Abstract
Nasal high flow (NHF) has been shown to increase expiratory pressure and reduce respiratory rate but the mechanisms involved remain unclear. Ten healthy participants [age, 22 ± 2 yr; body mass index (BMI), 24 ± 2 kg/m2] were recruited to determine ventilatory responses to NHF of air at 37°C and fully saturated with water. We conducted a randomized, controlled, cross-over study consisting of four separate ∼60-min visits, each 1 wk apart, to determine the effect of NHF on ventilation during wakefulness (NHF at 0, 15, 30, and 45 liters/min) and sleep (NHF at 0, 15, and 30 liters/min). In addition, a nasal cavity model was used to compare pressure/air-flow relationships of NHF and continuous positive airway pressure (CPAP) throughout simulated breathing. During wakefulness, NHF led to an increase in tidal volume from 0.7 ± 0.1 liter to 0.8 ± 0.2, 1.0 ± 0.2, and 1.3 ± 0.2 liters, and a reduction in respiratory rate (fR) from 16 ± 2 to 13 ± 3, 10 ± 3, and 8 ± 3 breaths/min (baseline to 15, 30, and 45 liters/min NHF, respectively; P < 0.01). In contrast, during sleep, NHF led to a ∼20% fall in minute ventilation due to a decrease in tidal volume and no change in fR. In the nasal cavity model, NHF increased expiratory but decreased inspiratory resistance depending on both the cannula size and the expiratory flow rate. The mechanisms of action for NHF differ from those of CPAP and are sleep/wake-state dependent. NHF may be utilized to increase tidal breathing during wakefulness and to relieve respiratory loads during sleep.
Keywords: CPAP, nasal high flow, ventilation, sleep, humidified air
respiratory failure is the most common complication of pulmonary and chest-wall disorders. Recently, an open nasal cannula system for delivering a high flow of oxygen or room air (nasal high flow, NHF) has been shown to assist ventilation in the acute setting in patients with chronic respiratory failure and in adults and children with sleep-disordered breathing (14, 15, 17, 18, 23, 29, 33). The mechanisms of ventilatory assistance of NHF, however, are not well understood.
Several studies in patient populations demonstrate that NHF increases end-expiratory pressure (7, 10, 15, 20, 26, 27) and improves in gas exchange (17, 27, 29, 33), but there is no data on ventilation in response to NHF. Although some reports indicate that ventilation may decrease in response to NHF, these conclusions were extrapolated from indirect measures such as improvements in gas exchange or reductions in respiratory rate (14, 17, 25, 27, 29). It remains unclear whether the observed physiological responses to NHF were confounded by disease-specific factors rather than being a systematic physiological response to NHF.
In general, respiratory pattern responses to NHF have been obtained during wakefulness (7, 10, 15, 20, 26, 27) rather than sleep (14, 15, 18). The sleep/wake state, however, is well known to modify ventilation, making it likely that observed results are partly due to wake vs. sleep-state effects rather than physiologic responses to NHF.
To address these issues, we conducted a randomized, controlled, cross-over trial in healthy individuals to determine the effect(s) of increasing flow rates of NHF on ventilation. In addition, to determine the mechanisms of NHF, we compared the ventilatory responses to NHF between wakefulness and sleep and determined the pressure/air-flow relationship in a physical model of the nasal orifice during different NHF test conditions. We hypothesized that 1) increasing flow rates of NHF would reduce the respiratory rate but not minute ventilation in normal individuals, and 2) ventilatory responses to NHF would be accentuated during wakefulness compared with sleep.
METHODS
Subjects
Normal subjects were recruited and considered eligible if they were nonsmoking adults between 18 and 30 yr of age and had a body mass index (BMI) of 20.0 to 35.0 kg/m2, and did not have abnormalities in sleep, including habitual snoring. Individuals were excluded if they had any abnormality in standard clinical examination or a pulmonary function test (as defined by the ratio of forced expiratory volume in 1 s to forced vital capacity <80% predicted, total lung capacity <80% predicted). Ten normal subjects [mean age, 22 SD 1.3 yr; mean BMI, 24 SD 2.4 kg/m2; with mean FEV1 of 102% (SD 7.0); predicted and mean FEV1/FVC of 109% (SD 7.0); predicted and mean FVC of 94 SD 9% predicted; for individual data, see Table 1] participated in the study, which was approved by the Massey University Human Ethics Committee with informed consent being obtained from each subject.
Table 1.
Anthropometric and spirometry data of subjects
| Subject | Age (yr) | Height (cm) | Weight (kg) | BMI (kg/m2) | FEV1 % predicted | FVC % predicted | FEV1/FVC % predicted | VDFowler (ml) |
|---|---|---|---|---|---|---|---|---|
| A* | 21 | 168 | 71 | 25 | 95 | 82 | 116 | 112 |
| B | 21 | 178 | 70 | 22 | 95 | 99 | 96 | 144 |
| C | 21 | 178 | 75 | 24 | 105 | 92 | 113 | 130 |
| D | 21 | 172 | 70 | 24 | 113 | 106 | 107 | 125 |
| E | 21 | 168 | 66 | 23 | 110 | 97 | 113 | 134 |
| F | 21 | 178 | 95 | 30 | 93 | 80 | 116 | 115 |
| G | 22 | 187 | 79 | 23 | 109 | 98 | 111 | 106 |
| H* | 22 | 172 | 75 | 25 | 98 | 84 | 115 | 108 |
| I* | 22 | 180 | 90 | 28 | 108 | 100 | 108 | 146 |
| J | 27 | 205 | 102 | 24 | 98 | 101 | 96 | 140 |
| Mean (SD) | 22 ± 1.9 | 179 ± 11 | 79.3 ± 12.2 | 24 ± 2.4 | 102 ± 7 | 94 ± 9 | 109 ± 7 | 126 ± 15.1 |
| Range | 21–27 | 168–205 | 66–102 | 22–30 | 93–113 | 82–100 | 96–116 | 106–146 |
BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; VDFowler, anatomical dead space.
