Abstract
Epidemiologic studies increasingly rely on improved exposure assessments to characterize pesticide exposures in agricultural populations. A subset of private pesticide applicators in the AHS epidemiological cohort was monitored around the time of their agricultural use of 2,4-D and chlorpyrifos to assess exposure levels and potential exposure factors. Measurements included pre- and post-application urine samples, and patch, hand wipe, and personal air samples. Broadcast or hand spray application methods were used by applicators for 2,4-D products. Chlorpyrifos products were applied using spray applications and in-furrow application of granular products. Geometric mean (GM) values for 69 2,4-D applicators were 7.8 and 25 µg/L in pre- and post-application urine, respectively (p < 0.05 for difference); 0.39 mg for estimated hand loading; 2.9 mg for estimated body loading; and 0.37 µg/m3 for concentration in personal air. Significant correlations were found between all media for 2,4-D. GM values for 17 chlorpyrifos applicators were 11 µg/L in both pre- and post-application urine for the 3,5,6-trichloro-2-pyridinol metabolite, 0.28 mg for body loading, and 0.49 µg/m3 for air concentration. Only 53% of the chlorpyrifos applicators had measureable hand loading results; their median hand loading was 0.02 mg. Factors associated with differences in 2,4-D measurements included application method and glove use; and, for hand spray applicators, use of adjuvants, equipment repair, duration of use, and contact with treated vegetation. Spray applications of liquid chlorpyrifos products were associated with higher measurements than in-furrow granular product applications. This study provides information on exposures and possible exposure determinants for several application methods commonly used by farmers in the cohort and will provide information to assess and refine exposure classification in the Agricultural Health Study. Results may also be of use in pesticide safety education for reducing exposures to applied pesticides.
Keywords: 2, 4-D; chlorpyrifos; exposure measurement; pesticide exposure; agricultural exposure; farm applicator; occupational exposure; Agricultural Health Study
INTRODUCTION
Despite low mortality and cancer incidence rates overall, farmers may experience excess risk of specific cancers and other adverse health outcomes (Alavanja et al., 2004; Zahm et al., 1997; Blair and Zahm, 1995; Fleming et al., 1999; Cordes and Rea, 1991). Farmers may be exposed to pesticides as well as a variety of other potentially hazardous substances including solvents, fuels, oils, vehicle exhaust, dust, mycotoxins, and agriculture-specific microbes. Epidemiological studies of agricultural pesticide applicators and agricultural workers have often been limited by inadequate or retrospective exposure information, leading to potential exposure misclassification (Dich et al., 1997; Sathiakumar and Delzell, 1997; Ritter et al., 2006). Exposure to pesticides used in agriculture may depend on many factors, including the amount and duration of chemical use, pesticide formulations, the physical and chemical properties of active ingredients, mixing/loading and application methods, and use of personal protective equipment. While recognized as important, few epidemiological studies have been able to incorporate these factors into their exposure estimates due to the complexities involved in obtaining complete and reliable information over time as well as in estimating their impact. Thus, epidemiological analyses often assume that exposure intensities resulting from agricultural uses are the same for all pesticides and all applicators.
Obtaining measurement information to directly determine exposures for all individuals would be ideal but is costly and not feasible for large longitudinal cohorts. Thus, studies often rely on questionnaires to provide information on pesticide use as a surrogate for exposure. Relatively few studies of farmers have included direct measurements to assess questionnaire-based exposure classification systems used in epidemiological investigations (Arbuckle et al. 2002; Baldi et al., 2006). Measurement data are needed to improve the understanding of relationships between patterns of chemical use and exposures and to evaluate questionnaire-based surrogates of exposure. To address this need in the Agricultural Health Study (AHS), a longitudinal cohort study of licensed pesticide applicators and their spouses, the Agricultural Health Study/Pesticide Exposure Study (AHS/PES) was designed to measure exposures from the agricultural use of two pesticides, 2,4-D and chlorpyrifos, with a high prevalence of use among farmers in the AHS cohort. AHS/PES objectives included measurement of farm applicator exposure via multiple routes to the selected pesticides, assessment of factors potentially associated with exposures, and evaluation of an algorithm that uses questionnaire information to estimate relative exposure intensities. In this paper we describe the study design; recruitment and monitoring outcomes; results for urinary biomarker, dermal, and air measurements; and assessment of some factors potentially related to exposures.
METHODS
AHS Study Background
The AHS is a prospective epidemiological study to evaluate the cancer and non-cancer risks in the agricultural community and to study relationships between agricultural exposures and disease. The cohort includes 52,395 licensed private pesticide applicators, 4,916 commercial applicators, and 32,347spouses in the states of North Carolina (NC) and Iowa (IA) in the USA. Participants enrolled in the study from 1993–1997 at pesticide certification classes and provided pesticide use information through self-administered questionnaires (Alavanja et al., 1996). Questionnaires were used at enrollment to collect information on the duration (years) and frequency (days per year) of use for 50 herbicides, insecticides, fungicides, and fumigants (Alavanja et al., 1996). Information was collected to characterize personal mixing/loading, application methods, use of protective equipment, equipment repair, and personal hygiene. Additional and updated information was obtained through computer assisted telephone interviews (CATI) conducted from 1999–2003. Questionnaire information is used in the AHS to stratify individual applicators with regard to their total lifetime days of use and intensity-adjusted lifetime days of use for specific pesticides using a previously published intensity algorithm (Dosemeci et al., 2002). Relative weighting levels for algorithm factors were based on literature reviews and information from the Pesticide Handlers Exposure Database (PHED, 1995) regarding relative exposure levels for different handling and use factors.
Selection of AHS/PES Target Chemicals
Two pesticides, 2,4-D (2,4-dichlorophenoxyacetic acid) and chlorpyrifos (O,O-diethyl-O-3,5,6-trichloro-2-pyridyl phosphorothioate), were selected for monitoring based on widespread use in the cohort and available analytical methods. A wide range of application methods and practices have been reported by the full study cohort; eight categories common for crop and non-crop applications are shown in Table 1. Pharmacokinetic data were also available to guide the timing of biomarker sample collections. The urinary elimination half-life of 2,4-D ranged from 10 – 28 h after oral dose (Sauerhoff et. al., 1977) and 18 – 68 h after dermal dose for 2,4-D and 18 – 87 h for 2,4-D dimethyl amine (Solomon and Harris, 1992). The reported half-life for 3,5,6-trichloro-2-pyridinol (TCP) in blood after oral dose ranged from 21- 34 h (Nolan et. al., 1984).
Table 1.
Common Product Application Scenarios and Reported Frequencies for Applicators in the AHS at Enrollment 1993–1997
| Application Scenario | Percenta,b |
|---|---|
| (A) Broadcast spray application, enclosed cab, rubber gloves | 26.6 |
| (B) Broadcast spray application, no enclosed cab, rubber gloves | 18.4 |
| (C) Broadcast spray application, enclosed cab, no rubber gloves | 4.1 |
| (D) Broadcast spray application, no enclosed cab, no rubber gloves | 10.0 |
| (E) Hand spray application, rubber gloves | 40.2 |
| (F) Hand spray application, no rubber gloves | 15.7 |
| (G) In-furrow/banded application, enclosed cab | 22.8 |
| (H) In-furrow/banded application, no enclosed cab | 17.0 |
Total exceeds 100% because applicators often reported multiple application methods.
Based on 19,658 – 20,967 respondents (depending on completeness of information for specific factors) reporting any pesticide use and providing information on the AHS take-home (Q1) questionnaire.
AHS/PES Participant Selection and Recruitment
Private pesticide applicators enrolled in the AHS cohort and who had completed the AHS CATI prior to the selection for each AHS/PES sampling year formed the sampling frame for this study. Applicators reporting recent use of 2,4-D or chlorpyrifos in one or more of the eight application scenarios (Table 1), and who resided in selected counties in IA or NC were selected for a telephone screening. Counties were chosen based on CATI-reported rates of target chemical use and driving proximity to field contractor facilities in Iowa City, IA and Research Triangle Park, NC. In IA, 22 central, eastern, and southeastern counties were included over three years (2000–2002). In NC, 22 eastern and central counties were included over two years (2001–2002). Prior to screening in 2001 a random sample of IA hand spray 2,4-D applicators (Table 1 category E) was selected for contact due to the large numbers reporting use of that method. Applicators reporting only hand spray 2,4-D applications were not selected for screening in IA in 2002.
All applicators completing the screening contact and reporting that they would or might use a product containing 2,4-D or chlorpyrifos on their farm in the coming season using broadcast, in-furrow/banded, or hand spray application methods were eligible for recruitment. Applicators reporting only orchard, forestry, or residential lawn/garden uses were not eligible. Field study representatives met with eligible applicators. Those consenting to participate were asked to contact the field study staff prior to a planned application of one of the target chemicals. Applicators were asked to participate in another monitoring visit, either in the same or the following year. Participating applicators received $100, with an additional $50 for those providing four additional 24-h urine samples. This was an observational research study, as defined in 40 CFR Part 26.402. The study was reviewed and approved by the Institutional Review Boards at the National Cancer Institute, the University of Iowa, Battelle Memorial Institute, and the Research Triangle Institute.
