Abstract
We performed high throughput transcriptomic profiling with RNA sequencing (RNA-Seq) to uncover network responses in human THP-1 monocytes treated with high glucose (HG). Our data analyses revealed that interferon (IFN) signaling, pattern recognition receptors, and activated interferon regulatory factors (IRFs) were enriched among the HG-upregulated genes. Motif analysis identified an HG-responsive IRF-mediated network in which interferon-stimulated genes (ISGs) were enriched. Notably, this network showed strong overlap with a recently discovered IRF7-driven network relevant to Type 1 diabetes. We next examined if the HG-regulated genes possessed any characteristic chromatin features in the basal state by profiling 15 active and repressive chromatin marks under normal glucose conditions using chromatin immunoprecipitation linked to promoter microarrays. Composite profiles revealed higher histone H3 lysine-9-acetylation levels around the promoters of HG-upregulated genes compared with all RefSeq promoters. Interestingly, within the HG-upregulated genes, active chromatin marks were enriched not only at high CpG content promoters, but surprisingly also at low CpG content promoters. Similar results were obtained with peripheral blood monocytes exposed to HG. These new results reveal a novel mechanism by which HG can exercise IFN-α-like effects in monocytes by upregulating a set of ISGs poised for activation with multiple chromatin marks.
Keywords: high glucose, monocytes, diabetic complications, interferon regulatory factors, chromatin state
chronic hyperglycemia or glucose toxicity associated with both Type 1 (T1D) and Type 2 diabetes has been attributed to the development of various complications including cardiovascular disease, neuropathy, nephropathy, and retinopathy (7, 8, 17, 48). These complications can result from the adverse effects of high glucose (HG) on many fundamental biological processes in target tissues and cells. As the soaring rates of diabetes and its complications have become major health care issues, it is imperative to examine the underlying mechanisms by evaluating signaling molecules, networks, genetic, and epigenetic elements that contribute to the relevant deregulated pathways using emerging new technologies.
Several biochemical mechanisms have been implicated in hyperglycemia-induced cellular damage (7, 8), including increased oxidant stress, activation of protein kinase C and other signaling kinases, as well as formation of advanced glycation end products. Evidence also shows that the expression of inflammatory cytokines and chemokines, Toll-like receptors, and many signaling molecules are affected by HG in primary human monocytes and THP-1 monocytes (5, 10, 11, 13, 20, 24, 32, 45–47, 53) and that this leads to the impairment of key biological pathways. Despite these efforts, our understanding of the genome-wide effects of HG in target cells remains incomplete, largely because majority of these studies were based on the evaluation of candidate genes, which therefore leaves a vast number of genes unexplored.
In recent years, high-throughput technologies such as RNA sequencing (RNA-Seq), chromatin immunoprecipitation linked to microarrays (ChIP-chip), ChIP-Sequencing (ChIP-seq), and bioinformatics analysis tools have yielded unprecedented insights into the transcriptome and epigenome and significantly enhanced biomedical research (21, 39). RNA-Seq can identify all transcripts in a cell in an unbiased manner, including novel protein coding and noncoding genes previously missed by traditional microarray expression profiling. It has emerged as a powerful tool to uncover previously unknown details of transcriptome changes under disease conditions (30, 51). In addition, ChIP-chip or ChIP-Seq and other related tools allow us to map histone posttranslational modifications (PTMs), DNA methylation, and the other components of the epigenome, as well as regulatory elements on a genome-wide scale (16, 42, 54). Because of the fundamental importance of HG to diabetes and diabetes complications, it is worthwhile to examine the genome-wide effects of HG in the context of these new technologies. By profiling key epigenetic histone PTM marks in HG-treated human monocytes using ChIP-chips, we previously identified genes and chromatin regions in which histone methylation levels were altered by chronic HG as well as their correlation with gene expression(34). Using the same ChIP-chip technology in peripheral blood monocytes and lymphocytes obtained from T1D patients and healthy controls, we also found genomic regions that had differential levels of several histones PTMs (31, 33). Another study using next-generation sequencing reported genome-wide changes in DNA methylation and histone H3 lysine 9/14 acetylation (H3K9/K14Ac) in HG-treated human endothelial cells, which revealed several known and novel genes and pathways associated with endothelial dysfunction (38).
In this study, we used RNA-Seq to investigate changes in the transcriptome in response to HG in human THP1 monocytes. Data analyses revealed key previously unidentified genes, transcription factors (TFs), and networks among the HG-upregulated transcripts. In addition, by profiling 15 selected histone PTM marks using ChIP-promoter arrays, we explored the chromatin features at the promoters of HG-upregulated genes in the basal unstimulated state. Key validations were also performed in primary human blood monocytes treated with HG. Overall, these approaches have uncovered new genome regions targeted by HG, as well as their related chromatin states in cells related to diabetic complications, which could lead to the identification of much-needed new therapeutic targets for subjects with debilitating complications of diabetes.
MATERIALS AND METHODS
THP-1 cell culture and human blood monocytes.
THP-1 cells (from ATCC) were cultured in RPMI 1640 medium containing normal glucose (NG, 5.5 mM) or HG (25 mM) and 10% serum for 72 h. Endotoxin levels were monitored with the Limulus Amebocyte Lysate QCL-1000 kit (#50-647U, Lonza). Peripheral blood mononuclear cells were isolated from healthy donors using endotoxin-free Ficoll-Paque Plus (GE Healthcare 17-1440-03) according to an approved Institutional Review Board protocol. Monocyte CD14+ cells were purified with enrichment kits (STEMCELL Technologies cat. no. 19059).
mRNA preparation for RNA-Seq.
Total RNA and polyA RNA were prepared by standard protocols. PolyA RNA was depleted of rRNA with RiboMinus kits (Invitrogen), and then RNA-Seq was performed after library preparation in our Sequencing Core according to the Illumina protocols.
Real-time quantitative PCR.
RT-QPCR (QPCR) for quantifying gene expression were performed with cDNA prepared from THP1 cell and human monocyte total RNAs. The following PCR primers were used: STAT2 (5′-CTTTGTGAGTCGGAGCCAGGAG-3′ and 5′-CTACTCTTGTACGGTTGCACAGAGTAG-3′), STAT1 (5′-CGAATACTTTCC CTGA CATCATTCG-3′ and 5′-TCCTTTAGGGCCATCAAGTTCC-3′), ISG15 (5′-GCGAACTCATCTTTGCCAGT-3′ and 5′-AGCATCTTCACCGTCAGGTC-3′), IFIT3 (5′-GCAGAGACACAGAGGGCAGT-3′ and 5′-TGGCATTTCAGCTGTGGA-3′), OAS2 (5′-CGGTCCAACTTTGAAGATGAGACCG-3′ and 5′-TGAAGCAGGGAGAGGATAACCA-3′), IRF7 (5′-AGCTGT GCTGGCGAGAAG-3′ and 5′-CATGTGTGTGTGCCAGGAA-3′), IFI44(5′-GTGAGCCTGTGAGGTCCAA-3′ and5′-TCCTTTACAGGGTCCAGCTC-3′), IFI27(5′-CCAAGCTTAAGACGGTGAGG-3′ and 5′-CCGTGGCCTAGAGAGTAAGAGA-3′), TNF (5′-CGAGTGACAAGCCTGTAG-3′ and 5′-GACCTGGGAGTAGATGAG-3′), ALOX5 (5′-CGGTTAGAACCTGTTCATCAACCG-3′ and 5′-GCCATTCAGGAACTGGTAGCC-3′), IFN-β (5′-CGACACTGTTCGTGTTGTCA-3′ and 5′-GAAGCACAACAGGAGAGCAA-3′), IL-1β (5′-TACCTGTCCTGCGTGTTGAA-3′ and 5′-TCTTTGGGTAATTTTTGGGATCT-3′), PCNA (5′-TGGAGAACTTGGAAATGGAAA-3′ and 5′-GAACTGGTTCATTCATCTCTATGG-3′).
ChIP and ChIP-chip experiments.
THP1 cells cultured in NG were cross-linked in 1% formaldehyde and sonicated, and ChIPs were performed with antibodies against histone PTMs as described (32, 34). ChIP without antibody was prepared as control. Small aliquots of DNA before ChIP (Input) and the ChIP-enriched DNA samples were saved for follow-up ChIP-QPCR validations of ChIP-chip data. The remaining ChIP-enriched DNA and no antibody controls were amplified, purified, and used for microarray hybridization as previously described (33, 34) using NimbleGen human 385K or 720K RefSeq promoter tiling arrays at our Functional Genomics Core. The following antibodies were used for ChIPs: anti-histone H3K4me3 (Millipore 07-473), H3K9me2 (Millipore 07-441), H3K27me3 (Millipore 07-449), H3K9Ac (Millipore 06-942), H3K56Ac (Millipore 07-677), KDM5A (JARID1A) (Abcam ab26049), KDM1 (LSD1) (Abcam ab17721), KDM2A (JHDM1A) (Abcam ab31739), KDM3A (JHDM2A) (Abcam ab91252), KDM3A (JHDM3A), (Abcam ab24545), SIN3A (Millipore 06-913), SNF2H (Millipore ABD22), BRG1 (Millipore 07-478), RNA polymerase II-CTD phosphoS5 (Abcam ab5131), and RNA polymerase II (Abcam ab817).