Individuals who also participated in a sleep study (see Fig. 4).
Study Design
NHF was delivered at 37°C and fully saturated with water by a high-flow humidification system (AIRVO; Fisher & Paykel Healthcare, New Zealand) through a nasal cannula (Optiflow OPT844 Medium; Fisher & Paykel Healthcare) as illustrated in Fig. 1. Prior to conducting the study, a brief introductory session was held to familiarize all subjects with the NHF interventions that they would be receiving in their subsequent four visits. The study design consisted of two parts: one for wakefulness; the other for sleep.
Fig. 1.
Illustration of nasal high-flow system and nasal cannula used in this study.
Wakefulness.
We conducted a randomized, controlled, cross-over study consisting of four separate ∼60-min visits, each 1 wk apart, to cover three different NHF interventions (15, 30, and 45 liters/min) and one control without NHF. Each subject received the interventions and control period (no NHF) in a randomized order (cross-over design).
Sleep.
To ensure continuity of ∼3-h non-rapid-eye movement sleep, NHF interventions were limited to 15 and 30 liters/min and one control period without NHF.
Subjects maintained a semirecumbent position throughout the wakefulness experiments, and a supine position throughout the sleep experiments. To compare NHF responses between wakefulness to sleep, ventilatory measures for wakefulness were repeated prior to all sleep studies in the supine position at NHF of 15 and 30 liters/min and no NHF.
Materials
Ventilation was monitored by a noninvasive respiratory inductance plethysmography (RIP) device (Respitrace QDC; Viasys Services, Lakeland, Florida), which was capable of self-calibration on the basis of natural breathing with the QDC method outlined by Sackner et al. (28). Nasal air-flow was measured by a heated, low-resistance pneumotachograph No.4 (Fleisch, Lausanne, Switzerland) and differential pressure transducer carrier-amplifier system (Validyne, Northridge, CA) connected to a full-face mask (RT041M; Fisher & Paykel Healthcare). The resulting flow was integrated into volume and calibrated against a 3-liter calibration syringe (Hans Rudolph).
Baseline polysomnography.
A standard baseline sleep study including infrared video cameras monitoring (Somologica; Medcare, Buffalo, NY) was performed to stage sleep and score respiratory events according to the American Academy of Sleep Medicine criteria (1).
Anatomical airway dead space of all subjects was evaluated by the method of single breath-test volumetric capnography with nasal passage breathing only. A single expiration was collected by a nasal pillow (Fisher & Paykel Healthcare) connected to the same pneumotachograph described above with a CO2 mainstream sensor (TG950P CO2 sensor and OLG-2800 CO2 monitor; Nihon Kohden) inserted in between. Five repeated capnography tests were recorded for all subjects.
Analog signals were digitized by an accurate, 16-bit ADC converter (ADI Powerlab 8/30; ADInstrument, Dunedin, New Zealand) and recorded by special software running on a laptop computer (ADI Labchart software; ADInstrument).
Experimental Protocol
Noninvasive plethysmography measurements were obtained with one belt placed at the nipple line and one at the level of the umbilicus. Subjects were instructed to maintain the semirecumbent posture throughout all wakefulness protocols and the supine position throughout all sleep/wake comparisons. Prior to all experiments the following procedures were used to calibrate the RIP device: first, all subjects were instructed to maintain natural nasal breathing for at least 5 min to assist the Respitrace QDC self-calibration process. Once the self-calibration had reached a satisfactory level, validated by visual inspections of the sum channel, baseline RIP gains were then determined during 5 min of spontaneous breathing with a full-face mask attached to a pneumotachograph. This was followed by another 5-min session without the full-face mask, which served as the baseline period for each intervention. During wakefulness, each intervention lasted 15 min with the predetermined NHF rate administered through the nasal cannula interface. During sleep, the duration of each intervention was 10 min. All visits were concluded with an additional 5 min of spontaneous breathing through the full-face mask with the pneumotachograph to perform RIP drift analysis.
A control period, consisting of 25 min of normal nasal breathing without the cannula interface, was performed to demonstrate no change in ventilation over time.
