Abstract
Bronchial asthma and COPD (chronic obstructive pulmonary disease) are obstructive pulmonary diseases that affected millions of people all over the world. Asthma is a serious global health problem with an estimated 300 million affected individuals. COPD is one of the major causes of chronic morbidity and mortality and one of the major public health problems worldwide. COPD is the fourth leading cause of death in the world and further increases in its prevalence and mortality can be predicted. Although asthma and COPD have many similarities, they also have many differences. They are two different diseases with differences in etiology, symptoms, type of airway inflammation, inflammatory cells, mediators, consequences of inflammation, response to therapy, course. Some similarities in airway inflammation in severe asthma and COPD and good response to combined therapy in both of these diseases suggest that they have some similar patophysiologic characteristics. The aim of this article is to show similarities and differences between these two diseases. Today asthma and COPD are not fully curable, not identified enough and not treated enough and the therapy is still developing. But in future better understanding of pathology, adequate identifying and treatment, may be and new drugs, will provide a much better quality of life, reduced morbidity and mortality of these patients.
Key words: asthma, COPD, differences, similarities.
1. INTRODUCTION
Bronchial asthma and COPD (Chronic Obstructive Pulmonary Disease) are obstructive pulmonary diseases that affected millions of people all over the world. These two illnesses have many similarities and many differences which may sometimes confuse therapists in the diagnostics and management of these diseases which affect more and more people every year worldwide.
Although asthma and COPD have many similarities, they also have many differences. COPD is not asthma. Asthma is not COPD. They have:
Different etiology;
Different symptoms;
Different type of airway inflammation;
Different inflammatory cells;
Different mediators;
Different consequences of inflammation;
Different response to therapy;
Different course.
2. ASTHMA
Asthma is a chronic inflammatory disorder of the airways (1).The chronic inflammation is associated with airway hyperresponsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness and coughing, particularly at night or in early morning (1, 2). These episodes are usually associated with widespread, but variable, airflow obstruction within lung that is often reversible either spontaneously or with the treatment (1).
Asthma is a serious global health problem with an estimated 300 million affected individuals (2, 3). People of all ages are affected by this illness that, when uncontrolled, can place severe limits on daily life and is sometimes fatal (1). The prevalence of asthma is increasing in most countries (3). Clinical manifestations of asthma can be controlled with appropriate treatment. When asthma is controlled severe exacerbations should be rare (1). The clinical spectrum of asthma is highly variable, but the airway inflammation remains a consistent feature (1).
Factors that influence the risk of asthma can be divided into those that cause the development of asthma and those that trigger asthma symptoms; some do both. The first include host factors (which are primarily genetic) and second are usually environmental factors (4, 5, 6).
Factors influencing the development and expression of asthma
Host Factors
Genetic, e.g..,
Genes pre-disposing to atopy
Genes pre-disposing to airway hyperresponsiveness
Obesity, Sex
Environmental factors
Allergens
Indoor: domestic mites, furred animals ( dogs, cats, mice), cockroach allergen, fungi ,molds, yeasts,
Outdoor: Pollens, fungi, molds, yeasts
Infections (predominantly viral)
Occupational sensitizes
Tobacco smoke (active smoking, passive smoking)
Outdoor/Indoor Air Pollution
Diet
2.1. Airway inflammation in asthma
The airway inflammation in asthma is persistent even though symptoms are episodic, and the relationship between the severity of asthma and the intensity of inflammation is not clearly established (1).The inflammation affects all airways including in most patients the upper respiratory tract and nose but its physiological effects are most pronounced in medium sized bronchi (1). The pattern of inflammation in the airways appears to be similar in all clinical form of asthma, whether allergic, non-allergic, or aspirin-induced and at all ages (1).
2.2. Inflammatory Cells in Asthmatic Airways
Mast cells -activated mucosal mast cells release bronchoconstrictor mediators–histamine, cysteinyl leukotriens, prostaglandin D2. They are activated by allergens through IgE receptors or by osmotic stimuli (7). Eosinophils are in increased number in airways, release basic proteins that may damage epithelial cells, and have a role in releasing a growth factors and airway remodeling (8) , T lymphocytes are in increased number and release specific cytokines, including IL-4, IL-5, IL-9, IL-13 that orchestrate eosinophilic inflammation and IgE production by B lymphocytes (9). There may also be an increase in inKT cells which release large amounts of T helper: Th1 and Th2 cytokines (10, 11). Dendritic cells,Macrophages are in increased number, and release inflammatory mediators and cytokines that amplify the inflammatory response (12, 13). Nutrophils are in increased number in airways and sputum of patients with severe asthma and in smoking asthmatics, but the role of these cells is uncertain and their increase may even be due to steroid therapy (12, 13, 14).
2.3. Inflammatory Mediators Involved in Asthma
Chemokines are important in the recruitment of inflammatory cells into the airways and are mainly expressed in airway epithelial cells (15, 16, 17). Eotaxin is selective for eosinophils, whereas thymus and activationregulated chemokines (TARC) and macrophage-derived chemokines (MDC) recruit Th2 cells (16, 17). Cysteinyl leukotrienes are potent bronchoconstrictors and proinflammatory mediators mainly derived from mast cells and eosinophils (18). Cytokines orchestrate the inflammatory response in asthma. Key cytokines include IL-1β and TNFα, and GM-CSF. Th2-derived cytokines include IL-5, which is required for eosinophil differentiation and survival; IL-4, which is important for Th2 cell differentiation; and IL-13, needed for IgE formation (19). Histamine is released from mast cells and contributes to bronchoconstriction and inflammation (15, 16). Nitric oxide (NO), a potent vasodilator, is produced from syntheses in airway epithelial cells (20) . Exhaled NO is increasingly being used to monitor the effectiveness of asthma treatment (21). Prostaglandin D2 is a bronchoconstrictor derived predominantly from mast cells and is involved in Th2 cell recruitment to the airways (7).
Airway structural cells involved in the pathogenesis of asthma are: airway epithelial cells, airway smooth muscle cells, endothelial cells, fibroblasts and myofibroblasts and airway nerves (13, 14, 22, 23).
3. COPD
COPD is one of the major causes of chronic morbidity and mortality worldwide. Many people suffer from this disease for years and die prematurely from its complications. COPD is the fourth leading cause of death in the world (24), and further increases in its prevalence and mortality can be predicted in the coming decades (25,26).
COPD is a pulmonary disease with some significant extrapulmonary effects that may contribute to the severity in individual patient. Its pulmonary component is characterized by airflow limitation that is not fully reversible (27). The airflow limitation is usually progressive and associated with an abnormal inflammatory response of the lung to noxious particles or gases (24).
