Abstract
Clinical and experimental studies have suggested benefit of treatment with intravenous glucose-insulin-potassium (GIK) in acute myocardial infarction. However, patients hospitalized with acute coronary syndromes often experience recurrent myocardial ischemia without infarction that may cause progressive left ventricular (LV) dysfunction. This study tested the hypothesis that anticipatory treatment with GIK attenuates both systolic and diastolic LV dysfunction resulting from ischemia and reperfusion without infarction in vivo. Open-chest, anesthetized pigs underwent 90 min of moderate regional ischemia (mean subendocardial blood flow 0.3 ml·g−1·min−1) and 90 min reperfusion. Eight pigs were treated with GIK (300 g/l glucose, 50 U/l insulin, and 80 meq/l KCl; infused at 2 ml·kg−1·h−1) beginning 30 min before ischemia and continuing through reperfusion. Eight untreated pigs comprised the control group. Regional LV wall area was measured with orthogonal pairs of sonomicrometry crystals. GIK significantly increased myocardial glucose uptake and lactate release during ischemia. After reperfusion, indexes of regional systolic function (external work and fractional systolic wall area reduction), regional diastolic function (maximum rate of diastolic wall area expansion), and global LV function (LV positive and negative maximum rate of change in pressure with respect to time) recovered to a significantly greater extent in GIK-treated pigs than in control pigs (all P < 0.05). The findings suggest that the clinical utility of GIK may extend beyond treatment of acute myocardial infarction to anticipatory metabolic protection of myocardium in patients at risk for recurrent episodes of ischemia.
Keywords: ventricular function, energy metabolism, substrates
Interventions that enhance the availability and utilization of glucose by the ischemic heart may attenuate ischemic injury and postischemic ventricular dysfunction (7). A mixture of glucose, insulin, and potassium (GIK) was first shown to limit electrocardiographic changes in acute myocardial infarction by Sodi-Pallares and colleagues in 1962 (33). Subsequent experimental studies demonstrated a salutary effect of GIK in limiting infarct size and improving recovery of mechanical function in models of myocardial infarction (1, 21, 27). Initial clinical trials in the 1960s and 1970s suggested that GIK reduced morbidity and mortality in acute myocardial infarction (3, 22, 29, 31), but the results were inconclusive. For the most part, the potential therapeutic utility of GIK was ignored for two succeeding decades.
Recently, there has been a resurgence of interest in GIK as a treatment for patients with acute myocardial infarction. In a meta-analysis of nine prior clinical studies, Fath-Ordoubadi and Beatt (10) came to the remarkable conclusion that GIK reduced in-hospital mortality from acute myocardial infarction by 28%. The magnitude of this mortality reduction is comparable to that achieved with thrombolytic therapy and suggests that metabolic protection of ischemic myocardium may be as important as reperfusion itself. The DIGAMI study (20) in diabetic patients showed that insulin-glucose infusion early in the course of acute myocardial infarction, followed by a multidose insulin regimen, reduced mortality compared with conventional therapy. In a multicenter study in acute myocardial infarction (8), treatment with GIK and a concurrent reperfusion strategy resulted in a 34% reduction in mortality compared with a control group of patients who did not receive GIK. GIK has also been shown to be effective in improving cardiac function after cardiopulmonary bypass and cardioplegic arrest (16, 24).
Despite the renewed interest in GIK as a clinical intervention in acute myocardial infarction, there is a paucity of data regarding the utility of GIK in acute myocardial ischemia without infarction. Patients hospitalized with acute coronary syndromes frequently experience recurrent episodes of myocardial ischemia without infarction that may cause progressive left ventricular (LV) dysfunction. Such patients may therefore benefit from a strategy of anticipatory metabolic protection. Indeed, when isolated, perfused hearts are subjected to moderate ischemia followed by reperfusion, the addition of extra glucose and insulin to the perfusate attenuates both contractile and lusitropic dysfunction (2, 9). To date, however, there have been no analogous data from in vivo models.
Beneficial effects of GIK in myocardial ischemia may result from increased myocardial glucose uptake and glycogen content, providing additional substrate for glycolytic energy generation. Benefit may also be due to reduced circulating levels and myocardial uptake of free fatty acids (FFA; see Ref. 28). However, there are at least two theoretical reasons why GIK might exert deleterious effects during ischemia. First, incremental myocardial glucose uptake due to GIK may result in increased glycogen synthesis (energy consuming) and/or increased glycolysis (energy producing). If, during ischemia, the rate of glycogen synthesis is increased to a greater extent than the rate of glycolysis (13), the net energetic consequence could be unfavorable. Second, glycolysis results in formation of protons, whose accumulation may promote intracellular calcium overload and reperfusion injury. Provision of additional glycolytic substrate during ischemia could exacerbate this mechanism of injury.
This study tested the hypothesis that anticipatory treatment with GIK attenuates both systolic and diastolic dysfunction resulting from myocardial ischemia and reperfusion without infarction in an intact, porcine model.
MATERIALS AND METHODS
Experimental preparation
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the United States National Institutes of Health. The experimental preparation and instrumentation of the heart have been described previously (32, 37). Nineteen female Yorkshire-Landrace pigs, weighing 28–38 kg, were premedicated with ketamine hydrochloride (25 mg/kg im). Anesthesia was induced with 100 mg/kg iv α-chloralose and was maintained with 40 mg·kg−1·h−1 iv α-chloralose. After endotracheal intubation via a tracheotomy, pigs were mechanically ventilated with an air-oxygen mixture. Respiratory rate and tidal volume were adjusted on the basis of frequent arterial blood gas analyses. To prevent hypothermia, pigs were wrapped in recirculating warm water blankets and insulated pads. Indomethacin (25 mg iv) was given to prevent subsequent hemodynamic response to the injection of fluorescent microspheres suspended in diluted Tween solution. Propranolol (1 mg/kg iv) and atropine (0.2 mg/kg iv) were given to prevent activation of autonomic reflexes.