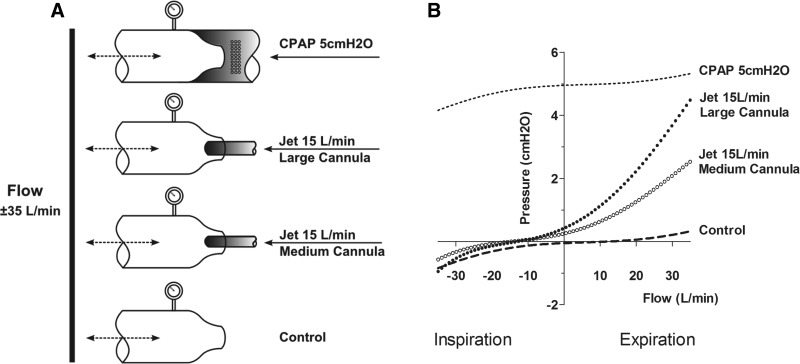
Bench Model for Studying the Jet Effect
A simple nasal cavity model was created to examine the pressure characteristics inside a narrowed opening of 9.6 mm simulating the nasal valve area as illustrated in Fig. 6A. A variable flow was cycled from −35 liters/min to +35 liters/min (simulating inspiratory and expiratory phase, respectively) from a 22-mm tube attached to the wide-open side of the model. Three experimental conditions were created at the nasal valve area: first, a 15 liter/min jet was delivered via either a 6.2-mm or 4.3-mm cannula with wall thickness 0.5 and 0.4 mm, respectively. The cannula used in the model represented typical Optiflow interfaces (OPT844 and OPT846) used for NHF in adults. Second, the nasal orifice was left open with no cannula in place (control). Third, an elastic tube was tightly fitted to the outer rim of the entrance of the nasal valve area. The tube was connected via an elbow of a typical nasal mask to a CPAP machine (HC600; Fisher & Paykel Healthcare), which provided 24 liters/min biased flow at pressure 5 cmH2O.
Fig. 6.
Experimental setup for experiments with the nasal cavity model. A: two streams of air, one bidirectional flow at the inlet was varied in 5 liters/min increments from −35 to +35 liters/min, simulating inspiratory and expiratory phases, respectively, and another unidirectional NHF jet, fixed at 15 liters/min, were generated and delivered at the outlet of the nasal cavity model through inelastic tubes (22- to 9.6-mm opening for variable flow and 6-mm for the jet) with rigid walls. B: pressure/air-flow relationship of NHF compared with control (open outlet) and CPAP.
This setup results in the following air-flow dynamics: during the negative (inspiratory) phase, air would be drawn from both the NHF jet cannula and from the narrow opening when inspiratory flow exceeds flow from the cannula (15 liters/min). During the positive (expiratory) phase, air-flow can exit only through the narrow openings while NHF or CPAP is delivered. Pressure/air-flow relationships were constructed over the range of inspiratory and expiratory phase for each condition.
Data Analysis
All respiratory parameters were extracted and computed from the RIP sum signal on a breath-by-breath basis. Effects of NHF were examined by comparing the average of the last 5-min steady-state RIP signals recorded in the intervention stage with the 5-min session baseline. Two separate volumetric scaling gains were calculated; one from measurements taken before the intervention and one from after the intervention. The volumetric scaling gain was significantly larger after the intervention than at the beginning of the experiment for all subjects (P = 0.0005), with a relative increase by +14% (SD 2.44) resulting in a drift of 4.65 ml/min (SD 0.73). Ventilation for baseline and intervention were then determined from linear drift-corrected gains calculated from two reference gains. Respiratory rate and tidal volume were computed for every minute of each experiment, and the mean of the last 5 min of each condition was taken for statistical analysis.
Anatomical airway dead space was determined by Fowler's equal area method (5). Reproducibility of volumetric capnography results was improved by a pneumotachograph built-in CO2 sensor synchronization algorithm similar to the one described by Verschuren et al. (31).
Results are expressed as the mean (SD) unless otherwise stated. Effects of intervention were examined by one-way ANOVA with a Dunnett's post hoc test for comparisons against the last 5 min of the control period (baseline 2). Paired t-tests were also performed to compare treatment against session baselines. The threshold for statistical significance was set at P < 0.05.
RESULTS
Ventilatory Responses during Wakefulness
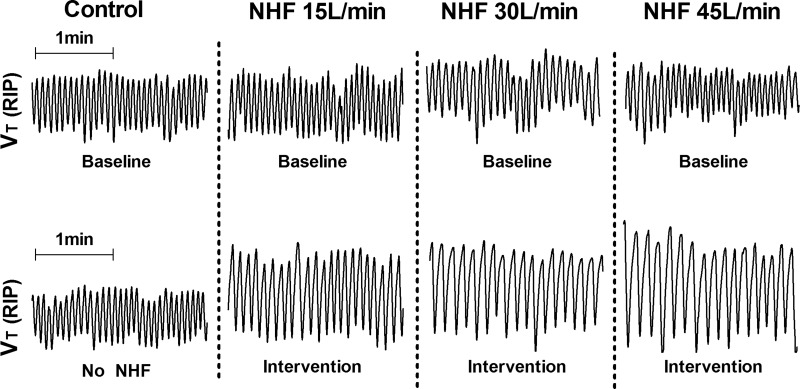
Figure 2 shows recording examples of the RIP sum signal (VT RIP) from a single subject. Compared with control, there was a reduction in respiratory rate and an increase in tidal volume between baseline (Fig. 2, top) and NHF interventions of 15, 30 and 45 liters/min (Fig. 2, bottom).
Fig. 2.
Representative 2-min trace from one subject illustrating tidal volume (VT) at baseline (top) and against NHF at control, 15, 30, and 45 liters/min (bottom).
