Abstract
Background
This study determined whether treatment of normal (nondiabetic) pigs with the insulin-sensitizing agent troglitazone improves recovery of left ventricular (LV) function after acute ischemia and whether such effects are associated with altered myocardial substrate metabolism.
Methods and Results
Juvenile pigs (n=6) were treated with troglitazone (75 mg · kg−1 · d−1 PO) for 8 weeks. Untreated pigs (n=8) served as controls. Under anesthetized, open-chest conditions, pigs underwent 90 minutes of moderate regional LV ischemia and 90 minutes of reperfusion. Regional LV function was assessed with subendocardial sonomicrometry crystals. Fasting plasma insulin and free fatty acid levels were lower in troglitazone-treated pigs than in untreated pigs, whereas blood glucose did not differ between groups. These findings suggest that treatment enhanced systemic insulin sensitivity. Baseline hemodynamics and regional LV function did not differ between groups. After ischemia and reperfusion, systolic function (external work) of the ischemic region recovered to 44±6% of baseline in troglitazone-treated pigs versus 18±6% of baseline in untreated pigs (P<0.05). Regional diastolic function (maximum rate of wall expansion) recovered to 78±7% of baseline in treated pigs versus 52±7% of baseline in untreated pigs (P<0.05). Recovery of global LV systolic and diastolic function was also significantly greater in treated pigs. Myocardial glucose uptake did not differ between groups under any condition; however, net myocardial lactate uptake after reperfusion was 7 times greater in troglitazone-treated pigs than in untreated pigs, suggesting that treatment enhanced myocardial carbohydrate oxidation after reperfusion.
Conclusions
In nondiabetic pigs, chronic troglitazone treatment improves recovery of LV systolic and diastolic function after acute ischemia.
Keywords: metabolism, drugs, ischemia, myocardial contraction, diastole
Pharmacological agents that enhance myocardial carbohydrate utilization have the potential to ameliorate ischemic and postischemic contractile and lusitropic dysfunction. Examples of such strategies include infusion of glucose, insulin, and potassium during ischemia1 or treatment with an activator of pyruvate dehydrogenase during reperfusion.2,3
Troglitazone is a thiazolidinedione drug that is used clinically to treat type II diabetes mellitus. In diabetic patients, thiazolidinediones act as insulin sensitizers, increasing insulin-mediated glucose disposal without increasing insulin release4 and reducing circulating free fatty acid (FFA) levels.5 However, it is unclear whether thiazolidinediones exert similar effects in normal (nondiabetic) animals.6,7 Furthermore, effects of thiazolidinediones on myocardial substrate metabolism, mechanical function, and response to ischemia and reperfusion have not been investigated in vivo.
The present study determined whether chronic treatment of normal (nondiabetic) pigs with troglitazone improves recovery of left ventricular (LV) function after acute ischemia and reperfusion, and if so, whether such effects are associated with changes in myocardial substrate metabolism.
Methods
Experimental Preparation
Experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health. The troglitazone group consisted of 6 Yorkshire-Landrace pigs that weighed 9±0 kg before troglitazone treatment. These pigs were treated with troglitazone 75 mg · kg−1 · d−1 orally for 8 weeks, along with a diet of dry pig chow. Peripheral venous blood samples were obtained under fasting conditions before treatment and after 3 and 8 weeks of treatment for measurement of glucose, insulin, and FFA and for liver function tests. Samples obtained during treatment were also assayed for trough plasma concentrations of troglitazone and its principal metabolites by a precipitation extraction technique, liquid chromatography, and mass spectrometry. The control group consisted of 8 Yorkshire-Landrace pigs that were fed dry pig chow but received no drug treatment. After 8 weeks, both groups of pigs weighed 31±1 kg. After an overnight fast, pigs were subjected to an acute ischemic protocol, described below.
Surgical Preparation, Instrumentation, and Hemodynamic Data Acquisition
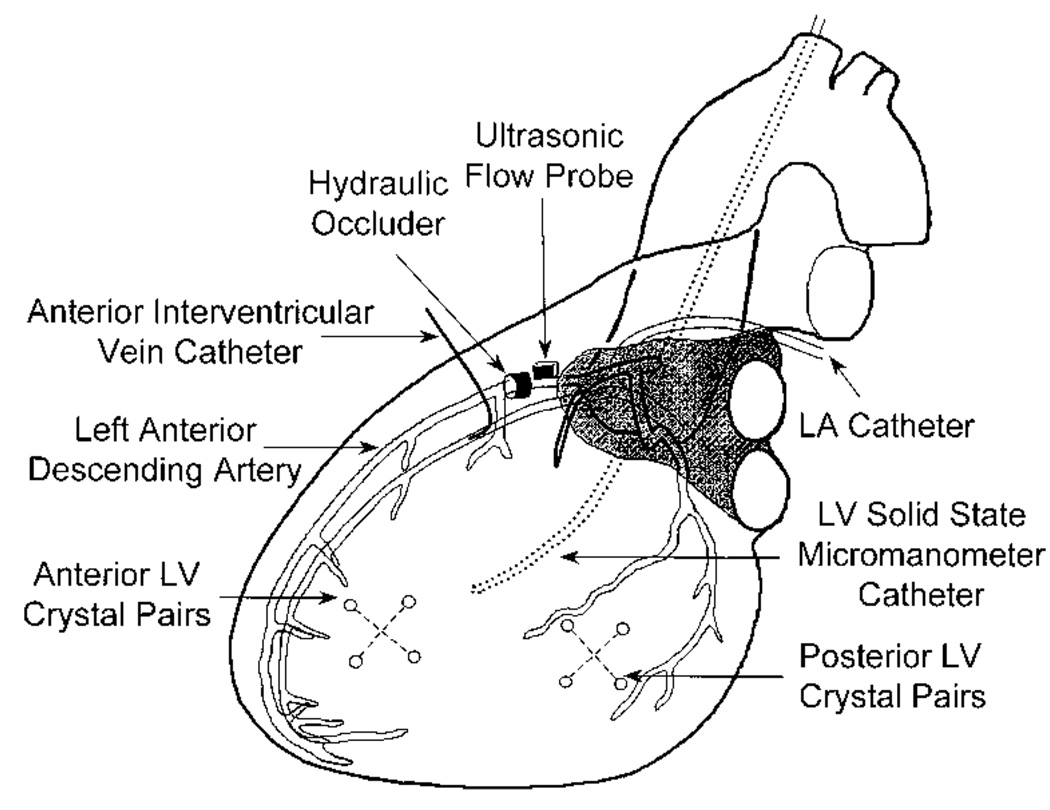
Pigs were anesthetized with α-chloralose and mechanically ventilated as described in previous communications.8,9 Indomethacin (25 mg IV) was given to prevent subsequent hemodynamic response to the injection of fluorescent microspheres suspended in dilute Tween solution. Propanolol (1 mg/kg IV) and atropine (0.2 mg/kg IV) were given to prevent activation of autonomic reflexes. Instrumentation of the heart is illustrated in Figure 1. Through a median sternotomy, fluid-filled catheters were placed in the aortic arch and left atrium, and a micromanometer catheter was placed in the LV. An adjustable hydraulic occluder (In Vivo Metric) and ultrasonic flow probe (Transonic Systems) were placed around the left anterior descending coronary artery (LAD) distal to the first diagonal branch to produce and monitor graded ischemia of the anterior LV. A catheter was inserted in the anterior interventricular vein at a site distal to the LAD occluder to sample coronary venous blood from the ischemic region. An array of 4 subendocardial sonomicrometry crystals (2 orthogonal pairs) was implanted in the center of the ischemic region; a similar array was implanted in the posterior LV (nonischemic region). Each crystal array measured regional myocardial wall area (the instantaneous product of 2 orthogonal segment lengths). Hemodynamic data were recorded and digitized during brief suspension of ventilation under steady-state hemodynamic conditions and during brief occlusion of the venae cavae. The latter data were used to derive regional preload-recruitable stroke work (PRSW) relations. At least 5 sets of data were collected and averaged under each experimental condition. Regional transmural myocardial blood flow was measured with fluorescent microspheres, as previously described.8
