Abstract
The stromal reaction surrounding tumors leads to the formation of a tumor-specific microenvironment, which may play either a restrictive role or a supportive role in the growth and progression of the tumors. Lumican, a small leucine-rich proteoglycan (SLRP) of the extracellular matrix (ECM), regulates collagen fibrillogenesis. Recently, lumican has also been shown to regulate cell behavior during embryonic development, tissue repair and tumor progression. The role of lumican in cancer varies according to the type of tumor. In this study we analyze the role of lumican in the pathogenesis of prostate cancer both in vivo and in vitro. Overall lumican up-regulation was observed in the primary tumors analyzed through both real-time PCR and immunostaining. The increase in lumican expression was observed in the reactive stroma surrounding prostate primary tumors with fibrotic deposition surrounding the acinar glands. In vitro analysis demonstrated that lumican inhibited both the migration and invasion of metastatic prostate cancer cells isolated from lymph node, bone and brain. Moreover, prostate cancer cells seeded on lumican presented a decrease in the formation of cellular projections, lamellipodia detected by a decreased rearrangement in ZO-1, keratin 8/18, integrin β1 and MT1-MMP, and invadopodia detected by disruption of α-smooth muscle actin, cortactin and N-WASP. Moreover, a significant increase in prostate cancer cell invasion was observed through the peritoneum of lumican knockout mice, further demonstrating the restrictive role lumican present in the ECM has on prostate cancer invasion. In conclusion, lumican present in the reactive stroma surrounding prostate primary tumors plays a restrictive role on cancer progression, and we therefore postulate that lumican could be a valuable marker in prostate cancer staging.
Keywords: Lumican, desmoplasia, tumor markers, prostate cancer, cytoskeleton, cell migration
Introduction
During tumor progression, cancer cells come in contact with the surrounding stroma triggering a stromal reaction. The result of this tumor-stroma interplay is the formation of a tumor-specific microenvironment, which may play either a restrictive role or a supportive role in the growth and progression of the tumor [1]. The composition and integrity of the remodeled extracellular matrix (ECM) can influence tumor progression and its ability to metastasize [2–5]. Proteoglycans, which are ubiquitous molecules of the ECM and cell surface, play a role in tumor progression and can be up- or down regulated and structurally altered in the tumoral reactive stroma [6].
Lumican belongs to the small leucine-rich proteoglycan (SLRP) family [7]. The generation of knockout mice has proven the role of lumican in the regulation of collagen fibrillar network formation [8]. Lumican contains four major domains: (i) a signal peptide of 16 residues; (ii) a negatively charged N-terminal domain containing sulfated tyrosine and disulphide bond(s); (iii) a signature characteristic of the SLRP family, the leucine-rich repeats domain (LXXLXLXXNXLSLXL) [9], mediating the binding of lumican to other components such as collagen; and (iv) a C-terminal domain of 50 amino acids containing two conserved cysteines [10]. The core protein adopts a unique horseshoe or arch conformation, which favors protein-protein interaction [11]. Lumican has four potential sites for the substitution of N-linked keratan sulfate (KS) or oligosaccharides [12–14]. In the cornea, lumican is present as a proteoglycan, containing covalently linked KS side chains [15], where it regulates collagenous matrix assembly and plays a vital role in cell migration and proliferation during embryonic development and tissue repair [16–19]. High levels of lumican have also been reported to occur in the human dermis [20], artery [21], aorta [22], lung [23], kidney [24], intervertebral discs [25], and connective tissue [26]. In these tissues and in the tumor stroma lumican is present as a glycoprotein containing short oligosaccharides, poorly sulfated or non-sulfated polylactosamine side chains [10, 27]. The physiological functions of lumican are greatly determined by the glycosylation pattern; for example, macrophages solely adhere to lumican bearing low sulfated KS side chains [28].
Several studies have demonstrated the vital role lumican plays in regulating cell behavior, embryonic development, tissue repair and tumor growth [29–31]. Lumican is not normally expressed by the corneal epithelium, however, it is expressed following injury, promoting corneal wound healing by both enhancing epithelial cell proliferation and promoting epithelial cell migration [17]. Lumican also plays a vital role in dermal wound healing, which is compromised in lumican knockout mice [32]. Studies have shown that the reactive stroma present during wounding and tissue repair is similar to the reactive stroma surrounding tumors [33]. Lumican has been detected in the reactive stroma surrounding tumors [6] and effects the adhesion, migration and proliferation of cancer cells [34, 35].
Lumican expression is up-regulated in pancreatic, colorectal, breast and uterine cervical cancers [27, 36–38]. Low expression levels of lumican are associated with poor outcome of invasive breast carcinoma [39]. Lumican is observed in the dermis and tumoral stroma of malignant melanoma and inhibits melanoma progression in mouse experimental models [40, 41]. Lumican is also observed in melanoma cell lines in vitro, but not in normal melanocytes [42]. Lumican inhibits the migration of melanoma cells via cytoskeleton remodeling and decreased pFAK phosphorylation [43]. In addition, direct binding of the lumican core protein to the α2 subunit of α2β1 integrin has been shown to inhibit melanoma cell migration [44]. On the other hand, in human colon cells extracellular lumican can promote actin cytoskeleton remodeling which leads to an increase in cell migration [45]. In colorectal cancer tissues, lumican is expressed in the cancer cells, adjacent fibroblasts, and epithelial cells, whereas in normal colorectal tissues, it is found in fibroblasts and stromal cells [30]. In osteosarcoma cells lumican inhibits TGFβ2 activity which results in the modulation of pSmad 2, integrin β1 and pFAK which consequently decreases cell adhesion [46].
The aim of this study was to analyze the role of lumican in prostate cancer. Lumican disposition was analyzed in vivo and the role of lumican on prostate cancer cells was tested in vitro using three metastatic prostate cancer cell lines. Our results show that during the early stages of prostate cancer tumorigenesis there is an increase in lumican expression in the surrounding stroma which actively inhibits prostate cancer cell invasion.
Material and Methods
Human prostate samples
Fresh tissue samples affected by adenocarcinoma of the prostate were obtained directly following prostate excision. Tumors were located and removed in collaboration with the Department of Pathology, UNIFESP, São Paulo, Brazil; the tissues were frozen and locally stained using hematoxylin/eosin for histological determination of neoplastic and non-neoplastic regions. Following confirmation, neoplastic and non-neoplastic tissues were cut in half; one fragment was allocated for fixing with 4% paraformaldehyde for immunohistochemistry assays, and the other fragment for RNA extraction. Tissue samples were obtained following the patients’ consent as recommended by national guidelines and the Declaration of Helsinki, and the study of human tissues was approved by the UNIFESP ethics committee (1796/08).
Animals
Lumican knockout mice (Lum−/−) were obtained by breeding heterozygous mice (Lum+/−) and all animals were genotyped prior to use [31]. Peritoneal tissue isolated from Lum−/− mice was used to determine the invasiveness of prostate cancer cells in the absence of lumican. Lum+/− were used to isolate control peritoneal tissue. Lum+/− mice did not exhibit any macroscopic abnormalities in the skin, peritoneum or vital organs when compared to wild type mice. All animal protocols were approved by both the Institutional Animal Care and Use Committee (IACUC) of the University of Cincinnati and the Federal University of São Paulo (CEUA).
Lumican purification
Two human amniotic membranes were obtained from consented donations to the Amparo Maternal, São Paulo, Brazil. Approximately 100 g of tissue was finely chopped and homogenized in 400 mL 4 M guanidinium chloride in 0.05 M sodium acetate buffer (pH 6), with the addition of protease inhibitor mixture (100 mM ε-aminocaproic acid, 6.5 mM benzamidine, 5.5 mM iodoacetamide and 0.1 mM phenylmethylsulfonyl fluoride (PMSF), final concentrations), and the mixture agitated at 4°C for 24 h. This homogenate was then filtered through surgical gauze and thereafter dialyzed against distilled water containing protease inhibitor mixture, at 4°C for 24 h. The material was subsequently lyophilized, suspended in 200 mL 7 M urea in 0.05 M sodium acetate buffer (pH 6) containing protease inhibitor mixture, triturated and agitated at 4°C for 24 h. The homogenate was filtered using a sintered glass funnel, dialyzed against distilled water and lyophilized. Next, the material was suspended in 0.1 M NaCl and submitted to ion-exchange chromatography using Q-Sepharose resin (GE Healthcare) and an Äkta Purifier (GE Healthcare) with a stepwise isocratic elution at a flow rate of 1 mL/min. Elution involved using NaCl at the following increasing molarities: 0.3 M, 0.5 M, 0.7 M and 1 M. The fractions collected were dialyzed against distilled water, lyophilized and the presence of lumican was confirmed in fractions 0.5 M and 0.7 M through ELISA assay using anti-lumican and thereafter Western blot analysis using monoclonal antibodies produced at our laboratory; anti-lumican and anti-KS (poorly sulfated KS) [47] (Supplementary Figure 1A). The mouse monoclonal anti-lumican was raised against a peptide designed based on the N-terminal region of lumican and recognizes all glycosylated forms of lumican, however, presents no cross-reactivity to other members of the SLRP family. The fractions that were positive for lumican were subjected to affinity-column chromatography (anti-lumican) previously equilibrated in PBS buffer, pH 8.6. The lumican positive fractions were incubated with anti-lumican conjugated beads and left gently shaking at 4°C for 16 h. Thereafter, lumican was eluted with 0.1 M glycine-HCl buffer (pH 3), immediately neutralized with 1 M Tris-HCl (pH 9) and concentrated using centricon microcentrators (pore size 3 kDa; Millipore Corporation) against PBS buffer. The total protein concentration of elutes was quantified using Coomassie Brilliant Blue R-250 (Bradford, 1976). The purified lumican was analyzed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) 10% and revealed with silver staining (Supplementary Figure 1B).
Finally, in order to analyze whether glycosylation was required for the effects of lumican on prostate cancer cells, recombinant lumican core protein was used. Briefly, recombinant GST-lumican fusion core protein was expressed using the origami (DE3) E.coli strain and the core protein devoid of glycosylation of approximately 40 kDa was purified using an affinity glutathione sepharose column.
Cell culture
Osteotropic human prostatic adenocarcinoma (PC3) cells, prostate carcinoma cells derived from brain metastasis (DU145) and prostate carcinoma cells derived from lymph node metastasis (LnCAP) were obtained from ATCC (CRL-1435, HTB-81 and CRL-174, respectively), and maintained in RPMI medium (GibcoBRL, Life Technologies Inc., Grand Island, NY)/10% fetal bovine serum (FBS, Cultilab, Campinas, Brazil), 100 units/ml penicillin and 100 µg/ml streptomycin, at 37°C in a 5% CO2 humidified environment. All experimental designs were previously approved by the Ethics Committee according to national and international guidelines at both institutions involved.