COPD has variable natural history and not all individuals follow the same course. However COPD is generally a progressive disease, especially if a patient’s exposure to noxious agents continues. The impact of COPD on an individual patient depends on the severity of symptoms (especially breathlessness and decreased exercise capacity, cough, mucous production), systemic effects, and any co-morbidity the patient may have -not just on the degree of airflow limitation (24, 27, 28). COPD is characterized by chronic airflow limitation and a range of pathological changes in the lung, some significant extra-pulmonary effects and important co-morbidities which may contribute to the severity of the disease in individual patient (27, 28). Systemic manifestations and co-morbidities in COPD are body weight loss, skeletal muscle wasting, cachexia, osteoporosis, pulmonary heart-cor pulmonale, heart failure, cardiac ischemia, cardiac arrhythmias, anemia, hypoalbuminemia, diabetes, cognitive deficits, depression (28,29) . Co-morbidities are common for people with COPD because organ systems work differently when they do not receive enough oxygen. Thus, COPD should be regarded as a pulmonary disease, but these significant co-morbidities must be taken into account in a diagnostic assessment of severity and in determining appropriate treatment (27, 28, 29). Cigarette smoking is the most commonly encountered risk factor for COPD, although in many countries, air pollution resulting from the burning of wood and other biomasses fuels has also been identified as a COPD risk factor (24, 27,30).
3.1. Inflammatory Cells in COPD
Neutrophils are present in sputum of smokers but increased in COPD and related to disease severity. They may be important in mucus hypersecretion and through release of proteases. Macrophages: big numbers are in airway lumen, lung parenchyma, and bronchoalveolar lavage fluid. They produce increased inflammatory mediators and proteases and may show defective phagocytosis. T lymphocytes: both CD4+ and CD8+ cells are increased in the airway wall and lung parenchyma, with big CD8+/CD4+ ratio. Increased is the number of CD8+ T cells (Tc1) and Th1 cells which secrete interferon-γ and express the chemokine receptor CXCR3. CD8+ cells may be cytotoxic to alveolar cells. B lymphocytes: are increased in peripheral airways and within lymphoid follicles, possibly as a response to colonization and infection. Eosinophils: increased eosinophil proteins in sputum and eosinophils in airway wall during exacerbations. Epithelial cells: May be activated by cigarette smoke to produce inflammatory mediators (31, 32, 33, 34, 35, 36).
3.2. Inflammatory Mediators Involved in COPD
Chemotactic factors: Lipid mediators: e.g., leukotriene B4 (LTB4) attracts neutrophils and T lymphocytes, Chemokines: e.g., interleukin-8 (IL-8) attracts neutrophils and monocytes. Proinflammatory cytokines: e.g., tumor necrosis factor- (TNF-α), IL-1 β, and IL-6 amplify the inflammatory process and may contribute to some of the systemic effects of COPD. Growth factors: e.g., transforming growth factor-ß (TGF-ß) may induce fibrosis in small airways (31, 32, 34, 37).
3.3. Airflow limitation in COPD
The chronic airflow limitation of COPD is caused by a mixture of small airway disease (obstructive bronchiolitis) and parenchymal destruction (emphysema), the relative contributions of which vary from person to person (24). Chronic inflammation causes structural changes and narrowing of small airways. Destruction of the lung parenchyma, also by inflammatory processes, leads to the loss of alveolar attachments to the small airways and decreases lung elastic recoil; in turn these changes diminish the ability of the airways to remain open during expiration (32, 34, 35).
So in COPD inflammation causes small airway disease (airway inflammation, airway remodeling) and parenchymal destruction (loss of alveolar attachments and decrease of elastic recoil) that all lead to airflow limitation (32,34,35).
4. ASTHMA AND COPD
COPD can coexist with asthma; both are characterized by an underlying airway inflammation. The underlying chronic airway inflammation is very different in these two diseases (32, 38) (Table 1). However, individuals with asthma who are exposed to noxious agents, particularly cigarette smoke may develop fixed airflow limitation and a mixture of “asthma -like” and “COPD-like” inflammation (38). Furthermore, there is epidemiologic evidence that longstanding asthma on its own can lead to fixed airflow limitation (38,39). Other patients with COPD may have features of asthma such as a mixed inflammatory pattern with increased eosinophils (31). Thus, while asthma can usually be distinguished from COPD, in some individuals with chronic respiratory symptoms and fixed airflow limitation it remains difficult to differentiate the two diseases.
Table 1.
Differences in cause, inflammation and airflow limitation between asthma and COPD
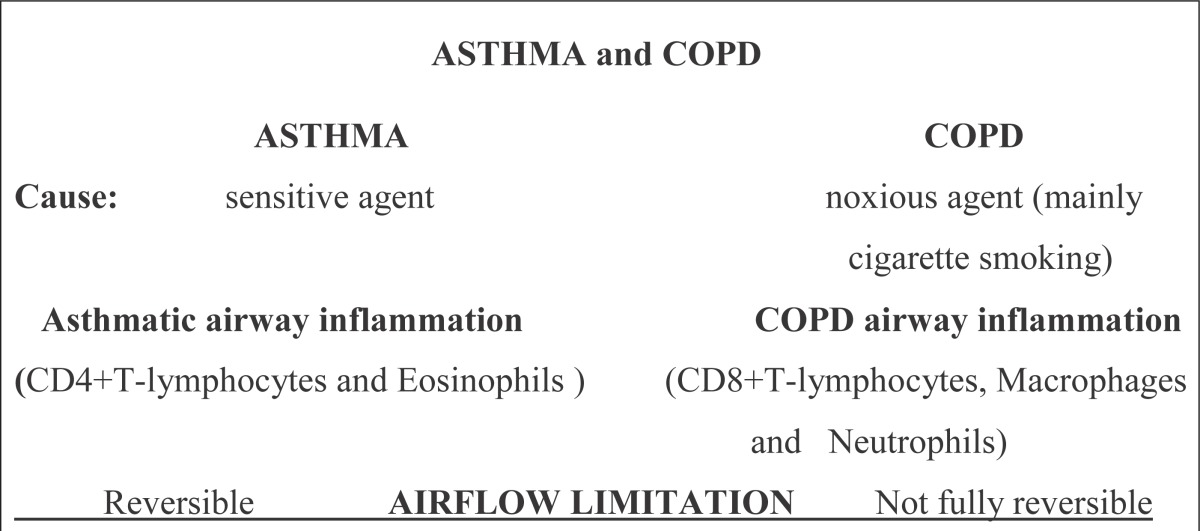 |
There are differences between cause, airway inflammation and airflow limitation between asthma and COPD.
The primary differences in pulmonary inflammation between asthma and COPD are shown in Table 2.
Table 2.
Differences in pulmonary inflammation between asthma and COPD. No. = nitric oxide, b/d = bronchodilatator (revised table from GOLD 2006.)