Figure 1.
Instrumentation of the heart. LA indicates left atrium.
Oxygen Consumption and Substrate Uptake
Paired arterial and coronary venous blood samples were withdrawn for measurement of oxygen, glucose, lactate, and FFA content, as previously described.9 Uptake of these substances by the anterior LV free wall was calculated as the product of mean transmural blood flow and coronary arteriovenous concentration difference. Circulating insulin was measured by radioimmunoassay.
Ischemic Protocol
After 30 minutes of stabilization, baseline measurements of hemodynamics, regional LV function, myocardial blood flow, substrate uptake, and circulating insulin were obtained. Ischemia was then induced by gradual inflation of the LAD occluder until LAD flow rate (monitored by the flowmeter) was reduced to 50% of baseline. This degree of flow reduction was maintained for 90 minutes. Ischemia of this severity and duration in this model does not cause myocardial infarction.10 A second set of measurements was made during the final 15 minutes of the ischemic period. The LAD constriction was then released, and a third set of measurements was made between 75 and 90 minutes of reperfusion.
After euthanasia, myocardium from the central ischemic and nonischemic regions was excised for analysis of regional blood flow by the microsphere method. In addition, a transmural section from center of the ischemic region was incubated in 1% triphenyl tetrazolium chloride for 20 minutes and examined for evidence of nonstaining, which is indicative of myocardial infarction.
Analysis of Systolic Function
LV pressure versus wall-area loops were analyzed in both ischemic and nonischemic regions. Steady-state regional external work was calculated as the average area of loops recorded without occlusion of the venae cavae. Regional PRSW relations were determined during brief occlusion of the venae cavae by plotting the area of each LV pressure versus wall-area loop against its corresponding end-diastolic wall area. End diastole was defined by the initial upstroke of a regional intramyocardial electrogram. Because regional wall area depends in part on how far apart each pair of crystals is implanted (ie, a source of experimental rather than physiological variability), external work and PRSW data were normalized to baseline values in each experiment. An additional measure of regional systolic function, fractional systolic wall-area reduction (FAR), was calculated as FAR = (EDA–ESA)/EDA, where EDA and ESA are end-diastolic and end-systolic wall area, respectively. FAR is a 2D analog to 1-dimensional fractional systolic segment shortening. Global LV contractility was assessed by peak positive LV dP/dt (+dP/dtmax).
Analysis of Diastolic Function
Regional diastolic function was assessed by the maximum rate of wall-area expansion (+dA/dtmax), a regional analog to global LV peak filling rate. +dA/dtmax was also normalized to its baseline value in each experiment. Global LV relaxation was assessed by peak negative LV dP/dt (−dP/dtmax).
Statistical Analysis
Data were expressed as mean±SEM. To determine the significance of a difference between groups in the response of a variable during the ischemic protocol, 2-way repeated-measures ANOVA was used, followed by unpaired t tests to compare the 2 groups under specific experimental conditions. To determine the significance of changes in a variable between baseline and subsequent conditions within the same group, a 1-way repeated-measures ANOVA was used, followed by Dunnett’s procedure. If data were not normally distributed, the Friedman repeated-measures ANOVA on ranks and Mann-Whitney test were substituted.
Results
Effects of Chronic Troglitazone Treatment
There were no discernible adverse effects of troglitazone treatment. Weight gain, activity, and liver function tests were normal in all treated pigs. Fasting blood glucose did not differ between groups (Table 1). Circulating insulin levels were unchanged after 3 weeks of treatment, but after 8 weeks of treatment, they were lower than baseline (P<0.05) and lower than those in the age- and weight-matched untreated control pigs (P<0.05). Plasma FFA levels after 8 weeks of treatment were also lower than baseline (P<0.05) and lower than those in untreated control pigs (P=0.06). These findings suggest that 8 weeks’ treatment with troglitazone enhanced systemic insulin sensitivity to a greater-than-normal level. Trough plasma concentrations of troglitazone and its metabolites are shown in Table 2.
