Abstract
The human airway epithelium is a pseudostratified heterogenous layer comprised of ciliated, secretory, intermediate, and basal cells. As the stem/progenitor population of the airway epithelium, airway basal cells differentiate into ciliated and secretory cells to replenish the airway epithelium during physiological turnover and repair. Transcriptome analysis of airway basal cells revealed high expression of vascular endothelial growth factor A (VEGFA), a gene not typically associated with the function of this cell type. Using cultures of primary human airway basal cells, we demonstrate that basal cells express all of the three major isoforms of VEGFA (121, 165 and 189) but lack functional expression of the classical VEGFA receptors VEGFR1 and VEGFR2. The VEGFA is actively secreted by basal cells and while it appears to have no direct autocrine function on basal cell growth and proliferation, it functions in a paracrine manner to activate MAPK signaling cascades in endothelium via VEGFR2-dependent signaling pathways. Using a cytokine- and serum-free co-culture system of primary human airway basal cells and human endothelial cells revealed that basal cell-secreted VEGFA activated endothelium to express mediators that, in turn, stimulate and support basal cell proliferation and growth. These data demonstrate novel VEGFA-mediated cross-talk between airway basal cells and endothelium, the purpose of which is to modulate endothelial activation and in turn stimulate and sustain basal cell growth.
Keywords: Basal cell, Endothelium, VEGF-A, Airway, Cross-talk, Paracrine
Introduction
The human bronchial tree is a branching structure of up to 23 generations that functions as a conduit of air to and from the alveoli [1, 2]. The bronchial tree is lined with a pseudostratified heterogeneous epithelium composed of four major cell types: ciliated, secretory, intermediate, and basal [3–5]. The classic role of the basal cell population is to function as stem/progenitor cells that, with appropriate signals, differentiate into intermediate cells and finally the specialized ciliated and secretory cells [6–11]. Utilizing methodology developed in our laboratory to culture pure populations of human airway basal cells from the complete airway epithelium obtained by brushing the airway epithelium of healthy non-smokers, we recently characterized the transcriptome of basal cells of healthy individuals [11].
Analysis of the human airway basal cell transcriptome uncovered expression of a variety of genes/pathways linked to the known stem/progenitor cell function of these cells, but also identified that basal cells express genes coding for molecules not typically associated with epithelial structure and function [11]. Among these genes was vascular endothelial growth factor A (VEGFA), the product of which is primarily associated with vascular endothelial growth and function [12, 13]. The VEGF family of receptors and ligands are critical regulators of vascular and lymphatic function during development and in health and disease [13–16]. There are five structurally related mammalian VEGF ligands [VEGFA, B, C, and D, and placenta growth factor (PLGF)], three receptors (VEGFR1, 2 and 3) and two co-receptors (neuropilin-1 and 2) that interact in various combinations to modulate vascular-related biological processes [12–14, 17]. Vascular endothelial growth factor A functions as a highly potent pro-angiogenic factor [12], and its signaling is mediated through direct binding of the ligand to the tyrosine kinase receptors VEGFR1, VEGFR2, and subsequent activation of downstream kinase signaling cascades [13, 16, 18].
Together, these observations lead to the hypothesis that airway basal cells may have a novel function beyond the role as stem/progenitor cells, i.e., do human airway basal cells support the structure and function of lung endothelial cells by expressing and secreting VEGFA? Using cultures of primary human airway basal cells and human endothelial cells alone and together, the data demonstrate that human airway basal cells express all of the three major isoforms of VEGFA (121, 165, and 189) but lack functional expression of the classical VEGFA receptors VEGFR1 and 2. The VEGFA is actively secreted by basal cells and while it appears to have no direct autocrine function on basal cell growth and proliferation, it functions in a paracrine manner to activate MAPK signaling cascades in endothelium via VEGFR2-dependent signaling pathways, with consequent endothelial cell-mediated reciprocal activation of basal cell proliferation. Overall, these data suggest a novel function of human airway basal cells to regulate activation of endothelium in a paracrine manner via secretion of VEGFA. In turn, activated endothelium express mediators that stimulate and support basal cell proliferation. Regulation of this molecular cross-talk between basal and endothelial cells may play an important role in health and disease.
Methods
Sampling the airway epithelium
Subjects were evaluated at the Department of Genetic Medicine Clinical Research Facility and the Weill Cornell NIH Clinical Translational Science Center (CTSC) or the Rockefeller University CTSC using Institutional Review Board-approved clinical protocols. All subjects were confirmed to be nonsmokers by urine levels of nicotine (<2 ng/ml) and cotinine (<5 ng/ml) with normal pulmonary functions tests and chest X-ray. After obtaining written informed consent, flexible bronchoscopy was used to collect large airway epithelial cells by brushing the epithelium as previously described [19–21]. Cells were detached from the brush by flicking into 5 ml of ice-cold Bronchial Epithelium Basal Medium (BEGM, Lonza, Basel, Switzerland). An aliquot of 0.5 ml was used for differential cell count. The remainder (4.5 ml) was processed immediately for either immediate RNA extraction or basal cell culture. The number of cells recovered by brushing was determined by counting on a hemocytometer. To quantify the percentage of epithelial and inflammatory cells and the proportions of basal, ciliated, secretory, and intermediate cells recovered, cells were prepared by centrifugation (Cytospin 11, Shandon Instruments, Pittsburgh, PA) and stained with Diff-Quik (Baxter Healthcare, Miami, FL). In all samples, the epithelial cells represented >97% of the cell population. The proportions of epithelial cells were as previously reported [19, 21].
Culture and characterization of primary human airway basal cells
Pure populations of human airway basal cells were obtained and characterized using the detailed protocols described in Hackett et al. [11]. Briefly, airway epithelial cells collected by brushing were pelleted by centrifugation (250 × g, 5 min) and disaggregated by resuspension in 0.05% trypsin–ethylenediaminetetraacetic acid (EDTA) for 5 min, at 37°C. Trypsinization was stopped by addition of HEPES buffered saline (Lonza, Basel, Switzerland), supplemented with 15% fetal bovine serum (FBS; GIBCO-Invitrogen, Carlsbad, CA), and the cells were again pelleted at 250 × g, 5 min. The pellet was resuspended with 5 ml of phosphate buffered saline, pH 7.4 (PBS), at 23°C, then centrifuged at 250 × g, 5 min. Following centrifugation, the PBS was removed, the cells resuspended in 5 ml of BEGM and 5 × 105 cells were cultured in T25 flasks in BEGM (Lonza, Basel, Switzerland), supplemented with growth factors according to the manufacturer’s instructions. The antibiotics supplied by the manufacturer were replaced with gentamicin (50 μg/ml; Sigma, St. Louis, MO), amphotericin B (1.25 μg/ml; Invitrogen, Carlsbad, CA), and penicillin–streptomycin (50 μg/ml; Invitrogen, Carlsbad, CA). Cultures were maintained in a humidified atmosphere of 5% CO2, 37°C. Unattached cells were removed by changing medium after 12 h. Thereafter, media was changed every 2 days until time of harvest at day 7 of culture, when the cells had reached 70–80% confluence. For sub-culturing, the cells were seeded at a density of 104 cells/cm2 and maintained in an identical manner. At day 7 of the initial culture, the basal cells were trypsinized and cytospin slides prepared for characterization by immunohistochemistry, as described below, using the following cell-type-specific markers: cytokeratin 5 (basal cell; 1/50; Thermo Scientific, Rockford, IL); p63 (basal cell; 1/50; Santa Cruz Biotechnology Inc., Santa Cruz, CA); CD151 (basal cell; 1/200; Leica Microsystems Inc., Bannockburn, IL); N-cadherin (mesenchymal cell; 1/2,500; Invitrogen, Carlsbad, CA); mucin 5AC (secretory cell; 1/50; Vector Laboratories, Burlingame, CA); TFF3 (secretory cell; 1/1,000; Santa Cruz Biotechnology Inc., Santa Cruz, CA); β-tubulin IV (ciliated cell; 1/2,000 dilution; Biogenex, San Ramon, CA); chromogranin A (neuroendocrine cell; 1/5,000; Thermo Scientific, Rockford, IL) and CGRP (neuroendocrine cell; 1/500; Sigma, St. Louis, MO). Only cultures that were >95% positive for basal cell markers and negative for other cell types were used in this study. To maintain consistency, air–liquid interface (ALI) and proliferation experiments were performed using passage two cells.
Gene expression
Genome-wide gene expression analysis of basal cells and large airway epithelium was assessed using the HG-U133 Plus 2.0 array (Affymetrix, Santa Clara, CA) as previously described [11]. TaqMan real-time RT-PCR was performed on RNA samples from the complete large airway epithelium of healthy non-smokers, cultured basal cells derived from the same region and human umbilical cord vein endothelial cells (HUVECs). cDNA was synthesized from 1 µg RNA in a 50-µl reaction volume, using the TaqMan reverse transcriptase reaction kit (Applied Biosystems, Foster City, CA), with random hexamers as primers. Two dilutions, 1:20 and 1:200, were made from each sample, and duplicate wells were run for each sample. TaqMan PCR reactions were carried out using the following gene-specific expression kits from Applied Biosystems: VEGFA all isoforms (Hs00900054_m1); VEGFA-121 (Hs03929005_m1); VEGFA-165 (Hs00900057_m1); VEGFA-189 (Hs00903129_m1); VEGFR1 (Hs01052961_m1); VEGFR2 (Hs 00911700_m1) and NRP-1 (Hs 00826128_m1). The endogenous control was human 18S rRNA (Applied Biosystems). Relative expression levels were determined using the ΔΔC t method, with the average value of expression in complete airway epithelium as the normalizer [22]. The PCR reactions were run in an Applied Biosystems Sequence Detection System 7500, and the relative quantity was determined using the algorithm provided by the manufacturer.