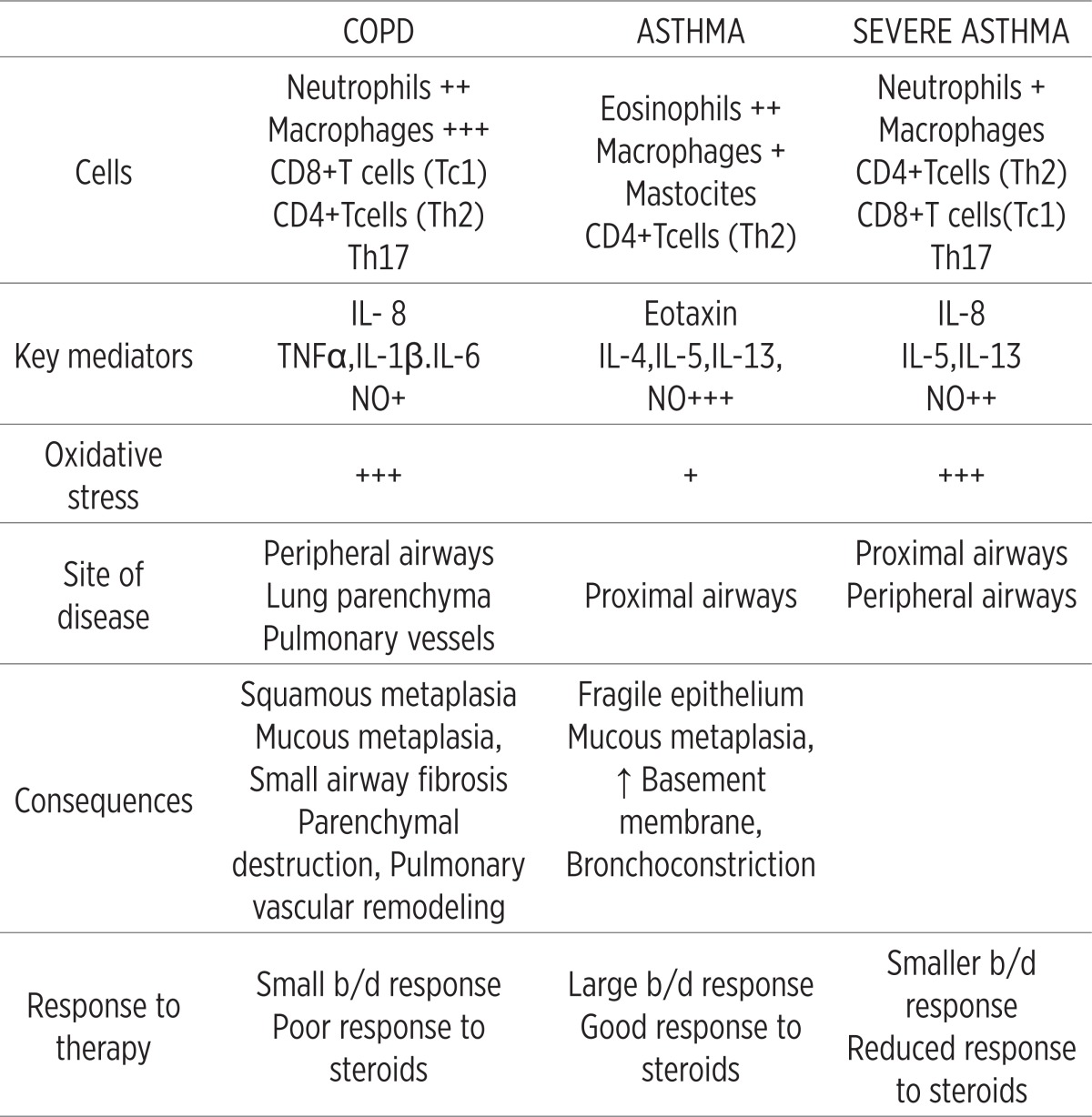 |
Some basic differences between asthma and COPD are seen in Table 3.
Table 3.
Asthma versus COPD
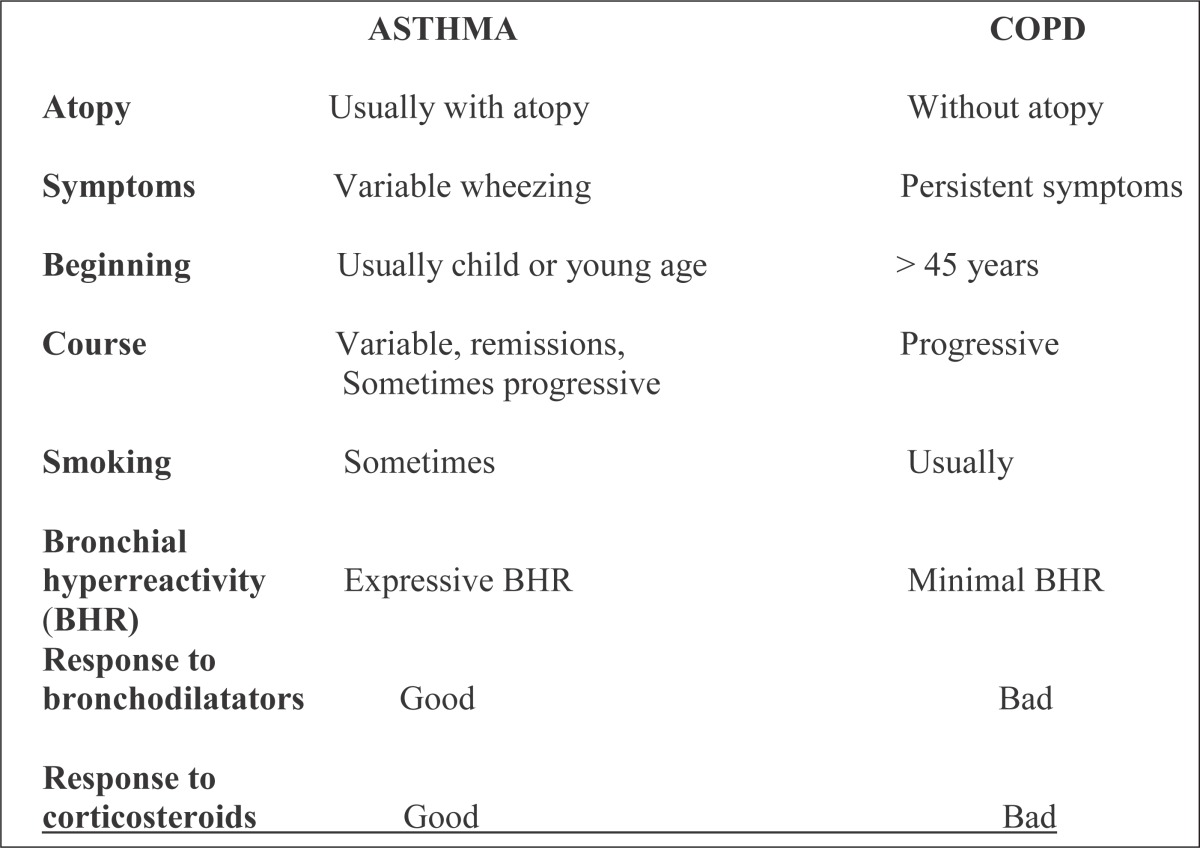 |
Asthma and COPD are usually differentiated (Table 4):
Different inflammatory cells
Different inflammatory mediators
Different response to therapy
Table 4.
