Abstract
Multinucleated myofibers are the functional contractile units of skeletal muscle. In adult muscle, mononuclear satellite cells, located between the basal lamina and the plasmalemma of the myofiber, are the primary myogenic stem cells. This chapter describes protocols for isolation, culturing and immunostaining of myofibers from mouse skeletal muscle. Myofibers are isolated intact and retain their associated satellite cells. The first protocol discusses myofiber isolation from the flexor digitorum brevis (FDB) muscle. These short myofibers are cultured in dishes coated with PureCol collagen (formerly known as Vitrogen) using a serum replacement medium. Employing such culture conditions, satellite cells remain associated with the myofibers, undergoing proliferation and differentiation on the myofiber surface. The second protocol discusses the isolation of longer myofibers from the extensor digitorum longus (EDL) muscle. Different from the FDB preparation, where multiple myofibers are processed together, the longer EDL myofibers are typically processed and cultured individually in dishes coated with Matrigel using a growth factor rich medium. Under these conditions, satellite cells initially remain associated with the parent myofiber and later migrate away, giving rise to proliferating and differentiating progeny. Myofibers from other types of muscles, such as diaphragm, masseter, and extraocular muscles can also be isolated and analyzed using protocols described herein. Overall, cultures of isolated myofibers provide essential tools for studying the interplay between the parent myofiber and its associated satellite cells. The current chapter provides background, procedural, and reagent updates, and step-by-step images of FDB and EDL muscle isolations, not included in our 2005 publication in this series.
Keywords: Skeletal muscle, satellite cells, stem cells, collagen, Matrigel, myofiber isolation, flexor digitorum brevis, extensor digitorum longus, diaphragm, masseter, extraocular, mouse, immunostaining, Pax7
1. Introduction
Myofibers are the functional contractile units of skeletal muscle. While myofibers are established during embryogenesis by fusion of myoblasts into myotubes, processes involved in their growth and repair continue throughout life. These processes are supported by myogenic progenitors known as satellite cells that are located between the basal lamina and the plasmalemma of the myofiber (1,2);; for a schematic and electron microscope image see Fig. 1. In a growing muscle at least some of the satellite cells are proliferating, and contribute myonuclei to the enlarging myofibers, whereas in intact adult muscles most satellite cells are quiescent. In response to a variety of conditions, ranging from increased muscle utilization to muscle injury, satellite cells can enter the cell cycle, producing progeny that fuse into existing myofibers, or form new myofibers (3,4);. Satellite cells are considered stem cells because in addition to giving rise to progeny needed for myofiber repair, they can self-renew (5,6);. It is not known, however, if all satellite cells are identical with regard to their amplification and renewal potential (6,7);. Insights into the cascade of cellular and molecular events controlling satellite cell myogenesis are essential for understanding the mechanisms controlling muscle maintenance as well as for developing strategies to enhance muscle repair after trauma or in myopathic diseases (8–11);.
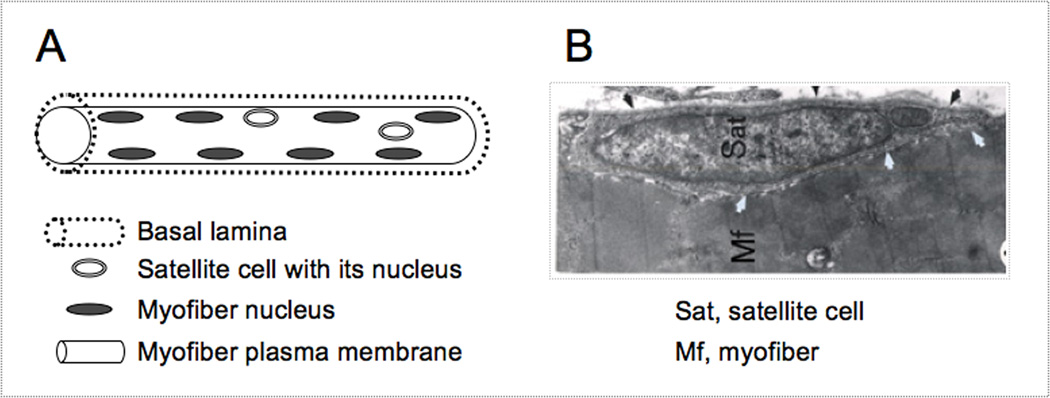
Fig. 1.
A schematic A and EM micrograph B of satellite cell location. The myofiber basement and plasma membranes have been routinely detected by immunostaining with antibodies against laminin and dystrophin, respectively. In panel A, myofiber nuclei depicted at the myofiber periphery represent the state of healthy adult myofibers (while immature myofibers present in regenerating muscles display centralized myofiber nuclei; not shown). In panel B, black arrows depict the basal lamina, white arrows depict apposing satellite cell and myofiber membranes; note the sarcomeric organization within the myofiber.
Satellite cells were initially identified using electron microscopy by their location under the myofiber basal lamina (1,12,13); (Fig. 1). More recently it has become possible to monitor satellite cells by light microscopy based on the expression of a range of markers that can be detected by immunostaining (14);. In particular, the specific expression of the paired box transcription factor Pax7 by satellite cells and the availability of an excellent antibody for immunodetection of this protein provide a uniform means to identify satellite cells in their native position in a range of species, such as mouse, rat, and chicken (15–18);. In humans, however, Pax7 expression may not necessarily identify all satellite cells (14);. Additionally, genetically manipulated reporter mice permit direct detection of satellite cells based on specific expression of a fluorescent tag or of beta-galactosidase (7,16,19,20);. We demonstrated that transgenic expression of GFP under the control of nestin regulatory elements (NES-GFP) allows detection of satellite cells in freshly isolated myofibers. NES-GFP mice also facilitate the isolation of satellite cells using fluorescent-activated cell sorting (FACS) and subsequent studies of purified populations (7,16);.
Satellite cell progeny can be distinguished from their quiescent progenitors based on distinctive gene expression patterns (2,4,7);. In particular, the myogenic regulatory factors MyoD and myogenin have been used extensively to monitor progeny of satellite cells (21–24);. Proliferating progeny (myoblasts) continue to express Pax7, but distinctly from their quiescent progenitors, also express MyoD. A decline in Pax7 along with the induction of the muscle-specific transcription factor myogenin mark myoblasts that have entered into the differentiation phase and subsequently fuse into myotubes. Re-emergence of cells that express Pax7, but not MyoD (reserve cells), define a self-renewing population of satellite cells (2,5–7,22–24);.
Two main cell culture approaches have been employed in the study of satellite cells: (i) primary myogenic cultures prepared from mononucleated cells dissociated from whole muscle; and (ii) cultures of isolated myofibers (also referred to below as “fibers”) where the satellite cells remain in their in situ position underneath the myofiber basal lamina. Protocols for obtaining primary myogenic cultures involve releasing satellite cells from their niche. Steps of mincing, enzymatic digestion and repetitive triturations of the muscle are required for breaking down both the connective tissue network and the myofibers in order to release the satellite cells from the muscle bulk. These steps are followed by procedures for removing tissue debris and reducing the contribution of non-myogenic cells typically present in primary isolates of myogenic cells (6,16,22,25–29);. In contrast, protocols for isolating individual muscle fibers result in the release of intact myofibers that retain satellite cells in their native position underneath the basal lamina (16,21–23,26);. These protocols allow the study of satellite cells and their progeny in their in situ position on the myofiber, and after they migrate from the parent myofiber.
This chapter describes two protocols used in our laboratory for isolation and culture of single myofibers from mouse skeletal muscles (22,30);. One protocol, first introduced by Bekoff and Betz (31); and further developed by Bischoff (32,33);, has been adopted by us for studies of satellite cells in isolated myofibers from both rats (21,26,34); and mice (22,35,36);. In this case, single myofibers are isolated from the flexor digitorum brevis (FDB) muscle of the hind feet. Because these FDB myofibers are short and do not get tangled, typically multiple myofibers are processed and cultured together. A second approach, introduced by Rosenblatt and colleagues (37,38);, allows isolation of longer myofibers from a variety of muscles, including extensor digitorum longus (EDL), tibialis anterior (TA) and soleus (5,20,37,38);, and has been used extensively by our laboratory as well (2,7,16,22,39);. These longer myofibers can get tangled, and therefore, when working with muscles such as the EDL, the released myofibers are typically processed and cultured individually. The EDL single myofiber isolation procedure described here has also been adapted in our laboratory for diaphragm, masseter and extraocular muscles. Both the short and long myofibers are cultured in dishes that have been pre-coated with commercially available matrices that facilitate rapid and firm adherence of the myofibers to the dish surface, as detailed below. It is worth noting, however, that in addition to the matrix-attached myofiber cultures described herein, other laboratories have introduced approaches where isolated myofibers are cultured in suspension (23,40);.
The current chapter contains background, procedural and reagent updates for FDB and EDL myofiber isolation. We also include figures depicting step-by-step “real live” images of respective muscle dissection and harvesting, not illustrated in our previous 2005 report on myofiber isolation and culture (30);. In addition, new to this chapter is a description of myofiber isolation from diaphragm, masseter and extraocular muscles. Table 1 compares the two approaches of myofiber isolation from FDB and EDL muscles and the specific use of each procedure, while representative micrographs of FDB and EDL myofiber cultures are shown in Figs. 2 and 3, respectively. Figs. 4 and 5 are presented later in the method section to assist the investigator in the dissection of the FDB and the EDL muscles. Protocols for immunocytochemical analysis of satellite cells in cultures of FDB and EDL myofibers and of freshly isolated myofibers are also included in the chapter.
Table 1.
Characteristics of Myofiber Cultures from FDB and EDL Muscles of Adult Mice
| Donor muscle | Flexor digitorum brevis (FDB) | Extensor digitorum longus (EDL) |
|---|---|---|
| Relative myofiber length | Short | Long |
| Number of fibers per culture | ~30–50 | 1 |
| Typical tissue culture dish | 35-mm dish | 24-well multiwell dish |
| Dish coating | PureCol. Thick, gel-like layer of native collagen type I prepared from bovine hide (Advanced BioMatrix; formerly known as Vitrogen; see Note 1);. | Thin coating of diluted, growth factor reduced Matrigel. Matrigel is a basement membrane preparation isolated from a mouse tumor (BD Biosciences; see Note 2);. |
| Medium | Dulbecco’s modified Eagle’s medium (DMEM)-based, mitogen-depleted serum; specific exogenous growth factors are added to study their effect on satellite cell activation, proliferation, and differentiation (21,22,35,36,46);. | DMEM-based, serum-rich / mitogen-rich; medium can be modified to a serum-poor / mitogen-poor to allow analysis of satellite cell activation (7,16,22,37,38,43);. |
| Satellite cell profile after culturing | Satellite cells remain at the surface of the parent myofiber as they proliferate and differentiate. Satellite cells undergo a limited number of proliferative cycles and rapidly differentiate without fusing with the parent myofiber. | Satellite cells emigrate from the parent myofiber and undergo multiple rounds of proliferation, giving rise to an elaborate network of myotubes, resembling regular primary cultures of cells dissociated from whole muscle. |
|
Summary |
Cultures may model in vivo behavior of satellite cells in intact fibers during growth and routine muscle utilization. Cultures typically are maintained short-term and can be employed for studying satellite cell activation and entry into the cell cycle. Steps of proliferation and differentiation are highly synchronous (21,22,35,36);. Cultures can be further used to study cells emigrating from the myofibers as described for the EDL fiber cultures. Satellite cells can be monitored in freshly isolated (Time 0) myofibers. |
Cultures may model events after muscle trauma where new myofibers are formed. Cultures typically are maintained long-term and employed in studies of myogenic cells and progeny of satellite cells that emigrate from the myofiber to the myofiber surroundings (7,16,22,37); . Cultures can also be used for analysis of molecular and cellular events associated with the first round of satellite cell proliferation, as in FDB cultures (43);. Satellite cells can be monitored in freshly isolated (Time 0) myofibers (7,22);. |
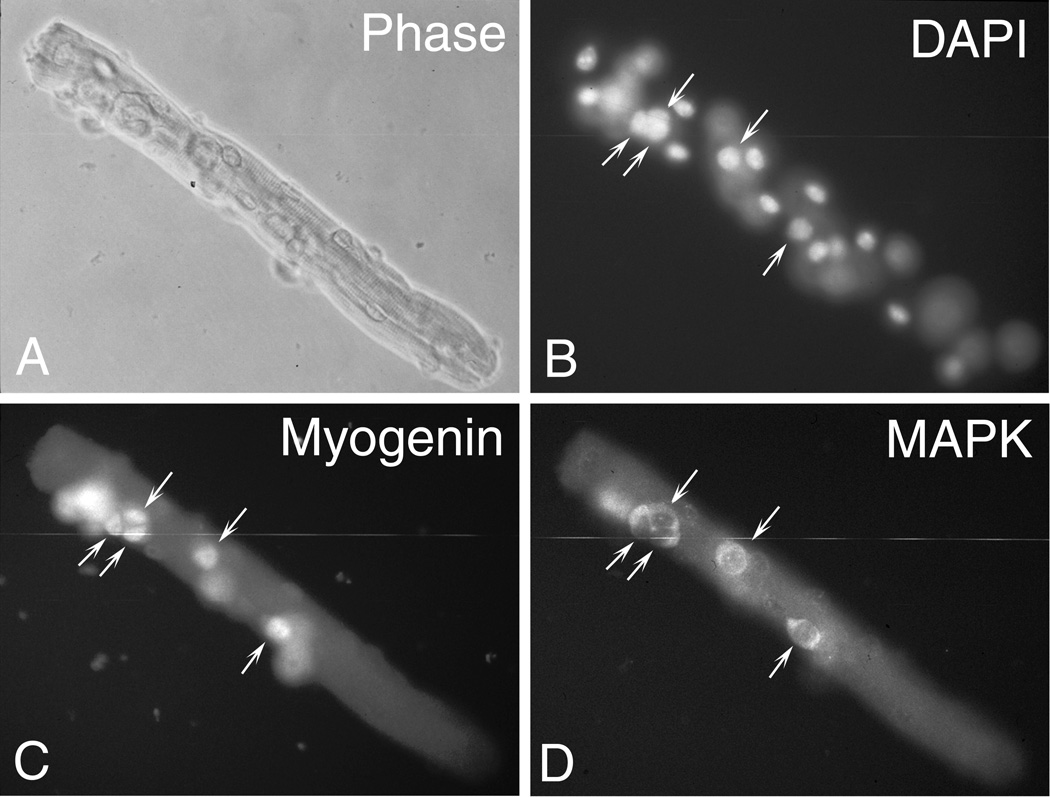
Fig. 2.