TABLE 1.
Effects of Troglitazone on Blood Glucose, Insulin, and FFA
| Troglitazone-Treated Pigs | Untreated Control Pigs |
|||
|---|---|---|---|---|
| Pretreatment | 3 Wk | 8 Wk | ||
| Body weight, kg | 9±0 | 16±1 | 31±1 | 31±1 |
| Glucose, fasting, µmol/mL |
4.7±0.4 | 3.7±0.3 | 4.9±0.2 | 4.8±0.4 |
| Insulin, µU/mL | 3.2±1.0 | 2.6±0.7 | 0.5±0.1*† | 5.8±0.9 |
| FFA, µmol/mL | 0.63±0.15 | 0.24±0.08† | 0.17±0.02† | 0.36±0.08 |
| (P=0.06 vs untreated control pigs) |
||||
All values are mean±SEM; n=6 (troglitazone), n=8 (control). Glucose and insulin values are reported per milliliter of serum; FFA is reported per milliliter of plasma. Values determined in peripheral venous blood samples.
P<0.05 vs untreated control pigs,
P<0.05 vs pretreatment.
TABLE 2.
Plasma Concentrations of Troglitazone and Its Principal Metabolites
| Duration of Treatment | ||
|---|---|---|
| 3 Wk | 8 Wk | |
| Troglitazone | 470±124 | 486±144 |
| M1 (sulfate) | 967±255 | 1407±298 |
| M2 (glucuronide) | 123±31 | 84±13 |
All concentrations are ng/mL, mean±SEM, n=6, determined 20 hours after preceding dose. The quinone metabolite was undetectable in most pigs.
Effects of Ischemia/Reperfusion
All 6 troglitazone-treated pigs completed the ischemic protocol. Two of 10 pigs in the control group sustained ventricular fibrillation during ischemia; results are reported for 8 pigs that survived the complete protocol. Regional myocardial blood flow did not differ between control and troglitazone groups under any experimental condition (Table 3). In particular, the severity of ischemia was nearly equal in both groups. Examination of myocardium after staining with triphenyl tetrazolium chloride revealed no evidence of infarction in any pig.
TABLE 3.
Regional Myocardial Blood Flow
| Baseline | Ischemia | Reperfusion | ||||
|---|---|---|---|---|---|---|
| Control | Troglitazone | Control | Troglitazone | Control | Troglitazone | |
| Flowmeter-determined blood flow, mL/min |
||||||
| LAD flow | 20±2 | 22±2 | 10±1* | 11±1* | 23±3 | 25±2 |
| Microsphere-determined blood flow, mL · g−1 · min−1 |
||||||
| Anterior LV subepicardium | 1.04±0.18 | 1.01±0.18 | 0.54±0.11* | 0.48±0.08* | 0.99±0.11 | 0.94±0.07 |
| Anterior LV midmyocardium | 1.03±0.19 | 1.17±0.24 | 0.34±0.15* | 0.40±0.07* | 0.85±0.15 | 0.99±0.16 |
| Anterior LV subendocardium | 1.27±0.23 | 1.11±0.22 | 0.32±0.15* | 0.25±0.04* | 0.97±0.20 | 0.80±0.12 |
| Posterior LV (transmural mean) | 1.18±0.24 | 1.02±0.27 | 1.10±0.08 | 0.90±0.22 | 0.96±0.18 | 0.95±0.23 |
All values are mean±SEM; n=8 (control), n=6 (troglitazone). There were no significant differences between groups.
P<0.05 vs baseline within same group.
LV Systolic Function
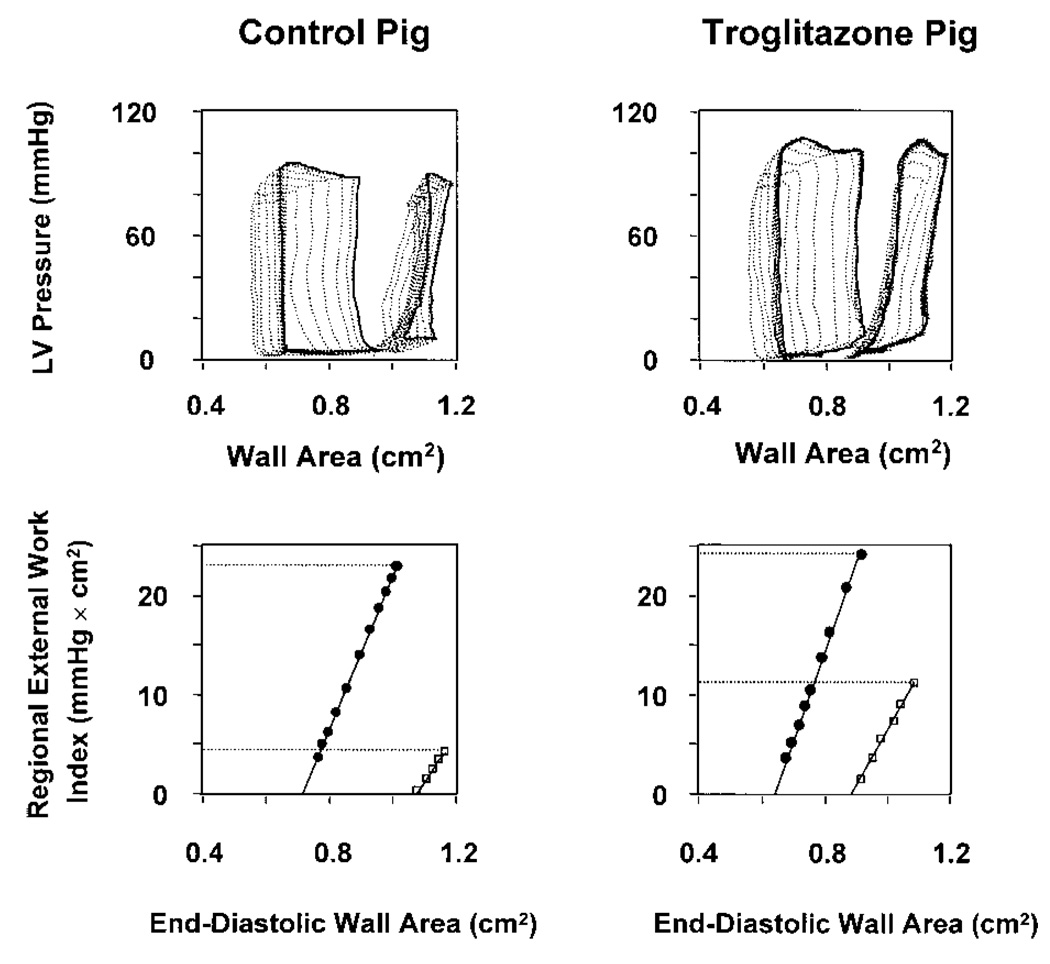
At baseline, there were no significant differences in hemodynamics (Table 4) or regional LV function (Table 5) between groups. During ischemia, regional systolic function (external work and FAR) of the anterior ischemic region diminished in both groups, but FAR remained greater in troglitazone-treated pigs. After reperfusion, troglitazone-treated pigs demonstrated greater recovery of both FAR and external work (Figure 2 and Table 5). For example, the latter variable recovered to 0.44±0.06 times baseline