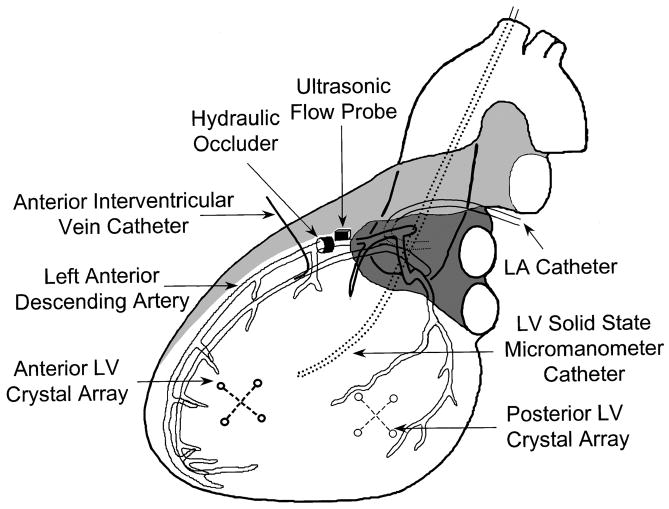
Figure 1 shows the instrumentation of the heart. The chest was opened via a median sternotomy. A bipolar pacing electrode was affixed to the left atrial appendage and was used to maintain heart rate slightly above the spontaneous rate. Fluid-filled catheters were inserted in the aortic arch via a carotid artery and in the left atrium via its appendage. A 5-Fr Millar catheter (Millar Instruments, Houston, TX) was inserted in the LV cavity through a carotid artery. To produce dynamic alteration in preload, hydraulic cuff occluders (In Vivo Metric, Healdsburg, CA) were placed around the inferior and superior venae cavae. A segment of the left anterior descending coronary artery (LAD) between its first and second diagonal branches was dissected free of surrounding tissue. An ultrasonic transit time flow probe (Transonic Systems, Ithaca, NY) was placed around the vessel to monitor coronary flow. A hydraulic cuff occluder was placed just distal to the flow probe to produce graded myocardial ischemia. To obtain coronary venous blood samples from the ischemic region, a catheter was inserted in the anterior interventricular vein at a site distal to that of the LAD occluder, with the tip of the catheter directed toward the apex of the heart. Regional LV function was measured in the central portion of the anterior free wall (ischemic region) and in the posterolateral free wall (nonischemic region). In each region, an array of four sonomicrometry crystals (2 orthogonal pairs) was implanted in the subendocardium. Each crystal array was used to calculate regional myocardial wall area as the instantaneous product of the two orthogonal segment lengths (17).
Fig. 1.
Instrumentation of the heart. LV, left ventricular; LA, left atrial.
Myocardial blood flow, oxygen consumption, and substrate uptake
Transmural myocardial blood flow measurements were made under each experimental condition using left atrial injection of fluorescent microspheres, as previously described (32). Oxygen, glucose, lactate, and FFA contents were measured in paired arterial and anterior interventricular venous blood samples, as previously described (32, 35). Uptakes of each of these substances by the anterior LV free wall were calculated as the product of the mean transmural blood flow and the coronary arteriovenous concentration difference. In pigs, at least 90% of anterior interventricular venous blood is derived from myocardium perfused by the LAD, even under ischemic conditions (4). Insulin and potassium were measured in arterial plasma by RIAand potentiometry, respectively.
Experimental protocol
The following four sets of measurements were made in each pig: baseline, preischemic treatment, ischemia, and reperfusion. After a 30-min period of stabilization, baseline measurements of hemodynamics, regional LV function, myocardial blood flow, myocardial oxygen and substrate uptake, and plasma insulin and potassium were obtained. Pigs were then alternately assigned to one of two groups. The control group (n = 8) received an intravenous infusion of normal saline (2 ml·kg−1·h−1), whereas the GIK group (n = 8) received an intravenous infusion of GIK (glucose: 300 g/l; insulin: 50 U/l; potassium: 80 meq/l) infused at 2 ml·kg−1·h−1. The infusions were continued for the remaining duration of the experimental protocol. After saline or GIK had been infused for 30 min under nonischemic conditions, the second set of measurements was obtained (preischemic treatment). Low-flow ischemia was then induced by gradual inflation of the LAD occluder until LAD flow rate (monitored by the transit-time flowmeter) was reduced to 50% of baseline. This degree of constriction was then maintained for 90 min; slight adjustments of the coronary occluder were made as necessary to maintain constant 50% reduction of LAD flow. We have previously shown that regional ischemia of this severity and duration in this model results in a reproducible reduction of subendocardial blood flow and regional external work to 25–35% of baseline, without histochemical or electron micrographic evidence of myocardial infarction (19). Between 60 and 90 min of ischemia, the third set of measurements was obtained. After 90 min ischemia, the coronary occluder was released. The fourth and final set of measurements was performed between 60 and 90 min of reperfusion. The control group of this study also served as the control group for another contemporaneous study (37).
After euthanasia, transmural specimens of myocardium were excised from ischemic and nonischemic regions of the LV for analysis of regional blood flow. In addition, a transmural sample of myocardium from the center of the ischemic region was incubated in warm 1% triphenyltetrazolium chloride (TTC) for 20 min and was examined for evidence of nonstaining indicative of myocardial infarction (11).
Hemodynamic data collection and analysis of ventricular function
Hemodynamic and sonomicrometry data were digitized and analyzed as previously described (19, 37). Data were collected both under steady-state hemodynamic conditions and during brief occlusion of the venae cavae with temporary suspension of mechanical ventilation. At least five sets of data were collected under each experimental condition.
LV pressure versus wall area loops were analyzed. The area of each loop was used as an index of the regional external work performed in that cardiac cycle. Steady-state regional external work was calculated as the average loop area recorded without occlusion of the venae cavae. Regional preload recruitable stroke work (PRSW) relations (12) were determined during brief occlusion of the venae cavae by plotting the area of each LV pressure versus wall area loop against its corresponding end-diastolic wall area. End diastole was defined by the initial upstroke of an intramyocardial electrogram recorded using the intramyocardial electrocardiogram capability of the sonomicrometer. PRSW slope and interecept were determined by linear regression. Because regional wall area depends in part upon how far apart each pair of crystals is implanted (i.e., a source of experimental rather than physiological variability), external work and PRSW data were normalized to their baseline values in each experiment. Systolic function was also assessed by fractional systolic wall area reduction (FAR), a two-dimensional analog to fractional systolic segment shortening, calculated as
where EDA and ESA are end-diastolic and end-systolic wall area, respectively. Peak positive change in LV pressure with respect to time (+dP/dtmax) was calculated as an index of global LV contractility.
Regional LV relaxation was assessed by the maximum rate of wall area expansion (peak positive dA/dt or +dA/dtmax) occurring during early diastole. This is a regional analog to peak LV filling rate. +dA/dtmax was normalized to baseline in each experiment. Passive diastolic properties were assessed by examining LV end-diastolic pressure versus regional wall area relations, using a monoexponential curve-fitting technique described previously (19). Global LV relaxation was assessed by peak negative LV dP/dt (−dP/dtmax).
Statistical analysis
To determine if the response of a variable differed between groups during the experimental protocol, two-way repeated-measures ANOVA was employed, followed by unpaired t-tests to compare the value of the variable between groups under specific experimental conditions. To determine the significance of changes in a variable between baseline and subsequent conditions within the same group, one-way repeated-measures ANOVA was employed, followed by Dunnett’s procedure. Data are expressed as means ± SE. Statistical analysis was performed using Sigma-Stat software (Jandel Scientific, San Rafael, CA).