Parallel phase and immunofluorescent micrographs of an isolated FDB myofiber with associated satellite cells undergoing myogenesis. Myofibers were isolated from a 3-month-old mouse and cultured in 35-mm tissue culture dishes coated with isotonic Vitrogen collagen in solution (now known as PureCol). Cultures were maintained for 4 days in basal medium containing fibroblast growth factor 2 (FGF2, 2 ng/mL) and fixed with methanol as described in Subheading 3.3.1. A & B Phase and DAPI stained images (both myofiber nuclei and satellite cell nuclei are labeled with DAPI). C & D Myofiber culture reacted by double immunofluorescence with a monoclonal antibody against myogenin (identifies the nuclei of differentiated myogenic cells) and a polyclonal antibody immunostaining against ERK1/ERK2 mitogen activated protein kinases (MAPK) (identifies the cytoplasm of all fiber-associated cells). Reactivity with the monoclonal and polyclonal antibodies was traced with fluorescein- and rhodamine-labeled secondary antibodies, respectively. Arrows in parallel panels point to the location of the same cell. Additional immunopositive cells present on the myofiber are not shown, as not all positive nuclei or cells on the fibers are in the same focal plane. All micrographs were taken with a 400x magnification. Additional details regarding the source of the antibodies and the rationale of using these antibodies are provided in our previous publications (22,35,36,46);.
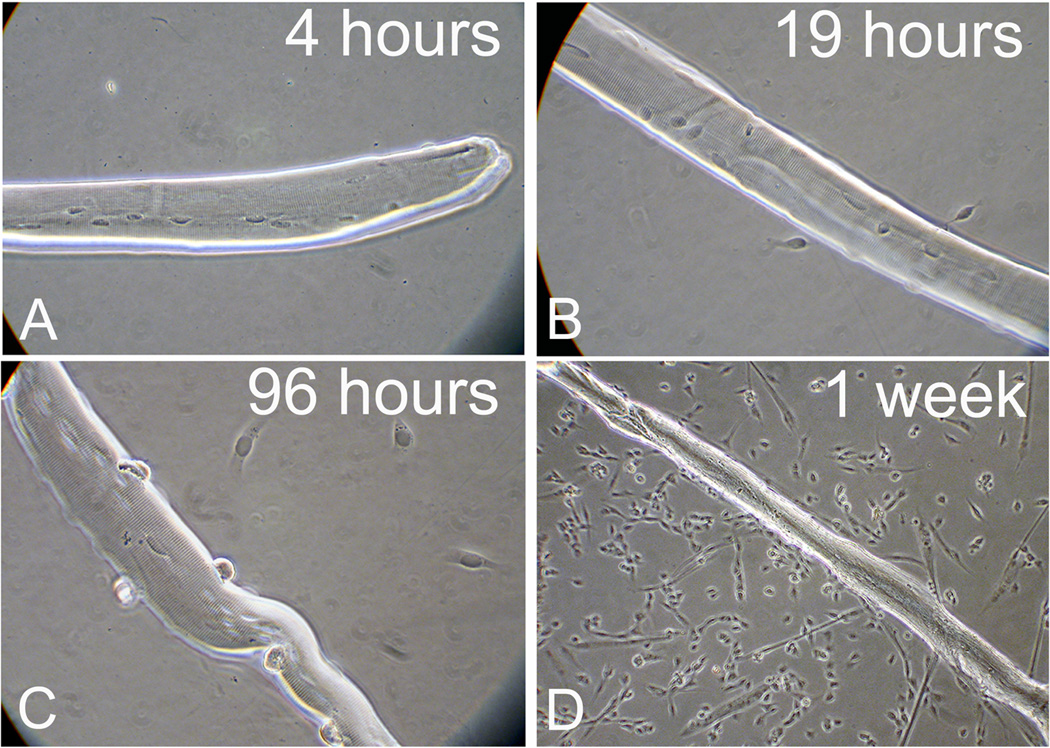
Fig. 3.
Phase micrographs of EDL myofibers depicting the temporal development of myogenic cultures from cells emanating from individual myofibers. Myofibers were isolated from 3 month-old mice and cultured individually in 24-well multiwell tissue culture dishes coated with Matrigel. Cultures were maintained in serum-rich/mitogen-rich growth medium and fixed with paraformaldehyde, as described in Subheading 3.3.2. Satellite cells begin to emigrate from the myofiber within the first day in culture and continue to emigrate during subsequent days. Progeny of satellite cells that have emigrated from the myofibers proliferate, differentiate and fuse into myotubes, establishing a dense myogenic culture. A Satellite cells remained attached to the muscle fiber during the first hours after culturing. B Nineteen hours after culturing, 2–3 cells detached from the fiber but remained in close proximity to the fiber. C Four days following culturing more cells are seen in the vicinity of the myofibers (at least 4 cells are visible). D By day 7, progeny of satellite cells that emigrated from the myofiber have established a culture containing mostly proliferating myoblasts and some myotubes. Micrographs in panels A-C were taken with a 400x magnification to show details of the few cells that emigrated from the myofiber, while the micrograph in panel D was taken with a 100x magnification to show the establishment of a dense myogenic culture. See our published study for additional details about growth of satellite cell progeny in long-term EDL myofiber cultures (22);.
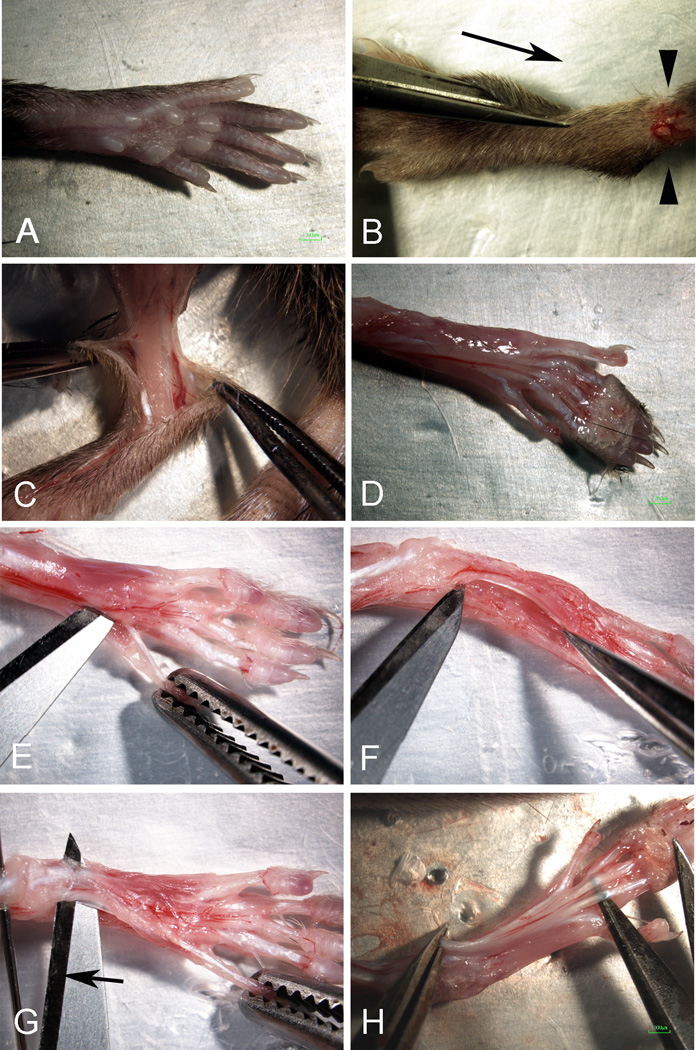
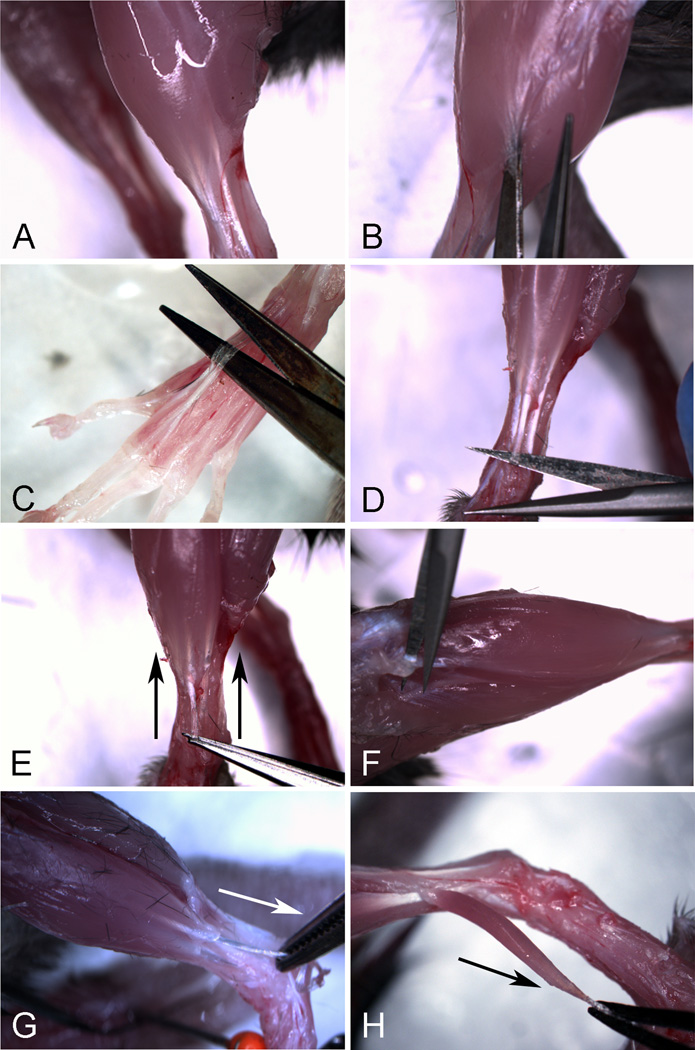
Fig. 4.