RNA-Seq data analysis.
Raw sequences were aligned to the human hg18 genome assembly using Bowtie. The aligned reads were imported to Partek Genomic Suite (version 6.5 beta; Partek, St. Louis, MO) to obtain the expression level for each RefSeq transcript by summarizing the number of reads located within each gene's exon(s). Expression data at the single gene level were calculated by combining the reads within all transcripts of the same gene. Fisher's exact test was applied to gene level expression data to compare HG and NG conditions, and the resulting P values were adjusted by the Bonferroni method. Gene level expression data were further normalized to obtain reads per kb per millions of aligned reads (RPKM) and the fold changes between HG and NG calculated based on RPKM data. For a gene with more than one transcript, gene length was defined as the length of the longest transcript. Differentially expressed genes between HG and NG were identified by the following criteria: 1) RPKM ≥10 in either one cell culture condition and 2) fold change between the samples ≥2.
Motif analysis.
The promoter regions [−500 bp to 100 bp relative to the transcription start site (TSS)] of the HG-upregulated genes identified by RNA-Seq in this study were examined for the enrichment of TF binding motifs, defined in the Transfac's nonredundant vertebrate database using Biobase TF Explain analysis system (version 2.4.2). The background gene set was defined as the 1,000 most commonly expressed genes under HG and NG conditions that have the largest P values and whose RPKM ≥10 in at least one sample. The motifs that are enriched in HG-upregulated genes were identified with Biobase's MATCH function. The motifs with P ≤ 1×10−5 and an enrichment fold, defined as average number of matched motifs per promoter in HG-upregulated genes versus that in background genes, of no less than 1.25 were considered enriched in HG-upregulated genes.
Promoter CpG-content based gene classification.
HG-upregulated genes were classified into groups consisting of those with of high CpG content promoters (HCP), intermediate CpG content promoters (ICP), and low CpG content promoters (LCP) based on the promoter CpG content (−500 bp to 500 bp relative to TSS). The sequence of each gene's promoter based on the hg18 genome assembly was scanned using a sliding window of 500 bp in length with a 5 bp step. HCPs were defined as promoters containing at least one 500 bp region with CpG ratio >0.75 and GC content >55%, while LCPs were defined as promoters without a 500-bp region of CpG ratio >0.48. ICPs were those not classified into either HCP or LCP (52). For convenience, the HCP and ICP were combined as HCP/ICP in this study.
ChIP-chip data heat map and composite profile generation.
For ChIP-chip data analyses, the promoter regions of each transcript in gene sets (presented in Figs. 4 and 5) were divided into nonoverlapping 100 bp windows, each as a positional bin. For each chromatin mark, log2 enrichment ratios of the microarray probes falling into each transcript's promoter region were retrieved, aligned, and assigned to the positional bin, which resulted in a data matrix containing the PTM level of a specific chromatin mark with each row representing one RefSeq transcript's promoter and each column representing each bin. Then the rows of the matrix were ordered by the expression levels of their corresponding genes [either measured by Affymetrix expression data from our earlier study (28) or RNA-Seq expression data (from the current study); see the figure legends for more details] and visualized by Java TreeView v1.1.3 to generate the heat map. For each gene set, averages of the data in each column were then calculated and plotted to generate composite profiles as also described earlier (31).
Calculation of gene-level H3K4me3 enrichment at defined promoter region.
For specific candidate genes of interest, the average H3K4me3 level at its defined promoter region was calculated using all the probes located in the region of the corresponding gene. For genes with more than one isoform, the H3K4me3 level was the average level of all its isoforms.
Data deposition.
The data reported in this study is deposited in the NCBI Gene Expression Omnibus database (GSE43871).
RESULTS
RNA-Seq analysis of gene expression in THP-1 cells under HG conditions.
We mimicked diabetic conditions by culturing THP-1 monocytes in HG (25 mM) relative to NG (5.5 mM) for 72 h (half-life of human monocytes). mRNA isolation and RNA-Seq were performed as described under materials and methods. We first compared the RNA-Seq data of THP-1 cells grown in NG to the gene expression dataset obtained previously using Affymetrix microarrays (28) and observed close correlations between these two approaches (Pearson's r = 0.75, Fig. 1), consistent with published results (Spearman's rho = 0.73–0.75) (29). For the RNA-Seq data, using the criteria of minimum RPKM normalized value ≥ 10 in either sample and fold change ≥2, a total of 337 HG-upregulated genes and 326 HG-downregulated genes were selected (Fig. 2A). The full gene list is provided in Table 1 and Table 2. Ingenuity Pathway Analysis (Ingenuity Systems, http://www.IPA.com) showed that interferon (IFN) signaling, virus entry pathway, cytosolic pattern recognition receptors, activated interferon regulatory factors (IRFs) and the ERK/MAPK signaling pathways were among the top canonical pathways enriched (P < 1×10−4) in the HG-upregulated genes (Fig. 2B). This suggested a strong connection between HG effects and IFN-related pathways. Within the downregulated genes, enriched pathways included mitotic cell cycle, protein complex assembly, DNA metabolic process, DNA repair, ribosome biogenesis, rRNA processing, oxidative phosphorylation, and mitochondrial electron transport (Fig. 2B).
Fig. 1.
Comparison of RefSeq gene expression measured by RNA-Seq with Affymetrix gene chip data. RefSeq gene expression profiles of THP1 cells grown in normal glucose (NG) condition were measured by RNA-Seq in the current study, as well as by Affymetrix GeneChip Human Genome U133 plus version 2.0 array in triplicate previously (28). For RNA-Seq, the sequences were processed and normalized with reads per kb per million (RPKM) as described in materials and methods. The RPKM expression level of each transcript was log2 transformed with offset 1. For Affymetrix data, raw intensity measurements were processed by RMA. The expression level of each transcript cluster across 3 replicates was then averaged. For a transcript represented by multiple transcript clusters, its expression level was the average level of all these clusters. A total of 18,029 common RefSeq transcripts shared by both platforms were plotted. Each dot represents the expression levels of 1 transcript, with data from Affymetrix arrays represented on the y-axis and RNA-Seq data on the x-axis. Pearson's correlation coefficient (R) between the 2 platforms is also shown.
Fig. 2.
RNA-Seq mediated identification of differentially expressed genes in THP-1 treated with high glucose (HG) vs. NG. A: gene expression in THP-1 cells grown under HG and NG conditions. Total reads located within the exons of each RefSeq gene (21,298 total) were summarized, RPKM-normalized, and transformed into log2 (reads #+1) format. Each point represents the expression level of 1 gene in NG and HG culture conditions. Differentially expressed genes were identified by the following criteria: 1) minimum RPKM normalized reads ≥ 10 in either cell culture condition; 2) fold change ≥ 2. The triangles represent 337 genes that were upregulated in HG, while squares represent the 326 genes that were downregulated in HG. The remaining dots represent genes that were not differentially expressed as per these described criteria. B: Ingenuity Pathway Analysis report of HG-upregulated and downregulated genes with enrichments of P < 1× 10−4.
Table 1.