RNA extraction from tissues and real-time reverse transcription-PCR analysis
Neoplastic and non-neoplastic tissue fragments identified at the Department of Pathology, UNIFESP, São Paulo, Brazil, and intended for RNA extraction were frozen in dry ice and maintained at −80°C until RNA extraction. For extracting RNA, the tissue fragments were weighed and pulverized using a mortar and pestle and liquid nitrogen. Total RNA was extracted by homogenizing the fine powder and adding Trizol® Reagent (Invitrogen, Carlsbad, CA) and following the manufacturer’s protocol. The concentration and purity of the RNA in each sample was determined using a spectrophotometer at 260 and 280 nm. First strand cDNA was reverse transcribed using 1 µg of total RNA and the kit Improm-II™ Reverse Transcriptase (Promega, Madison, WI), according to the manufacturer’s protocol. Quantitative RT-PCR amplification was performed on 2 µl of the cDNA (1:5) with specific primers for lumican and the kit SYBR® Green Master Mix (Applied Biosystems, Foster City, CA) in a 7500 Real-Time PCR System (Applied Biosystems, Warrington, UK), using the activation cycle of 95°C for 10 min, 40 cycles of 95°C for 15 sec, 61°C for 1 min and 72°C for 30 sec. The specificity of the amplified products was analyzed through dissociation curves generated by the equipment yielding single peaks. Negative controls were used in parallel to confirm the absence of any form of contamination in the reaction. Analysis of the data was carried out using the 2−ΔCt method [48] using the 7500 Real-Time PCR System’s software. The primer combination used for lumican was forward: 5’TGGCATTGATTGGTGGTACCAGTG3’ and reverse: 3’TGGGTAGCTTTCAGGGCAGTTACA5’. Expression values were normalized to the housekeeping gene ribosomal protein S29 (RPS29) using the primer combination forward: 5’CCTGGAGGAGAAGAGHAAAGAGA3’ and reverse: 3’TTGAGGACCTCTGTGTATTTGTCAA5’.
Cell proliferation assay
The effect of lumican on prostate cancer cell proliferation was assayed using a colorimetric bromodeoxyuridine (BrdU) incorporation assay (Kit III, Roche Applied Science) performed according to the manufacturer’s protocol. Briefly, 1 × 104 prostate cancer cells (PC3, DU145 or LNCaP) were seeded in 96-well microtiter plates (Corning Inc., Corning, NY). After 24 h, the medium was removed, and the cells were treated with lumican (0, 1, 12.5 or 25 µg/ml), GST-lumican (25 µg/ml) or GST (25 µg/ml) in RPMI supplemented with 10% FBS for 16 h at 37°C in a 5% CO2 atmosphere. Anti-lumican antibody was used as a control in order to block lumican activity. BrdU solution was added to the medium for the final 4 h. Thereafter, the cells were fixed and DNA was denatured in one step by adding the nuclease solution provided. Incorporated BrdU was detected by an anti-BrdU peroxidase-conjugated antibody. The immune complex was detected by a subsequent substrate colorimetric reaction and quantified by measuring the absorbance at 405 nm. All experimental points were performed in triplicate.
Cell migration assay
The effect of lumican on the migration of prostate cancer cells was analyzed using the “scratch assay”. 24-well polystyrene culture dishes (Costar 3516; Corning) or glass cover slips placed inside 24-well polystyrene culture dishes were incubated in a solution of 10 µg/mL lumican, 10 µg/mL GST-lumican or 10 µg/mL GST in EBSS, left for 2 h in a sterile atmosphere (flow hood) and then washed 3 times in EBSS. Naïve culture dishes and cover slips were used as controls. After culture dish treatment, 2 × 104 prostate cancer cells (PC3, DU145 and LNCaP) were seeded in RPMI containing L-glutamine/penicillin/streptomycin and 10% FBS, and incubated for 24 h at 37°C in a 5% CO2 humidified environment. A scratch was performed in the cells on the culture dish using a 100 µL pipette tip. Cells were washed three times with EBSS in order to remove any loose cells, the culture was maintained for a further 12 h (37°C, 5% CO2) and subsequently, photos were captured every 4 h using phase contrast (Axiovert 40 CFL with Axiocam MRC, Zeiss). The scratch width was measured using Image-Pro software (Media Cybernetics, USA) and the percentage of wound area calculated. The experiments were performed in triplicate, carried out thrice and the results analyzed by two separate observers. For the immunocytochemistry assay cells were fixed 4 h after wounding with 4% buffered paraformaldehyde. In order to assess invadopodia formation coverslips were sequentially treated with 1 µg/mL collagen I (purified) for 1 h in a sterile atmosphere (flow hood) followed by 10 µg/mL lumican for 2 h in a sterile atmosphere (flow hood). Coverslips treated with 1 µg/mL collagen I were used as controls.
Cell Invasion assay
The effect of lumican on the invasion of prostate cancer cells was analyzed using culture plate inserts of 8 µm pore size (12 mm, Millicell®-PCF, Millipore Corp., Bedford, MA), which were placed one per well in 24-well polystyrene culture dishes (Costar 3516; Corning) in a solution of 1 µg/mL, 5 µg/mL or 10 µg/mL lumican in EBSS, left for 2 h in a sterile atmosphere (flow hood) and then washed 3 times in EBSS. Control transwell inserts were treated with BSA. After insert treatment they were placed in new 24-well polystyrene culture dishes and subsequently 2 × 104 prostate cancer cells (PC3 and DU145) were plated in the insert in RPMI containing L-glutamine/penicillin/streptomycin in the absence of FBS, and incubated for 4 h at 37°C in a 5% CO2 humidified environment. Following the 4 h incubation, RPMI enriched with 10% FBS was added to the bottom compartment of the system to serve as a stimulus for cell invasion and the cells were incubated for a further 4 h (37°C, 5% CO2). After this incubation period, the inserts were washed twice with PBS buffer and fixed with a buffered 4% paraformaldehyde solution for 15 min. The inserts were then covered with crystal violet solution (0.2% in distilled water) for staining the cells. The cells that had not migrated towards the bottom compartment, i.e. on the top surface of the insert, were removed using a Q-tip, and the cells that had migrated were counted using a light microscope (Axiovert 40 CFL with Axiocam MRC, Zeiss), by three independent observers. The experiment was performed in quadruplicate and repeated twice.
Cell Invasion assay through lumican KO mice peritoneum
In order to elucidate the role of ECM lumican on prostate cancer cell invasiveness and metastasis an invasion assay was performed using peritoneal tissue isolated from lumican KO mice. Mice were euthanized by CO2 inhalation and the peritoneum was immediately removed and washed 3 times in HBSS (Gibco). Peritoneal tissue was placed in a 6 well microplate covering the surface of the well. Prostate cancer cells (PC3) were labeled with DiI for 30 minutes, washed 3 times in HBSS and, subsequently, 3 × 105 prostate cancer cells (PC3) were placed upon the visceral side of the peritoneal tissue in 1:1 RPMI/DMEM supplemented with 10% FBS. The cells were allowed to invade for 24 h and subsequently the tissue was washed 3 times in sterile PBS and fixed overnight in buffered 4% paraformaldehyde. The peritoneal tissue was embedded in tissue freezing medium (Tissue Tek, Sakura) and cut into 4 µm sections using a cryostat (Leica). The number of adhered or invaded cells was counted in every fifth slide. The invasion of PC3 cells was analyzed using three independent peritoneal tissues isolated from lumican KO and heterozygous tissues. Invaded cells were counted and images captured using a Zeiss Observer Z1 microscope with an ApoTome and images analyzed using LSM Image Browser 3.2 software (Zeiss, Germany).
Immunohistochemistry, Immunocytochemistry and Microscopy
Human prostate tissues or mouse peritoneum following the invasion assay were immersion fixed in 4% buffered paraformaldehyde for 4 h or 12 h, respectively. Subsequently, tissues were washed 3 times in PBS placed in 30% sucrose for 48 h and embedded in tissue freezing medium (Electron Microscopy Sciences). The embedded tissue was sliced into 4 µm sections using a cryostat (Leica). The tissue slices were collected on silanized slides for immunofluorescence staining. Cells cultured on cover slips were sequentially immersion fixed in 4% paraformaldehyde in PBS for 30 min and acetone:methanol 1:1 for 2 min and subsequently washed 3 times in PBS (15 min each wash). For immunofluorescence the tissue slices and coverslips were incubated in blocking solution (5% FBS) at room temperature for 1 h. Tissue slices and coverslips were then incubated with primary antibodies overnight at 4°C. Primary antibodies used were: mouse monoclonal anti-lumican (produced in our laboratory), anti-Keratin 8 (2032-1 Epitomics), anti-Keratin 18 (1924-1 Epitomics), anti-ZO-1 (40-2200 Invitrogen), mouse anti-smooth muscle α-actin conjugated with Cy3 (clone 1A4, Sigma-Aldrich, St. Louis, MI), N-WASP or goat anti-cortactin (G-18, Santa Cruz Biotechnology), anti-integrinβ1 (Millipore MAB2247) anti-MT1-MMP (Millipore AB8345) and rabbit anti-N-WASP (H-100, Santa Cruz Biotechnology). Actin filaments were stained with Phalloidin 488 (Life Technologies). Afterwards, the cover slips were washed 3 times in PBS and then incubated for 1 h at room temperature with appropriate fluorescent secondary antibodies produced in donkey conjugated to Alexa Fluor® 488 or Alexa Fluor® 594 (Molecular Probes/Invitrogen, Eugene, OR). In order to investigate the possible co-localization of compounds, a second incubation with primary and secondary antibodies was performed. After incubation with the antibodies, the cover slips were washed 3 times in PBS, incubated with 4,6-diamidino-2-phenylindole (DAPI) in PBS (300 ng/ml) (Sigma) for 30 minutes, washed 3 times in PBS, mounted on glass slides in Fluoromount G (2:1 in PBS, Electron Microscopy Sciences, Hatfield, PA) and sealed with nail polish. Negative control immunostaining was performed with omission of each primary antibody and did not yield specific immunostaining (not shown). Cover slips were examined using a Zeiss LSM510 scanning confocal inverted microscope and images analyzed using LSM Image Browser 3.2 software (Zeiss, Germany).
Statistics
All values are presented as mean ± standard deviation of the mean. The difference between two groups was compared by unpaired Mann-Whitney test. P < 0.05 was considered to be statistically significant. Statistical analysis was performed with the GraphPad Prism version 5 software package (GraphPad Software, San Diego, CA).