Monitoring Design
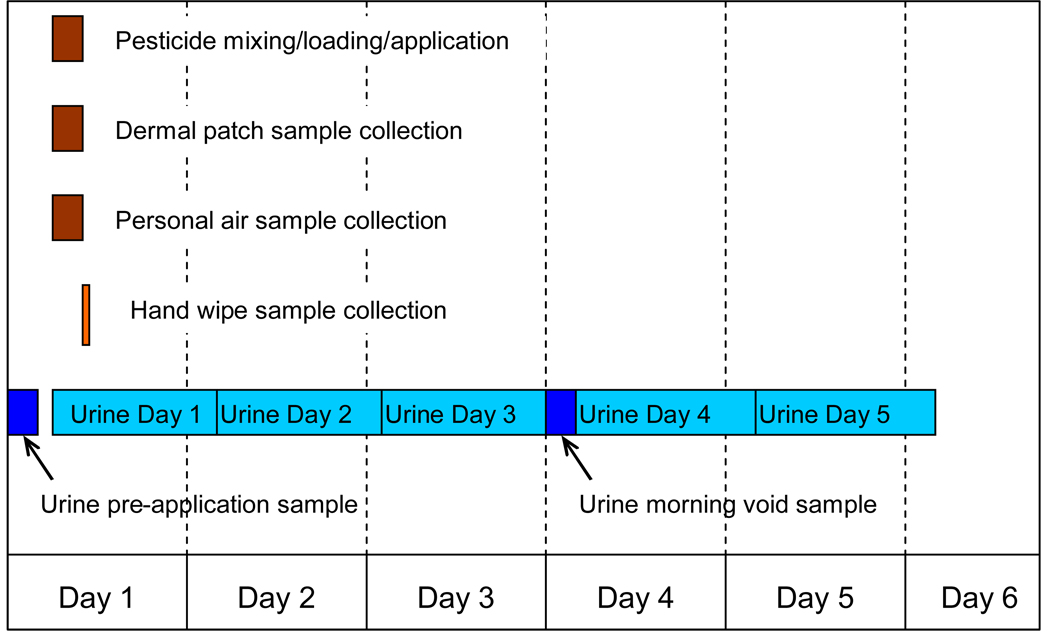
Monitoring was performed around a pesticide mixing, loading, and application (MLA) activity on one day. Applicators followed their usual pesticide handling and application practices. The measurement strategy was based on using methods that could be applied within a working agricultural cohort. Urine samples were collected before, during, and after a monitored pesticide use; and patch, hand wipe, and personal air samples were collected during the monitored activity (Figure 1). Where possible, applicators were monitored during their first use of the target pesticide for the year or during a use separated by several days from other uses. Given the time requirements and high farm activity levels during the planting season this could not always be achieved, so the timing for collecting urine samples was designed to both minimize burden and reduce the potential impact on urinary biomarker measurements from target chemical uses on the days before and after the monitored use. Dermal (patch and hand wipe) and air measurements were made to assess potential routes of exposure.
Figure 1.
Timeline for collection of samples in the AHS Pesticide Exposure Study (Day 2 – 5 urine samples were optional).
Applicators were asked to collect a first morning void urine sample prior to pesticide handling on the day of the monitored activity. Field staff applied the sampling patches and attached a personal air sampler prior to the start of pesticide handling activities. Hand wipe samples were collected and patch and air samples were retrieved at the end of the monitored activity. In several cases two hand wipe, patch, or air samples were collected for an individual monitoring day either due to a break in work activities (for example, between mixing/loading and application) or when they would normally wash. In these cases the analysis results were combined to produce a single measurement. Field staff recorded information about the MLA activity on a structured data collection from. The pesticide use component of the AHS Phase II CATI, modified for single-day use, was interviewer-administered to the applicator upon completing the monitored activity. Field staff made a final visit to the applicator’s home to collect urine samples and to administer a questionnaire to collect additional information about the applicator as well as farm and home activities and conditions.
Sample Collection and Analysis
Urine Samples
Applicators were provided 470-mL polyethylene containers for a collecting a pre-application sample as a single void and a third-day post-application first morning void. Applicators were provided with larger 3-L polyethylene containers for collecting the Day-1 post-application composite sample. For applicators agreeing to collect four additional 24-h urine samples, the sample collections were scheduled to start with the void after the first morning void and through the first morning void on the following day, continuing this pattern for four days. Applicators were provided with sample collection instructions that included how to wash hands, the need to avoid touching the inside of the container and lid, and to place lids inside-up while samples were collected. The applicator was asked to record the collection time and date, and the time of the previous void prior to collecting the sample. Total void volumes were recorded for each sample. Samples were stored under refrigerated conditions in the field. Aliquots were prepared and frozen after transport to the field contractor laboratories. Prior to analysis, samples were hydrolized, solvent extracted, and derivatized. Derivatized extracts were analyzed by gas chromatography/mass spectrometry (GC/MS) to determine 2,4-D or TCP concentrations. The recovery of isotopically labeled 2,4-D and TCP surrogate standards added to each urine sample was used to correct the analyte concentration. A separate aliquot of each urine sample was analyzed for creatinine. Because urinary biomarker concentrations can be affected by variable urine volumes resulting from different fluid intake rates, excretion rates were also calculated for 2,4-D and TCP by multiplying the concentration by the sample volume and then dividing by the duration between the previous void time prior to sample collection through the final collection time.
Hand Wipe Samples
Dermal wipe samples were collected from the pesticide applicator’s hands at a break in work when hands were washed and/or at completion of the MLA activity. Twelve predefined locations (3 × 1 cm) on each hand were thoroughly wiped using polyurethane foam-tipped swabs wetted with isopropanol. Samples were solvent extracted and analyzed by GC/MS for the neutral analytes 2,4-D ethylhexyl ester, 2,4-D butoxyethylester, and chlorpyrifos or by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) for 2,4-D acid and 2,4-D dimethylamine (Gardner et al, 2005). Hand loadings were estimated by multiplying the measured mg/72 cm2 by the total estimated area (cm2) for both hands estimated from hand tracings.
Patch Samples
Patches were applied to 10 locations on the applicator’s chest, back, arms, and legs. Chromatography paper patches were used for liquid formulations while cotton gauze patches were used for dry formulations. The surface area of each patch was proportional to standard surface areas of the body location the patch represented (U.S. EPA 1996). Patches were placed on regular clothing or skin and were placed under any personal protective equipment worn by the applicator. The applicator wore the patches during the MLA activity. Patches were removed and combined for analysis; separate compositing and analysis was performed for patches placed on skin. Samples were solvent extracted and neutral target analytes were measured by GC/MS while the 2,4-D acid or dimethylamine were measured using LC/MS/MS. An estimate of total body loading (except for the hand areas) was made based on the overall combined patch loading value (mg/cm2) multiplied by a total standard body area (20,320 cm2, U.S. EPA 1996).
Air Samples
Air samples were collected in the applicator’s breathing zone for the duration of the MLA activity. An OSHA Versatile Sampler (OVS SKC No. 226-58) containing a quartz filter and XAD-2 resin was clipped to the applicator’s collar and connected to a battery operated pump operated at 1.0 L/min. Flow calibration checks were performed prior to and at the completion of the sampling period. Samples were solvent extracted and analyzed by LC/MS/MS for the applied target pesticide. Analyte concentrations were calculated by dividing the collected mass by the air sampling volume determined from the sampling flow rate and sample collection duration.
Quality Control
Quality control (QC) samples and analyses were applied to assess recovery, background, and precision. Unfortified sampling media (field blanks) and media fortified with the target analytes (field controls) were prepared, transported, stored, and analyzed with samples to assess background and recovery for hand wipe, patch, and air samples. Pooled urine from commercial sources was obtained each year to prepare field blanks and controls and endogenous levels of 2,4-D and TCP were subtracted to calculate recoveries. Aliquots of the spiking solutions used to prepare field controls were also spiked into vials containing solvent for analysis along with each type of sample; their recoveries were used to adjust for differences in field control recoveries resulting from spiking and spiking solution differences. Surrogate standards were added to each sample prior to extraction and analysis. The isotopic analogs 13C6-2,4-D or 13C2-15N-TCP were added as surrogate recovery standards for urine samples. For hand wipe, patch, and air samples 13C6-2,4-D was used as the surrogate standard for acid analyses and parathion-d10 was used for neutrals analyses. Repeated analyses of sample extracts were used to assess analytical precision. Measurement results were not adjusted for field blank background or field control recovery. Urine values were adjusted for surrogate standard recoveries on an individual sample basis.
In order to evaluate completeness of urine sample collection, a separate aliquot of each urine sample was analyzed for creatinine. Estimated 24-h creatinine excretion was calculated for each sample that had complete collection time and volume information. Creatinine excretion rates were evaluated against literature values, and when multiple 24-h samples were available for the individual, excretion rates were also examined for internal consistency. The completeness and accuracy of previous void time, sample collection, and sample volume information was used to determine suitability for calculation of target analyte urinary excretion rates.
Data Analysis
2,4-D Acid Equivalents
Amounts of 2,4-D 2-ethylhexyl ester and 2,4-D butoxyethyl ester measured in hand wipe, patch, and personal air samples were converted to 2,4-D acid equivalents using molecular weight ratio multipliers of 0.664 and 0.688 respectively. When two or more 2,4-D species were present in a sample, the acid equivalents for each analyte were added together. All results are reported as 2,4-D acid equivalents (a.e.).
Amount of Active Ingredient
The amount of active ingredient (a.i.) handled during the MLA was estimated based on the concentration in the product and the amount of product used. These amounts were used to calculate a.i.-adjusted measurement results for hand wipe, patch, and air sample data by dividing the measurement by the kg of a.i. used. For 55% of 2,4-D and 71% of chlorpyrifos monitoring days the applicator used additional product later in the day after the field team departed. Estimates of the total amount of a.i. used for the entire day were based on the amount of a.i. used per acre or per hour during the monitored application and the applicator-reported total acres or time of use for the entire day. These amounts were used to calculate a.i.-adjusted Day 1 post-application urine concentrations.
Method Detection Limits and Replacement of Non-Detects
Method detection limits (MDL) were calculated as analyte mass/sample from the standard deviation of analyte amounts measured on field blanks multiplied by the Student’s t-value at the 0.99 level. If analytes were not detected on any field blanks the MDL was calculated as one-half of the lowest analytical calibration level. This approach was also used for urine samples which had variable endogenous levels of the target compounds in field blanks. If any amount of target analyte lower than the MDL was detected in a sample the reported value was used in data analysis (Clayton et al., 2003). If no target analyte was detected it was replaced with the value of the MDL/. For hand wipe, patch, and air samples with multiple 2,4-D analytes the acid equivalent of the highest MDL value obtained from field blank measurements was used for replacement.