Dissection of FDB muscle from the rear foot of adult mouse. A Rear foot before dissection. B Cutting of “T” toward the ankle, left to right); arrowheads identify the circumferential cut at the ankle and arrow shows the direction of cutting. C Peeling the skin back from the ankle exposing the muscles and tendons. D Digit tendons of the FDB exposed on the sole of the foot. E Cutting the connective tissue under the FDB toward the heal of the foot. F Freeing the FDB from the underlying connective tissue. G Cutting the FDB at the heal origin; arrow indicates direction of cutting. (H) Preparing the release of the FDB from its tendon insertion points at the digits.
Fig. 5.
Dissection of EDL muscle from the hindlimb of adult mouse. A Anterior lower hindlimb with skin removed. B Facia covering the anterior lower hindlimb muscles is removed to allow access to tendons. C The four foot tendon insertion points of the EDL are isolated and cut. D The common tendon of the EDL is carefully exposed and isolated at the ankle. E Once isolated and foot insertions are cut, the EDL tendons are pulled proximally up from the foot; arrows indicate the direction of pulling. The tendons should easily slide underneath the connective tissue sheath at the ankle up from the foot. If the tendons do not easily slide out, then reexamine the foot tendons to ensure that they have been cut. F Origin of the EDL is exposed then cut at the lateral surface of the tibia condyle head. (G Grasping only the EDL tendon (do not grasp the muscle as it can easily be damaged), carefully pull distally toward the toes to remove the EDL muscle; arrow indicates the direction of pulling. H) The EDL should slide underneath the TA muscle and should pull out easily. It is important to pull gently and there should be little resistance; if the muscle does not slide out easily, one or both tendons at the muscle origin may still be attached to the bone. In this case identify the attached tendon and cut it.
Altogether, to achieve the isolation of intact myofibers, it is of utmost importance to delicately manipulate the muscle of interest. Following the procedures and protocol notes detailed in this chapter, investigators can successfully isolate, culture and analyze myofibers from well studied EDL and FDB fibers, and also use these protocols as a framework for the study of other muscles.
2. Materials
2.1. General Comments
As a general rule, only sterile materials and supplies are to be used. All solutions, unless otherwise noted, are sterilized by filtering through 0.22-µm filters, all glassware and dissection tools are sterilized by autoclaving, and all cell culture procedures are performed using sterile techniques.
Cultures are maintained at 37°C and 5% CO2 in a humidified tissue culture incubator.
All culture media are stored at 4°C and used within 3 weeks from preparation.
Before starting the isolation procedure, tissue culture medium is pre-warmed to 37°C and then held at room temperature throughout the procedures. Before transferring solutions/media into the tissue culture hood, spray the glass/plastic containers with 70% ethanol.
The quantities of glassware, media and reagents as well as the time intervals for enzymatic digestion described in this chapter are appropriate for the isolation of myofibers from one adult mouse of the age and strain detailed below (see Note 3);.
2.2. General Equipment
The following facilities are required for the cultures described in this chapter:
Standard humidified tissue culture incubator (37°C, 5% CO2 in air).
Tissue-culture hood.
Stereo dissecting microscope with transmitted light base (microscope is either placed inside a tissue culture hood or in an isolation box/clean area).
Bunsen or alcohol burner.
Water bath (37°C).
pH meter and pH paper strips (e.g., EMD, colorpHast strips).
Inverted phase contrast microscope for monitoring cell culture.
2.3. Surgical Tools
Straight operating scissors: V. Mueller, fine-tipped, Sharp/Sharp stainless steel, 165-mm (6½"), for delicate cutting and fine incisions.
Dissecting scissors: stainless steel, 140-mm (5½") length; both blades blunt, to protect the surrounding tissue from any unwanted nicks.
Dressing forceps: V. Mueller, serrated, stainless steel, rounded points, and 140-mm (5½") length.
Two, very fine point forceps: extra-fine tips, smooth spring action, stainless steel. Straight, 110-mm (4¼") length.
Microscissors, Vannas type: 8-cm long, straight 5-mm blades, 0.1-mm tips.
Scalpel handle and blades: size 3 handle for blade numbers 10– 15 and sterile blades (#10)
Two straight, 5" hemostatic forceps.
Dissecting board with tissue pins.
2.4. Animals
C57BL/6 mice, 2– 5 months old, maintained according to institutional animal care regulations. Aged mice (up to 33 months old) and other mouse strains have also been used in our studies following the same myofiber isolation procedures (e.g., (7,22);; see Note 3);. When harvesting muscles for fiber preparation, we prefer cervical dislocation for euthanizing mice as this method is more rapid and minimizes muscle stiffening that occurs after death. Muscle stiffening can make the isolation of single fibers more difficult and decrease overall fiber yield.
2.5. Plastic and Glassware for Myofiber Isolation and Culture
2.5.1. FDB Myofiber Isolation and Culture
Standard 9” glass Pasteur pipettes; fire polish the ends to avoid damage to myofibers, which are transferred using these pipettes. As noted above in item 1 in Subheading 2.1., all Pasteur pipettes are sterilized by autoclaving before use.
Standard 5” glass Pasteur pipettes. Prepare three gradually narrower-bore pipettes from standard 5” Pasteur pipettes. Use a file or a diamond knife to prepare a set of pipettes with bore diameter of approximately 3, 2, and 1 mm. Shake the pipette to remove any glass fragments and fire polish the sharp ends. These pipettes are used to triturate the digested muscle in order to release single myofibers.
Syringe filters, 0.22-µm PVDF low protein binding filters (Millipore is recommended).
3- or 10-cc disposable plastic syringes.
Bottle top filters, 0.22 µm.
Polypropylene conical centrifuge tubes, sterile, 15 and 50 mL
Three glass Corex tubes, 15 mL (Sorvall centrifuge tubes; or alternatively 15 mL bicarbonate Sorvall tubes).
Wide-bore 100-µl micropipette tips. Trim 100-µl micropipette tips approximately 3 mm from the end. Use of these trimmed micropipettes minimizes myofiber shearing when transferring or dispensing FDB myofibers.
Tissue culture dishes, 35-mm.
Two L-shape bent pipette spreaders prepared from standard 9” Pasteur pipettes. Use flame to first seal the distal end, then flame about 2 cm from the sealed end until the pipette starts to bend. The bent pipettes are used to spread the coating solution on the tissue culture dishes; the length of the bent end is designed for working with the 35-mm culture dishes for FDB myofiber cultures. Spreaders should be prepared in advance and allowed to cool before use.
2.5.2. EDL Myofiber Isolation and Culture
Standard 9” and 5” sterile Pasteur pipettes and syringe filters listed and treated as described in items 1–4 in Subheading 2.5.1.
Plastic petri dishes 60×15 mm and 100×15 mm (for muscle and myofiber rinsing), 35-mm tissue culture dishes.
Twenty-four well, multiwell tissue culture dishes (see Note 4);.
Bottle filters and conical tubes and as in items 5 and 6 in Subheading 2.5.1.
1-mL serological glass pipettes (used for Matrigel aliquoting, see Note 2);.
Cryogenic vials sealed with O-rings (for storing Matrigel aliquots, see Note 2);.
2.6. Media, Enzymes, and Cell Culture Reagents
2.6.1. FDB Myofiber Isolation and Culture
DMEM/high glucose (Dulbecco’s Modified Eagle Medium with 4500 mg/L glucose, 4.0 mM L-glutamine, and 110 mg/L sodium pyruvate; readily available from multiple vendors), supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin.
Horse serum (HS); standard, not heat inactivated (see Note 5);. Original bottles are stored at −80°C; once thawed and aliquoted, store at −20°C.
Controlled Process Serum Replacement (CPSR, Sigma-Aldrich, stored at −80°C; once thawed and aliquoted, store at −20°C; see Note 6 for product composition and availability). Alternative serum replacement products (e.g., Sigma-Aldrich, cat. no. S0638 or S9388 (41);) can also be used depending on experimental requirements (see Note 6);.
FDB myofiber culture medium: DMEM/high glucose (supplemented with antibiotics), 20% Controlled Process Serum Replacement, and 1% HS.
PureCol collagen (Advanced BioMatrix, cat. no. 5005-B; this collagen in solution was formerly known as Vitrogen when sold by Cohesion Technologies) for coating 35-mm tissue culture dishes (see Note 1);.
7X DMEM made from powder DMEM (Sigma-Aldrich, cat. no. D5648); used to prepare isotonic PureCol collagen (see Note 1);.
Collagenase (type I, Sigma-Aldrich, cat. no. C-0130). The final working solution is prepared as described in item 3 in Subheading 3.1.1.
100 mL of DMEM containing 10% HS. HS is freshly filtered on the day of use through a 0.22-µm filter. This DMEM-10% HS medium is used for FDB myofiber purification as detailed in Subheading 3.1.5. Also, all Pasteur pipettes and micropipette tips are pre-flushed with this DMEM-10% HS medium to prevent sticking of myofibers during manipulation.
2.6.2. EDL Myofiber Isolation and Culture
DMEM and horse serum (HS) as listed and prepared in items 1 and 2 in Subheading 2.6.1.
Fetal bovine serum (FBS; standard, not heat inactivated; see Note 7);. Original bottles are stored at −80°C; once thawed and aliquoted, stored at −20°C.
Chicken embryo extract (CEE; see Notes 8 and 9);; stored at −80°C for long term or −20°C when aliquoted.
EDL myofiber culture medium: DMEM/high glucose (same formulation as in item 1 in Subheading 2.6.1. for FDB fibers and supplemented with 100 U/mL penicillin and 100 µg/mL streptomycin), 20% fetal bovine serum, 10% HS, and 1% CEE.
Matrigel (BD Biosciences; see Note 2); for coating 24-well, multiwell dishes. We typically dispense Matrigel into aliquots of 100–200 µl and freeze back at −20°C. See Note 2 for all handling details.
Collagenase, as listed in item 7 in Subheading 2.6.1 working solution is prepared as in item 1 in Subheading 3.2.3.
HS, 10 ml, freshly filtered on day of use with 22-µm syringe filter. Used to coat petri dishes and pre-flush Pasteur pipettes to minimize potential sticking of myofibers during isolation procedure.
2.7. Reagents and Solutions for Fixing and Immunostaining Myofiber Cultures
2.7.1. FDB Myofiber Cultures
Pre-fixation rinse solution: DMEM as in item 1 in Subheading 2.6.1.
Fixative: ice-cold 100% methanol (see Note 10);.
Rinse solution: Tris-buffered saline (TBS); 0.05 M Tris, 0.15 M NaCl, pH 7.4 (see Note 11);.
Detergent: Tween 20.
Detergent solution: TBS containing 0.05% Tween 20 (TBS-TW20).
Blocking reagent: Normal goat serum (standard, e.g., Invitrogen, cat. no. 16210-072). Can be stored at −80°C; once thawed and aliquoted, store at −20°C.
Blocking Solution: TBS containing 1% normal goat serum (TBS-NGS).
DAPI solution (4',6-diamidino-2-phenylindole, dihydrochloride); stock concentration 10 mg/mL and a working concentration of 1 µg/mL diluted in TBS-NGS prior to use (see Note 12);.
Mounting medium: Vectashield (Vector Laboratories, cat. no. H-1000); store at 4°C.
Cover glass, 22 mm2.
2.7.2. EDL Myofiber Cultures
Fixative: 4% paraformaldehyde in a sodium phosphate buffer containing 0.03 M sucrose (see Notes 13 and 14 for specific buffer details and preparation). Store at 4°C, pre-warm to room temperature before use. To maintain quality and effectiveness of fixative, pre-warm only the volume that is required for immediate use.
Rinse solution: TBS as in item 3 in Subheading 2.7.1.
Detergents: Triton X-100 and Tween 20.
Detergent solution: TBS containing 0.5% Triton X-100 (TBS-TRX100); TBS-TW20 as in item 5 in Subheading 2.7.1.
Blocking reagent (NGS) and solution (TBS-NGS), same as items 6 and 7 in Subheading 2.7.1.