The mRNA levels of specific VEGFA isoforms were assessed by RT-PCR using forward (5′-TGCAGACCAAAGAAAGATAGAGCAAGA-3′) and reverse (5′-CCCTGAGGGAGGCTCCTTCCT-3′) primers that bind within exons 5 and 8a of VEGFA, respectively. These primers give rise to an expected product of 86 bp for VEGFA-121, 218 bp for VEGFA-165, and 290 bp for VEGFA-189. Basal cell cDNA was synthesized using the method described above. All reactions were performed using Platinum® PCR Supermix (Invitrogen, Carlsbad, CA) in a 50-µl reaction volume and 40 cycles of amplification as follows: denature 94°C, 30 s; anneal. 55°C, 30 s; and extend 72°C, 1 min.
Immunohistochemistry analysis of VEGFA expression
To analyze VEGFA expression in basal cell cultures by immunohistochemistry, the cells were trypsinized, and cytospin slide preparation fixed in 4% paraformaldehyde for 15 min. To enhance staining, an antigen recovery step was carried out by steaming the samples for 15 min in citrate buffer solution (Labvision, Fremont, CA) followed by cooling at 23°C, 20 min. Endogenous peroxidase activity was quenched using 0.3% H2O2, and normal serum matched secondary antibody was used for 20 min to reduce background staining. Samples were incubated overnight at 4°C with the primary antibody, mouse monoclonal anti-human VEGF antibody (1/50; MAB293, R&D Biosystems, Minneapolis, MN). Isotype-matched IgG (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) was the negative control. Vectastain Elite ABC kit and AEC substrate kit (Dako North America Inc., Carpinteria, CA) were used to visualize antibody binding. The sections were counterstained with Mayer’s hematoxylin (Polysciences Inc., Warrington, PA) and mounted using faramount mounting medium (Dako North America Inc.). Bright field microscopy was done using a Nikon Microphot microscope equipped with a Plan 40× numerical aperture (NA) 0.70 objective lens. Images were captured with an Olympus DP70 CCD camera.
Elisa
The secretion of VEGFA by basal cells was assessed by ELISA (R&D Biosystems, Minneapolis, MN). Bronchial Epithelium Basal Medium growth media exposed to basal cell cultures for 2 days was removed and then centrifuged at 250 × g, 5 min to pellet cellular debris. The supernatant was then analyzed by ELISA using the manufacturer’s instructions. Basal (BEBM) and growth (BEGM) media not exposed to basal cells were used as a negative control. To determine whether basal-cell-derived VEGFA is secreted apically or basolateral, ALI cultures of basal cells were established as described below. At day 12 of ALI culture, when tight junctions are established, fresh media was exclusively added to either the lower or upper chamber of the cultures. Two days post-incubation, the media was removed and processed to determine the levels of secreted VEGFA as described above. ALI media not exposed to basal cells were used as a negative control.
Air–liquid interface culture
Primary airway basal cells were trypsinized and seeded at a density of 6 × 105 cells/cm2 onto a 0.4-µm pore-sized Costar™ Transwell inserts (Corning Incorporated, Corning, NY) pre-coated with type IV collagen (Sigma, St. Louis, MO). The initial culture medium consisted of a 1:1 mixture of DMEM and Ham’s F-12 medium (GIBCO-Invitrogen, Carlsbad, CA) containing 100 U/ml penicillin, 5% fetal bovine serum, 100 µg/ml streptomycin, 0.1% gentamicin, and 0.5% amphotericin B. The following day, the medium was changed to 1:1 DMEM/Ham’s F-12 (including antibiotics described above) with 2% Ultroser G serum substitute (BioSerpa SA, Cergy-Saint-Christophe, France). Two days post-seeding, once the cells had reached confluence on the membrane, the media was removed from the upper chamber to expose the apical surface to air and establish the ALI (referred to as ALI “day 0”). The cells were then grown at 37°C, 8% CO2, and the culture medium was changed every other day. Following 5 days on ALI, the cells were grown at 37°C, 5% CO2 until required.
Basal cell proliferation
Proliferation assays were used to assess the ability of specific blocking antibodies to inhibit basal cell proliferation under growth factor-rich culture conditions. Basal cells were (2 × 104) seeded into each well of a 12-well plate in BEGM growth media. The next day (termed day 0) the media was removed and the cells were washed twice with PBS before the addition of fresh BEGM. For inhibition of basal cell proliferation, anti-VEGFA antibody (Bevacizumab; 0.1 μg/ml; Genentech), human VEGFR2 neutralizing antibody 1121 (1 μg/ml; ImClone, New York, NY) or IgG isotype control was added to the media at the desired concentration. Fresh media and antibody was added every 2 days of culture throughout the course of the experiment. At the desired time points, cells were trypsinized and total cell numbers were measured with a hemocytometer and the viability assessed by counting of Trypan blue dye-excluded cells.
Western-blot analysis
Cells were trypsinized and lysed in radioimmunoprecipitation lysis (RIPA) buffer (Sigma, St. Louis, MO) plus complete protease inhibitor cocktail (Roche, Mannheim, Germany) and halt phosphatase inhibitor cocktail (Pierce, Rockford, IL), and incubated on ice for 30 min. Lysates were clarified by centrifugation at 22,500 × g for 10 min in an Eppendorf 5415C microcentrifuge at 4°C. Total protein concentration was measured using the Bio-Rad (Hercules, CA) protein assay to the manufacturer’s guidelines. NuPAGE® LDS sample buffer (4×; Invitrogen, Carlsbad, CA) supplemented with 200 mM dithiothreitol (DTT) was added to each sample before boiling for 10 min and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). For analysis of protein phosphorylation, cells were lysed directly in the dish using 1× NuPAGE LDS sample buffer (diluted in RIPA buffer containing complete protease inhibitor cocktail (Roche, Mannheim, Germany), halt phosphatase inhibitor cocktail (Pierce, Rockford, IL) and 50 mM DTT). Once lysed, the samples were then transferred to a 1.5-ml Eppendorf tube before boiling for 10 min and SDS-PAGE. All proteins were analyzed using NuPAGE 4–12% Bis–Tris gradient gels (Invitrogen, Carlsbad, CA) and subsequently transferred onto nitrocellulose membranes with a Bio-Rad semi-dry apparatus before Western analysis. The membranes were then blocked overnight at 4°C in 4% blocking reagent made in PBS containing 0.1% Tween-20 (PBST). Nonfat milk was used as a standard blocking reagent for general protein analysis. This was replaced with bovine serum albumin for analysis of protein phosphorylation. After blocking the membranes overnight, immobilized proteins were reacted with cell-type-specific antibodies in 4% blocking reagent for 1 h, 23°C with shaking. Following the primary antibody incubation, membranes were washed three times for 5 min each with PBST, incubated with an anti-rabbit or anti-mouse antibody conjugated to horseradish peroxidase in 4% blocking reagent for 1 h, at 23°C with shaking. Upon completion of secondary antibody incubation, the membranes were washed again three times for 5 min with PBST and twice with PBS, and antibodies were visualized after the addition of ECL Western blotting detection reagents (GE Healthcare Biosciences, Pittsburgh, PA) by exposure to X-ray film.
The primary antibodies used for Western analysis included: rabbit polyclonal anti-human VEGFR1 (1/1,000, #2893, Cell Signaling Technology, Danvers, MA); rabbit monoclonal anti-human VEGFR2 (55B11; 1/1,000, #2479, Cell Signaling Technology, Danvers, MA); rabbit monoclonal anti-human phospho-VEGFR2 (Tyr 1,175; 19A10; 1/1,000, #2478, Cell Signaling Technology, Danvers, MA); rabbit monoclonal anti-human neuropilin-1 (NRP-1; 1/1,000, ab81321, Abcam, Cambridge, MA); rabbit polyclonal anti-human p44/42 MAPK (Erk1/2; 1/1,000, #9102, Cell Signaling Technology, Danvers, MA); rabbit polyclonal anti-human phospho-p44/42 MAPK (Erk1/2; Thr202/Tyr204; 1/1,000, #9101, Cell Signaling Technology, Danvers, MA); rabbit polyclonal anti-human p38 MAPK (1/1,000, #9212, Cell Signaling Technology, Danvers, MA); rabbit polyclonal anti-human phospho-p38 MAPK (Thr180/Tyr182; 1/1,000, #9211, Cell Signaling Technology, Danvers, MA); mouse monoclonal anti-human β-actin (1/10,000; Santa Cruz Biotechnology) and mouse monoclonal anti-human α-tubulin (1/10,000; Santa Cruz Biotechnology).
Analysis of MAPK activation
Equal numbers of HUVEC and basal cells were seeded per dish in the appropriate growth media. The following day, cells were washed twice with PBS and then serum-starved for at least 6 h in the appropriate cell-type-specific serum-free media. Following serum starvation, the cells were stimulated for 15 min with either: (1) base media (BEBM); (2) conditioned media (BEBM exposed overnight to cultured basal cells) or (3) base media supplemented with recombinant VEGFA-165 (50 ng/ml; R&D Biosystems). After stimulation, the media was aspirated from the cells and the cells were washed once with PBS. Following removal of the PBS, the cells were lysed directly in the dish with 1× NuPAGE LDS sample buffer (diluted in RIPA buffer containing complete protease inhibitor cocktail, halt phosphatase inhibitor cocktail and 50 mM DTT) and processed for Western analysis as described above. Vascular endothelial growth factor receptor 2-dependent MAPK activation was evaluated using both phosphor- and pan-specific antibodies targeted against VEGFR2, p44/p42 MAPK, and p38 MAPK. The data shown are representative of four independent experiments.
Culture and maintenance of HUVEC
Human umbilical cord vein endothelial cells (HUVECs) were isolated as previously described [23]. Cells were cultured in endothelial cell growth medium (medium 199; Sigma, St. Louis, MO), 20% (v/v) fetal bovine serum, 20 μg/ml endothelial cell growth supplement (Hallway), 1% (v/v) antibiotics (Hallway), and 20 units/ml heparin (Sigma, St. Louis, MO). HUVEC-Akt cells were generated as previously described [23]. Briefly, HUVEC cells were transduced with a lentivirus expressing myristoylated Akt and a GFP marker at a multiplicity of infection of ten and maintained in an identical manner to HUVEC.