Results
Lumican expression and localization in human prostate cancer and non-neoplastic tissues
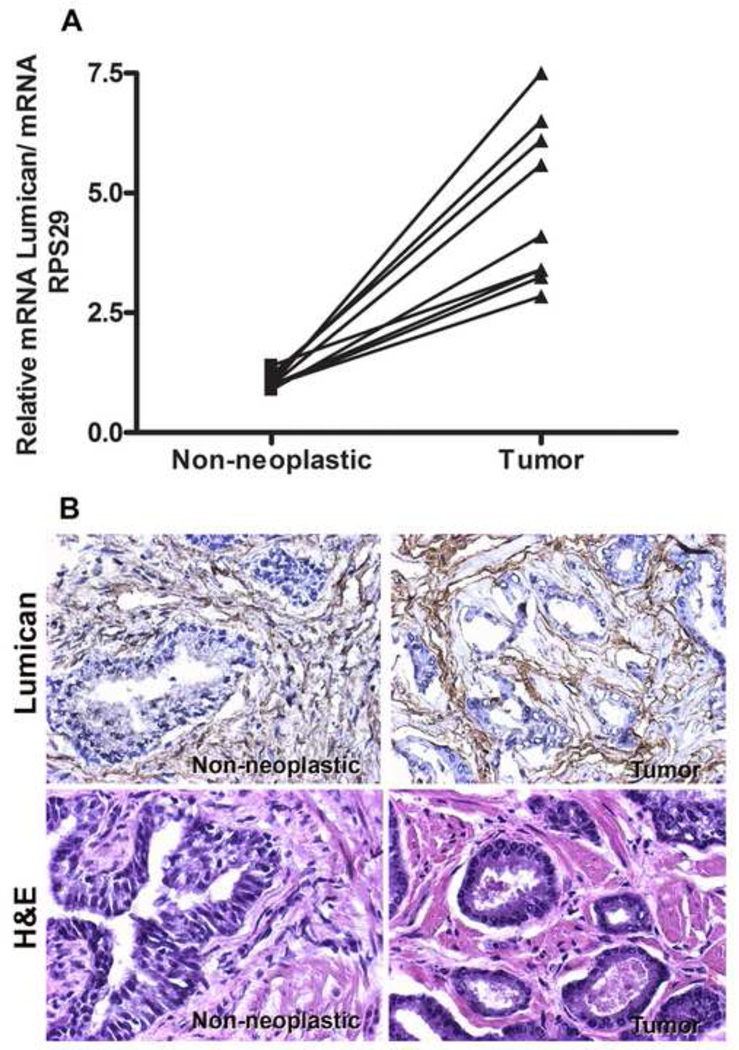
In non-neoplastic prostate tissues lumican staining was mainly observed in the muscular stroma, in a distribution pattern that was parallel to the muscle fibers (Figure 1B). Lumican staining was also detected in the stroma surrounding the acini. Weak lumican staining was detected in epithelial cells of the acini as previously described [49]. Interestingly, quantification of the immunohistochemistry images showed a 4-fold increase in the lumican surrounding neoplastic acini. The lumican in neoplastic prostate tissue was arranged as concentric circles around the acini with a fibrotic disposition, in all ten samples analyzed (Figure 1B). Accordingly, a 4.5±1.5-fold increase in lumican expression was detected by real-time PCR in neoplastic prostate tissues compared to contralateral non-neoplastic prostate tissues (Figure 1A).
Figure 1.
Expression and localization of Lumican in human neoplastic and non-neoplastic prostate. Fresh neoplastic and contralateral non-neoplastic specimens were isolated from human prostate primary tumors; the specimens were frozen and stained using hematoxylin/eosin for histological determination of neoplastic and non-neoplastic regions. Following confirmation, neoplastic and non-neoplastic tissues were cut in half; one fragment was allocated for RNA extraction and real-time PCR analysis (A), and the other fragment was fixed with 4% paraformaldehyde for immunohistochemistry assays (B and C). An increase in lumican expression was observed both through real-time PCR (A) and immunohistochemistry (B and C). Lumican in neoplastic tissues displayed characteristic fibrotic disposition surrounding neoplastic acinar glands. Gene expression was normalized against ribosomal protein S29 (RPS29). *p<0.05.
Purified lumican
Lumican was isolated from human amniotic membranes and purified using an ion-exchange chromatography Q-Sepharose resin. Two populations of lumican, of approximately 40 and 55 kDa, were isolated in fractions 0.5 M and 0.7 M of NaCl, respectively. The lumican core protein was recognized as a 40 kDa population using an anti-lumican antibody and lumican glycoprotein was recognized a 55 kDa population with both the anti-lumican antibody and the anti-KS antibody which recognizes poorly sulfated polylactosamine side chains (Supplementary Figure 1A). The fractions that were positive for lumican were pooled and further submitted to affinity chromatography using anti-lumican conjugated beads. The purified lumican populations, of 40 and 55 kDa, were exclusively visualized by SDS-PAGE developed with silver staining (Supplementary Figure 1B). The purification of the two lumican populations from the amniotic membrane was in accordance with those previously described [17]. Lumican core protein and the glycosylated form have been detected together in the reactive stroma surrounding tumors and exert effects on the adhesion, migration and proliferation of cancer cells [30, 34, 35]. Therefore, in order to mimic the lumican content present in the prostatic reactive stroma both populations of lumican were used in conjunction. Thereafter, the importance of glycosylation was investigated by analyzing the effect of solely the lumican core protein devoid of glycosylation (GST-lumican) on prostate cancer cells.
Role of lumican in prostate tumor cell viability and proliferation
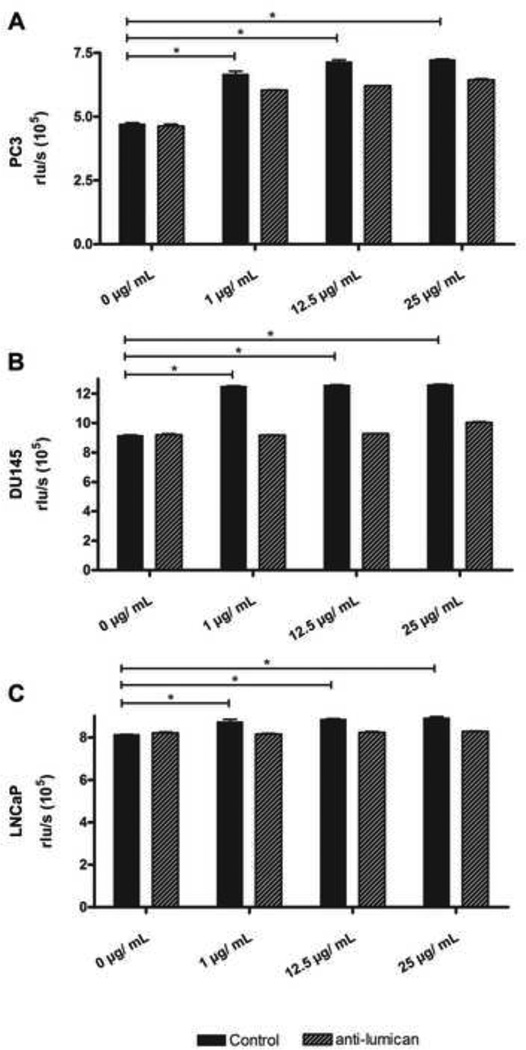
As a result of the prostate stromal reaction, lumican expression is up-regulated surrounding prostate primary tumors. In order to elucidate the effect this lumican has on prostate cancer cells we assessed the role of lumican on cell viability and proliferation of the prostate cancer cell lines PC3, DU145 and LNCaP. Interestingly, lumican and GST-lumican had no effect on the viability of the three prostate cancer cell lines (results not shown). However, an effect of lumican was observed on prostate cancer cell proliferation, which increased by approximately 15, 25 and 10% for PC3, DU145 and LnCAP, respectively (Figure 2). The addition of anti-lumican completely abolished the effect of lumican on DU145 and LnCAP cell proliferation; however, it did not completely abolish the effect of lumican on PC3 cell proliferation. Similar results were obtained for the effect of GST-lumican on the proliferation of PC3 and DU145 (results not shown).
Figure 2.
Effect of Lumican on prostate cancer cell proliferation. Prostate cancer cells (PC3 (A), DU145 (B) and LnCAP (C)) were treated with increasing concentrations of lumican extracted from amniotic membrane for 24 h. Cell proliferation was measured using the BrdU assay. Treatment of cells with anti-lumican blocked the effect of lumican on cell proliferation. Error bars indicate S.D. of six samples. (*) p<0.05 versus control (0 µg/mL).
Lumican inhibits the migration of prostate tumor cells
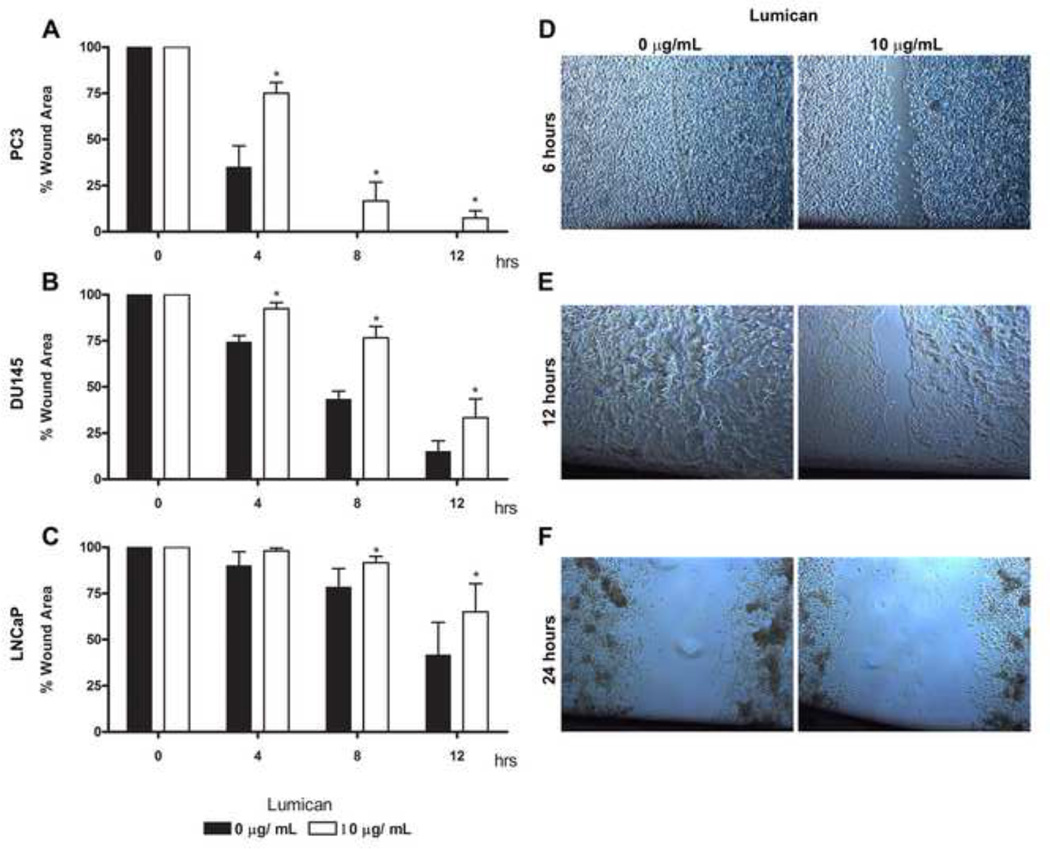
The immunohistochemistry results showed that lumican has a fibrotic disposition concentrically arranged around neoplastic acini, possibly as a stromal reaction to the primary prostate tumor and potentially forming a restrictive barrier around compromised tissue. We therefore assessed a possible role of lumican in prostate cancer cell migration using a scratch wound assay of cultured cells. Prostate cancer cells were seeded on culture dishes previously treated with lumican and naïve dishes were used as controls. A decrease in the migration of all three prostate cancer cell lines (PC3, DU145 and LNCaP) was observed when cells were seeded on lumican, however the effect on PC3 cells was more pronounced (Figure 3A). Four hours after seeding the cells on a lumican coat there was an overall significant decrease in metastatic prostate cancer cell migration; approximately 50% and 20% for PC3 and DU145, respectively. A significant decrease in the migration of LNCaP was only evident after 8 h. Lumican decreased LNCaP cell migration by 35% by 12 h when compared to control cells seeded in the absence of a coat. PC3 control cells seeded in the absence of a coat had completely closed the wound by 8 h, whereas cells seeded on lumican coats still had not completely closed the wound by 12 h. The role of GST-lumican was tested on PC3 and DU145 prostate cancer cells. GST-lumican decreased the migration of both PC3 and DU145, by approximately 45% and 20% respectively, after 6 hours (results not shown). Therefore, lumican significantly inhibits the migration of prostate tumor cells.