in troglitazone-treated pigs but to only 0.18±0.06 times baseline in control pigs (P<0.05). PRSW slope, an index of contractility, recovered to a significantly greater extent in treated pigs. Treatment effect could not be attributed to a difference in loading conditions, because neither end-diastolic wall area (regional preload) nor LV systolic pressure differed between groups under any condition. Regional function of the posterior (nonischemic) region did not differ between groups under any condition, indicating that the effect of troglitazone was limited to the ischemic region. Thus, troglitazone treatment caused a load-independent improvement in regional systolic function during ischemia and after reperfusion. This improvement was paralleled by better preservation of global LV systolic function, manifest by greater LV +dP/dtmax in treated pigs (P<0.01).
TABLE 4.
Hemodynamic Data
| Baseline | Ischemia | Reperfusion | ||||
|---|---|---|---|---|---|---|
| Control | Troglitazone | Control | Troglitazone | Control | Troglitazone | |
| Heart rate, bpm | 111±2 | 117±3 | 110±1 | 115±2 | 111±1 | 115±3 |
| Mean aortic pressure, mm Hg | 95±3 | 93±6 | 85±5 | 88±5 | 84±5 | 86±7 |
| LV systolic pressure, mm Hg | 104±4 | 108±3 | 94±5 | 100±2 | 90±4 | 97±3† |
| LV end-diastolic pressure, mm Hg | 8±1 | 8±1 | 14±2† | 11±1 | 14±2† | 11±1 |
| LA mean pressure, mm Hg | 4±1 | 3±2 | 7±2 | 4±3 | 9±2 | 5±3 |
| LV +dP/dtmax, fraction of baseline | 1 | 1 | 0.75±0.03† | 0.91±0.04* | 0.62±0.02† | 0.78±0.03*† |
| Absolute value, mm Hg · sec−1 | 1792±124 | 1924±193 | ||||
| LV −dP/dtmax, fraction of baseline | 1 | 1 | 0.68±0.04† | 0.84±0.02*† | 0.61±0.03† | 0.81±0.02*† |
| Absolute value, mm Hg · sec−1 | −2534±335 | −2487±176 | ||||
All values are mean±SEM; n= (control), n= (troglitazone).
P<0.01 vs control group under same condition.
P<0.05 vs baseline within same group.
TABLE 5.
Regional LV Function
| Baseline | Ischemia | Reperfusion | ||||
|---|---|---|---|---|---|---|
| Control | Troglitazone | Control | Troglitazone | Control | Troglitazone | |
| Anterior LV | ||||||
| Preload | ||||||
| Steady-state end-diastolic crystal area, fraction of baseline |
1 | 1 | 1.17±0.03‡ | 1.22±0.16 | 1.09±0.05 | 1.11±0.06 |
| Systolic function | ||||||
| Steady-state external work, fraction of baseline |
1 | 1 | 0.21±0.05‡ | 0.33±0.02‡ | 0.18±0.06‡ | 0.44±0.06*‡ |
| Fractional systolic crystal area reduction |
0.28±0.02 | 0.26±0.03 | −0.02±0.02‡ | 0.11±0.01†‡ | 0.01±0.02‡ | 0.09±0.02†‡ |
| Diastolic function | ||||||
| dA/dtmax, fraction of baseline | 1 | 1 | 0.62±0.04‡ | 0.87±0.07† | 0.52±0.07‡ | 0.78±0.07*‡ |
| Posterior LV | ||||||
| Preload | ||||||
| Steady-state end-diastolic crystal area, fraction of baseline |
1 | 1 | 1.04±0.02 | 1.06±0.02‡ | 1.02±0.02 | 1.04±0.02 |
| Systolic function | ||||||
| Steady-state external work, fraction of baseline |
1 | 1 | 0.93±0.04 | 0.93±0.04 | 0.83±0.02‡ | 0.92±0.08 |
| Fractional systolic crystal area reduction |
0.27±0.03 | 0.29±0.03 | 0.32±0.03‡ | 0.30±0.02 | 0.29±0.06 | 0.28±0.02 |
| Diastolic function | ||||||
| dA/dtmax, fraction of baseline | 1 | 1 | 0.90±0.09 | 0.99±0.02 | 0.90±0.10 | 0.97±0.06 |
All values are mean±SEM; n=8 (control), n=6 (troglitazone).