Co-culture proliferation assays
Co-culture proliferation assays were used to assess the ability of endothelial cells to support basal cell proliferation in cytokine- and serum-free conditions. HUVEC-Akt cells (5 × 104) were seeded into each well of a 12-well plate in HUVEC growth media. The next day the media was removed and the cells washed twice with PBS followed by seeding 2 × 104 basal cells into each well in BEGM growth media. The next day (termed day 0), the media was removed and the cells were washed twice with PBS before the addition of cytokine- and serum-free BEBM media. The cells were then incubated and subsequently harvested and counted at the desired time points. As a control, basal cells were seeded into wells containing no HUVEC-Akt controls and subsequently treated in an identical manner to those in co-culture. For inhibition of basal cell proliferation in co-culture, human VEGFR2-neutralizing antibody 1121 (ImClone, New York, NY) or IgG isotype control was added to the media at a concentration of 1 μg/ml. Fresh media and antibody was added every 2 days of culture throughout the course of the experiment.
At the desired time points, cells were trypsinized and total cell numbers were measured with a hemocytometer and the viability assessed by counting of Trypan blue dye-excluded cells. The population of GFP-labeled HUVEC-Akt cells in the harvested sample was determined as the GFP+VE-cadherin+ population by flow cytometric analysis, and the GFP−VE-cadherin− population quantified as expanded basal cells.
Results
Expression of VEGFA in human airway basal cells
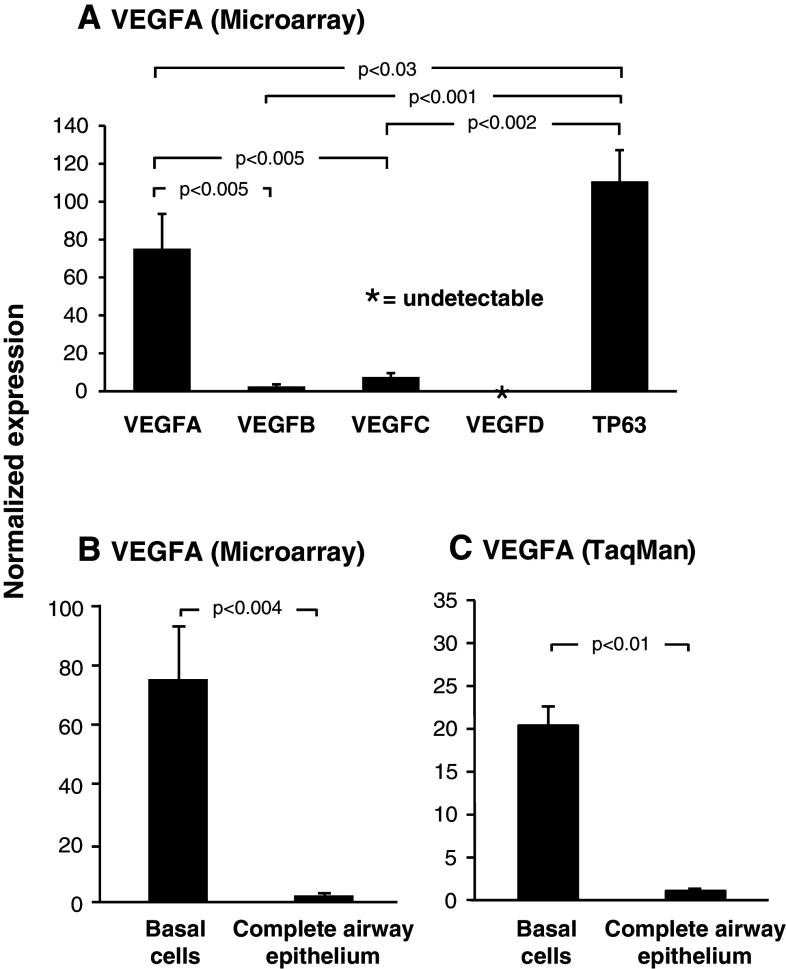
Microarray analysis of VEGF ligand (VEGFA, VEGFB, VEGFC, and VEGFD) expression in airway basal cells revealed differential abundance of each. VEGFA was the most highly expressed ligand, while both VEGFB (p < 0.005) and VEGFC (p < 0.005) were expressed at significantly lower levels compared to VEGFA (Fig. 1a). Analysis of multiple probe sets for VEGFD confirmed that the ligand was not expressed in basal cells. Comparison of each ligand’s expression level with that of the basal cell specific gene TP63 revealed each was expressed at significantly lower levels (VEGFA, 1.5-fold lower, p < 0.03; VEGFB, 43.5-fold lower, p < 0.001 and VEGFC, 14.7-fold lower, p < 0.002; Fig. 1a). Due to the high expression level of VEGFA in basal cells compared to the other ligands and its known role in lung biology, we focused the remainder of our study on this ligand.
Fig. 1.
Vascular endothelial growth factor A is highly expressed in cultured human airway basal cells. a Microarray analysis of VEGF ligand expression VEGFA (probeset: 212171_x_at), VEGFB (probeset: 203683_s_at), VEGFC (probeset: 209946_at) and VEGFD (probset: 206742_at) in basal cells (n = 4). For comparison, expression levels of the basal-cell-specific gene TP63 (probeset: 209863_s_at) is included. b Microarray analysis of VEGFA expression (probeset: 212171_x_at) in basal cells (n = 4) compared to complete airway epithelium (n = 22). c TaqMan analysis of VEGFA expression (all isoforms) in basal cells (n = 3) compared to complete airway epithelium (n = 3)
Further analysis of VEGFA revealed that it was highly expressed in cultured human basal cells relative to the complete airway epithelium, with a relative difference of 75.2-fold (p < 0.004; Fig. 1b). TaqMan quantitative PCR using specific primers and probe for VEGFA confirmed the microarray data and demonstrated VEGFA was expressed 20.2-fold (p < 0.01) higher in basal cells relative to the complete airway epithelium (Fig. 1c).
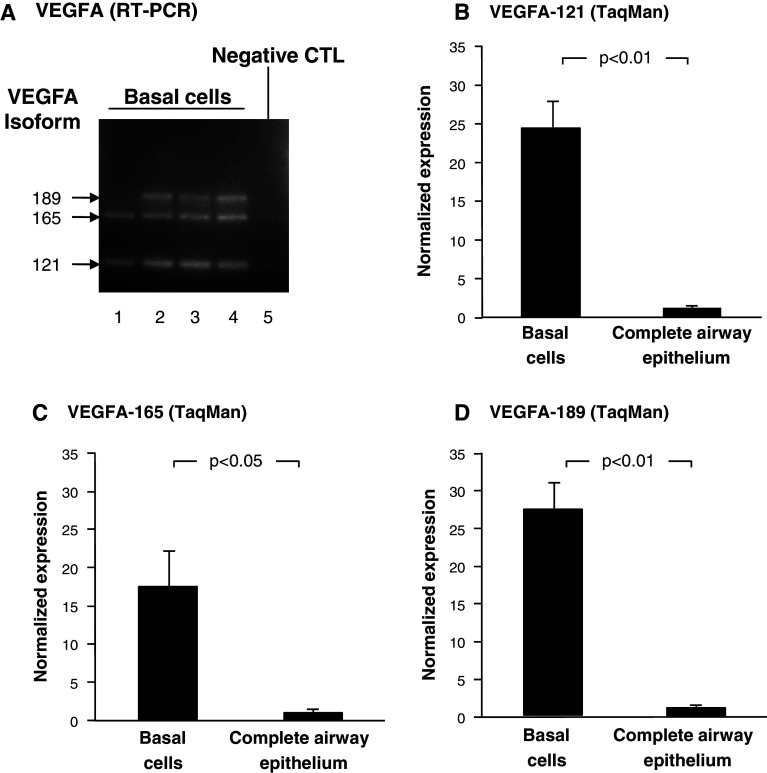
The gene encoding VEGFA has three major splice variants, including VEGFA-121, 165, and 189 [12, 17]. To determine which VEGFA isoforms were expressed by human airway basal cells, two approaches were employed. First, forward and reverse PCR primers were designed to amplify products of a unique size for each isoform. RT-PCR analysis revealed that human airway basal cells predominantly express all three major VEGFA isoforms 121, 165, and 189 (Fig. 2a, lanes 1–4). No PCR products were present in the negative control, confirming the specificity of the result (Fig. 2a, lane 5). Second, TaqMan quantitative PCR was carried out using specific primers and probe for each VEGFA isoform (Fig. 2b–d). As expected, VEGFA-121 (Fig. 2b), 165 (Fig. 2c) and 189 (Fig. 2d) were highly expressed in basal cells relative to the complete airway epithelium. Vascular endothelial growth factor A-121 expression was 24.4-fold higher in basal cells relative to the complete airway epithelium (p < 0.01). Whereas, VEGFA-165 and 189 were 17.3-fold (p < 0.05) and 27.5-fold (p < 0.01) higher, respectively.
Fig. 2.
