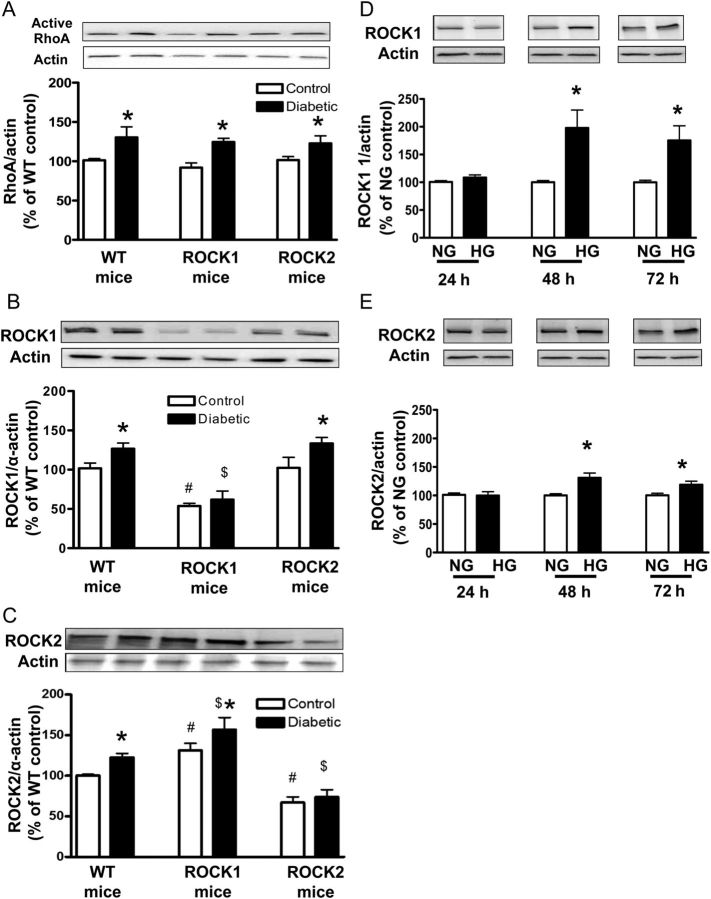
To investigate the role of the RhoA/ROCK pathway in diabetes-induced EDVD, we determined the effect of diabetes and HG on levels of active RhoA in aortas from all animal groups and expression of ROCK1 and ROCK2 in mice and HG-treated BAECs. Diabetes significantly increased the level of active RhoA compared with their respective controls in all experiment groups (Figure 1A). Diabetic WT aortas showed increased ROCK1 and ROCK2 protein levels compared with non-diabetic WT aortas (Figure 1B and C). Similarly, diabetes significantly increased the ROCK1 expression in ROCK2 KO aortas and the ROCK2 expression in ROCK1 KO aortas, compared with their respective controls. Expression of ROCK1 and ROCK2 were both reduced by ∼50% in aortas from the respective ROCK1 and ROCK2 KO control and diabetic mice compared with WT control (Figure 1B and C). No difference of ROCK1 expression was observed between ROCK2 KO and WT aortas in non-diabetic or diabetic groups (Figure 1B). However, both ROCK1 KO control and diabetic aortas had higher ROCK2 expression than WT control and diabetic aortas (Figure 1C).
Figure 1.
Levels of active RhoA expression (A) and ROCK1 (B) and ROCK2 (C) expression in aortas from WT and ROCK1+/− (ROCK1) and ROCK2+/− (ROCK2) control and diabetic mice. Data are expressed as percentage of WT. Values are represented as means ± SEM, n = 4–6 mice/group. *P < 0.05, diabetic mice vs. control mice, #P < 0.05, ROCK1 or ROCK2 control mice vs. WT control mice; $P < 0.05, ROCK1-D or ROCK2-D mice vs. WT-D mice. Levels of ROCK1 (D) and ROCK2 (E) expression in HG (25 mM)-treated BAECs. BAECs were cultured in HG media for indicated time periods. Results were quantified by densitometry and both ROCK isoforms were normalized to actin levels. Values for normal glucose (NG, 5.5 mM) at respective time points were considered as 100% and data are represented as percentage of respective NG control. Values are expressed as means ± SEM, n = 4–6/group. *P < 0.05 vs. its NG control.
Treatment of BAECs with HG (25 mM) for 48 h also caused significant increases in ROCK1 and ROCK2 levels above control levels, by 2- and 1.3-fold, respectively. The enhancement in both proteins was maintained through 72 h (Figure 1D and E).
3.3. ROCK deletion prevents diabetes-induced decrease in NO production in aortas
DAF-2 fluorescence intensity (indicator of available NO) was assessed in previously frozen aortic sections (Figure 2). Intensity of DAF-2 fluorescence was significantly reduced in diabetic WT compared with the control WT group. This reduction in NO production was reversed by treatment with the arginase inhibitor BEC (10−4 M, 1 h) (Figure 2A and B). In non-diabetic groups, aortas of both ROCK haplo-insufficient (H-I) mice presented a much higher fluorescence intensity compared with WT mice. Additionally, the diabetes-induced reduction in NO levels was prevented in ROCK1 aortas, and partially prevented in ROCK2 aortas. Arginase inhibitor BEC further elevated the intensity level in diabetic ROCK2 aortas (Figure 2A and B). L-NAME pre-treatment blocked DAF-2 fluorescence in control ROCK2 aortas (Figure 2A).
Figure 2.