Figure 3.
Role of lumican on prostate cancer cell migration. 24 well polystyrene culture dishes were incubated in a solution of 10 µg/mL lumican and left for 2 h in a sterile atmosphere (flow hood) and then washed 3 times in EBSS. Untreated culture dishes were used as controls. After culture dish treatment, 2 × 104 prostate cancer cells (PC3 (A and D), DU145 (B and E) and LNCaP (C and F)) were seeded in RPMI containing L-glutamine/penicillin/streptomycin and 10% FBS, and incubated for 24 h at 37°C in a 5% CO2 humidified environment. A scratch was performed in the cells on the culture dish using a 100 µL pipette tip. Cells were washed three times with EBSS and maintained for a further 24 h (37°C, 5% CO2), images being captured every 4 h. Photos were taken using phase contrast. Measurements were calculated using Image-pro.
Lumican inhibits the invasion of prostate tumor cells
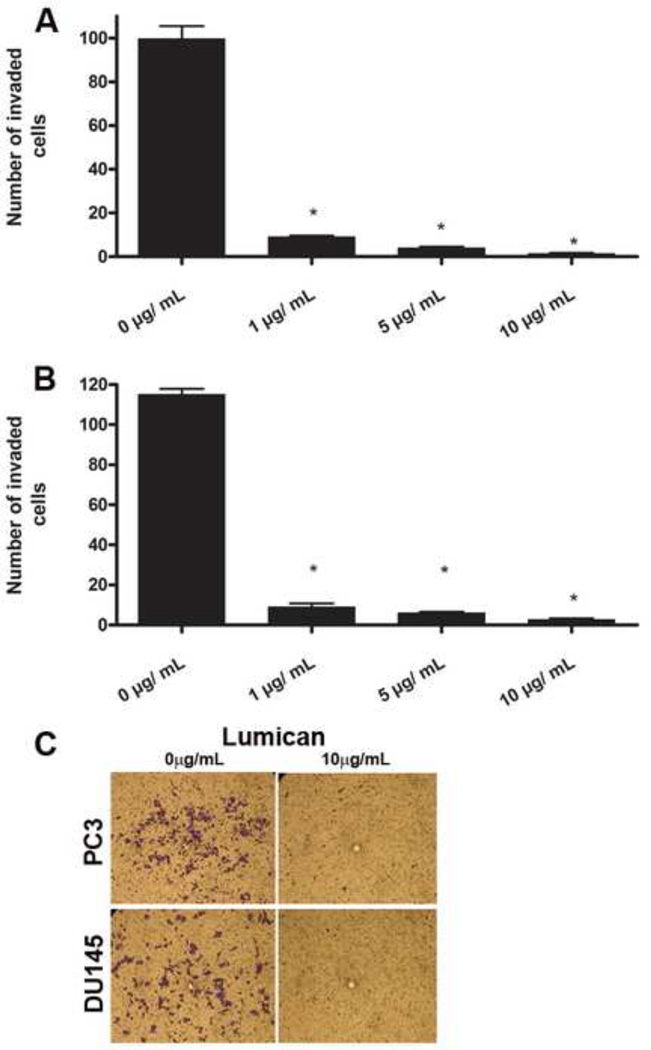
The role of lumican in prostate cancer cell invasion was evaluated using the transwell insert system. Transwell inserts were treated with lumican, or BSA as the control. Subsequently, prostate cancer cells were seeded in the upper chamber with medium supplemented with 10% FBS in the lower chamber as an attractant for cell invasion into the lower chamber. Lumican treatment (10 µg/mL) reduced prostate cancer cell invasion into the lower chamber by 98% and 80% for PC3 and DU145, respectively, compared to BSA treated control inserts (Figure 4). Therefore, lumican treatment drastically inhibits the invasion of prostate cancer cells.
Figure 4.
Role of lumican on prostate cancer cell invasion. The effect of lumican on the invasion of prostate cancer cells was analyzed using culture plate inserts (8 µm pore size) previously treated with 1 µg/mL, 5 µg/mL or 10 µg/mL of lumican purified from amniotic membrane for 2 h and subsequently washed 3 times in EBSS. Control transwell inserts were treated with BSA. After insert treatment, 2 × 104 prostate cancer cells (PC3 (A) and DU145 (B)) were seeded in the insert in RPMI containing L-glutamine/penicillin/streptomycin in the absence of FBS, and incubated for 4 h at 37°C in a 5% CO2 humidified environment. Thereafter, RPMI enriched with 10% FBS was added to the bottom compartment and the cells were incubated for a further 2 h (37°C, 5% CO2), and subsequently the inserts were washed, fixed and stained with crystal violet solution (0.2% in distilled water). Cells in the top compartment were removed using a Q-tip, and migrated cells (C) were counted (A and B).
Effect of lumican on the cytoskeleton and formation of cellular projections in prostate tumor cells
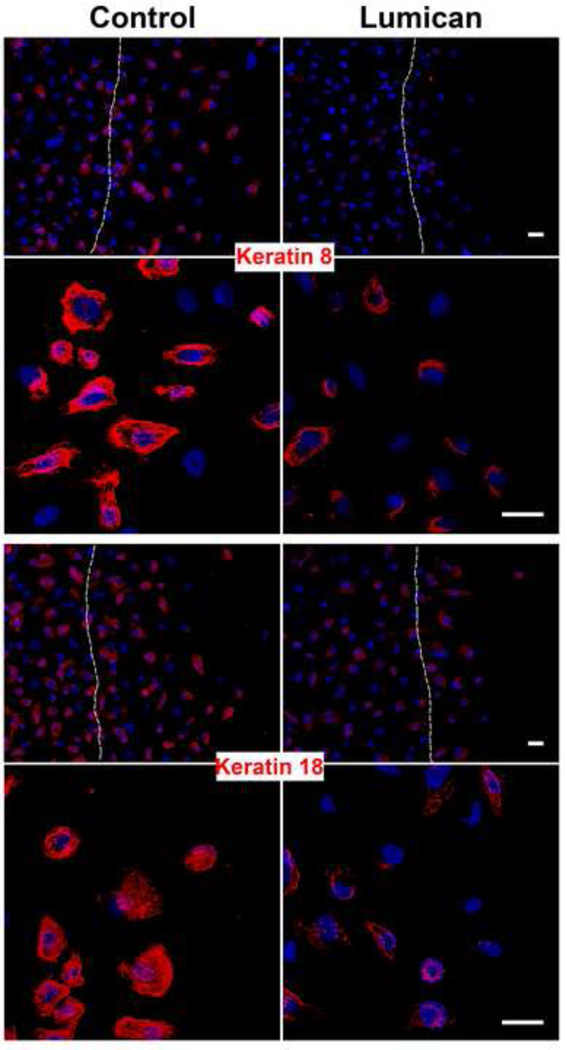
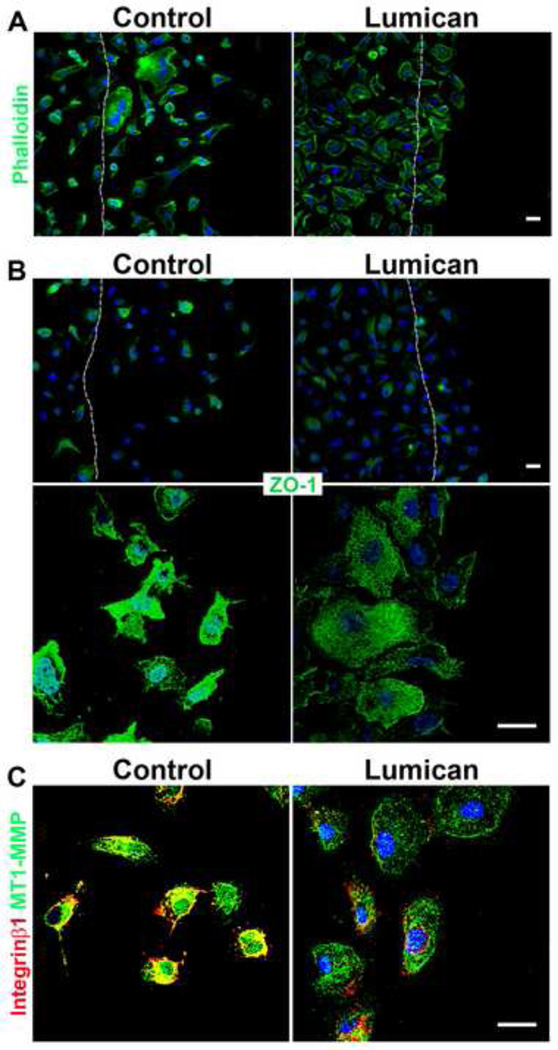
Considering the drastic effect lumican had on prostate cancer cell invasion we proceeded to investigate the mechanism by which lumican inhibits both the migration and invasion of prostate cancer cells. PC3 cells were used due to the more pronounced effect lumican had on PC3 cell migration and invasion. Previous studies have demonstrated that lumican may induce cytoskeleton re-organization, therefore potential cytoskeletal alterations were studied in prostate cancer cells. The disposition of the intermediate filaments keratin 8 and 18, which have altered expression patterns in prostatic neoplasic epithelial cells, was analyzed in prostate cancer cells seeded upon lumican coats. Lumican lead to a decrease in both keratin 8 and 18 expressions in prostate cancer cells (Figure 5). Moreover, the localization of keratin 8 and 18 in control PC3 cells is organized and the keratin filaments extend throughout cellular protrusions, lamellipodia. When PC3 cells were seeded upon lumican coated cover slips the disposition of keratin changes to a perinuclear localization resulting in prostate cancer cells that have a more rounded cell shape (Figure 5). Phalloidin staining revealed elongated control prostate cancer cells with cellular projections parallel to the migrating front, whereas prostate cancer cells seeded upon lumican present a more rounded cell shape. ZO-1 and integrins, in concert with the cytoskeleton, regulate polarized cell migration. Prostate cancer cells seeded upon lumican coated coverslips presented a decrease in both ZO-1 and integrin β1 expression and loss of ZO-1 and integrin β1 rich cellular projections (Figure 6B and 6C). Moreover, control prostate cancer cells presented nuclear ZO-1 staining; however, when the cells were seeded upon lumican they no longer presented nuclear ZO-1 staining (Figure 6B). Prostate cancer cells presented strong MT1-MMP expression which co-localized with integrin β1 in the cellular projections, whereas, when prostate cancer cells were seeded upon lumican coats a drastic decrease in MT1-MMP expression was observed which no longer localized to cellular projections (Figure 6C).