Expression of VEGFA isoforms 121, 165, and 189 in cultured human airway basal cells. a PCR amplification of VEGFA isoforms in basal cells (n = 4). Lanes 1–4: examples of four independent basal cell cultures. VEGFA-121, VEGFA-165, and VEGFA-189 are expressed. Lane 5: negative control (no DNA added). b–d TaqMan analysis of VEGFA isoform expression in basal cells (n = 3) compared to complete airway epithelium (n = 3) using isoform specific probes. b VEGFA-121, c VEGFA-165, and d VEGFA-189
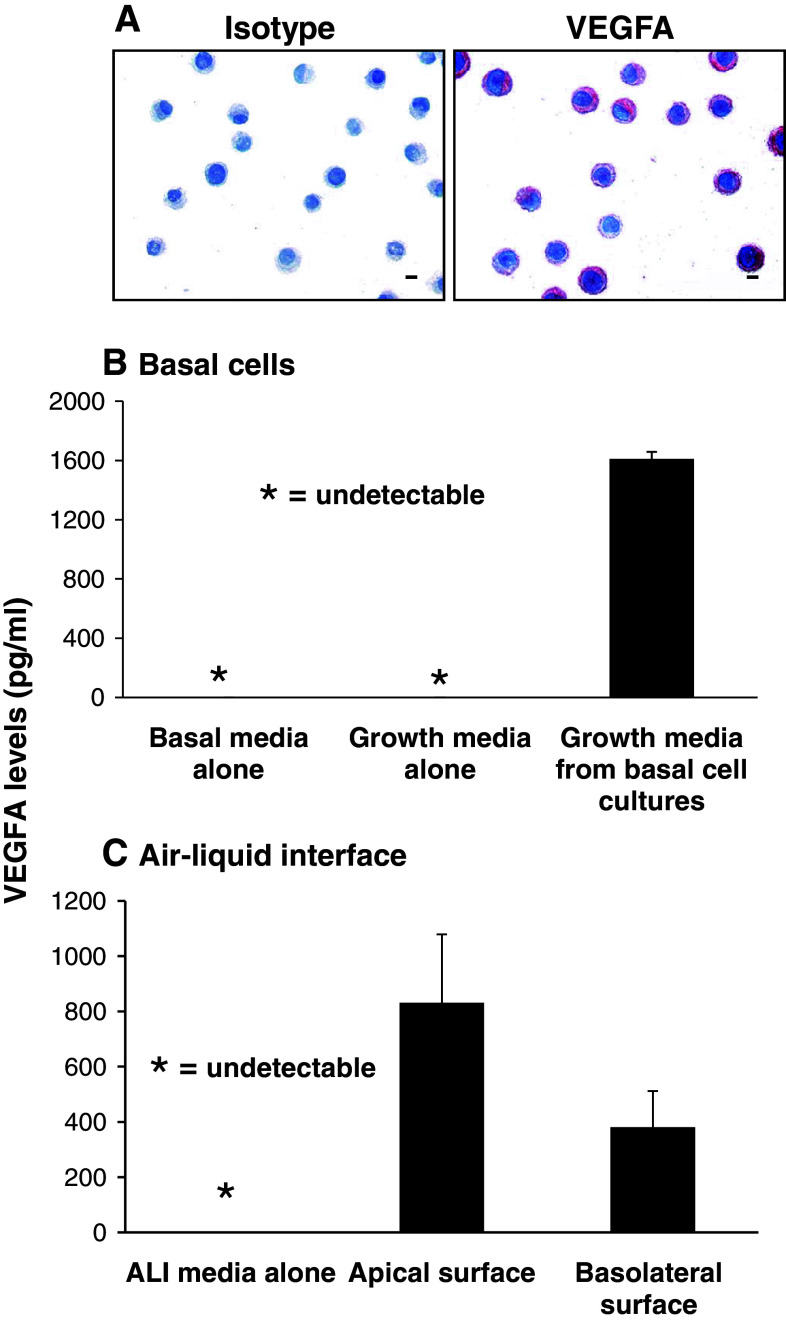
To further confirm the gene expression data, anti-VEGFA immunohistochemistry was carried out on cytospin preparations of cultured basal cells with the appropriate isotype used as a negative control (Fig. 3a). Consistent with the gene expression, immunohistochemical staining confirmed expression of VEGFA in human airway basal cells with all cells staining positive.
Fig. 3.
Expression and secretion of VEGFA by cultured human airway basal cells. a Immunohistochemical staining of VEGFA in human airway basal cells (bar = 10 μm). b VEGFA secretion by human airway basal cells. VEGFA levels assessed by ELISA in basal media, growth media, and growth media from basal cell cultures (n = 9). c VEGFA secretion by human airway basal cells during air–liquid interface culture (ALI). VEGFA levels assessed by ELISA in ALI media, and ALI media exclusively exposed to the apical surface (upper chamber) or basolateral surface (lower chamber) of basal cells during ALI culture (n = 3)
VEGFA is actively secreted by human airway basal cells
The secretion of VEGFA by human airway basal cells was assessed using ELISA (Fig. 3b). Growth media exposed to basal cell cultures for 2 days was removed and processed as described in the Methods section. While basal and growth media were negative for VEGFA, growth media exposed to basal cell cultures contained high levels of VEGFA. Analysis for nine independent cultures revealed an average level of 1,600 pg/ml of VEGFA. To determine whether VEGFA was secreted from the apical or basolateral surface of basal cells, ALI cultures of basal cells were established as described in the materials and methods. At day 12 of ALI culture, when tight junctions had been established, ALI media was added exclusively to the apical surface (upper chamber) or basolateral surface (lower chamber) of basal cells and following 2 days of incubation, removed and processed. As expected, the ALI media was negative for VEGFA; however, media exposed to both the apical and basolateral surface of basal cells were positive for VEGFA (Fig. 3c). Analysis of three independent cultures revealed an average level of 830 pg/ml of VEGFA on the apical surface vs. an average level of 381 pg/ml of VEGFA on the basolateral surface.
Expression of VEGFA-associated receptors in human airway basal cells
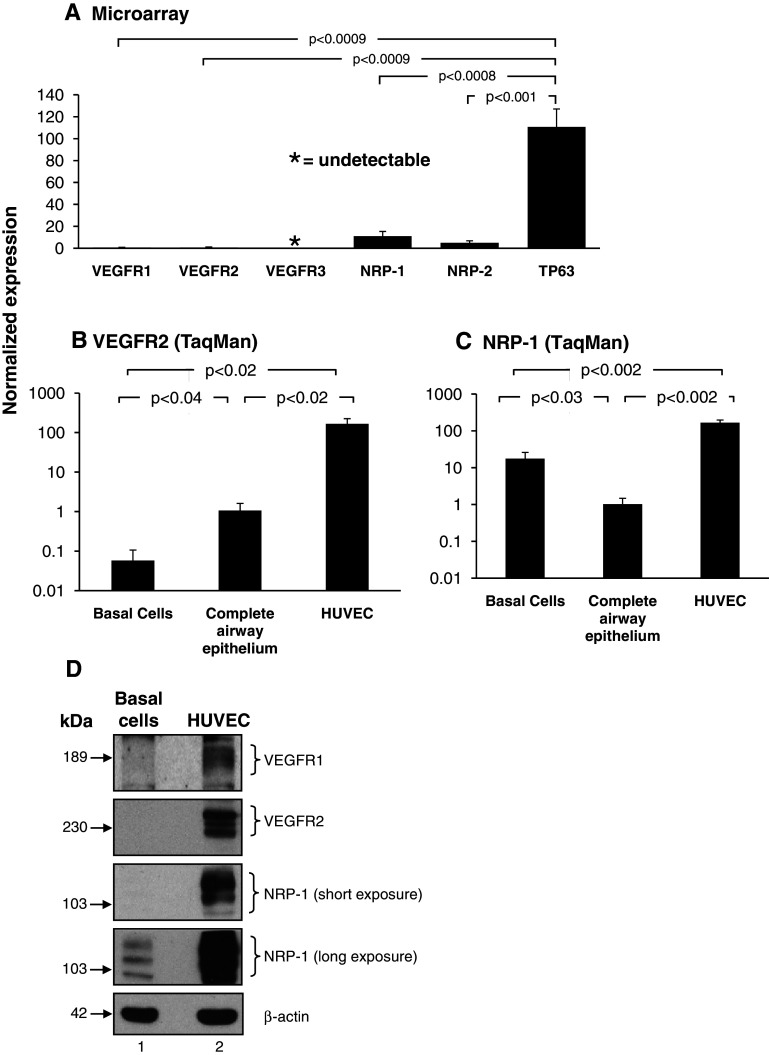
To further characterize the VEGF signaling pathway in basal cells, we assessed the expression of the VEGF receptors (VEGFR1, VEGFR2, and VEGFR3) and co-receptors (NRP-1 and NRP2). Microarray analysis revealed moderate expression of NRP-1 and NRP-2 in cultured human basal cells, whereas VEGFR1 and VEGFR2 were almost undetectable, with each demonstrating a normalized expression value of less than 1. Vascular endothelial growth factor receptor 3 was undetected in all the probe sets analyzed (Fig. 4a). Compared to the basal-cell-specific gene TP63, all receptors and co-receptors were expressed at significantly lower levels (VEGFR1, 173-fold lower, p < 0.0009; VEGFR2, 176-fold lower, p < 0.0009, NRP-1, tenfold lower, p < 0.0008 and NRP-2, 22.3-fold lower, p < 0.001; Fig. 4a).
Fig. 4.
Expression of VEGF receptors and co-receptors in cultured human airway basal cells. a Microarray analysis of VEGFR1 (probeset: 226497_s_at), VEGFR2 (probeset: 203934_at), VEGFR3 (probeset: 210316_at), NRP-1 (probeset: 212298_at) and NRP-2 (probeset: 229225_at) expression in basal cells (n = 4). For comparison, expression levels of the basal-cell-specific gene TP63 (probeset: 209863_s_at) are included. b–c TaqMan analysis of VEGFR2 and NRP-1 expression in basal cells (n = 4) and human umbilical vein endothelial cells (HUVEC) (n = 4) compared to complete airway epithelium (n = 4) using specific probes. b VEGFR2, c NRP-1. d Western-blot analysis of VEGFR1, VEGFR2, and NRP-1 in basal cells compared to HUVEC. Lane 1: basal cells; lane 2: HUVEC. For both cell types, shown is expression of VEGFR1, VEGFR2, NRP-1 (short and long exposure) and β-actin as a loading control
Vascular endothelial growth factor A signaling is mediated through direct binding of the ligand to the tyrosine kinase receptors VEGFR1, VEGFR2, and subsequent activation of downstream kinase-signaling cascades [12, 13, 16–18]. The ability of VEGFA to bind and activate these receptors is regulated by the co-receptor neuropilin-1 (NRP-1), which can directly bind VEGFA and function as a bridging molecule between ligand and receptor [14, 24]. To further validate the microarray expression data for the VEGFA-dependent receptors and co-receptors, TaqMan quantitative PCR using specific primers and probe for VEGFR1, VEGFR2, and NRP-1 was performed (Fig. 4b–c). The results showed that VEGFR2 was expressed in basal cells; however, as expected, the levels were extremely low and significantly lower relative to the complete airway epithelium (18.7-fold lower, p < 0.04, Fig. 4b) and human umbilical vein endothelial cells (HUVEC) (2,912-fold lower, p < 0.02, Fig. 4b). Direct comparison of VEGFR2 expression in the complete airway epithelium with HUVEC cells demonstrated a significantly lower level of epithelial expression (156-fold lower, p < 0.02). Vascular endothelial growth factor receptor 1 expression was undetected in most complete airway epithelium samples tested (three out of four tested) and undetected in all basal cell samples (not shown). These low levels of VEGFR1 expression in complete airway epithelium are consistent with a previous study [25]. In contrast to VEGFR1 and VEGFR2, NRP-1 was expressed significantly higher in basal cells compared to the complete airway epithelium (17.2-fold higher, p < 0.03; Fig. 4c). However, when compared to HUVEC cells, the levels were significantly lower (9.5-fold lower, p < 0.002, Fig. 4c). Western-blot analysis of human airway basal cells using antibodies directed against VEGFR1, VEGFR2, and NRP-1, with HUVEC as a positive control, demonstrated that VEGFR1 and VEGFR2 protein were undetected in airway basal cells even following long exposures, whereas NRP-1 was expressed and readily detectable (Fig. 4d, lane 1). The VEGFR2 Western-blot analysis data suggests that even though basal cells express a low level of VEGFR2 mRNA, the resulting transcripts are not translated into detectable levels of protein. As expected HUVEC cells expressed high levels of all three proteins (Fig. 4d, lane 2). Overall, the gene expression and Western analysis data demonstrate that even though basal cells express moderate levels of the co-receptor NRP-1, they lack functional expression of the VEGFA-dependent signaling receptors VEGFR1 and VEGFR2.