P<0.05,
P<0.01 vs control group under same condition;
P<0.05 vs baseline within same group.
Figure 2.
Assessment of regional systolic function. Top, LV pressure versus regional wall-area loops under baseline conditions (larger loops) and after 90 minutes’ ischemia and 90 minutes’ reperfusion (smaller loops). Data from pig in control group are shown on left and from troglitazone-treated pig on right. Bold loops were recorded under steady-state conditions, whereas lighter loops were recorded during brief occlusion of the venae cavae. Area of each loop is an index of regional external work. In both pigs, contractile dysfunction after reperfusion is manifest by reduced loop area compared with baseline. However, recovery of steady-state loop area is greater in troglitazone-treated pig. Bottom, Regional PRSW relations derived from data in top panels by plotting area of each loop against its corresponding end-diastolic wall area (regional preload). Relations under baseline conditions (●) and after 90 minutes’ ischemia and 90 minutes’ reperfusion (□) are shown. Uppermost point of each PRSW relation reflects steady-state condition, corresponding to bold loops in top panels. After reperfusion, there is less rightward shift of PRSW relation in troglitazone-treated pig, indicating greater recovery of regional external work.
LV Diastolic Function
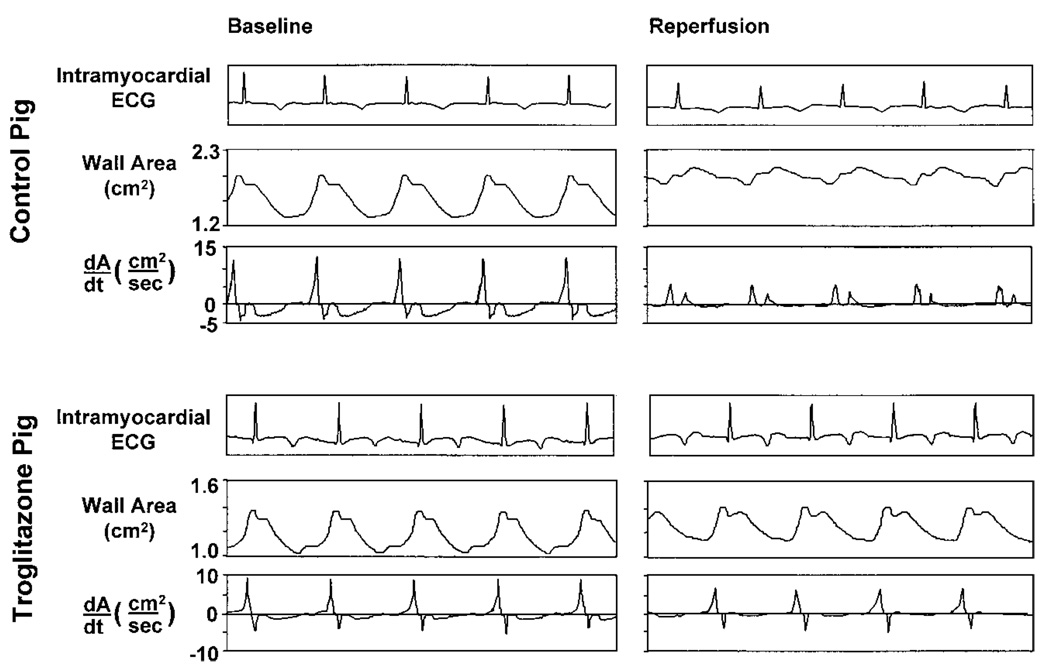
Troglitazone treatment also preserved regional diastolic function during ischemia and reperfusion (Figure 3, Table 4 and Table 5). After reperfusion, the maximum rate of diastolic wall area expansion (+dA/dtmax) recovered to 78±7% of baseline in treated pigs compared with 52±7% in untreated pigs (P<0.05). Recovery of global LV relaxation (LV −dP/dtmax) was also improved in troglitazone-treated pigs (P<0.01). Treatment effect could not be attributed to a difference in left atrial pressure between groups, nor was there evidence for a direct lusitropic effect of troglitazone, because LV −dP/dtmax did not differ between groups at baseline.
Figure 3.
Assessment of regional diastolic function. Top, Recordings from ischemic zone of pig from control group. Bottom, Recordings from ischemic zone of pig from troglitazone group. For both pigs, recordings of regional wall area and first time derivative of wall area (dA/dt) are shown under baseline conditions (left) and after 90 minutes’ low-flow ischemia and 90 minutes’ reperfusion (right). In top right panel, postischemic diastolic dysfunction in control pig is manifest by reduced +dA/dtmax. Early systolic wall area expansion is also evident. In lower right panel, troglitazone-treated pig demonstrates relatively greater recovery of +dA/dtmax and less early systolic wall-area expansion. Absolute values of wall area and dA/dt depend on distances between sonomicrometry crystals at implantation, which vary between experiments.
Myocardial Oxygen Consumption and Substrate Uptake
Myocardial oxygen consumption did not differ between groups under any condition. Circulating insulin levels were lower in the troglitazone group under each condition (P<0.05), but arterial blood glucose and myocardial glucose uptake did not differ between groups under any condition (Table 6). Thus, the beneficial effects of troglitazone treatment on LV function were not due to increased myocardial glucose uptake.
TABLE 6.