Figure 5.
Role of lumican on keratin 8 and 18 filaments in prostate cancer cells. Coverslips placed in 24 well polystyrene culture dishes were incubated in a solution of lumican (10 µg/mL) and untreated culture dishes were used as controls. After culture dish treatment, 2 × 104 prostate cancer cells, PC3, were seeded in RPMI containing L-glutamine/penicillin/streptomycin and 10% FBS, and incubated for 24 h at 37°C in a 5% CO2 humidified environment. A scratch was performed in the cells on the culture dish using a 100 µL pipette tip. Cells were washed three times with EBSS, maintained for a further 4 h (37°C, 5% CO2) and fixed for immunocytochemistry. The cells were labeled with anti-keratin 8 and anti-keratin 18 and the nuclei were visualized with DAPI. Dashed line represents the original wound edge. Images were captured using a Zeiss LSM510 scanning Confocal inverted microscope and Zeiss Observer Z1 microscope coupled with an ApoTome.
Figure 6.
Role of lumican on the expression and localization of actin filaments, ZO-1, integrin β1 and MT1-MMP in prostate cancer cells. Coverslips placed in 24 well polystyrene culture dishes were incubated in a solution of lumican (10 µg/mL) and untreated culture dishes were used as controls. After culture dish treatment, 2 × 104 prostate cancer cells, PC3, were seeded in RPMI containing L-glutamine/penicillin/streptomycin and 10% FBS, and incubated for 24 h at 37°C in a 5% CO2 humidified environment. A scratch was performed in the cells on the culture dish using a 100 µL pipette tip. Dashed line represents the original wound edge. Cells were washed three times with EBSS, maintained for a further 4 h (37°C, 5% CO2) and fixed for immunocytochemistry. The cells were labeled with phalloidin (A) and anti-ZO-1 (B) and the nuclei were visualized with DAPI. Images were captured using a Zeiss LSM510 scanning Confocal inverted microscope and Zeiss Observer Z1 microscope coupled with an ApoTome.
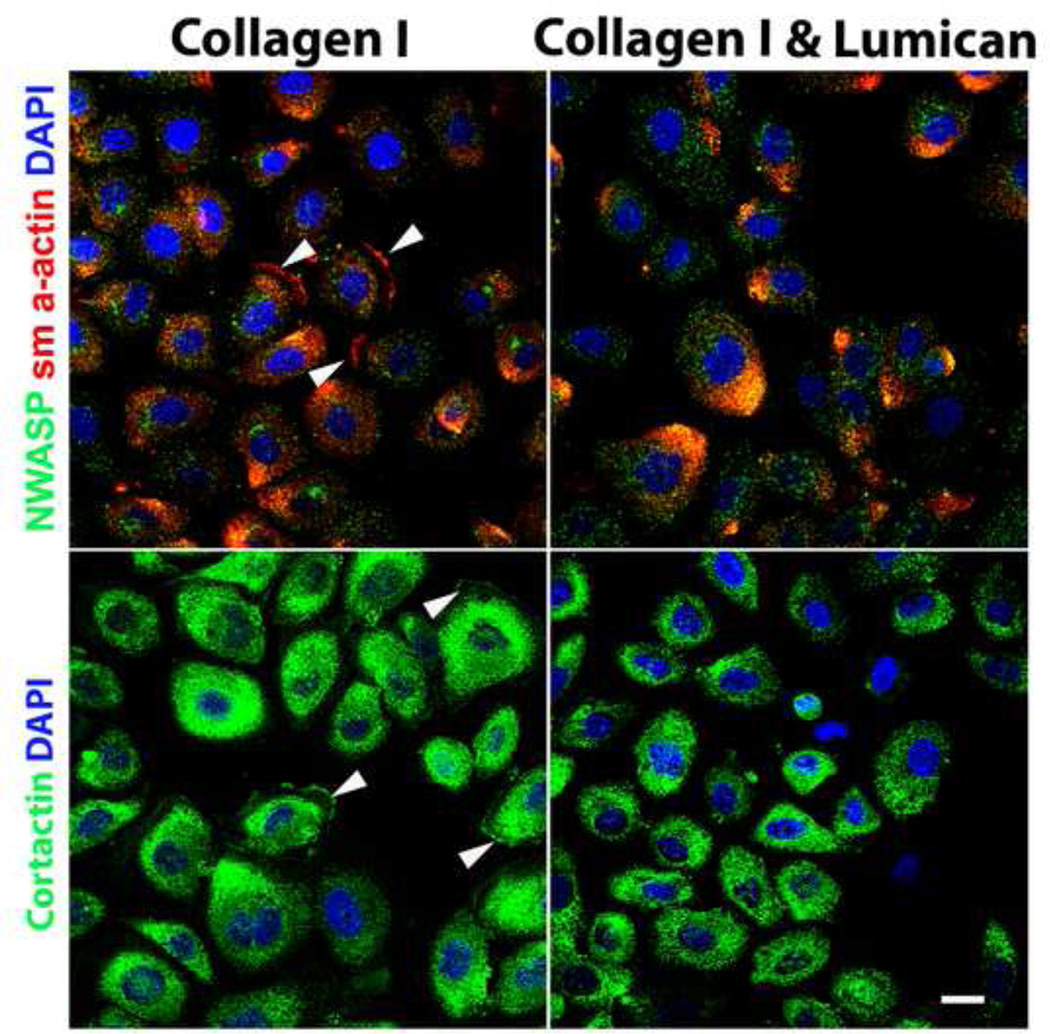
In order to investigate whether prostate cancer cells present invadopodia and, more importantly, whether lumican disrupts the formation of invadopodia, prostate cancer cells (PC3) were seeded on collagen or a bi-layer of collagen I and lumican and, thereafter, subjected to the scratch assay, fixed after 4 h and analyzed by immunocytochemistry. The cells were stained for α-SMA, N-WASP and cortactin. In prostate cancer cells seeded directly on collagen I coated cover slips an invadopodia-like α-SMA and cortactin distribution was observed at protruding edges of the cell membrane (Figure 7). When prostate cancer cells were seeded on a bi-layer coating formed by sequential collagen I and lumican treatment, drastically unorganized α-SMA was observed. Invadopodia-like structures were no longer detected by α-SMA and cortactin staining. Therefore, lumican inhibits the formation of actin-based structures at the edges of membrane protrusions, invadopodia, which are responsible for active cell movement.
Figure 7.
Role of lumican on the expression and distribution of N-WASP, cortactin and sm α-actin in prostate cancer cells. Coverslips placed in 24 well polystyrene culture dishes were incubated in a solution of either 1 µg/mL collagen I or sequential 1 µg/mL collagen I and 10 µg/mL lumican. After culture dish treatment, 2 × 104 prostate cancer cells, PC3 (A) or DU145 (B), were seeded in RPMI containing L-glutamine/penicillin/streptomycin and 10% FBS, and incubated for 24 h at 37°C in a 5% CO2 humidified environment. A scratch was performed in the cells on the culture dish using a 100 µL pipette tip. Cells were washed three times with EBSS, maintained for a further 4 h (37°C, 5% CO2) and fixed for immunocytochemistry. The cells were labeled with anti-N-WASP, anti-sm α-actin, anti-cortactin and DAPI and analyzed using a Zeiss LSM510 scanning Confocal inverted microscope.