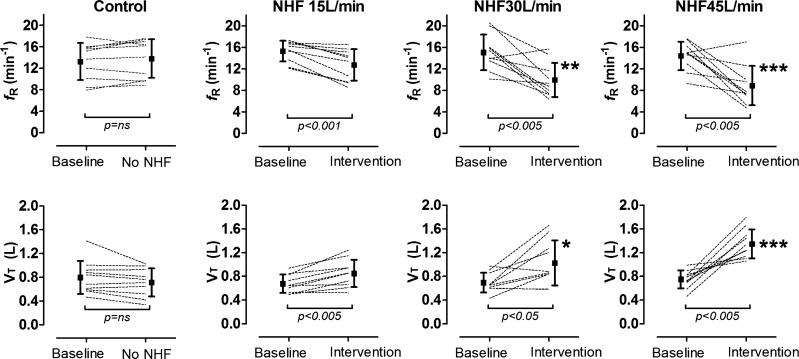
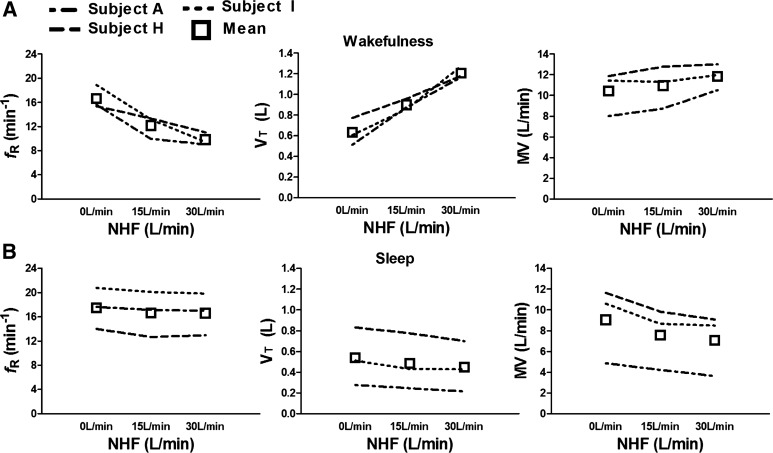
Figure 3 shows individual and pooled data for respiratory rate (top) and tidal volume (bottom) between baseline and no NHF (far left) and intervention of 15, 30, and 45 liters/min (second left to right). NHF led to a marked increase in tidal volume that was offset by a reduction in respiratory rate. There was no change in overall minute ventilation between NHF compared with baseline. The reduction in respiratory rate was largely due to a marked increase in expiratory time (TE) from 3.1 ± 0.8 s at control to 4.9 ± 1.5 s at 30 liters/min and 6.0 ± 2.3 s at 45 liters/min NHF (P < 0.01 and P < 0.001, respectively; one-way ANOVA with Dunnett's post hoc test). VDFowler/VT reduced by ∼50% from 0.2 ± 0.05 at control to 0.1 ± 0.06 at 45 liters/min (P < 0.001; one-way ANOVA with Dunnett's post hoc test).
Fig. 3.
Individual and mean (± SD) data for respiratory rate (fR, top) and tidal volume (VT, bottom) at baseline and following NHF at 15, 30, and 45 liters/min. *P < 0.05, **P < 0.01, and ***P < 0.001 indicate significant differences between control, no NHF, and intervention.
There was no difference in mean respiratory rate and mean tidal volumes between each of the four baseline periods during wakefulness and between the baseline and the control period (no NHF).
Ventilatory Responses during Sleep
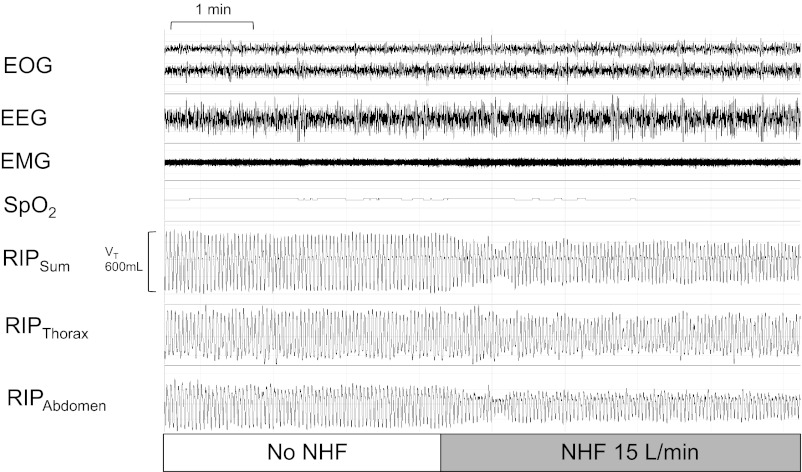
Three subjects underwent repeat experiments during sleep. There was a marked difference in ventilatory responses to NHF between wakefulness and sleep. During the wakefulness just prior to sleep (Fig. 4, top), increasing NHF flow rates were associated with a fall in respiratory rate and a rise in tidal volume, leading to a slight increase in minute ventilation. In contrast, during sleep (Fig. 4, bottom), there was a fall in minute ventilation due to a decrease in tidal volume without a change in respiratory rate. The reduction in tidal volume with NHF was associated with a stable oxyhemoglobin level (SaO2) and reductions in the excursions of the inductive plethysmography belts of the thorax and the abdomen (see RIPThorax and RIPAbdomen in recording example of Fig. 5). There was no difference in respiratory rate and tidal volumes between wakefulness in the semirecumbent position and the supine.
Fig. 4.
Individual and mean data for ventilatory responses during wakefulness (top) and sleep (bottom) in a subset of 3 individuals at baseline and following NHF at 15 and 30 liters/min.
Fig. 5.
Representative ∼10-min trace from one individual during non-rapid-eye movement stage N3 sleep illustrating a reduction in tidal volume [VT(RIP)], stable oxyhemoglobin, and reduced respiratory effort with NHF at 15 liters/min compared with baseline.