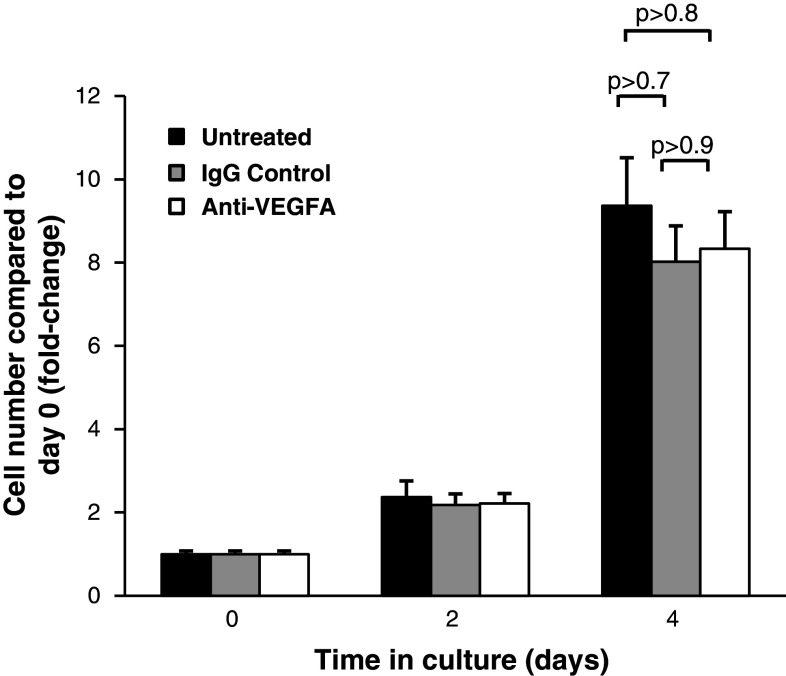
Inhibition of VEGFA has no effect on proliferation of airway basal cells
To further dissect the role of VEGFA in basal cell biology, we next investigated the effect of specifically inhibiting VEGFA on basal cell growth and proliferation. Basal cells were cultured alone in regular growth media in the absence and presence of VEGFA blocking antibody or IgG control (Fig. 5). In three independent experiments, there was a minor insignificant decrease in basal cell growth following 4 days of culture in the presence of control IgG relative to untreated cells (eightfold vs. 9.3-fold increase in cell numbers compared to day 0, p > 0.7). Treatment of cells with anti-VEGFA antibody also resulted in a minor insignificant decrease in basal cell growth compared to untreated cells (8.3-fold vs. 9.3-fold increase in cell numbers at day 4 compared to day 0, p > 0.8); however, this was identical to that observed with IgG control. Therefore, inhibition of VEGFA signaling has no specific direct effects on basal cell growth and proliferation, suggesting that basal-cell-derived VEGFA functions in a paracrine rather than autocrine manner.
Fig. 5.
Inhibition of VEGFA signaling has no effect on proliferation of airway basal cells. Human airway basal cells were cultured in growth media and incubated with control IgG or blocking monoclonal antibody against VEGFA. Data shown is the average of three independent experiments. Untreated (black), IgG control (gray), and anti-VEGFR2 (white)
Activation of endothelial cells by basal-cell-derived VEGFA
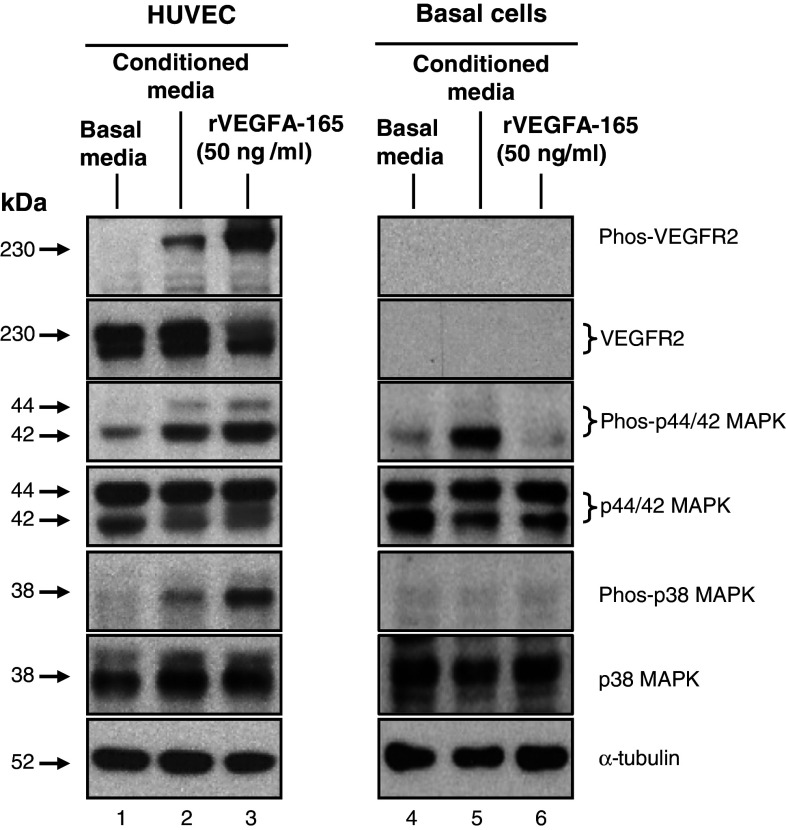
If the function of basal-cell-secreted VEGFA is to regulate activation of signaling in other cell types in a paracrine manner, then which cell types are targeted? Basal cells secrete VEGFA both apically and basolaterally (Fig. 3c). Therefore, it is possible that basal cells can signal to additional cell types of the complete airway epithelium or the underlying stroma. Considering the anatomical location of airway basal cells and their close proximity to the vasculature [6, 9], we investigated whether basal-cell-derived VEGFA could activate signaling cascades in endothelium. To assess this question, HUVEC exposed to basal-cell-derived media were analyzed for the activation of the VEGFR2 receptor by examining the phosphorylation of VEGFR2 and its downstream activated kinases p44/42 MAPK and p38 MAPK [13, 16, 18]. Following serum starvation, HUVEC were stimulated with either basal media, conditioned media (basal media exposed overnight to cultured basal cells) or basal media supplemented with recombinant VEGFA-165. After stimulation, the media was aspirated from the cells and total cell lysates were generated for subsequent Western-blot analysis. In cells stimulated with basal media, no phosphorylated VEGFR2 was detected, demonstrating that classical VEGFA signaling was not initiated (Fig. 6, lane 1). This was further confirmed by low levels of basal phosphorylation of the downstream activated kinases p44/p42 MAPK and p38 MAPK. In contrast, HUVEC stimulated with basal-cell-conditioned media (Fig. 6, lane 2) or basal media containing recombinant VEGFA-165 (Fig. 6, lane 3) showed robust levels of phosphorylated VEGFR2, p44/p42 MAPK, and p38 MAPK relative to the control. To confirm the differences in phosphorylated protein levels between samples that were not the result of differences in total proteins levels, each membrane was stripped and re-probed with antibodies that recognize total cellular VEGFR2, p42/p44 MAPK, and p38 MAPK. In addition, α-tubulin levels were analyzed as a loading control. As expected, the levels of total protein for each were equal for each sample.
Fig. 6.
Secreted VEGFA from airway basal cells activates endothelium via VEGFR2-mediated signaling. Human umbilical vein endothelial cells (HUVEC) and human airway basal cells were serum-starved for 6 h and then stimulated with basal media (without serum or cytokines), basal media conditioned with basal cells, or, as a positive control, basal media containing recombinant VEGFA-165 (50 ng/ml). Following stimulation, cell lysates were prepared and the activation of endothelium and basal cells was evaluated by Western-blot analysis and staining for phosphorylated VEGFR2 (Phos-VEGFR2), phosphorylated p44/42 MAPK (Phos-p44/44 MAPK) and phosphorylated p38 MAPK (Phos-p38 MAPK). The levels of total VEGFR2, p44/42 MAPK, and p38 MAPK were also evaluated. α-tubulin was used as a loading control. Lane 1: lysates of HUVEC exposed to basal cell media alone; lane 2: HUVEC exposed to conditioned media; lane 3: recombinant VEGFA-165; lanes 4–6: identical to lanes 1–3, but with lysates of basal cells
To further investigate the specificity and function of basal-cell-derived VEGFA, the above experiment was repeated, but this time stimulating serum-starved cultured basal cells under the same conditions. The data demonstrated that stimulation of basal cells with recombinant VEGFA (Fig. 6, lane 6) showed no increase in the levels of phosphorylated p44/p42 MAPK and p38 MAPK relative to the basal media stimulated control (Fig. 6, lane 4). As expected, no VEGFR2 or phosphorylated VEGFR2 was detected under any condition (Fig. 6, lanes 4–6). These data show that VEGFA does not activate classical VEGFR2-mediated signaling cascades in basal cells. Interestingly, in cells stimulated with basal cell-conditioned media (Fig. 6, lane 5), a small increase in the level of phosphorylated p44/p42 MAPK relative to control (Fig. 6, lane 4) was observed, suggesting that airway basal cells secrete additional factors that stimulate MAPK activation via VEGFR2 independent mechanisms. Overall, the data confirms that basal-cell-secreted VEGFA is biologically active and functions in a paracrine, rather than autocrine, manner, to activate VEGFR2-mediated signaling cascades of the endothelium.