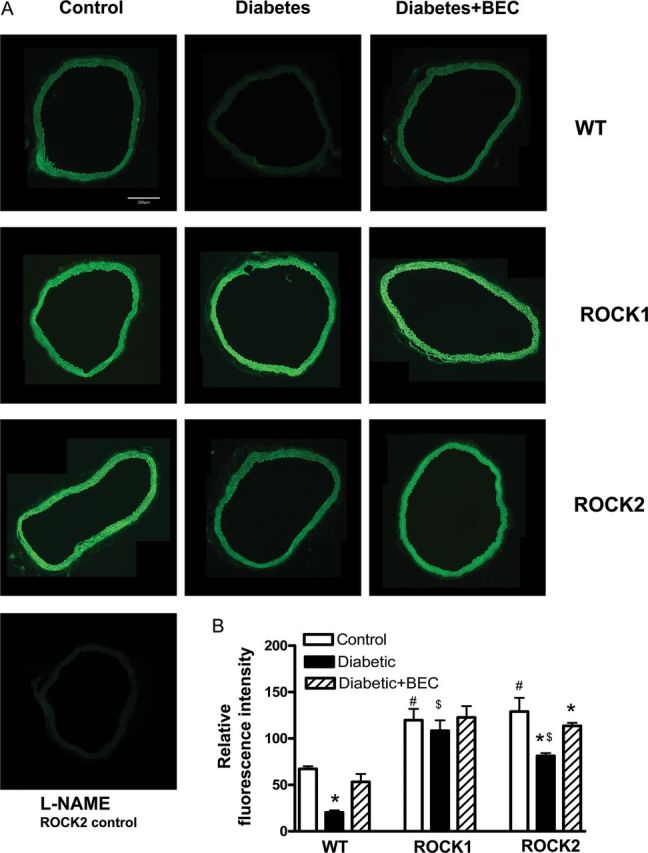
NO production assessment by DAF-2 in aortic rings from WT, ROCK1+/− (ROCK1) and ROCK2+/− (ROCK2) control, diabetic (D) mice and diabetic mice treated with arginase inhibitor BEC (10−4 M, 1 h). Representative DAF-2 fluorescence images were taken in aortic rings from all groups, corrected for fluorescence in the presence of L-NAME (A). Fluorometric analysis of DAF-2-loaded aortic rings in all groups is in (B). Values are represented as means ± SEM, n = 4–6 mice group. *P < 0.05 vs. its non-diabetic mice; #P < 0.05, ROCK1 or ROCK2 control mice vs. WT control mice; $P < 0.05, ROCK1-D or ROCK2-D mice vs. WT-D mice.
3.4. Prevention of diabetes-induced endothelial-EDVD by ROCK deletion
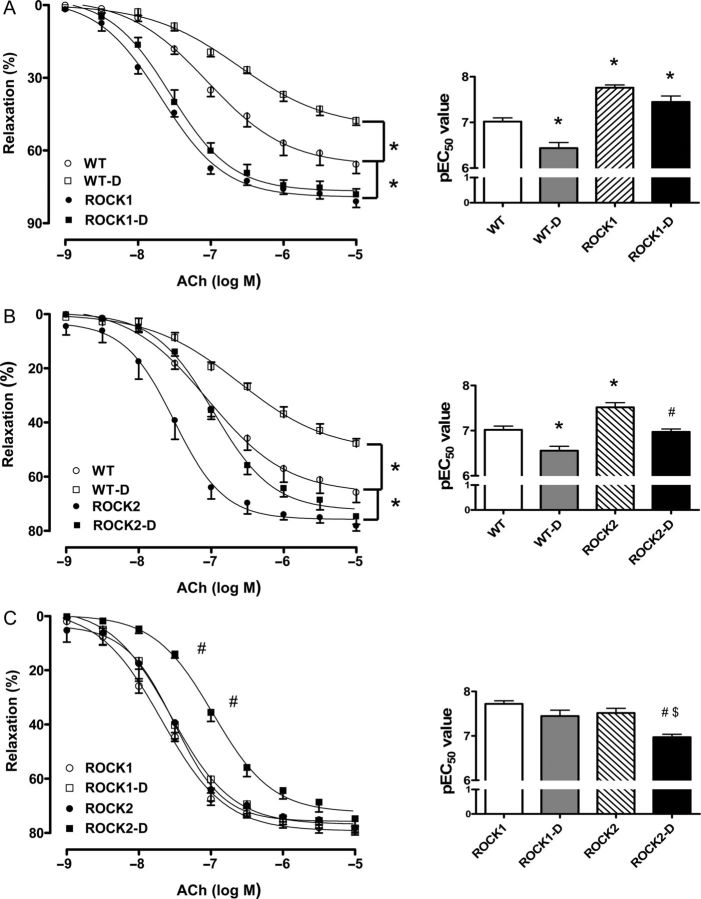
To determine the role of ROCK isoforms in diabetes-induced EDVD, aortic relaxation responses to ACh were assessed in aortas of STZ-induced diabetic and control WT and H-I ROCK1 or ROCK2 mice (Figure 3). Comparison of vasorelaxation responses in the WT diabetic vs. WT non-diabetic group revealed a significant EDVD as evident by an increased EC50 and decreased maximal response (Emax) (Figure 3A).
Figure 3.
Endothelium-dependent vasorelaxation in aortas of WT, ROCK1+/− (ROCK1) and ROCK2+/− (ROCK2) control, and diabetic (D) mice. Endothelium-mediated relaxation to ACh (10−9 to 10−5M) was assessed in aortic rings from all groups. (A–C) Comparisons between genotypes. Data were calculated as changes from the contraction produced by PE (10−7 to 10−6 M) in each tissue, which was taken as 100%. To the right side of each relaxation curve are pEC50 values (negative logarithm of the concentration of ACh effective in producing 50% of the maximum relaxation). Data are represented as means ± SEM, n = 5–8 mice/group. *P < 0.05 vs. WT control mice; #P < 0.05 vs. ROCK2 control mice; $P < 0.05 vs. ROCK1-D mice.
Vasorelaxation responses in non-diabetic ROCK1 and ROCK2 aortas were both markedly enhanced compared with the non-diabetic WT tissue as indicated by a decreased EC50 and a greater Emax value (Figure 3A and B). Furthermore, diabetes had no effect on vasorelaxation responses of aortas of ROCK1 mice, but increased EC50 in the ROCK2 aortas compared with their respective controls (Figure 3A and B). Although no differences were observed in Emax values between the ROCK1 and ROCK2 diabetic and non-diabetic groups, the ROCK1 diabetic group displayed a decreased EC50 compared with the ROCK2 diabetic group (Figure 3C). This finding indicates a greater role of ROCK1 in diabetes-induced EDVD.
