Abstract
Emotional processes are enhanced in aging, such that aging is characterized by superior emotional regulation. This article provides a brief review of the neural bases supporting this effect with a focus on functional neuroimaging studies of perception and episodic memory. The most consistent finding across these studies is that older adults show an alteration in the recruitment of the amygdala, but greater recruitment of the frontal cortex. These Fronto-amygdalar Age-related Differences in Emotion (FADE) may reflect emotional regulation strategies mediated by frontal brain regions that dampen emotion-related activations in the amygdala.
Keywords: fMRI, Affect, Frontal cortex, Amygdala, Older adults, Emotional regulation
INTRODUCTION
In the past decade, the number of empirical studies examining emotion and aging has risen substantially. Despite age-related declines in many cognitive domains (Dennis & Cabeza, 2008), healthy aging has less of an impact on emotion (Kensinger, 2008) and has been characterized by superior emotional regulation, the ability to exert control over emotional responses (Ochsner & Gross, 2005). According to socioemotional selective theory, aging is associated with motivational differences in allocating attention to emotional information. This leads to increases in mood and well-being, and potentially greater likelihood that positive events will be attended to and remembered (positivity shift; Mather & Carstensen, 2005). In sum, there are a substantial number of behavioral studies demonstrating that emotional processes are maintained and sometimes enhanced in aging. However, it is critical to also understand the neural mechanisms underlying the effects of aging on emotion to distinguish potential age-related differences in emotional regulation strategies and to dissociate memory encoding versus retrieval processes. The present article provides a brief review of the small, but growing literature on functional neuroimaging studies investigating the effects of aging on emotional perception and episodic memory. Although the field is complex, and sometimes contradictory due to many differences between studies (e.g., stimuli, particular contrast & task, and so on; see Table 1), there is a consistent pattern whereby aging is associated with alterations in amygdalar activity coupled with an increase in frontal activity, particularly in medial frontal regions associated with emotional regulation processes. Table 1 summarizes the results of age-related functional neuroimaging studies of emotional perception and episodic memory by showing both age-related differences and age-invariant activations elicited across several brain regions. We call this pattern Fronto-amygdalar Age-related Differences in Emotion (FADE). Before turning to the evidence supporting or opposing the FADE pattern we begin with an overview of the neural systems involved in emotional processing and the impact of aging.
Table 1.
Results of Functional Neuroimaging Studies of Aging and Emotion
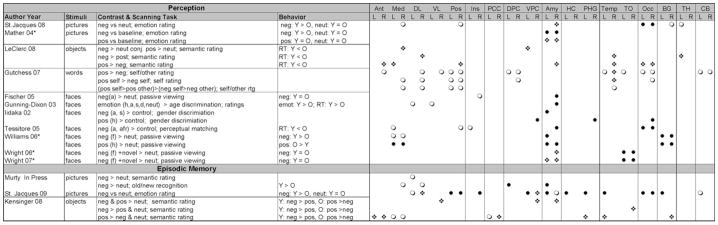
|
● Young > Older; ❍ Older > Young; ❖ Young = Older
Contrast: neg = negative, pos = positive, neut = neutral; h = happy, a = angry, s = sad, d = disgust, f = fear, afr = afraid
Behavior: Unless indicated the behavior results refer to subjective emotion ratings; RT = response time
Brain Regions: Frontal Cortex: Ant = anterior, Med = medial, DL = dorsolateral, VL = ventrolateral, Pos = posterior; Ins = Insula; PCC = posterior cingulate cortex; DPC = dorsal parietal cortex, VPC = ventral parietal cortex Amy = amygdala; HC = hippocampus, PHG = parahippocampal gyrus; Temp = temporal cortex; TO = temporal occipital cortex; Occ = occipital cortex; BG = basal ganglia; TH = thalamus; CB = cerebellum
Indicates Region of Interest Analysis
THE EMOTIONAL BRAIN AND AGING
The neural processing of emotionally salient events involves many brain regions (Phan, Wager, Taylor, & Liberzon, 2002) including automatic processes in the amygdala and controlled processes in the prefrontal cortex (PFC). The amygdala is critical for the detection of emotions and the generation of physiological responses. Furthermore, the amygdala optimizes perception and memory of emotional events through its dense connections with cortical and subcortical areas (Amaral & Price, 1984), including the modulation of memory consolidation (LaBar & Cabeza, 2006). In contrast, the PFC exerts control over emotions (Ochsner & Gross, 2005), with medial and lateral subregions supporting different neural processes. First, medial PFC (Brodmann’s Area 10), including nearby anterior cingulate cortex (ACC; Brodmann’s Area 32, 24), coordinates the generation and regulation of affect (Phan et al., 2002). Anterior medial PFC regions (particularly BA 10) are also associated with self-referential processes (Amodio & Frith, 2006), which refers to relating information to one’s self. Medial PFC is part of the default network, which refers to a set of regions that tend to be deactivated during attention demanding tasks and activated during internally focused tasks, such as during rest (Gusnard, Akbudak, Shulman, & Raichle, 2001). The default network is implicated in emotionally meaningful events (Cabeza & St Jacques, 2007) and malfunctions in depressed patients (Mayberg, 1997; Sheline et al., 2009). Second, lateral PFC regions (Brodmann’s Area 44/46, 46/9) are more generally related to controlled processes (Miller & Cohen, 2001) that contribute to emotional regulation (Ochsner & Gross, 2005).