Circulating Insulin and Substrate Concentrations; Myocardial Substrate Uptake and Oxygen Consumption
| Baseline | Ischemia | Reperfusion | ||||
|---|---|---|---|---|---|---|
| Control | Troglitazone | Control | Troglitazone | Control | Troglitazone | |
| Insulin, µU/mL plasma | 6±1 | 2±1* | 5±1 | 2±1* | 5±1 | 2±1* |
| Arterial glucose, µmol/mL blood | 3.8±0.4 | 3.7±0.5 | 3.9±0.2 | 4.2±0.4 | 3.7±0.2 | 3.7±0.3 |
| Anterior LV glucose uptake, µmol · g−1 · min−1 |
0.04±0.05 | 0.11±0.03 | 0.29±0.08 | 0.25±0.07 | 0.23±0.08 | 0.21±0.02 |
| Arterial lactate, µmol/mL blood | 1.6±0.3 | 1.6±0.1 | 1.1±0.1‡ | 1.6±0.1* | 0.9±0.1‡ | 1.3±0.2* |
| Anterior LV lactate uptake, µmol · g−1 · min−1 |
0.79±0.21 | 0.96±0.16 | −0.22±0.07‡ | −0.14±0.04‡ | 0.06±0.07‡ | 0.40±0.03†‡ |
| Arterial FFA, µmol/mL blood | 0.27±0.06 | 0.14±0.01 | 0.20±0.03 | 0.13±0.02 | 0.19±0.03 | 0.09±0.01*‡ |
| Anterior LV FFA uptake, µmol · g−1 · min−1 |
0.058±0.054 | 0.035±0.024 | 0.009±0.005 | 0.004±0.002 | 0.025±0.013 | 0.012±0.007 |
| Anterior LV MVO2, µmol · g−1 · min−1 |
5.2±0.7 | 4.1±0.8 | 1.8±0.4‡ | 1.6±0.2‡ | 2.7±0.4‡ | 2.5±0.3‡ |
All values are mean±SEM; n=8 (control), n=6 (troglitazone).
P<0.05
P<0.01 vs control group under same condition;
P<0.05 vs baseline within same group.
Myocardial lactate uptake did not differ between groups under baseline conditions. During ischemia, both groups demonstrated a shift from net lactate uptake to net lactate release, consistent with an increase in anaerobic glycolytic metabolism. However, during reperfusion, myocardial lactate uptake was substantially greater in troglitazone-treated pigs than in untreated control pigs (0.40 versus 0.06 µmol · g−1 · min−1; P<0.01).
Arterial FFA concentrations were lower in the troglitazone group than in the control group, and this difference achieved statistical significance during reperfusion. These differences were paralleled by a trend to lower myocardial FFA uptake in the troglitazone group under all 3 experimental conditions.
Discussion
New Findings of This Study
There are 3 key findings of this study. First, chronic troglitazone treatment improved recovery of systolic and diastolic LV function after ischemia and reperfusion in nondiabetic pigs. Second, improved recovery of LV function was not due to increased myocardial glucose uptake but was associated with greater myocardial lactate uptake during reperfusion. Third, troglitazone treatment reduced fasting blood insulin and FFA levels without affecting blood glucose, suggesting that treatment enhanced systemic insulin sensitivity.
Although a positive inotropic effect of troglitazone has been observed in isolated rat hearts11 and increased cardiac output has been observed in diabetic patients treated with the drug,12 a direct of effect of troglitazone on LV function is unlikely in the present experiments because there were no differences between groups in preischemic values of any hemodynamic variable. Furthermore, there were no differences in myocardial blood flow or oxygen consumption between groups under any condition.
Shimabukuro et al13 observed that chronic troglitazone treatment improved recovery of LV function after ischemia in isolated hearts of diabetic rats; however, no benefit of treatment was observed in hearts of nondiabetic rats. The present study is the first to examine effects of a thiazolidinedione compound on recovery of LV function after ischemia in vivo and the first to demonstrate a salutary effect of treatment in nondiabetic animals.
Effects of Troglitazone on Myocardial Substrate Metabolism
Bähr et al14 showed that when cultured cardiomyocytes from normal rats were exposed to troglitazone in vitro, content of glucose transporter proteins and uptake of glucose increased. These findings suggest that troglitazone may have the potential to affect myocardial glucose metabolism in nondiabetic animals. However, we found that the protective effect of troglitazone in vivo was not associated with increased myocardial glucose uptake.
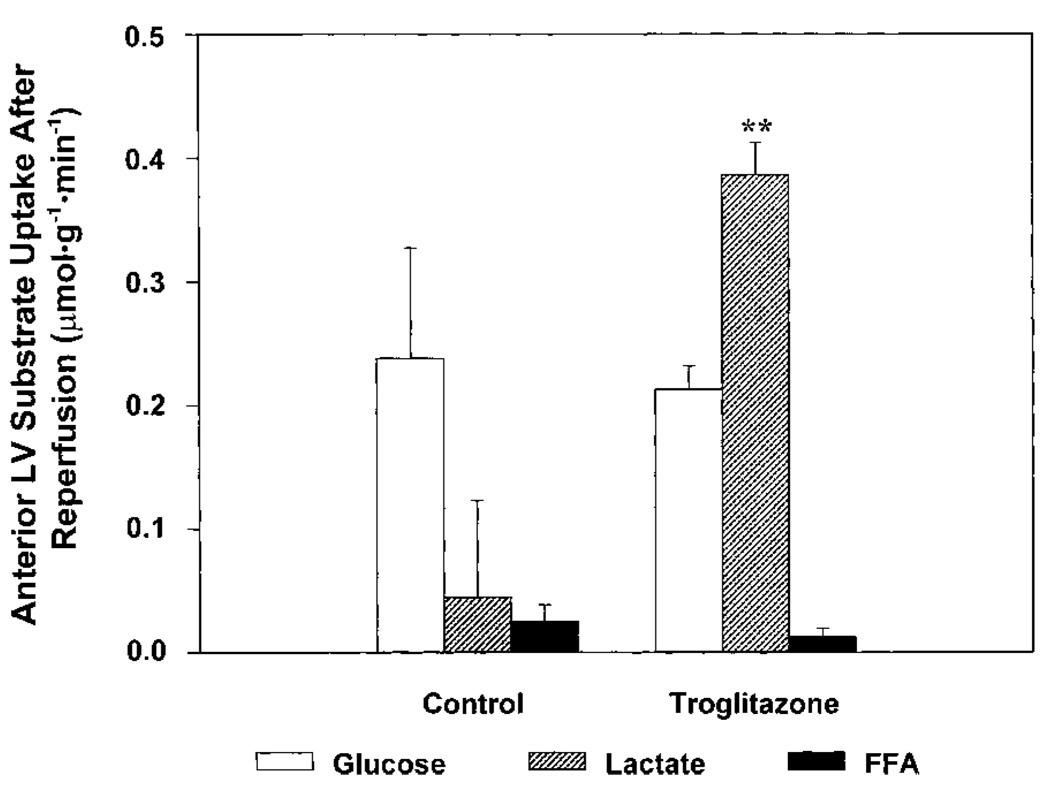