RESULTS
Three pigs sustained ventricular fibrillation during ischemia (2 in the control group and 1 in the GIK group) and were not resuscitated. Hemodynamic and metabolic data from 16 pigs that completed the experimental protocol (8 in each group) are reported below.
Regional myocardial blood flow
During ischemia, total LAD flow (measured by the ultrasonic flow probe) was regulated to maintain a 50% reduction from baseline. This degree of flow reduction was associated with moderate anterior LV subendocardial ischemia (~25% of baseline perfusion) and milder subepicardial ischemia (~60% of baseline perfusion) as indicated in Table 1. Perfusion of the posterior LV did not change significantly throughout the experimental protocol. There were no significant differences between groups in either LAD blood flow or regional myocardial perfusion under any experimental condition. Incubation of myocardium from the ischemic region in TTC resulted in homogeneous, transmural red staining, consistent with the absence of myocardial infarction.
Table 1.
Regional myocardial blood flow
| Baseline |
Preischemic Treatment |
Ischemia |
Reperfusion |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | GIK | Control | GIK | Control | GIK | Control | GIK | |
| Flowmeter-determined blood flow, ml/min | ||||||||
| LAD blood flow | 20±2 | 22±2 | 20±2 | 22±2 | 10±1* | 11±1* | 23±3 | 26±2* |
| Microsphere-determined blood flow, ml·g−1·min−1 | ||||||||
| Anterior LV subepicardium | 1.04±0.18 | 1.01±0.18 | 1.28±0.22 | 1.29±0.39 | 0.54±0.11 | 0.67±0.15 | 0.99±0.11 | 1.25±0.43 |
| Anterior LV midmyocardium | 1.03±0.19 | 1.16±0.19 | 1.34±0.23 | 1.41±0.37 | 0.34±0.15* | 0.45±0.17* | 0.85±0.15 | 1.22±0.39 |
| Anterior LV subendocardium | 1.27±0.23 | 1.04±0.09 | 1.31±0.21 | 1.26±0.19 | 0.32±0.15* | 0.24±0.06* | 0.97±0.20 | 1.01±0.26 |
| Posterior LV subepicardium | 1.12±0.28 | 0.92±0.07 | 1.38±0.22 | 1.10±0.14 | 1.01±0.10 | 1.00±0.15 | 0.92±0.20 | 1.07±0.15 |
| Posterior LV midmyocardium | 1.23±0.25 | 1.08±0.13 | 1.31±0.25 | 1.30±0.19 | 1.11±0.05 | 1.12±0.19 | 1.02±0.18 | 1.14±0.17 |
| Posterior LV subendocardium | 1.19±0.23 | 1.08±0.14 | 1.29±0.25 | 1.27±0.21 | 1.19±0.08 | 1.02±0.17 | 0.93±0.16 | 1.13±0.27 |
Values are means ± SE; n = 8 pigs in each group. GIK, glucose-insulin-potassium; LAD, left anterior descending coronary artery; LV, left ventricular.
P < 0.05 vs. baseline within same group.
Circulating substrate and insulin levels and substrate uptake of the anterior LV
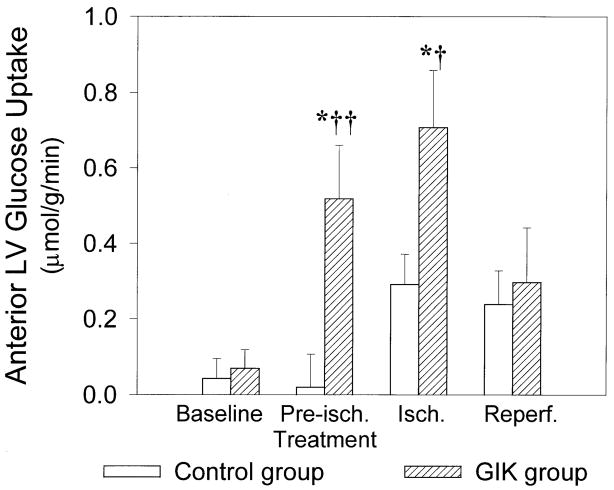
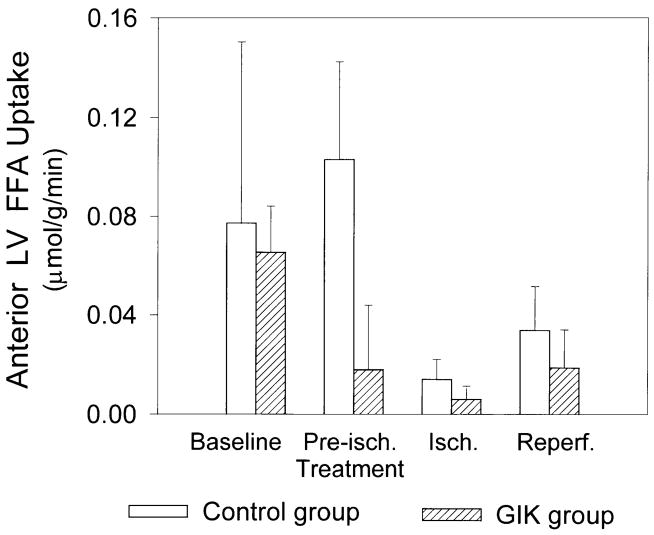
At baseline, there were no significant differences between groups in arterial substrate, insulin, or potassium concentrations, myocardial substrate uptake, or oxygen consumption (Table 2). Infusion of GIK increased arterial glucose and insulin concentrations, decreased arterial FFA concentration, and did not alter plasma potassium concentration. GIK significantly increased anterior LV glucose uptake during both preischemic treatment and ischemia (Fig. 2). During ischemia, there was net lactate release in both groups; however, the GIK-treated group released significantly more lactate, indicating enhanced anaerobic glycolysis (Fig. 3). There was a trend to diminished myocardial FFA uptake with GIK during both preischemic treatment and ischemic periods; however, this trend did not achieve statistical significance (Fig. 4). Anterior LV oxygen consumption declined during ischemia and remained reduced during reperfusion, but there were no significant differences between groups. During ischemia, the coronary arteriovenous pH difference (reflecting transmyocardial production of hydrogen ions) was significantly greater in the GIK group than in the control group, consistent with a higher rate of anaerobic glycolytic metabolism. During reperfusion, the coronary arteriovenous pH difference returned to baseline in both groups (Table 2).
Table 2.