3.5. Prevention of diabetes-induced EDVD by acute inhibition of arginase
Acute treatment of aortic rings of WT diabetic mice with arginase inhibitor BEC (100 μM, 1 h) improved EDVR, as shown by a marked increase in the Emax value and decrease in EC50 in response to ACh (Figure 4A). BEC treatment also improved EDVR in the ROCK2 diabetic tissues as shown by a decreased EC50 (Figure 4C). However, no significant effect of BEC treatment was observed in ROCK1 diabetic aortas (Figure 4B) or in non-diabetic WT, ROCK1, or ROCK2 aortas (data not shown). These data indicate that aortas of ROCK1 diabetic mice and all non-diabetic mice have adequate l-arginine available for normal EC-dependent vasorelaxation, suggesting that no appreciable competition exists between arginase and NOS for their substrate.
Figure 4.
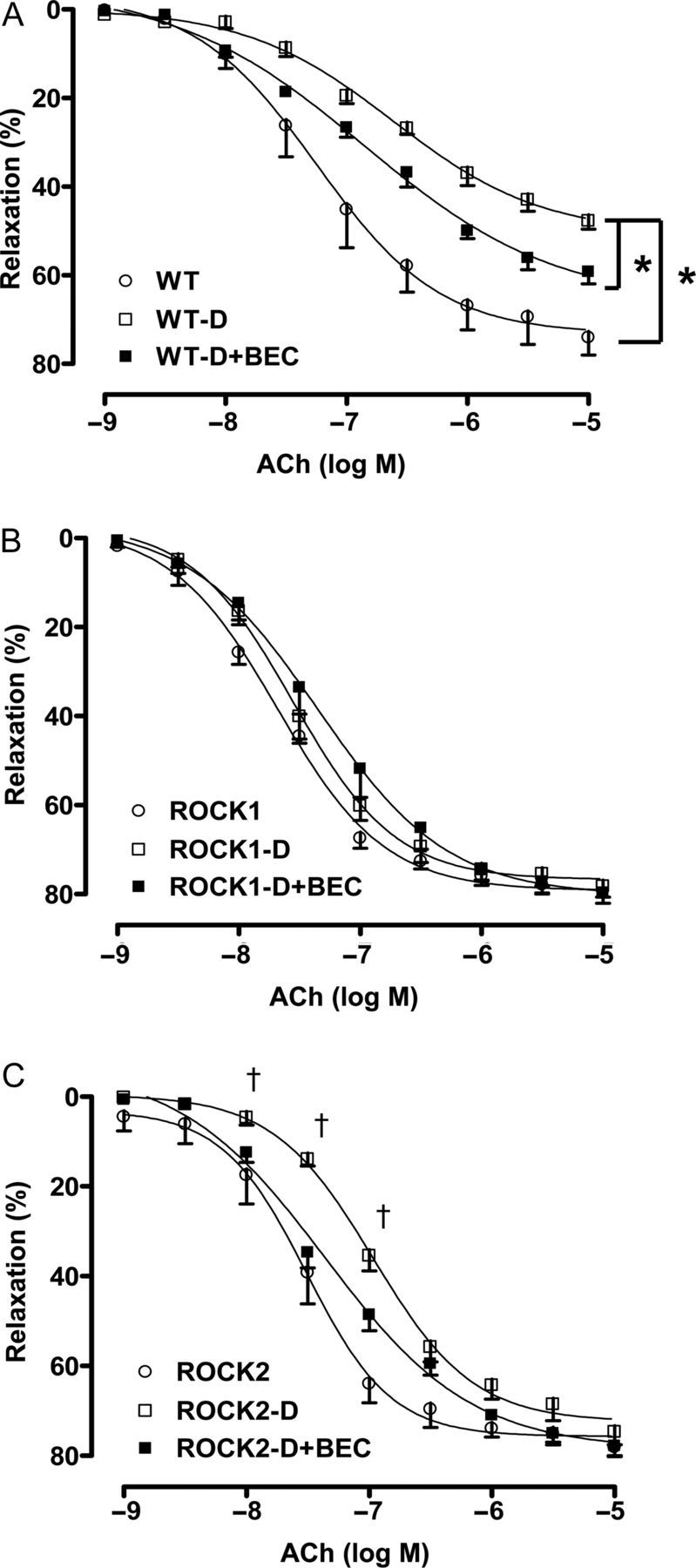
Effect of arginase inhibition on endothelium-dependent vasorelaxation. ACh (10−9 to 10−5 M)-mediated relaxation was determined before and after the application of arginase inhibitor, BEC (100 μM) into the baths containing the aortic rings from ROCK1+/− (ROCK1), ROCK2+/− (ROCK2), and WT diabetic (D) mice. Data were calculated relative to the contraction produced by PE (10−7 to 10−6 M) in each tissue, which was taken as 100%. Data are represented as means ± SEM, n = 5–8 mice/group. *P < 0.05 vs. WT-D mice; †P < 0.05 ROCK2-D mice vs. ROCK2-D + BEC mice.
3.6. SNP-induced aortic relaxation
The EC50 and Emax values for relaxation to the NO donor SNP were not different among all the groups (data not shown). This suggests that, in our diabetic model, partial expression of the ROCK1 or ROCK2 gene reduces vascular endothelial dysfunction only and has no direct effect on smooth muscle relaxation.
3.7. Reduction of diabetes-induced enhanced contractile responses by ROCK deletion
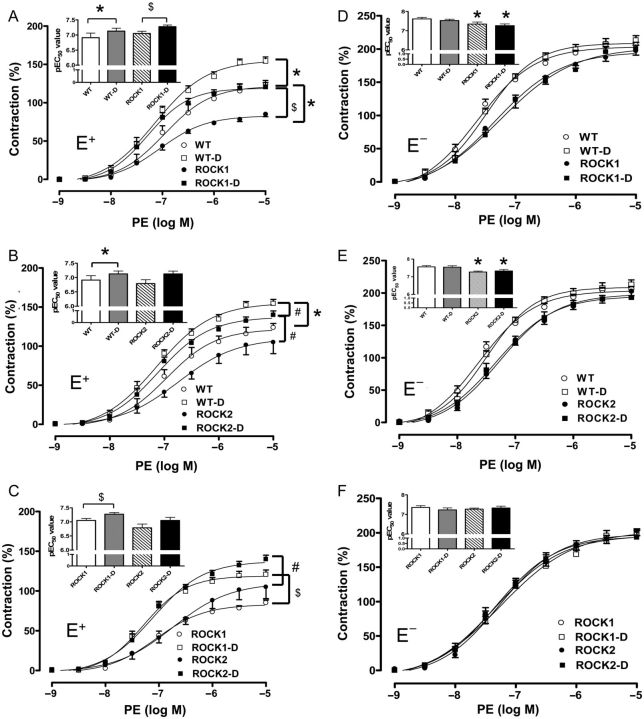
Treatment of intact (E+) and endothelium-denuded (E−) aortic rings with phenylepherine (PE) caused concentration-dependent contractions in all groups.