HG-upregulated gene list (337) by RNA-Seq in THP1 cells
| Gene Name | Gene Name | Gene Name | Gene Name | Gene Name | Gene Name | Gene Name |
|---|---|---|---|---|---|---|
| ABHD4 | CCDC88B | FURIN | JUNB | OASL | RHOC | TIMP3 |
| ACCN2 | CD300A | FYN | KCNE1 | OBSL1 | RNF213 | TKTL1 |
| ACSL1 | CD82 | G0S2 | KCNG1 | OGFR | RNF24 | TLE3 |
| ADAMTS2 | CDC42EP1 | GAB2 | KCNK5 | OGT | RNF41 | TMCO4 |
| ADAP1 | CDC42EP3 | GAS7 | KIAA1618 | ORM1 | RNPEPL1 | TMEM63A |
| ADAR | CDKN1A | GDF15 | KIAA1958 | ORM2 | ROGDI | TNF |
| ADM2 | CEACAM19 | GIPC1 | KIF21B | P2RX4 | RPS19BP1 | TNFRSF10B |
| ADRB1 | CEBPB | GPR132 | KIFC3 | P2RY11 | RPS6KA2 | TNFRSF1B |
| AGRN | CHAC1 | GPT2 | KLF10 | PACSIN2 | S100P | TOX2 |
| AHNAK | CHD2 | GRB10 | KLHL13 | PAG1 | SAMD9L | TRIB3 |
| AKNA | CHPF | GTPBP2 | LAT | PARP10 | SDC2 | TRIM22 |
| ALDH1L2 | CKAP4 | H1F0 | LBH | PARP12 | SDC3 | TRIM56 |
| ALOX5 | CLTB | HAX1 | LGALS3BP | PARP14 | SEMA4C | TRIM8 |
| ALOX5AP | CMPK2 | HBEGF | LIMD1 | PARP9 | SERPINE2 | TSC22D3 |
| ANGPTL6 | CORO1A | HDAC5 | LIMD2 | PCBP4 | SESN2 | TSC22D4 |
| ANKRD11 | COX19 | HERPUD1 | LMNA | PCK2 | SH3BP1 | TSSC4 |
| AP2A2 | CREBBP | HES6 | LOC153684 | PCNX | SH3BP2 | TTYH3 |
| APBA2 | CSF3R | HLA-A | LOC728855 | PDLIM7 | SH3GL1 | TWF2 |
| APOL6 | CST7 | HLA-E | LOC728875 | PDZD7 | SHISA5 | TXNIP |
| ARAP1 | CTSD | HLA-F | LOC90246 | PEA15 | SHMT2 | TYMP |
| ARHGEF10L | CXXC5 | HLA-H | LONP1 | PEX6 | SIGLEC1 | UBA7 |
| ARRDC4 | DBN1 | HMBOX1 | LRP10 | PGAM2 | SLC12A8 | UBE2L6 |
| ASNS | DDAH2 | HMHA1 | LRRC3B | PHC2 | SLC16A3 | UBXN6 |
| ASS1 | DDIT4 | HOMER3 | LY6E | PHGDH | SLC1A5 | UHMK1 |
| ATF5 | DNM1 | HPCAL1 | MALAT1 | PKD1 | SLC27A3 | USP18 |
| ATG4D | DRAM1 | HSH2D | MAP1B | PLEC1 | SLC31A1 | VEGFA |
| AXL | DTX3L | HSPB7 | METRNL | PLEK | SLC37A2 | WARS |
| BARX1 | EEF1A2 | IER2 | MFAP4 | PLSCR1 | SLC3A2 | WDR45 |
| BATF3 | EHBP1L1 | IFI27 | MGC12982 | PLXNA1 | SLC43A1 | XAF1 |
| BCL2L11 | EIF2AK2 | IFI35 | MICAL2 | PPAN-P2RY11 | SLC6A9 | ZBTB7B |
| BEST1 | EIF2C2 | IFI44 | MID1IP1 | PPARD | SLC7A5 | ZFAND3 |
| BRPF3 | EIF4EBP1 | IFI44L | MKNK2 | PPM1M | SMG5 | ZFP36L1 |
| C10orf54 | EPSTI1 | IFI6 | MLST8 | PPP1R3C | SNTB1 | ZNF513 |
| C12orf44 | ESRP1 | IFIH1 | MMP14 | PRPSAP1 | SP110 | ZNF516 |
| C14orf49 | ESRRA | IFIT1 | MOV10 | PSAT1 | SPNS3 | ZNF740 |
| C16orf79 | ETS1 | IFIT2 | MRC2 | PSTPIP1 | SPSB3 | ZNFX1 |
| C19orf22 | F11R | IFIT3 | MX1 | PTP4A3 | SQSTM1 | ZXDC |
| C19orf66 | FAM125A | IFITM1 | MX2 | PUS1 | SSBP2 | |
| C1orf84 | FAM134B | IFITM3 | MYH9 | PVR | ST3GAL1 | |
| C1RL | FAM84B | INHBE | MYO15B | PYCR1 | ST6GALNAC3 | |
| C3AR1 | FBXO6 | IRF1 | NCAM1 | QSOX2 | STAT2 | |
| C3orf54 | FCER2 | IRF7 | NFE2L1 | RAB3D | STC2 | |
| C5AR1 | FEZ1 | IRF9 | NFIL3 | RARA | STK10 | |
| C9orf37 | FGD3 | ISG15 | NFKBIL1 | RBCK1 | SYTL1 | |
| C9orf89 | FGR | ITGAL | NINJ1 | RCAN1 | TAP1 | |
| C9orf91 | FLJ35024 | ITGB2 | NR1H2 | REC8 | TBX15 | |
| CAMTA2 | FLJ39739 | ITGB7 | NUPR1 | RELL2 | TESC | |
| CAP2 | FLNA | ITPRIP | OAS1 | RFXANK | TFEB | |
| CBX4 | FLRT1 | JDP2 | OAS2 | RGMA | THTPA | |
| CCDC69 | FOSL2 | JUB | OAS3 | RGS16 | TIMM44 |
HG, high glucose.
Table 2.
HG-downregulated gene list (326) by RNA-Seq in THP1 cells
| Gene Name | Gene Name | Gene Name | Gene Name | Gene Name | Gene Name | Gene Name |
|---|---|---|---|---|---|---|
| HSD11B1 | UBE2T | DUT | PRDX6 | CDCA3 | METTL7B | SNRPG |
| HLA-DRA | KPNA2 | PAQR8 | RNASE2 | FAM83D | C15orf57 | PCYOX1L |
| BNIP3 | GLA | BUB1B | NUDT7 | NDUFS5 | RPS23 | RPL22 |
| SERPINB2 | ARL6IP1 | HSP90B1 | RBBP7 | MANF | PARP1 | ATP6V1C2 |
| DEFB1 | AIG1 | PRR11 | PRIM1 | GINS1 | RPS24 | PSMG2 |
| MS4A6A | IGFBP7 | NUTF2 | NP | SFRS2 | C11orf1 | CKAP2 |
| SERPINB10 | RRM1 | TMEM106C | C4orf46 | HSP90B3P | CCNB2 | PPIAL4B |
| CEACAM6 | SLC25A5 | ROMO1 | CAPNS1 | CCDC99 | UQCRH | LTA4H |
| GPR160 | CCNA2 | GPI | PLK1 | CACNA2D3 | MRPS28 | BLM |
| ALDOC | C10orf75 | CACYBP | OAT | ANXA4 | COX6C | NEIL3 |
| S100A8 | CCNE2 | DHCR7 | CAT | RPS17 | RAN | STRA13 |
| HSPE1 | CENPF | RPL7A | TUBA1A | HPRT1 | RAB11A | IPO5 |
| NDUFB6 | UBE2C | NUSAP1 | CEP55 | RPSAP58 | RPL39L | ENOSF1 |
| CD302 | KIF11 | RPS25 | RPS3A | HAUS1 | RPLP2 | ADSS |
| GLO1 | PTTG3P | PAICS | GPC3 | CCNG1 | C11orf82 | C9orf100 |
| GAL | HMGB1L1 | ZWINT | RFC3 | C14orf19 | TPI1 | SRI |
| HMMR | EBPL | TRIP13 | RPL39 | HSP90AB1 | C13orf27 | PRC1 |
| MS4A3 | SC4MOL | EIF3E | MS4A7 | RANBP1 | C6orf129 | FEN1 |
| KIF20A | PCNA | RPL5 | RPS4X | RPL23P8 | EAF2 | RNASE3 |
| ACOT7 | TUBA1B | FDFT1 | PENK | NMNAT3 | NDUFAB1 | GNL3 |
| PTTG1 | CCL23 | NXT2 | COX6B1 | THOC4 | INSIG1 | RPL26 |
| HMGCS1 | RNPEP | HMGCR | ECT2 | NME2 | NUP37 | GTF3C6 |
| FABP5 | SERPINB8 | SNRNP25 | BEX1 | GNG10 | RPS7 | DDT |
| CALR | B3GNT7 | KIF23 | HISPPD1 | HIBADH | CETN3 | NCAPH |
| TFRC | BUB1 | PLK4 | C20orf103 | CYB5A | PPIAL4A | NGFRAP1 |
| PRTN3 | TUBA1C | MND1 | HNRPA1L-2 | HNRNPA1 | APOBEC3B | C14orf147 |
| ACAT2 | C8orf45 | NEK2 | NUF2 | FH | NTAN1 | |
| GCSH | TYMS | TTK | CETN2 | KHDRBS1 | PPIAL4C | |
| SNHG6 | FBXO5 | CSTB | RPS15A | MTCH2 | CALM2 | |
| NPC2 | TECR | C16orf73 | C18orf10 | DHFR | RPS14 | |
| KIAA0101 | AKR1B1 | WBP5 | CBX1 | RPL23AP64 | PBK | |
| NUCB2 | SKP2 | THBS4 | NCAPG | GATM | CERKL | |
| CDKN3 | RPL21 | RPL14 | RPL30 | RPL23A | THOC3 | |
| LDHA | TXN | HSPD1 | NDUFA1 | SLC25A3 | C7orf30 | |
| DLGAP5 | RPL21P28 | PKIB | UCP2 | PPA1 | SYNC | |
| MT1G | HMGB1 | RPL7 | RPA3 | HNRNPR | HSP90AB2P | |
| CCNB1 | ACAT1 | DEPDC1B | MLF1IP | HLA-DQB1 | MCM3 | |
| C6orf173 | CXCR4 | HEXB | HMGB2 | DNAJC25-GNG10 | TBCA | |
| HINT1 | NME1 | NDUFA12 | GBE1 | RPSA | CENPH | |
| DCK | RPL11 | LYZ | SKP1 | PSRC1 | MRPL36 | |
| MAD2L1 | EEF1B2 | SF3A3 | NDUFA4 | GSTM3 | HJURP | |
| MNDA | TMEM97 | KYNU | C2orf79 | RPL9 | GPNMB | |
| STMN1 | NDUFB8 | SNRPE | C1orf54 | CNN3 | AURKA | |
| GAMT | PPA2 | RPS26P11 | ATP5G3 | PIR | EIF3L | |
| ASPM | CDC20 | MYEOV2 | ANLN | DNAJC5B | EPR1 | |
| CDCA7 | CDC2 | IDI1 | TPX2 | P4HA1 | NPM1 | |
| PRDX1 | GGH | NDC80 | RRM2 | E2F8 | S100A10 | |
| TOP2A | RPL23 | EEF1G | NDUFA7 | LOC728554 | EIF2A | |
| TUBB | ARPC5 | HSP90AA1 | AP1S3 | SNRPF | C10orf58 | |
| DARS2 | SOD1 | FAM166A | NME1-NME2 | CENPA | WDR54 |
Promoter motif analysis indicates that IRFs participate in HG-upregulated gene expression in THP1 cells.