In the present study, the most striking effect of troglitazone on myocardial substrate metabolism was an increase in net lactate uptake after reperfusion (Figure 4), to a level 7 times greater than that in untreated pigs, despite similar glucose uptake in both groups. The difference in lactate uptake cannot be explained by the modest difference in arterial lactate concentration between groups. Rather, this observation suggests that troglitazone treatment increased carbohydrate (glucose and lactate) oxidation in reperfused myocardium. The basis for this explanation is as follows: Net myocardial lactate uptake is the sum of uptake of exogenous lactate and release of intracellular lactate formed by anaerobic glycolysis. These processes occur simultaneously.15 In untreated control pigs after reperfusion, net myocardial lactate uptake was depressed, whereas glucose uptake remained robust. This pattern of substrate uptake is consistent with depressed myocardial carbohydrate oxidation, as previously described in reperfused myocardium.16 Under such conditions, an increased fraction of myocardial glucose undergoes anaerobic metabolism to lactate, resulting in increased lactate release and decreased net myocardial lactate uptake. In contrast, the pattern of substrate uptake observed after reperfusion in troglitazone-treated pigs is consistent with a stimulatory effect of treatment on myocardial carbohydrate oxidation, resulting in decreased lactate release from anaerobic glycolysis, increased oxidation of exogenous lactate, and increased net myocardial lactate uptake. Increased oxidation of carbohydrate substrates may have been offset by decreased oxidation of FFA, resulting in no net difference in myocardial oxygen consumption between groups. The mechanism by which troglitazone may have stimulated myocardial carbohydrate oxidation is unclear. One possibility is that lower myocardial FFA uptake during ischemia and reperfusion treatment allowed greater activation of pyruvate dehydrogenase complex in troglitazone-treated pigs.17 An increase in myocardial carbohydrate oxidation after reperfusion may be a mechanism for improved recovery of contractile function, because other agents that activate pyruvate dehydrogenase, such as dichloroacetate, also improve postischemic contractile function.2,3
Figure 4.
Myocardial substrate uptake after reperfusion. Data are mean±SEM, n=8 (control group) and n=6 (troglitazone group). Uptake of glucose and FFA did not differ significantly between groups, but there was a marked difference in lactate uptake. In control pigs, net lactate uptake was very low, and glucose was the predominant exogenous substrate. In contrast, lactate was the predominant exogenous substrate in troglitazone-treated pigs. These findings suggest greater carbohydrate oxidation in troglitazone-treated pigs, as discussed in text. **P<0.01 vs control group under same condition.
Alternatively, the benefit of troglitazone treatment may have been unrelated to effects on myocardial substrate metabolism. In particular, thiazolidinediones may exert antioxidant effects18 and calcium channel antagonism,19 both actions that may be protective during myocardial ischemia and reperfusion.
Effect of Chronic Troglitazone Treatment on Systemic Insulin Sensitivity
Although troglitazone and other thiazolidinedione drugs enhance insulin sensitivity in diabetic animals and patients,4,5 effects on insulin sensitivity in nondiabetic animals have been less clear.6,7 To the best of our knowledge, the current findings of normal fasting blood glucose in the face of diminished insulin levels and reduced circulating FFA constitute the first evidence that troglitazone may enhance insulin sensitivity in a large animal without preexisting insulin resistance.
We chose an 8-week duration of treatment because this is the approximate length of time required to observe maximal insulin-sensitizing and FFA-lowering effects of troglitazone in diabetic patients.20,21 We chose a troglitazone dose of 75 mg · kg−1 · d−1 because this lies within the range of doses that have been studied in intact animals without adverse effects,7 but it is within 1 order of magnitude of clinical doses.
Significance
Pharmacological agents that alter myocardial energy substrate metabolism have the potential to mitigate the adverse effects of ischemia and reperfusion on myocardial function. To date, however, such strategies have had limited clinical application, in part owing to lack of suitable agents for chronic, oral administration. Troglitazone is a drug that is already in widespread clinical use for treatment of diabetes. The present data indicate that it can also attenuate the severity of postischemic dysfunction of the nondiabetic heart in vivo. Therefore, troglitazone may be a potential new tool in the management of ischemic heart disease in both nondiabetic and diabetic patients.
Acknowledgments
This study was supported by National Institutes of Health grant HL-49944 (Dr Schwartz), a postdoctoral research fellowship of the American Heart Association, California Affiliate (Dr Lu), and the Medical Research Service of the Department of Veterans Affairs. The authors thank Jon Ebright and Erick Kindt of Parke-Davis Pharmaceutical Research for analysis of plasma troglitazone concentrations.