Insulin, potassium, substrate concentrations and myocardial oxygen consumption
| Baseline |
Preischemic Treatment |
Ischemia |
Reperfusion |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | GIK | Control | GIK | Control | GIK | Control | GIK | |
| Insulin, μU/ml plasma | 6±1 | 7±1 | 7±2 | 64±7*‡ | 5±1 | 84±7*‡ | 5±1 | 79±10*‡ |
| Potassium, mmol/l plasma | 4.1±0.2 | 3.9±0.1 | 3.9±0.2 | 3.9±0.2 | 3.9±0.1 | 3.9±0.1 | 4.0±0.1 | 4.2±0.2 |
| Arterial glucose, μmol/ml blood | 3.8±0.4 | 3.7±0.5 | 3.9±0.3 | 8.1±0.4*‡ | 3.9±0.2 | 9.1±0.7*‡ | 3.7±0.2 | 7.1±0.8*‡ |
| Arterial lactate, μmol/ml blood | 1.6±0.3 | 1.5±0.2 | 1.3±0.2* | 1.4±0.1 | 1.1±0.1* | 1.5±0.1† | 0.9±0.1* | 1.2±0.1 |
| Arterial FFA, μmol/ml blood | 0.36±0.08 | 0.31±0.05 | 0.41±0.09 | 0.20±0.03*† | 0.27±0.04 | 0.11±0.02*‡ | 0.26±0.04 | 0.11±0.02*‡ |
| Anterior LV MV̇O2, μmol·g−1·min−1 | 5.2±0.7 | 4.7±0.4 | 5.6±0.7 | 5.4±1.1 | 1.8±0.4* | 2.0±0.4* | 2.7±0.4* | 2.9±0.5* |
| Coronary venous pH | 7.37±0.02 | 7.34±0.03 | 7.37±0.03 | 7.34±0.02 | 7.28±0.04* | 7.18±0.04* | 7.29±0.03* | 7.24±0.02* |
| Coronary arteriovenous pH difference | 0.06±0.01 | 0.05±0.01 | 0.07±0.01 | 0.09±0.01*‡ | 0.11±0.01* | 0.17±0.03*† | 0.06±0.01 | 0.06±0.01 |
Values are means ± SE; n = 8 in each group. FFA, free fatty acid; MV̇O2, myocardial O2 consumption.
P < 0.05 vs. baseline within same group.
P ≤ 0.05 and
P < 0.01 vs. control group under same condition.
Fig. 2.
Ischemic zone glucose uptake. Data are means ± SE, n = 8 pigs in each group. Isch, ischemia; reperf, reperfusion. Infusion of glucose-insulin-potassium (GIK) significantly increased anterior LV glucose uptake during preischemic treatment and ischemia. *P < 0.05 vs. baseline within same group. †P < 0.05 and ††P < 0.01 vs. control group under same condition.
Fig. 3.
Ischemic zone lactate uptake. Data are means ± SE; n = 8 in each group. During ischemia, there was net lactate release (negative lactate uptake) in both groups; however, the GIK-treated group released significantly more lactate. *P < 0.05 vs. baseline within same group. †P < 0.05 vs. control group under same condition.
Fig. 4.
Ischemic zone free fatty acid (FFA) uptake. Data are means ± SE; n = 8 in each group. There was a trend to diminished FFA uptake with GIK in both the preischemic treatment and ischemic periods compared with the control group. However, this trend did not achieve statistical significance.
Regional systolic function
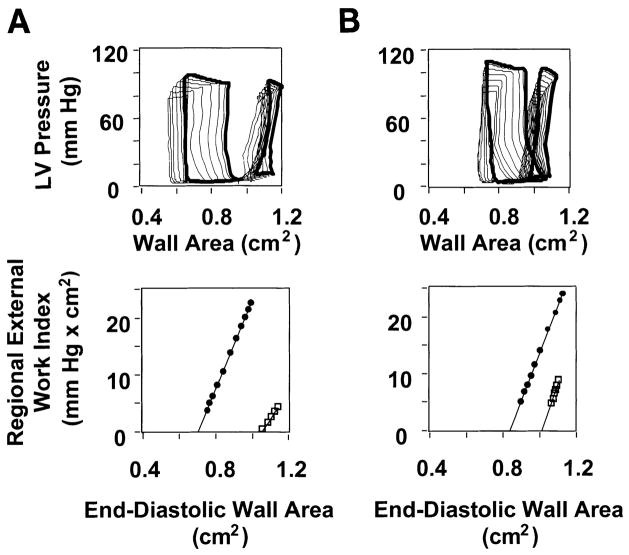
Administration of GIK during the preischemic treatment period had no discernible effect on indexes of regional systolic function. As expected, indexes of systolic function in the anterior LV declined during ischemia and remained depressed during reperfusion in both groups. However, GIK-treated pigs demonstrated significantly greater recovery of systolic function with reperfusion (Fig. 5). Both steady-state regional external work and FAR recovered to significantly greater values in the GIK group. For example, regional external work recovered to a mean level 38% of baseline with GIK but only to 18% of baseline in the control group. Similarly, FAR recovered to 38% of baseline in the GIK group but to only 4% of baseline in the control group. The improved recovery of systolic function with GIK was not attributable to a difference in loading conditions, since end-diastolic wall area (preload) and aortic systolic pressure did not differ between groups (Table 3). Analysis of anterior LV PRSW relations also revealed a beneficial effect of GIK. After reperfusion, PRSW slope (the increment in regional external work for a given increment in preload) recovered to 81 ± 7% of baseline in the GIK group compared with 61 ± 5% of baseline in the control group (P < 0.05). However, there was no difference between groups in PRSW intercept, which increased significantly with ischemia and remained increased from baseline during reperfusion. The effects of anterior LV ischemia on systolic function of the posterior LV were minor and did not differ significantly between groups (Table 3).
Fig. 5.
Typical ischemic zone LV pressure vs. wall area loops in control and GIK-treated pigs. A and B, top: LV pressure vs. wall area loops were obtained during 10-s occlusion of the venae cavae under baseline conditions (large loops) and after 90 min reperfusion after 90 min ischemia (small loops). Data from a pig in the control group are shown in A, and data from a pig in the GIK group are shown in B. In each example, a loop recorded under steady-state conditions (i.e., before occlusion of the venae cavae) is shown in bold, while loops recorded during occlusion of the venae cavae are shown as light lines. Both pigs exhibit contractile dysfunction after reperfusion, manifest by reduced loop area (an index of regional external work) compared with baseline. However, the recovery of loop area after reperfusion is greater in the GIK-treated pig. A and B, bottom: preload-recruitable stroke work (PRSW) relations derived from data in top by plotting the area of each loop against its corresponding end-diastolic wall area under baseline conditions (●) and after 90 min reperfusion after 90 min ischemia (□). Top point of each PRSW relation indicates steady-state condition, corresponding to the bold loops in top. Data demonstrate that recovery of regional external work is greater in the GIK-treated pig than in the untreated (control group) pig.