Absorbed Dose Estimation
Estimates of absorbed dose were calculated for the subset of 2,4-D applicators from any visit in which all standard and optional urine samples were obtained and no other reported uses of a product containing the target chemical in the four days preceding or following the monitored use were reported. The total amount of 2,4-D excreted across the five-day post-application period (mean 118 ± 13 h) was determined from measured concentrations and urine volumes. An absorbed dose was estimated by dividing the amount excreted by a factor of 0.90, based on the 0.95 fraction of urinary excretion over 144 h (Sauerhoff et al., 1977) and the fractions of 0.85 for 2,4-D acid and 0.77 for 2,4-D dimethylamine over 96 h (Harris and Solomon et al., 1992). Absorbed dose estimates were not made for chlorpyrifos applicators because only two users met the conditions described above, and in both cases the pre-application urinary TCP concentrations exceeded post-application levels.
Statistical Analysis
Analyses were performed using results from the first visit for applicators that had more than one monitoring visit. Results from another monitoring day were included in two cases in which the same participant used a different chemical (2,4-D vs. chlorpyrifos) and one case in which a different application method (broadcast vs. hand spray) was used. Univariate analyses of natural log (ln)-transformed measurement results were performed for each analyte/sample combination. The hypothesis of a normal distribution of ln-transformed data was not rejected based on the Shapiro-Wilk test (0.05 level) except for chlorpyrifos in hand wipe samples, which had a high percentage of non-detect values. Geometric means and geometric standard deviations were calculated and reported for the exposure measurements, except for chlorpyrifos hand loading estimates. Differences between pre- and post-application urine levels were evaluated using paired one-tailed t-tests. Spearman correlations were calculated to examine associations between measures for each chemical. Measurement results were grouped into eight categories (Table 1) based on the applicator’s use during the monitoring period. Differences in GM values across the categories were assessed using an F-test of the differences in the least-square means of the ln-transformed values. Bivariate analyses were performed for selected chemical handling and application conditions using F-tests for variables with discrete response categories and regression analysis for continuous variables. An assessment of within- and between-applicator variability based on a subset of applicators with repeat visits using the same chemical and application method was performed using regression and covariance parameter estimation. Analyses were performed using SAS V9.1 (SAS Institute, Cary, NC).
RESULTS
Recruitment and Monitoring
Screening, recruitment, and monitoring results are shown in Table 2. A total of 108 monitoring visits (84 in IA, 24 in NC) were completed for 84 different applicators. One NC applicator applied only atrazine and is not included in subsequent analyses. Repeat monitoring was performed 24 times, with twenty-one visits in which the same chemical and methods were used, and three visits in which a different chemical or application method was used. All applicators were male and the average age at monitoring was 52 ± 11 years. A comparison of several characteristics based on information provided upon enrollment in the AHS is shown in Table 3 for those selected for screening and those monitored. Applicators participating in monitoring were younger, had more education, were less likely to be current smokers, and had a slightly higher rate of applying pesticides greater than 40 days per year at enrollment. The groups had similar years of experience applying pesticides.
Table 2.
Screening, Recruitment, and Monitoring Information for the AHS/PES
| IA | NC | ||
|---|---|---|---|
| 2000 – 2002 | 2001 – 2002 | Total | |
| Screening | |||
| Selected for screening | 824 | 619 | 1443 |
| Screening not performed | 48 | 6 | 54a |
| Refusal or no contact | 90 | 75 | 165 |
| Ineligible based on screening | 254 | 269 | 523 |
| Potentially eligible | 432 | 268 | 700 |
| Recruitment | |||
| Selected for recruitment | 432 | 233b | 665 |
| Ineligible for monitoring | 68 | 36 | 104 |
| Total potentially eligible | 364 | 197 | 561 |
| Refusal or no contact | 233 | 86 | 319 |
| Total consented to participate | 131 | 111 | 242 |
| Monitoring | |||
| Applicators monitored first time | 60 | 24c | 84 |
| Repeated monitoring visits | 24 | 0 | 24 |
| Total Monitoring visits | 84 | 24 | 108 |
40 applicators in Iowa 2002 were not screened when field team capacity was reached; eight were deceased; six were not screened for other reasons.
35 North Carolina applicators with early season uses were not recruited in 2001.
Includes one applicator that used only atrazine during monitoring visit; not included in data analyses.
Table 3.
Comparison of Several Demographic, Lifestyle, and Work Characteristics for Participants Selected for Screening and Those Monitored in the AHS/PESa
| Characteristic | Selected for Screening (n = 1443) % |
Monitored (n = 84) % |
|---|---|---|
| Age | ||
| ≤ 30 years | 11.8 | 14.3 |
| 31 – 50 | 50.1 | 56.0 |
| >50 | 38.0 | 29.8 |
| Gender | ||
| Male | 98.8 | 100.0 |
| Female | 1.2 | 0.0 |
| Race | ||
| White | 99.2 | 96.4 |
| Other | 0.1 | 0.0 |
| Missing | 0.8 | 3.6 |
| Education | ||
| High school or lower | 53.7 | 45.3 |
| Greater than high school | 43.8 | 50.0 |
| Missing | 2.5 | 4.8 |
| Smoking status | ||
| Never smoked | 60.4 | 64.3 |
| Past smoker | 27.4 | 27.4 |
| Current smoker | 10.9 | 4.8 |
| Missing | 1.4 | 3.6 |
| Years applying pesticides | ||
| ≤ 5 | 14.0 | 14.3 |
| 6 – 20 | 46.4 | 46.5 |
| > 20 | 34.7 | 33.3 |
| Missing | 4.8 | 6.0 |
| Days per year personally apply pesticides | ||
| ≤ 9 | 40.0 | 36.9 |
| 10 –39 | 49.6 | 48.8 |
| ≥ 40 | 5.5 | 8.4 |
| Missing | 5.0 | 6.0 |
| Number of acres planted previous year | ||
| ≤ 199 | 19.0 | 22.7 |
| 200 – 499 | 34.3 | 29.8 |
| ≥ 500 | 43.4 | 39.3 |
| Missing | 3.3 | 8.3 |
As reported on the AHS enrollment private applicator questionnaire (1993 – 1997).
Product Use
Products containing 2,4-D or chlorpyrifos were used 69 and 17 times, respectively, in the first-visit applications. More than one 2,4-D compound was present in some products used by the applicators, and in several cases different products containing 2,4-D active ingredients were tank-mixed. Surfactants or other adjuvants were added in 24 applications. The participating applicator personally performed mixing/loading operations for all but four monitored applications; in three other cases the mixing/loading activities were performed by the participating applicator prior to the arrival of the field team.
Broadcast spray applications of 2,4-D in IA were for soybean pre-planting burn-down, field corn, hay, and pasture. Broadcast applications of 2,4-D in NC were made for wheat, field corn, forage crops (hay and Bermuda grass), and pasture. All 2,4-D broadcast spray applications were made with tractor-mounted boom sprayers except for one truck-mounted boom sprayer and one highboy application in IA. North Carolina 2,4-D applicators were more likely to use open-cab vehicles for broadcast spray than IA applicators (69% vs. 26%). Hand spray uses of 2,4-D were primarily non-crop applications for weed control on fence-rows and other farm areas and all but one application occurred in IA. Different combinations of tractor and all terrain vehicle (ATV) mounted systems were used for most hand spraying but five portable sprayer uses were also monitored. In one case, the applicator alternated between boom and hand spraying. The different 2,4-D chemicals sprayed, and their proportion of the 69 monitored uses, were 2,4-D acid (8.7%), 2,4-D 2-ethylhexyl ester (62%), 2,4-D butoxyethyl ester (14%), and 2,4-D dimethlyamine salt (28%); the total exceeds 100% because of product and tank mixes. All 11 chlorpyrifos applications in IA involved in-furrow planter box application of a granular product for corn. One NC chlorpyrifos use was an over-the-row cultivator application of a granular product for peanuts (this use was grouped with the 11 in-furrow granular chlorpyrifos applications for data analysis). The remaining five NC chlorpyrifos applications used liquid products for tobacco, sod, and sweet potatoes. One tobacco application was made by air-blast, the sweet potato application was made using a cultivator, and the remaining three applications were by boom spray.
Information on the amounts of active ingredient mixed/loaded, time of pesticide handling and application, and the area treated is reported in Table 4 by target chemical and application method. Although the duration of 2,4-D broadcast spray applications made with enclosed-cab vehicles was about 1.3 times higher than open-cab tractors, the amount of a.i. used was about 2.2 times greater, and areas treated were about 4.1 times higher on average. For 2,4-D hand spray applicators, those applying from tractors used more a.i. on average than those using ATVs, who in turn used more than applicators not spraying from a vehicle.
Table 4.
Product Use Information for the Monitored Mixing/Loading/Application and Estimates for the Full Day
| Monitored Use |
Estimates for the Full Day |
||||||
|---|---|---|---|---|---|---|---|
| Amount a.i.a Applied |
Time of Use |
Area Treated |
Amount a.i. Applied |
Time of Use |
Area Treated |
||
| N | kg | h | acres | kg | h | acres | |
| Mean (standard deviation) with range in italics | |||||||
| Broadcast Application - Liquid Products | |||||||
| 2,4-D | |||||||
| Open Tractor Cab | 18 | 3.0 (3.4) 0.7 – 16 |
1.5 (0.9) 0.6 – 4.1 |
11 (9.9) 2.5 – 46 |
7.3 (7.6) 1.3 –32 |
3.9 (2.6) 1.0 –8.0 |
21 (17) 2.5 – 50 |
| Enclosed Tractor Cabb | 25 | 7.1 (4.9) 1.6 –22 |
2.0 (0.8) 0.9 – 3.6 |
37 (25) 8.0 – 100 |
15.9 (11) 1.6 – 46 |
5.1 (3.2) 1.0 – 12 |
87 (71) 8.0 – 280 |
| Chlorpyrifos | |||||||
| Open Tractor Cab | 3 | 7.5 (2.6) 4.5 – 9.2 |
1.9 (1.2) 0.9 – 3.3 |
8.2 (2.8) 5.0 –10 |
11 (2.6) 9.0 – 14 |
3.5 (0.5) 3.0 – 4.0 |
10 (5.0) 5.0 – 15 |
| Enclosed Tractor Cabc | 2 | 6.1 (4.2) 3.2 – 9.1 |
1.6 (1.6) 0.5 – 2.7 |
8.5 (2.1) 7.0 – 10 |
8.4 (7.4) 3.2 – 14 |
3.1 (3.0) 1.0 –5.2 |
11 (5.7) 7.0 – 15 |
| In-Furrow/Banded Application - Granular Products | |||||||
| Chlorpyrifos | |||||||
| Open Tractor Cab | 7 | 6.4 (3.2) 1.6 – 12 |
3.1 (1.6) 1.4 – 5.4 |
14 (9.8) 3.5 – 34 |
14 (5.5) 6.8 – 20 |
5.3 (1.9) 1.5 –7.0 |
21 (9.7) 4.8 – 34 |
| Enclosed Tractor Cab | 5 | 9.7 (2.8) 6.8 – 13 |
2.1 (0.7) 1.0 – 2.6 |
15 (1.7) 14 – 18 |
19 (8.5) 8.5 – 31 |
5.5 (2.6) 3.0 – 8.5 |
27 (11) 12 – 38 |
| Hand Spray Application – Liquid Products | |||||||
| 2,4-D | |||||||
| From Tractord | 14 | 2.0 (1.6) 0.08 – 4.9 |
1.5 (0.8) 0.4 – 3.2 |
NAe | 3.3 (2.7) 0.1 – 10 |
2.9 (2.2) 1.0 – 8.0 |
NA |
| From ATV | 7 | 0.49 (0.31) 0.09 – 0.9 |
1.0 (0.4) 0.6 – 1.7 |
NA | 0.85 (0.85) 0.09 –2.7 |
2.1 (2.3) 0.2 – 7.0 |
NA |
| No Vehicle | 5 | 0.19 (0.24) 0.01 –0.6 |
1.1 (0.4) 0.6 –1.6 |
NA | 0.27 (0.32) 0.01 –0.7 |
1.3 (0.9) 0.5 –2.5 |
NA |
Amount of active ingredient; for 2,4-D the amount is calculated as acid equivalents.