Airway inflammation
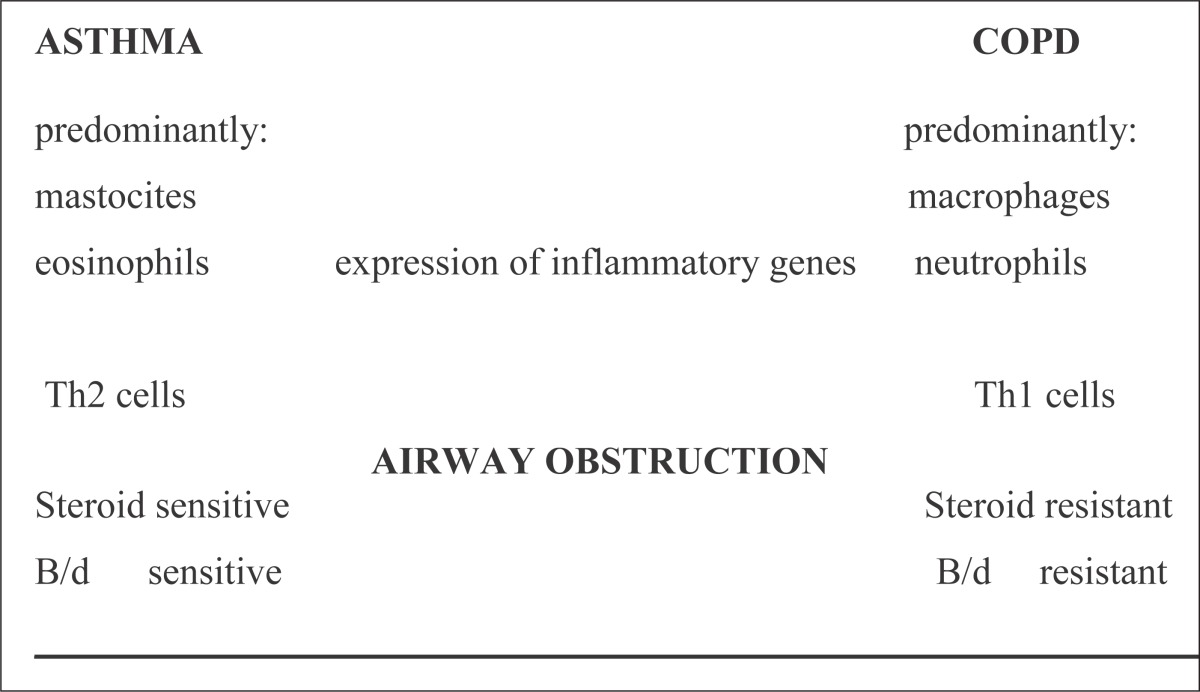 |
Asthma and COPD are usually similar:
“Reversible COPD” (asthma coexists)
Severe asthma
Asthma in smokers
Neutrophil asthma (asthma in smokers, non-allergic asthma)
allergic asthma
acute exacerbation
Similarities in airway inflammation and obstruction between asthma and COPD are showen on Table 4 and 5). Inflamatory cells are included in airway inflammation.
Table 5.
Inflammatory cells included in airway inflammation in Asthma and COPD
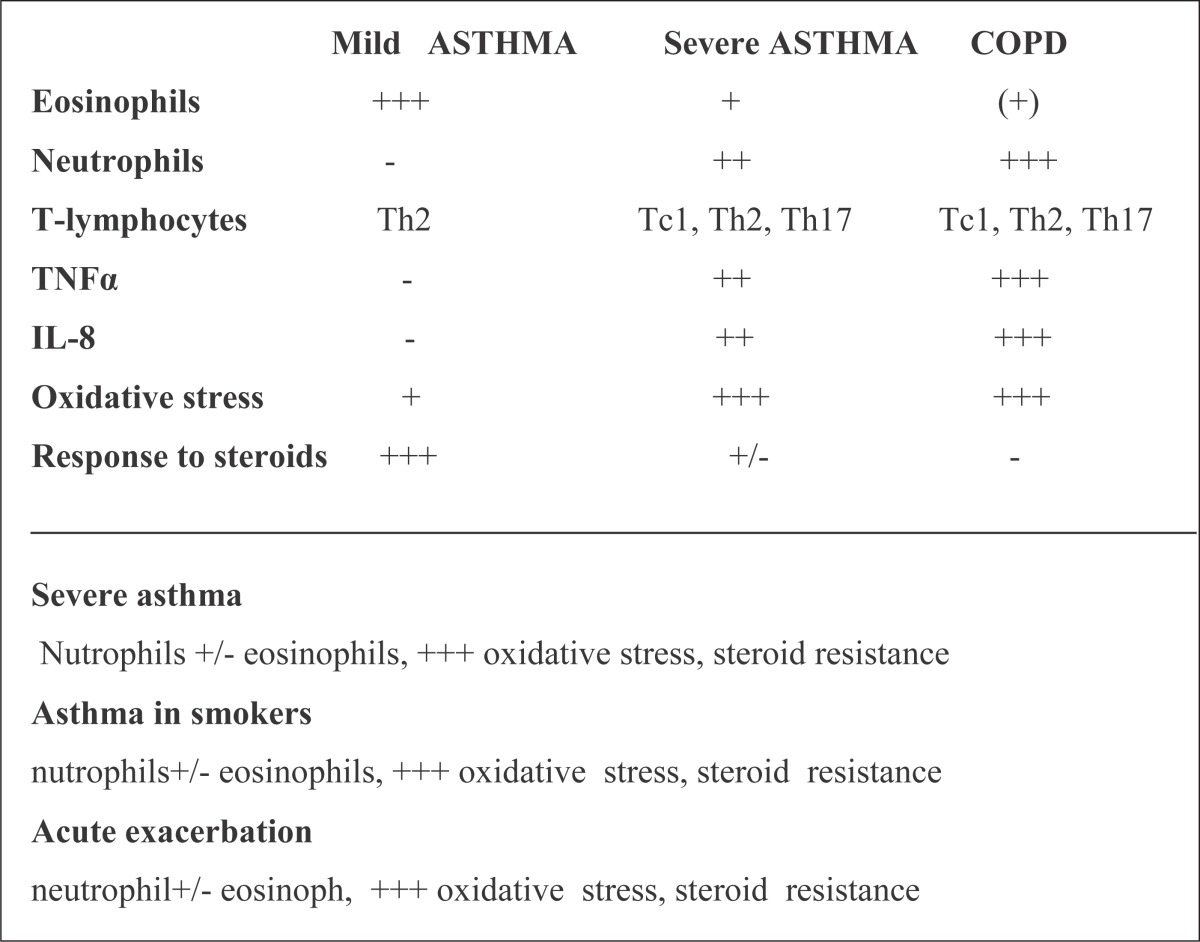 |
Causes of airway obstruction in asthma and COPD are different. There are some similarities and differences in pathological changes in airways in severe asthma and COPD (Table 6 and 7).
Table 6.
