Abstract
Intravascular adenosine triphosphate (ATP) evokes vasodilation and is implicated in the regulation of skeletal muscle blood flow during exercise. Mechanical stresses to erythrocytes and endothelial cells stimulate ATP release in vitro. How mechanical effects of muscle contractions contribute to increased plasma ATP during exercise is largely unexplored. We tested the hypothesis that simulated mechanical effects of muscle contractions increase [ATP]venous and ATP effluent in vivo, independent of changes in tissue metabolic demand, and further increase plasma ATP when superimposed with mild-intensity exercise. In young healthy adults, we measured forearm blood flow (FBF) (Doppler ultrasound) and plasma [ATP]v (luciferin-luciferase assay), then calculated forearm ATP effluent (FBF×[ATP]v) during rhythmic forearm compressions (RFC) via a blood pressure cuff at three graded pressures (50, 100, and 200 mmHg; Protocol 1; n = 10) and during RFC at 100 mmHg, 5% maximal voluntary contraction rhythmic handgrip exercise (RHG), and combined RFC + RHG (Protocol 2; n = 10). [ATP]v increased from rest with each cuff pressure (range 144–161 vs. 64 ± 13 nmol/l), and ATP effluent was graded with pressure. In Protocol 2, [ATP]v increased in each condition compared with rest (RFC: 123 ± 33; RHG: 51 ± 9; RFC + RHG: 96 ± 23 vs. Mean Rest: 42 ± 4 nmol/l; P < 0.05), and ATP effluent was greatest with RFC + RHG (RFC: 5.3 ± 1.4; RHG: 5.3 ± 1.1; RFC + RHG: 11.6 ± 2.7 vs. Mean Rest: 1.2 ± 0.1 nmol/min; P < 0.05). We conclude that the mechanical effects of muscle contraction can 1) independently elevate intravascular ATP draining quiescent skeletal muscle without changes in local metabolism and 2) further augment intravascular ATP during mild exercise associated with increases in metabolism and local deoxygenation; therefore, it is likely one stimulus for increasing intravascular ATP during exercise in humans.
Keywords: muscle compression, exercise, blood flow, vasodilation, adenosine triphosphate
accumulating evidence indicates that intravascular adenosine triphosphate (ATP) evokes local vasodilation and contributes to skeletal muscle blood flow regulation during exercise (16). Unlike many of the other candidate substances that have been investigated to date in regards to skeletal muscle blood flow regulation, intravascular ATP is a potent vasodilator that is capable of causing vasodilation to a level similar to that seen during maximal exercise (16) and is uniquely capable of blunting sympathetically mediated vasoconstriction in a graded manner (29, 36). An increase in venous plasma [ATP] draining skeletal muscle in response to muscle contractions has been observed in both early (13) and more recent human studies (16, 33), including those performed in our own laboratory (27). What particular stimuli and cellular sources account for the increase in venous plasma [ATP] during exercise have yet to be fully elucidated.
Many different cell types possess mechanisms for ATP release and can contribute to the observed level of plasma ATP in vivo. Two primary cellular sources of plasma ATP appear to be endothelial cells (2) and erythrocytes (9). Although both skeletal myocytes and sympathetic nerve terminals can release ATP, the blood vessel itself provides an effective barrier for passage of ATP into the intraluminal space, and thus it is thought that neither of these sources appears to contribute to ATP levels in the plasma (16, 30, 32, 33). In vitro studies using endothelial cells and erythrocytes have identified a variety of experimental stimuli that can increase ATP release, including shear stress on the endothelial surface (2), deoxygenation (9, 23, 27), mechanical deformation (40, 44), and acidosis and hypercapnia (1, 9). Limited studies have explored the combined influence of these stimuli, in particular deoxygenation and mechanical stress, to further augment ATP release (3, 10). When considering potential stimuli for ATP release during exercise, the local milieu of contracting muscle provides an integrative stimulus for ATP release, including reductions in hemoglobin (Hb) oxygenation, acidosis, as well as potential mechanical effects of muscle contraction. With respect to the latter, extravascular pressure increases in a graded fashion with the level of muscle tension generated during contractions (20, 38) causing mechanical compression/deformation of the vasculature and traversing red blood cells, and thus it is plausible that this may serve as an independent stimulus for elevating intravascular ATP during exercise.
Despite the overlap of experimental stimuli that elicit ATP release in vitro and the physiological changes that occur with exercise, relatively few studies have attempted to isolate the influence of any of these factors to gain an understanding of how plasma ATP increases during exercise in humans (15–17). Previously, we and others (28, 43) have mimicked the mechanical effects of muscle contraction in the human forearm via rhythmic mechanical tissue deformation. Using this experimental approach as well as low-intensity handgrip exercise, we sought to determine whether the mechanical effects of muscle contraction independently contribute to the observed increase in plasma ATP during exercise (27). We hypothesized that graded simulated mechanical effects of muscle contractions evoke a graded increase in venous [ATP] and ATP effluent in vivo, independent of changes in tissue metabolic demand. Furthermore, we hypothesized that augmenting the mechanical effects of muscle contractions during mild-intensity exercise (via superimposed mechanical deformation) elicits a greater increase in venous [ATP] and ATP effluent than contractions or mechanical deformation alone due to greater stimuli for ATP release (e.g., Hb deoxygenation) as a result of increased tissue metabolism.
MATERIALS AND METHODS
Subjects
With Institutional Review Board approval and after written informed consent, a total of 20 young healthy adults (18 men, 2 women; age = 23 ± 1 yr; weight = 76.9 ± 2.4 kg; height = 177 ± 2 cm; body mass index = 24.6 ± 0.6 kg/m2; means ± se) participated in the present study. Ten different subjects participated in each protocol. All subjects were nonsmokers, nonobese, normotensive (resting blood pressure <140/90 mmHg), and not taking any medications. Studies were performed after a 4-h fast and 24-h abstention from caffeine and exercise with subjects in the supine position. Female subjects were studied during the early follicular phase of their menstrual cycle to minimize any potential cardiovascular effects of sex-specific hormones. All studies were performed according to the Declaration of Helsinki.
Arterial Blood Pressure and Heart Rate
Resting arterial blood pressure was measured noninvasively over the brachial artery of the control arm after 30 min of supine rest before any experimental trials and just before each experimental trial after the study began (Cardiocap/5; Datex-Ohmeda, Louisville, CO). Beat-by-beat arterial blood pressure was measured at heart level by finger photoplethysmography (Finometer 1; Finapres Medical Systems BV, Amsterdam, The Netherlands) on the middle finger of the control hand during all experimental trials. Heart rate was determined using a three-lead ECG (Cardiocap/5, Datex-Ohmeda) (27).