Activated endothelial cells support the growth of airway basal cells in the absence of growth factors
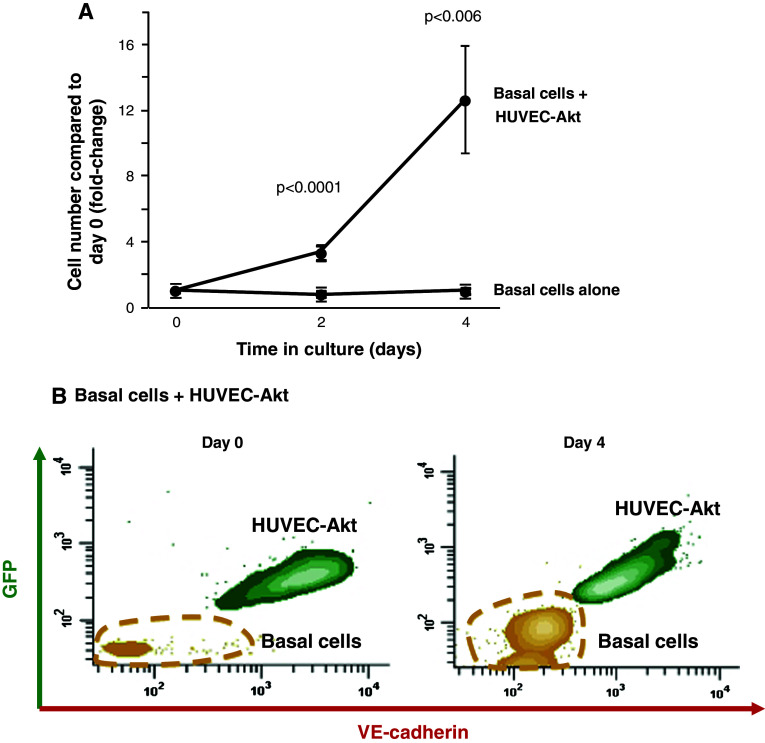
Following the confirmation that basal-cell-derived VEGFA activates endothelium via VEGFR2-dependent signaling, we next asked if this activation was reciprocal and whether activated endothelium could support the growth of basal cells. To answer this question, a cytokine- and serum-free co-culture system was used to examine the growth of airway basal cell in the absence and presence of endothelial cells. Primary endothelial cells require growth factor-enriched media for maintenance in vitro, the deprivation of which results in rapid cell death [26]. To circumvent this issue, we utilized modified HUVEC constitutively expressing Akt activity (HUVEC-Akt) that are capable of surviving cytokine- and serum-free conditions for extended periods of time [23]. Basal cells were cultured alone or in co-culture with HUVEC-Akt cells (in growth factor-negative media) and proliferation was quantified every 2 days. When cultured alone in the absence of growth factors, no basal cell proliferation was observed over the course of 4 days, and cell numbers remained constant relative to day 0 (Fig. 7a). However, when co-cultured with HUVEC-Akt cells, at 4 days post-culture, basal cell proliferation was observed and the total cell number increased 12.7-fold relative to day 0. This increase in cell number was statistically significant when compared to the number of basal cells at day 4 when grown alone (p < 0.006). Expansion of the basal cell population at day 4 of culture vs. day 0 was demonstrated using flow cytometric analysis (Fig. 7b). To investigate if the mitogenic effects of HUVEC cells on basal cell growth are secreted, we performed co-culture experiments in the absence of cell-to-cell contact using the ALI system. In growth factor-negative media, basal cells were cultured in the upper chamber with HUVEC-Akt cells in the lower chamber and proliferation was quantified every 2 days. From two independent experiments, no endothelial-dependent growth support of basal cells was observed (not shown). These data suggest that the mitogenic effects of HUVEC cells are not secreted; however, the distance between the cells in these experiments is large and non-physiological. Therefore, it is possible that factors are secreted but the concentrations are too low to function over this distance.
Fig. 7.
Endothelial cells support the growth of airway basal cells in the absence of growth factors. a Human airway basal cells were cultured alone or in co-culture with Akt-activated human umbilical vein endothelial cells (HUVEC-Akt) in cytokine- and serum-free conditions. At the desired time points, cells were harvested and the GFP-labeled HUVEC-Akt cells was determined as the GFP+VE-cadherin+ population by flow cytometric analysis, and the GFP−VE-cadherin− population quantified as expanded basal cells. Data shown is the average of four independent experiments. b Representative flow cytometric analysis of human airway basal cell and HUVEC-Akt populations at day 0 and day 4 of co-culture. HUVEC-Akt cells were determined as the GFP+VE-cadherin+ population, and the GFP−VE-cadherin− population quantified as expanded basal cells
Overall, these data demonstrate that activated endothelial cells can support and sustain airway basal cell proliferation in the absence of exogenous growth factors.
Inhibition of VEGFR2 signaling suppresses endothelial cell-dependent proliferation of airway basal cells
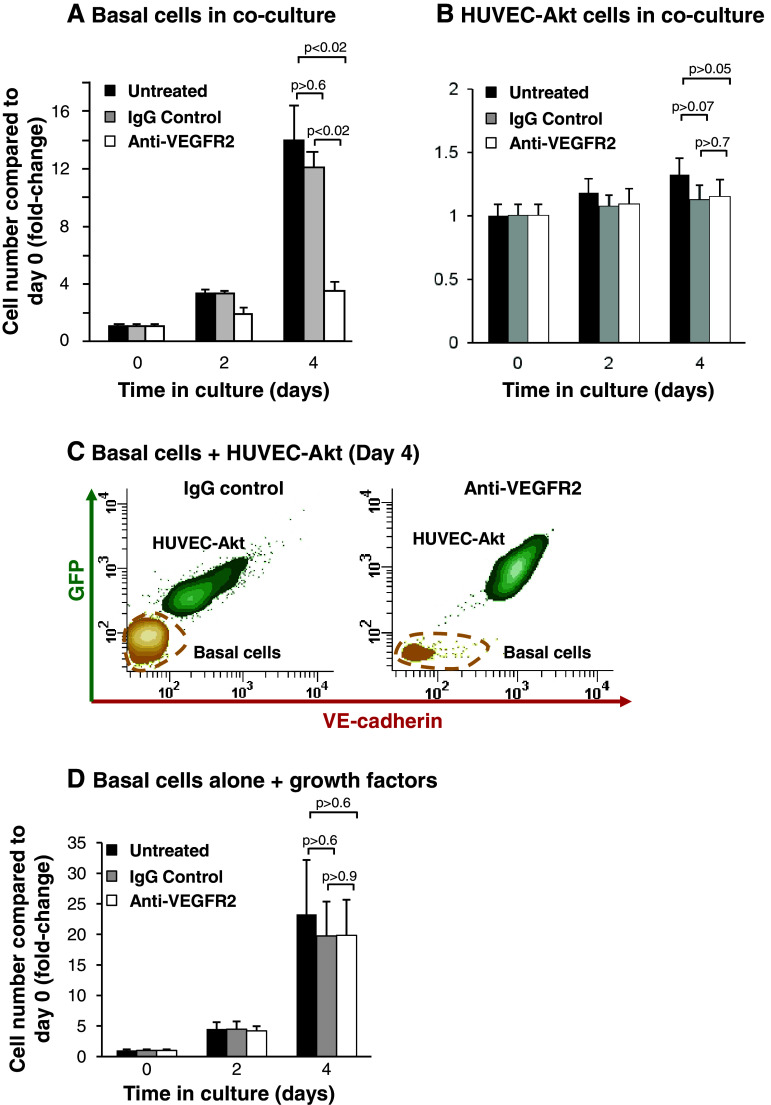
To further characterize the mechanisms regulating endothelial cell-dependent proliferation of airway basal cells, we asked if VEGFA-dependent activation of endothelium cells was required. Using the co-culture system described above, co-cultures of basal and HUVEC-Akt cells were untreated, or incubated with an antibody against VEGFR2 which blocks VEGFA dependent signaling through this receptor. To control for non-specific off-target effects of the antibody, cells were also incubated with an appropriate IgG isotype. As expected, over 4 days of culture, untreated basal cells proliferated with a 14-fold increase in cell numbers compared to day 0 (Fig. 8a). Incubation of cells with IgG had no significant effect (p > 0.6) on basal cell proliferation over 4 days (12.1-fold increase at day 4 compared to day 0) relative to untreated cells (Fig. 8a). However, incubation of cells with a blocking antibody against VEGFR2 significantly suppressed basal cell growth compared to untreated (p < 0.02) and IgG-treated cells (p < 0.02). We next analyzed the effect of VEGFR2 inhibition on the HUVEC-Akt cell population in the same experiments. Over 4 days of culture, the HUVEC-Akt cell population proliferated in co-culture with airway basal cells with a 1.3-fold increase in cell numbers compared to day 0 (Fig. 8b). Incubation of cells with IgG and anti-VEGFR2 resulted in a small decrease in HUVEC-Akt cells numbers (1.1-fold increase at day 4 compared to day 0 for IgG and 1.2-fold increase at day 4 compared to day 0 for anti-VEGFR2, Fig. 8b) relative to untreated cells. However, in both cases, the decreases were insignificant (p > 0.05 for IgG and p > 0.07 for anti-VEGFR2). A reduction in basal cell numbers following treatment with VEGFR2-blocking antibody vs. IgG control at day 4 of culture was demonstrated using flow cytometric analysis (Fig. 8c).