Airway obstruction in asthma and COPD
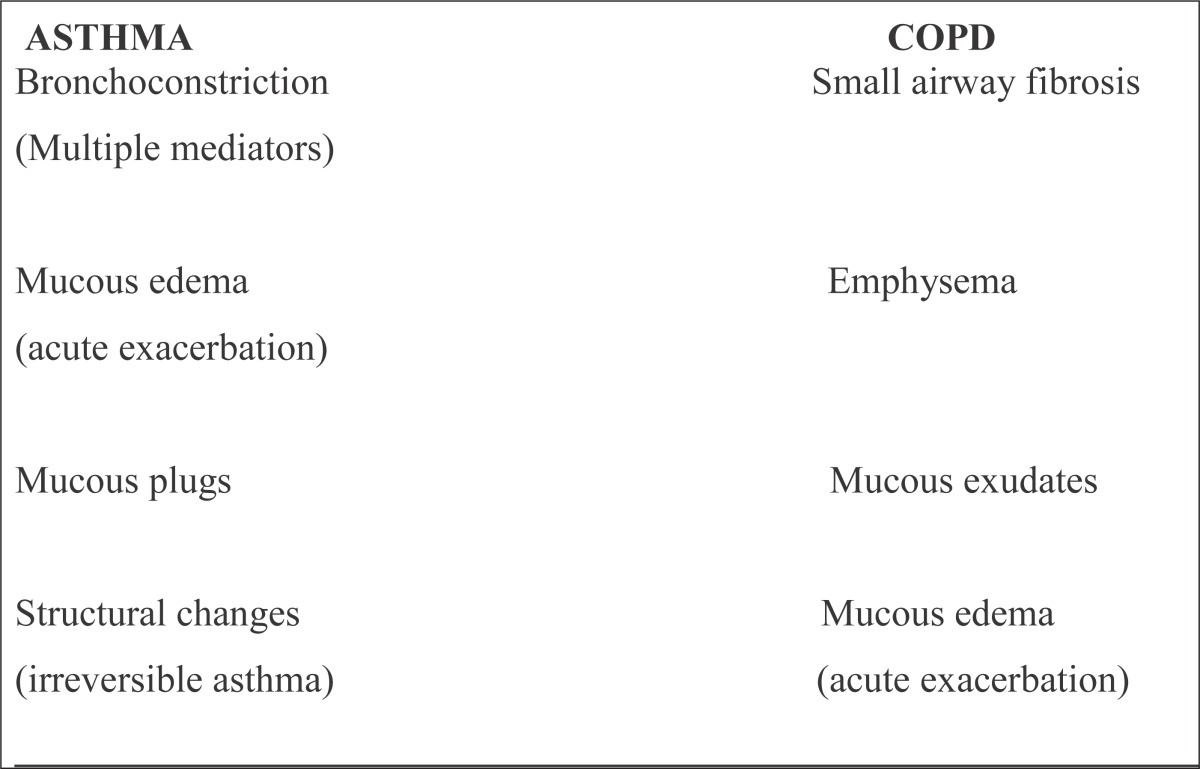 |
Table 7.
Severe (fatal) Asthma Severe COPD
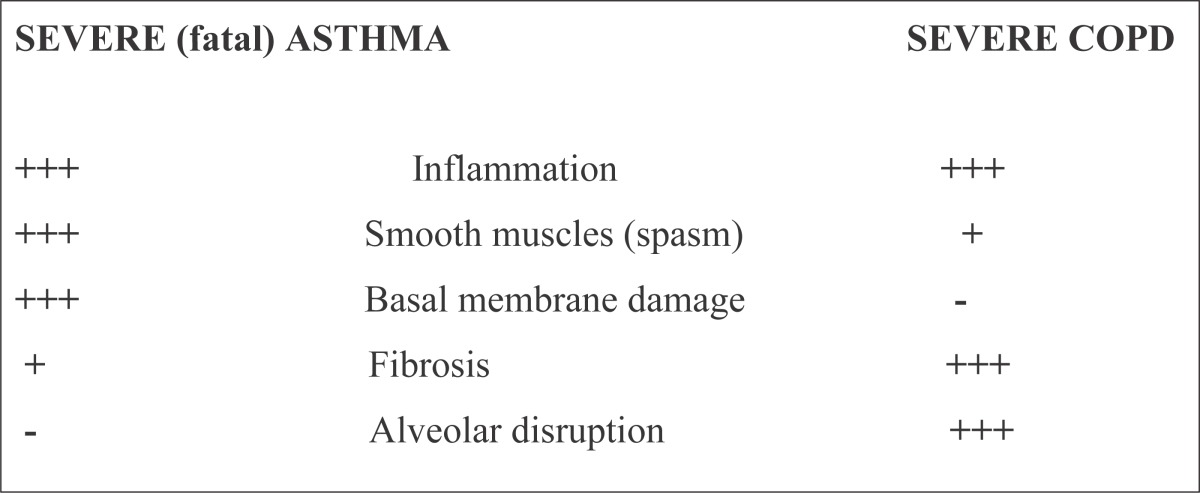 |
Functional pulmonary testing (spirometry, body-pletizmography) is most important for diagnosis, determining of severity and management of both asthma and COPD. Characteristics of functional pulmonary testing are shown on Table 8.
Table 8.
Characteristics of functional pulmonary testing
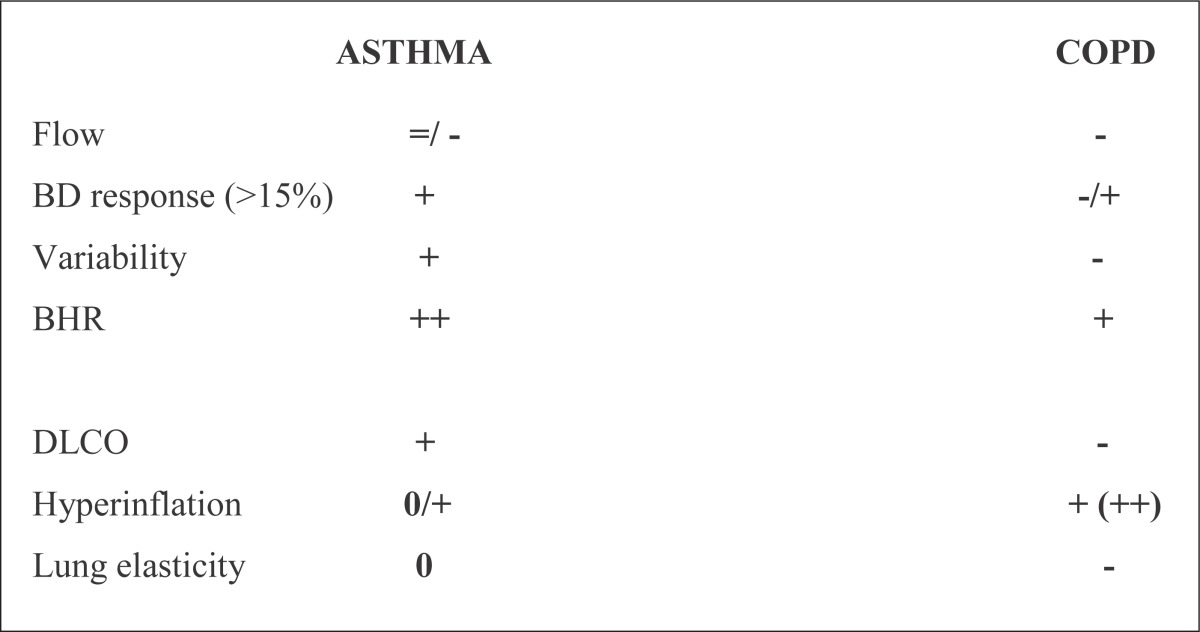 |
There are differences in functional pulmonary testing between asthma and COPD, especially between “typical reversible” asthma and COPD.