Forearm Blood Flow and Vascular Conductance
A 12-MHz linear-array ultrasound probe (Vivid 7; General Electric, Milwaukee, WI) was used to determine brachial artery mean blood velocity (MBV) and brachial artery diameter. The probe was securely fixed to the skin over the brachial artery proximal to the catheter insertion site as previously described (6). For blood velocity measurements, the probe insonation angle was maintained at <60 degrees, and the frequency used was 5 MHz. The Doppler shift frequency spectrum was analyzed via a Multigon 500M TCD (Multigon Industries, Mt. Vernon, NY) spectral analyzer from which mean velocity was determined as a weighted mean of the spectrum of Doppler shift frequencies. Triplicate brachial artery diameter measurements were made in duplex mode at end-diastole of a single cardiac cycle and between contractions during the last 30 s of steady-state conditions. Forearm blood flow (FBF) was calculated as: FBF = MBV × π(brachial artery diameter/2)2 × 60, where the FBF is in ml/min, the MBV is in cm/s, the brachial diameter is in cm, and 60 was used to convert from ml/s to ml/min. Forearm vascular conductance (FVC) was calculated as (FBF/mean arterial pressure) × 100, and expressed as ml/min/100 mmHg.
Venous Catheterization
An 18- or 20-gauge (depending on visual inspection of vein size) 3.8-cm catheter was inserted in retrograde fashion into an antecubital vein of the experimental arm for deep venous blood samples (5). The catheter was connected to a three-way stopcock with one connection to an intravenous solution set for continuous flushing with saline and the other to a 10- or 3-ml syringe for blood sampling (27). We have previously demonstrated that arterial plasma [ATP] does not increase during handgrip exercise (27), and ATP in the arterial circulation is undetectable in venous samples given the rapid (<1 s) breakdown by nucleotidases (27, 33). Given these findings and the fact that the milieu (Po2, Pco2, pH) of venous blood is more closely representative of the microcirculatory environment conducive to ATP release, we focused our investigation on plasma [ATP] within venous blood draining the skeletal muscle.
Blood Sampling and Measurement of Plasma [ATP] and [Hb]
In general, our blood sampling, preparation, and subsequent ATP assay follow those procedures established by Gorman and colleagues for the measurement of ATP in human plasma (18, 19) and recently performed in our laboratory (27). Briefly, venous blood samples were drawn through the deep venous catheter directly into a preheparinized 10-ml syringe. Immediately, 2 ml of blood was gently expelled down the side of a test tube containing 2.7 ml of the ATP-stabilizing solution to inhibit degradation of ATP via nucleotidases and additional ATP release. Samples were then centrifuged at 4,000 revolution/min for 3 min at 22°C and then directly analyzed for plasma [ATP] via luciferin-luciferase assay. An ATP standard curve was created on the day of the experiment before all experimental trials and in plasma medium from each subject studied.
Small amounts of red blood cell hemolysis can lead to significant increases in ATP, and thus, to account for venous concentrations of ATP induced from hemolysis, 1 ml of plasma supernatant from the same blood:diluent samples used for plasma [ATP] measurements was analyzed for plasma Hb via spectrophotometry (SpectraMax; Molecular Devices, Sunnyvale, CA). This plasma [Hb] reading was used to account for a given [ATP] from hemolysis, as done previously by our laboratory (27). To prevent samples with high hemolysis from sample handling influencing our ATP measures, any sample with greater than 0.03% hemolysis was discarded and data from that subject were not used for any analysis.
At the same time that blood sampling for ATP determination occurred, a 2-ml blood sample was also drawn from the venous catheter into a preheparinized 3-ml syringe for cooximetry blood gas parameters, including hematocrit measured via blood gas analyzer (Rapid Point 400 Series Automatic Blood Gas System; Siemens Healthcare Diagnostics, Deerfield, IL).
Rhythmic Forearm Compressions
Subjects were instrumented with a blood pressure cuff that was wrapped around the experimental forearm. To mimic the mechanical effects of exercise, rhythmic forearm compressions were performed with an inflation/deflation cycle of 1 s/2 s (20 inflations/min), which is the same duty cycle used for the exercise trials in the present study and in our previous study on exercise-induced increases in plasma [ATP] (27). The cuff was inflated and deflated via a rapid cuff inflation unit (E20; Hokanson, Bellevue, WA) to pressures of 50 mmHg, 100 mmHg, and 200 mmHg in Protocol 1 and to 100 mmHg in Protocol 2. In Protocol 1, we chose these three cuff pressures in an attempt to mimic the graded increase in extravascular pressure that occurs with graded rhythmic handgrip exercise. Although the exact mechanical distortions that occur with contractions have not been well established in this muscle bed, it has been shown in the biceps brachii and rectus femoris that there is a relatively linear increase in intramuscular tissue pressure during progressive intensity contractions, and externally applied pressure can approximately mimic these intramuscular values (38). We estimate that the pressures we utilize represent intensities of ∼10%, ∼50%, and ∼90% maximal voluntary contraction (MVC) (25). In Protocol 2, we utilized 100 mmHg to repeat a level of pressure tested in Protocol 1 and as a pressure we had previously used in combination with low-workload muscle contractions to elicit vasodilation (28). Studies were performed with the forearm positioned slightly above heart level to minimize the influence of the muscle pump on forearm hemodynamics.
Rhythmic Handgrip Exercise
MVC (mean 48.4 ± 4.4 kg, range 25.0–76.3 kg) was determined for the experimental arm in those subjects that participated in Protocol 2 as the average of three maximal squeezes of a handgrip dynamometer (Stoelting, Chicago, IL) that were within 3% of each other. Rhythmic handgrip exercise in Protocol 2 was performed with weight corresponding to 5% MVC (mean 2.4 ± 0.2 kg, range 1.3–3.8 kg) attached to a pulley system and lifted 4–5 cm over the pulley at a duty cycle of 1-s contraction/2-s relaxation (20 contractions per minute) using both visual and auditory feedback to insure the correct timing (6). We chose this mild workload to increase metabolic demand and evoke Hb deoxygenation while minimizing increases in extravascular pressure from muscle contraction. Additionally, in our previous study, we observed that plasma [ATP] and ATP effluent was significantly increased from rest at this workload (27). Finally, this workload can be performed without reflex increases in sympathetic nervous system activity and thus isolates the local effects of muscle contraction on vascular tone (28).