Includes one high-boy and one truck mounted boom sprayer.
Includes one air blast sprayer.
Includes one use with both hand and broadcast spray.
Not applicable; includes applications for fence rows and other non-crop uses.
Quality Control
Endogenous levels of 2,4-D and TCP in the urine purchased for field blank preparation were 0.6 ± 0.9 µg/L (n = 43) and 4.1 ± 3.4 µg/L (n = 22) respectively. For the four acid and neutral analytes, the mean amounts on field blanks (n = 16 to 28) ranged from 0 to 0.52 ± 2.4 µg/sample (hand wipe), 0.17 ± 0.92 to 0.35 ± 1.3 µg/sample (patch), and 0 – 0.0001 ± 0.0002 µg/sample (air). Mean recoveries of surrogate standards across all applicator urine samples were 73 ± 16% (n = 429) for 2,4-D and 79 ±13% (n = 102) for TCP. Mean surrogate standard recoveries for hand wipe, patch, and air samples ranged from 93 ± 8% to 112 ± 19% (n = 23 to 141). Mean surrogate-corrected recoveries of 2,4-D and TCP from urine field controls were 99 ± 34% (n = 34) and 108 ± 28% (n = 18) respectively. Two 2,4-D field controls with recoveries >400% and two TCP field controls with recoveries >250% prepared at the same time and location were not included. Mean recoveries from field controls across all analytes for hand wipe, patch, and air samples ranged from 103 – 118% (n = 13 to 25); the associated standard deviations ranged from 18 – 24% for hand wipe and patch samples but were 38 –91% for air samples. Repeated extract analysis resulted in 1.1 to 10% mean percent relative standard deviations (RSD) of paired results for most analytes and media. Exceptions were 2,4-D butoxyethyl ester in patch samples (26% RSD) that included one pair with and without a detectable result, and for neutral analytes in air samples (30 – 37% RSDs).
One Day-1 urine sample was excluded from data analysis because the sample volume for the designated 24-h period was 180 mL (less than the single void pre- and post-application samples) and the estimated 24-h creatinine excretion was 250 mg, suggesting incomplete collection. Two optional 24-h samples that had greater than a three-fold difference of creatinine excretion from the average of other 24-h samples provided by the participant were excluded. Results were not used for two samples in which the collection time for the last sample in the series of optional 24-h samples was <5 h. Previous void times and/or final collection times were not recorded by the applicator, or the information was judged to be uncertain, for about 15% of the urine samples and excretion rate values were not used.
Measurements
A summary of pre- and post application urine sample measurement results for the monitored applicators is shown in Table 5. Geometric mean (GM) urinary 2,4-D concentrations were 7.8 and 25 µg/L in the pre-application and the Day1 post-application samples, respectively. Post-application 2,4-D levels were significantly higher than pre-application levels at the Day1 and Day4 morning void time points for unadjusted concentrations, and at several post-application times for estimated excretion rates. Eight 2,4-D applicators had pre-application concentrations that were higher than post-application levels and in four of these cases the applicator reported use of a 2,4-D product within two days preceding the monitoring day. Twenty-five applicators reported additional uses of products containing 2,4-D on the two days following the monitoring day. The mean estimate of absorbed dose calculated for fourteen 2,4-D broadcast and spray applications was 0.0027 ± 0.0044 mg/kg/day (GM = 0.0016; range 0.00032 to 0.018). Biomarker measurements and estimates of absorbed dose reflect exposures from all sources, including the agricultural use plus any exposures occurring through dietary ingestion or contact with surfaces containing 2,4-D residues in the home or around the farm.
Table 5.
Summary Pre- and Post-Application Urine Measurement Results for 2,4-D and Chlorpyrifos Applicators in the AHS/PES
| 2,4-D | Chlorpyrifos metabolite TCPa | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urine Collection Period | N | % Detectb |
% >MDLc |
GM | GSD | Min. | Max. | N | % Detect |
% >MDL |
GM | GSD | Min. | Max. |
| Unadjusted Concentrations (µg/L) | ||||||||||||||
| Pre-application | 68 | 96 | 93 | 7.8 | 4.7 | NDd | 210 | 15 | 100 | 100 | 11 | 2.9 | 2.1 | 63 |
| Day 1 | 68 | 100 | 100 | 25e | 4.1 | 1.6 | 970 | 16 | 100 | 100 | 11 | 2.3 | 2.5 | 80 |
| Day 2 | 28 | 100 | 100 | 26 | 3.7 | 2.2 | 1000 | 8 | 100 | 88 | 13 | 2.5 | 2.8 | 71 |
| Day 3 | 27 | 100 | 100 | 23 | 4.1 | 1.3 | 840 | 9 | 100 | 89 | 15 | 3.2 | 2.5 | 130 |
| Day 4 (morning void) | 66 | 100 | 100 | 27e | 4.4 | 1.1 | 1700 | 16 | 100 | 100 | 16 | 3.1 | 3.1 | 170 |
| Day 4 | 28 | 100 | 100 | 20 | 4.3 | 1.2 | 1200 | 10 | 100 | 90 | 11 | 2.6 | 2.9 | 110 |
| Day 5 | 26 | 100 | 100 | 17 | 5.5 | 0.8 | 2500 | 9 | 100 | 100 | 12 | 2.6 | 2.4 | 75 |
| Excretion Rates (µg/h) | ||||||||||||||
| Pre-application | 60 | 95 | 92 | 0.29 | 4.9 | ND | 12 | 14 | 100 | 100 | 0.52 | 2.4 | 0.10 | 2.0 |
| Day 1 | 59 | 100 | 100 | 1.3e | 4.0 | 0.07 | 22 | 15 | 100 | 100 | 0.44 | 1.8 | 0.18 | 1.6 |
| Day 2 | 25 | 100 | 100 | 1.4e | 3.2 | 0.08 | 30 | 7 | 100 | 86 | 0.62 | 1.9 | 0.18 | 1.5 |
| Day 3 | 20 | 100 | 100 | 1.2 | 3.7 | 0.09 | 26 | 8 | 100 | 88 | 0.72 | 2.1 | 0.19 | 1.8 |
| Day 4 (morning void) | 56 | 100 | 100 | 1.3e | 4.0 | 0.06 | 19 | 14 | 100 | 100 | 0.61 | 2.0 | 0.24 | 2.6 |
| Day 4 | 18 | 100 | 100 | 1.5e | 3.3 | 0.22 | 11 | 7 | 100 | 86 | 0.50 | 1.7 | 0.18 | 0.9 |
| Day 5 | 24 | 100 | 100 | 1.0 | 5.0 | 0.06 | 63 | 7 | 100 | 100 | 0.81 | 1.6 | 0.39 | 1.3 |
3,5,6-Trichloro-2-pyridinol.
Percent of participants for which a target analyte was detected.
Percent of participants for which a target analyte amount was greater than the method detection limit.
Not detected.
Significantly higher than pre-application concentration based on one-tailed paired t-test, α = 0.05.
The GM TCP concentration for chlorpyrifos applicators was the same (11 µg/L) in the pre-application and Day1 post-application measurements. Differences in pre- and post-application levels were not significant across the applicators in this study. Three applicators reported using a product containing chlorpyrifos in the four days preceding, and six applicators reported uses in the two days following the monitored application. However, applicators with the seven highest pre-application urinary TCP concentrations did not report any chlorpyrifos product use in the four days preceding monitoring and pre-application levels often exceeded Day1 post application levels.
Summaries of measurement results for estimated hand loading, estimated body loading, and air concentration are shown in Table 6 for 2,4-D and chlorpyrifos applicators. Large ranges were measured for hand and body loading estimates, with higher variability as compared to the urine measurement results. Dermal measurements were higher for 2,4-D applicators compared to chlorpyrifos applicators in this study, largely as a result of higher values for 2,4-D hand spray applicators and lower dermal loadings measured for chlorpyrifos granular product applicators. Spearman correlations between measures are shown in Table 7. Estimated 2,4-D hand loading values had the highest correlations with urine biomarker concentrations, but all 2,4-D measures were significantly correlated. Estimated body loading had the highest correlation with urine biomarker levels for chlorpyrifos, but the strongest correlations were between estimated hand and body loadings.
Table 6.