In the E+ aortas, diabetes increased the Emax value in vessels from the WT, ROCK1, and ROCK2 mice and decreased EC50 in WT and ROCK1 aortas when compared with their respective controls. The EC50 in the ROCK2 diabetic group also tended to be lower than ROCK2 control, but it is not significantly different (Figure 5A and B). The Emax value was significantly blunted in the ROCK1 non-diabetic aortas when compared with WT non-diabetic aortas, but not in the ROCK2 non-diabetic aortas (Figure 5A and B). There was no significant difference between contractile responses of the ROCK1 and ROCK2 vessels in either control or diabetic conditions (Figure 5C).
Figure 5.
Contractile-response curve with PE in E+ and E− aortas of WT, ROCK1+/− (ROCK1) and ROCK2+/− (ROCK2) non-diabetic and diabetic (D) mice. Contraction to α1-adrenergic receptor agonist PE (10−9 to 10−5 M) was assessed in aortic rings from all groups. (A–C and D–F) Comparisons between genotypes. Data were calculated relative to the maximal changes from the contraction produced by KCl (80 mM), which was taken as 100%. To the left top side of each contraction curve are pEC50 values (negative logarithm of the concentration of PE effective in producing 50% of the maximum contraction). Data are represented as means ± SEM, n = 5–8 mice/group. *P < 0.05 vs. WT control mice; $P < 0.05 vs. ROCK1 control mice; #P < 0.05 vs. ROCK2-D mice.
In the E− aortas, diabetes did not change the Emax and EC50 level compared with their respective controls in all the experiment groups (Figure 5D–F). However, ROCK1 and ROCK2 mice exhibited higher EC50 compared with WT mice in either control or diabetic conditions (Figure 5D and E). There was no difference in EC50 between no-diabetic WT, ROCK1, and ROCK2 vessels (Figure 5F).
3.8. Attenuation of diabetes/HG-stimulated arginase activation by deletion or inhibition of ROCK
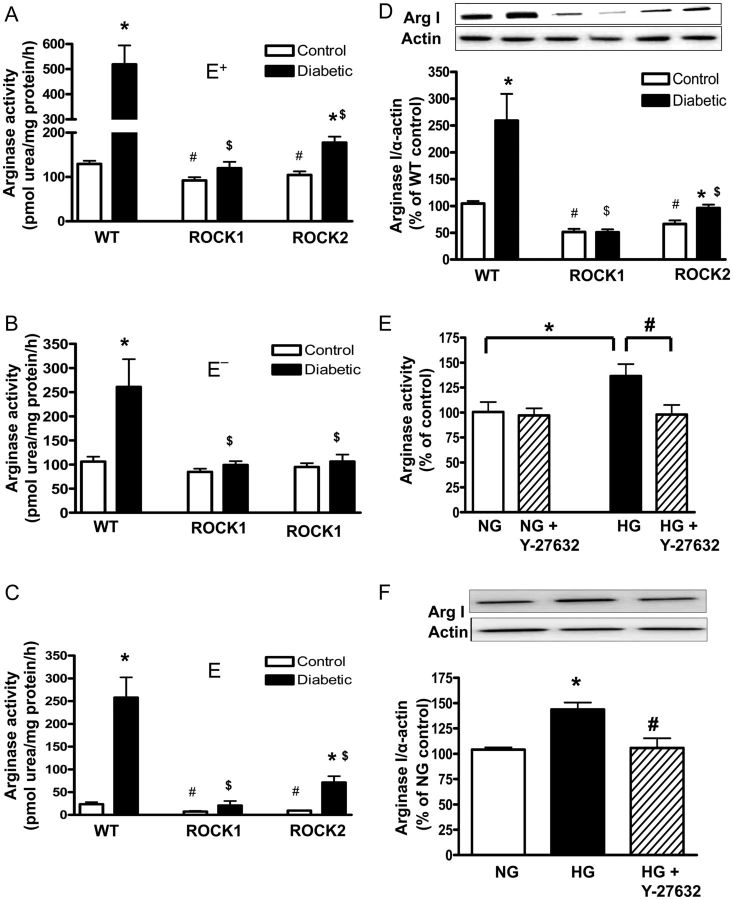
To assess the role of total vascular- and endothelium-dependent RhoA/ROCK-induced activation of arginase in diabetes-induced EDVD, arginase activities were analysed in both E+ and E− aortas. The arginase activity in the endothelium was calculated by the subtraction of values for the E− aorta from values for E+ tissues.
In the E+ aorta and in the endothelium itself, diabetes induced a much higher arginase activity as shown by 4-fold and 10-fold increase in the WT aorta compared with non-diabetic WT tissues, respectively (Figure 6A and C). The diabetes-induced elevation of arginase activity was absent in aortas of ROCK1 mice and markedly diminished in ROCK2 mice. Arginase activity in both E+ tissues and endothelia of non-diabetic ROCK1 and ROCK2 mice was also significantly lower than in non-diabetic WT aortas.
Figure 6.