Experimental Protocols
Protocol 1: graded rhythmic forearm compressions.
The purpose of Protocol 1 was to investigate the effects of graded rhythmic forearm compressions on plasma [ATP] and ATP effluent. After 2 min of baseline measurements, 4 min of rhythmic forearm compressions occurred at 50 mmHg, 100 mmHg, and 200 mmHg in succession. The total time of forearm compressions was 12 min. Venous blood was sampled for blood gases and plasma [ATP] and [Hb] at the end of rest and the end of each level of forearm compressions. Immediately before blood sampling, FBF was determined at rest and each cuff pressure.
Protocol 2: rhythmic forearm compressions and muscle contraction.
The purpose of Protocol 2 was to determine the potential interaction of muscle contractions and associated changes in the local mileu (e.g., Hb deoxygenation) due to greater metabolic demand with an enhanced mechanical stimulus on plasma [ATP] and ATP effluent. To do this, we performed three separate trials. The first trial always consisted of 2 min of baseline measurements followed by 4 min of rhythmic forearm compressions at 100 mmHg. After 45 min of rest, the second trial occurred. Again, 2 min of resting baseline measures were made, and then either rhythmic handgrip exercise at 5% MVC was performed alone or performed with simultaneous rhythmic forearm compressions at 100 mmHg superimposed on the muscle contractions. The third trial, consisting of whichever experimental manipulation was not performed in the second trial, was then performed following 45 min of rest. The order of the last two trials (handgrip exercise alone and handgrip exercise with superimposed forearm compressions) was counterbalanced between subjects. Similar to Protocol 1, venous blood was sampled for blood gases and plasma [ATP] and [Hb] at the end of rest and the end of each trial (e.g., forearm compressions, handgrip exercise, combined forearm compressions, and handgrip exercise). Immediately before blood sampling, FBF was determined at rest and the end of each trial.
Data Acquisition and Analysis
Data were collected and stored on a computer at 250 Hz and were analyzed offline with signal-processing software (WinDaq; DATAQ Instruments, Akron, OH). Mean arterial pressure was determined from the Finometer pressure waveform. FBF, vascular conductance, heart rate, and mean arterial pressure represent an average of the last 30 s of the appropriate time period. Blood gas values were determined from blood samples obtained during each condition. From the blood gas data, arteriovenous oxygen difference (a-V̇o2) was calculated as the difference between arterial and venous oxygen content, where 20 mg/dl is the assumed arterial oxygen content (22, 35). Importantly, arterial oxygen content is not altered during forearm exercise, and thus changes in oxygen consumption reflect changes in blood flow and venous oxygen content (5, 35). Oxygen consumption across the forearm (v̇o2) was calculated as: (FBF × a-V̇o2 difference) and expressed in ml/min.
To account for changes in FBF and its impact on [ATP] concentration measurements and to quantify the rate of total ATP draining the active muscle, ATP effluent was calculated as: FBF × [ATP]/1,000, similar to what we have done previously (27) and how others have quantified data when blood flow is altered (14, 16, 34). Furthermore, given that bulk erythrocyte delivery is impacted by blood flow and erythrocytes may be a primary source of ATP in circulation, ATP effluent is a relevant calculation (27).
Statistics
Data are presented as means ± se. Differences between resting and stimulus conditions were determined with paired two-tailed Student's t-tests for all variables except systemic hemodynamics (heart rate and mean arterial pressure), which were analyzed by one-way repeated-measures ANOVA and Fisher's least significant difference post hoc testing when significance was observed. Significance was set a priori at P < 0.05.
RESULTS
Protocol 1: Effects of Graded Rhythmic Forearm Compressions
Forearm and systemic hemodynamics.
As expected, there were significant increases in FBF and FVC at each of the rhythmic forearm compression pressures (Fig. 1, A and B; P < 0.05). Furthermore, at the highest level of forearm compressions (200 mmHg), FBF and FVC were significantly greater than at both 50 mmHg and 100 mmHg (P < 0.05), consistent with a graded effect of compressions on the forearm vasculature. Systemic hemodynamics for Protocol 1 are presented in Table 1. A small but significant increase in heart rate (∼3–4 beats/min) was observed during the 100-mmHg and 200-mmHg levels of forearm compressions (P < 0.05); however, mean arterial pressure was not different across all conditions (P = 0.65).
Fig. 1.
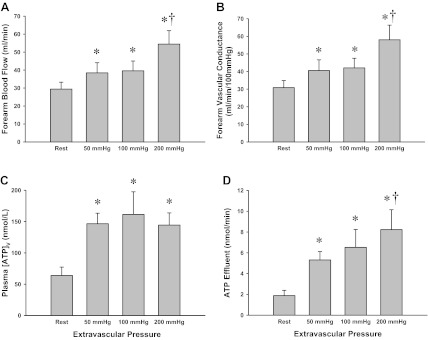
Protocol 1: forearm hemodynamics and plasma adenosine triphosphate (ATP) measures during graded rhythmic forearm compressions (RFCs). Forearm blood flow (FBF) (A) and forearm vascular conductance (FVC) (B) across all conditions. Significant increases from rest were observed in both FBF and FVC with all levels of RFC, and were significantly greater at 200 mmHg RFC than at 50 mmHg and 100 mmHg. C: venous plasma [ATP] significantly increased from rest with all levels of RFC. D: ATP effluent represents the combined influence of changes in FBF and venous plasma [ATP]. RFC increased ATP effluent from rest across all pressures and was significantly greater at 200 mmHg than that at 50 or 100 mmHg. *P < 0.05 vs. Rest; †P < 0.05 vs. 50 and 100 mmHg via 2-tailed paired Student's t-test.
Table 1.
Systemic hemodynamics during Protocol 1
| HR, beats/min | MAP, mmHg | |
|---|---|---|
| Protocol 1 | ||
| Rest | 58 ± 3 | 95 ± 2 |
| 50 mmHg | 60 ± 4 | 95 ± 2 |
| 100 mmHg | 62 ± 4* | 93 ± 2 |
| 200 mmHg | 61 ± 4* | 95 ± 2 |
Applicable values are means ± SE. HR, heart rate; MAP, mean arterial pressure.
P < 0.05 vs. Rest.
Venous plasma [ATP] and ATP effluent.