DAPI working solution and Vectashield; same as items 8 and 9 in Subheading 2.7.1.
Sterile glycerol solution: 25% glycerol in TBS, store at 4°C.
3. Methods
3.1. Isolation of Single Myofibers from the Flexor Digitorum Brevis (FDB) Muscle
The information in this introductory section is provided to assist in the identification of the FDB muscles. The FDB is a superficial, multipennate, broad and thin muscle of the foot and paw (33,42);; it arises from the tendon of the plantaris as three slender muscles converging into long tendons. At the base of the first phalanx it divides into two, passes around the tendon of the flexor hallucis longus obliquely across the dorsum of the foot, and ends as the tendons insert into the second phalanx of the 2nd through the 5th digits. As the FDB contracts, digits 2–5 are flexed. For additional details about the anatomy of the FDB muscle see Note 15.
For uniformity, we typically use only the hindlimb muscles in our studies. Fig. 4 depicts “real-live” images of steps in FDB muscle isolation that emphasize the location of the specific tendons that are handled during the process. It is of utmost importance to delicately manipulate the muscle of interest only at the tendons during its excision and further processing.
3.1.1. Initial Steps Prior to Harvesting the Muscle and Preparation of Digestive Enzyme
Add 3 mL of DMEM to six 35-mm tissue culture dishes and place the dishes in the tissue culture incubator until muscle dissection begins.
Add 3 mL of DMEM containing 10% HS to three 35-mm tissue culture dishes and place them in the tissue culture incubator until needed for the isolated single myofibers.
Add 6 mg of collagenase type I to 3 mL of DMEM in order to prepare 0.2% (w/v) collagenase type I solution. Use a 0.22-µm syringe filter attached to a 3- or 10-cc syringe to filter the collagenase solution into a 35-mm tissue culture dish (see Note 16);. We prepare this solution fresh for each experiment.
3.1.2. Dissection of FDB Muscle (Fig. 4);
Euthanize one mouse according to institute regulations.
Spray the hind foot (Fig. 4A); lightly with 70% ethanol.
All the following steps, until the muscle is dissected out, are carried in an enclosure dedicated for this procedure in order to limit contamination.
Secure the mouse, lying on its back, to the dissecting board by pinning down the forelimb diagonally across from the limb being dissected.
Use a scalpel to carefully cut the skin circumferentially just above the ankle joint, so that the skin above and below the cut site are completely separated (after this circular cut, the skin below resembles a sock).
Using scissors, cut the skin in a straight line along the center of the ventral part of the foot almost all the way to the digits (the cut as viewed from the front of the foot should resemble a “T”) (Fig. 4B);.
Using a hemostat, grasp one of the upper corners of the cut tissue (at the junction of the circular and longitudinal cuts) and reflect the skin away from the foot (Fig. 4C);.
Hold the scalpel with its blade parallel to the longitudinal axis of the partially exposed muscle and carefully separate the skin from the connective tissue. Be especially careful not to cut into the muscle tissue at the back of the leg, as the FDB is the most superficial muscle of the back of the foot.
Clamp a second hemostat to the other corner of the cut tissue and repeat step 8.
When the skin is completely cut away from the foot, the FDB should be exposed all the way to the tendons reaching the digits (Fig. 4D);.
Turn the mouse over so that it lies on its stomach, and identify the FDB. During the next steps of the dissection, be careful not to injure the small medial plantar artery that supplies blood to the FDB to limit blood cell contamination of the myofiber preparation. This artery passes along the medial part of the sole of the foot and branches into the digits.
Carefully run the tip of the scalpel along each side of the FDB to dissect the connective tissue holding the muscle in place (Fig. 4E);.
When the FDB is separated from the surrounding muscles, carefully lift the FDB by inserting one arm of your smooth forceps or a fine blunt probe underneath the FDB so that the flat side of the scalpel may be inserted horizontally underneath it.
With the blade of the scalpel underneath the muscle, running horizontal and parallel to the muscle, cut away the underlying connective tissue (Fig. 4F);. It is best to cut towards the heel and only lift that portion of the muscle directly over the scalpel.
Cut underneath the tendon to separate the muscle and a large portion of its tendon from the heel bone (Fig. 4G);.
Grasp the freed tendon as far as possible from the muscle tissue with a hemostat and gently lift the FDB away from the leg. While lifting the FDB, use the scalpel, running parallel to the muscle, to cut through the connective tissue while holding the foot down.
Continue cutting through the connective tissue until the tendons that connect the FDB muscle to the digits have been exposed (Fig. 4H);. When about half the length of the three tendons has been exposed, cut the tendons and release the entire muscle from the leg. The fourth small lateral tendon (attached to the 5th digit) and its attached myofibers can be trimmed off.
Retrieve from the incubator three 35-mm tissue culture dishes containing DMEM and place them close to the dissection area.
Place the harvested FDB in one of the 35-mm tissue culture dishes.
For harvesting the FDB from the other hind foot repeat steps 4–18 and place the muscle in a second 35-mm tissue culture dish.
Place the 35-mm tissue culture dishes, one at a time, under the stereo dissecting microscope.
Use fine point forceps to pull the connective tissue perpendicular to the line of the muscle and use fine dissection scissors to cut it off.
Once the muscle is clean, shorten the tendons but do not cut all of them off.
Use a wide-bore Pasteur pipette to transfer the cleaned muscle to another 35-mm tissue culture dish containing DMEM.
Repeat steps 21–24 to clean the second FDB muscle.
3.1.3. Enzymatic Digestion
Working in the tissue culture hood transfer the two cleaned FDB muscles to a 35-mm tissue culture dish containing 1.5ml of the 0.2% collagenase I solution.
Place this 35-mm tissue culture dish inside the tissue culture incubator for 2.5 h (see Notes 3 and 16);. Gently swirl the dish every 15–20 min during digestion or, if available, one can use a low speed agitator placed inside the tissue culture incubator. In the latter case, the speed should be adjusted to the lowest possible speed for minimal agitation, to avoid damage to the myofibers.
At the end of the digestion period, transfer each muscle to a 35-mm tissue culture dish containing 10% HS.
3.1.4. Separation of the Three Tendons and Release of Myofibers
Pre-flush all Pasteur pipettes with 10% HS, prepared as described in item 8 in Subheading 2.6.1.
Place one muscle at a time under the stereo dissecting microscope.
Identify the two grooves running between the three tendons separating the middle from the two lateral tendons.
Being careful not to touch the muscle, insert the tip of a pair of forceps into one of the grooves and hold the muscle in place by securing the connective tissue between the tendons to the dish.
Use another pair of forceps to gently pull away the connective tissue that holds the tendons and their attached muscle tissue together.
Continue removing the connective tissue until the lateral tendons are separated from the middle tendon and its attached myofibers.
Holding the muscle only at its tendons, transfer the muscle preparation to a 35-mm dish containing 3 mL of DMEM containing 10% HS.
While grasping one end of the middle tendon with a pair of forceps, use a second pair of forceps to grip its surrounding connective tissue sheath and pull gently. If the sheath does not come off easily, use fine point forceps to pull the connective tissue perpendicular to the line of the muscle and cut it off.
Repeat steps 1–7 with the second FDB muscle until all six tendons and their attached myofibers are in the 35-mm tissue culture dish containing 10% HS.
For one tendon at a time: hold one end of the tendon with a pair of forceps and with the tip of a second pair gently separate the myofibers from the tendon. The liberation of the myofibers from the two lateral tendons should be easy, while the middle tendon requires patience since the myofibers are attached to it more firmly.
Use a wide-bore Pasteur pipette to gently triturate the clumps of myofibers until they disengage into single myofibers. The number of trituration rounds can vary, but it may take at least 5 times. Excessive trituation can lead to fiber damage (see Note 3);.
Remaining clumps should be transferred to another 35-mm tissue culture dish containing 10% HS and further triturated until disengaged into single myofibers.
Set the stereo dissecting microscope magnification so that the small pieces of connective tissue floating around in the suspension are visible and use fine forceps (or standard narrow-bore Pasteur pipette, fire-polished) to pick them out. Continue until the myofiber suspension is clean of any connective tissue debris.
Triturate the myofiber suspension 10 more times using a 9” Pasteur pipette with a fire-polished tip to further separate small clumps of myofibers.
3.1.5. Further Purification of FDB Myofibers
Add 10 mL of DMEM containing 10% HS to each of the three glass Corex tubes.
Using the trimmed 100-µl pipette tip, transfer the myofiber suspension to the top of the 10% HS column in the first Corex tube. Allow the myofibers to settle (at 1g) through the HS column for 15 min at room temperature (see Note 17);. This step is important for purifying the myofibers from free mononucleated cells, debris, and occasional damaged myofibers.
As soon as the myofibers are settled, aspirate about 11 mL of the supernatant (leaving about 1–1.5 mL). Triturate the myofiber suspension gently with a 5” fire-polished Pasteur pipette and transfer the suspension to the next Corex tube as described in step 2.
Allow myofibers to settle and transfer the myofiber suspension to the third Corex tube as in steps 2 and 3.
Allow myofibers to settle and harvest the final myofiber suspension. Following the third purification, the residual volume of medium to be left with the myofiber suspension depends on the number of culture dishes and the desired myofiber number per dish. Typically in our studies the volume of the final myofiber suspension is 300 µl, which is sufficient for culturing 4–6 dishes.
3.1.6. Preparation of Isotonic PureCol Collagen
Isotonic PureCol collagen can be prepared during the settling of myofibers. The isotonic mixture should be kept on ice. Stock PureCol is an acidic solution, and when made isotonic, it gels rapidly if not maintained at 4°C (see Note 1);.
Place the PureCol collagen stock bottle, the 7X DMEM, and one 15-mL conical tube on ice.
On ice: Add 1 volume of 7X DMEM and 6 volumes of PureCol to the 15-mL conical tube and mix gently. Calculate the volume of stock PureCol needed for the experiment based on using 120-µl isotonic PureCol collagen to coat each 35-mm tissue culture dish. Use pH paper strips to ensure a neutral pH of the PureCol collagen in DMEM solution. The pH of this solution rises slightly after coating the culture dish. If the pH remains acidic after coating a test dish, add 1–2 drops of 1 M NaOH to the PureCol collagen in DMEM solution.
3.1.7. Coating Culture Dishes with Isotonic PureCol Collagen and Myofiber Culturing
On ice: Transfer 120 µl of isotonic PureCol collagen to the center of a 35-mm culture dish and immediately use the L-shape spreader to coat the dish evenly. The coated culture plates need to be kept on ice until used as detailed below, to avoid premature matrix gelling.
Gently swirl the myofiber suspension (in the 15-mL tube) for even distribution of myofibers throughout the residual medium.
Remove one culture dish at a time from ice to allow rapid warming to room temperature.
Use a wide-bore, 100-µl micropipette tip to dispense about 50 µl of the myofiber suspension per each culture dish.
Gently swirl the culture dish to allow even distribution of the myofibers.
Repeat steps 2–5 one dish at a time, for additional culture dishes.
Transfer the culture dishes to the tissue culture incubator for a minimum of 20–30 min to allow the formation of PureCol collagen matrix and the adherence of the myofibers to the matrix.
Remove dishes from the incubator. Gently add 1 mL of myofiber culture medium to each dish without agitating the myofibers and return dishes to the incubator. When the effect of growth factors on satellite cell proliferation / differentiation is investigated, parallel cultures are maintained in myofiber culture medium with / without additives, and the medium is replaced every 24 h to ensure that growth factors do not become rate limiting. These cultures can be used for monitoring satellite cells and their progeny in live cultures and for fixed/immunostained cultures as detailed in Fig. 2 and in Subheading 3.3.
3.2. Isolation of Single Myofibers from the Extensor Digitorum Longus (EDL) Muscle
The information in this introductory section is provided to assist in the identification of the EDL muscles. The EDL muscle is situated at the ventral-lateral aspect of the hindlimb, running from the knee to the ankle, extending to the 2nd – 5th digits (42);. The EDL actually consists of four combined muscle bellies and their tendons; the bellies arise from the lateral condyle of the tibia and the front edge of the fibula (2 tendons at the origin of the muscle). The tendons lie close to each other and appear as one glistening white tendon that continues down to the surface of the ankle. At the ankle joint it separates to 4 tendons, each attached to one of the 2nd-5th digits. As the EDL contracts, the 4 digits are extended. For additional details about the anatomy of the EDL muscle see Note 15.