But pulmonary functional testing is very similar in fixed “non-reversible” progressive asthma and COPD (1, 24, 31, 32, 38, 39).
4.1. Similaraties and differences in acute exacerbation of asthma and COPD
Pathology is different in exacerbation of asthma and COPD
Causes of acute exacerbation of asthma and COPD are different.
Different role of LABA ( long-acting β-2 agonists) and ICS (inhalatory corticosteroids) in prophylaxis of exacerbation of asthma and COPD.
Treatment of acute exacerbation is similar in asthma and COPD.
Acute exacerbation of Asthma
Triggers of acute exacerbation of asthma are usually: allergens, infections (respiratory viruses, sometimes bacterial infections), GE (gastro-esophageal) reflux, other triggers, sometimes and co-morbidity (1, 4, 5, 6).
Pharmacotherapy of acute asthma exacerbation
(inhalatory) Bronchodilators (A);
β-2 agonists and/or anticholinergics;
(systemic /oral) corticosteroids (A). Other therapy
oxygen therapy (A);
metilxantins (B);
non -invasive mechanical ventilation (A);
antibiotics;
epinephrine (adrenalin) –rarely in a very serious asthma attack;
He/Ox(helium/oxygen inhalation) rarely and MgSO4 intravenously rarely.
Acute exacerbation of COPD
Triggers of acute exacerbation of COPD are usually: infections (respiratory viruses, bacterial infections), airpollution, GE (gastro –esophageal) reflux, sometimes and co-morbidity (24, 30).
Pharmacotherapy of acute COPD exacerbation:
(inhalatory) Bronchodilators (A);
β-2 agonists and/or anticholinergics;
(systemic /oral) corticosteroids (A);
antibiotics in patients with severe exacerbation (b) Other therapy:
oxygen therapy (A);
metilxantins (B);
non -invasive mechanical ventilation (A).
4.2. Similarities and differences in regular standard treatment of asthma and copd
In both diseases the adequate treatment may reduce symptoms and number of exacerbations and improve the quality of life.
Treatment of asthma is characterized by suppression of inflammation.
Treatment of COPD is characterized by decreasing of symptoms.
The GOAL of treatment in ASTHMA is to: reduce inflammation and to achieve¸total control (1). The GOAL of treatment in COPD is to: reduce symptoms, prevent exacerbations and decrease mortality (24). In both asthma and COPD almost the same drugs are used, but not in the same order and the same efficiency in treatment.
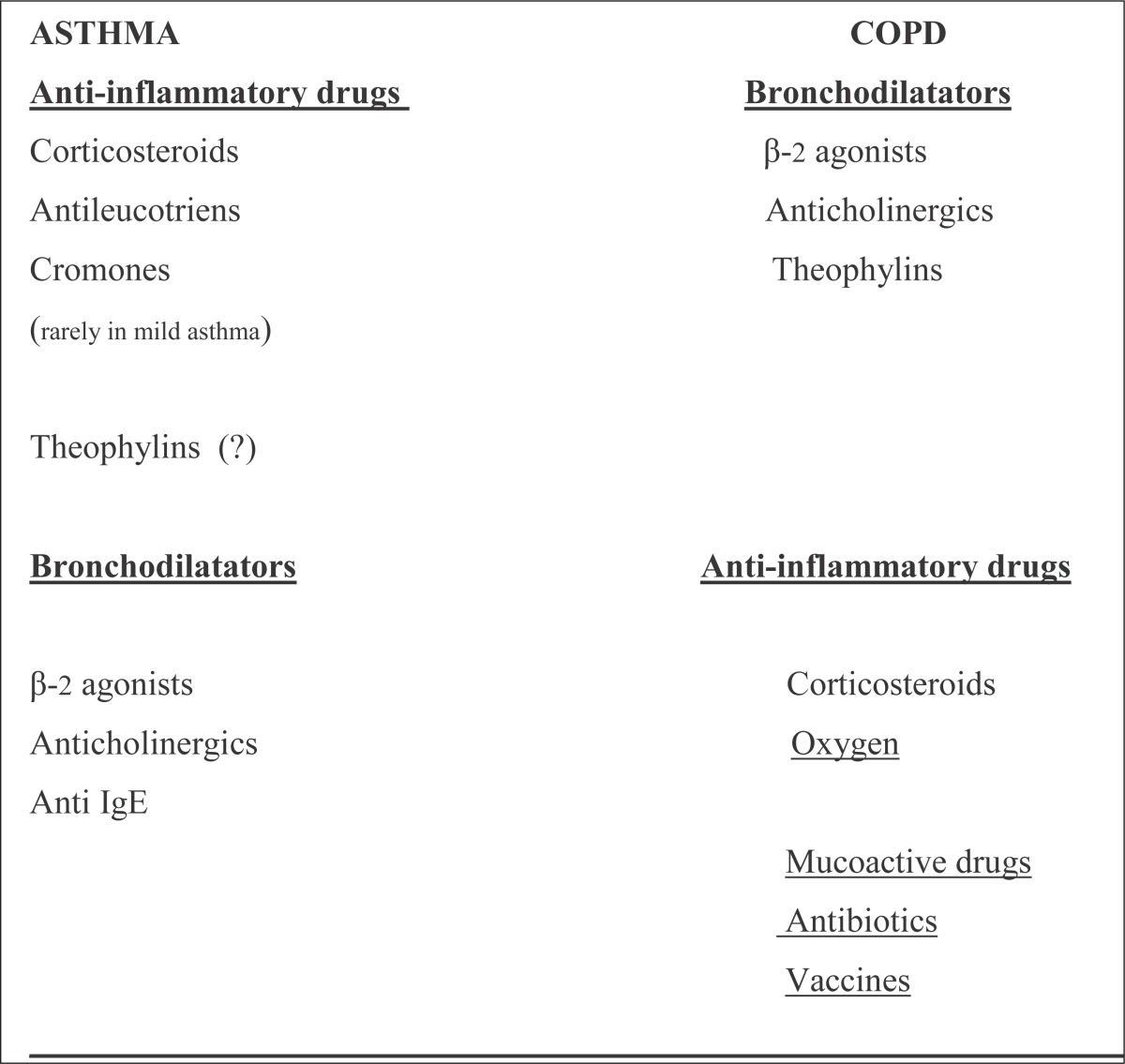
In Asthma, ICS (inhalatory corticosteroids), decrease number of exacerbations, improve pulmonary function for long time , slow the decreasing of pulmonary function, decrease re-modulation of airways and reduce needs for additional medications (1, 43-48). In COPD, ICS are useful in patients with COPD with bigger degree of bronchodilatator response, who have notes about allergic or inflammatory response, repeated exacerbations or variable course of illness and in advanced COPD (24, 45, 49-54). The treatment with combined therapy: LABA/ICS (long-acting β-2 agonists/ inhalatory corticosteroids) is effective in both: asthma and COPD (46-48, 50, 52-54) which suggests that these two diseases have some similar patophysiologic characteristics. In both asthma and COPD early treatment can influence on: morbidity and mortality -reduced, quality of life –improved, costs of treatment –reduced (1, 24, 45-48, 50, 52-54). According to the course of illness it is unknown in asthma, but in COPD only the cut off smoking can have a positive influence (24). There is no therapy that can fully modify: re-modulation of small airways in asthma, and re-modulation of small airways, loss of alveolar connections, and collagen/ elastin destruction in COPD (1, 24) (Table 9).