Table 3.
Regional left ventricular function
| Baseline |
Preischemic Treatment |
Ischemia |
Reperfusion |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | GIK | Control | GIK | Control | GIK | Control | GIK | |
| Anterior LV | ||||||||
| Preload | ||||||||
| Steady-state end-diastolic crystal area | 1 | 1 | 1.00±0.01 | 1.00±0.01 | 1.17±0.03* | 1.13±0.02* | 1.09±0.05 | 1.10±0.04* |
| Systolic function | ||||||||
| Steady-state external work | 1 | 1 | 0.97±0.03 | 0.95±0.03 | 0.21±0.05* | 0.32±0.04* | 0.18±0.06* | 0.38±0.05*† |
| Fractional systolic wall area reduction | 0.28±0.02 | 0.29±0.02 | 0.28±0.02 | 0.29±0.02 | −0.02±0.02* | 0.08±0.01*‡ | 0.01±0.02* | 0.11±0.01*‡ |
| Diastolic function | ||||||||
| dA/dtmax | 1 | 1 | 0.99±0.04 | 1.01±0.06 | 0.62±0.04* | 0.92±0.11† | 0.52±0.07* | 0.77±0.05*† |
| Posterior LV | ||||||||
| Preload | ||||||||
| Steady-state end-diastolic crystal area | 1 | 1 | 0.97±0.02 | 1.00±0.01 | 1.04±0.02 | 1.09±0.03* | 1.02±0.02 | 1.07±0.03* |
| Systolic function | ||||||||
| Steady-state external work | 1 | 1 | 0.99±0.04 | 0.99±0.02 | 0.93±0.04 | 0.95±0.05 | 0.83±0.02* | 0.87±0.07 |
| Fractional systolic wall area reduction | 0.27±0.03 | 0.29±0.02 | 0.26±0.03 | 0.28±0.02 | 0.32±0.03* | 0.34±0.01* | 0.29±0.06 | 0.31±0.01 |
| Diastolic function | ||||||||
| dA/dtmax | 1 | 1 | 0.94±0.04 | 0.98±0.03 | 0.90±0.09 | 0.90±0.08 | 0.90±0.10 | 0.90±0.09 |
Values are means ± SE; n = 8 in each group. dA/dtmax, maximum rate of diastolic wall area expansion with respect to time. Except for fractional systolic wall area reduction, all values are expressed as fraction of baseline.
P < 0.05 vs. baseline within same group.
P < 0.05 and
P < 0.01 vs. control group under same condition.
Regional diastolic function
Administration of GIK during the preischemic treatment period had no discernible effect on indexes of regional diastolic function. In the control group during both ischemia and reperfusion, the maximum rate of diastolic wall area expansion (+dA/dtmax) was reduced in the anterior LV. The duration of early diastolic wall expansion was shortened, and early systolic wall area expansion was prominent. In contrast in the GIK-treated group, +dA/dtmax did not change significantly between baseline and ischemia and remained significantly greater than in the control group during both ischemia and reperfusion (Fig. 6, Table 3). Furthermore, the duration of early diastolic wall area expansion remained longer, and early systolic wall expansion was less prominent compared with the control group (Fig. 6). The beneficial effect of GIK on early diastolic wall expansion was not attributable to a difference in left atrial driving pressure, which did not differ between groups. Treatment with GIK did not alter the effects of ischemia and reperfusion on passive (end-diastolic) LV pressure versus wall area relations. In both groups, these relations shifted to the right compared with baseline at low LV end-diastolic pressures (0–3 mmHg) and became steeper (stiffer) compared with baseline at higher end-diastolic pressures. Neither early nor late diastolic function of the posterior (nonischemic) LV changed significantly under any of the experimental conditions in either group (Table 3).
Fig. 6.
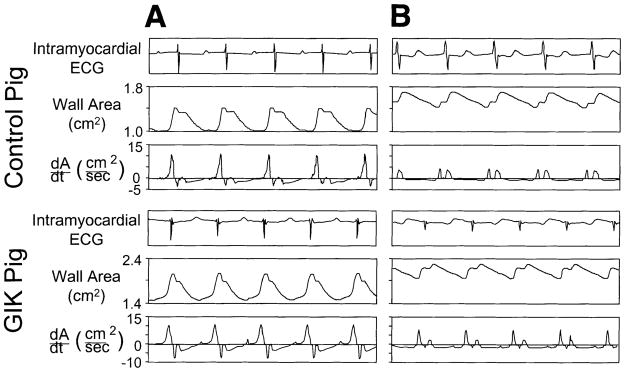
Typical recordings of regional wall area and the first derivative of wall area with respect to time (+dA/dt). Top: recordings from ischemic zone of a pig from the control group. Bottom: recordings from ischemic zone of a pig from the GIK group. For both pigs, recordings are shown under baseline conditions (A) and after 90 min reperfusion after 90 min ischemia (B). Under baseline conditions, both pigs exhibit rapid diastolic wall expansion and no evidence of early systolic wall expansion. After reperfusion, the pig from the control group exhibits impaired early diastolic wall expansion (reduced +dA/dtmax); early systolic wall expansion is also evident. In comparsion, the GIK-treated pig exhibits greater recovery of +dA/dtmax and less prominent early systolic wall expansion. Note that absolute values of wall area and dA/dt vary between pigs because of differences in distance between pairs of ultrasonic crystals at implantation.
Global LV function
LV end-diastolic pressure was elevated during ischemia and reperfusion in both groups. During ischemia, LV +dP/dtmax fell significantly in the control group but not in the GIK group. With reperfusion, LV +dP/dt and dP/dtmax remained significantly greater in the GIK group than in the control group (Table 4), suggesting that global LV contractility was better preserved with GIK. LV −dP/dtmax decreased during ischemia in both groups and remained reduced from baseline during reperfusion; however, the values of LV −dP/dtmax during ischemia and reperfusion remained significantly greater in GIK pigs (Table 4), indicating that abnormal LV relaxation was mitigated by GIK. Interpretation of changes in LV dP/dt depends upon concurrent loading conditions. Although LV end-diastolic volume and systolic wall stress were not measured, surrogate measures of preload and afterload (LV end-diastolic pressure and peak systolic pressure) did not differ between groups. Thus the data indicate that GIK helped to preserve global LV systolic and diastolic function in parallel with its salutary effects on regional function of the ischemic zone.
Table 4.