Summary of Hand Loading, Body Loading, and Personal Air Measurement Results for 2,4-D and Chlorpyrifos Applicators in the AHS/PES
| 2,4-D | Chlorpyrifos | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measurement Type | N | % Detecta |
% >MDLb |
GM | GSD | Min. | Max. | N | % Detect |
% >MDL |
GM | GSD | Min. | Max. |
| Estimated hand loading (mg) | 68 | 97 | 84 | 0.39 | 9.2 | NDc | 22 | 17 | 53 | 53 | 0.02d | - | ND | 0.93 |
| Estimated body loading (mg)e | 69 | 100 | 91 | 2.9 | 12 | 0.02 | 880 | 17 | 94 | 59 | 0.28 | 5.1 | ND | 5.8 |
| Personal air (µg/m3) | 68 | 85 | 66 | 0.37 | 5.8 | ND | 10 | 17 | 100 | 100 | 0.49 | 3.0 | 0.048 | 2.0 |
Percent of participants for which a target analyte was detected.
Percent of participants for which a target analyte amount was greater than the method detection limit.
Not detected.
Median value reported for chlorpyrifos hand loading due to the number of samples with no analyte detected.
The estimated total body loading does not include the hands.
Table 7.
Spearman Correlations Between Measurements
| 2,4-D | Chlorpyrifos/TCP | |||||
|---|---|---|---|---|---|---|
| Measures (all urine values are Day1) | N | r | p | N | r | p |
| Urine (µg/L) and Urine (µg/h) | 59 | 0.93 | <0.001 | 15 | 0.59 | 0.020 |
| Urine (µg/L) and Hand Loading | 67 | 0.74 | <0.001 | 16 | 0.19a | 0.471 |
| Urine (µg/L) and Body Loading | 68 | 0.43 | <0.001 | 16 | 0.41 | 0.110 |
| Urine (µg/L) and Air Concentration | 67 | 0.40 | <0.001 | 16 | 0.02 | 0.940 |
| Hand Loading and Body Loading | 68 | 0.61 | <0.001 | 17 | 0.70a | 0.002 |
| Hand Loading and Air Concentration | 67 | 0.34 | 0.006 | 17 | 0.13a | 0.619 |
| Body Loading and Air Concentration | 68 | 0.62 | <0.001 | 17 | 0.04 | 0.874 |
Only 53% of chlorpyrifos hand loading samples had detectable levels.
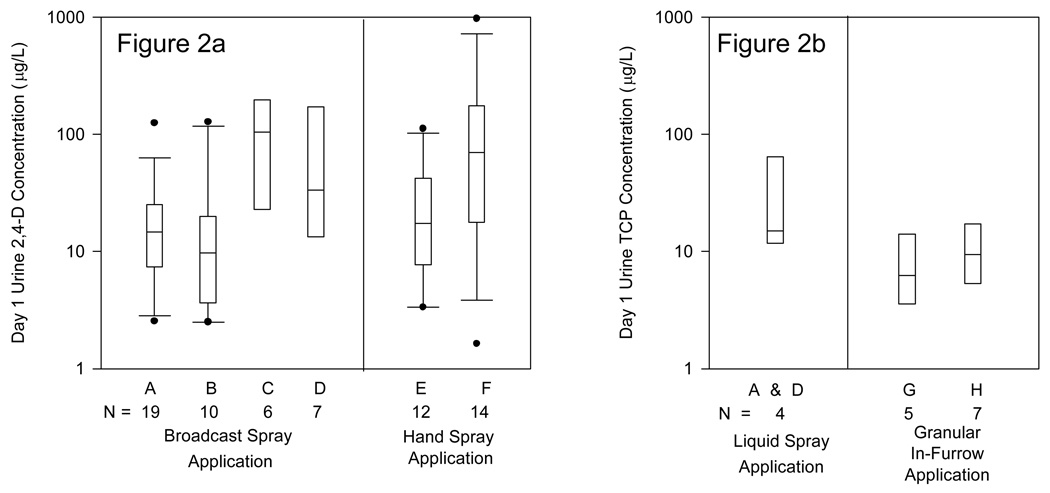
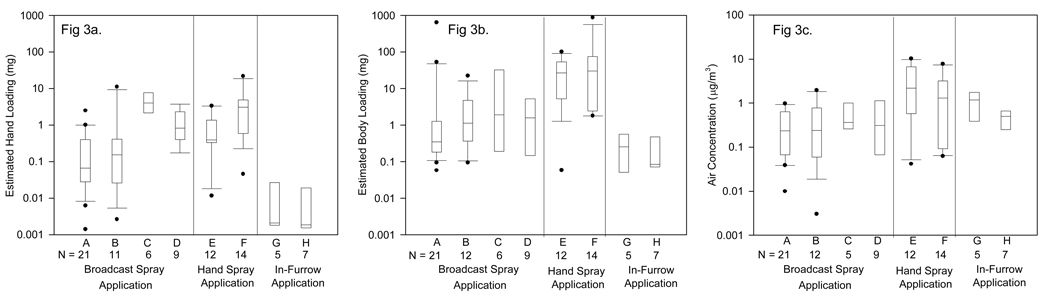
Distributions of Day1 post-application urinary biomarker concentrations in eight categories are shown in Figure 2 separately for 2,4-D and chlorpyrifos applicators, with the four chlorpyrifos liquid spray applicators collapsed into one category. Urine results are reported separately for 2,4-D and chlorpyrifos because of differences in absorption, metabolism, and excretion. Distributions for hand loading, body loading, and personal air measurements are shown in Figure 3 with measurements for 2,4-D and chlorpyrifos applicators combined. Tests for overall differences across categories were significant for urine 2,4-D concentrations and excretion rates (data not shown) with F-values of 3.8 – 4.2 (p < 0.006), and for the hand loading, body loading, and air measurements with a range of F-values from 2.8 – 17 (p <0.02). There was little difference in measurement distributions between those using open and enclosed-cab tractors, but 2,4-D applicators using rubber gloves tended to have lower measurement levels for urine and estimated hand loading. Hand spray applicators had higher estimated body loading and air concentrations than broadcast spray applicators. No significant differences were found across the three categories for urine TCP concentrations or excretion rates but the number of observations was small for each group. Applicators using in-furrow application of a granular product had lower estimated hand and body loading measurement distributions than for other application methods. There was overlap between many of the categorical distributions and considerable within-category variability, suggesting that other factors were often important at the individual level.
Figure 2.
Distributions of Day 1 urine biomarker concentrations for 2,4-D applicators (Figure 2a) and chlorpyrifos applicators (Figure 2b) for eight pesticide use categories described in Table 1. Boxes show the median, 25th, and 75th percentile intervals; whiskers show the 10th and 90th percentile intervals for those with sufficient numbers of observations, and dots show high and low values outside the percentile ranges.
Figure 3.
Distributions of estimated hand loading (Figure 3a), estimated body loading (Figure 3b), and personal air concentrations (Figure 3c) for eight pesticide use categories described in Table 1. Results for both 2,4-D and chlorpyrifos are included. Boxes show the median, 25th, and 75th percentile intervals; whiskers show the 10th and 90th percentile intervals for those with sufficient numbers of observations, and dots show high and low values outside the percentile ranges.
Bivariate analyses were performed for selected handling and application conditions for 2,4-D broadcast spray applicators, 2,4-D hand spray applicators, and chlorpyrifos applicators (Table 8). Several factors were found to be potentially associated with differences in measurement results. Hand, body, and air GM levels were significantly lower for broadcast spray applicators compared to hand spray applicators.
Table 8.