Gene networks triggered by dysregulated TFs can significantly amplify signals that drive cells into abnormal states. Earlier studies have shown that increased activity of NF-κB TF is associated with HG effects in monocytes (10, 11, 20, 32, 46). In our current RNA-Seq data, we also observed the induction of several known NF-κB target genes including TNFα and CCL2 (data not shown). To further identify specific TFs that regulate genes upregulated by HG in THP-1 cells, we performed a motif analysis of the 337 upregulated genes (Table 1) for TF binding sites using Biobase Explain software. Results showed that IRF motifs were significantly enriched in the promoter regions of HG-upregulated genes identified in this study (Table 3). Notably, this unbiased in silico analysis indicated for the first time that IRFs and their target genes are the most highly enriched gene sets induced by HG, indicating the power of RNA-Seq to uncover previously underestimated transcripts, TF regulated pathways and related factors affected by HG.
Table 3.
List of overrepresented motifs in the promoters of HG-upregulated genes
| TF Matrix | Gene | Enrichment Ratio | P Value |
|---|---|---|---|
| V$IRF_Q6 | IRF1, IRF2, IRF3, IRF4, IRF5, IRF6, IRF7, IRF8, IRF9 | 6.1891 | 3.92E-21 |
| V$IRF2_01 | IRF1, IRF2 | 4.5087 | 7.36E-15 |
| V$ISRE_01 | IRF7, IRF8, IRF9, STAT1, STAT2 | 3.1134 | 8.22E-13 |
| V$LRF_Q2 | ZBTB7A | 1.3964 | 4.50E-14 |
| V$VDR_Q3 | VDR | 1.2975 | 7.32E-21 |
| V$MAZ_Q6 | MAZ | 1.2861 | 1.50E-09 |
Each motif represents the corresponding transcription factor(s) listed in the “Gene” column. Enrichment ratio for each matrix represents the ratio of the average number of matched motif binding sites per 1,000 bp in the promoters of HG-upregulated genes vs. background genes (see materials and methods for background gene set selection). “TF Matrix”column shows the names of Transfac Position Weight Matrices (PWM) motifs. Significance of the representation is measured by the P value derived from binomial distribution. Motifs with P < 1×10−5 and enrichment ratio >1.25 are listed. The motifs in boldface (in the top 3 rows) are considered as highly enriched with P < 1E-12 and enrichment ratio > 3.
We next assessed whether specific IRFs were differentially expressed in response to HG by RNA-Seq and found that IRF1, IRF2, IRF7, and IRF9 were all highly upregulated (Table 4). To further identify specific target genes and networks regulated by IRFs, we scanned the promoter regions of the 337 HG-upregulated genes (−500 bp to 100 bp relative to TSS) (Table 1) to detect those containing at least one binding site of the IRF-related matrices. As a result, 81 potential IRF target genes were identified among the HG-upregulated genes that we designated as high glucose-induced IRF network (HGIN) (81 gene list provided in Supplementary Table S1 in Supplementary Materials).1 Notably, this HGIN was highly enriched in typical antiviral response interferon-stimulated genes (ISGs) driven by IRFs. To verify the RNA-Seq data, we selected key HGIN genes (ISG15, STAT2, IFI27, IFI44, IFIT3, IRF7, OAS2, and ALOX5), STAT1 as well as positive controls TNF-α and IL-1β for further validation by RT-QPCR using separate RNA obtained from THP-1 cells treated with HG or osmotic control (19.5 mM mannitol + NG). Results showed that all these genes, except STAT2 and the negative control PCNA, were upregulated by HG, but not by mannitol (Fig. 3). Importantly, we also verified that the culture medium had no detectable LPS/endotoxin contamination.
Table 4.
Selected IRF and STAT genes in RNA-Seq data from THP1 cells cultured under NG and HG conditions
| Gene Name | RPKM in HG | RPKM in NG | Fold Change |
|---|---|---|---|
| IRF7 | 47.21 | 4.9 | 9.63 |
| STAT2 | 40.85 | 16.06 | 2.54 |
| IRF1 | 19.24 | 7.69 | 2.5 |
| STAT1 | 53.83 | 28.74 | 1.87 |
| IRF2 | 17.15 | 10.15 | 1.69 |
| IRF9 | 62.42 | 19.74 | 1.61 |
| IRF3 | 23.09 | 18.54 | 1.25 |
| IRF5 | 40.24 | 34.19 | 1.18 |
| IRF8 | 103.95 | 157.9 | 0.66 |
Transcription factors corresponding to the 3 highly enriched motifs (P < 1E-12 and enrichment fold > 3) are listed. The gene-level expression in THP1 grown under normal glucose (NG) and HG levels were measured by RNA-Seq with reads per kb per million (RPKM) normalization. Genes with RPKM normalized reads ≥1 in THP1 cells grown in either 1 condition and expression level fold change between HG vs. NG no less than1.5 are considered differentially expressed. IRF, interferon regulatory factor.
Fig. 3.
RT-QPCR validation of RNA-Seq data of HG-upregulated gene expression. A: THP1 cells were cultured in 5.5 mM glucose (NG), 25 mM glucose (HG), or osmotic control mannitol (5.5 mM glucose + 20 mM mannitol) for 72 h. Total RNA samples were prepared for mRNA quantification by standard RT-QPCR. β-Actin was used as the internal control. Results shown (means ± SE) are from 2 sets of PCRs with each sample run in duplicate. *P <0.001 HG vs. NG, by Student's t-tests.
A recent integrated study of datasets from genome-wide gene expression and sequence variations in rat tissues identified an IRF7-driven inflammatory network (IDIN) that was also enriched in human monocytes and was shown to contribute to T1D risk (22). Both this reported IDIN and our current HGIN gene networks are regulated by IRF, and we found a significant overlap between them (χ2 P < 2.2 × 10−16). Twenty-eight out of our 81 HGIN genes (Supplementary Table S1) were also present in IDIN (Table 5), suggesting a strong internal correlation between the two networks. A closer examination of these 28 genes revealed that this correlation was primarily represented by the type 1 IFN response since the signature molecules of ISGs such as ISG15, OASL, MOV10, IFI44L, IFITM3, MX1, and EIF2AK2 were included (44). Interestingly, there were no detectable reads for IFN-α in the RNA-Seq data, while IFN-β reads were slightly increased by HG (data not shown). We then confirmed by RT-QPCR that HG induces IFN-β expression (Fig. 3). Collectively, these results suggest that HG itself can upregulate an antiviral transcription network in monocytes possibly through the induction of IFN-β.
Table 5.
Expression levels of common genes present in both HGIN and IDIN gene sets in THP1 cells
| Symbol | CpG Content | Representative Accession # | HG, RPKM | NG, RPKM | Fold Change |
|---|---|---|---|---|---|
| EPSTI1 | HCP | NM_001002264 | 16.05 | 2.40 | 6.69 |
| IRF7 | HCP | NM_004029 | 47.21 | 4.90 | 9.63 |
| EIF2AK2 | HCP | NM_001135651 | 20.14 | 6.45 | 3.12 |
| MX1 | HCP | NM_002462 | 73.22 | 9.75 | 7.51 |
| PLSCR1 | HCP | NM_021105 | 51.88 | 18.30 | 2.84 |
| WARS | HCP | NM_173701 | 179.09 | 49.38 | 3.63 |
| ADAR | HCP | NM_015841 | 150.15 | 62.64 | 2.40 |
| HAX1 | HCP | NM_001018837 | 194.39 | 88.09 | 2.21 |
| PARP12 | ICP | NM_022750 | 16.77 | 4.21 | 3.99 |
| PARP9 | ICP | NM_001146105 | 15.96 | 4.92 | 3.24 |
| MOV10 | ICP | NM_001130079 | 31.88 | 12.23 | 2.61 |
| ISG15 | ICP | NM_005101 | 277.21 | 16.04 | 17.28 |
| IFITM3 | ICP | NM_021034 | 333.06 | 126.32 | 2.64 |
| IFI44L | LCP | NM_006820 | 46.07 | 0.91 | 50.59 |
| IFIT2 | LCP | NM_001547 | 10.33 | 1.73 | 5.96 |
| OASL | LCP | NM_003733 | 11.93 | 2.13 | 5.61 |
| IFI44 | LCP | NM_006417 | 68.89 | 2.73 | 25.23 |
| IFI27 | LCP | NM_005532 | 70.44 | 2.95 | 23.84 |
| IFIT1 | LCP | NM_001548 | 25.50 | 3.13 | 8.15 |
| IFIT3 | LCP | NM_001549 | 34.20 | 4.00 | 8.55 |
| PARP10 | LCP | NM_032789 | 42.14 | 6.03 | 6.99 |
| SP110 | LCP | NM_001185015 | 23.40 | 6.46 | 3.62 |
| OAS1 | LCP | NM_002534 | 76.30 | 8.70 | 8.77 |
| OAS2 | LCP | NM_001032731 | 59.23 | 9.58 | 6.19 |
| IFI35 | LCP | NM_005533 | 54.55 | 11.43 | 4.77 |
| XAF1 | LCP | NM_017523 | 37.42 | 11.74 | 3.19 |
| IFI6 | LCP | NM_022872 | 401.23 | 56.91 | 7.05 |
| IFITM1 | LCP | NM_003641 | 279.64 | 59.18 | 4.73 |
Twenty-eight genes shared in both high glucose-induced IRF network (HGIN) and IRF7-driven inflammatory network (IDIN) were obtained by comparing our HGIN with the published IDIN. These genes were further classified into high CpG content promoters (HCP), intermediate CpG content promoters (ICP), and low CpG content promoters (LCP) based on promoter CpG content (see materials and methods for classification criteria). For genes with multiple transcripts, 1 transcript was randomly chosen whose accession number is listed in the “Representative Accession #” column for each gene. The expression levels of the 28 genes and fold increase in HG are also shown.