Pressure Responses to NHF in a Nasal Cavity Model
Figure 6A shows the nasal cavity model and pressure/air-flow relationships to delivering NHF and CPAP at the flow-restricted side of the model compared with control. In the control experiment (dashed line), pressure fell slightly below the atmospheric pressure level during the inspiratory phase and, in a similar but mirrored fashion, above atmosphere during the expiratory phase, indicating a similar resistance in the nasal cavity for both the inspiratory and expiratory phase. In contrast, NHF of 15 liters/min increased resistance during expiration but decreased it during inspiration. Large cannula (closed circles) produced slightly higher expiratory and inspiratory pressure than the medium cannula (open circles). During expiration this difference was more significant than during inspiration. Note that inspiratory pressure remained positive until inspiratory flow exceeded −15 liters/min, which was the NHF rate. When the inspiratory flow exceeded NHF the large cannula caused slightly more negative pressure than the medium cannula.
CPAP led to a similar pressure/air-flow relationship as control at atmosphere, indicating that CPAP did not change inspiratory and expiratory resistance in this nasal cavity model. Of note, expiratory pressure rose markedly more with NHF, indicating that the mechanism of expiratory pressure buildup differs between NHF and CPAP.
DISCUSSION
In the current study we determined ventilatory responses to nasal high flow, warmed and humidified air through a nasal cannula (NHF) in normal subjects. There were two major new findings: first, ventilatory responses to NHF were markedly dependent on the sleep/wake state. During wakefulness, respiratory rate slowed and tidal volume increased in response to NHF, whereas during sleep there was no change in respiratory rate but a reduction in tidal volume. Of note, the respiratory-pattern response during wakefulness preserved minute ventilation, whereas NHF during sleep was associated with a ∼20% decrease in minute ventilation. Second, in a nasal cavity model, we demonstrated that the effect of NHF on intranasal pressure is different compared with that during the use of CPAP. Although NHF increased expiratory but decreased inspiratory resistance, CPAP did not alter resistances throughout the respiratory cycle compared with normal breathing. Thus, pressure/air-flow dynamics markedly differed between NHF and CPAP, indicating that the mechanical effects of NHF on the upper airways differ from that of CPAP. Taken together, both the wakefulness response and sleep response to NHF may support rehabilitation efforts to increase tidal breathing in patients with respiratory or cardiac dysfunction during wakefulness and appear to relieve respiratory loads during sleep.
Mechanisms of Action: NHF Differs from CPAP
Previous data demonstrate that NHF increases pharyngeal pressure of ∼0.5 to 1 cmH2O per 10 liters/min NHF (7, 10, 15, 20, 26, 27). The rise in pressure is most likely due to increases in expiratory resistance of the nose that is created by either the size of the cannula or the in-going jet flow against exhaled expiratory air. To address the effect of cannula size on air-flow dynamics we created a simple benchmark nasal cavity model (see Fig. 6A) that allowed us to measure the pressure and air-flow characteristics comparing NHF and CPAP (15 liters/min through one cannula). As can be seen from the pressure at zero air-flow, which corresponds to end-inspiratory and end-expiratory pressure (PEEP), respectively, PEEP is higher when NHF is delivered through the large-size compared with the medium-size cannula. PEEP at a given NHF rate, therefore, may reflect the contribution of the cannula size/nasal valve area. In contrast, the rise in peak expiratory pressure (PEP) during the expiratory phase depends on both the size of the cannula and the expiratory flow rate. Thus, although PEP depends on the peak expiratory flow rate and the cannula size, the predominant contribution to PEP, however, appears to be the expiratory flow rate. Taken together, our model predicts that NHF would markedly increase expiratory pressure depending on both the size of NHF cannula in relation to the cross-sectional area of the nasal valve and the patient's expiratory flow rate.
Although NHF increased the expiratory pressure, it differs from CPAP during the expiratory phase. As illustrated in Fig. 6B, NHF increased the expiratory resistance compared with control and CPAP, which had similar expiratory resistances. Of note, our model confirms previous findings that CPAP via a nasal mask does not alter expiratory resistance of the upper airway (12). There are several explanations for the difference in expiratory resistance responses between NHF and CPAP. First, NHF may exert a jet-flow effect that creates a pressure gradient across the flow-restricted nose segment (zero at the nares and positive inside the nasal cavity), whereas CPAP increases the pressure at nares without creating a further pressure gradient across the valve area. Second, as illustrated in Fig. 6, CPAP increases expiratory pressure only minimally with increasing expiratory flow rates, indicating that air-flow resistance during expiration remained relatively constant with CPAP. Thus, the mechanisms of increasing expiratory pressure differ between NHF and CPAP. Third, NHF may have triggered alae nasae muscle activation, thereby stiffening the airway (6). Stiffening of the nasal passage may also contribute to a greater expiratory resistance with increasing expiratory air flow. Regardless of the mechanism, our data indicate that NHF is not like minimal CPAP; rather, it serves as a means to increase resistance to expiratory air.
Our model also predicts that NHF alters inspiratory air-flow dynamics. Without NHF, the inspiratory pressure in the nasal cavity becomes negative with onset of inspiration. In contrast, when NHF is present, the pressure at onset of inspiration remained above atmosphere for most of the inspiratory phase (see Fig. 6B). This increase would raise the driving pressure for inspiration (ΔPN-T) according to Ohm's law (ΔPN-T = R·ΔV), where ΔPN-T is the pressure gradient between the nose and the trachea, R is the resistance of the airway, and ΔV is the inspiratory air flow. Taken together, the mechanisms of action of NHF appear to be through improvements in inspiratory air-flow dynamics and increases in expiratory resistance, both of which make NHF a distinctly different form of ventilatory assistance compared with CPAP.