Compared with structural and functional decline in other brain regions, the amygdala and some subregions of the PFC might be less vulnerable to aging. First, the structural integrity of the amygdala remains relatively preserved in aging (Good et al., 2001; Grieve, Clark, Williams, Peduto, & Gordon, 2005), as does the functional recruitment of this region to novelty (Wright et al., 2008; Wright, Wedig, Williams, Rauch, & Albert, 2006). Second, there is evidence for structural integrity of the medial PFC and ACC (Salat, Kaye, & Janowsky, 2001; also see Raz & Rodrigue, 2006; although see Petit-Taboue, Landeau, Desson, Desgranges, & Baron, 1998). In fact, older adults sometimes show less deactivation in medial PFC regions during cognitive tasks (Grady, Springer, Hongwanishkul, McIntosh, & Winocur, 2006), which has been linked to their difficulty in disengaging default mode activity during these attention demanding tasks. One interpretation of this finding is that older adults chronically engage self-referential processes (Kensinger & Leclerc, 2009), which might interfere or benefit performance depending upon the particular cognitive task (Gutchess, Kensinger, Yoon, & Schacter, 2007b). In contrast to the medial PFC, aging has a large impact on the structural integrity of the lateral PFC (Raz et al., 2005). Functional neuroimaging studies have shown that lateral PFC regions are over-recruited in aging (Grady, 2008). In particular, greater age-related recruitment of the PFC is typically coupled with a reduction in the recruitment of posterior regions (Posterior-Anterior Shift in Aging, PASA; Dennis & Cabeza, 2008), a pattern of activation linked to compensation (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008).
In summary, the relative preservation of the structure and function of the amygdala and specific frontal regions in older adults might underlie the maintenance and enhancement of emotional processes in aging.
AGING AND EMOTIONAL PERCEPTION
Functional neuroimaging studies of age-related differences in emotional perception are generally consistent with the FADE pattern. Below we discuss the findings relevant to the amygdala and frontal cortices and the interaction between these regions.
One of the most consistent findings from functional neuroimaging studies of emotional perception is an age-related reduction in amygdala activation for negative stimuli (see Table 1). For example, in the first functional magnetic resonance imaging (fMRI) study of emotion and aging, Iidaka et al. (2002) found less left amygdala activity for negative faces in older adults when compared with young adults, and other studies have since replicated this finding (Fischer et al., 2005; Gunning-Dixon et al., 2003; Tessitore et al., 2005; also see Erk, Walter, Abler, 2008). However, in the aforementioned studies (Gunning-Dixon et al., 2003; Iidaka et al., 2002; Tessitore et al., 2005), older adults reported negative stimuli valence ratings that were less negative than the ones given by young adults and associated with the standardized ratings (Lang, Bradley, & Cuthbert, 1997). Thus, one possible interpretation is that the age-related reduction in amygdala activity reflects an inability of the stimuli to elicit strong negative emotions in older adults rather than a deficit in amygdala function. Consistent with this idea, others and we have found that amygdala activity can be as strong in older as in young adults when stimuli were classified according to participants’ own ratings (Leclerc & Kensinger, 2008a; Mather et al., 2004; St Jacques, Dolcos, & Cabeza, 2008; Wright et al., 2006) or when there were no age-related differences in emotional ratings (Fischer et al., 2005). Furthermore, we (St Jacques et al., 2008) found that amygdala activity was indeed reduced for negative stimuli that older adults subjectively rated as neutral. Interestingly, age-invariant activity in the amygdala is typically observed for positive stimuli (Gutchess, Kensinger, & Schacter, 2007a; Leclerc & Kensinger, 2008b; Mather et al., 2004; although see Williams et al., 2006) and might reflect a shift in the preference of amygdala activation in aging (Mather et al., 2004; also see Wright et al., 2006; Wright, Dickerson, Feczko, Negeira, & Williams, 2007). In sum, the function of the amygdala remains intact in aging. Below we discuss age-related findings in the frontal cortex and how this might interact with the amygdala findings.