Hemodynamic data
| Baseline |
Preischemic Treatment |
Ischemia |
Reperfusion |
|||||
|---|---|---|---|---|---|---|---|---|
| Control | GIK | Control | GIK | Control | GIK | Control | GIK | |
| Heart rate, min−1 | 111±2 | 112±2 | 110±1 | 112±2 | 110±1 | 113±2 | 111±1 | 113±2 |
| Mean aortic pressure, mmHg | 98±3 | 97±1 | 94±5 | 100±4 | 87±5 | 86±7 | 85±5 | 85±8 |
| LV systolic pressure, mmHg | 104±4 | 112±5 | 102±5 | 112±5 | 94±5 | 96±5 | 90±4 | 93±6 |
| LV end-diastolic pressure, mmHg | 8±1 | 8±1 | 8±1 | 8±1 | 14±2* | 11±1* | 14±2* | 11±2* |
| LA mean pressure, mmHg | 4±1 | 5±1 | 4±1 | 5±1 | 7±2 | 8±1 | 9±2 | 9±1 |
| LV +dP/dtmax Fraction of baseline, mmHg/s | 1 (1,792±124) | 1 (1,534±112) | 1.02±0.01 | 0.99±0.01 | 0.75±0.03* | 0.92±0.02‡ | 0.62±0.02* | 0.82±0.04*† |
| LV −dP/dtmax Fraction of baseline, mmHg/s | 1 (−2,534±335) | 1 (−2,118±205) | 0.98±0.02 | 1.03±0.02 | 0.68±0.04* | 0.81±0.04*† | 0.61±0.03* | 0.82±0.07*† |
Values are means = SE; n = 8 in each group. LA, left atrial; dP/dtmax, maximum change in pressure with respect to time.
P < 0.05 vs. baseline within same group.
P < 0.05 and
P < 0.01 vs. control group under same condition.
DISCUSSION
New findings of this study
This study demonstrates that infusion of GIK exerts salutary effects on LV function during and after ischemia without infarction in vivo. These effects were determined using rigorous methods to analyze regional LV function. Both regional systolic function (external work, FAR, and PRSW slope) and diastolic function (maximal rate of diastolic wall area expansion) were better preserved during ischemia and reperfusion in GIK-treated pigs compared with untreated pigs. Furthermore, these effects were paralleled by higher values of LV +dP/dtmax and −dP/dtmax after ischemia and reperfusion, implying improved recovery of global LV contractility and relaxation. These findings suggest that the clinical utility of GIK may extend beyond its prior application in acute myocardial infarction (8, 10) to the treatment of episodic myocardial ischemia and unstable coronary syndromes.
Effects of GIK on myocardial substrate metabolism
The effects of GIK on myocardial substrate metabolism were examined during preischemic, ischemic, and reperfusion periods. In the preischemic period, GIK-treated hearts demonstrated increased glucose uptake, a trend to decreased FFA uptake, and no difference in lactate uptake compared with untreated hearts. Approximately two-thirds of the additional glucose uptake by GIK-treated hearts can be explained by a shift in oxidative substrate from FFA to glucose, since oxidation of 4 mol of glucose consumes as much oxygen as oxidation of 1 mol of FFA, and oxygen consumption did not differ between groups. The remainder of the increased preischemic glucose uptake by GIK-treated hearts was likely used for synthesis and accumulation of additional myocardial glycogen (15). In isolated, perfused hearts, a high preischemic glycogen content has been associated with improved functional recovery from moderate myocardial ischemia (6), and this may be a mechanism of benefit of GIK in the current study.
During ischemia, myocardial glucose uptake in GIK-treated pigs was greater than that in untreated pigs by an average of 0.42 μmol·g−1·min−1. Approximately two-thirds of this difference (0.27 μmol·g−1·min−1) may be explained by increased anaerobic glycolysis (i.e., nonoxidative metabolism of glucose to lactate), based on the observation that mean lactate release from the ischemic myocardial region was 0.54 μmol·g−1·min−1 greater in GIK-treated pigs than in untreated pigs (0.75 vs. 0.21 μmol·g−1·min−1) and that 2 mol of lactate are produced from 1 mol of glucose through glycolysis.
The effect of GIK on myocardial ATP synthesis during ischemia can be estimated as follows. Each mole of net lactate release is associated with 1 mol of ATP synthesis by anaerobic glycolysis. Accordingly, anaerobic ATP production during ischemia can be estimated at 0.75 μmol·g−1·min−1 in GIK-treated pigs and 0.21 μmol·g−1·min−1 in untreated pigs. Furthermore, assuming that 6 mol of ATP are synthesized for every mole of oxygen consumed, the contribution of anaerobic ATP production to total transmural ATP production averaged 8% in GIK-treated pigs but only 2% in untreated pigs. In the subendocardium, the relative contribution of anaerobic ATP production is likely to have been even greater. ATP production by anaerobic glycolysis may help to maintain vital cellular processes during ischemia. In ischemic isolated perfused hearts, the incremental ATP production facilitated by supplemental glucose and insulin attenuates the decline of myocardial high-energy phosphates and leads to improved postischemic recovery of contractile function (9). Furthermore, glycolytic ATP production during ischemia may play an important role in maintaining LV relaxation by limiting intracellular calcium accumulation (36).
Approximately one-third of the difference in glucose uptake between GIK-treated and untreated hearts during ischemia cannot be accounted for by increased anaerobic glycolysis and lactate release and is most likely explained by increased oxidation of glucose in lieu of FFA and/or by increased glycogen synthesis. A beneficial effect of GIK due to one or more of the above mechanisms was evident before reperfusion, since end-ischemic indexes of regional and global systolic and diastolic LV function were significantly better in GIK-treated pigs than in untreated pigs.
During reperfusion, GIK did not affect the pattern of myocardial substrate uptake or oxygen consumption. In both groups, glucose uptake was greater than baseline, but net lactate uptake was much less than baseline. These findings imply a persistently increased rate of lactate production from anaerobic glycolysis despite normal myocardial perfusion and oxygen delivery and are consistent with previous observations that glucose oxidation is impaired in reperfused myocardium (18). Although interventions that stimulate glucose oxidation in reperfused hearts have been shown to improve functional recovery (18), the lack of any effect of GIK on substrate uptake or oxygen consumption during reperfusion suggests that this was not the mechanism of benefit of GIK in the current study.
Accumulation of fatty acyl metabolites during ischemia may be toxic to cardiac myocytes, causing mitochondrial damage and alterations in membrane ion channels (26). One of the potential salutary effects of GIK is an insulin-mediated reduction in circulating FFA concentrations, with consequent reduction in myocardial FFA uptake (30). In this porcine model, baseline levels of circulating FFA are low (mean arterial plasma concentration 0.3 μmol/ml) compared with mean values of ~0.8 μmol/ml in healthy human volunteers (14). Nonetheless, GIK caused significant reductions in arterial FFA concentration in our model (to ~0.1 μmol/ml) and a trend toward reduced FFA uptake during ischemia. Therefore, it is possible that reduced uptake of FFA during ischemia contributed to the beneficial effects of GIK on regional LV function in this model.