As detailed in Subheading 3.1. we typically use only the hindlimb muscles in our studies. Fig. 5 depicts “real-live” images of the steps in EDL muscle isolation with emphasis on the location of the specific tendons that are handled during the process. It is of utmost importance to delicately manipulate the muscle of interest only at the tendons during its excision and further processing.
The EDL single myofiber isolation procedure described here has also been adapted in our laboratory for the isolation of myofibers from the diaphragm, masseter and extraocular muscles (see Note 18);.
3.2.1. – 3.2.3. Initial Steps Prior to Harvesting the Muscle and Preparation of Digestive Enzyme
3.2.1. Preparation of Matrigel Working Mixture and Coating 24-well Tissue Culture Dishes with Matrigel
Steps 1–6 are done on ice, unless otherwise noted.
Thaw the required amount of Matrigel by placing frozen aliquot(s) on ice for at least 30 min and as much as 1.5 h to allow the Matrigel stock to completely liquefy for subsequent dilution to the working solution (see Note 2);.
Pre-chill a 50-mL conical tube on ice and transfer the thawed Matrigel into the tube. Add ice-cold DMEM to dilute the Matrigel to a final concentration of 1 mg/mL. Gently mix the Matrigel and DMEM by several repetitive drawings through a 1-mL glass pipette. An optimal Matrigel stock is at ~10 mg/mL protein concentration, further diluted at 1:10 for the working Matrigel solution. Stock protein concentration can vary greatly from lot to lot and should be monitored. Allow the diluted Matrigel solution to cool on ice for at least 15 min.
After 15 min, use a chilled 1-mL glass pipette to draw up the diluted Matrigel solution and coat wells with an appropriate volume (250 to 300 µl per well for a 24-well plate). In our experience, 2 mL of working Matrigel solution can be used to coat an entire 24-well plate; we typically coat 6–8 wells at a time as detailed next.
Per each series of wells, leave the culture plate coated with the Matrigel working solution on ice for 7 min, then use the same pipette as before (held cooled in a tube on ice) to remove the Matrigel solution and place it back in the 50-mL conical tube that is kept on ice. This will leave a thin coat of Matrigel at the bottom of the wells.
Once all of Matrigel solution has been placed back in the tube, use the same pipette to coat the next set of wells. Leave the diluted Matrigel in each well for 7 min.
Having coated all the desired wells per 1 tray, tilt the tray and use a 20-µl pipette tip to carefully remove residual Matrigel and place it back in the 50-ml conical tube that is kept on ice (see Note 19)
Incubate the Matrigel-coated multiwell dishes in the tissue culture incubator for at least 1 h.
About 10 min before culturing myofibers, take the Matrigel-coated, 24-well dish out of the incubator to the tissue culture hood and open the lid. This will allow evaporation of water that otherwise will condense on the underside of the lid when moving the dish from the warm incubator to room temperature. If allowed to form, the condensation will drip into the well, disturbing the Matrigel coating.
3.2.2. Coating Glassware and Plasticware Dishes with Horse Serum (HS)
This is done to minimize adherence of myofibers to plasticware and glassware used during the isolation process.
For each EDL muscle being processed, coat six plastic 100-mm petri dishes and 1–2 60-mm petri dishes with filtered HS. Successively transfer a volume of HS to each Petri dish that is sufficient to cover the bottom of the plate, then swirl the dish to coat evenly. Allow each dish to sit with HS for about 2–3 min at room temperature and then remove HS and apply it to the next dish. After all dishes have been coated, add 9–12 mL of DMEM to each 100-mm petri dish and 3–5 mL to each 60-mL petri dish. One may consider processing a pair of EDLs together (which reduces usage of materials and supplies), but we typically process each EDL alone to allow for better separation of fibers with less debris.
Incubate the petri dishes in the tissue culture incubator until needed following muscle digestion.
Coat the fire-polished Pasteur pipettes by flushing HS solution through the pipettes several times. The coated pipettes are then placed vertically in sterile plastic tubes (e.g., 5mL Falcon tubes) to maintain sterility and also for reflushing HS through the pipettes to refresh the coating.
3.2.3. Preparation of the Digesting Enzyme Solution and Post-digestion Rinse Plates
Prepare 0.4% collagenase type I solution by dissolving 0.012 g of collagenase in 3 mL of DMEM. Use a 0.22-µm syringe filter attached to a 3- or 10-cc syringe to filter the collagenase solution into a 35-mm tissue culture dish. We prepare this solution fresh for each experiment (see Note 16);.
Fill three 100-mm petri dishes with 9-mL DMEM and place in tissue culture incubator to warm dishes for later use as rinse dishes.
3.2.4. Dissection of EDL Muscle (Fig. 5);
Euthanize one mouse according to institute regulations.
Spray the hindlimbs with 70% ethanol.
Secure the mouse, lying on its back, to the dissecting board by pinning down the forelimb diagonal to the hindlimb to be dissected.
Use straight rounded-tip scissors to cut through the skin, opening a small incision above the knee.
Holding the skin with fine forceps, insert the rounded-tip scissors beneath the skin and carefully open the scissors to loosen the skin from the underlying muscles.
Extend the incision to a point just in front of the digits.
Loosen the skin as you go, being careful not to cut the underlying muscles or blood vessels.
Cut and remove the skin from the knee to the paw (Fig. 5A) and cut the fascia (thin connective tissue layer that covers the muscles) that overlays the EDL and TA muscles (Fig. 5B). This will facilitate the identification of the tendons.
Identify the four tendons in the foot at the insertion of the EDL, each extending to one of the digits but not the large toe.
Use the microscissors to cut all four tendons (Fig. 5C).
Using fine forceps, gently isolate and pull the portion of the tendon before its division (into 4 tendons) at the ankle up from the paw (Fig. 5D and 5E); the tendon should slide up and out from under the connective tissue sheath at the ankle, with the four divisions trailing behind it. Carefully work the tendon of the EDL out from underneath the TA tendon and pull the tendon out of the ankle with the four divisions trailing behind it (Fig. 5E).
Identify the two tendons that are located by the knee cap, facing the lateral part of the leg (i.e., opposite to the midline of the body).
Use microscissors to cut these tendons as far as possible from the muscle itself (Fig. 5F).
Grasp the four tendons and carefully pull distally toward the toes to remove the EDL muscle.
The EDL should slide underneath the TA muscle and should pull out easily (Fig. 5G and 5H). It is important not to apply too much force. If the muscle does not slide out easily, one or both tendons at the muscle origin at the knee may still be attached to the bone. In this case, identify the yet attached tendon and cut it.
The muscle should be handled only by its tendons to prevent damage to the myofibers. Be careful not to injure the anterior tibial artery that supplies blood to the EDL, to avoid blood cell contamination of the myofiber preparation.
3.2.5. Enzymatic Digestion
Holding the muscle by its 4 tendons, place the EDL in a 35-mm tissue culture dish containing warm DMEM to rinse. Next, transfer the muscle to the 35-mm tissue culture dish containing 0.4% collagenase I solution. A pair of EDLs can be digested in the same dish.
Place the dish inside the tissue culture incubator for 45–60 min (see Notes 3 and 16);. Gently swirl the dish every 15–20 min during digestion (alternatively, one can use a low speed agitator placed inside the tissue culture incubator) to facilitate muscle dissociation.
3.2.6. Liberation of Single Myofibers from Muscle Bulk
Use a stereo dissecting microscope throughout the procedure, which involves rinses of the digested muscle bulk and a 3-step sequence of muscle bulk trituration to release myofibers. All Pasteur pipettes used in this process should be fire-polished. It is recommended to spend no more than 5–7 min at a time per each trituration step. When processing multiple EDLs it is a good strategy to alternate between muscle bulks so that only one EDL is outside of the incubator at a time in order to minimize muscle cooling. Additionally, the recommended number of rinses of the digested muscle and of individual myofibers as detailed in this section should not be overlooked. The myofiber rinses are essential for minimizing the contribution of non-myogenic cells that are released from the muscle bulk during the enzymatic digestion. Unless myofibers are well rinsed, such non-myogenic cells will be co-isolated with the myofibers and eventually produce many progeny in the rich culture conditions.
Inspect the muscle under the stereo dissecting microscope to make sure that the myofibers are loosened from the muscle bulk; the muscle should look like a loose skein of yarn. If the myofibers are not loosened, continue enzymatic digestion for another 10 min and check again.
Retrieve from the incubator the three 100-mm petri dishes containing 9-mL DMEM (rinse plates). Use the widest bore Pasteur pipette to transfer the muscle bulk from the collagenase solution to the first DMEM rinse plate to wash away the collagenase and debris that might have dissociated from the muscle during digestion. Transfer the muscle to the second then third petri dish for further dilution of any possible collagenase that may remain. These rinses must be performed with great care; limit the amount of mechanical manipulation of the muscle or swirling of the dish until the trituration step is reached.
Retrieve from the incubator one of the six 100-mm petri dishes that were pre-coated with HS and filled with DMEM (this will be the holding dish for the muscle bulk and will be used in several of the steps described below). Transfer the rinsed muscle bulk to the holding dish. Place the dish in the incubator for approximately 10 minutes to allow the tissue to warm up.
Retrieve from the incubator a second HS-coated, DMEM containing 100-mm dish. Using the same widest-bore pipette, transfer the muscle bulk to this dish (1st trituration dish). Return the holding dish to the incubator to warm.
Use another HS-coated Pasteur pipette (tip diameter: approx 3–4 mm) to triturate the muscle along its length. This orientation of the EDL muscle during triturations is critical to prevent damage to the myofibers.
When single myofibers are liberated from the muscle, its diameter decreases. Therefore, use a narrower bore pipette for subsequent triturations.
When 10–15 viable single myofibers are released, retrieve from the incubator the holding plate, transfer the muscle bulk into it and place it back in the incubator. Additionally, place the dish with the single myofibers in the tissue culture incubator to keep the fibers warm (typically the fibers from this 1st trituration round are not used, but save the plate in case it is needed). Allow the holding plate with muscle bulk to warm up for at least five to ten minutes in the incubator before the next round of trituration.
Retrieve from the incubator a third HS-coated, DMEM containing 100-mm dish (2nd trituration dish) and the holding plate with muscle bulk. Transfer the muscle bulk to the 2nd trituration dish using the same widest-bore pipette used in the 1st trituration dish. Using a wide bore-pipette with a smaller diameter, triturate the tissue until 30–50 myofibers are obtained (but do not triturate the tissue for more than 5–7 min). Transfer the muscle bulk back to the holding dish and place both the holding dish and the dish with released myofibers back in the incubator.
Follow the pattern of moving the muscle bulk as described in items 7 and 8 create a 3rd trituration dish; triturate the muscle bulk until approximately 100 myofibers have been released. When the 3rd trituration step is complete, transfer the tissue back into the holding dish and place both the holding dish and the dish with released myofibers back into the incubator. Typically, three rounds of triturations are sufficient to dissociate the muscle bulk entirely.
- Using a HS-coated 9” Pasteur pipette (standard bore size) begin to transfer individual fibers from the 2rd and 3nd trituration plates to the remaining two HS-coated, DMEM containing 100-mm petri dishes (collection plates). Refresh the HS coating of the pipette before each fiber transfer so that fibers do not adhere to the glass. Alternate between (at least) two collection plates to minimize cooling of the myofibers. As a general scheme:
- Transfer 10 fibers from the 2nd trituration dish to one of the collection plates and then move both plates back to the incubator.
- Remove the 3rd trituration dish from the incubator and transfer 10 fibers to a second collection plate. Try to avoid using the first trituration dish as the fibers from this trituration are much more fragile and often have more non-myogenic cells attached to them.
- Repeat this process when triturating the second EDL, alternating with the first EDL throughout the processing. If using only one EDL, always allow the plates to rest for 10 minutes in the incubator before repeating the process. Collect those fibers that are relatively straight and are not partially contracted.