Basal chromatin profiles of HG up- and downregulated genes.
Chromatin is composed of DNA wrapped by an octamer of histones, modified and unmodified, as well as nonhistone proteins including TFs, cofactors, and RNA polymerase complexes. Together, these components define the chromatin state and accessibility, and therefore, mapping their occupancies on genomic DNA is essential to understand of the role of chromatin in gene regulation (42). To explore the basal chromatin features of genes that were regulated by HG, we profiled 15 selected chromatin modifications using ChIP-promoter arrays containing ∼24,000 human promoters as described (33, 34) in unstimulated THP1 cells grown in NG. These 15 marks fall into five categories: histone PTMs (histone H3K9Ac, H3K56Ac, H3K4me3, H3K9me2, H3K27me3); histone demethylases (JARD1A, LSD1, JHDM1A, JHDM2A, and JHDM3A), a co-repressor (Sin3A); chromatin remodeling factors (SNF2H and BRG1), and gene transcription machinery (RNA Pol II, RNA Pol II-Ser5P). The rationale is based on the documented role of these marks in gene regulation (9, 54). Histone H3K4me3, H3K9Ac, and H3K56Ac are associated with promoters and active genes, while H3K9me2 and H3K27me3 are associated with repressed regions and inactive genes (6, 54). Histone demethylases affect gene regulation by erasing histone modifications (35). SIN3A is a transcriptional repressor (18), while SNF2H and BRGI are components of ATP-dependent chromatin remodeling complexes (41, 43). RNA PolII marks transcriptionally active chromatin (16, 19, 54). As anticipated, the heat map of all RefSeq genes (in the basal unstimulated state) of these chromatin marks around promoter regions (Fig. 4A) shows that histone H3K4me3, H3K9Ac, H3K56Ac, RNA Pol II, and RNA Pol II Ser-5′P marks were generally associated with active genes, while H3K27me3 and H3K9me2 were associated with repressed genes.
Fig. 4.
Chromatin profiles at RefSeq gene promoter regions in THP-1 cells in the basal unstimulated state. A: heat map of 15 chromatin marks at RefSeq gene promoter regions in THP-1 cells. ChIP-chip profiling with antibodies to the indicated 15 chromatin marks was performed in cells grown in NG using human promoter tiling arrays. RefSeq transcripts covered in both ChIP-promoter tiling array and Affymetrix human expression arrays are represented. Only 1 transcript was retained if multiple transcripts shared the same gene symbol and transcription start site (TSS). Enrichment levels of each posttranslational modification (PTM) at gene promoter regions were calculated (see materials and methods for details) and sorted by their Affymetrix array gene expression levels (28) from the lowest (top) to highest (bottom). Red represents enrichment, green represents depletion, and black represents no enrichment or missing data due to lack of probes. B–P: composite profiles of basal chromatin states at promoters of HG-regulated genes in THP-1 cells. HG-upregulated and -downregulated genes were identified from RNA-Seq data. The basal enrichment of the chromatin marks at these genes sets were then evaluated from the ChIP-chip data. Each panel represents the average enrichment level of 1 chromatin mark for 3 groups of genes, namely, HG-upregulated genes (red), HG-downregulated genes (blue), and all RefSeq genes (green). Identities of chromatin marks are listed at the top of each panel: RNA Pol II (B), RNA Pol II Ser-5P (C), H3K4me3 (D), H3K9Ac (E), H3K56Ac (F), H3K9me2 (G), H3K27me3 (H), JARID1A (I), LSD1 (J), JHDM1A (K), JHDM2A (L), JHDM3A (M), SIN3A (N), SNF2H (O), BRGI (P). The average log2 enrichment ratios of the genes measured by ChIP-chip assay across the promoter region and defined on the NimbleGen tiling array were plotted at 100 bp resolution.
We next examined the ChIP-chip data of each chromatin mark at the promoter/enhancer regions of the 337 genes upregulated by HG (Table 1) and 326 genes downregulated by HG (Table 2) and compared them with that of all RefSeq genes. From the composite profiles of the 15 marks tested (Fig. 4, B–P), only histone H3K9Ac depicted higher enrichment at the promoter/enhancer regions of HG-upregulated genes relative to all RefSeq genes (Fig. 4E). Since H3K9Ac levels generally have a positive correlation with gene expression, these data suggest that H3K9Ac might be indispensable for the poised “open” chromatin status required for both basal gene expression and induction by HG. Interestingly, the HG-downregulated gene set also had higher levels of H3K9Ac (Fig. 4E) as well as other active chromatin marks including RNA Pol II (Fig. 4B), RNA Pol II Ser5P (Fig. 4C), H3K4me3 (Fig. 4D), and H3K56Ac (Fig. 4F). The reason for this is not entirely clear, but further examination indicated that most of these HG-downregulated genes are normally highly expressed genes involved in key cellular functions (Fig. 2) and hence would be expected to be enriched in active chromatin modifications.
HG-upregulated genes with LCP are in a poised state with multiple active chromatin marks.
We next examined the promoters of the HG-upregulated genes more closely to determine if they had any distinctive chromatin modifications related to their upregulation in response to HG. Increasing evidence shows that promoter CpG islands play a central role in gene regulation, particularly in transcription initiation (12). Notably, >60% of human genes have CpG islands located near the TSS, termed HCP, in contrast to LCP. In general, HCP genes tend to have greater enrichment of active chromatin marks and higher expression levels than LCP genes (25).
To explore the role of promoter CpG islands in HG-induced gene regulation, we compared the basal state profiles of the 15 chromatin marks at the promoters of the 337 HG-upregulated genes between the HCP/ICP and LCP gene sets. Specifically, the ChIP-promoter array datasets of the 15 chromatin modifications at the 337 HG-upregulated genes were extracted and divided into HCP/ICP and LCP genes based on their promoter CpG content as described in materials and methods. Heat maps of chromatin features were generated based on log2 enrichment ratios of the microarray probes located at the promoter/enhancer regions of the 337 HG-upregulated genes (Fig. 5A), which showed that HCP/ICP and LCP genes have distinct chromatin patterns of key histone PTMs and other chromatin components. This is more clearly demonstrated in the chromatin composite profiles, which show the average enrichment levels of each mark at HG-upregulated genes with HCP/ICP (solid red line) and LCP (solid blue line) (Fig. 5, B–P). Compared with LCP, it is evident that, in the promoter regions of HG-upregulated genes, HCP/ICP had markedly higher enrichment signals of active marks RNA Pol II (Fig. 5B), RNA Pol II ser-5P (Fig. 5C), histone H3K4me3 (Fig. 5D), and H3K56Ac (Fig. 5F). Slightly higher enrichments of JARID1A (Fig. 5I), LSD1(Fig. 5J), JHDM1A (Fig. 5K), SIN3A (Fig. 5N), and SNF2H (Fig. 5O) were also seen, and very little differences in the levels of H3K9me2 (Fig. 5G), H3K27me3 (Fig. 5H), JHDM2A (Fig. 5L), JHDM3A (Fig. 5M), and BRG1 (Fig. 5P). In parallel, we also generated composite profiles of all RefSeq genes with HCP/ICP (Fig. 5, B–P, dashed red line) and LCP (Fig. 5, B–P, dashed blue line) for each chromatin mark. For all RefSeq genes, the differences in composite profiles between HCP/ICP and LCP followed a pattern similar to the HG-upregulated genes (Fig. 5, B–P, dashed red line vs. dashed blue line), with HCP/ICP in general depicting enhanced enrichment of active marks, and lower or no changes in repressive marks like H3K9me2, H3K27me3, JHDM3A, Sin3A, and BRG1.
Fig. 5.