Effect of lumican on prostate tumor cell invasion and metastasis
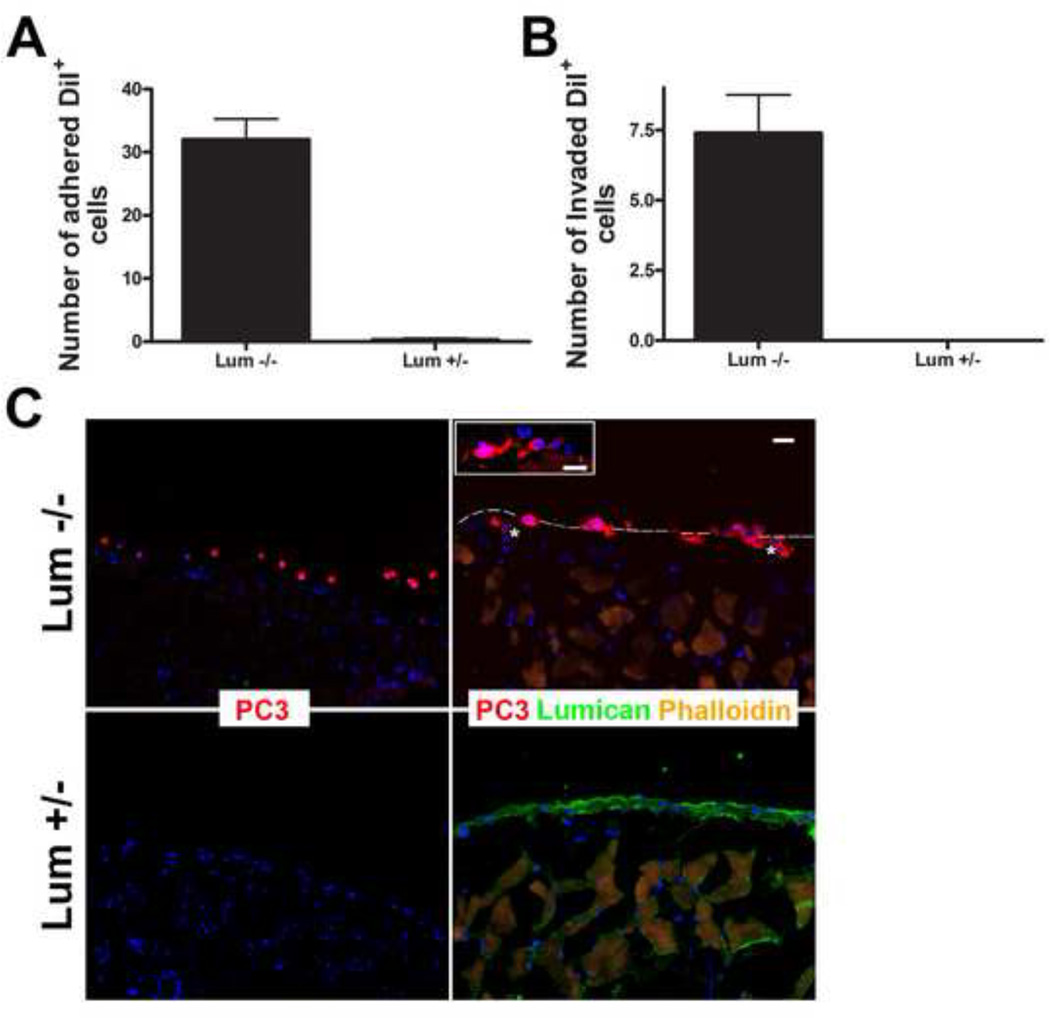
In order for cancer cells to metastasize they must invade the surrounding tissue which normally includes a serous membrane. A serous membrane is composed of a secretory epithelial layer and a connective tissue layer. The peritoneal membrane, the serous membrane which surrounds the abdominal cavity, is often used as a model for analyzing the invasive capability of tumor cells. In order to evaluate the role lumican present in the ECM has on the capability of prostate cancer cells to invade, PC3 cells were seeded upon the peritoneal membrane of lumican KO and heterozygous mice and left to invade for 24 h. PC3 cells adhered to and invaded the peritoneum isolated from lumican KO mice, whereas, few cells adhered to the heterozygous control peritoneal membrane (Figure 8). Entire PC3 cells were able to invade the lumican KO peritoneal membrane, to such an extent that they were fully submerged in the membrane. On the other hand, of the few cells that adhered to the heterozygous membrane none had more than 30% of the cell body within the peritoneal tissue. Therefore, prostate cancer cells readily adhered to and invaded the peritoneal membrane in the absence of lumican in only 24 h, whereas, in the presence of lumican few cells were able to adhere to and invade the peritoneal membrane in the same timeframe.
Figure 8.
Role of lumican on the capacity of prostate cancer cells to invade adjacent tissue. Prostate cancer cells (PC3) previously labeled with DiI (red) were seeded upon peritoneal tissue isolated from either lumican KO mice or littermate heterozygous controls and left to invade for 24 h. The peritoneum was washed and fixed and the invaded cells were visualized under a fluorescent microscope. The total number of DiI positive cells which had penetrated either lumican KO or littermate control tissue were counted and represented in a graph (A). PC3 cells adhered to and invaded the lumican KO peritoneal tissue, whereas few cells adhered to the heterozygous tissue and no cells fully invaded the heterozygous tissue (B). PC3 cellular projections (asterisk) protrude into the lumican KO peritoneal tissue.
Discussion
Our group has previously shown the important role stromal reaction plays in cancer progression [2, 3]. Colorectal stromal reaction at the site of the primary tumor presents an up-regulation of collagen I, collagen III, collagen IV and collagen V both in vivo and in vitro. This up-regulation restricts the invasion of colorectal tumor cells [2]. On the other hand, prostate metastatic cancer cells down-regulate the expression of ECM components such as collagens I, II, III, and IV, and the SLRPs decorin, biglycan, lumican, and fibromodulin in stromal fibroblasts in vitro, leading to a more tumorigenic phenotype in vivo [3]. Lumican, a SLRP, has attracted attention as a molecule of the extracellular matrix, however, lumican also presents chemokine properties affecting cell behavior [50]. Lumican promotes corneal epithelium wound healing and maintains corneal homeostasis by modulating gene expression in normal and diseased tissues [10, 17, 51]. Moreover, lumican has been shown to play an important role in cancer progression, displaying anti-tumor activity in human malignant melanoma [40].
The up-regulation of lumican has been described in pancreatic, colorectal, breast and uterine cervical cancers [27, 36–38]. We analyzed the expression of lumican in primary prostate tumors in vivo. Immunohistochemistry results show that non-neoplastic prostate tissues have a significant amount of lumican distributed throughout the stroma, suggesting it plays an important role in normal prostate physiology. An increase in lumican was observed in the neoplastic prostate tissues when compared to non-neoplastic tissues. The distribution of lumican in the neoplastic tissues assumes a periacinar disposition typical of desmoplasia. This increase in expression was confirmed through real-time PCR analysis of neoplastic tissues compared to non-neoplastic tissues. Therefore, the stroma surrounding prostate primary tumors up-regulates lumican expression forming a lumican rich barrier surrounding the tumors.
The stroma surrounding tumors consists of ECM, fibroblasts, endothelial cells, inflammatory cells and neurons. Accumulating evidence supports the important role the stromal compartment plays on cancer tumorgenesis. A vital step in tumor progression is the transformation of surrounding fibroblasts into tumor associated fibroblasts (TAF) which promote tumor growth by producing cytokines and aid tumor invasion by modifying the ECM [2, 3]. Thus, we further investigated whether the up-regulation of lumican surrounding human primary prostate tumors would have a tumor promoting or restricting function on prostate cancer cells. In order to do this, the effect of lumican on prostate cancer cells was tested in vitro using three prostate cancer cell lines (PC3, DU145 and LnCAP). Previously, lumican has been shown to inhibit cell migration and proliferation of murine melanoma cells [41]. Moreover, lumican has been shown to increase the invasiveness of murine pancreatic adenocarcinoma cells, however, decreases the invasiveness of murine fibrosarcoma cells, demonstrating a tumor-specific effect of lumican [52]. To our knowledge the role of lumican in prostate cancer to date remains elusive. Our results show that lumican core protein has no effect on prostate cancer cell viability and no significant effect on cell proliferation, however inhibits the migration and invasion of prostate cancer cells.
Cell migration and invasion are largely dependent on the complex organization of the various cytoskeletal components. We postulated that the inhibitory role lumican plays on prostate cancer cell migration and invasion could be due to a reorganization of the cytoskeleton, which has previously been shown in melanoma cells. Melanoma cells grown on lumican coats present altered cell morphology, loss of stress fibers and rearranged actin filament localization that assumes a condensed sub-membrane distribution [53]. Bone derived metastatic prostate cancer cells seeded upon lumican coats presented a loss in both elongated cell shape and cellular projections parallel to the migratory path at the wound edge, shown by phalloidin staining. Intermediate filaments such as the keratin cytoskeleton together with actin filaments and microtubules play a vital role in cell motility. Previous studies have shown that keratin 8 re-organization leads to enhanced migration of human epithelial tumor cells [54]. An increased expression of both keratin 8 and keratin 18 expression has been associated with neoplastic progression, invasion and poor prognosis in squamous cell carcinomas (SCCs) [55, 56]. Moreover, the loss of keratin 8 and keratin 18 has been linked to a decrease in α6β4 integrin expression, cancer cell motility and cancer cell invasion [57]. Keratin 8 and keratin 18, are not expressed in normal keratinocytes, however both forms of keratins are co-expressed in metastatic keratinocytes forming keratin8/18 filaments. The co-expression of keratin 8/18 filaments in normal keratinocytes enables these cells to efficiently invade an artificial basement membrane [58]. Our studies revealed a decrease in both keratin 8 and 18 expressions in prostate cancer cells seeded upon lumican, as well as, a change in the disposition of keratin 8 and 18 from organized filaments extending throughout the cellular protrusions, lamellipodia, to a perinuclear localization. The important role keratin 8 and 18 play in lamellipodia dynamics has been previously shown [59]. Cell migration involves the formation of cytoskeletal projections such as lamellipodia, filopodia and, in some cell types, such as cancer cells, invadopodia. The loss of lamellipodia in prostate cancer cells seeded upon lumican coats was observed through phalloidin, integrin β1 and keratin staining which no longer extended through cellular projections. Proteolytic activity of cancer cells is normally focused on plasma membrane protrusions, such as lamellipodia and invadopodia [60, 61]. A drastic decrease in MT1-MMP was observed in prostate cancer cells seeded upon lumican which was no longer detected in cellular projections. Lamellipodia and invadopodia are formed at the leading edge of migrating cells, such as breast cancer cells which present both lamellipodia and invadopodia, and jointly enable their migration and invasion [60, 62]. Our results further revealed that lumican also induces a loss of invadopodia formation in prostate cancer cells. Sm α-actin and N-WASP were down-regulated in prostate cancer cells seeded on lumican. Moreover, the cellular distribution of sm α-actin, N-WASP and cortactin changed from protruding invadopodia at the cell surface to a perinuclear disposition.
Cell migration and invasion are also dependent upon the formation of adhesion complexes. The interaction between lumican and melanoma cells has been shown to induce the destabilization of focal adhesion complexes inhibiting cell migration [43]. Zona occluden protein 1 (ZO-1) is an adaptor proteins involved in intercellular tight junctions. Recent studies have localized ZO-1 to lamellipodia and shown it may play an active role in cell migration and carcinogenesis that extends beyond the maintenance of epithelial tight junction integrity. ZO-1 has been reported to exist at the leading edge of migrating fibroblasts during wound healing [63]. ZO-1 associates with integrin α5β1 and participates in the initiation of the integrin-dependent adhesion complexes [63, 64]. Therefore, ZO-1 and integrins, in concert with the cytoskeleton, regulate polarized cell migration. Moreover, protein kinase C regulation of an alpha5 integrin-ZO-1 complex controls lamellae formation in migrating cancer cells, where lamellipodial ZO-1 co-localizes with G-actin, α-actinin, and cortactin [64]. ZO-1 has also been shown to re-localize from tight junctions to the nucleus during epithelial-mesenchymal transition (EMT) [65]. Prostate cancer cells seeded upon lumican coated coverslips presented a decrease in ZO-1 expression, with ZO-1 evenly distributed throughout the cell membrane, and not concentrated and localized in cellular projections. Moreover, prostate cancer cells seeded upon lumican no longer presented nuclear ZO-1 staining, as observed in control prostate cancer cells.
Based on our data, lumican expression is increased in the stroma surrounding human primary prostate cancer. Moreover, lumican inhibits prostate cancer invasion via cytoskeletal rearrangement and disruption of lamellipodia and invadopodia. Therefore, up-regulation of lumican surrounding prostate tumors plays a restrictive role in prostate tumorigenesis. Thus, we hypothesize that during prostate cancer progression two events must take place: (1) prostate cancer cells must increase the secretion of MMPs to degrade stromal lumican and/or (2) prostate cancer cells must elicit a stromal reaction that leads to down-regulation of ECM components, as previously shown [3]. Moreover, we postulate that lumican could be a valuable marker in prostate cancer staging.