Arginase activity in the E+ (A) and E− aortic tissue (B) and Arginase I expression in E+ (D) from WT, ROCK1+/− (ROCK1) and ROCK2+/− (ROCK2) control and diabetic mice. Arginase activity was measured in E+ and E− aortas from all groups. The values of arginase activity in the endothelium (C) are calculated by the subtraction of the activity values in E− from those in E+. Values are represented as means ± SEM, n = 3–8/group. *P < 0.05 vs. its control mice; #P < 0.05, ROCK1 or ROCK2 control mice vs. WT control mice; $P < 0.05, ROCK1-D or ROCK2-D mice vs. WT-D mice. Arginase activity (E) and expression (F) in HG (25 mM)-treated BAECs. Some BAECs were pretreated with the ROCK inhibitor, Y-27632 (10 μM) for 1 h and then treated with normal glucose (NG, 5.5 mM) or HG for 72 h. Arginase activity and arginase I expression were analysed in these cells. Values are expressed as means ± SEM, n = 4 mice/group; *P < 0.05, HG vs. NG; #P < 0.05, Y-27632 vs. HG.
Furthermore, E− aortas of WT diabetic mice showed two-fold higher arginase activity than those of WT controls (Figure 6B). The diabetes-induced elevation of arginase activity was absent in both ROCK1- and ROCK2-deficient E− aortas. However, no differences in arginase activity were observed in E− aortas from non-diabetic ROCK1, ROCK2, and WT mice.
In association with elevated arginase activity in E+ aortas, arginase I protein levels were also increased in diabetic WT aortas (Figure 6D). This diabetes-induced increase was not observed in ROCK1 aortas and was greatly blunted in ROCK2 aortas. Furthermore, basal levels of aortic arginase I expression in the non-diabetic KO mice were also reduced when compared with WT mice. Arginase II expression was not different among the groups (data not shown).
To further determine the involvement of ROCK activity in hyperglycaemia-induced elevation of arginase function in the vascular endothelium, we measured arginase activity and expression in BAECs exposed to normal or HG (25 mM) media in the presence or absence of ROCK inhibitor Y27632 (Figure 6E). Under HG conditions, both arginase activity and arginase I expression were elevated by 1.4-fold vs. normal glucose; these effects were completely blocked by co-treatment with the ROCK inhibitor Y27632 (Figure 6F). These data indicate that HG-induced elevation of arginase function in aortic EC is mediated through the RhoA/ROCK pathway.
4. Discussion
We and others have previously observed that diabetes or HG raises arginase activity/expression and levels of active RhoA in human and bovine coronary ECs and rat vessels.5,20 Inhibitors of ROCK and RhoA were both shown to prevent this rise in arginase activity and expression.5,20,29 Our present study demonstrates that in HG-treated bovine aortic ECs, the increase of arginase activation was abolished by the ROCK inhibitor Y-27632, further confirming that RhoA/ROCK are upstream regulators of HG-induced arginase activation. Western blot analysis showed significant increases in levels of both ROCK isoforms in the HG-treated ECs. Partial deficiency of ROCK1 has been reported to completely block the increase of ROCK activity in HG-treated murine lung ECs.30 Consistent with this observation, our studies of HG-treated BAECs show that the relative increase in ROCK1 was 0.7-fold greater than that of ROCK2. We also find that elevation of active RhoA and ROCK1/ROCK2 expression in whole aortas from diabetic WT mice is associated with increased arginase I expression and arginase activity and that these effects are lacking in ROCK-deficient mice.
These findings support the involvement of both RhoA and ROCK in diabetes-induced increases in arginase activity. Furthermore, ROCK1 expression is reduced ∼50% in ROCK1 KO mice, with no compensatory change in ROCK2. A reduction of ROCK2 expression is observed in ROCK2 KO mice. However, ROCK2 expression was significantly increased in ROCK1 KO non-diabetic and diabetic mice, a seemingly compensatory effect. Further, diabetes-induced increases of arginase I expression and arginase activity in WT aortas are absent in ROCK1-deficient tissue. These results indicate that the ROCK2 gene has less effect on the regulation of arginase than the ROCK1 gene.
The two isoforms of ROCK, ROCK1 and ROCK2, have been reported to differentially regulate vascular functions in both tissue culture and animal models.24,30–32 For example, both ROCK isoforms, but ROCK2 to a greater extent, are up-regulated by angiotensin II (Ang II) and IL-1β in human coronary vascular SMCs. This effect is mediated by PKC and NF-kappa B.24 In contrast, HG-induced elevation of plasminogen activator inhibitor-1 protein levels can be completely blocked in heterozygous ROCK1 KO mouse ECs.30 Similarly, ROCK1, but not ROCK2, KO mice have substantially reduced vascular inflammation and neointima formation after flow cessation-induced vascular injury in a ligated carotid artery model.32 Our study used ROCK1 and ROCK2 KO mice in a model of type 1 diabetes to determine for the first time the specific role of ROCK1 and ROCK2 in mediating vascular endothelial-dependent dysfunction via the activation of arginase.
Several studies have shown that ROCK is an important mediator of insulin signalling and glucose metabolism.33–35 In our study, deletion of one copy of the ROCK1 or ROCK2 genes did not prevent glucose elevation or reduced growth in STZ-induced mice, and no difference in these variables was noted among the non-diabetic groups. However, diabetes-induced elevation of BP seen in the WT mice was not observed in either the ROCK1 or ROCK2 diabetic mice. In addition, lower basal BP levels were observed in non-diabetic ROCK1 and ROCK2 mice compared with WT. This finding is in agreement with earlier studies showing that inhibition of ROCK decreases BP level in various experimental models, including normotensive animals.36–38 We also observed lower levels of arginase expression and activity in the whole aorta from both diabetic and control ROCK-deficient mice compared with WT mice. There is a 10-fold elevation of arginase activity in the endothelium of the WT diabetic aorta, compared with WT control. This increase is six-fold higher than in the E+ aorta and 7.5-fold higher than in the E− aorta. These data indicate that diabetes mainly targets EC elevation of arginase activity in aortic tissue.
Although emerging evidence shows that ROCK inhibitors such as fasudil and Y27632 can prevent vascular dysfunction in diabetic animal models,29,39,40 isoform involvement has not been determined, as no isoform-specific inhibitors are available. In our study, endothelium-dependent vascular relaxation (EDVR) was evaluated in aortic tissues from WT, ROCK1, and ROCK2 mice. Severe impairment of EDVR observed in 8-week diabetic WT mice was completely prevented in ROCK1 and partially rescued in ROCK2 mice. These effects are associated with the prevention of diabetes-induced reduction of aortic NO levels in both ROCK1 and ROCK2 mice. Furthermore, the rise of arginase activity and arginase I protein levels by diabetes was absent in ROCK1 and markedly blunted in ROCK2 aorta. These results suggest that ROCK1 plays a dominant role in diabetes-induced enhancement of aortic arginase activity/expression.