Ventilatory Reponses Are Sleep/Wake-State Dependent
Stimulation of the nasal mucosa is known to provoke profound cardiovascular and respiratory reflexes (32). However, a recent study demonstrated that there was no significant effect on ventilation during exercise when NHF was delivered at 25 liters/min (13). In contrast, recent studies show that individuals slow down respiration during wakefulness but not during sleep in response to increases in expiratory pressure and resistance (8, 12, 21). We observed marked reductions in respiratory rate and increases in tidal volume during wakefulness. During sleep, all three subjects demonstrated a decrease in tidal volume that led to a substantial fall in minute ventilation. Although the physiologic mechanisms for the wakefulness ventilatory responses observed with NHF remain unclear, our data indicate that the sleep/wake-state responses to NHF are markedly different.
NHF Reduces the Proportion of Dead-Space Ventilation during Wakefulness and Sleep
The increase in tidal volume with NHF during wakefulness decreased the proportion of dead-space volume, thus improving breathing efficiency as defined by the ratio of dead space volume (VD) to tidal volume (VT). Most likely the decrease in the VD/VT ratio by NHF during wakefulness was largely due to a significant increase in VT rather than a wash-out effect of the nasal cavity.
The shallower breathing during sleep, however, does not appear to increase the proportion of dead-space volume for the following reasons. First, we observed a significant reduction in tidal excursions of the thorax and abdomen with NHF (see Fig. 5). The reductions in minute ventilation and respiratory movements were associated with a stable breathing pattern without sleep fragmentation. The mechanism for maintaining a stable breathing pattern with lower tidal volumes could be due to improvements in inspiratory air-flow dynamics with NHF, as mentioned above. Lowering inspiratory airway resistance during sleep has been shown to reduce respiratory drive but not respiratory rate (19). Alternatively, it is possible that NHF washed out the nasal or nasopharyngeal dead-space volume. A wash-out in nasal dead space volume would resemble unidirectional breathing, which is known to reduce inspired dead-space volume of ∼50 to 70 ml (9). Finally, the delivery of warm and humidified air with NHF could have reduced the energy expenditure of heating and humidifying inspired air. The reductions in minute ventilation with NHF may be partly due to a lower CO2 production. Although we cannot determine which of these mechanisms were responsible for the decrease in ventilation during sleep, these observations are consistent with the notion that NHF did not worsen breathing efficiency during sleep.
The Sleep/Wake-Dependent Responses Explain Findings of Clinical Case Reports
The findings of the current physiologic experiments allow explanation of the results of several NHF clinical studies that warm and humidified air improved oxygenation, patient comfort, and adherence to the therapy over a wide range of patients with hypoxic respiratory failure (2–4, 11, 22, 27, 30). Whereas most studies demonstrate a marked improvement in oxygenation with increasing air-flow rates (27, 29, 33), inconsistencies exist in regard to the respiratory pattern response to NHF (3, 17, 27, 24, 29, 33). We now propose an explanation of the controversial results: the majority of studies tested responses to NHF during wakefulness. Although some patients may have stayed awake throughout the protocols, others may have dozed off given the comfort and relief of respiratory stress with NHF. As shown above, ventilatory responses are sleep/wake-state dependent and may account for the heterogeneous responses between and even within a study population. Nevertheless, we recognize that disease-specific phenotypes influence both blood gas and respiratory-pattern response to NHF, but that these responses are best examined during sleep or sedation (or both), rather than at times of wakefulness.
Strengths and Limitations
There are several strengths in the current study. The first strength is our study design, which compared baseline breathing with control (no NHF) and NHF in a randomized, controlled, cross-over approach. Second, the assessment of tidal volume did not interfere with the NHF equipment nor did it create a discomfort that would have changed the respiratory pattern. Third, our comparison of NHF responses between sleep and wakefulness allowed us to determine that wakefulness markedly modifies ventilatory responses to NHF.
The major limitations were as follows: first, all subjects were young men with BMI varying from 22 to 30 kg/m2. Some showed atypically high minute ventilation at rest. In fact, we observed two subjects (both of whom were athletes) who had a high respiratory rate or high tidal volume at baseline and during some but not all NHF experiments. Nevertheless, even these volunteers demonstrated similar differences between baseline and at least one NHF intervention, indicating that respiratory responses are consistent despite a potential differential physiological background. Second, we did not include arterial blood gas measures that could have helped to better define steady-state periods. These measures were excluded because it would have introduced too many interruptions throughout the protocols. Third, our observation of a decrease in the VD/VT ratio by NHF during wakefulness was likely due to a marked increase in VT rather than a wash-out effect of the nasal cavity. The reduction in minute ventilation (and VT) during sleep, however, might be related to a reduction in dead space volume, but sleep prevented us from applying the Fowler technique. Fourth, increases in end- and peak-expiratory pressure with NHF may have increased functional residual capacity and altered the ventilation/perfusion relationship, both of which are known to change arterial blood gases that potentially influence breathing patterns. Taken together, more physiologic experiments would be necessary to determine which of the proposed mechanisms are responsible for the changes in ventilation with NHF.