Fig. 8.
Inhibition of VEGFR2 signaling suppresses endothelial cell-dependent proliferation of airway basal cells. a–b Human airway basal cells were co-cultured with human umbilical vein endothelial cells activated with Akt (HUVEC-Akt) in cytokine- and serum-free conditions and incubated with control IgG or blocking monoclonal antibodies against VEGFR2. Data shown is the average of three independent experiments. a Basal cells, b HUVEC-Akt cells, c representative flow cytometric analysis of human airway basal cell and HUVEC-Akt cell populations at day 4 of co-culture following incubation with control IgG or anti-VEGFR2. HUVEC-Akt cells were determined as the GFP+VE-cadherin+ population, and the GFP−VE-cadherin− population quantified as basal cells, and d human airway basal cells were cultured in growth media and incubated with control IgG or blocking monoclonal antibodies against VEGFR2. Data shown is the average of three independent experiments. For all panels, shown are untreated (black), IgG control (gray), and anti-VEGFR2 (white)
To confirm the effect is due to specific inhibition of VEGFR2 signaling cascades in endothelium and not due to direct effects of the VEGFR2 antibody on basal cells, basal cells were cultured alone in regular growth media in the absence and presence of VEGFR2-blocking antibody or IgG control (Fig. 8d). In three independent experiments, there was a minor insignificant decrease in basal cell growth following 4 days of culture in the presence of control IgG relative to untreated cells (19.7-fold vs. 23.3-fold increase in cell numbers compared to day 0, p > 0.6). Treatment of cells with anti-VEGFR2 antibody also resulted in a minor insignificant decrease in basal cell growth compared to untreated cells (19.8-fold vs. 23.3-fold increase in cell numbers at day 4 compared to day 0, p > 0.6); however, this was identical to that observed with IgG control. Therefore, anti-VEGFR2 treatment has no specific direct effects on basal cell proliferation and growth, further confirming the endothelial specific targeting of the antibody in the co-culture system. Overall, these data demonstrate that activation of endothelial cells via VEGFR2-dependent signaling cascades is essential for efficient endothelial cell-dependent proliferation of airway basal cells.
Discussion
Human airway basal cells function as the stem/progenitor population of the airway epithelium, capable of differentiation into specialized ciliated and secretory cells during physiological turnover and repair [6–11]. A previous study in our laboratory characterizing the transcriptome of human airway basal cells identified that basal cells likely have other functions, including the expression of VEGFA [11]. The focus of the present study was to characterize the expression of VEGFA in human airway basal cells and elucidate its function.
Expression and function of VEGFA in the lung
VEGFA levels in respiratory epithelial lining fluid are 500 times higher than in plasma [27]. Consistent with this observation, a variety of studies have shown that VEGFA is expressed in several types of pulmonary cells, including endothelium, smooth muscle, fibroblasts, alveolar type II epithelial cells, and airway epithelium [15, 28–33]. Our study characterizing the human airway basal cells transcriptome demonstrated that, on a per-cell basis, airway basal cells express 8.3-fold greater VEGFA than the complete airway epithelium [11]. The present study extends these observations, demonstrating that human airway basal cells express high levels of all three major VEGFA isoforms 121, 165, and 189, and that VEGFA is actively secreted by the basal cells. Interestingly, however, although the airway basal cells express high levels of VEGFA, they lack functionally detectable levels of the receptors VEGFR1 and VEGFR2, thus preventing their activation upon exposure to VEGFA. Based on this observation, we hypothesized that basal-cell-derived VEGFA functions via a paracrine, rather than autocrine, manner to signal other cell types of the airway, i.e., that basal cells have other functions beyond their role as stem/progenitor cells for the airway epithelium.
Basal cell–endothelial cell cross-talk
Airway basal cells play a central role in anchoring the epithelium to the basement membrane and matrix, helping to protect the underlying airway cell types (including endothelium, smooth muscle, and fibroblasts) from the external environment [3–5]. The positioning of basal cells allows for potential paracrine signaling of these other airway cell types, as well as the other airway cell types regulating basal cell function. Considering the anatomical location of airway basal cells, their close proximity to the vasculature [6, 9], and the knowledge that endothelial cells express VEGFA receptors [12], we investigated whether basal-cell-derived VEGFA could activate endothelium. The data demonstrates that basal-cell-derived VEGFA activates VEGFR2-mediated signaling cascades within the endothelium, confirming that basal cell-secreted VEGFA functions in a paracrine manner. Utilizing a cytokine- and serum-free basal cell–endothelial cell co-culture system, we observed that endothelial cells can support and sustain airway basal cell proliferation in the absence of exogenous growth factors. Interestingly, the endothelium activated by basal-cell-derived VEGFA talks back to the basal cells, supporting basal cell growth. In this context, inhibition of VEGFR2 activation on endothelium significantly suppressing endothelial-dependent basal cell growth, i.e., VEGFA–VEGFR2 cross-talk between these two cell types plays a role in activating the endothelium, but also in regulating the growth of basal cells.
Consistent with these observations, we recently demonstrated in mice that epithelial–endothelial cross-talk plays an important role in promoting alveologenesis following unilateral pneumonectomy [34]. The study showed that pneumonectomy stimulates pulmonary capillary endothelial cells via VEGFR2 and FGFR1 signaling-dependent mechanisms to produce angiocrine growth factors that induce proliferation of epithelial progenitor cells supporting alveologenesis. In addition to its role in repair processes, murine studies have demonstrated that epithelial–endothelial cross-talk plays an important role in lung morphogenesis [35, 36]. In these studies, epithelial expression of VEGFA is critical in mediating branching morphogenesis and distal septae formation in the developing lung [35, 36]. In concordance with these observations, Franzdottir et al. [37], have demonstrated that in the presence of endothelial cells, an immortalized human lung epithelial cell line can be induced to form branching bronchoalveolar-like structures in 3D culture. Studies from other organ systems including bone marrow, brain, liver, and pancreas suggest endothelial cell interactions play a pivotal role in regulating organogenesis, tissue maintenance, and stem cell fate [38]. In this context, studies of both neural and hematopoietic stem cells demonstrate that endothelial cells stimulate self-renewal of stem cells at the expense of differentiation [23, 39, 40]. These data, together with the data from the present study, suggest that the adult lung basal-to-endothelial cross-talk may play a role in the maintenance of basal cell growth in the steady state and also in the initial stages of the repair process, whereby there is an initial expansion of the basal cell population to re-populate the injured site, followed by subsequent differentiation to replenish the pseudostratified epithelium [8, 10].
Possible roles in health and disease
It is well recognized that airway basal cells have a central role in homeostasis of the normal airway epithelium and regeneration following injury [8, 10]. However, there is increasing evidence that airway basal cells contribute to the pathogenesis of chronic obstructive pulmonary disease (COPD) and lung cancer [9]. Smoking is the most common insult to the airways and a known contributor to the risk of developing both COPD and lung cancer [41–43]. The earliest abnormality in the airway epithelium associated with smoking is hyperplasia of basal cells [44]. The identification of airway VEGFA/endothelial cell VEGFR2-mediated cross-talk between airway basal and endothelial cells raises the question regarding the role of this process in smoking-induced lung disorders. In support of this concept, there is evidence of over-expression of VEGFA in bronchial dysplasia’s of smokers and smokers with COPD relative to the normal epithelium [28, 45]. In addition to the epithelial remodeling associated with COPD [46], the airways in COPD have an increased capillary number compared to healthy controls [47]. Therefore, one may postulate that if the elevated levels of VEGFA observed in the bronchial epithelium are basal-cell-derived, it may increase activation of endothelium, resulting in enhanced proliferation and angiogenesis. In addition to the direct effects of VEGFA, additional indirect factors may regulate cross-talk between basal and endothelial cells during homeostasis and the disease state. Soltani et al. [48] has demonstrated increased fragmentation of the basement membrane and altered distribution of vessels in the airway of smokers and smokers with COPD compared to healthy non-smokers. In such circumstances, it is likely that increased fragmentation of the basement membrane alters the local microenvironment, allowing closer interaction of the basal and endothelial cells, further enhancing the cross-talk. Based on these concepts, if indeed enhanced basal-endothelial cell cross-talk influences the development of smoking-induced diseases such as basal cell hyperplasia, then inhibition of this cross-talk via specific targeting of VEGFA or VEGFR2 may result in therapeutic benefits and a slowing of disease progression.
Acknowledgments
We thank R. Zwick for technical assistance, M. Staudt and J. Fuller for coordinating sample collection and N. Mohamed, D.N. McCarthy and R. Hamid for help in preparing this manuscript. These studies were supported, in part, by P50 HL084936, 1R01HL107882, UL1-RR024996 and UL1-RR024143.