Endothelium-derived NO is a key mediator of EDVR.41 In ECs, NO is produced by the activity of eNOS on its substrate l-arginine. In addition to enhanced level of arginase, activation of RhoA and ROCK can decrease levels of eNOS expression in ECs by decreasing the stability of eNOS mRNA.42 In addition, RhoA/ROCK can negatively regulate Akt (protein kinase B) and eNOS, leading to reduced NO production.14,43 Thus, ROCK-deficient mice may have higher eNOS function than WT mice through multiple mechanisms including lower arginase activity, higher Akt signalling, and decreased eNOS expression. We have not studied this pathway in the current study.
Activation of RhoA/ROCK pathway also has been reported to enhance vascular SMCs contraction by phosphorylating the regulatory subunit of myosin light chain (MLC) phosphatase, and thereby inhibiting MLC phosphatase activity and increasing levels of phospho-MLC.37 Our present results show that PE-induced E+ aortic contractions are increased in 8-week diabetic WT mice, and that these contractions are attenuated in both diabetic ROCK1 and ROCK2 mice. In E− aorta, the EC50 values for PE-induced contractions are higher in both ROCK1 and ROCK2 aorta compared with WT aorta in either control or diabetic conditions. Maximal contractions are the same in all six groups. These results proved that in addition to the lower levels of phospho-MLC, the increased vascular NO production is the main factor to cause the reduction of the aortic contraction in both ROCK1 and ROCK2 diabetic mice compared with diabetic WT mice.
Numerous studies have shown that mitogen-activated protein kinases (MAPK), such as p38 MAPK, can be activated by hyperglycaemia, Ang II, and ROS in ECs, aortic tissue, and nerve tissue of type 1 and type 2 diabetes.44–46 Additionally, the elevation of arginase activity and expression is reported to be mediated by the p38 MAPK signalling in macrophages.47 Our recent studies have shown that inhibitors of RhoA activation (simvastatin) or ROCK (Y-27632) prevented both Ang II-induced elevation of phosphorylation of p38 MAPK and arginase activity in ECs.27 These findings strongly indicate that reduced function of RhoA/ROCK downregulates p38 MAPK and arginase activity/arginase I expression. These events could reduce or prevent vascular endothelial dysfunction in STZ-diabetic model.
In summary, our present study provides evidence for a role of both isoforms of ROCK in diabetes- and HG-induced vascular dysfunction. In particular, partial knockout of ROCK1, not ROCK2, can completely prevent elevation of arginase expression/activity and normalize endothelium-dependent vascular function in a model of type 1 diabetes. Thus, targeting ROCK1 may present a novel therapeutic means for preventing diabetes-induced endothelial dysfunction.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
This study was supported by the National Institute Health grants—RO1 HL-70215 and RO1 EY-11766 to R.B.C. and R.W.C.
Acknowledgements
We thank Dr James K. Liao (Chief of Cardiology, University of Chicago School of Medicine) for providing breeding pairs of the ROCK1 and 2 KO mice.
Conflict of interest: none declared.
References
- 1.Cosentino F, Luscher TF. Endothelial dysfunction in diabetes mellitus. J Cardiovasc Pharmacol. 1998;32(Suppl. 3):S54–S61. [PubMed] [Google Scholar]
- 2.John S, Schmieder RE. Impaired endothelial function in arterial hypertension and hypercholesterolemia: potential mechanisms and differences. J Hypertens. 2000;18:363–374. doi: 10.1097/00004872-200018040-00002. doi:10.1097/00004872-200018040-00002. [DOI] [PubMed] [Google Scholar]
- 3.Luscher TF, Creager MA, Beckman JA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part II. Circulation. 2003;108:1655–1661. doi: 10.1161/01.CIR.0000089189.70578.E2. doi:10.1161/01.CIR.0000089189.70578.E2. [DOI] [PubMed] [Google Scholar]
- 4.Chang CI, Liao JC, Kuo L. Arginase modulates nitric oxide production in activated macrophages. Am J Physiol Heart Circ Physiol. 1998;274:H342–H348. doi: 10.1152/ajpheart.1998.274.1.H342. [DOI] [PubMed] [Google Scholar]
- 5.Romero MJ, Platt DH, Tawfik HE, Labazi M, El-Remessy AB, Bartoli M, et al. Diabetes-induced coronary vascular dysfunction involves increased arginase activity. Circ Res. 2008;102:95–102. doi: 10.1161/CIRCRESAHA.107.155028. doi:10.1161/CIRCRESAHA.107.155028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim JH, Bugaj LJ, Oh YJ, Bivalacqua TJ, Ryoo S, Soucy KG, et al. Arginase inhibition restores NOS coupling and reverses endothelial dysfunction and vascular stiffness in old rats. J Appl Physiol. 2009;107:1249–1257. doi: 10.1152/japplphysiol.91393.2008. doi:10.1152/japplphysiol.91393.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson FK, Johnson RA, Peyton KJ, Durante W. Arginase inhibition restores arteriolar endothelial function in Dahl rats with salt-induced hypertension. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1057–R1062. doi: 10.1152/ajpregu.00758.2004. doi:10.1152/ajpregu.00758.2004. [DOI] [PubMed] [Google Scholar]