Measurement Result Comparisons for Selected Handling and Use Conditions
| Day 1 Post- Application Urine |
Estimated Hand Loading |
Estimated Body Loading |
Air Concentration | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | GM (GSD) | p | GM (GSD) | p | GM (GSD) | p | GM (GSD) | p | |
| µg/L | mg | mg | µg/m3 | ||||||
| All 2,4-D Applicators | |||||||||
| Broadcast Spray Application | 42–43a | 21 (1.2) | 0.181 | 0.22 (1.4) | 0.007 | 0.99 (1.4) | <0.001 | 0.21 (1.3) | <0.001 |
| Hand Spray Applicationb | 26 | 33 (1.3) | 0.97 (1.5) | 17 (1.5) | 0.97 (1.4) | ||||
| 2,4-D Broadcast Spray Applicators | |||||||||
| Used adjuvant | 14–15 | 26 (1.4) | 0.392 | 0.36 (1.8) | 0.317 | 1.8 (1.7) | 0.191 | 0.32 (1.5) | 0.165 |
| No adjuvant used | 27–28 | 18 (1.3) | 0.17 (1.6) | 0.86 (1.5) | 0.16 (1.3) | ||||
| One tank load | 34–35 | -c | 0.19 (1.5) | 0.453 | 0.99 (1.5) | 0.991 | 0.19 (1.3) | 0.601 | |
| Two tank loads | 8 | 0.39 (2.3) | 0.98 (2.2) | 0.26 (1.7) | |||||
| Used rubber gloves | 29–30 | 13 (1.2) | <0.001 | 0.090 (1.4) | <0.001 | 0.95 (1.5) | 0.834 | 0.21 (1.3) | 0.304 |
| Did not use rubber gloves | 12–13 | 57 (1.4) | 1.6 (1.7) | 1.1 (1.8) | 0.30 (1.5) | ||||
| Repaired equipment | 17 | 19 (1.4) | 0.739 | 0.25 (1.8) | 0.900 | 2.6 (1.6) | 0.066 | 0.25 (1.5) | 0.640 |
| No equipment repair | 21–22 | 22 (1.3) | 0.23 (1.7) | 0.77 (1.5) | 0.20 (1.4) | ||||
| Minor splashes/leaks/drips | 11–12 | 25 (1.5) | 0.543 | 0.73 (1.9) | 0.042 | 2.9 (1.8) | 0.046 | 0.14 (1.5) | 0.272 |
| No minor splashes/leaks/drips | 30–31 | 19 (1.3) | 0.14 (1.5) | 0.66 (1.5) | 0.24 (1.3) | ||||
| Enclosed tractor cab | 23–24 | 24 (1.3) | 0.495 | 0.20 (1.6) | 0.660 | 1.1 (1.6) | 0.717 | 0.24 (1.4) | 0.460 |
| Open tractor cab | 17–18 | 18 (1.4) | 0.28 (1.8) | 0.87 (1.7) | 0.17 (1.4) | ||||
| Adjusted for amount a.i. mixed | µg/L/kg a.i. | mg/kg a.i | mg/kg a.i. | µg/m3/kg a.i. | |||||
| Enclosed tractor cab | 23–24 | 2.0 (1.4) | 0.153 | 0.033 (1.6) | 0.068 | 0.18 (1.5) | 0.297 | 0.04 (1.4) | 0.302 |
| Open tractor cab | 17–18 | 4.1 (1.4) | 0.12 (1.7) | 0.37 (1.6) | 0.07 (1.5) | ||||
| 2,4-D Hand Spray Applicators | µg/L | mg | mg | µg/m3 | |||||
| Used adjuvant | 8 | 87 (1.7) | 0.031 | 2.1 (1.8) | 0.142 | 27 (2.0) | 0.473 | 1.1 (1.9) | 0.832 |
| No adjuvant used | 18 | 21 (1.4) | 0.69 (1.5) | 14 (1.6) | 0.92 (1.5) | ||||
| One tank load | 17 | -c | 1.1 (1.6) | 0.577 | 24 (1.6) | 0.218 | 1.3 (1.5) | 0.290 | |
| Two tank loads | 9 | 0.74 (1.8) | 9.0 (1.9) | 0.58 (1.8) | |||||
| Used rubber gloves | 12 | 18 (1.5) | 0.059 | 0.43 (1.6) | 0.026 | 14 (1.8) | 0.610 | 0.97 (1.7) | 0.378 |
| Did not use rubber gloves | 14 | 56 (1.5) | 2.0 (1.6) | 21 (1.7) | 0.72 (1.6) | ||||
| Repaired equipment | 6 | 130 (1.8) | 0.011 | 4.5 (1.9) | 0.013 | 120 (2.0) | 0.004 | 1.9 (2.1) | 0.304 |
| No equipment repair | 20 | 22 (1.4) | 0.61 (1.4) | 9.9 (1.5) | 0.79 (1.5) | ||||
| Minor splashes/leaks/drips | 16 | 41 (1.5) | 0.397 | 1.1 (1.6) | 0.595 | 27 (1.6) | 0.157 | 2.5 (1.4) | <0.001 |
| No minor splashes/leaks/drips | 10 | 24 (1.6) | 0.76 (1.8) | 8.7 (1.8) | 0.21 (1.5) | ||||
| Contact sprayed vegetation | 5 | 100 (1.9) | 0.070 | 2.2 (2.2) | 0.263 | 61 (2.3) | 0.115 | 2.4 (2.2) | 0.207 |
| No contact sprayed vegetation | 21 | 25 (1.4) | 0.80 (1.5) | 13 (1.5) | 0.78 (1.5) | ||||
| No vehicle used | 5 | 18 (2.0) | 0.385 | 0.76 (2.3) | 0.703 | 18 (2.1) | 0.008 | 0.38 (2.2) | 0.240 |
| ATV used | 7 | 24 (1.8) | 0.66 (2.0) | 2.9 (1.9) | 0.66 (1.9) | ||||
| Tractor used | 14 | 49 (1.5) | 1.3 (1.6) | 42 (1.6) | 1.6 (1.6) | ||||
| Adjusted for amount a.i. mixed | µg/L/kg a.i. | mg/kg a.i | mg/kg a.i | µg/m3/kg a.i. | |||||
| No vehicle used | 5 | 200 (2.1) | 0.051 | 11 (2.3) | 0.042 | 250 (2.1) | 0.005 | 5.4 (2.2) | 0.199 |
| ATV used | 7 | 42 (1.9) | 1.7 (2.1) | 7.4 (1.9) | 1.6 (2.0) | ||||
| Tractor used | 14 | 21 (1.6) | 0.73 (1.7) | 24 (1.6) | 0.94 (1.6) | ||||
| Chlorpyrifos Applicators | µg/L | mgd | mg | µg/m3 | |||||
| In-furrow, granular product | 12 | 8.3 (1.2) | 0.045 | 0.002 | - | 0.17 (1.5) | 0.033 | 0.52 (1.4) | 0.736 |
| Spray, liquid product | 4–5 | 21 (1.4) | 0.27 | 1.0 (1.9) | 0.42 (1.7) | ||||
| Adjusted for amount a.i. mixed | µg/L/kg a.i. | mg/kg a.i | mg/kg a.i. | µg/m3/kg a.i. | |||||
| In-furrow, granular product | 12 | 0.57 (1.2) | 0.002 | 0.0003 | - | 0.015 (1.5) | 0.004 | 0.05 (1.4) | 0.604 |
| Spray, liquid product | 4–5 | 2.5 (1.4) | 0.042 | 0.16 (1.8) | 0.07 (1.6) | ||||
Number of measurements may vary by sample type because of missing data.
Includes one applicator that used both broadcast and hand spray.
Number of tank loads was counted only for period with dermal and air measurements.
Median values reported for chlorpyrifos hand loading, no tests of differences due to number of non-detects.
For 2,4-D broadcast spray applicators minor spills, splashes, drips, or leaks were associated with higher GM hand and body loadings and equipment repair was associated with higher body loading (Table 8). Broadcast spray applicators using and adjuvant had higher GM levels for all media but the differences were not significant. Lower GM urinary biomarker and hand loading levels were associated with use of rubber gloves. Only after adjustment for amount of a.i. used did 2,4-D broadcast applicators using enclosed-cab tractors have lower GM values than those using open-cab tractors, but the differences were not significant at the p = 0.05 level. Broadcast spray applicators in NC used open cab tractors more often than applicators in IA, but had lower Day1 GM urine levels (16 vs. 23 µg/L) and estimated hand loading values (0.11 vs. 0.33 mg) than IA applicators. The differences could be related to the lower amounts of a.i. used in NC compared to IA (mean 5.8 vs. 16 kg).
Use of an adjuvant by 2,4-D hand spray applicators was associated with higher Day1 urine GM levels; equipment repair was associated with higher GM levels for all media except air (Table 8). GM levels were higher across all median for those with minor spills, splashes, drips, or, leaks or contact with sprayed vegetation, but in most cases the differences were not significant at the p = 0.05 level. Lower urine and hand loading GM levels were found for the use of rubber gloves, but the result was significant at the p = 0.05 level only for hand loading. Day1 urine and air levels showed large but not significant differences in the order of no vehicle < ATV < tractor. Body loadings were lowest for ATV users.
Overall, the amount of a.i. used by broadcast spray applicators was not associated with urine concentrations or excretion rates but was weakly associated with hand and body loadings, with r2 values of 0.09 (p = 0.060) and 0.15 (p = 0.010), respectively. For 2,4-D hand spray applicators the Day1 urine concentrations increased with increasing duration of use (r2 = 0.30, p = 0.004) but were not significantly associated with the amount of a.i. used (r2 = 0.07, p = 0.189).
Chlorpyrifos applicators using a granular product and in-furrow or over-the-row application had significantly lower GM Day-1 urine and body loading levels than those performing spray applications of liquid mixtures (Table 8). Hand loading measurements were also much lower for those applying a granular chlorpyrifos product. This application method/formulation combination was the predominant predictor of measurement difference for chlorpyrifos applicators in this study. While this may reflect different exposure potential based on formulation type, the differences between crops, application methods, or other factors in the two states may also contribute to this result since all of the liquid spray applications were performed in NC. Across the three application methods for 2,4-D and chlorpyrifos, the GM Day1 urine, hand loading, and body loading levels were in the order of in-furrow <broadcast spray < hand spray. GM air concentrations fell in the order of broadcast spray < in-furrow <hand spray.
Approximately half of the repeat measurement visits were within the same year and half in a following year. All but one set of repeat visits involved 2,4-D and included one in-furrow, twelve hand spray, four boom spray, and two hand and boom spray applications. Results are shown in Table 9 for correlations between first and second visits across applicators and for within- and between-person variances. Correlations for urine measures were higher than those for hand, body, and air measures. Within-person variance was lower than between-person variance for urine and estimated body loading measures while the opposite was true for estimated hand loading and personal air concentrations. In two cases adjustment for amount of a.i. used decreased within-person differences to less than two-fold, but in two other cases this adjustment increased differences to greater than two-fold. Five of the thirteen applicators who had a two-fold or greater difference in estimated hand loading measurements at different visits also had differences in glove use. Other factors that differed between visits such as performing repairs, contact with sprayed vegetation or equipment, and equipment clean-up may have contributed to within-person variability for some individuals.
Table 9.
Correlation and Variance for the Subset of Applicators with Repeat Measurementsa
| Correlationb |
Variancec |
|||
|---|---|---|---|---|
| Measurement | N | r2 | σ2W | σ2B |
| Urine Day 1 concentration | 19 | 0.66 | 0.37 | 1.79 |
| Urine Day 1 excretion rate | 16 | 0.57 | 0.44 | 1.49 |
| Estimated hand loading | 18 | 0.21 | 1.61 | 1.27 |
| Estimated body loading | 19 | 0.43 | 1.63 | 3.32 |
| Personal air | 19 | 0.09 | 2.10 | 0.81 |
For a subset of Iowa applicators with repeat visits in the same (48%) or different (52%) year.
Regression of Visit1 and Visit2 natural log of measurement results across applicators with repeat visits.
Within (σ2W) and between (σ2B) person variance, based on natural log of measurement values.
DISCUSSION
Some exposure assessment limitations in previous agricultural studies are being addressed through the Agricultural Health Study. The AHS Pesticide Exposure Study was performed to assess exposures that may occur in the cohort for two widely used pesticides and to provide information to assess some potential exposure factors. Information from the study can also provide information on exposure classification strategies used in the AHS and other epidemiological studies and the applicability of measurement approaches that might be used in other cohort studies.