Comparison of basal chromatin status between HG-upregulated genes with high CpG content promoter/intermediate CpG content promoter (HCP/ICP) and low CPG content promoter (LCP) in THP1 cells. A: heat map of indicated chromatin marks in the basal NG state of HG-upregulated genes with HCP/ICP and LCP in THP1 cells. HG-upregulated genes were classified into genes with HCP/ICP and LCP based on the CpG content within ±500 bp relative to the TSS. For each mark, the average PTM levels of HCP/ICP and LCP genes across their promoter region (indicated on the x-axis) at 100 bp resolution are shown. Genes were ordered by the expression level in NG condition measured by RNA-Seq, with the highest on top and lowest at bottom. B–P: composite profiles of the basal chromatin states of HG-upregulated genes with HCP/ICP and ICP. RefSeq genes were classified into genes with HCP/ICP and LCP based on the CpG content within the region of ±500 bp relative to the TSS. The average log2 enrichment ratios across the promoter regions of 4 gene sets were plotted at 100 bp resolution. These gene sets are: HG-upregulated genes with HCP/ICP (red solid line), HG-upregulated genes with LCP (blue solid line), all RefSeq genes with HCP/ICP (red dashed line), and all RefSeq with LCP (blue dashed line). Each panel represents the average enrichment of 1 chromatin mark: RNA Pol II (B), RNA Pol II Ser-5P (C), H3K4me3 (D), H3K9Ac (E), H3K56Ac (F), H3K9me2 (G), H3K27me3 (H), JARID1A (I), LSD1 (J), JHDM1A (K), JHDM2A (L), JHDM3A (M), SIN3A (N), SNF2H (O), BRGI (P).
Although the basal chromatin state of HG-upregulated genes with HCP/ICP was in general very similar to that of all RefSeq HCP/LCP genes (solid vs. dashed red lines), which is in line with the known features of HCP/ICP, it was important to note that the chromatin state of HG-upregulated genes with LCP clearly showed marked differences from that of all RefSeq genes with LCP (solid vs. dashed blue lines). More specifically, we observed that: 1) HG-upregulated genes with LCP depict markedly higher enrichment levels of active marks including RNA Pol II, RNA PolI ser-5P, histone H3K4me3, H3K9Ac, and H3K56Ac compared with all RefSeq genes with LCP (Fig. 5, B–F, solid blue lines vs. dashed blue lines); 2) although the enrichment levels of H3K4me3 (Fig. 5D) and H3K56Ac (Fig. 5F) at HG-upregulated genes with LCP were lower than those of HG-upregulated genes with HCP/ICP in the upstream regions of TSS, they increased sharply soon after TSS (0 to +500 bp) to reach levels similar to HCP/ICP (Fig. 5, D and F, solid blue line vs. solid red line); 3) the levels of histone H3K9Ac were very similar at HG-upregulated genes with LCP as well as with HCP/ICP in the entire promoter region (Fig. 5E, solid blue line vs. solid red line). Together, these high-resolution data demonstrate that, overall, HG-upregulated genes with LCP depict greater enrichment levels of active chromatin marks in contrast to the remaining RefSeq genes with LCP where low levels of active chromatin marks were observed as expected. This indicates that an open promoter configuration at LCP genes, similar to HCP/ICP, is necessary for upregulation by HG.
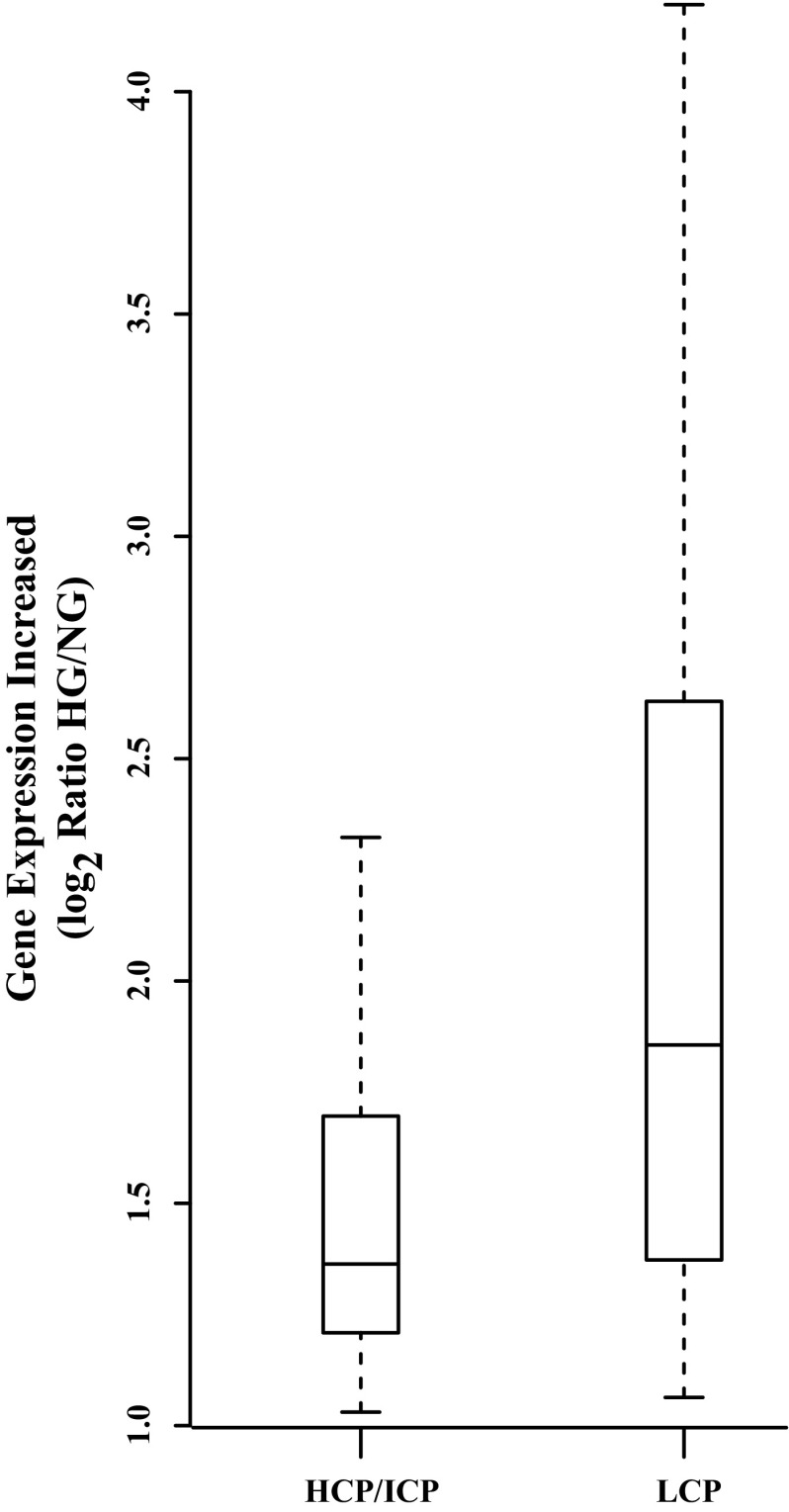
More specifically, we observed that, out of the 28 common genes shared between HGIN and IDIN (Table 5), 15 were LCP genes. The twofold enrichment of LCP genes within the 28 genes is noteworthy (54% compared with 28% in all RefSeq genes, χ2 P < 0.0018). In fact, all these 15 genes were ISGs with critical antiviral biological functions suggesting that these LCP genes have functionally important roles relative to the remaining HG-induced genes. In addition, as seen in Fig. 6, their promoter H3K4me3 (active mark) levels were markedly greater than the average levels at RefSeq LCP genes (∼0.17, Fig. 6, last bar). Moreover, we compare the increased expression levels of HG-upregulated genes with HCP/ICP and LCP. The average induction of LCP genes by HG was fourfold compared with 2.5-fold for HCP/LCP genes (Fig. 7), suggesting, in general, that regulation of LCP genes could be biologically important. Collectively, our data suggest that the promoters of HG-upregulated genes with LCP need to be in a poised and permissive state to be selected for subsequent active transcription and induction by HG. This poised state is enriched in multiple active chromatin marks such as histones H3K4me3, H3K9Ac, H3K56Ac, RNA Pol II ser-5P, and perhaps RNA Pol II. HG-upregulated genes with LCP likely play important roles in the HGIN.
Fig. 6.
Histone H3K4me3 enrichment levels near the TSS of common genes with LCP shared by high glucose-induced IRF network (HGIN) and IRF7-driven inflammatory network (IDIN). Histone H3K4me3 data were extracted from the ChIP-chip results. Among the 28 genes shared in HGIN and IDIN, 15 genes shown here had at least 1 transcript isoform containing LCP. The average H3K4me3 level at the defined region (TSS to 500 bp downstream of TSS) of each gene was calculated from all the probes located in the region and presented in the form of log2 ratios. For genes having >1 isoform with LCP promoter, the H3K4me3 levels of all the isoforms of the gene were averaged and presented.
Fig. 7.
Comparison of the increased expression levels of HG-upregulated genes with HCP/ICP and LCP. The expression level increase of each gene was calculated by the ratio of RPKM normalized reads between HG and NG in log2 scale shown on the y-axis. The distributions of expression level increase of HCP/ICP and LCP genes are summarized and presented as box plots.
Relevance to primary human monocytes.