Table 9.
DRUGS FOR ASTHMA AND COPD
 |
5. CONCLUSION
Bronchial asthma and COPD are obstructive pulmonary diseases that affected millions of people all over the world. Although asthma and COPD have many differences they also have some similarities. They are two different diseases with differences in etiology, symptoms, type of airway inflammation, inflammatory cells, mediators, consequences of inflammation, response to therapy, course. Some similarities in airway inflammation in severe asthma and COPD and good response to combined therapy (LABA/ ICS) in both these diseases suggest that they have similar patophysiologic characteristics. Today asthma and COPD are not fully curable, not identified enough and not treated enough and the therapy is still developing. But in future better understanding of pathology, adequate identifying and treatment, perhaps and new drugs will provide a much better quality of life, reduced morbidity and mortality of these patients.
CONFLICT OF INTEREST
None declared.
REFERENCES
- 1.GINA (Global Initiative for Asthma) – global strategy for asthma management and prevention, revised. http://www.ginasthma.org . 2006. pp. 1–92.
- 2.Masoli M, Fabian D, Holt S, Beasley R. The global burden of asthma: executive summaryof the GINA Dissemination Committee report. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Beasley R. The Global Burden of Asthma Report, Global Initiative for Asthma (GINA) Available from http://www.ginasthma.org . 2004.
- 4.Holgate ST. Genetic and environmental interaction in allergy and asthma. J Allergy Clin Immunol. 1999;104(6):1139–1146. doi: 10.1016/S0091-6749(99)70005-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holloway JW, Beghe B, Holgate ST. The genetic basis of atopic asthma. Clin Exp Allergy. 1999;29(8):1023–1032. doi: 10.1046/j.1365-2222.1999.00599.x. [DOI] [PubMed] [Google Scholar]
- 6.Wiesch DG, Meyers DA, Bleecker ER. Genetics of asthma. J Allergy Clin Immunol. 1999;104(5):895–901. doi: 10.1016/s0091-6749(99)70065-5. [DOI] [PubMed] [Google Scholar]
- 7.Robinson DS. The role of the mast cell in asthma: induction of airway hyperresponsiviness by interaction with smooth muscle? J Allergy Clin Immunol. 2004;114(1):58–65. doi: 10.1016/j.jaci.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 8.Kay AB, Phipps S, Robinson DS. A role for eosinophils in airway remodelling in asthma. Trends Immunol. 2004;25(9):477–482. doi: 10.1016/j.it.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Larche M, Robinson DS, Kay AB. The role of T lymphocytes in the pathogenesis of asthma. J Allergy Clin Immunol. 2003;111(3):450–463. doi: 10.1067/mai.2003.169. [DOI] [PubMed] [Google Scholar]
- 10.Akbari O, Faul JL, Hoyte EG, Berry GJ, Wahlstrom J, Kronenberg M, et al. CD4+ invariant T-cell-receptor+ natural killer T cells in bronchial asthma. N Engl J Med. 2006;354(11):1117–1129. doi: 10.1056/NEJMoa053614. [DOI] [PubMed] [Google Scholar]
- 11.Kuipers H, Lambrecht BN. The interplay of dendritic cells, Th2 cells and regulatory T cells in asthma. Curr Opin Immunol. 2004;16(6):702–708. doi: 10.1016/j.coi.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Peters-Golden M. The alveolar macrophage: the forgotten cell in asthma. Am J Respir Cell Mol Biol. 2004;31(1):3–7. doi: 10.1165/rcmb.f279. [DOI] [PubMed] [Google Scholar]
- 13.Wenzel S. Mechanisms of severe asthma. Clin Exp Allergy. 2003;33(12):1622–1628. doi: 10.1111/j.1365-2222.2003.01799.x. [DOI] [PubMed] [Google Scholar]
- 14.Chung KF. Airway smooth muscle cells: contributing to and regulating airway mucosal inflammation? Eur Respir J. 2000;15(5):961–968. doi: 10.1034/j.1399-3003.2000.15e26.x. [DOI] [PubMed] [Google Scholar]
- 15.Groneberg DA, Quarcoo D, Frossard N, Fischer A. Neurogenic mechanisms in bronchial inflammatory diseases. Allergy. 2004;59(11):1139–1152. doi: 10.1111/j.1398-9995.2004.00665.x. [DOI] [PubMed] [Google Scholar]
- 16.Barnes PJ, Chung KF, Page CP. Inflammatory mediators of asthma: an update. Pharmacol Rev. 1998;50(4):515–596. [PubMed] [Google Scholar]
- 17.Miller AL, Lukacs NW. Chemokine receptors: understanding their role in asthmatic disease. Immunol Allergy Clin North Am. 2004;24(4):667–683. doi: 10.1016/j.iac.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 18.Leff AR. Regulation of leukotrienes in the management of asthma: biology and clinical therapy. Annu Med Rev. 2001;52:1–14. doi: 10.1146/annurev.med.52.1.1. [DOI] [PubMed] [Google Scholar]
- 19.Barnes PJ. Cytokine modulators as novel therapies for asthma. Annu Rev Pharmacol Toxicol. 2002;42:81–98. doi: 10.1146/annurev.pharmtox.42.111901.111143. [DOI] [PubMed] [Google Scholar]
- 20.Ricciardolo FL, Sterk PJ, Gaston B, Folkerts G. Nitric oxide in health and disease of the respiratory system. Physiol Rev. 2004;84(3):731–765. doi: 10.1152/physrev.00034.2003. [DOI] [PubMed] [Google Scholar]
- 21.Smith AD, Taylor DR. Is exhaled nitric oxide measurement a useful clinical test in asthma? Curr Opin Allergy Clin Immunol. 2005;5(1):49–56. doi: 10.1097/00130832-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 22.James A. Airway remodeling in asthma. Curr Opin Pulm Med. 2005;11(1):1–6. doi: 10.1097/01.mcp.0000146779.26339.d8. [DOI] [PubMed] [Google Scholar]
- 23.Vignola AM, Mirabella F, Costanzo G, Di Giorgi R, Gjomarkaj M, Bellia V, et al. Airway remodeling in asthma. Chest. 2003;123(3):417S–422S. doi: 10.1378/chest.123.3_suppl.417s. [DOI] [PubMed] [Google Scholar]
- 24.GOLD (Global Initiative for Chronic Obstructive Lung Disease) - global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease, revised. http://www.goldcopd.org . 2006. pp. 1–100.