Venous plasma [ATP] was significantly greater than rest at all levels of the rhythmic forearm compressions (P < 0.05; Fig. 1C). There were no significant differences in venous plasma [ATP] between the different forearm compression pressures (P = 0.45–0.92). When changes in blood flow were accounted for, ATP effluent was significantly greater than rest at all levels of forearm compressions (P < 0.05; Fig. 2D). In addition, ATP effluent at the 200-mmHg level of forearm compressions was significantly greater than at 50 mmHg (P < 0.05) but not statistically greater than at 100 mmHg (P = 0.26).
Fig. 2.
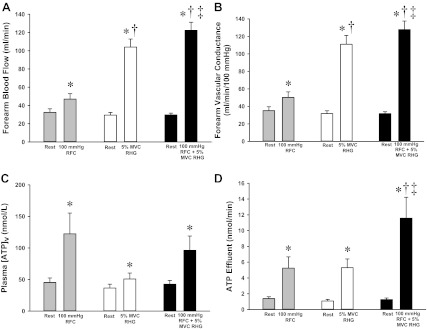
Protocol 2: forearm hemodynamics and plasma ATP measures during RFCs and rhythmic handgrip exercise (RHG). FBF (A) and FVC (B) across all conditions. All conditions were greater than rest, and RHG at 5% MVC was greater than that observed with RFC. The combination of RFC and RHG resulted in greater FBF and FVC than either RFC or RHG alone. C: venous plasma [ATP] significantly increased from rest with RFC, RHG, and RFC + RHG. D: ATP effluent represents the combined influence of changes in FBF and venous plasma [ATP]. RFC, RHG, and RFC + RHG all increased ATP effluent from rest, and the ATP effluent with RFC + RHG was significantly greater than that of RFC or RHG alone. *P < 0.05 vs. Rest; †P < 0.05 vs. RFC; ‡P < 0.05 vs. RHG via 2-tailed paired Student's t-test.
Blood gas parameters.
Technical difficulties precluded complete blood gas parameter analysis on all samples in 3 of the 10 subjects. Therefore, blood gas parameters represent n = 7. As shown in Table 2, in general, there were no significant differences in the blood gas values, and V̇o2 was not significantly different across conditions (P = 0.2); however, Po2 was significantly greater during rhythmic forearm compressions of 200 mmHg than all other conditions due to the elevation in FBF to the resting (quiescent) muscle (P < 0.05).
Table 2.
Venous blood gas and hemoglobin parameters during Protocol 1
| pH | pO2, mmHg | pCO2, mmHg | CtO2, ml/dl | CtCO2, ml/dl | FO2Hb, % | VO2, ml/min | Hct, % | tHb, g/dl | |
|---|---|---|---|---|---|---|---|---|---|
| Protocol 1 (n = 7) | |||||||||
| Rest | 7.36 ± 0.01 | 29.3 ± 1.8 | 45.5 ± 1.1 | 10.9 ± 1.1 | 27.1 ± 0.6 | 52.6 ± 4.3 | 2.2 ± 0.4 | 43.0 ± 1.9 | 14.6 ± 0.7 |
| 50 mmHg | 7.38 ± 0.01 | 31.0 ± 2.4 | 45.3 ± 1.1 | 11.3 ± 1.5 | 26.8 ± 0.4 | 54.2 ± 5.7 | 2.7 ± 0.4 | 42.9 ± 2.0 | 14.6 ± 0.7 |
| 100 mmHg | 7.36 ± 0.01 | 32.1 ± 2.1 | 44.8 ± 0.9 | 11.4 ± 1.3 | 26.0 ± 0.5 | 55.0 ± 4.7 | 2.7 ± 0.4 | 42.6 ± 2.1 | 14.6 ± 0.7 |
| 200 mmHg | 7.37 ± 0.01 | 35.3 ± 1.8*†‡ | 44.5 ± 1.2 | 12.8 ± 1.2 | 26.2 ± 0.6 | 61.4 ± 3.6‡ | 3.0 ± 0.4 | 43.1 ± 2.2‡ | 14.8 ± 0.7 |
Values are means ± SE. p, partial pressure; Ct, content; F, fraction; Hb, hemoglobin; VO2, oxygen consumption; Hct, hematocrit; t, total.
P < 0.05 vs. Rest;
P < 0.05 vs. 50;
P < 0.05 vs. 100 mmHg.
Protocol 2: Effects of Rhythmic Forearm Compressions and Muscle Contraction
Forearm and systemic hemodynamics.
As expected, there were significant increases in FBF and FVC from rest during all experimental conditions (Fig. 2, A and B; P < 0.05). Furthermore, during the combination of rhythmic forearm compressions and rhythmic handgrip exercise, FBF and FVC were significantly greater than with either forearm compressions or handgrip exercise alone (P < 0.05). There were no significant differences in FBF or FVC at rest between the three different trials (P = 0.30–0.31). Systemic hemodynamics for Protocol 2 are presented in Table 3. A small but significant increase in heart rate was observed with combined forearm compressions and handgrip exercise (P < 0.05); however, MAP was not different across all conditions (P = 0.16).
Table 3.
Systemic hemodynamics during Protocol 2
| HR, beats/min | MAP, mmHg | |
|---|---|---|
| Protocol 2 | ||
| Rest | 52 ± 4 | 93 ± 2 |
| 100 mmHg RFC | 54 ± 4 | 94 ± 2 |
| Rest | 54 ± 4 | 93 ± 2 |
| 5% MVC RHG | 56 ± 4 | 95 ± 2 |
| Rest | 55 ± 3 | 94 ± 3 |
| RFC + RHG | 57 ± 3* | 96 ± 3 |
Values are means ± SE. RFC, rhythmic forearm compression; MVC, maximal voluntary contraction; RHG, rhythmic handgrip exercise.
P < 0.05 vs. Rest.
Venous plasma [ATP] and ATP effluent.
There were no differences in venous plasma [ATP] or ATP effluent at rest across conditions. Similar to Protocol 1, rhythmic forearm compressions at 100 mmHg significantly increased plasma [ATP] from rest (Fig. 2C; P < 0.05). Additionally, plasma [ATP] increased above rest during 5% MVC handgrip exercise alone as well as during combined forearm compressions and handgrip exercise (Fig. 2C). Although trends were observed for plasma [ATP] to be greater during forearm compressions alone and the combination of exercise and compressions compared with exercise alone, these differences did not achieve statistical significance (both P = 0.08). When changes in blood flow were taken into account, ATP effluent was significantly greater than rest with all three manipulations (Fig. 2D; P < 0.05). In contrast to plasma [ATP], ATP effluent was the greatest with the combined stimulus of forearm compressions superimposed with handgrip exercise compared with either stimulus alone (P < 0.05).
Blood gas parameters.