Footnotes
Reprints: Information about reprints can be found online at http://www.lww.com/reprints
References
- 1.Eberli FR, Weinberg EO, Grice WN, Horowitz GL, Apstein CS. Protective effect of increased glycolytic substrate against systolic and diastolic dysfunction and increased coronary resistance prolonged global underperfusion and reperfusion in isolated rabbit hearts perfused with erythrocyte suspensions. Circ Res. 1991;68:466–481. doi: 10.1161/01.res.68.2.466. [DOI] [PubMed] [Google Scholar]
- 2.Okuda K, Nohara R, Fujita M, Tamaki N, Konishi J, Sasayama S. Improvement in myocardial ischemic dysfunction with dichloroacetic acid: experimental study by repeated ischemia in dogs. J Cardiovasc Pharmacol. 1995;26:990–999. doi: 10.1097/00005344-199512000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Lewandowski ED, White LT. Pyruvate dehydrogenase influences postischemic heart function. Circulation. 1995;91:2071–2079. doi: 10.1161/01.cir.91.7.2071. [DOI] [PubMed] [Google Scholar]
- 4.Sparano N, Seaton TL. Troglitazone in type II diabetes mellitus. Pharmacotherapy. 1998;18:539–548. [PubMed] [Google Scholar]
- 5.Komers R, Vrana A. Thiazolidinediones: tools for the research of metabolic syndrome X. Physiol Res. 1998;47:215–225. [PubMed] [Google Scholar]
- 6.Lee MK, Olefsky JM. Acute effects of troglitazone on in vivo insulin action in normal rats. Metab Clin Exp. 1995;44:1166–1169. doi: 10.1016/0026-0495(95)90010-1. [DOI] [PubMed] [Google Scholar]
- 7.Yagi N, Takasu N, Higa S, Ishikawa K, Murakami K, Mimura G. Effect of troglitazone, a new oral antidiabetic agent, on fructose-induced insulin resistance. Horm Metab Res. 1995;27:439–441. doi: 10.1055/s-2007-979997. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz GG, Xu Y, Greyson C, Cohen J, Lu L. Low-dose inotropic stimulation during left ventricular ischaemia does not worsen post-ischaemic dysfunction. Cardiovasc Res. 1996;32:1024–1037. doi: 10.1016/s0008-6363(96)00150-2. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GG, Greyson CR, Wisneski JA, Garcia J, Steinman S. Relation among regional O2 consumption, high-energy phosphates, and substrate uptake in porcine right ventricle. Am J Physiol. 1994;266:H521–H530. doi: 10.1152/ajpheart.1994.266.2.H521. [DOI] [PubMed] [Google Scholar]
- 10.Lu L, Xu Y, Greyson CR, Ursell PC, Schwartz GG. Non-elastic deformation of myocardium in low-flow ischemia and reperfusion: ultrastructure-function relations. J Mol Cell Cardiol. 1999;31:1157–1169. doi: 10.1006/jmcc.1999.0948. [DOI] [PubMed] [Google Scholar]
- 11.Shimoyama M, Ogino K, Tanaka Y, Ikeda T, Hisatome I. Hemodynamic basis for the acute cardiac effects of troglitazone in isolated perfused rat hearts. Diabetes. 1999;48:609–615. doi: 10.2337/diabetes.48.3.609. [DOI] [PubMed] [Google Scholar]
- 12.the Troglitazone Study Group. Ghazzi MN, Perez JE, Antonucci TK, Driscoll JH, Saling MH, Faja BW, Whitcomb RW. Cardiac and glycemic benefits of troglitazone treatment in NIDDM. Diabetes. 1997;46:433–439. doi: 10.2337/diab.46.3.433. [DOI] [PubMed] [Google Scholar]
- 13.Shimabukuro M, Higa S, Shinzato T, Nagamine F, Komiya I, Takasu N. Cardioprotective effects of troglitazone in streptozotocin-induced diabetic rats. Metabolism. 1996;45:1168–1173. doi: 10.1016/s0026-0495(96)90018-9. [DOI] [PubMed] [Google Scholar]
- 14.Bähr M, Spelleken M, Bock M, von Holtey M, Kiehn R, Eckel J. Acute and chronic effects of troglitazone (CS-045) on isolated rat ventricular cardiocytes. Diabetologia. 1996;39:766–774. doi: 10.1007/s001250050509. [DOI] [PubMed] [Google Scholar]
- 15.Wisneski JA, Gertz EW, Neese RA, Gruenke LD, Morris LD, Craig JC. Metabolic fate of extracted glucose in normal human myocardium. J Clin Invest. 1985;76:1819–1827. doi: 10.1172/JCI112174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopaschuk GD, Stanley WC. Glucose metabolism in the ischemic heart. Circulation. 1997;95:313–315. doi: 10.1161/01.cir.95.2.313. [DOI] [PubMed] [Google Scholar]
- 17.Randle PJ. Regulation of glycolysis and pyruvate oxidation in cardiac muscle. Circ Res. 1976;38(suppl I):I8–I15. [PubMed] [Google Scholar]
- 18.Inoue I, Katayama S, Takahashi K, Negishi K, Miyazaki T, Sonoda M, Komoda T. Troglitazone has a scavenging effect on reactive oxygen species. Biochem Biophys Res Commun. 1997;235:113–116. doi: 10.1006/bbrc.1997.6512. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura Y, Ohya Y, Onaka U, Fujii K, Abe I, Fujishima M. Inhibitory action of insulin-sensitizing agents on calcium channels in smooth muscle cells from resistance arteries of guinea-pig. Br J Pharmacol. 1998;123:675–682. doi: 10.1038/sj.bjp.0701669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Troglitazone and Exogenous Insulin Study Group. Schwartz S, Raskin P, Fonseca V, Graveline JF. Effect of troglitazone in insulin-treated patients with type II diabetes mellitus. N Engl J Med. 1998;338:861–866. doi: 10.1056/NEJM199803263381302. [DOI] [PubMed] [Google Scholar]
- 21.Inzucchi SE, Maggs DG, Spollett GR, Page SL, Rife FS, Walton V, Shulman GI. Efficacy and metabolic effects of metformin and troglitazone in type II diabetes mellitus. N Engl J Med. 1998;338:867–872. doi: 10.1056/NEJM199803263381303. [DOI] [PubMed] [Google Scholar]