- 8.Ryoo S, Gupta G, Benjo A, Lim HK, Camara A, Sikka G, et al. Endothelial arginase II: a novel target for the treatment of atherosclerosis. Circ Res. 2008;102:923–932. doi: 10.1161/CIRCRESAHA.107.169573. doi:10.1161/CIRCRESAHA.107.169573. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Baban B, Rojas M, Tofigh S, Virmani SK, Patel C, et al. Arginase activity mediates retinal inflammation in endotoxin-induced uveitis. Am J Pathol. 2009;175:891–902. doi: 10.2353/ajpath.2009.081115. doi:10.2353/ajpath.2009.081115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris SM, Jr, Gao T, Cooper TK, Kepka-Lenhart D, Awad AS. Arginase-2 mediates diabetic renal injury. Diabetes. 2011;60:3015–3022. doi: 10.2337/db11-0901. doi:10.2337/db11-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romero MJ, Iddings JA, Platt DH, Ali MI, Cederbaum SD, Stepp DW, et al. Diabetes-induced vascular dysfunction involves arginase I. Am J Physiol Heart Circ Physiol. 2012;302:H159–H166. doi: 10.1152/ajpheart.00774.2011. doi:10.1152/ajpheart.00774.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Toque HA, Tostes RC, Yao L, Xu Z, Webb RC, Caldwell RB, et al. Arginase II deletion increases corpora cavernosa relaxation in diabetic mice. J Sex Med. 2010;8:722–733. doi: 10.1111/j.1743-6109.2010.02098.x. doi:10.1111/j.1743-6109.2010.02098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laufs U, Liao JK. Post-transcriptional regulation of endothelial nitric oxide synthase mRNA stability by Rho GTPase. J Biol Chem. 1998;273:24266–24271. doi: 10.1074/jbc.273.37.24266. doi:10.1074/jbc.273.37.24266. [DOI] [PubMed] [Google Scholar]
- 14.Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, et al. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467–8477. doi: 10.1128/MCB.22.24.8467-8477.2002. doi:10.1128/MCB.22.24.8467-8477.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Higashi M, Shimokawa H, Hattori T, Hiroki J, Mukai Y, Morikawa K, et al. Long-term inhibition of Rho-kinase suppresses angiotensin II-induced cardiovascular hypertrophy in rats in vivo: effect on endothelial NAD(P)H oxidase system. Circ Res. 2003;93:767–775. doi: 10.1161/01.RES.0000096650.91688.28. doi:10.1161/01.RES.0000096650.91688.28. [DOI] [PubMed] [Google Scholar]
- 16.Shah DI, Singh M. Effect of fasudil on macrovascular disorder-induced endothelial dysfunction. Can J Physiol Pharmacol. 2006;84:835–845. doi: 10.1139/y06-036. doi:10.1139/Y06-036. [DOI] [PubMed] [Google Scholar]
- 17.Arita R, Hata Y, Nakao S, Kita T, Miura M, Kawahara S, et al. Rho kinase inhibition by fasudil ameliorates diabetes-induced microvascular damage. Diabetes. 2009;58:215–226. doi: 10.2337/db08-0762. doi:10.2337/db08-0762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bivalacqua TJ, Champion HC, Usta MF, Cellek S, Chitaley K, Webb RC, et al. RhoA/Rho-kinase suppresses endothelial nitric oxide synthase in the penis: a mechanism for diabetes-associated erectile dysfunction. Proc Natl Acad Sci USA. 2004;101:9121–9126. doi: 10.1073/pnas.0400520101. doi:10.1073/pnas.0400520101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horowitz S, Binion DG, Nelson VM, Kanaa Y, Javadi P, Lazarova Z, et al. Increased arginase activity and endothelial dysfunction in human inflammatory bowel disease. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1323–G1336. doi: 10.1152/ajpgi.00499.2006. doi:10.1152/ajpgi.00499.2006. [DOI] [PubMed] [Google Scholar]
- 20.Ming XF, Barandier C, Viswambharan H, Kwak BR, Mach F, Mazzolai L, et al. Thrombin stimulates human endothelial arginase enzymatic activity via RhoA/ROCK pathway: implications for atherosclerotic endothelial dysfunction. Circulation. 2004;110:3708–3714. doi: 10.1161/01.CIR.0000142867.26182.32. doi:10.1161/01.CIR.0000142867.26182.32. [DOI] [PubMed] [Google Scholar]
- 21.Chandra S, Romero M, Shatanawi A, Alkilany A, Caldwell R, Caldwell R. Oxidative species increase arginase activity in endothelial cells through the RhoA/Rho kinase pathway. Br J Pharmacol. 2012;165:506–519. doi: 10.1111/j.1476-5381.2011.01584.x. doi:10.1111/j.1476-5381.2011.01584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei L, Roberts W, Wang L, Yamada M, Zhang S, Zhao Z, et al. Rho kinases play an obligatory role in vertebrate embryonic organogenesis. Development. 2001;128:2953–2962. doi: 10.1242/dev.128.15.2953. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa O, Fujisawa K, Ishizaki T, Saito Y, Nakao K, Narumiya S. ROCK-I and ROCK-II, two isoforms of Rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996;392:189–193. doi: 10.1016/0014-5793(96)00811-3. doi:10.1016/0014-5793(96)00811-3. [DOI] [PubMed] [Google Scholar]
- 24.Hiroki J, Shimokawa H, Higashi M, Morikawa K, Kandabashi T, Kawamura N, et al. Inflammatory stimuli upregulate Rho-kinase in human coronary vascular smooth muscle cells. J Mol Cell Cardiol. 2004;37:537–546. doi: 10.1016/j.yjmcc.2004.05.008. doi:10.1016/j.yjmcc.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 25.Shimada H, Rajagopalan LE. Rho kinase-2 activation in human endothelial cells drives lysophosphatidic acid-mediated expression of cell adhesion molecules via NF-kappaB p65. J Biol Chem. 2010;285:12536–12542. doi: 10.1074/jbc.M109.099630. doi:10.1074/jbc.M109.099630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Somoza B, Gonzalez MC, Gonzalez JM, Abderrahim F, Arribas SM, Fernandez-Alfonso MS. Modulatory role of the adventitia on noradrenaline and angiotensin II responses role of endothelium and AT2 receptors. Cardiovasc Res. 2005;65:478–486. doi: 10.1016/j.cardiores.2004.10.007. doi:10.1016/j.cardiores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 27.Shatanawi A, Romero MJ, Iddings JA, Chandra S, Umapathy NS, Verin AD, et al. Angiotensin II-induced vascular endothelial dysfunction through RhoA/Rho kinase/p38 mitogen-activated protein kinase/arginase pathway. Am J Physiol Cell Physiol. 