Once a large enough number of fibers has been collected (generally 20–30 per collection plate) begin selecting fibers that will be used for analysis. Remove the 100-mm collection plates one at a time and visually inspect the fibers under the highest magnification available. Avoid fibers that have visible associated debris, also avoid those that are kinked or partially contracted (see Note 3);. Transfer fibers that pass these criteria to another HS-coated 60-mm dish (final dish). Try not to place too many fibers into one single plate as the fibers may become entangled with each other or associated with debris that may have been carried over in the transfer (as an approximation, no more than 3 fibers/1 cm2 of dish surface area). Although including this step of fiber selection and transfer to the final plate requires extra time, it allows for another wash step to remove non-myogenic cells that may have been carried over during trituration, thereby ensuring a more optimal fiber preparation.
3.2.7. Culturing Single Myofibers in 24-well Multiwell Dishes
This section describes how to establish and maintain EDL myofiber cultures. We also harvest freshly isolated myofibers for satellite cell analysis (2,7,16,22); (see Subheading 3.3.3.).
Transfer a Matrigel-coated, 24-well multiwell dish from the incubator to the tissue culture hood and open its lid to allow moisture, generated during the incubation period, to evaporate. Add 500 µl of pre-warmed, culture medium (see item 4 in Subheading 2.6.2.) to each well.
Bring the 60-mm petri dish containing single fibers (final dish) to the dissection microscope along with the 24-well plate.
Under the dissection microscope, use a fire-polished, HS-coated 9" Pasteur pipette to select fibers that are free of associated debris or connective tissue. Transfer one fiber at a time with minimal residual medium and gently release the myofiber into the bottom of the well as close to the center as possible. After myofibers are dispensed to the desired number of wells, check again under the stereo dissecting microscope to ensure that indeed there is a myofiber in each well. This step is necessary since occasionally myofibers adhere to the Pasteur pipette and are not released into the well or the fiber becomes damaged in the transfer. Avoid excessive agitation of the fibers.
If needed, add a myofiber to empty well(s) or replace with an intact fiber. Minimize the length of time the final plate and mutliwell dish are held at room temperature; transfer dishes back to the incubator after 10 min to warm while continuing to dispense isolated fibers.
When the desired number of fibers has been plated, place the 24-well multiwell dish in the tissue culture incubator. Avoid handling the plate (i.e., to inspect fibers) for a minimum of 18 h (overnight). Myofibers can also be cultured for early time points (e.g., to analyze satellite cell numbers from freshly isolated fibers; see Subheading 3.3.3.), however, extra special care should be exercised when handling such early time points for microscopic examination or immunostaining because the fibers are only loosely adhered and too much manipulation can damage the fibers and cause contraction.
After the fibers have been in culture for three days, gently add an additional 500 µl of complete media to the fibers. After three more culture days, replace the entire old medium with 500 µl of fresh growth medium. Continue changing the media every three days. We typically maintain myofiber cultures for 10–14 days without any apparent decline in culture quality. Depending on the goal of the project, we also have maintained fiber cultures for up to 3 weeks, but Matrigel may be partially degraded by then, and myotubes may detach from the plate. Moreover, the medium change schedule may need to be more frequent for longer culture periods.
3.3. Immunolabeling of FDB and EDL Myofiber Cultures
This section details current protocols used in our laboratory to fix myofiber cultures for immunofluorescent studies of satellite cells and their progeny. FDB myofiber cultures are typically fixed with ice-cold methanol (the preferred fixative when working with dishes coated with PureCol collagen), whereas the EDL myofiber cultures are typically fixed with paraformaldehyde that is pre-warmed to room temperature. Ideal fixatives for FDB or EDL myofiber cultures are not necessarily the optimal fixatives for specific antigen detection. Thus, when analyzing single myofibers via immunofluorescence, fixatives should be optimized for both preserving the myofibers and the antigens being analyzed. Fixation protocols described in this section are also appropriate for detecting proliferating satellite cells in single myofibers by autoradiography following labeling with 3H-thymidine (32,34); or when analyzing proliferation using bromodeoxyuridine (2,16,41,43);. All steps are done in a sterile manner. Handling antibodies strictly in the tissue culture hood minimizes possible bacterial contamination and helps maintain antibody stocks for years.
3.3.1. Fixing and Immunofluorescent Staining of Isolated FDB Myofiber Cultures
Warm DMEM in a water bath set at 37°C.
Rinse cultures with 500 µl warm DMEM three times. Following the final rinse add 1-mL ice-cold 100% methanol to each 35-mm tissue culture dish and transfer the dishes to 4°C for 10 min.
Return dishes to room temperature, aspirate the methanol and allow the dishes to air-dry for 10–15 min in the tissue culture hood (see Note 20);.
Add 1.5 mL of blocking solution (TBS-NGS) to each culture dish, to block nonspecific antibody binding.
Cultures are then kept at 4°C for at least overnight and up to 2 weeks. Bring cultures to room temperature when ready to start antibody labeling.
Dilute the appropriate primary antibody in the NGS-TBS blocking solution. If not otherwise published, before diluting your antibody, test a range of dilutions to determine the lowest concentration of antibody that gives a clear specific signal without non-specific background.
Rinse the cultures three times with 500 µl TBS-TW20.
Remove the final TBS-TW20 rinse and add 100 µl of the primary antibody solution. Incubate for 1 hour at room temperature followed by an overnight incubation at 4°C in a humidified chamber (see Notes 21 and 22);. Primary and secondary antibodies are applied at the center of the dish followed by a light swirling on a flat surface to ensure optimal spreading of the antibody across the dish. This approach allows using just 100 µl antibody solution, which is beneficial for conserving antibody stocks.
Dilute the appropriate secondary antibody in the NGS-TBS blocking solution. Secondary antibodies are often diluted at 1:1000 or greater, but the researcher needs to determine the optimal dilutions for their specific study.
Rinse cultures with 500 µl TBS-TW20 three times.
Remove the final TBS-TW20 rinse and add 100 µl of the diluted secondary antibody. Incubate for 1–2 h at room temperature.
Remove the secondary antibody and wash three times with 500 µl TBS-TW20. For nuclear visualization, add at least 100 µl of DAPI working solution (1 µg/mL, diluted in TBS-NGS prior to use; see item 8 in Subheading 2.7.1.) and incubate for 30 min at room temperature.
Rinse the cultures twice with 500 µl TBS-TW20 followed by a final rinse with 500 µl TBS.
Remove the TBS and mount in Vectashield mounting medium. Add 1 drop at the center of each culture dish and cover with a cover slip. Cultures should be viewed as soon as possible, but if not, then stored at 4°C sealed in Parafilm, covered with aluminum foil to protect from light, and viewed within a week after immunostaining to avoid fading.
3.3.2. Fixing and Immunostaining Long-Term EDL Myofiber Cultures
EDL myofiber cultures are fixed by slightly different approaches when fixing long term cultures (detailed in this section) or when fixing freshly isolated (Time 0; T0 fibers; detailed in the following section). Importantly, when fixing T0 cultures and early time points, use a stereo dissecting microscope throughout the procedure to ensure that the fibers are not lost or become damaged. All additional wash steps should be performed using a 9” fire-polished Pasteur pipette. At later time points, when fibers and emanating cells are adhering strongly to the matrix, one may not necessarily require the aid of a microscope when fixing or rinsing the cultures.
Warm the needed volume of the 4% paraformaldehyde fixative solution to room temperature (according to the number of wells to be fixed, and using about 500 µl per well).
While observing each myofiber under the stereo dissecting microscope, use a Pipetman to gently, without agitating the culture or touching the myofiber, add an equal volume (500 µl) of the 4% paraformaldehyde fixative solution to the culture medium in each well in the 24-well dish. Allow 10 min at room temperature for the fixation, then carefully remove (by aspiration or using a pipette) the culture medium-paraformaldehyde fixative mixture and rinse each well three times with 500 µl TBS.
Add 500 µl of TBS-TRX100 for 5 min at room temperature. Alternatively, Triton X-100 can be omitted (but cultures can be treated with it later) as some antigens may be more optimally detected if Triton X-100 has not been used.
Add 500 µl of blocking solution (TBS-NGS) to each of the 24 wells, to block nonspecific antibody binding.
Follow steps 5–13 as described in Subheading 3.3.1. However, when exposed to antibodies, the 24-well multiwell trays should be continuously and gently swirled as described in Note 22 as uneven antibody staining can otherwise occur.
Remove the final TBS rinse and add 1 drop of Vectashield mounting medium as in step 14 Subheading 3.3.1. We prefer not to use cover slips when working with 24-well, multiwell trays. Instead, we add 300 µl of the glycerol mounting solution (25% glycerol in TBS) following the initial drop of Vectashield to allow sufficient mounting medium coverage of individual wells in 24-multiwell trays. Trays should be viewed as soon as possible. If they cannot be viewed immediately, they should be stored at 4°C sealed in Parafilm, covered with aluminum foil to protect from light, and viewed within a week after imunostaining to avoid fading.
3.3.3. Fixing and Immunostaining Freshly Isolated (T0) EDL Fibers
Plate EDL fibers as previously described in Subheading 3.2.7. but instead of plating the fiber in a well containing 500-µl medium, transfer the fiber with residual DMEM (~150 µl) into the center of a Matrigel-coated well that has not received growth medium. The fiber should be sitting in a droplet of DMEM, on top of the Matrigel to ensure that it does not dry out. After the desired number of fibers has been dispensed (1 per well), place the plate back in the incubator for 3 h to allow the fibers to adhere to the Matrigel. Minimize the amount of time that the fibers remain outside of the incubator and do not subject the plate to sudden motion as this can cause the fibers to contract or lose contact with the plate substrate.
Use a fire-polished Pasteur pipette to slowly add the 4% paraformaldehyde fixative solution (pre-warmed to room temperature) until the droplet containing the fiber has approximately doubled in volume. Allow the fiber to sit in the fixative solution for 10 min at room temperature.
Follow steps 2–5 as described in Subheading 3.3.2.
Remove the final TBS rinse and add one drop of Vectashield plus 300 µl 25% glycerol-TBS.
Observe and analyze the fibers under the microscope then seal the multiwell tray with Parafilm and store at 4°C and in the dark (e.g., can be stored wrapped with aluminum foil) when not in use. Typically, we aim to complete analyses within a week following fiber harvesting.
4. Notes
PureCol collagen (formally known as Vitrogen), is a sterile solution of purified, pepsin-solubilized, bovine hide collagen (97% Type I, 3% Type III) dissolved in 0.01 N HCl and stored at 4°C until used (vendor: Advanced BioMatrix). In our studies, PureCol collagen is made isotonic by mixing 6 volumes of stock PureCol collagen with 1 volume of 7x DMEM. The isotonic solution is prepared just prior to coating dishes because it gels rapidly at room temperature. To obtain consistent coating, the culture dishes should be pre-cooled and coated on ice. When removed from the ice, these dishes warm up rapidly and are ready for myofiber addition. Preparations of collagen Type I from other sources (e.g., Sigma-Aldrich) have been used by some laboratories as an alternative to PureCol collagen. The use of alternative sources would require pre-screening to ensure compatibility; we only have experience with the bovine-derived product.
Matrigel (BD Biosciences) is a solubilized basement membrane preparation extracted from the Engelbreth-Holm-Swarm mouse sarcoma, a tumor rich in extracellular matrix proteins. Its major component is laminin, followed by collagen IV, entactin, and heparan sulfate proteoglycan (44);. Matrigel is shipped on dry ice and is stored at −20°C until aliquoted. Matrigel should be thawed on ice; never use at a warmer temperature, as it will prematurely gel. To ensure Matrigel stability, we follow the manufacturer’s handling instructions, thawing the product on ice (overnight in an ice bucket placed at 4°C). Once liquefied, Matrigel is aliquoted with pre-chilled 1-mL serological glass pipettes into tubes chilled on ice. Typically, we aliquot 200 µl each into 2-mL cryogenic vials sealed with O-rings. These aliquots are stored at −20°C. We have observed some batch-to-batch variation in the time it takes to thaw the aliquots for final dish coating, therefore, for consistency, we typically allow Matrigel aliquots to thaw for 1.5 h. Matrigel can be purchased in its standard format (BD Biosciences, cat. no. 354234) or in its growth factor reduced format (BD Biosciences, cat. no. 354230). We have typically used the growth factor reduced format, but more recently have begun using the standard format for routine studies in rich growth medium. Invitrogen carries Matrigel-like products that might be useful as an alternative to Matrigel (e.g., Geltrex; cat. no. A11343); however, we do not have sufficient experience with the latter product for detailed recommendations.