Technical difficulties precluded complete blood gas parameter analysis on all samples in 1 of the 10 subjects. Therefore, blood gas parameters represent n = 9. Complete blood gas parameters for each of the trials are presented in Table 4. Many significant differences exist due to the increased metabolism associated with handgrip exercise and combined forearm compressions and handgrip exercise. Importantly, there were no significant differences between handgrip exercise alone and exercise in combination with forearm compressions except for a slightly greater V̇o2 with combined forearm compressions and handgrip exercise compared with handgrip exercise alone (P < 0.05).
Table 4.
Blood gas and hemoglobin parameters during Protocol 2
| pH | pO2, mmHg | pCO2, mmHg | CtO2, ml/dl | CtCO2, ml/dl | FO2Hb, % | VO2, ml/min | Hct, % | tHb, g/dl | |
|---|---|---|---|---|---|---|---|---|---|
| Protocol 2 (n = 9) | |||||||||
| Rest | 7.37 ± 0.02 | 28.4 ± 1.8 | 48.3 ± 2.5 | 10.2 ± 0.8 | 28.7 ± 0.8 | 48.6 ± 3.9 | 3.1 ± 0.3 | 44.0 ± 1.1 | 14.9 ± 0.4 |
| 100 mmHg RFC | 7.37 ± 0.01 | 36.9 ± 3.9* | 46.7 ± 1.6 | 13.4 ± 1.4* | 27.7 ± 0.6 | 62.4 ± 5.5* | 2.6 ± 0.4 | 44.8 ± 1.3 | 15.2 ± 0.4* |
| Rest | 7.36 ± 0.01 | 28.5 ± 1.7 | 49.0 ± 2.2 | 10.1 ± 0.9 | 28.4 ± 1.7 | 48.2 ± 3.8 | 2.9 ± 0.3 | 43.8 ± 1.2 | 14.9 ± 0.4 |
| 5% MVC RHG | 7.33 ± 0.01*† | 21.9 ± 0.6*† | 51.1 ± 2.3 | 6.1 ± 0.5*† | 28.4 ± 1.3 | 30.8 ± 1.9*† | 14.3 ± 1.3*† | 43.2 ± 1.2† | 14.7 ± 0.4*† |
| Rest | 7.37 ± 0.01 | 28.9 ± 1.9 | 46.8 ± 1.4 | 10.3 ± 1.2 | 27.5 ± 0.6 | 48.6 ± 4.5 | 2.9 ± 0.4 | 43.7 ± 1.5 | 14.8 ± 0.5 |
| RFC + RHG | 7.34 ± 0.01*† | 21.9 ± 0.6*† | 51.8 ± 1.4*† | 6.7 ± 0.6*† | 29.0 ± 0.8* | 31.9 ± 2.0*† | 16.1 ± 1.3*†‡ | 43.3 ± 1.3† | 14.7 ± 0.4† |
Values are means ± SE.
P < 0.05 vs. Rest;
P < 0.05 vs. RFC;
P = 0.052 vs. RHG.
DISCUSSION
In the present study, we were interested in whether simulated mechanical effects of muscle contractions increase venous [ATP] and ATP effluent draining the local muscle tissue in vivo, independent of changes in tissue metabolic demand, and whether mechanical effects of muscle contractions further increase circulating ATP when superimposed with exercise. The primary novel findings of the present study are as follows. First, graded increases in extravascular pressure, via mechanical tissue compression, elicit significant increases in plasma [ATP] and ATP effluent. Second, the increase in plasma [ATP] is robust at mild levels of extravascular pressure (50 mmHg), and this does not increase further with pressures up to 200 mmHg. In contrast, the circulating rate of ATP (i.e., ATP effluent) is graded with increasing levels of extravascular pressure. Third, although mechanical deformation, mild rhythmic handgrip exercise, and the combination of the two stimuli all increase plasma [ATP] similarly, the combination of augmented superimposed mechanical deformation during muscle contractions results in a significantly greater increase in ATP effluent than either stimulus alone. Taken together, our data indicate that the mechanical effects of muscle contraction are a stimulus for ATP release in vivo, independent of any change in muscle metabolism, and thus are likely one stimulus for increasing intravascular ATP during exercise in humans.
Circulating ATP During Exercise in Humans
Interest has been growing in the role of intravascular (circulating) ATP in the regulation of skeletal muscle blood flow during exercise. We and others have observed a significant increase in venous plasma [ATP] draining contracting skeletal muscle tissue (11–13, 16, 27, 33, 45, 46), whereas few studies have reported no change in plasma [ATP] (7, 15, 31). In the present study, we were interested in investigating the mechanical compression associated with muscle contractions as a possible contributor to the observed increase in plasma ATP during exercise.
To address whether mechanical effects of muscle contraction could contribute to increased plasma [ATP] during exercise, we first set out to determine whether mechanical deformation alone, independent of any change in tissue metabolism, could elevate circulating ATP, as a variety of mechanical stimuli have been shown to increase ATP release from erythrocytes and endothelial cells in vitro (2, 3, 39, 41). Indeed, in Protocol 1 of the present study, we observed a significant increase in venous plasma [ATP] at each level of extravascular pressure (via cuff compression) we employed to mimic the mechanical effects of rhythmic muscle contraction (Fig. 2). Our results agree with the only previous investigation into this topic that we are aware of, a single level of high-pressure compressions (∼250 mmHg) of the thigh to increase femoral venous [ATP] as measured by intravascular microdialysis (33). Interestingly, in our study, plasma [ATP] increased approximately threefold at mild levels of extravascular pressure (50 mmHg) and remained elevated without significantly increasing further with progressive elevations in extravascular pressure (100 and 200 mmHg). When taking into account tissue blood flow, ATP effluent (i.e., the circulating rate of ATP) also was significantly elevated above resting levels, and this displayed a graded increase with increasing levels of extravascular pressure such that ATP effluent was greatest during forearm compressions at 200 mmHg (Fig. 2D). Interestingly, it appears that the greatest effect on ATP was a result of increasing extravascular pressure from rest to superimposed 50 mmHg with less effect of additional increases in extravascular pressure to 100 and 200 mmHg. This observation may imply that a relatively modest stimulus is needed to stimulate mechanically induced mechanisms of ATP release. It is possible that additional release at greater levels of extravascular pressure may correspond with small increases in blood flow and thus erythrocyte delivery rather than increased pressure (26, 27). Importantly, during this protocol, our blood gas data indicate that we were successful in our attempt to cause increased mechanical deformation with no change in metabolism and thus isolate mechanical influences on intravascular ATP (Table 2).