2011;300:C1181–C1192. doi: 10.1152/ajpcell.00328.2010. doi:10.1152/ajpcell.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toque HA, Romero MJ, Tostes RC, Shatanawi A, Chandra S, Carneiro ZN, et al. p38 Mitogen-activated protein kinase (MAPK) increases arginase activity and contributes to endothelial dysfunction in corpora cavernosa from angiotensin-II-treated mice. J Sex Med. 2010;7:3857–3867. doi: 10.1111/j.1743-6109.2010.01996.x. doi:10.1111/j.1743-6109.2010.01996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.El-Remessy AB, Tawfik HE, Matragoon S, Pillai B, Caldwell RB, Caldwell RW. Peroxynitrite mediates diabetes-induced endothelial dysfunction: possible role of Rho kinase activation. Exp Diabetes Res. 2010;2010:247861. doi: 10.1155/2010/247861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rikitake Y, Liao JK. Rho-kinase mediates hyperglycemia-induced plasminogen activator inhibitor-1 expression in vascular endothelial cells. Circulation. 2005;111:3261–3268. doi: 10.1161/CIRCULATIONAHA.105.534024. doi:10.1161/CIRCULATIONAHA.105.534024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoneda A, Multhaupt HA, Couchman JR. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol. 2005;170:443–453. doi: 10.1083/jcb.200412043. doi:10.1083/jcb.200412043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, et al. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Immunol. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furukawa N, Ongusaha P, Jahng WJ, Araki K, Choi CS, Kim HJ, et al. Role of Rho-kinase in regulation of insulin action and glucose homeostasis. Cell Metab. 2005;2:119–129. doi: 10.1016/j.cmet.2005.06.011. doi:10.1016/j.cmet.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 34.Begum N, Sandu OA, Ito M, Lohmann SM, Smolenski A. Active Rho kinase (ROK-alpha) associates with insulin receptor substrate-1 and inhibits insulin signaling in vascular smooth muscle cells. J Biol Chem. 2002;277:6214–6222. doi: 10.1074/jbc.M110508200. doi:10.1074/jbc.M110508200. [DOI] [PubMed] [Google Scholar]
- 35.Kanda T, Wakino S, Homma K, Yoshioka K, Tatematsu S, Hasegawa K, et al. Rho-kinase as a molecular target for insulin resistance and hypertension. FASEB J. 2006;20:169–171. doi: 10.1096/fj.05-4197fje. [DOI] [PubMed] [Google Scholar]
- 36.Komers R, Oyama TT, Beard DR, Anderson S. Effects of systemic inhibition of Rho kinase on blood pressure and renal haemodynamics in diabetic rats. Br J Pharmacol. 2011;162:163–174. doi: 10.1111/j.1476-5381.2010.01031.x. doi:10.1111/j.1476-5381.2010.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uehata M, Ishizaki T, Satoh H, Ono T, Kawahara T, Morishita T, et al. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. doi:10.1038/40187. [DOI] [PubMed] [Google Scholar]
- 38.Winaver J, Ovcharenko E, Rubinstein I, Gurbanov K, Pollesello P, Bishara B, et al. Involvement of Rho kinase pathway in the mechanism of renal vasoconstriction and cardiac hypertrophy in rats with experimental heart failure. Am J Physiol Heart Circ Physiol. 2006;290:H2007–H2014. doi: 10.1152/ajpheart.00600.2005. doi:10.1152/ajpheart.00600.2005. [DOI] [PubMed] [Google Scholar]
- 39.Nuno DW, Lamping KG. The role of rho kinase in sex-dependent vascular dysfunction in type 1 diabetes. Exp Diabetes Res. 2010;2010:176361. doi: 10.1155/2010/176361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shah DI, Singh M. Involvement of Rho-kinase in experimental vascular endothelial dysfunction. Mol Cell Biochem. 2006;283:191–199. doi: 10.1007/s11010-006-2679-6. doi:10.1007/s11010-006-2679-6. [DOI] [PubMed] [Google Scholar]
- 41.Rongen GA, Smits P, Thien T. Endothelium and the regulation of vascular tone with emphasis on the role of nitric oxide. Physiology, pathophysiology and clinical implications. Neth J Med. 1994;44:26–35. [PubMed] [Google Scholar]
- 42.Eto M, Barandier C, Rathgeb L, Kozai T, Joch H, Yang Z, et al. Thrombin suppresses endothelial nitric oxide synthase and upregulates endothelin-converting enzyme-1 expression by distinct pathways: role of Rho/ROCK and mitogen-activated protein kinase. Circ Res. 2001;89:583–590. doi: 10.1161/hh1901.097084. doi:10.1161/hh1901.097084. [DOI] [PubMed] [Google Scholar]
- 43.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. doi:10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, et al. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999;103:185–195. doi: 10.1172/JCI3326. doi:10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Costanzo A, Moretti F, Burgio VL, Bravi C, Guido F, Levrero M, et al. Endothelial activation by angiotensin II through NFkappaB and p38 pathways: involvement of NFkappaB-inducible kinase (NIK), free oxygen radicals, and selective inhibition by aspirin. J Cell Physiol. 2003;195:402–410. doi: 10.1002/jcp.10191. doi:10.1002/jcp.10191. [DOI] [PubMed] [Google Scholar]
- 46.Purves T, Middlemas A, Agthong S, Jude EB, Boulton AJ, Fernyhough P, et al. A role for mitogen-activated protein kinases in the etiology of diabetic neuropathy. FASEB J. 2001;15:2508–2514. doi: 10.1096/fj.01-0253hyp. doi:10.1096/fj.01-0253hyp. [DOI] [PubMed] [Google Scholar]
- 47.Chang CI, Zoghi B, Liao JC, Kuo L. The involvement of tyrosine kinases, cyclic AMP/protein kinase A, and p38 mitogen-activated protein kinase in IL-13-mediated arginase I induction in macrophages: its implications in IL-13-inhibited nitric oxide production. J Immunol. 2000;165:2134–2141. doi: 10.4049/jimmunol.165.4.2134. [DOI] [PubMed] [Google Scholar]