Footnotes
G. Curradi, M. S. Walters, and B. S. Ding contributed equally to this study.
References
- 1.Breeze RG, Wheeldon EB. The cells of the pulmonary airways. Am Rev Respir Dis. 1977;116:705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- 2.Mercer RR, Russell ML, Roggli VL, Crapo JD. Cell number and distribution in human and rat airways. Am J Respir Cell Mol Biol. 1994;10:613–624. doi: 10.1165/ajrcmb.10.6.8003339. [DOI] [PubMed] [Google Scholar]
- 3.Knight DA, Holgate ST. The airway epithelium: structural and functional properties in health and disease. Respirology. 2003;8:432–446. doi: 10.1046/j.1440-1843.2003.00493.x. [DOI] [PubMed] [Google Scholar]
- 4.Thompson AB, Robbins RA, Romberger DJ, Sisson JH, Spurzem JR, Teschler H, et al. Immunological functions of the pulmonary epithelium. Eur Respir J. 1995;8:127–149. doi: 10.1183/09031936.95.08010127. [DOI] [PubMed] [Google Scholar]
- 5.Crystal RG, Randell SH, Engelhardt JF, Voynow J, Sunday ME. Airway epithelial cells: current concepts and challenges. Proc Am Thorac Soc. 2008;5:772–777. doi: 10.1513/pats.200805-041HR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans MJ, Van Winkle LS, Fanucchi MV, Plopper CG. Cellular and molecular characteristics of basal cells in airway epithelium. Exp Lung Res. 2001;27:401–415. doi: 10.1080/019021401300317125. [DOI] [PubMed] [Google Scholar]
- 7.Hajj R, Baranek T, Le NR, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25:139–148. doi: 10.1634/stemcells.2006-0288. [DOI] [PubMed] [Google Scholar]
- 8.Rock JR, Onaitis MW, Rawlins EL, Lu Y, Clark CP, Xue Y, et al. Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc Natl Acad Sci USA. 2009;106:12771–12775. doi: 10.1073/pnas.0906850106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Model Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rock JR, Gao X, Xue Y, Randell SH, Kong YY, Hogan BL. Notch-dependent differentiation of adult airway basal stem cells. Cell Stem Cell. 2011;8:639–648. doi: 10.1016/j.stem.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hackett NR, Shaykhiev R, Walters MS, Wang R, Zwick RK, Ferris B, et al. The human airway epithelial basal cell transcriptome. PLoS One. 2011;6:e18378. doi: 10.1371/journal.pone.0018378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 13.Grunewald FS, Prota AE, Giese A, Ballmer-Hofer K. Structure–function analysis of VEGF receptor activation and the role of coreceptors in angiogenic signaling. Biochim Biophys Acta. 2010;1804:567–580. doi: 10.1016/j.bbapap.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 14.Tugues S, Koch S, Gualandi L, Li X, Claesson-Welsh L. Vascular endothelial growth factors and receptors: anti-angiogenic therapy in the treatment of cancer. Mol Aspects Med. 2011;32:88–111. doi: 10.1016/j.mam.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Mura M, dos Santos CC, Stewart D, Liu M. Vascular endothelial growth factor and related molecules in acute lung injury. J Appl Physiol. 2004;97:1605–1617. doi: 10.1152/japplphysiol.00202.2004. [DOI] [PubMed] [Google Scholar]
- 16.Roy H, Bhardwaj S, Yla-Herttuala S. Biology of vascular endothelial growth factors. FEBS Lett. 2006;580:2879–2887. doi: 10.1016/j.febslet.2006.03.087. [DOI] [PubMed] [Google Scholar]
- 17.Woolard J, Bevan HS, Harper SJ, Bates DO. Molecular diversity of VEGF-A as a regulator of its biological activity. Microcirculation. 2009;16:572–592. doi: 10.1080/10739680902997333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akeson A, Herman A, Wiginton D, Greenberg J. Endothelial cell activation in a VEGF-A gradient: relevance to cell fate decisions. Microvasc Res. 2010;80:65–74. doi: 10.1016/j.mvr.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hackett NR, Heguy A, Harvey BG, O’Connor TP, Luettich K, Flieder DB, et al. Variability of antioxidant-related gene expression in the airway epithelium of cigarette smokers. Am J Respir Cell Mol Biol. 2003;29:331–343. doi: 10.1165/rcmb.2002-0321OC. [DOI] [PubMed] [Google Scholar]
- 20.Harvey BG, Heguy A, Leopold PL, Carolan BJ, Ferris B, Crystal RG. Modification of gene expression of the small airway epithelium in response to cigarette smoking. J Mol Med (Berl) 2007;85:39–53. doi: 10.1007/s00109-006-0103-z. [DOI] [PubMed] [Google Scholar]
- 21.Heguy A, Harvey BG, Leopold PL, Dolgalev I, Raman T, Crystal RG. Responses of the human airway epithelium transcriptome to in vivo injury. Physiol Genomics. 2007;29:139–148. doi: 10.1152/physiolgenomics.00167.2006. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi H, Butler JM, O’Donnell R, Kobayashi M, Ding BS, Bonner B, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/S0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki M, Betsuyaku T, Nagai K, Fuke S, Nasuhara Y, Kaga K, et al. Decreased airway expression of vascular endothelial growth factor in cigarette smoke-induced emphysema in mice and COPD patients. Inhal Toxicol. 2008;20:349–359. doi: 10.1080/08958370701866412. [DOI] [PubMed] [Google Scholar]
- 26.Seandel M, Butler JM, Kobayashi H, Hooper AT, White IA, Zhang F, et al. Generation of a functional and durable vascular niche by the adenoviral E4ORF1 gene. Proc Natl Acad Sci USA. 2008;105:19288–19293. doi: 10.1073/pnas.0805980105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaner RJ, Crystal RG. Compartmentalization of vascular endothelial growth factor to the epithelial surface of the human lung. Mol Med. 2001;7:240–246. [PMC free article] [PubMed] [Google Scholar]
- 28.Kranenburg AR, de Boer WI, Alagappan VK, Sterk PJ, Sharma HS. Enhanced bronchial expression of vascular endothelial growth factor and receptors (Flk-1 and Flt-1) in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:106–113. doi: 10.1136/thx.2004.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boussat S, Eddahibi S, Coste A, Fataccioli V, Gouge M, Housset B, et al. Expression and regulation of vascular endothelial growth factor in human pulmonary epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L371–L378. doi: 10.1152/ajplung.2000.279.2.L371. [DOI] [PubMed] [Google Scholar]
- 30.Medford AR, Douglas SK, Godinho SI, Uppington KM, Armstrong L, Gillespie KM, et al. Vascular endothelial growth factor (VEGF) isoform expression and activity in human and murine lung injury. Respir Res. 2009;10:27. doi: 10.1186/1465-9921-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alagappan VK, Willems-Widyastuti A, Seynhaeve AL, Garrelds IM, ten Hagen TL, Saxena PR, et al. Vasoactive peptides upregulate mRNA expression and secretion of vascular endothelial growth factor in human airway smooth muscle cells. Cell Biochem Biophys. 2007;47:109–118. doi: 10.1385/cbb:47:1:109. [DOI] [PubMed] [Google Scholar]
- 32.Kamio K, Sato T, Liu X, Sugiura H, Togo S, Kobayashi T, et al. Prostacyclin analogs stimulate VEGF production from human lung fibroblasts in culture. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1226–L1232. doi: 10.1152/ajplung.00129.2007. [DOI] [PubMed] [Google Scholar]
- 33.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–L221. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 34.Ding BS, Nolan DJ, Guo P, Babazadeh AO, Cao Z, Rosenwaks Z, et al. Endothelial-derived angiocrine signals induce and sustain regenerative lung alveolarization. Cell. 2011;147:539–553. doi: 10.1016/j.cell.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Moral PM, Sala FG, Tefft D, Shi W, Keshet E, Bellusci S, et al. VEGF-A signaling through Flk-1 is a critical facilitator of early embryonic lung epithelial to endothelial crosstalk and branching morphogenesis. Dev Biol. 2006;290:177–188. doi: 10.1016/j.ydbio.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 36.Yamamoto H, Yun EJ, Gerber HP, Ferrara N, Whitsett JA, Vu TH. Epithelial–vascular cross-talk mediated by VEGF-A and HGF signaling directs primary septae formation during distal lung morphogenesis. Dev Biol. 2007;308:44–53. doi: 10.1016/j.ydbio.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 37.Franzdottir SR, Axelsson IT, Arason AJ, Baldursson O, Gudjonsson T, Magnusson MK. Airway branching morphogenesis in three dimensional culture. Respir Res. 2010;11:162. doi: 10.1186/1465-9921-11-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Red-Horse K, Crawford Y, Shojaei F, Ferrara N. Endothelium-microenvironment interactions in the developing embryo and in the adult. Dev Cell. 2007;12:181–194. doi: 10.1016/j.devcel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 39.Butler JM, Nolan DJ, Vertes EL, Varnum-Finney B, Kobayashi H, Hooper AT, et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell. 2010;6:251–264. doi: 10.1016/j.stem.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 2004;304:1338–1340. doi: 10.1126/science.1095505. [DOI] [PubMed] [Google Scholar]
- 41.Shopland DR. Tobacco use and its contribution to early cancer mortality with a special emphasis on cigarette smoking. Environ Health Perspect. 1995;103(Suppl 8):131–142. doi: 10.1289/ehp.95103s8131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hylkema MN, Sterk PJ, de Boer WI, Postma DS. Tobacco use in relation to COPD and asthma. Eur Respir J. 2007;29:438–445. doi: 10.1183/09031936.00124506. [DOI] [PubMed] [Google Scholar]
- 43.Barnes PJ. Chronic obstructive pulmonary disease: a growing but neglected global epidemic. PLoS Med. 2007;4:e112. doi: 10.1371/journal.pmed.0040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auerbach O, Gere JB, Forman JB, Petrick TG, Smolin HJ, Muehsam GE, et al. Changes in the bronchial epithelium in relation to smoking and cancer of the lung a report of progress. N Engl J Med. 1957;256:97–104. doi: 10.1056/NEJM195701172560301. [DOI] [PubMed] [Google Scholar]
- 45.Merrick DT, Haney J, Petrunich S, Sugita M, Miller YE, Keith RL, et al. Overexpression of vascular endothelial growth factor and its receptors in bronchial dysplasia demonstrated by quantitative RT-PCR analysis. Lung Cancer. 2005;48:31–45. doi: 10.1016/j.lungcan.2004.07.049. [DOI] [PubMed] [Google Scholar]
- 46.Zanini A, Chetta A, Imperatori AS, Spanevello A, Olivieri D. The role of the bronchial microvasculature in the airway remodelling in asthma and COPD. Respir Res. 2010;11:132. doi: 10.1186/1465-9921-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Calabrese C, Bocchino V, Vatrella A, Marzo C, Guarino C, Mascitti S, et al. Evidence of angiogenesis in bronchial biopsies of smokers with and without airway obstruction. Respir Med. 2006;100:1415–1422. doi: 10.1016/j.rmed.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 48.Soltani A, Reid DW, Sohal SS, Wood-Baker R, Weston S, Muller HK, et al. Basement membrane and vascular remodelling in smokers and chronic obstructive pulmonary disease: a cross-sectional study. Respir Res. 2010;11:105. doi: 10.1186/1465-9921-11-105. [DOI] [PMC free article] [PubMed] [Google Scholar]