Physiologic and Clinical Implications
There are several implications as a result of our findings. First, the outcomes provide evidence that NHF significantly alters respiratory pattern during wakefulness in normal subjects. If these responses are present in those with cardiorespiratory diseases, it may help to manage these patients as follows: for example, patients with chronic obstructive pulmonary disease (COPD) often adopt pursed-lip breathing to lower their respiratory rate and prolong expiratory time to alleviate expiratory flow limitation and dynamic hyperinflation. Pursed-lip breathing is, however, associated with an increased work of breathing and patients cannot maintain this pattern over a longer time period. NHF responses, in fact, resemble the breathing pattern of pursed-lip breathing. Thus, NHF may provide a therapeutic benefit for patients who cannot or will not adopt a slow and deep breathing pattern. Second, preventing atelectasis by adoption of a deep and slow breathing pattern is a main strategy of preventive rehabilitation medicine. Both the positive expiratory pressure in conjunction with the deep and slow breathing pattern response to NHF during wakefulness might be utilized as a simple-to-use respiratory support in the rehabilitation of patients with respiratory or cardiac dysfunction.
NHF may also be beneficial for subjects who have high dead space ventilation due to tachypnea or a rapid, shallow breathing pattern, particularly during sleep. NHF may help to prevent development of respiratory failure in patients who suffer from increased ventilatory loads during sleep.
In summary, NHF lowers inspiratory resistance but increases expiratory resistance in the nose. In response to NHF, normal individuals reduced ventilation during sleep while they maintained ventilation during wakefulness by adopting a slow and deep breathing pattern. Such ventilatory responses to NHF can explain the improvements in gas exchange with NHF in patients with chronic and acute respiratory failure.
GRANTS
The study was sponsored by National Heart, Lung, and Blood Institute Grants HL-105546 and P50-HL-084945-01. Support for this study, by provision of NHF equipment and direct project costs only, was also provided by Fisher & Paykel Healthcare to T.M.
DISCLOSURES
The authors have reported to the Journal the following competing of interest: materials for the study described in this article were provided by Fisher and Paykel Healthcare. H.S.: Dr. Schneider is also a paid consultant to Fisher and Paykel Healthcare. The terms of this arrangement are being managed by the Johns Hopkins University in accordance with its competing interest policies. T.M.: other than the provision of humidification equipment and financial support of direct project costs only by Fisher and Paykel Healthcare (to T.M.), Dr. Mündel reports no competing interests.
AUTHOR CONTRIBUTIONS
Author contributions: T.M., S.F., S.T., and H.S. conceived and designed research; T.M., S.F., and S.T. performed experiments; T.M., S.F., S.T., and H.S. analyzed data; T.M., S.F., S.T., and H.S. interpreted results of experiments; T.M., S.F., S.T., and H.S. prepared figures; T.M., S.F., S.T., and H.S. drafted manuscript; T.M., S.F., S.T., and H.S. edited and revised manuscript; T.M., S.F., S.T., and H.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Kimble Dunster and Sian Reynolds for their assistance with the RIP and data collection.
REFERENCES
- 1. American Academy of Sleep Medicine Manual for the Scoring of Sleep, and Associated Events. American Academy of Sleep Medicine. Darien, IL: 2011 [Google Scholar]
- 2. Boyer A, Vargas F, Delacre M, Saint-Léger M, Clouzeau B, Hilbert G, Gruson D. Prognostic impact of high-flow nasal cannula oxygen supply in an ICU patient with pulmonary fibrosis complicated by acute respiratory failure. Intensive Care Med 37: 558–559, 2011 [DOI] [PubMed] [Google Scholar]
- 3. Corley A, Caruana LR, Barnett AG, Tronstad O, Fraser JF. Oxygen delivery through high-flow nasal cannulae increase end-expiratory lung volume and reduce respiratory rate in post-cardiac surgical patients. Br J Anaesth 107: 998–1004, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Cuquemelle E, Pham T, Papon JF, Louis B, Danin PE, Brochard L. Heated and humidified high-flow oxygen therapy reduces discomfort during hypoxemic respiratory failure. Respir Care 57: 1571–1577, 2012 [DOI] [PubMed] [Google Scholar]
- 5. Fowler WS, Comroe JH. Lung function studies. I. The rate of increase of arterial oxygen saturation during the inhalation of 100 percent O2. J Clin Invest 27: 327–334, 1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gold AR, Smith PL, Schwartz AR. Effect of alae nasi activation on maximal nasal inspiratory air-flow in humans. J Appl Physiol 84: 2115–2122, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Groves N, Tobin A. High-flow nasal oxygen generates positive airway pressure in adult volunteers. Aust Crit Care 20: 126–131, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Iber C, Berssenbrugge A, Skatrud JB, Dempsey JA. Ventilatory adaptations to resistive loading during wakefulness and non-REM sleep. J Appl Physiol 52: 607–614, 1982 [DOI] [PubMed] [Google Scholar]
- 9. Jiang Y, Liang Y, Kacmarek RM. The principle of upper airway unidirectional flow facilitates breathing in humans. J Appl Physiol 105: 854–858, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Kubicka ZJ, Limauro J, Darnall RA. Heated, humidified high-flow nasal cannula therapy: yet another way to deliver continuous positive airway pressure? Pediatrics 121: 82–88, 2008 [DOI] [PubMed] [Google Scholar]