Increased myocardial acidosis due to accelerated glycolytic generation of hydrogen ions is a potential concern over the use of GIK in myocardial ischemia (6). Although intracellular pH was not measured in the current study, coronary venous pH was lower and the coronary arteriovenous pH difference was significantly greater in the GIK group than in the control group during ischemia. Therefore, improved systolic and diastolic LV function with GIK occurred despite increased myocardial hydrogen ion generation during ischemia.
Insulin has been purported to increase cardiac output and exert direct positive inotropic effects in some experimental models (34). Administration of GIK before the onset of ischemia enabled us to determine if such direct inotropic effects were evident in this model. We found no discernible effects of GIK on either systolic or diastolic LV function during the preischemic treatment period. Therefore, the improvement in postischemic function with GIK cannot be attributed to a direct inotropic effect of insulin.
Limitations
Postischemic LV dysfunction is generally more severe in anesthetized, open-chest animals than in conscious animals (5). The protective effect of GIK may also depend on these conditions. Pretreatment of pigs with indomethacin and propranolol may also have influenced the severity of postischemic LV dysfunction (23, 25); however, any effects due to these agents would be expected in both experimental groups. Changes in myocardial glycogen and high-energy phosphate content with GIK have been measured previously and may be related to therapeutic effect in ischemia (28); however, these substances were not measured in the current study. We examined the anticipatory use of GIK (i.e., treatment begun before ischemia) but did not determine whether a similar benefit can be achieved by treatment initiated at or after the onset of ischemia.
Implications
Many patients who are admitted to hospital with unstable coronary syndromes experience early, recurrent episodes of myocardial ischemia. These episodes can cause both systolic and diastolic LV dysfunction, even in the absence of myocardial infarction and thereby contribute to morbidity and mortality. Just as such patients are treated with a combination of antianginal, antiplatelet, and anticoagulant drugs to prevent or mitigate the effects of further ischemic episodes, a strategy of anticipatory metabolic protection with GIK may also be valuable. Based on data from this study in a model of moderate myocardial ischemia without irreversible injury, the clinical benefits of GIK may extend beyond its prior application in patients with myocardial infarction to patients with intermediate coronary syndromes, such as unstable angina, who are at high risk for early, recurrent episodes of ischemia.
Acknowledgments
This work was supported by National Heart, Lung, and Blood Institute Grants HL-49944 (G. G. Schwartz) and K08-HL-03475 (C. Greyson), a postdoctoral research fellowship of the American Heart Association, California Affiliate (L. Lu), and the Medical Research Service of the Dept. of Veterans Affairs.
References
- 1.Ahmed SS, Lee CH, Oldwurtel HA, Regan TJ. Sustained effect of glucose-insulin-potassium on myocardial performance during regional ischemia. J Clin Invest. 1978;61:1123–1135. doi: 10.1172/JCI109027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apstein CS, Gravino FN, Haudenschild CC. Determinants of a protective effect of glucose and insulin on the ischemic myocardium. Effects on contractile function, diastolic compliance, metabolism, and ultrastructure during ischemia and reperfusion. Circ Res. 1983;52:515–526. doi: 10.1161/01.res.52.5.515. [DOI] [PubMed] [Google Scholar]
- 3.Arnott WM, Barritt DW, Douglas AS, Hunter B, Kitchin AH, Laurie J, Macnon J, Richards RL, Scarborough R, Shillingford JP, Herland I, Swan WGA, Vessey MP, Whitaker W, Wilson JMG, Fletcher RF. Potassium, glucose, and insulin treatment for acute myocardial infarction. Lancet. 1968;2:1355–1360. [PubMed] [Google Scholar]
- 4.Bier J, Sharaf B, Gewirtz H. Origin of anterior interventricular vein blood in domestic swine. Am J Physiol Heart Circ Physiol. 1991;260:H1732–H1736. doi: 10.1152/ajpheart.1991.260.5.H1732. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R. Basic and clinical aspects of myocardial stunning. Prog Cardiovasc Dis. 1998;40:477–516. doi: 10.1016/s0033-0620(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 6.Cross HR, Opie LH, Radda GK, Clarke K. Is a high glycogen content beneficial or detrimental to the ischemic rat heart? A controversy resolved. Circ Res. 1996;78:482–491. doi: 10.1161/01.res.78.3.482. [DOI] [PubMed] [Google Scholar]
- 7.Depre C, Vanoverschelde JL, Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–588. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 8.Diaz R, Paolasso EA, Piegas LS, Tajer CD, Moreno MG, Corvalan R, Isea JE, Romero G. Metabolic modulation of acute myocardial infarction: the ECLA glucose-insulin-potassium pilot trial. Circulation. 1998;98:2227–2234. doi: 10.1161/01.cir.98.21.2227. [DOI] [PubMed] [Google Scholar]
- 9.Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance from prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res. 1991;68:466–481. doi: 10.1161/01.res.68.2.466. [DOI] [PubMed] [Google Scholar]
- 10.Fath-Ordoubadi F, Beatt KJ. Glucose-insulin-potassium therapy for treatment of acute myocardial infarction: an overview of randomized placebo-controlled trials. Circulation. 1997;96:1152–1156. doi: 10.1161/01.cir.96.4.1152. [DOI] [PubMed] [Google Scholar]
- 11.Fishbein MC, Meerbaum S, Rit J, Lando U, Kanmatsuse K, Mercier JC, Corday E, Ganz W. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Am Heart J. 1981;101:593–600. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 12.Glower DD, Spratt JA, Kabas JS, Davis JW, Rankin JS. Quantification of regional myocardial dysfunction after acute ischemic injury. Am J Physiol Heart Circ Physiol. 1988;255:H85–H93. doi: 10.1152/ajpheart.1988.255.1.H85. [DOI] [PubMed] [Google Scholar]