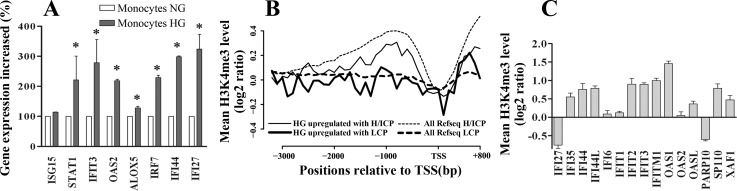
THP1 cells are widely used to investigate the functions of human monocytes (40). We next examined whether the results obtained in THP-1 cells can be recapitulated in peripheral blood CD14+ monocytes obtained from normal human volunteers cultured in HG or NG for 72 h. We observed that, among eight candidate genes tested in Fig. 3, seven were also significantly upregulated by HG in human monocytes (Fig. 8A). Furthermore, composite files from ChIP-chip data performed with these primary monocytes in NG (Fig. 8B) revealed that the average promoter enrichment levels of H3K4me3 (mark of active gene promoters) was similar to that seen in THP-1 cells (Fig. 5D), including the marked increases at regions downstream of TSS in LCP HG-upregulated genes compared with all RefSeq genes with LCP (Fig. 8B). Moreover, the majority of the LCP genes in HGIN exhibited high H3K4me3 levels also in primary monocytes (Fig. 8C), similar to THP1 cells (Fig. 6). Overall, these results indicate that the upregulation of antiviral response genes by HG can also occur in primary human peripheral blood monocytes.
Fig. 8.
Experiments in peripheral blood monocytes from human donors. A: purified human monocytes from normal healthy donors were cultured in 5.5 mM glucose (NG) or 25 mM glucose(HG) for 72 h. Total RNA samples were prepared for mRNA quantification by standard RT-QPCRs. β-Actin was used as the internal control. Results shown (means ± SE) are from 2 donor samples and 2 sets of PCRs with each sample run in triplicate; *P <0.001 HG vs. NG, by Student's t-tests. B: histone H3K4me3 composite profiles of HG-upregulated genes in human monocytes. RefSeq genes were classified into genes with HCP/ICP and LCP based on the CpG content within the ±500 bp region relative to the TSS. The average log2 enrichment ratios across the promoter region of 4 gene sets were plotted at 100-bp resolution. These gene sets are HG-upregulated genes with HCP/ICP (thin solid line), HG-upregulated genes with LCP (heavy solid line), all RefSeq genes with HCP/ICP (thin dashed line), and all RefSeq with LCP (heavy dashed line). C: histone H3K4me3 enrichment levels near the TSS of the 15 HGIN and IDIN shared genes with LCPs in human monocytes. The data were extracted from the ChIP-chip experiments run with monocytes from 3 separate donors. The average H3K4me3 level at the defined region (TSS to 500 bp downstream of TSS) of each gene was calculated and presented in the form of log2 ratios. The log2 enrichment ratios of the 3 samples at each probe were averaged to represent the H3K4me3 levels at RefSeq gene promoters in human primary monocytes as shown.
DISCUSSION
The Diabetes Complication and Control Trial and the follow-up observational Epidemiology of Diabetes Interventions and Complications Study have demonstrated that strict glycemic control can significantly reduce the progression of micro- and macrovascular complications in people with T1D (1, 2, 15, 26). They also identified the “metabolic memory” phenomenon, which suggests that prior exposure to hyperglycemia due to nonintensive glycemic control can result in persistently increased risk for diabetic complications even long after glucose normalization instituted by intensive glycemic control. Despite major efforts, the mechanisms behind these observations are still not fully clear.
To better understand the effects of hyperglycemia, we adopted the RNA-Seq approach because of its potential to reveal unprecedented insights into the diabetes-induced misregulated transcriptome. Indeed, this led to the discovery of a novel antiviral response network, HGIN, mediated by IRFs in HG-treated monocytes that was not identified in previous studies. ISGs were core components of this network, which, interestingly, depicted marked overlap with the IDIN network implicated in T1D risk (22). Further evaluation of the basal chromatin features of the HG-upregulated genes revealed that an open chromatin state (premarked with active histone modifications and Pol II) at promoter regions of these genes was a prerequisite for regulation by HG. Importantly, within the HG-upregulated gene set, similar to the HCP genes, the LCP genes were also poised for induction since they were also enriched with active chromatin marks, despite the “general rule” that LCPs have lower active chromatin marks. This clearly demonstrates the importance of chromatin states of promoter regions for gene upregulation by HG in monocytes.
The well-studied type 1 IFN pathway functions as a host defense against pathogens and triggers numerous genes collectively termed the antiviral response program. This occurs through the JAK-STAT1/2 pathway to activate the IRFs (49). IRFs interact directly with histone acetylase/deacetylases to control the balance of histone PTMs at target genes (3). IRF7 is the master regulator of type 1 IFN-dependent immune responses (23), which are critical for immunity against viruses. In this study, we demonstrated that HG has an IFN-α-like effect, triggering an ISG network likely through IFN-β, which therefore could be involved in initiating human T1D. The connection between IFN-α/β and T1D is well known including in human and rodent models (4, 50). IFN-α initiates T1D through upregulation IFN-α target genes in CD4+ cells in nonobese diabetic mice (27). Moreover, T1D occasionally appears during IFN-α therapy for unrelated diseases such as chronic hepatitis C. Interestingly, patients who developed IFN-α-induced T1D were genetically susceptible to T1D (36, 37). Our study clearly showed that HG upregulates an ISG network in THP1 cells as well as in human primary monocytes. Although it is well known that HG contributes to diabetic complications through upregulating cytokines such as TNF-α, IL-1β, IL-6, and MCP-1 in monocytes (10, 14, 20, 24, 32, 45, 46), our results reveal the IFN-α pathway as another major player in this scenario. Overall, this study has revealed for the first time that HG treatment of monocytes can trigger an IRF-mediated HGIN featured by ISGs, which can be implicated in T1D and its complications. Furthermore, epigenomic profiling uncovered key distinctive chromatin features of the HG-upregulated genes. These results provide novel new insights into the role of HG in the pathology of diabetes and its complications as well as chromatin states in HG-regulated gene expression.
GRANTS
This work was supported by National Institutes of Health Grants R01 DK-065073, R01 DK-081705, and R01 HL-087864 and the Juvenile Diabetes Research Foundation (to R. Natarajan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: F.M. and R.N. conception and design of research; F.M., L.Z., J.W., and H.G. performed experiments; F.M., Z.C., and X.W. analyzed data; F.M., Z.C., X.W., and R.N. interpreted results of experiments; F.M. and Z.C. prepared figures; F.M., Z.C., and R.N. drafted manuscript; F.M., Z.C., X.W., and R.N. edited and revised manuscript; F.M., Z.C., L.Z., J.W., H.G., X.W., and R.N. approved final version of manuscript.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to Margaret Morgan (Beckman Research Institute of City of Hope) for assistance with the manuscript.
Footnotes
The online version of this article contains supplemental material.