Adjustments, such as concentration of collagenase, length of muscle digestion, and extent of muscle trituration for releasing myofibers, may be needed when isolating myofibers from younger/older mice, other mouse strains, mutant mice, or other rodents such as rats. Prolonged digestion and extensive trituration of the muscle bulk will result in poor yields of intact myofibers. Myofibers that are damaged in the course of the isolation can be distinguished from the intact myofibers since they typically hypercontract. Bent myofibers are also damaged to some degree and should not be collected when preparing myofiber cultures.
Falcon Primaria 24-well multiwell dishes (BD Biosciences; cat. no. 353847) were initially used for single myofiber cultures; however, we find that the standard, less expensive, Falcon 24-well, multiwell dishes (cat. no. 353047) are as good.
Horse serum (HS) is used for tissue culture medium and for coating plastic and glassware. HS used for tissue culture media should be pre-characterized by comparing sera from various suppliers (e.g., over years of studies, our preferred serum lots came typically from Invitrogen, HyClone, or Sigma-Aldrich). We select HS based on its capacity to support proliferation and differentiation of primary chicken myoblasts cultured at standard and clonal densities (45);. One may consider replacing HS with bovine serum albumin (BSA) for coating plastic and glassware to further minimize any possible activation of satellite cells during myofiber isolation. However, attention should be given to the purity of the BSA as some lots may contain growth-promoting factors.
The Controlled Processed Serum Replacement 2 (CPSR-2; Sigma-Aldrich) that had been routinely used in our myofiber culture studies (21,22,26,34–36,46); has been discontinued. The source of this discontinued CPSR-2 was dialyzed bovine plasma. This product was further processed in a manner that also reduced lipids. Another alternative serum replacement product, serum replacement 2 (50x) (Sigma-Aldrich; cat. C9388) contains highly purified bovine serum albumin, insulin, and transferrin, and its use for mouse myofiber cultures has been previously described (41);.
Fetal bovine serum (FBS) should be pre-characterized by comparing sera from several suppliers (e.g., over years of studies, our preferred serum lots came from Invitrogen, HyClone, or JR Scientific). We select FBS based on the capacity of the serum to support proliferation and differentiation of mouse primary myoblasts cultured at various cell concentrations. Only sera able to support growth and differentiation over a wide range of concentrations, down to a clonal density, are employed in our studies. Primary myogenic cultures are prepared according to our published procedures (26,27,29);.
Chicken embryo extract (CEE) is available commercially from several sources with which we have no experience. We prepare CEE in our laboratory using 10-day old White Leghorn embryos (47);. The procedure is similar to a previously described method (48); but uses the entire embryo. We recommend this approach over purchasing CEE if the investigator can obtain embryonated chicken eggs, as the quality is thought to be higher and the cost lower than that of purchased CEE.
- Preparation of chicken embryo extract:
- Embryonated chicken eggs (8 dozen, White Leghorn; from Charles River) are maintained in a standard egg incubator (incubation conditions: a dry temperature of 38°C, a wet temperature of 30°C and relative humidity of 56%). The following egg incubator is well suited for basic research use: Marsh Automatic Incubator, model # PROFI, cat. no. 910-028, manufactured by Lyon Technologies, Chula Vista, CA.
- After 10 days, batches of 15–30 eggs are removed from the incubator and transferred into the tissue culture hood. All steps from here on are performed in a sterile manner.
- Place the eggs lengthwise in the rack and spray with 70% ethanol to sterilize. Wait for several minutes until the ethanol evaporates.
- Crack open one egg at a time into a 150-mm petri dish.
- Remove the embryo from surrounding membranes by piercing it with fine forceps. Rinse the embryo by transferring it through three 150-mm petri dishes containing DMEM supplemented with antibiotics (see item 1 in Subheading 2.6.1.). Swirl embryo a few times in each dish for a good rinse.
- Empty the egg remains from the initial 150-mm dish (described in step d) into a waste beaker and repeat steps d–f until the final rinse dish contains about 30 embryos.
- The embryos are transferred with fine forceps into a 60-mL disposable syringe, forced through with the syringe plunger, and the suspension is collected into a 500-mL sterile glass bottle.
- The extract is diluted with approximately an equal volume of DMEM (supplemented with antibiotics as detailed in item 1 in Subheading 2.6.1.) and gently agitated for 2 h at room temperature. To ensure good agitation, keep the maximum volume to one-half bottle capacity and place the bottle at a 45° angle during the agitation.
- The extract is frozen at −80°C for a minimum of 48 h. It is then thawed, dispensed into 50-mL conical tubes, and centrifuged at approximately 500g for 10 min to remove residual tissue.
- The supernatant is pooled, divided into 40-mL aliquots and kept frozen at −80°C for long-term storage. For short-term storage, we typically prepare aliquots of 2.5 mL that are kept frozen at −20°C.
- Prior to use, the CEE-thawed aliquot should again be centrifuged at about 800–1000g for 10 min to remove aggregates. The supernatant is then collected and added to the DMEM-based medium to prepare the rich growth medium for EDL myofiber cultures. The growth medium is then passed through a sterile 0.22-µm filter (to clear remaining particles and sterilize). All details of supplies for generating the medium are in Subheading 2.6.2. To ensure optimal cell growth conditions, we typically prepare only 250-mL medium each time, and use it up within a few weeks.
Methanol is a colorless flammable liquid with an alcohol-like odor. Use nitrile gloves, safety goggles, and a fume hood when handling. It is important to refer to the MSDS instructions and institutional regulations for further information regarding storage, handling and first-aid.
Preparation 1 L of 10X Tris-buffered saline (TBS): Weigh 60.5 g of Tris-Base into a beaker and add 700-mL deionized water. Stir on a magnetic stirrer until the powder has dissolved and adjust the pH to 7.4. Add deionized water to bring the volume up to 1 L, mix well, then autoclave or sterilize by passing it through a 0.22-µm filter, and store at 4°C. To make 1 L of 1XTBS: Weigh 8.766 g NaCl into a beaker and add 100 mL of 10X TB. Mix vigorously until the powder has dissolved. Add deionized water to bring the volume up to 1 L, mix well, then sterile filter and store at 4°C.
DAPI is potentially harmful. Avoid prolonged or repeated exposure. We typically dissolve the entire powder in its original container and generate a concentrated stock solution. Alternatively, a ready-made DAPI reagent is available from Molecular Probes. It is important to refer to the MSDS instructions and institutional regulations for further information regarding storage and handling.
Paraformaldehyde is a white powder with a formaldehyde-like odor. It is a rapid fixative and a potential carcinogen. When handling paraformaldehyde, wear gloves, a mask, and goggles. It is important to refer to the MSDS instructions and institutional regulations for further information regarding storage, handling and first-aid.
Preparation of 100 mL of 4% paraformaldehyde with 0.03 M sucrose: In a fume hood mix 4 g of paraformaldehyde powder and 80 mL of deionized water in a glass beaker. Warm the solution to 60°C with continuous stirring to dissolve the powder. Allow the solution to cool to room temperature. Add 1–4 drops of 1 N NaOH, until the opaque color of the solution clears. Add 10-mL 1 M sodium phosphate. Adjust the pH to 7.2–7.4 using concentrated HCl and color pH strips. Add 1.026 g of sucrose. Bring the volume to 100 mL and filter through a 0.22 µm disposable filter unit (Millipore; cat. no. SCGPT01RE) into a bottle. Store at 4°C in an aluminum foil-wrapped bottle for no more than 1 month.
-
For additional details about the FDB-muscle anatomy refer to:For additional details about the EDL muscle anatomy refer to:
We recommend these links as good resources for anatomical description and schematic images of the muscles although they refer to human muscles.
Collagenase concentration, as well as the optimal time for enzymatic digestion, should be adjusted for younger or older mice and for other muscle groups. The enzyme sold by Sigma-Aldrich tends to have consistent specific activity between batches, but attention should be given to the specific activity with each batch. The volume needed for the preparation should be evaluated based on the size of the tissue, so that the tissue will be fully covered by the collagenase solution (e.g., 1.5 mL is sufficient to cover an EDL muscle but more will be needed to cover a TA).
The time required for the myofiber suspension to settle (at 1g) through 10 mL of 10% HS can vary between 5 and 15 min and the investigator should adjust this time accordingly. A prolonged period results in a preparation with more debris and residual single-cell carryover (not necessarily myofiber-associated) released from the digested tissue. Depending on mouse age, the number of rounds of myofiber settling in the 15-mL glass Corex tubes, as well as the amount of medium in the tube, may also need to be adjusted.
- Isolation of myofibers from Masseter, Diaphragm, and Extraocular muscles:
- General Comment:
- Details in this section are provided in brief and focus mainly on muscle harvesting and dissociation. All reagents are as described for EDL myofiber isolation and culture in earlier sections of this chapter. For all muscles, tissue is dissociated with 0.4% collagenase (type I, source as listed item 7, Subheading 2.6.1. and preparation as in item 1, Subheading 3.2.3.).
- The Diaphragm Muscle:
- The diaphragm muscle consists of two portions; the costal muscle, radially arrayed from a central non-contractile tendon (the central tendon), and the crural diaphragm through which the aorta, thoracic duct, and esophagus are transmitted. When isolating single myofibers from the diaphragm (49);, we have found that at the risk of contaminating the preparation with a greater amount of debris and shorter intercostal fibers of the ribcage, removal of the diaphragm in whole with its immediate supporting ribcage structure provides the greatest fiber yield. After removing the diaphragm, rinse the muscle in a dish containing pre-warmed DMEM. Observe the diaphragm under the dissection scope and without touching the muscle, carefully remove any obvious fat or connective tissue, otherwise this material can foul the prep. Other steps that ensure higher and purer yield of fibers are the addition a wash step post-enzymatic digest and not “over-digesting” the muscle. Digesting for 45 – 60 min in 0.4% collagenase is recommended. The muscle is triturated centrally from the position of the central tendon and ends of the ribcage using the largest bore, fire-polished Pasteur pipette. Expect no more that 50 ideal fibers (undamaged and without associated debris) per diaphragm.
- The Masseter Muscle:
- The masseter muscle of the jaw (like the diaphragm) requires harvesting some of the supporting skeletal structure along with the muscle for the highest fiber yields. The masseter muscle is a multilayered muscle with complex and extensive investment geometry. We have found that including the origin and investment surfaces in the enzymatic digest, rather than risking damage to the muscle at the tendon-bone interface ensures more intact muscle fibers. Once removing the masseter muscles with the associated bones, rinse and remove debris in a pre-warmed 100-mm petri dish containing DMEM. Digest the masseter muscle en bulk with jaw and skull bone attachments in 0.4% collagenase for 45 min followed by careful washes and triturations. This preparation typically yields 30–100 fibers.
- The Extraocular Muscles (EOM):
- EOM are a unique set of muscles that control eye movements. There are 6 EOMs per eye accessed by first bisecting the skull from the top of the head and removing the brain to expose the bone at top of the eye socket. Once exposed, the eye socket is broken along its suture lines to expose the eye. Following removal of the lacrimal gland, the entire eye with its associated muscles is then removed “en bulk” by first cutting the optic nerve at a point just behind the annulus of Zinn (the point where 5 of the 6 EOM muscles meet). The isolation is completed by cutting the remaining soft tissue from around the eye, freeing the eye from the socket. The eye, with the muscles still attached, is placed in 0.4 % collagenase digest for 90 min at 37°C and then transferred to fresh 0.4% collagenase for an additional 45–60 min digestion. Gentle swirling of the digest every 15 min will help increase the yield. The rinsing and trituration steps that follow are similar to those described for the EDL, except with less vigor. This procedure yields an array of short and long, thick and thin EOM myofibers for analysis; 30–100 fibers are typically derived per preparation.