- 13.Kashiwaya Y, King MT, Veech RL. Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart. Am J Cardiol. 1997;80:50A–64A. doi: 10.1016/s0002-9149(97)00458-x. [DOI] [PubMed] [Google Scholar]
- 14.Lassers BW, Kaijser L, Carlson LA. Myocardial lipid and carbohydrate metabolism in healthy, fasting men at rest: studies during continuous infusion of 3H-palmitate. Eur J Clin Invest. 1972;2:348–358. doi: 10.1111/j.1365-2362.1972.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 15.Laughlin MR, Taylor JF, Chesnick AS, Balaban RS. Regulation of glycogen metabolism in canine myocardium: effects of insulin and epinephrine in vivo. Am J Physiol Endocrinol Metab. 1992;262:E875–E883. doi: 10.1152/ajpendo.1992.262.6.E875. [DOI] [PubMed] [Google Scholar]
- 16.Lazar HL, Zhang X, Rivers S, Bernard S, Shemin RJ. Limiting ischemic myocardial damage using glucose-insulin-potassium solutions. Ann Thorac Surg. 1995;60:411–416. doi: 10.1016/0003-4975(95)00402-7. [DOI] [PubMed] [Google Scholar]
- 17.Lew WYW, LeWinter MM. Regional comparison of midwall segment and area shortening in the canine left ventricle. Circ Res. 1986;58:678–691. doi: 10.1161/01.res.58.5.678. [DOI] [PubMed] [Google Scholar]
- 18.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–315. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Xu Y, Greyson CR, Ursell PC, Schwartz GG. Non-elastic deformation of myocardium in low-flow ischemia and reperfusion: Ultrastructure-function relations. J Mol Cell Cardiol. 1999;31:1157–1169. doi: 10.1006/jmcc.1999.0948. [DOI] [PubMed] [Google Scholar]
- 20.Malmberg K, Ryden L, Efendic S, Herlitz J, Nicol P, Waldenstrom A, Wedel H, Welin L. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 21.Maroko PR, Libby P, Sobel BE, Bloor CM, Sybers HD, Shell WE, Covell JW, Braunwald E. Effect of glucose-insulin-potassium infusion on myocardial infarction following experimental coronary artery occlusion. Circulation. 1972;45:1160–1175. doi: 10.1161/01.cir.45.6.1160. [DOI] [PubMed] [Google Scholar]
- 22.Mittra B, Agra MB. Potassium, glucose, and insulin in treatment of myocardial infarction. Lancet. 1965;2:607–609. doi: 10.1016/s0140-6736(65)90516-7. [DOI] [PubMed] [Google Scholar]
- 23.Mobert J, Becker BF. Cyclooxygenase inhibition aggravates ischemia-reperfusion injury in the perfused guinea pig heart: involvement of isoprostanes. J Am Coll Cardiol. 1998;31:1687–1694. doi: 10.1016/s0735-1097(98)00158-2. [DOI] [PubMed] [Google Scholar]
- 24.Nishida H, Grooters RK, Thieman KC, Soltanzadeh H, Schneider RF, Merkley DF. Enhanced ventricular recovery from high dose glucose, insulin and potassium with cardiopulmonary bypass support prior to cardioplegic arrest. Eur J Cardiothorac Surg. 1993;7:409–413. doi: 10.1016/1010-7940(93)90004-u. [DOI] [PubMed] [Google Scholar]
- 25.Norris RM. Beta-blockers and infarct size. J Mol Cell Cardiol. 1986;18(Suppl 4):99–103. doi: 10.1016/s0022-2828(86)80033-5. [DOI] [PubMed] [Google Scholar]
- 26.Oliver MF, Opie LH. Effects of glucose and fatty acids on myocardial ischemia and arrhythmias. Lancet. 1994;343:155–158. doi: 10.1016/s0140-6736(94)90939-3. [DOI] [PubMed] [Google Scholar]
- 27.Opie LH, Bruyneel K, Owen P. Effects of glucose, insulin and potassium infusion on tissue metabolic changes within first hour of myocardial infarction in the baboon. Circulation. 1975;52:49–57. doi: 10.1161/01.cir.52.1.49. [DOI] [PubMed] [Google Scholar]
- 28.Opie LH, Owen P. Effect of glucose-insulin-potassium infusions on arteriovenous differences of glucose and of free fatty acids and on tissue metabolic changes in dogs with developing myocardial infarction. Am J Cardiol. 1976;38:310–321. doi: 10.1016/0002-9149(76)90173-9. [DOI] [PubMed] [Google Scholar]
- 29.Rackley CE, Russell RO, Jr, Rogers WJ, Mantle JA, McDaniel HG, Papapietro SE. Clinical experience with glucose-insulin-potassium therapy in acute myocardial infarction. Am Heart J. 1981;102:1038–1048. doi: 10.1016/0002-8703(81)90488-9. [DOI] [PubMed] [Google Scholar]
- 30.Rogers WJ, Russell RO, Jr, McDaniel HG, Rackley CE. Acute effects of glucose-insulin-potassium infusion on myocardial substrates, coronary blood flow and oxygen consumption in man. Am J Cardiol. 1977;40:421–428. doi: 10.1016/0002-9149(77)90166-7. [DOI] [PubMed] [Google Scholar]
- 31.Rogers WJ, Stanley AW, Jr, Breinig JB, Prather JW, McDaniel HG, Moraski RE, Mantle JA, Russell RO, Jr, Rackley CE. Reduction of hospital mortality rate of acute myocardial infarction with glucose-insulin-potassium infusion. Am Heart J. 1976;92:441–454. doi: 10.1016/s0002-8703(76)80043-9. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz GG, Xu Y, Greyson C, Cohen J, Lu L. Low-dose inotropic stimulation during left ventricular ischaemia does not worsen post-ischaemic dysfunction. Cardiovasc Res. 1996;32:1024–1037. doi: 10.1016/s0008-6363(96)00150-2. [DOI] [PubMed] [Google Scholar]
- 33.Sodi-Pallares D, Testelli M, Fishleder F. Effects of an intravenous infusion of a glucose-potassium-insulin solution on the electrocardiographic signs of myocardial infarction. Am J Cardiol. 1962;9:166–181. doi: 10.1016/0002-9149(62)90035-8. [DOI] [PubMed] [Google Scholar]
- 34.Weissler AM, Altschuld RA, Gibb LE, Pollack ME, Kruger FA. Effect of insulin on the performance and metabolism of the anoxic isolated perfused rat heart. Circ Res. 1973;32:108–116. doi: 10.1161/01.res.32.1.108. [DOI] [PubMed] [Google Scholar]
- 35.Wisneski JA, Gertz EW, Neese RA, Mayr M. Myocardial metabolism of free fatty acids. Studies with 14C labeled substrates in humans. J Clin Invest. 1987;79:359–366. doi: 10.1172/JCI112820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu HY, Zweier JL, Becker LC. Functional coupling between glycolysis and sarcoplasmic reticulum Ca2+ transport. Circ Res. 1995;77:88–97. doi: 10.1161/01.res.77.1.88. [DOI] [PubMed] [Google Scholar]
- 37.Zhu P, Lu L, Xu Y, Schwartz GC. Troglitazone improves recovery of ventricular function after regional ischemia in pigs. Circulation. doi: 10.1161/01.cir.101.10.1165. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]