Although our goal in Protocol 1 was to mimic the mechanical effects of muscle contraction in the absence of changes in tissue metabolism and associated blood gas parameters (e.g., hemoglobin oxygenation, pH), the normal local milieu of contracting muscle includes alterations in many stimuli that could be involved in the net integrated response resulting in elevated intravascular ATP. Thus in Protocol 2 we set out to determine whether increased mechanical deformation beyond that which occurs during low-intensity muscle contraction could further elevate circulating ATP. Specifically, we were interested in investigating how the combination of deoxygenation (resulting from increased metabolism of exercise) and mechanical deformation (resulting from muscle contraction during exercise and enhanced with superimposed rhythmic cuff inflation) interact to increase plasma ATP. In vitro experiments using both endothelial cells and erythrocytes have shown that deoxygenation (9, 23) and mechanical deformation (40, 44) can both independently and in combination (3, 10) stimulate ATP release. By performing three trials, one each of mechanical deformation, handgrip exercise, and the combination of increased mechanical deformation during handgrip exercise, we were able to address these potential interactions.
Our results from Protocol 2 confirm the findings from Protocol 1 and our previously published data (27) that mechanical deformation and low-intensity handgrip exercise, respectively, can significantly increase [ATP] and ATP effluent (Fig. 2). We also found a similar increase in plasma [ATP] during the combined stimulus of forearm compressions and handgrip exercise. In this protocol, the trial of combined forearm compressions and handgrip exercise resulted in a significantly greater increase in ATP effluent than either the compression or exercise trial alone. This finding suggests that the further increase in ATP effluent that occurs with progressive intensity exercise [as in our previous study (27)] could partially be the result of the increased extravascular pressure, even to a modest degree, and subsequent mechanical deformation that occurs with higher-intensity exercise (38).
Plasma ATP and FBF
The collective observations from the present set of experiments deserve mention regarding the magnitude of changes in plasma [ATP], FBF, as well as the circulating rate of ATP (i.e., effluent). We would be remiss to not acknowledge that the conditions of mechanical compression alone resulted in the greatest increase in plasma [ATP] yet the lowest FBF responses (see Figs. 1 and 2). This is seemingly paradoxical given the previous work from our laboratory and others (4, 16, 29, 37) demonstrating that intravascular ATP is a very potent vasodilator. However, there are several possible explanations that should be discussed. First, mechanical compression alone of the forearm tissue increases blood flow without changes in metabolic demand, thus resulting in “overperfusion” of a quiescent tissue and washing out of vasoactive metabolites produced at rest that normally contribute to basal vascular tone and can increase blood flow. Second, there is a large retrograde flow associated with the physical impedance caused by the cuff inflation that does not occur with low levels of handgrip exercise and thus decreases the mean blood velocity (and thus blood flow) across cardiac cycles during the mechanical stimulus. Third, it is also possible that the diffuse nature of our cuff compressions cause ATP release from cells outside the site of blood flow control within the vascular tree or provide a greater stimulus for ATP release than the mechanical effect of actual forearm contractions, increasing sampled [ATP]. Fourth, during exercise, nucleotidase activity is elevated (27, 46), which can rapidly degrade ATP, thus lowering the amount detectable in our venous blood sample and potentially somewhat underestimating the release of ATP during muscle contractions. Finally, we must recognize that multiple signals contribute to the increase in muscle blood flow that occurs with exercise, even at a relatively low intensity (24). Thus, although we propose that ATP contributes to the increase in blood flow observed during handgrip exercise, we do not intend to imply that it is the only signal for vasodilation in this condition.
As stated in materials and methods, we have provided rationale that ATP effluent is an appropriate quantification in addition to plasma [ATP], and this is especially relevant in the current study, where large differences in FBF occur across conditions and may impact the interpretation of our data. ATP effluent takes into account FBF and thus is a means to quantify the total circulating rate of ATP at rest and in response to the experimental conditions. As previously discussed (27), it is also physiologically relevant because the number of red cells perfusing the forearm are a primary source of intravascular ATP (8, 26, 42). Our data indicate that ATP effluent during forearm compressions alone and mild handgrip exercise is nearly identical (P = 0.98), yet FBF is greater during handgrip exercise. This again highlights the fact that multiple signals for vasodilation during exercise contribute to this increased muscle blood flow. In contrast to plasma [ATP], data obtained during the combined stimulus of mechanical deformation and mild exercise indicate that ATP effluent was augmented compared with either stimulus alone. Taken together, our data indicate that the mechanical effects of muscle contraction can be a stimulus for increased plasma ATP and ATP effluent during exercise.
Experimental Considerations
By design, in Protocol 2, the handgrip exercise was performed at a low intensity (5% MVC) that stimulated primarily deoxygenation (e.g., decreased venous FO2Hb) with minimal changes in other metabolic byproducts (e.g., pH, Pco2) that have been shown to be independent stimuli of ATP release in vitro (1, 9). This allowed us to be able to investigate the combined effects of mechanical deformation (via superimposed cuff inflation) and primarily deoxygenation during our combined forearm compressions and handgrip exercise trial. Whether the combination of deoxygenation and increased mechanical stress creates an additive or a synergistic release of ATP is difficult to address. In vitro studies of erythrocytes suggest that deoxygenation- and mechanically induced release of ATP is mediated by a similar intracellular signaling and release cascade (42), and thus it is not likely that these two factors interact synergistically. The present data support this idea in that the increase in plasma [ATP] or ATP effluent during the combined forearm compression and handgrip exercise trial does not appear to be greater than the sum of the responses during forearm compressions and handgrip exercise alone (Fig. 2).
It is important to acknowledge the diffuse nature of our mechanical stimulus utilized in the present protocols. As previously discussed, our rhythmic forearm compressions via a blood pressure cuff wrapped around the entire forearm result in a significant increase in mechanical stress of the forearm tissue (muscle and skin), as well as blood vessels and traversing blood cells. Although we are unable to precisely quantify this stress or identify the exact nature of the physical strain or cell types undergoing this stimulus, we speculate that increases in intravascular ATP reflects release from erythrocytes or vascular endothelial cells. We have previously used this type of compression stimulus to mimic the mechanical influence of muscle contractions in humans (25, 28) and feel that, despite these limitations, this technique lends novel insight into the contribution of the mechanical effects of muscle contractions on plasma [ATP] and ATP effluent in the human forearm.