- 11. Lucangelo U, Vassallo FG, Marras E, Ferluga M, Beziza E, Comuzzi L, Berlot G, Zin WA. High-flow nasal interface improves oxygenation in patients undergoing bronchoscopy. Crit Care Res Pract 2012: 506382, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Masdeu MJ, Seelall V, Patel AV, Ayappa I, Rapoport DM. Awake measures of nasal resistance and upper airway resistance on CPAP during sleep. J Clin Sleep Med 7: 31–40, 2011 [PMC free article] [PubMed] [Google Scholar]
- 13. Mason R, Sharp K, Muendel T, Tatkov S, Pack RJ. The sustained effect of nasal insufflations on cardio-respiratory, metabolic and performance measures in athletes under respiratory stress. Medicina Sportiva 14: 50–55, 2010 [Google Scholar]
- 14. McGinley B, Halbower A, Schwartz AR, Smith PL, Patil SP, Schneider H. Effect of a high-flow open nasal cannula system on obstructive sleep apnea in children. Pediatrics 124: 179–188, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGinley BM, Patil SP, Kirkness JP, Smith PL, Schwartz AR, Schneider H. A nasal cannulae can be used to treat obstructive sleep apnea. Am J Respir Crit Care Med 176: 194–200, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nilius G, Franke KJ, Domanski U, Rühle KH, Kirkness JP, Schneider H. Effects of nasal insufflation on arterial gas exchange and breathing pattern in patients with chronic obstructive pulmonary disease and hypercapnic respiratory failure. Adv Exp Med Biol 755: 27–34, 2013 [DOI] [PubMed] [Google Scholar]
- 18. Nilius G, Wessendorf T, Maurer J, Stoohs R, Patil SP, Schubert N, Schneider H. Predictors for treating obstructive sleep apnea with an open nasal cannula system (transnasal insufflation). Chest 137: 521–528; 2010 [DOI] [PubMed] [Google Scholar]
- 19. O'Donoghue FJ, Catcheside PG, Eckert DJ, McEvoy RD. Changes in respiration in NREM sleep in hypercapnic chronic obstructive pulmonary disease. J Physiol 559: 663–673, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Parke R, McGuinness S, Eccleston M. Nasal high-flow therapy delivers low-level positive airway pressure. Br J Anaesth 103: 886–890, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Patel AV, Hwang D, Masdeu MJ, Chen GM, Rapoport DM, Ayappa I. Predictors of response to a nasal expiratory resistor device and its potential mechanisms of action for treatment of obstructive sleep apnea. J Clin Sleep Med 7: 13–22, 2011 [PMC free article] [PubMed] [Google Scholar]
- 22. Peters SG, Holets SR, Gay PC. Nasal high flow oxygen therapy in do-not-intubate patients with hypoxemic respiratory distress. Respir Care 2012. July 10 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 23. Rea H, McAuley S, Jayaram L, Garrett J, Hockey H, Storey L, O'Donnell G, Haru L, Payton M, O'Donnell K. The clinical utility of long-term humidification therapy in chronic airway disease. Respir Med 104: 525–533, 2010 [DOI] [PubMed] [Google Scholar]
- 24. Rello J, Pérez M, Roca O, Poulakou G, Souto J, Laborda C, Balcells J, Serra J, Masclans JR; CRIPS Investigators High-flow nasal therapy in adults with severe acute respiratory infection: a cohort study in patients with 2009 influenza A/H1N1v. J Crit Care 27: 434–439, 2012 [DOI] [PubMed] [Google Scholar]
- 25. Riera J, Pérez P, Cortés J, Roca O, Masclans JR, Rello J. Effect of high-flow nasal cannula and body position on end-expiratory lung volume. A cohort study using electrical impedance tomography. Respir Care 2012. October 8 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26. Ritchie JE, Williams AB, Gerard C, Hockey H. Evaluation of a humidified nasal high-flow oxygen system, using oxygraphy, capnography and measurement of upper airway pressures. Anaesth Intensive Care 39: 1103–1110, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Roca O, Riera J, Torres F, Masclans JR. High-flow oxygen therapy in acute respiratory failure. Respir Care 55: 408–413, 2010 [PubMed] [Google Scholar]
- 28. Sackner MA, Watson H, Belsito AS, Feinerman D, Suarez M, Gonzalez G, Bizousky F, Krieger B. Calibration of respiratory inductive plethysmograph during natural breathing. J Appl Physiol 66: 410–420, 1989 [DOI] [PubMed] [Google Scholar]
- 29. Sztrymf B, Messika J, Bertrand F, Hurel D, Leon R, Dreyfuss D, Ricard JD. Beneficial effects of humidified high-flow nasal oxygen in critical care patients: a prospective pilot study. Intensive Care Med 37: 1780–1786, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Sztrymf B, Messika J, Mayot T, Lenglet H, Dreyfuss D, Ricard JD. Impact of high-flow nasal cannula oxygen therapy on intensive care unit patients with acute respiratory failure: a prospective observational study. J Crit Care 27: 324–13, 2012 [DOI] [PubMed] [Google Scholar]
- 31. Verschuren F, Heinonen E, Clause D, Zech F, Reynaert MS, Liistro G. Volumetric capnography: reliability and reproducibility in spontaneously breathing patients. Clin Physiol Funct Imaging 25: 275–280, 2005 [DOI] [PubMed] [Google Scholar]
- 32. Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect 109 Suppl 4: 579–584, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Woodhead DD, Lambert DK, Clark JM, Christensen RD. Comparing two methods of delivering high-flow gas therapy by nasal cannula following endotracheal extubation: a prospective, randomized, masked, cross-over trial. J Perinatol 26: 481–485, 2006 [DOI] [PubMed] [Google Scholar]