Challenges were encountered in identifying, recruiting, and monitoring members of a cohort with a wide geographic dispersion and multiple crop protection product options. Many farmers agreed to participate in the study based on pre-growing season projections of the products they might use in the upcoming season. However, some farmers subsequently decided to use a different product or no product at all. Also, in some cases, application decisions were made in a time frame too short for a monitoring team to respond. Severe drought in NC during the spring and summer of 2002 led to decisions not to use products containing target chemicals by many of the farmers that had consented to participate early in the year. Fewer chlorpyrifos product uses were monitored than was anticipated based on information on previous frequency of use information in the AHS cohort. This may have been a result of decreased chlorpyrifos use in general; in IA for example an average of 8.3% of the corn acreage was treated with chlorpyrifos from 1993 – 1999, but dropped to 2.7% from 2000 – 2003 (NASS, 2006). Many previous uses of chlorpyrifos products were reported for tomato crops in NC but this use was rescinded in 2001.
Agricultural and forestry worker exposure to 2,4-D has been measured in earlier studies (Abbot et al., 1987; Draper and Street, 1982; Grover et al., 1986; Knopp and Glass, 1991; Lavy et al., 1987) but changes in products, equipment, and practices may have occurred since these studies were conducted. A wide range in 2,4-D urinary biomarker, estimated hand loading, estimated body loading, and air concentrations were measured for the applicators participating in this study and some of these results can be compared to more recent measurements made for other applicators. GM urinary 2,4-D levels for broadcast spray applicators in this study (GM 21 µg/L, range 2.5 – 270 µg/L for Day1 urine samples) were lower than those measured by Aquavella et al. (2006) (GM 64 µg/L, range 2 – 1856 µg/L) for 34 applicators and by Hines et al. (2003) (estimated 61 µg/L based on a regression model and assuming spraying only on the day prior to measurement) for 15 custom broadcast spray applicators. GM results from this study were higher than measurements reported by Arbuckle et al. (2002) (GM 5.4 µg/L, range 0.5 – 410 µg/L) for 43 Ontario farm applicators. Few recent comparable data are available for 2,4-D dermal and air levels. Hines et al. (2001), reported an adjusted GM hand loading of 2,4-D ethylhexyl ester of 0.16 mg/hand (0.21 mg for two hands calculated as 2,4-D a.e. with an assumption of equal loading on both hands), and air concentrations of 0.36 µg/m3 (0.24 µg/m3 as 2,4-D a.e.) across twelve spray days, similar to the GM values of 0.22 mg (both hands) and 0.21 µg/m3 for broadcast spray applicators in this study. No urinary 2,4-D biomarker data from studies of farmer non-crop hand spray applications were found for comparison. GM Day1 urinary levels for hand spray applicators in this study (GM 33 µg/L, range 1.6 – 1040 µg/L) were lower than those reported by Garry et al. (2001) (GM of 185 µg/L, range 28 – 1700 µg/L) for seven forestry workers using backpack sprayers. These professional forestry applicators likely sprayed more a.i. on a daily basis in their jobs than the farmers in this study. The range of chlorpyrifos measurement results in this study was more limited, based on a smaller number of participants and a different mix of application methods than for 2,4-D. The GM Day1 urine TCP levels in this study were 21 µg/L (range 11 – 80 µg/L) for spray applicators and 8.3 µg/L (range 2.5 – 29 µg/L) for in-furrow applications of granular products. Alexander et al. (2006) reported a GM of 31 µg/L following liquid spray applications and 11 µg/L following granular applications based on the maximum urine TCP concentrations in post-application 24-h samples.
Differences in exposure between and within different agricultural applicator populations are likely to be explained by a combination of factors. These may include differences in the amount and duration of pesticide use, handling and application methods and equipment, crops, and use of personal protective equipment. Differences in measurement methods and strategies may also lead to different results. Categorical presentation of results in Figures 2 and 3 reveal some important differences associated with application method and glove use in this study, but the wide range and overlap in many of the distributions also suggests multiple factors were affecting exposures at an individual level. In general, we found a pattern of increasing exposure from granular in-furrow, to broadcast liquid, to hand spray liquid applications. The use of rubber gloves was associated with lower GM urinary 2,4-D levels in this study, but distributions overlapped and glove use alone did not distinguish between urinary biomarker concentrations or excretion rates for many applicators. The use of enclosed tractor cabs was associated with lower 2,4-D exposures only when the measurements were adjusted for the amount of a.i. used. However, when considered alone, the amount of a.i. used was not significantly associated with urinary biomarker levels or excretion rates for 2,4-D broadcast spray applicators. Broadcast spray applicator exposures may occur primarily during the mixing/loading operation and may not depend on the amount of chemical used. Duration of use was modestly associated with urinary biomarker concentrations and excretion rates for 2,4-D hand spray applicators but not for 2,4-D broadcast spray applicators. Other factors associated with increased levels in some sample types and for some 2,4-D application methods included equipment repair, use of adjuvants, and minor spills, splashes, or leaks. Large differences were observed between those with and without contact with sprayed vegetation for 2,4-D hand spray applicators, but the number with such contact was small and the differences were not significant. Each of these factors can affect the amount of contact with the chemical or, in the case of an adjuvant, may affect contact time or dermal absorption. Combinations of these factors likely contribute to the overall range and variability in measured exposures.
Bivariate analyses of selected variables have been reported for broadcast spray applicators for glyphosate (Acquavella et al., 2004), chlorpyrifos (Alexander et al., 2006), atrazine (Perry et al., 2006) and for dithiocarbamates for vineyard workers (Baldi et al., 2006). In other cases a multivariate analysis approach has been taken for broadcast spray 2,4-D applicators (Hines et al., 2003 and 2001; Arbuckle et al., 2002) or professional turf 2,4-D applicators (Harris et al., 2002). Associations were seen in some studies between exposure and a measure of chemical use, such as number of tank loads, that may be related to the number of mixing/loading operations or the amount of chemical applied. Many of the studies report associations between exposure and skin or body contact with the chemical or potentially contaminated surfaces and many, but not all studies reported reduced exposures with use of protective gloves. Lower chlorpyrifos biomarker concentrations were found by Alexander et al. (2006) and in this study for both biomarker and dermal measures for applicators using granular products compared to those performing liquid spray applications. It is not clear whether this is due to the product formulation, the in-furrow application method, a combination of the two, or other crop and method factors that differ between IA and NC. However, chlorpyrifos air concentrations were not lower for the granular in-furrow applicators in this study, and particles becoming airborne during pouring of the product into planter bins may play a role. The small number of chlorpyrifos applicators limited additional assessments within these categories. Interpreting chlorpyrifos exposures using urinary biomarkers is also complicated because of the other sources of exposure to the pesticide or its metabolite (Morgan et al., 2005).
Estimates of absorbed dose may be the most relevant measure for assessing exposures related to agricultural chemical use. However, measuring and interpreting biomarker data can be difficult in an active farm population when products containing the active ingredient are likely to be used on the days before and after a monitored use. In this study there were 14 monitored applications for which there were no reported uses of products containing 2,4-D on the four days preceding and following the monitored application and the applicator provided complete sets of urine samples over five days. Estimated doses were compared to the 15 mg/kg/day intermediate-term dermal/inhalation toxicological endpoint for occupational risk assessment in the U.S. EPA reregistration eligibility decision (U.S. EPA, 2005), giving an estimated average margin of exposure of 5,600 (range 830 – 47,000). The long-term toxicological significance of these dose levels is less clear when considering that agricultural uses in this cohort typically occur only several days per year but may continue across multiple years. These dose estimates have some uncertainty due to individual differences in 2,4-D excretion rates and variability in sample collection durations when compared to the assumption of 90% excretion in 120 hours. The measurements may overestimate the dose ascribed to the monitored agricultural application because of existing body burden at the start of the collections and the possibility of additional occupational, residential, or dietary exposures during the measurement period.
Some limitations apply to this study. Measurements were made for only two chemicals and, although these chemicals and their application methods were selected because of their wide use in the cohort, chemicals with different formulations or physical/chemical properties may result in different internal exposures. Residential and dietary contributions to exposure were not assessed, which complicates interpretation of urinary biomarkers with regard to understanding important factors and sources that contribute to total exposures, particularly for chlorpyrifos. Measurements in this study were made only once or twice across one or two years and may not represent product uses in prior years and long-term exposures in the AHS cohort. The study sample was not selected to be representative of the full AHS cohort and extrapolation of measurement distribution parameters beyond the study sample is not recommended.
In summary, urinary biomarker, dermal, and air measurements were made for a subset of farmers in the AHS epidemiological cohort who used several application methods, including agricultural non-crop hand spraying which has not been widely reported on elsewhere. The multimedia monitoring approach provides information to assess different exposure routes and to make comparisons with measured biomarker levels. The study also provides information to assess possible exposure determinants for a range of pesticide handling and application methods commonly used by farmers in the cohort. Results from this study will inform development of future questionnaires for use in agricultural populations and will improve exposure classification in the AHS epidemiological study. Results may also be of use in pesticide safety education for reducing exposure to pesticides.
ACKNOWLEDGMENTS
The authors thank the AHS cohort members participating in this study for their considerable time and effort. Several EPA researchers provided significant contributions to the study design including Ross Highsmith, William Steen, Miles Okino and Ruth Allen. Paul Jones at EPA provided statistical support and Guadalupe Chapa assisted in data analysis. Joy Herrington, Nyla Logsden-Sackett, and Patti Gillette at the AHS Field Stations in IA and NC led participant screening activities. We thank Marcia Nishioka (Battelle Memorial Institute), Robin Helburn (RTI International) and David Camann (Southwest Research Institute) for leading sample analyses. This work has been supported in part by intramural research funds of the National Institutes of Health at NCI and NIEHS.
Abbreviations
- a.e.
2,4-D acid equivalents
- AHS
Agricultural Health Study
- a.i.
active ingredient
- ATV
all terrain vehicle
- CATI
Computer assisted telephone interview
- GC/MS
gas chromatography/mass spectrometry
- GM
geometric mean
- GSD
geometric standard deviation
- IA
Iowa
- LC/MS/MS
liquid chromatography/mass spectrometry/mass spectrometry
- MDL
method detection limit
- MLA
mixing, loading, and application
- NC
North Carolina
- PES
Pesticide Exposure Study
- PHED
Pesticide Handler Exposure Database
- TCP
3,5,6-trichloro-2-pyridinol
- U.S. EPA
United States Environmental Protection Agency
Footnotes
DISCLAIMER
This work has been funded in part by the U.S. Environmental Protection Agency under Contracts 68-D99-011 and 68-D99-012, and through Interagency Agreement DW-75-93912801-0. Mention of trade names or commercial products does not constitute endorsement or recommendation for use. This manuscript is now being subjected to external peer-review and has not been cleared for publication by the US Environmental Protection Agency.