Previous work suggests that the distribution of increased blood flow that occurs during muscle contractions is highly specific and is not necessarily mimicked by other conditions that increase blood flow, such as vasodilator infusion (21). We are unable to determine the heterogeneity of the changes in blood flow that we observed during our mechanical compression stimulus or handgrip exercise. Along these lines, blood obtained from the deep venous catheter provides a mixed sample and thus reflects the plasma outflow from a variety of tissues that may or may not present an increased stimulus for ATP release. Furthermore, although it may be argued that concentration is the only relevant quantification of vascular ATP if vascular receptors are responsive to local concentrations of an agonist, we are unable to determine concentrations at the microvascular level given our mixed venous sample and differences in blood flow heterogeneity. Our calculation of ATP effluent reflects the total circulating rates of ATP and provides additional insight into the changes in ATP and blood flow we observe during exercise and forearm compressions.
Perspectives and Conclusions
There is considerable momentum in support of ATP having a vital role in the regulation of skeletal muscle blood flow during exercise. Unfortunately, no specific antagonist for the purinergic receptors that mediate ATP vasodilation is available for human use, and because of this a direct role for ATP during exercise hyperemia in humans has not been established. In attempting to determine whether ATP may contribute to exercise hyperemia, it has become important to try to understand the various stimuli that occur during exercise and can elicit ATP release and subsequent vasodilation. In the present study, we have identified that the mechanical effects of muscle contraction can independently increase plasma [ATP] draining the human forearm and that ATP effluent is augmented when low-intensity exercise is combined with a superimposed mechanical stimulus. Thus we conclude that the mechanical effects of muscle contraction can independently elevate plasma [ATP] and ATP effluent draining quiescent skeletal muscle without changes in local metabolism and can further augment intravascular ATP during mild exercise associated with increases in metabolism and local deoxygenation. Collectively, our data indicate that the mechanical effects of muscle contraction are likely one stimulus for increasing intravascular ATP draining active skeletal muscle in humans.
GRANTS
This research was supported by National Institutes of Health awards HL087952 and HL102720 (F. Dinenno).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: A.R.C., B.S.K., and F.A.D. conception and design of research; A.R.C., B.S.K., J.C.R., and F.A.D. performed experiments; A.R.C. analyzed data; A.R.C., B.S.K., and F.A.D. interpreted results of experiments; A.R.C. prepared figures; A.R.C. and F.A.D. drafted manuscript; A.R.C., B.S.K., J.C.R., and F.A.D. edited and revised manuscript; A.R.C., B.S.K., J.C.R., and F.A.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Julia A. Davis, Leora J. Garcia, and Wyatt F. Voyles for assistance in data collection as well as the subjects who volunteered to participate in this study.
REFERENCES
- 1. Bergfeld GR, Forrester T. Release of ATP from human erythrocytes in response to a brief period of hypoxia and hypercapnia. Cardiovasc Res 26: 40–47, 1992 [DOI] [PubMed] [Google Scholar]
- 2. Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol 103: 1203–1205, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bodin P, Burnstock G. Synergistic effect of acute hypoxia on flow-induced release of ATP from cultured endothelial cells. Experientia 51: 256–259, 1995 [DOI] [PubMed] [Google Scholar]
- 4. Crecelius AR, Kirby BS, Richards JC, Garcia LJ, Voyles WF, Larson DG, Luckasen GJ, Dinenno FA. Mechanisms of ATP-mediated vasodilation in humans: modest role for nitric oxide and vasodilating prostaglandins. Am J Physiol Heart Circ Physiol 301: H1302–H1310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Augmented skeletal muscle hyperaemia during hypoxic exercise in humans is blunted by combined inhibition of nitric oxide and vasodilating prostaglandins. J Physiol 589: 3671–3683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Crecelius AR, Kirby BS, Voyles WF, Dinenno FA. Nitric oxide, but not vasodilating prostaglandins, contributes to the improvement of exercise hyperemia via ascorbic acid in healthy older adults. Am J Physiol Heart Circ Physiol 299: H1633–H1641, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dufour SP, Patel RP, Brandon A, Teng X, Pearson J, Barker H, Ali L, Yuen AH, Smolenski RT, Gonzalez-Alonso J. Erythrocyte-dependent regulation of human skeletal muscle blood flow: role of varied oxyhemoglobin and exercise on nitrite, S-nitrosohemoglobin, and ATP. Am J Physiol Heart Circ Physiol 299: H1936–H1946, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Edwards J, Sprung R, Sprague R, Spence D. Chemiluminescence detection of ATP release from red blood cells upon passage through microbore tubing. Analyst 126: 1257–1260, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Ellsworth ML, Forrester T, Ellis CG, Dietrich HH. The erythrocyte as a regulator of vascular tone. Am J Physiol Heart Circ Physiol 269: H2155–H2161, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Faris A, Spence DM. Measuring the simultaneous effects of hypoxia and deformation on ATP release from erythrocytes. Analyst 133: 678–682, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Forrester T. A quantitative estimation of adenosine triphosphate released from human forearm muscle during sustained exercise. J Physiol 221: 25P–26P, 1972 [PubMed] [Google Scholar]
- 12. Forrester T, Lind AR. Adenosine triphosphate in the venous effluent and its relationship to exercise. Fed Proc 28: 1280–1283, 1969 [PubMed] [Google Scholar]
- 13. Forrester T, Lind AR. Identification of adenosine triphosphate in human plasma and the concentration in the venous effluent of forearm muscles before, during and after sustained contractions. J Physiol 204: 347–364, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Giannarelli C, Virdis A, De Negri F, Magagna A, Duranti E, Salvetti A, Taddei S. Effect of sulfaphenazole on tissue plasminogen activator release in normotensive subjects and hypertensive patients. Circulation 119: 1625–1633, 2009 [DOI] [PubMed] [Google Scholar]
- 15. Gonzalez-Alonso J, Mortensen SP, Dawson EA, Secher NH, Damsgaard R. Erythrocytes and the regulation of human skeletal muscle blood flow and oxygen delivery: role of erythrocyte count and oxygenation state of haemoglobin. J Physiol 572: 295–305, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gonzalez-Alonso J, Olsen DB, Saltin B. Erythrocyte and the regulation of human skeletal muscle blood flow and oxygen delivery: role of circulating ATP. Circ Res 91: 1046–1055, 2002 [DOI] [PubMed] [Google Scholar]