- 25.World Health Report. Geneva: World Health Organization; 2000. Available from URL: http://www.who.int/whr/2000/en/statistics.htm . [Google Scholar]
- 26.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 27.Soriano JB, Visick GT, Muellerova H, Payvandi N, Hansell AL. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest. 2005;128(4):2099–2107. doi: 10.1378/chest.128.4.2099. [DOI] [PubMed] [Google Scholar]
- 28.Agusti AG. Systemic effects of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2005;2(4):367–370. doi: 10.1513/pats.200504-026SR. [DOI] [PubMed] [Google Scholar]
- 29.Van Weel C, Schellevis FG. Comorbidity and guidelines: conflicting interests. Lancet. 2006;367(9510):550–551. doi: 10.1016/S0140-6736(06)68198-1. [DOI] [PubMed] [Google Scholar]
- 30.Eisner MD, Balmes J, Katz BP, Trupin L, Yelin E, Blanc P. Lifetime environmental tobacco smoke exposure and the risk of chronic obstructive pulmonary disease. Environ Health Perspect. 2005;4:7–15. doi: 10.1186/1476-069X-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnes PJ, Shapiro SD, Pauwels RA. Chronic obstructive pulmonary disease: molecular and cellular mechanisms. Eur Respir J. 2003;22(4):672–688. doi: 10.1183/09031936.03.00040703. [DOI] [PubMed] [Google Scholar]
- 32.Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364(9435):709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- 33.Saetta M, Turato G, Maestrelli P, Mapp CE, Fabbri LM. Cellular and structural bases of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2001;163(6):1304–1309. doi: 10.1164/ajrccm.163.6.2009116. [DOI] [PubMed] [Google Scholar]
- 34.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 35.Cosio MG, Majo J. Inflammation of the airways and lung parenchyma in COPD: role of T cells. Chest. 2002;121(5):160S–165S. doi: 10.1378/chest.121.5_suppl.160s. [DOI] [PubMed] [Google Scholar]
- 36.Barnes PJ. Macrophages as orchestrators of COPD. J COPD. 2004;1:59–70. doi: 10.1081/COPD-120028701. [DOI] [PubMed] [Google Scholar]
- 37.Barnes PJ. Mediators of chronic obstructive pulmonary disease. Pharmacol Rev. 2004;56(4):515–548. doi: 10.1124/pr.56.4.2. [DOI] [PubMed] [Google Scholar]
- 38.Tillie-Leblond I, Gosset P, Tonnel AB. Inflammatory events in severe acute asthma. Allergy. 2005;60(1):23–29. doi: 10.1111/j.1398-9995.2005.00632.x. [DOI] [PubMed] [Google Scholar]
- 39.Bumbacea D, Campbell D, Nguyen L, Carr D, Barnes PJ, Robinson D, et al. Parameters associated with persistent airflow obstruction in chronic severe asthma. Eur Respir J. 2004;24(1):122–128. doi: 10.1183/09031936.04.00077803. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 41.Standardization of Spirometry, 1994. Update. American Thoracic Society. Am J Respir Crit Care Med. 1995;152(3):1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 42.Standardized lung function testing. Official statement of the European Respiratory Society. Eur Respir J Suppl. 1993;16:1–100. [PubMed] [Google Scholar]
- 43.Barnes PJ. Efficacy of inhaled corticosteroids in asthma. J Allergy Clin Immunol. 1998;102(4 Pt 1):531–538. doi: 10.1016/s0091-6749(98)70268-4. [DOI] [PubMed] [Google Scholar]
- 44.Kamada AK, Szefler SJ, Martin RJ, Boushey HA, Chinchilli VM, Drazen JM, et al. Issues in the use of inhaled glucocorticoids. The Asthma Clinical Research Network. Am J Respir Crit Care Med. 1996;153(6 Pt 1):1739–1748. doi: 10.1164/ajrccm.153.6.8665030. [DOI] [PubMed] [Google Scholar]
- 45.Newman SP. Inhaler treatment options in COPD. Eur Respir Rev. 2005;14(96):102–108. [Google Scholar]
- 46.National Asthma Education and Prevention Program. Bethesda, MD: National Heart, Lung and Blood Institute. National Institutes of Health; 1997. Guidelines for the diagnosis and management of asthma. [Google Scholar]
- 47.Lazarus SC, Boushey HA, Fahy JV, Chinchilli VM, Lemanske RF, Jr, Sorkness CA, et al. Long-acting beta2-agonist monotherapy vs continued therapy with inhaled corticosteroidsin patients with persistent asthma: a randomized controlled trial. JAMA. 2001;285(20):2583–2593. doi: 10.1001/jama.285.20.2583. [DOI] [PubMed] [Google Scholar]
- 48.Hondras MA, Linde K, Jones AP. Manual therapy for asthma. Cochrane Database Syst Rev. 2005;(2):CD001002. doi: 10.1002/14651858.CD001002.pub2. [DOI] [PubMed] [Google Scholar]
- 49.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA. Prednisolone response in patients with chronic obstructive pulmonary disease: results from the ISOLDE study. Thorax. 2003;58(8):654–658. doi: 10.1136/thorax.58.8.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calverley PM, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibilityt esting in chronic obstructive pulmonary disease. Thorax. 2003;58(8):659–664. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Burge PS, Calverley PM, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320(7245):1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vestbo J, Pauwels R, Anderson JA, Jones P, Calverley P. Early onset of effect of salmeterol and fluticasone propionate in chronic obstructive pulmonary disease. Thorax. 2005;60(4):301–304. doi: 10.1136/thx.2004.025411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guyatt GH, Townsend M, Pugsley SO, Keller JL, Short HD, Taylor DW, et al. Bronchodilators in chronic air-flow limitation. Effects on airway function, exercise capacity, and quality of life. Am Rev Respir Dis. 1987;135(5):1069–1074. doi: 10.1164/arrd.1987.135.5.1069. [DOI] [PubMed] [Google Scholar]
- 54.Calverley P, Pauwels R, Vestbo J, Jones P, Pride N, Gulsvik A, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomized controlled trial. Lancet. 2003;361(9356):449–456. doi: 10.1016/S0140-6736(03)12459-2. [DOI] [PubMed] [Google Scholar]