Supplementary Material
Lumican was purified from amniotic membrane. (A) Lumican was extracted from two human amniotic membranes (PL1 and PL2), lumican was enriched from the extract (E) using ion-exchange chromatography with Q-Sepharose resin (GE Healthcare) and the presence of lumican core protein and glycosylated lumican were detected in the fractions 0.5 M and 0.7M by Western blot analysis using monoclonal antibodies produced at our laboratory (anti-lumican and anti-KS). (B) Fractions were pooled and lumican was further purified using anti-lumican affinity-column chromatography and analyzed by polyacrylamide gel electrophoresis (SDS-PAGE) 10% followed by silver staining revealing lumican core protein (40 kDa) and glycosylated lumican (55 kDa) compared to the protein ladder (La).
Highlights.
Increased lumican expression was observed in prostate reactive stroma.
Lumican inhibited metastatic prostate cancer cell migration and invasion in vitro.
Lumican lead to prostate cancer cell cytoskeletal rearrangement.
Lumican inhibits the formation of cellular projections in prostate cancer cells.
Acknowledgements
This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo, FAPESP 2007/59801- 1 and 2010/52426-3, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and NIH/NEI EY 011845. We would like to thank Dr. Osamu Yamanaka and Carolina M. Vicente for their support throughout this study. We also acknowledge Caroline Z. Romera and Elizabeth N. Kanashiro (INFAR/UNIFESP, Brazil) for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Proia DA, Kuperwasser C. Stroma: tumor agonist or antagonist. Cell Cycle. 2005;4:1022–1025. doi: 10.4161/cc.4.8.1903. [DOI] [PubMed] [Google Scholar]
- 2.Coulson-Thomas VJ, Coulson-Thomas YM, Gesteira TF, de Paula CA, Mader AM, Waisberg J, Pinhal MA, Friedl A, Toma L, Nader HB. Colorectal cancer desmoplastic reaction up-regulates collagen synthesis and restricts cancer cell invasion. Cell Tissue Res. 2011;346:223–236. doi: 10.1007/s00441-011-1254-y. [DOI] [PubMed] [Google Scholar]
- 3.Coulson-Thomas VJ, Gesteira TF, Coulson-Thomas YM, Vicente CM, Tersariol IL, Nader HB, Toma L. Fibroblast and prostate tumor cell cross-talk: fibroblast differentiation, TGF-beta, and extracellular matrix down-regulation. Exp Cell Res. 2010;316:3207–3226. doi: 10.1016/j.yexcr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Lu P, Weaver VM, Werb Z. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. doi: 10.1016/s1470-2045(01)00620-9. [DOI] [PubMed] [Google Scholar]
- 6.Wegrowski Y, Maquart FX. Involvement of stromal proteoglycans in tumour progression. Crit Rev Oncol Hematol. 2004;49:259–268. doi: 10.1016/j.critrevonc.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- 8.Chakravarti S, Magnuson T, Lass JH, Jepsen KJ, LaMantia C, Carroll H. Lumican regulates collagen fibril assembly: skin fragility and corneal opacity in the absence of lumican. J Cell Biol. 1998;141:1277–1286. doi: 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol. 1997;136:729–743. doi: 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kao WW, Funderburgh JL, Xia Y, Liu CY, Conrad GW. Focus on molecules: lumican. Exp Eye Res. 2006;82:3–4. doi: 10.1016/j.exer.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iozzo RV. The family of the small leucine-rich proteoglycans: key regulators of matrix assembly and cellular growth. Crit Rev Biochem Mol Biol. 1997;32:141–174. doi: 10.3109/10409239709108551. [DOI] [PubMed] [Google Scholar]
- 12.Blochberger TC, Cornuet PK, Hassell JR. Isolation and partial characterization of lumican and decorin from adult chicken corneas. A keratan sulfate-containing isoform of decorin is developmentally regulated. J Biol Chem. 1992;267:20613–20619. [PubMed] [Google Scholar]
- 13.Funderburgh JL, Funderburgh ML, Brown SJ, Vergnes JP, Hassell JR, Mann MM, Conrad GW. Sequence and structural implications of a bovine corneal keratan sulfate proteoglycan core protein. Protein 37B represents bovine lumican and proteins 37A and 25 are unique. J Biol Chem. 1993;268:11874–11880. [PubMed] [Google Scholar]
- 14.Funderburgh JL, Funderburgh ML, Hevelone ND, Stech ME, Justice MJ, Liu CY, Kao WW, Conrad GW. Sequence, molecular properties, and chromosomal mapping of mouse lumican. Invest Ophthalmol Vis Sci. 1995;36:2296–2303. [PubMed] [Google Scholar]
- 15.Hayashida Y, Akama TO, Beecher N, Lewis P, Young RD, Meek KM, Kerr B, Hughes CE, Caterson B, Tanigami A, Nakayama J, Fukada MN, Tano Y, Nishida K, Quantock AJ. Matrix morphogenesis in cornea is mediated by the modification of keratan sulfate by GlcNAc 6-O-sulfotransferase. Proc Natl Acad Sci U S A. 2006;103:13333–13338. doi: 10.1073/pnas.0605441103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saika S, Miyamoto T, Tanaka S, Tanaka T, Ishida I, Ohnishi Y, Ooshima A, Ishiwata T, Asano G, Chikama T, Shiraishi A, Liu CY, Kao CW, Kao WW. Response of lens epithelial cells to injury: role of lumican in epithelial-mesenchymal transition. Invest Ophthalmol Vis Sci. 2003;44:2094–2102. doi: 10.1167/iovs.02-1059. [DOI] [PubMed] [Google Scholar]
- 17.Yeh LK, Chen WL, Li W, Espana EM, Ouyang J, Kawakita T, Kao WW, Tseng SC, Liu CY. Soluble lumican glycoprotein purified from human amniotic membrane promotes corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2005;46:479–486. doi: 10.1167/iovs.04-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Wu J, Zhou X, Chen Z, Zhou G. The impact of visible light on the immature retina: a model of early light exposure in neonatal mice. Brain Res Bull. 87:534–539. doi: 10.1016/j.brainresbull.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 19.Hassell JR, Newsome DA, Krachmer JH, Rodrigues MM. Macular corneal dystrophy: failure to synthesize a mature keratan sulfate proteoglycan. Proc Natl Acad Sci U S A. 1980;77:3705–3709. doi: 10.1073/pnas.77.6.3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakravarti S, Magnuson T. Localization of mouse lumican (keratan sulfate proteoglycan) to distal chromosome 10. Mamm Genome. 1995;6:367–368. doi: 10.1007/BF00364803. [DOI] [PubMed] [Google Scholar]
- 21.Funderburgh JL, Funderburgh ML, Mann MM, Conrad GW. Arterial lumican. Properties of a corneal-type keratan sulfate proteoglycan from bovine aorta. J Biol Chem. 1991;266:24773–24777. [PubMed] [Google Scholar]
- 22.Qin H, Ishiwata T, Asano G. Effects of the extracellular matrix on lumican expression in rat aortic smooth muscle cells in vitro. J Pathol. 2001;195:604–608. doi: 10.1002/path.994. [DOI] [PubMed] [Google Scholar]
- 23.Dolhnikoff M, Morin J, Roughley PJ, Ludwig MS. Expression of lumican in human lungs. Am J Respir Cell Mol Biol. 1998;19:582–587. doi: 10.1165/ajrcmb.19.4.2979. [DOI] [PubMed] [Google Scholar]
- 24.Schaefer L, Grone HJ, Raslik I, Robenek H, Ugorcakova J, Budny S, Schaefer RM, Kresse H. Small proteoglycans of normal adult human kidney: distinct expression patterns of decorin, biglycan, fibromodulin, and lumican. Kidney Int. 2000;58:1557–1568. doi: 10.1046/j.1523-1755.2000.00317.x. [DOI] [PubMed] [Google Scholar]
- 25.Sztrolovics R, Alini M, Mort JS, Roughley PJ. Age-related changes in fibromodulin and lumican in human intervertebral discs. Spine (Phila Pa 1976) 1999;24:1765–1771. doi: 10.1097/00007632-199909010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Grover J, Chen XN, Korenberg JR, Roughley PJ. The human lumican gene. Organization, chromosomal location, and expression in articular cartilage. J Biol Chem. 1995;270:21942–21949. doi: 10.1074/jbc.270.37.21942. [DOI] [PubMed] [Google Scholar]
- 27.Ping Lu Y, Ishiwata T, Asano G. Lumican expression in alpha cells of islets in pancreas and pancreatic cancer cells. J Pathol. 2002;196:324–330. doi: 10.1002/path.1037. [DOI] [PubMed] [Google Scholar]
- 28.Funderburgh JL, Mitschler RR, Funderburgh ML, Roth MR, Chapes SK, Conrad GW. Macrophage receptors for lumican. A corneal keratan sulfate proteoglycan. Invest Ophthalmol Vis Sci. 1997;38:1159–1167. [PubMed] [Google Scholar]
- 29.Baba H, Ishiwata T, Takashi E, Xu G, Asano G. Expression and localization of lumican in the ischemic and reperfused rat heart. Jpn Circ J. 2001;65:445–450. doi: 10.1253/jcj.65.445. [DOI] [PubMed] [Google Scholar]
- 30.Lu YP, Ishiwata T, Kawahara K, Watanabe M, Naito Z, Moriyama Y, Sugisaki Y, Asano G. Expression of lumican in human colorectal cancer cells. Pathol Int. 2002;52:519–526. doi: 10.1046/j.1440-1827.2002.01384.x. [DOI] [PubMed] [Google Scholar]
- 31.Saika S, Shiraishi A, Liu CY, Funderburgh JL, Kao CW, Converse RL, Kao WW. Role of lumican in the corneal epithelium during wound healing. J Biol Chem. 2000;275:2607–2612. doi: 10.1074/jbc.275.4.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeh JT, Yeh LK, Jung SM, Chang TJ, Wu HH, Shiu TF, Liu CY, Kao WW, Chu PH. Impaired skin wound healing in lumican-null mice. Br J Dermatol. 2010;163:1174–1180. doi: 10.1111/j.1365-2133.2010.10008.x. [DOI] [PubMed] [Google Scholar]
- 33.Rowley DR. What might a stromal response mean to prostate cancer progression? Cancer Metastasis Rev. 1998;17:411–419. doi: 10.1023/a:1006129420005. [DOI] [PubMed] [Google Scholar]
- 34.D'Onofrio MF, Brezillon S, Baranek T, Perreau C, Roughley PJ, Maquart FX, Wegrowski Y. Identification of beta1 integrin as mediator of melanoma cell adhesion to lumican. Biochem Biophys Res Commun. 2008;365:266–272. doi: 10.1016/j.bbrc.2007.10.155. [DOI] [PubMed] [Google Scholar]