- 17. Gonzalez-Alonso J, Richardson RS, Saltin B. Exercising skeletal muscle blood flow in humans responds to reduction in arterial oxyhaemoglobin, but not to altered free oxygen. J Physiol 530: 331–341, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gorman MW, Feigl EO, Buffington CW. Human plasma ATP concentration. Clin Chem 53: 318–325, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Gorman MW, Marble DR, Ogimoto K, Feigl EO. Measurement of adenine nucleotides in plasma. Luminescence 18: 173–181, 2003 [DOI] [PubMed] [Google Scholar]
- 20. Gray SD, Carlsson E, Staub NC. Site of increased vascular resistance during isometric muscle contraction. Am J Physiol 213: 683–689, 1967 [DOI] [PubMed] [Google Scholar]
- 21. Heinonen I, Kemppainen J, Kaskinoro K, Peltonen JE, Borra R, Lindroos MM, Oikonen V, Nuutila P, Knuuti J, Hellsten Y, Boushel R, Kalliokoski KK. Comparison of exogenous adenosine and voluntary exercise on human skeletal muscle perfusion and perfusion heterogeneity. J Appl Physiol 108: 378–386, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Holmgren A, Linderholm H. Oxygen and carbon dioxide tensions of arterial blood during heavy and exhaustive exercise. Acta Physiol Scand 44: 203–215, 1958 [DOI] [PubMed] [Google Scholar]
- 23. Jagger JE, Bateman RM, Ellsworth ML, Ellis CG. Role of erythrocyte in regulating local O2 delivery mediated by hemoglobin oxygenation. Am J Physiol Heart Circ Physiol 280: H2833–H2839, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Joyner MJ, Wilkins BW. Exercise hyperaemia: is anything obligatory but the hyperaemia? J Physiol 583: 855–860, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kirby BS, Carlson RE, Markwald RR, Voyles WF, Dinenno FA. Mechanical influences on skeletal muscle vascular tone in humans: insight into contraction-induced rapid vasodilatation. J Physiol 583: 861–874, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirby BS, Crecelius AR, Richards JC, Dinenno FA. Sources of intravascular ATP during exercise in humans: critical role for skeletal muscle perfusion. Exp Physiol. doi: 10.1113/expphysiol.2012.071555. In press. (DOI: 10.1113/expphysiol.2012.071555) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kirby BS, Crecelius AR, Voyles WF, Dinenno FA. Impaired skeletal muscle blood flow control with advancing age in humans: attenuated ATP release and local vasodilation during erythrocyte deoxygenation. Circ Res 111: 220–230, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kirby BS, Markwald RR, Smith EG, Dinenno FA. Mechanical effects of muscle contraction do not blunt sympathetic vasoconstriction in humans. Am J Physiol Heart Circ Physiol 289: H1610–H1617, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Kirby BS, Voyles WF, Carlson RE, Dinenno FA. Graded sympatholytic effect of exogenous ATP on postjunctional alpha-adrenergic vasoconstriction in the human forearm: implications for vascular control in contracting muscle. J Physiol 586: 4305–4316, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mo FM, Ballard HJ. The effect of systemic hypoxia on interstitial and blood adenosine, AMP, ADP and ATP in dog skeletal muscle. J Physiol 536: 593–603, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mortensen SP, Gonzalez-Alonso J, Damsgaard R, Saltin B, Hellsten Y. Inhibition of nitric oxide and prostaglandins, but not endothelial-derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol 581: 853–861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mortensen SP, Gonzalez-Alonso J, Nielsen JJ, Saltin B, Hellsten Y. Muscle interstitial ATP and norepinephrine concentrations in the human leg during exercise and ATP infusion. J Appl Physiol 107: 1757–1762, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Mortensen SP, Thaning P, Nyberg M, Saltin B, Hellsten Y. Local release of ATP into the arterial inflow and venous drainage of human skeletal muscle: insight from ATP determination with the intravascular microdialysis technique. J Physiol 589: 1847–1857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation 107: 579–585, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Richards JC, Crecelius AR, Kirby BS, Larson DG, Dinenno FA. Muscle contraction duration and fibre recruitment influence blood flow and VO2 independent of contractile work during steady-state exercise in humans. Exp Physiol 97: 750–761, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenmeier JB, Hansen J, Gonzalez-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol 558: 351–365, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Rosenmeier JB, Yegutkin GG, Gonzalez-Alonso J. Activation of ATP/UTP-selective receptors increases blood flow and blunts sympathetic vasoconstriction in human skeletal muscle. J Physiol 586: 4993–5002, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sadamoto T, Bonde-Petersen F, Suzuki Y. Skeletal muscle tension, flow, pressure, and EMG during sustained isometric contractions in humans. Eur J Appl Physiol Occup Physiol 51: 395–408, 1983 [DOI] [PubMed] [Google Scholar]
- 39. Sprague RS, Ellsworth ML, Stephenson AH, Kleinhenz ME, Lonigro AJ. Deformation-induced ATP release from red blood cells requires CFTR activity. Am J Physiol Heart Circ Physiol 275: H1726–H1732, 1998 [DOI] [PubMed] [Google Scholar]
- 40. Sprague RS, Ellsworth ML, Stephenson AH, Lonigro AJ. ATP: the red blood cell link to NO and local control of the pulmonary circulation. Am J Physiol Heart Circ Physiol 271: H2717–H2722, 1996 [DOI] [PubMed] [Google Scholar]
- 41. Sprung R, Sprague R, Spence D. Determination of ATP release from erythrocytes using microbore tubing as a model of resistance vessels in vivo. Anal Chem 74: 2274–2278, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Sridharan M, Sprague RS, Adderley SP, Bowles EA, Ellsworth ML, Stephenson AH. Diamide decreases deformability of rabbit erythrocytes and attenuates low oxygen tension-induced ATP release. Exp Biol Med (Maywood) 235: 1142–1148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tschakovsky ME, Shoemaker JK, Hughson RL. Vasodilation and muscle pump contribution to immediate exercise hyperemia. Am J Physiol Heart Circ Physiol 271: H1697–H1701, 1996 [DOI] [PubMed] [Google Scholar]
- 44. Wan J, Ristenpart WD, Stone HA. Dynamics of shear-induced ATP release from red blood cells. Proc Natl Acad Sci USA 105: 16432–16437, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wood RE, Wishart C, Walker PJ, Askew CD, Stewart IB. Plasma ATP concentration and venous oxygen content in the forearm during dynamic handgrip exercise. BMC Physiol 9: 24, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yegutkin GG, Samburski SS, Mortensen SP, Jalkanen S, Gonzalez-Alonso J. Intravascular ADP and soluble nucleotidases contribute to acute prothrombotic state during vigorous exercise in humans. J Physiol 579: 553–564, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]