REFERENCES
- 1. The effect of intensive treatment of diabetes on the development, and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329: 977–986, 1993. [DOI] [PubMed] [Google Scholar]
- 2. Retinopathy and nephropathy in patients with type 1 diabetes four years after a trial of intensive therapy. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. N Engl J Med 342: 381–389, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agalioti T, Chen G, Thanos D. Deciphering the transcriptional histone acetylation code for a human gene. Cell 111: 381–392, 2002. [DOI] [PubMed] [Google Scholar]
- 4. Baccala R, Kono DH, Theofilopoulos AN. Interferons as pathogenic effectors in autoimmunity. Immunol Rev 204: 9–26, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Bao W, Min D, Twigg SM, Shackel NA, Warner FJ, Yue DK, McLennan SV. Monocyte CD147 is induced by advanced glycation end products and high glucose concentration: possible role in diabetic complications. Am J Physiol Cell Physiol 299: C1212–C1219, 2010. [DOI] [PubMed] [Google Scholar]
- 6. Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, Wei G, Chepelev I, Zhao K. High-resolution profiling of histone methylations in the human genome. Cell 129: 823–837, 2007. [DOI] [PubMed] [Google Scholar]
- 7. Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature 414: 813–820, 2001. [DOI] [PubMed] [Google Scholar]
- 8. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54: 1615–1625, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Campos EI, Reinberg D. Histones: annotating chromatin. Annu Rev Genet 43: 559–599, 2009. [DOI] [PubMed] [Google Scholar]
- 10. Dasu MR, Devaraj S, Jialal I. High glucose induces IL-1beta expression in human monocytes: mechanistic insights. Am J Physiol Endocrinol Metab 293: E337–E346, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes 57: 3090–3098, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Deaton AM, Bird A. CpG islands and the regulation of transcription. Genes Dev 25: 1010–1022, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devaraj S, Jialal I. Increased secretion of IP-10 from monocytes under hyperglycemia is via the TLR2 and TLR4 pathway. Cytokine 47: 6–10, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Devaraj S, Venugopal SK, Singh U, Jialal I. Hyperglycemia induces monocytic release of interleukin-6 via induction of protein kinase c-alpha and -beta. Diabetes 54: 85–91, 2005. [DOI] [PubMed] [Google Scholar]
- 15. EDIC Writing Team. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 287: 2563–2569, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ernst J, Kheradpour P, Mikkelsen TS, Shoresh N, Ward LD, Epstein CB, Zhang X, Wang L, Issner R, Coyne M, Ku M, Durham T, Kellis M, Bernstein BE. Mapping and analysis of chromatin state dynamics in nine human cell types. Nature 473: 43–49, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Giaccari A, Sorice G, Muscogiuri G. Glucose toxicity: the leading actor in the pathogenesis and clinical history of type 2 diabetes - mechanisms and potentials for treatment. Nutr Metab Cardiovasc Dis 19: 365–377, 2009. [DOI] [PubMed] [Google Scholar]
- 18. Grzenda A, Lomberk G, Zhang JS, Urrutia R. Sin3: master scaffold and transcriptional corepressor. Biochim Biophys Acta 1789: 443–450, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell 130: 77–88, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guha M, Bai W, Nadler JL, Natarajan R. Molecular mechanisms of tumor necrosis factor alpha gene expression in monocytic cells via hyperglycemia-induced oxidant stress-dependent and -independent pathways. J Biol Chem 275: 17728–17739, 2000. [DOI] [PubMed] [Google Scholar]
- 21. Hawkins RD, Hon GC, Ren B. Next-generation genomics: an integrative approach. Nat Rev Genet 11: 476–486, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heinig M, Petretto E, Wallace C, Bottolo L, Rotival M, Lu H, Li Y, Sarwar R, Langley SR, Bauerfeind A, Hummel O, Lee YA, Paskas S, Rintisch C, Saar K, Cooper J, Buchan R, Gray EE, Cyster JG, Erdmann J, Hengstenberg C, Maouche S, Ouwehand WH, Rice CM, Samani NJ, Schunkert H, Goodall AH, Schulz H, Roider HG, Vingron M, Blankenberg S, Munzel T, Zeller T, Szymczak S, Ziegler A, Tiret L, Smyth DJ, Pravenec M, Aitman TJ, Cambien F, Clayton D, Todd JA, Hubner N, Cook SA. A trans-acting locus regulates an anti-viral expression network and type 1 diabetes risk. Nature 467: 460–464, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T, Shimada N, Ohba Y, Takaoka A, Yoshida N, Taniguchi T. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 434: 772–777, 2005. [DOI] [PubMed] [Google Scholar]
- 24. Jain SK, Rains JL, Croad JL. High glucose and ketosis (acetoacetate) increases, and chromium niacinate decreases, IL-6, IL-8, and MCP-1 secretion and oxidative stress in U937 monocytes. Antioxid Redox Signal 9: 1581–1590, 2007. [DOI] [PubMed] [Google Scholar]
- 25. Juven-Gershon T, Hsu JY, Theisen JW, Kadonaga JT. The RNA polymerase II core promoter - the gateway to transcription. Curr Opin Cell Biol 20: 253–259, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lasker RD. The diabetes control and complications trial. Implications for policy and practice. N Engl J Med 329: 1035–1036, 1993. [DOI] [PubMed] [Google Scholar]
- 27. Li Q, Xu B, Michie SA, Rubins KH, Schreriber RD, McDevitt HO. Interferon-alpha initiates type 1 diabetes in nonobese diabetic mice. Proc Natl Acad Sci USA 105: 12439–12444, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, Natarajan R. Role of the histone H3 lysine 4 methyltransferase, SET7/9, in the regulation of NF-kappaB-dependent inflammatory genes. Relevance to diabetes and inflammation. J Biol Chem 283: 26771–26781, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marioni JC, Mason CE, Mane SM, Stephens M, Gilad Y. RNA-seq: an assessment of technical reproducibility and comparison with gene expression arrays. Genome Res 18: 1509–1517, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matkovich SJ, Zhang Y, Van Booven DJ, Dorn GW., 2nd Deep mRNA sequencing for in vivo functional analysis of cardiac transcriptional regulators: application to Galphaq. Circ Res 106: 1459–1467, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Miao F, Chen Z, Zhang L, Liu Z, Wu X, Yuan YC, Natarajan R. Profiles of epigenetic histone post-translational modifications at type 1 diabetes susceptible genes. J Biol Chem 287: 16335–16345, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling events leading to inflammatory gene transcription under diabetic conditions. J Biol Chem 279: 18091–18097, 2004. [DOI] [PubMed] [Google Scholar]
- 33. Miao F, Smith DD, Zhang L, Min A, Feng W, Natarajan R. Lymphocytes from patients with type 1 diabetes display a distinct profile of chromatin histone H3 lysine 9 dimethylation: an epigenetic study in diabetes. Diabetes 57: 3189–3198, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Miao F, Wu X, Zhang L, Yuan YC, Riggs AD, Natarajan R. Genome-wide analysis of histone lysine methylation variations caused by diabetic conditions in human monocytes. J Biol Chem 282: 13854–13863, 2007. [DOI] [PubMed] [Google Scholar]
- 35. Mosammaparast N, Shi Y. Reversal of histone methylation: biochemical and molecular mechanisms of histone demethylases. Annu Rev Biochem 79: 155–179, 2010. [DOI] [PubMed] [Google Scholar]
- 36. Nakamura K, Kawasaki E, Imagawa A, Awata T, Ikegami H, Uchigata Y, Kobayashi T, Shimada A, Nakanishi K, Makino H, Maruyama T, Hanafusa T. Type 1 diabetes and interferon therapy: a nationwide survey in Japan. Diabetes Care 34: 2084–2089, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakanishi K, Saitoh S. Clinical and genetic characteristics of patients with type 1 diabetes associated with interferon therapy. Diabetes Care 34: 471–473, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pirola L, Balcerczyk A, Tothill RW, Haviv I, Kaspi A, Lunke S, Ziemann M, Karagiannis T, Tonna S, Kowalczyk A, Beresford-Smith B, Macintyre G, Kelong M, Hongyu Z, Zhu J, El-Osta A. Genome-wide analysis distinguishes hyperglycemia regulated epigenetic signatures of primary vascular cells. Genome Res 21: 1601–1615, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol 28: 1057–1068, 2010. [DOI] [PubMed] [Google Scholar]
- 40. Qin Z. The use of THP-1 cells as a model for mimicking the function and regulation of monocytes and macrophages in the vasculature. Atherosclerosis 221: 2–11, 2012. [DOI] [PubMed] [Google Scholar]
- 41. Racki LR, Yang JG, Naber N, Partensky PD, Acevedo A, Purcell TJ, Cooke R, Cheng Y, Narlikar GJ. The chromatin remodeller ACF acts as a dimeric motor to space nucleosomes. Nature 462: 1016–1021, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ren B, Robert F, Wyrick JJ, Aparicio O, Jennings EG, Simon I, Zeitlinger J, Schreiber J, Hannett N, Kanin E, Volkert TL, Wilson CJ, Bell SP, Young RA. Genome-wide location and function of DNA binding proteins. Science 290: 2306–2309, 2000. [DOI] [PubMed] [Google Scholar]
- 43. Ryan DP, Owen-Hughes T. Snf2-family proteins: chromatin remodellers for any occasion. Curr Opin Chem Biol 15: 649–656, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature 472: 481–485, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shanmugam N, Gaw Gonzalo IT, Natarajan R. Molecular mechanisms of high glucose-induced cyclooxygenase-2 expression in monocytes. Diabetes 53: 795–802, 2004. [DOI] [PubMed] [Google Scholar]
- 46. Shanmugam N, Reddy MA, Guha M, Natarajan R. High glucose-induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes 52: 1256–1264, 2003. [DOI] [PubMed] [Google Scholar]
- 47. Shanmugam N, Reddy MA, Natarajan R. Distinct roles of heterogeneous nuclear ribonuclear protein K and microRNA-16 in cyclooxygenase-2 RNA stability induced by S100b, a ligand of the receptor for advanced glycation end products. J Biol Chem 283: 36221–36233, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sheetz MJ, King GL. Molecular understanding of hyperglycemia's adverse effects for diabetic complications. JAMA 288: 2579–2588, 2002. [DOI] [PubMed] [Google Scholar]
- 49. Stark GR, Darnell JE., Jr The JAK-STAT pathway at twenty. Immunity 36: 503–514, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Theofilopoulos AN, Baccala R, Beutler B, Kono DH. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu Rev Immunol 23: 307–336, 2005. [DOI] [PubMed] [Google Scholar]
- 51. Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet 10: 57–63, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Weber M, Hellmann I, Stadler MB, Ramos L, Paabo S, Rebhan M, Schubeler D. Distribution, silencing potential and evolutionary impact of promoter DNA methylation in the human genome. Nat Genet 39: 457–466, 2007. [DOI] [PubMed] [Google Scholar]
- 53. Wu CH, Yeh CT, Shih PH, Yen GC. Dietary phenolic acids attenuate multiple stages of protein glycation and high-glucose-stimulated proinflammatory IL-1beta activation by interfering with chromatin remodeling and transcription in monocytes. Mol Nutr Food Res 54, Suppl 2: S127–S140, 2010. [DOI] [PubMed] [Google Scholar]
- 54. Zhou VW, Goren A, Bernstein BE. Charting histone modifications and the functional organization of mammalian genomes. Nat Rev Genet 12: 7–18, 2011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.