- 35.Seomun Y, Joo CK. Lumican induces human corneal epithelial cell migration and integrin expression via ERK 1/2 signaling. Biochem Biophys Res Commun. 2008;372:221–225. doi: 10.1016/j.bbrc.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Koninger J, Giese T, di Mola FF, Wente MN, Esposito I, Bachem MG, Giese NA, Buchler MW, Friess H. Pancreatic tumor cells influence the composition of the extracellular matrix. Biochem Biophys Res Commun. 2004;322:943–949. doi: 10.1016/j.bbrc.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Leygue E, Snell L, Dotzlaw H, Hole K, Hiller-Hitchcock T, Roughley PJ, Watson PH, Murphy LC. Expression of lumican in human breast carcinoma. Cancer Res. 1998;58:1348–1352. [PubMed] [Google Scholar]
- 38.Naito Z, Ishiwata T, Kurban G, Teduka K, Kawamoto Y, Kawahara K, Sugisaki Y. Expression and accumulation of lumican protein in uterine cervical cancer cells at the periphery of cancer nests. Int J Oncol. 2002;20:943–948. [PubMed] [Google Scholar]
- 39.Troup S, Njue C, Kliewer EV, Parisien M, Roskelley C, Chakravarti S, Roughley PJ, Murphy LC, Watson PH. Reduced expression of the small leucine-rich proteoglycans, lumican, and decorin is associated with poor outcome in node-negative invasive breast cancer. Clin Cancer Res. 2003;9:207–214. [PubMed] [Google Scholar]
- 40.Brezillon S, Venteo L, Ramont L, D'Onofrio MF, Perreau C, Pluot M, Maquart FX, Wegrowski Y. Expression of lumican, a small leucine-rich proteoglycan with antitumour activity, in human malignant melanoma. Clin Exp Dermatol. 2007;32:405–416. doi: 10.1111/j.1365-2230.2007.02437.x. [DOI] [PubMed] [Google Scholar]
- 41.Vuillermoz B, Khoruzhenko A, D'Onofrio MF, Ramont L, Venteo L, Perreau C, Antonicelli F, Maquart FX, Wegrowski Y. The small leucine-rich proteoglycan lumican inhibits melanoma progression. Exp Cell Res. 2004;296:294–306. doi: 10.1016/j.yexcr.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Sifaki M, Assouti M, Nikitovic D, Krasagakis K, Karamanos NK, Tzanakakis GN. Lumican, a small leucine-rich proteoglycan substituted with keratan sulfate chains is expressed and secreted by human melanoma cells and not normal melanocytes. IUBMB Life. 2006;58:606–610. doi: 10.1080/15216540600951605. [DOI] [PubMed] [Google Scholar]
- 43.Brezillon S, Radwanska A, Zeltz C, Malkowski A, Ploton D, Bobichon H, Perreau C, Malicka-Blaszkiewicz M, Maquart FX, Wegrowski Y. Lumican core protein inhibits melanoma cell migration via alterations of focal adhesion complexes. Cancer Lett. 2009;283:92–100. doi: 10.1016/j.canlet.2009.03.032. [DOI] [PubMed] [Google Scholar]
- 44.Zeltz C, Brezillon S, Kapyla J, Eble JA, Bobichon H, Terryn C, Perreau C, Franz CM, Heino J, Maquart FX, Wegrowski Y. Lumican inhibits cell migration through alpha2beta1 integrin. Exp Cell Res. 2010;316:2922–2931. doi: 10.1016/j.yexcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 45.Radwanska A, Litwin M, Nowak D, Baczynska D, Wegrowski Y, Maquart FX, Malicka-Blaszkiewicz M. Overexpression of lumican affects the migration of human colon cancer cells through up-regulation of gelsolin and filamentous actin reorganization. Exp Cell Res. 2012;318:2312–2323. doi: 10.1016/j.yexcr.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Nikitovic D, Chalkiadaki G, Berdiaki A, Aggelidakis J, Katonis P, Karamanos NK, Tzanakakis GN. Lumican regulates osteosarcoma cell adhesion by modulating TGFbeta2 activity. Int J Biochem Cell Biol. 2011;43:928–935. doi: 10.1016/j.biocel.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Alves ML, Straus AH, Takahashi HK, Michelacci YM. Production and characterization of monoclonal antibodies to shark cartilage proteoglycan. Braz J Med Biol Res. 1994;27:2103–2108. [PubMed] [Google Scholar]
- 48.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 49.Holland JW, Meehan KL, Redmond SL, Dawkins HJ. Purification of the keratan sulfate proteoglycan expressed in prostatic secretory cells and its identification as lumican. Prostate. 2004;59:252–259. doi: 10.1002/pros.20002. [DOI] [PubMed] [Google Scholar]
- 50.Fullwood NJ, Davies Y, Nieduszynski IA, Marcyniuk B, Ridgway AE, Quantock AJ. Cell surface-associated keratan sulfate on normal and migrating corneal endothelium. Invest Ophthalmol Vis Sci. 1996;37:1256–1270. [PubMed] [Google Scholar]
- 51.Kao WW. Ocular surface tissue morphogenesis in normal and disease states revealed by genetically modified mice. Cornea. 2006;25:S7–S19. doi: 10.1097/01.ico.0000247207.55520.a4. [DOI] [PubMed] [Google Scholar]
- 52.Williams KE, Fulford LA, Albig AR. Lumican reduces tumor growth via induction of fas-mediated endothelial cell apoptosis. Cancer Microenviron. 2011;4:115–126. doi: 10.1007/s12307-010-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Radwanska A, Baczynska D, Nowak D, Brezillon S, Popow A, Maquart FX, Wegrowski Y, Malicka-Blaszkiewicz M. Lumican affects actin cytoskeletal organization in human melanoma A375 cells. Life Sci. 2008;83:651–660. doi: 10.1016/j.lfs.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 54.Busch T, Armacki M, Eiseler T, Joodi G, Temme C, Jansen J, von Wichert G, Omary MB, Spatz J, Seufferlein T. Keratin 8 phosphorylation regulates keratin reorganization and migration of epithelial tumor cells. J Cell Sci. 125:2148–2159. doi: 10.1242/jcs.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fillies T, Werkmeister R, Packeisen J, Brandt B, Morin P, Weingart D, Joos U, Buerger H. Cytokeratin 8/18 expression indicates a poor prognosis in squamous cell carcinomas of the oral cavity. BMC Cancer. 2006;6:10. doi: 10.1186/1471-2407-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schaafsma HE, Van Der Velden LA, Manni JJ, Peters H, Link M, Rutter DJ, Ramaekers FC. Increased expression of cytokeratins 8, 18 and vimentin in the invasion front of mucosal squamous cell carcinoma. J Pathol. 1993;170:77–86. doi: 10.1002/path.1711700113. [DOI] [PubMed] [Google Scholar]
- 57.Alam H, Kundu ST, Dalal SN, Vaidya MM. Loss of keratins 8 and 18 leads to alterations in alpha6beta4-integrin-mediated signalling and decreased neoplastic progression in an oral-tumour-derived cell line. J Cell Sci. 124:2096–2106. doi: 10.1242/jcs.073585. [DOI] [PubMed] [Google Scholar]
- 58.Yamashiro Y, Takei K, Umikawa M, Asato T, Oshiro M, Uechi Y, Ishikawa T, Taira K, Uezato H, Kariya K. Ectopic coexpression of keratin 8 and 18 promotes invasion of transformed keratinocytes and is induced in patients with cutaneous squamous cell carcinoma. Biochem Biophys Res Commun. 399:365–372. doi: 10.1016/j.bbrc.2010.07.077. [DOI] [PubMed] [Google Scholar]
- 59.Woll S, Windoffer R, Leube RE. Dissection of keratin dynamics: different contributions of the actin and microtubule systems. Eur J Cell Biol. 2005;84:311–328. doi: 10.1016/j.ejcb.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 60.Jing J, Tarbutton E, Wilson G, Prekeris R. Rab11-FIP3 is a Rab11-binding protein that regulates breast cancer cell motility by modulating the actin cytoskeleton. Eur J Cell Biol. 2009;88:325–341. doi: 10.1016/j.ejcb.2009.02.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamada H, Abe T, Li SA, Masuoka Y, Isoda M, Watanabe M, Nasu Y, Kumon H, Asai A, Takei K. Dynasore, a dynamin inhibitor, suppresses lamellipodia formation and cancer cell invasion by destabilizing actin filaments. Biochem Biophys Res Commun. 2009;390:1142–1148. doi: 10.1016/j.bbrc.2009.10.105. [DOI] [PubMed] [Google Scholar]
- 62.Jaafar H, Sharif SE, Murtey MD. Distinctive features of advancing breast cancer cells and interactions with surrounding stroma observed under the scanning electron microscope. Asian Pac J Cancer Prev. 13:1305–1310. doi: 10.7314/apjcp.2012.13.4.1305. [DOI] [PubMed] [Google Scholar]
- 63.Taliana L, Benezra M, Greenberg RS, Masur SK, Bernstein AM. ZO-1: lamellipodial localization in a corneal fibroblast wound model. Invest Ophthalmol Vis Sci. 2005;46:96–103. doi: 10.1167/iovs.04-0145. [DOI] [PubMed] [Google Scholar]
- 64.Tuomi S, Mai A, Nevo J, Laine JO, Vilkki V, Ohman TJ, Gahmberg CG, Parker PJ, Ivaska J. PKCepsilon regulation of an alpha5 integrin-ZO-1 complex controls lamellae formation in migrating cancer cells. Sci Signal. 2009;2:ra32. doi: 10.1126/scisignal.2000135. [DOI] [PubMed] [Google Scholar]
- 65.Polette M, Mestdagt M, Bindels S, Nawrocki-Raby B, Hunziker W, Foidart JM, Birembaut P, Gilles C. Beta-catenin and ZO-1: shuttle molecules involved in tumor invasion-associated epithelial-mesenchymal transition processes. Cells Tissues Organs. 2007;185:61–65. doi: 10.1159/000101304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lumican was purified from amniotic membrane. (A) Lumican was extracted from two human amniotic membranes (PL1 and PL2), lumican was enriched from the extract (E) using ion-exchange chromatography with Q-Sepharose resin (GE Healthcare) and the presence of lumican core protein and glycosylated lumican were detected in the fractions 0.5 M and 0.7M by Western blot analysis using monoclonal antibodies produced at our laboratory (anti-lumican and anti-KS). (B) Fractions were pooled and lumican was further purified using anti-lumican affinity-column chromatography and analyzed by polyacrylamide gel electrophoresis (SDS-PAGE) 10% followed by silver staining revealing lumican core protein (40 kDa) and glycosylated lumican (55 kDa) compared to the protein ladder (La).