In contrast with the amygdala, functional neuroimaging studies of emotional perception have generally found an age-related increase in the recruitment of the frontal cortex, especially in medial PFC regions (see Table 1), although age-related increases in lateral PFC regions are also prevalent (Gunning-Dixon et al., 2003; Gutchess et al., 2007a; Tessitore et al., 2005). We and others (St Jacques et al., 2008; Williams et al., 2006) found an age-related increase in the recruitment of medial PFC during the perception of negative versus neutral pictures, while other studies have observed that the age-related increases in medial PFC recruitment are greater for positive versus negative stimuli (Gutchess et al., 2007a; Leclerc & Kensinger, 2008a). These apparent differences might be due to the dissociable effects of arousal and valence in dorsal versus ventral aspects of the medial PFC (e.g., Dolcos, LaBar, & Cabeza, 2004). For example, Leclerc and Kensinger (2008a) found age-invariant activity in dorsomedial regions reflecting emotional arousal, whereas, the age-related differences observed in ventromedial regions (specifically ventral ACC) reflected differences in positive versus negative valence. Future research is needed to tease apart potential age-related differences in the neural correlates subserving emotional arousal and valence.
In summary, consistent with the FADE pattern, emotional perception studies of aging converge on the observation of an age-related alteration in amygdala activation coupled with an increase in frontal cortex activation, which suggest that older adults engage more controlled processing during the perception of emotional stimuli. It is also critical to understand how the effects of aging on emotional processing ramify through cognitive operations, such as memory processes.
AGING AND EMOTIONAL EPISODIC MEMORY
In young adults, memory is usually better for emotional than neutral stimuli. Although this enhancement for emotional memory is relatively preserved in healthy aging, there is an age-related alteration in the extent of the enhancement, especially for negative information. Only recently have functional neuroimaging studies begun to explore the neural bases of the age-related changes in emotional memory, and the available evidence is consistent with FADE. Below we discuss the age-related findings in the amygdala and PFC.
Despite differences in methodology, all three available functional neuroimaging studies of memory encoding show age-invariant amygdala activity coupled with age-related increases in PFC activity. For example, we (St Jacques, Dolcos, & Cabeza, 2009) found that amygdala activity predicted subsequent memory of negative versus neutral pictures in both young and older adults, but that older adults recruited additional frontal activity to support memory formation as visual cortex activity declined. Similarly, Murty et al. (2008) found age-related increases in the recruitment of left dorsolateral PFC (DLPFC) during the encoding of negative versus neutral picture blocks, but they did not observe age-related differences in the amygdala. Consistent with behavioral findings of preserved enhancement for emotional memory in aging, Murty et al. (2008) suggested that the increased recruitment of the PFC might reflect compensation. Complementing these findings, Kensinger and Schacter (2008) observed age-invariant recruitment of the amygdala during successful memory encoding of objects, irrespective of the particular negative or positive valence, but older adults elicited greater medial PFC activity during successful encoding of positive objects. Furthermore, others and we have observed an age-related decrease in functional connectivity between the amygdala and the areas that typically support memory formation (i.e., hippocampus) but an age-related increase in functional connectivity with PFC areas involved in controlled processing (Murty et al., 2008; St Jacques et al., 2009). Currently, only one study (Murty et al., 2008) has examined age-related effects during memory retrieval, where they found that older adults showed a reduction in the recruitment of the amygdala during memory retrieval of negative versus neutral blocks coupled with an age-related increase in right DLPFC.