References
- Abbot IM, Bonsall JL, Chester G, Hart TB, Turnbull GJ. Worker exposure to a herbicide applied with ground sprayers in the United Kingdom. Am Ind Hyg Assoc J. 1987;48:167–175. doi: 10.1080/15298668791384571. [DOI] [PubMed] [Google Scholar]
- Acquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, Bleeke M. Glyphosate biomonitoring for farmers and their families: Results from the Farm Family Exposure Study. Environ Health Perspect. 2004;112:321–326. doi: 10.1289/ehp.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acquavella JF, Alexander BH, Mandel JS, Burns CJ, Gustin C. Exposure misclassification in studies of agricultural pesticides. Epidemiology. 2006;17(1):69–74. doi: 10.1097/01.ede.0000190603.52867.22. [DOI] [PubMed] [Google Scholar]
- Alavanja MC, Hoppin JA, Kamel F. Health Effects Of Chronic Pesticide Exposure: Cancer And Neurotoxicity. Annu Rev Public Health. 2004;25:155–197. doi: 10.1146/annurev.publhealth.25.101802.123020. [DOI] [PubMed] [Google Scholar]
- Alavanja MCR, Sandler D, McMaster S, Zahm S, McDonnell C, Lynch C, Pennybacker M, Rothman N, Dosemeci M, Bond A, Blair A. The Agricultural Health Study. Environ Health Perspect. 1996;104:362–369. doi: 10.1289/ehp.96104362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander BH, Burns CJ, Bartels MJ, Acquavella JF, Mandel JS, Gustin C, Baker BA. Chlorpyrifos exposure in farm families: Results for the farm family exposure study. J Expos Sci Environ Epidemiol. 2006;15(5):447–456. doi: 10.1038/sj.jes.7500475. [DOI] [PubMed] [Google Scholar]
- Arbuckle TE, Burnett R, Cole D, Teschke K, Dosemeci M, Bancej C, Zhang J. Predictors of herbicide exposure in farm applicators. Int Arch Occup Environ Health. 2002;75:406–414. doi: 10.1007/s00420-002-0323-7. [DOI] [PubMed] [Google Scholar]
- Baldi I, Lebailly P, Jean S, Rougetet L, Dulaurent S, Marquet P. Pesticide contamination of workers in vineyards in France. J Expos Sci Environ Epidemiol. 2006;16(2):115–124. doi: 10.1038/sj.jea.7500443. [DOI] [PubMed] [Google Scholar]
- Blair A, Zahm SH. Agricultural exposures and cancer. Environ Health Perspect. 1995;103(Suppl 8):205–208. doi: 10.1289/ehp.95103s8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton CA, Mosquin PL, Pellizzari ED, Quackenboss JJ. Limitations on the uses of multimedia exposure measurements for multipathway exposure assessment--Part I: handling observations below detection limits. Qual Assur. 2003;10(3–4):123–159. doi: 10.1080/10529410390892133. [DOI] [PubMed] [Google Scholar]
- Cordes DH, Rea DF. Occupational Medicine: State of the Art Reviews. 3. Vol. 6. Philadelphia, Pa: Hanley and Belfus; 1991. Health hazards of farming. [Google Scholar]
- Dich J, Zahm SH, Hanberg A, Adami HO. Pesticides and cancer. Cancer Causes Control. 1997;8(3):420–443. doi: 10.1023/a:1018413522959. [DOI] [PubMed] [Google Scholar]
- Dosemeci M, Alavanja MCR, Rowland AS, Mage D, Zahm SH, Rothman N, Lubin JH, Hoppin JA, Sandler DP, Blair A. A semi-quantitative approach for estimating exposure to pesticides in the Agricultural Health Study. Ann of Occup Hyg. 2002;46:245–260. doi: 10.1093/annhyg/mef011. [DOI] [PubMed] [Google Scholar]
- Draper WN, Street JC. Applicator exposure to 2,4-D, dicamba, and a dicamba isomer. J Environ Sci Health. 1982;B17(4):321–339. doi: 10.1080/03601238209372324. [DOI] [PubMed] [Google Scholar]
- Fleming LE, Bean JA, Rudolph M, Hamilton K. Cancer incidence in a cohort of licensed pesticide applicators in Florida. J Occup Environ Med. 1999;41(4):279–289. doi: 10.1097/00043764-199904000-00010. [DOI] [PubMed] [Google Scholar]
- Gardner M, Spruill-McCombs M, Beach J, Michael L, Thomas K, Helburn RS. Quantification of 2,4-D on solid-phase exposure sampling media by LC-MS-MS. J. Anal. Toxicol. 2005;29:188–192. doi: 10.1093/jat/29.3.188. [DOI] [PubMed] [Google Scholar]
- Garry VF, Tarone RE, Kirsch IR, Abdallah JM, Lombardi DP, Long LK, Burroughs BL, Barr DB, Kesner JS. Biomarker correlations of urinary 2,4-D levels in foresters: genomic instability and endocrine disruption. Environ Health Perspect. 2001;109(5):495–500. doi: 10.1289/ehp.01109495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover R, Cessna AJ, Muir NI, Riedel D, Franklin CA, Yoshida K. Factors affecting the exposure of ground-rig applicators to 2,4-D dimethylamine salt. Arch Environ Contam Toxicol. 1986;15:677–686. doi: 10.1007/BF01054915. [DOI] [PubMed] [Google Scholar]
- Harris SA, Sass-Kortsak AM, Corey PN, Purdham JT. Development of models to predict dose of pesticides in professional turf applicators. J Expos Anal Environ Epidemiol. 2002;12:130–144. doi: 10.1038/sj.jea.7500208. [DOI] [PubMed] [Google Scholar]
- Harris SA, Solomon KR. Percutaneous penetration of 2,4-dichlorophenoxyacetic acid and 2,4-D dimethylamine salt in human volunteers. J Toxicol Environ Health. 1992;36:233–240. doi: 10.1080/15287399209531634. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Deddens JA, Striley CAF, Biagini RE, Shoemaker DA, Brown KK, Mackenzie BA, Hull RD. Biological monitoring for selected herbicide biomarkers in the urine of exposed custom applicators: Application of mixed-effect models. Ann Occup Hyg. 2003;47:503–517. doi: 10.1093/annhyg/meg067. [DOI] [PubMed] [Google Scholar]
- Hines CJ, Deddens JA, Tucker SP, Hornung RW. Distributions and determinants of pre-emergent herbicide exposures among custom applicators. Ann Occup Hyg. 2001;45:227–239. doi: 10.1093/annhyg/45.3.227. [DOI] [PubMed] [Google Scholar]
- Knopp D, Glass S. Biological monitoring of 2,4-dichlorophenoxyacetic acid-exposed workers in agriculture and forestry. Int Arch Occup Environ Health. 1991;63:329–333. doi: 10.1007/BF00381583. [DOI] [PubMed] [Google Scholar]
- Morgan MK, Sheldon LS, Croghan CW, Jones PA, Robertson GL, Chuang JC, Wilson NK, Lyu CW. Exposures of preschool children to chlorpyrifos and its degradation product 2,5,6-trichloro-2-pyridinol in their everyday environments. J Expos Anal Environ Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- Lavy TL, Norris LA, Mattice JD, Marx DB. Exposure of forestry ground workers to 2,4-D, picloram and dichlorprop. Environ Toxicol Chem. 1987;6:209–224. [Google Scholar]
- NASS. National Agricultural Statistical Service. Database Agricultural Chemical Statistics. [accessed 5/12/2006]. http://www.pestmanagement.info/nass/app_graph.cfm.
- Nolan RJ, Rick DL, Freshour NL, Saunders JH. Chlorpyrifos: pharmacokinetics in human volunteers. Toxicol Appl Pharmacol. 1984;73:8–15. doi: 10.1016/0041-008x(84)90046-2. [DOI] [PubMed] [Google Scholar]
- Perry MJ, Marbella A, Layde PM. Nonpersistent pesticide exposure self-report versus biomonitoring in farm pesticide applicators. Ann Epidemiol. 2006;16:701–707. doi: 10.1016/j.annepidem.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Pesticide Handlers Exposure Database (PHED) Reference Manual Version 1.1. Springfield, VA: Versar, Inc; 1995. Feb, U.S. Environmental Protection Agency, Health and Welfare Canada, and the American Crop Protection Association. [Google Scholar]
- Ritter L, Goushelff NC, Arbuckle T, Cole D, Raizenne M. Addressing the linkage between exposure to pesticides and human health effects - research trends and priorities for research. J Toxicol Environ Health B Crit Rev. 2006;9(6):441–456. doi: 10.1080/10937400600755895. [DOI] [PubMed] [Google Scholar]
- Sathiakumar N, Delzell E. A review of epidemiologic studies of triazine herbicides and cancer. Crit Rev Toxicol. 1997;27(6):599–612. doi: 10.3109/10408449709084405. [DOI] [PubMed] [Google Scholar]
- Sauerhoff MW, Braun WH, Blau GE, Gehring PJ. The fate of 2,4-dichlorophenoxyacetic acid (2,4-D) following oral administration to man. Toxicol. 1977;8:3–11. doi: 10.1016/0300-483x(77)90018-x. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Occupational and Residential Exposure Test Guidelines: OPPTS 875.1000, Background for application exposure monitoring test guidelines. EPA712-C-96-261. 1996 [Google Scholar]
- U.S. EPA. Reregistration Eligibility Decision for 2,4-D. Prevention, Pesticides, and Toxic Substances. [accessed 10/9/2007];EPA 738-R-005-02. 2005 http://www.epa.gov/oppsrrd1/REDs/24d_red.pdf.
- Zahm SH, Ward MH, Blair A. Pesticides and Cancer. In: Keifer MC, editor. Human Health Effects of Pesticides, State of the Art Reviews, Occup. Med. 2. Vol. 12. 1997. pp. 269–290. [PubMed] [Google Scholar]