The working Matrigel solution can be used to coat additional trays after completing the first tray coating. Matrigel that has been used to coat too many dishes, however, is less effective in supporting myofiber adhesion. We typically limit reuse of diluted Matrigel to three rounds of coating and work with a larger volume of diluted Matrigel if coating more than 1 tray. Also, we only use Matrigel that has been diluted the day of the fiber isolation to maintain consistency.
The tissue culture dishes are dry when the bottom appears opaque white.
For some antibodies the cultures may be blocked for just 2–4 h at room temperature if overnight blocking is not desired.
For even and continuous distribution of the antibodies (both primary and secondary), it is recommended to place the dishes on a gyrating platform rotator (e.g., Lab-Line Maxi Rotator, model no. 4631R) when staining cultures in 24-well, multiwell dishes; without this agitation, the antibody solution tends to rapidly accumulate at the well periphery, leading to uneven staining across the culture.
Acknowledgements
The authors are grateful to the granting agencies that funded this study. Our current research is supported by grants to Z.Y.R. from the National Institutes of Health (AG021566; AG035377; AR057794) and the Muscular Dystrophy Association (135908). The development the FDB myofiber isolation protocol described in this chapter could not be possible without the valuable contribution of our former lab member, Anthony Rivera, and previous funding from the Muscular Dystrophy Association, the Cooperative State Research, Education and Extension Service / US Department of Agriculture (National Research Initiative), the National Institutes of Health, and the Nathan Shock Center of Excellence in the Basic Biology of Aging, University of Washington.
References
- 1.Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961;9:493–495. doi: 10.1083/jcb.9.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yablonka-Reuveni Z, Day K, Vine A, Shefer G. Defining the transcriptional signature of skeletal muscle stem cells. J Anim Sci. 2008;86:E207–E216. doi: 10.2527/jas.2007-0473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hawke TJ, Garry DJ. Myogenic satellite cells: physiology to molecular biology. J Appl Physiol. 2001;91:534–551. doi: 10.1152/jappl.2001.91.2.534. [DOI] [PubMed] [Google Scholar]
- 4.Zammit PS, Partridge TA, Yablonka-Reuveni Z. The skeletal muscle satellite cell: the stem cell that came in from the cold. J Histochem Cytochem. 2006;54:1177–1191. doi: 10.1369/jhc.6R6995.2006. [DOI] [PubMed] [Google Scholar]
- 5.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, Morgan JE. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Sacco A, Doyonnas R, Kraft P, Vitorovic S, Blau HM. Self-renewal and expansion of single transplanted muscle stem cells. Nature. 2008;456:502–506. doi: 10.1038/nature07384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Day K, Shefer G, Shearer A, Yablonka-Reuveni Z. The depletion of skeletal muscle satellite cells with age is concomitant with reduced capacity of single progenitors to produce reserve progeny. Dev Biol. 2010;340:330–343. doi: 10.1016/j.ydbio.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 9.Shefer G, Yablonka-Reuveni Z. Ins and outs of satellite cell myogenesis: the role of the ruling growth factors. In: Schiaffino S, Partridge T, editors. Skeletal Muscle Repair and Regeneration. Dordrecht, Netherlands: Springer; 2008. pp. 107–144. [Google Scholar]
- 10.Morgan JE, Zammit PS. Direct effects of the pathogenic mutation on satellite cell function in muscular dystrophy. Exp Cell Res. 2010;316:3100–3108. doi: 10.1016/j.yexcr.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Yablonka-Reuveni Z, Day K. S Skeletal muscle stem cells in the spotlight: the satellite cell. In: Cohen I, Gaudette G, editors. Regenerating the Heart: Stem Cells and the Cardiovascular System. Springer, Humana Press; 2011. pp. 173–200. [Google Scholar]
- 12.Muir AR, Kanji AH, Allbrook D. The structure of the satellite cells in skeletal muscle. J Anat. 1965;99:435–444. [PMC free article] [PubMed] [Google Scholar]
- 13.Yablonka-Reuveni Z. Development and postnatal regulation of adult myoblasts. Microsc Res Tech. 1995;30:366–380. doi: 10.1002/jemt.1070300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boldrin L, Muntoni F, Morgan JE. Are human and mouse satellite cells really the same? J Histochem Cytochem. 2010;58:941–955. doi: 10.1369/jhc.2010.956201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 16.Day K, Shefer G, Richardson JB, Enikolopov G, Yablonka-Reuveni Z. Nestin-GFP reporter expression defines the quiescent state of skeletal muscle satellite cells. Dev Biol. 2007;304:246–259. doi: 10.1016/j.ydbio.2006.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shefer G, Rauner G, Yablonka-Reuveni Z, Benayahu D. Reduced satellite cell numbers and myogenic capacity in aging can be alleviated by endurance exercise. PLos ONE. 2010;5:e13307. doi: 10.1371/journal.pone.0013307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allouh MZ, Yablonka-Reuveni Z, Rosser BW. Pax7 reveals a greater frequency and concentration of satellite cells at the ends of growing skeletal muscle fibers. J Histochem Cytochem. 2008;56:77–87. doi: 10.1369/jhc.7A7301.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montarras D, Morgan J, Collins C, Relaix F, Zaffran S, Cumano A, Partridge T, Buckingham M. Direct isolation of satellite cells for skeletal muscle regeneration. Science. 2005;309:2064–2067. doi: 10.1126/science.1114758. [DOI] [PubMed] [Google Scholar]
- 20.Beauchamp JR, Heslop L, Yu DS, Tajbakhsh S, Kelly RG, Wernig A, Buckingham ME, Partridge TA, Zammit PS. Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells. J Cell Biol. 2000;151:1221–1234. doi: 10.1083/jcb.151.6.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yablonka-Reuveni Z, Rivera AJ. Temporal expression of regulatory and structural muscle proteins during myogenesis of satellite cells on isolated adult rat fibers. Dev Biol. 1994;164:588–603. doi: 10.1006/dbio.1994.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shefer G, Van de Mark DP, Richardson JB, Yablonka-Reuveni Z. Satellite-cell pool size does matter: defining the myogenic potency of aging skeletal muscle. Dev Biol. 2006;294:50–66. doi: 10.1016/j.ydbio.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004;166:347–357. doi: 10.1083/jcb.200312007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day K, Paterson B, Yablonka-Reuveni Z. A distinct profile of myogenic regulatory factor detection within Pax7+ cells at S phase supports a unique role of Myf5 during posthatch chicken myogenesis. Dev Dyn. 2009;238:1001–1009. doi: 10.1002/dvdy.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yablonka-Reuveni Z, Quinn LS, Nameroff M. Isolation and clonal analysis of satellite cells from chicken pectoralis muscle. Dev Biol. 1987;119:252–259. doi: 10.1016/0012-1606(87)90226-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kastner S, Elias MC, Rivera AJ, Yablonka-Reuveni Z. Gene expression patterns of the fibroblast growth factors and their receptors during myogenesis of rat satellite cells. J Histochem Cytochem. 2000;48:1079–1096. doi: 10.1177/002215540004800805. [DOI] [PubMed] [Google Scholar]
- 27.Yablonka-Reuveni Z. Isolation and culture of myogenic stem cells. In: Lanza R, Blau D, Melton D, Moore M, Thomas ED, Verfaillie C, Weissman I, West M, editors. Handbook of Stem Cells - Vol 2: Adult and Fetal Stem Cells. San Diego: Elsevier: Academic Press; 2004. [Google Scholar]
- 28.Ieronimakis N, Balasundaram G, Rainey S, Srirangam K, Yablonka-Reuveni Z, Reyes M. Absence of CD34 on murine skeletal muscle satellite cells marks a reversible state of activation during acute injury. PLoS One. 2010;5:e10920. doi: 10.1371/journal.pone.0010920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danoviz ME, Yablonka-Reuveni Z. Skeletal muscle satellite cells: Background and methods for isolation and analysis in a primary culture system. Methods Mol Biol. 2011;798:21–52. doi: 10.1007/978-1-61779-343-1_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shefer G, Yablonka-Reuveni Z. Isolation and culture of skeletal muscle myofibers as a means to analyze satellite cells. Methods Mol Biol. 2005;290:281–304. doi: 10.1385/1-59259-838-2:281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bekoff A, Betz W. Properties of isolated adult rat muscle fibres maintained in tissue culture. J Physiol. 1977;271:537–547. doi: 10.1113/jphysiol.1977.sp012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bischoff R. Proliferation of muscle satellite cells on intact myofibers in culture. Dev Biol. 1986;115:129–139. doi: 10.1016/0012-1606(86)90234-4. [DOI] [PubMed] [Google Scholar]
- 33.Bischoff R. Analysis of muscle regeneration using single myofibers in culture. Med Sci Sports Exerc. 1989;21:S164–S172. [PubMed] [Google Scholar]
- 34.Yablonka-Reuveni Z, Rivera AJ. Proliferative dynamics and the role of FGF2 during myogenesis of rat satellite cells on isolated fibers. Basic Appl Myology (BAM) 1997;7:189–202. [PMC free article] [PubMed] [Google Scholar]
- 35.Yablonka-Reuveni Z, Anderson JE. Satellite cells from dystrophic (mdx) mice display accelerated differentiation in primary cultures and in isolated myofibers. Dev Dyn. 2006;235:203–212. doi: 10.1002/dvdy.20602. [DOI] [PubMed] [Google Scholar]
- 36.Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol. 1999;210:440–455. doi: 10.1006/dbio.1999.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rosenblatt JD, Lunt AI, Parry DJ, Partridge TA. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim. 1995;31:773–779. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblatt JD, Parry DJ, Partridge TA. Phenotype of adult mouse muscle myoblasts reflects their fiber type of origin. Differentiation. 1996;60:39–45. doi: 10.1046/j.1432-0436.1996.6010039.x. [DOI] [PubMed] [Google Scholar]
- 39.Shefer G, Wleklinski-Lee M, Yablonka-Reuveni Z. Skeletal muscle satellite cells can spontaneously enter an alternative mesenchymal pathway. J Cell Sci. 2004;117:5393–5404. doi: 10.1242/jcs.01419. [DOI] [PubMed] [Google Scholar]
- 40.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wozniak AC, Pilipowicz O, Yablonka-Reuveni Z, Greenway S, Craven S, Scott E, Anderson JE. C-Met expression and mechanical activation of satellite cells on cultured muscle fibers. J Histochem Cytochem. 2003;51:1437–1445. doi: 10.1177/002215540305101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greene EC. Anatomy of the rat. New York, NY: Hafner Publishing Company; 1963. [Google Scholar]
- 43.Shefer G, Partridge TA, Heslop L, Gross JG, Oron U, Halevy O. Low-energy laser irradiation promotes the survival and cell cycle entry of skeletal muscle satellite cells. J Cell Sci. 2002;115:1461–1469. doi: 10.1242/jcs.115.7.1461. [DOI] [PubMed] [Google Scholar]
- 44.Kleinman HK, McGarvey ML, Liotta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- 45.Yablonka-Reuveni Z, Seifert RA. Proliferation of chicken myoblasts is regulated by specific isoforms of platelet-derived growth factor: evidence for differences between myoblasts from mid and late stages of embryogenesis. Dev Biol. 1993;156:307–318. doi: 10.1006/dbio.1993.1079. [DOI] [PubMed] [Google Scholar]
- 46.Yablonka-Reuveni Z, Seger R, Rivera AJ. Fibroblast growth factor promotes recruitment of skeletal muscle satellite cells in young and old rats. J Histochem Cytochem. 1999;47:23–42. doi: 10.1177/002215549904700104. [DOI] [PubMed] [Google Scholar]
- 47.Yablonka-Reuveni Z. Myogenesis in the chicken: the onset of differentiation of adult myoblasts is influenced by tissue factors. Basic Appl. Myology (BAM) 1995;5:33–42. [PMC free article] [PubMed] [Google Scholar]
- 48.O'Neill MC, Stockdale FE. A kinetic analysis of myogenesis in vitro. J Cell Biol. 1972;52:52–65. doi: 10.1083/jcb.52.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stuelsatz P, Keire P, Almuly R, Yablonka-Reuveni Z. A contemporary atlas of the mouse diaphragm: myogenicity, vascularity and the Pax3 connection. J Histochem Cytochem. 2012;60:638–657. doi: 10.1369/0022155412452417. [DOI] [PMC free article] [PubMed] [Google Scholar]