In summary, the results of these initial fMRI studies suggest that aging leads to an increased reliance on controlled processes in the PFC that support and maintain enhanced memory for emotional materials.
THE FADE PATTERN
Evidence from age-related functional neuroimaging studies across both emotional perception and memory domains generally show a pattern of activity consistent with FADE, although some studies provide only partial support potentially due to methodological differences (Fischer et al., 2005; Gutchess et al., 2007a; Iidaka et al., 2002) and to focused region of interest analyses on the amygdala (Mather et al., 2004; Wright et al., 2006, 2007). Below we focus on the evidence that supports the FADE pattern and provide possible interpretations of this empirical regularity.
Although age-invariant activation in the amygdala was observed during both emotional perception and the encoding and formation of emotional memories, suggesting that the function of the amygdala remains intact in aging, the amount of amygdala activity elicited in older adults might differ depending upon the recruitment of controlled processes involving the frontal cortices. There are at least three potential interpretations of the increase in frontal recruitment associated with the FADE pattern. First, the frontal increase could be an instance of the posterior anterior shift in aging (PASA), which is frequently observed in nonemotional domains (Dennis & Cabeza, 2008). Consistent with this interpretation, others and we have found that the increase in frontal activity is coupled with a decrease in the recruitment of posterior regions (St Jacques et al., 2008, 2009; Tessitore et al., 2005; also see Gunning-Dixon et al., 2003; Iidaka et al., 2002). Furthermore, in keeping with the compensatory account of PASA, we also found a significant correlation between the age-related increase in frontal activity and the reduction in visual cortex activity during emotional perception (St Jacques et al., 2008) and that the age-related frontal increase was predictive of subsequent memory for negative stimuli (St Jacques et al., 2009).
Second, age-related increases in medial PFC could reflect an augmentation of self-referential processes (Kensinger & Leclerc, 2009), which are processes previously associated with this region (Northoff & Bermpohl, 2004). One line of evidence supporting this interpretation is that medial PFC recruitment in older adults has been shown to vary as a function of valence (Kensinger & Schacter, 2008; Leclerc & Kensinger, 2008a). For example, Leclerc and Kensinger (2008a) found an age-related reversal in the medial PFC, such that older adults recruited this region more for positive stimuli and less for negative stimuli (although see Williams et al., 2006). They suggested that older adults might interpret positive stimuli in a more self-relevant way. In keeping with this interpretation, a study directly interrogating age-related differences in self-referential processing for emotional stimuli found that older adults recruited medial PFC regions to a greater extent for self-related positive words (Gutchess et al., 2007a). Given that older adults tend to engage the default network more during cognitive tasks (Grady et al., 2006), one possibility is that the positivity shift observed in perception and memory studies might result from an age-related increase in the tendency to interpret information in a self-relevant manner (cf. Kensinger & Leclerc, 2009).
Third, age-related frontal increases could reflect emotional regulation. Consistent with FADE, in many studies, the age-related increase in frontal activity was coupled with a reduction in amygdala activity during perception (Gunning-Dixon et al., 2003; Tessitore et al., 2005; also see Fischer et al., 2005; Iidaka et al., 2002; Samanez-Larkin et al., 2007; St Jacques et al., 2008; although see Williams et al., 2006) and retrieval (Murty et al., 2008) of negative stimuli. Thus, one possibility is that older adults’ enhanced emotional regulation strategies lead to the recruitment of PFC-mediated control processes that dampen amygdala responses for negative stimuli. For example, we examined age-related differences in the functional connectivity with the amygdala and found an age-related increase in the functional connectivity between the ventral ACC and the amygdala (St Jacques et al., 2008). Importantly, we found a negative correlation between these regions during the perception of negative pictures that older adults subjectively rated as neutral and a subsequent decrease in amygdala activity, which suggests the engagement of emotional regulation. Urry et al. (2006) directly asked older adults to regulate emotions while viewing negative pictures and found that compared with passive viewing, when older adults were asked to decrease their emotional responses they recruited greater ventromedial PFC activity coupled with a reduction in the recruitment of the amygdala. In fact, emotional well-being in aging has been associated with a shift from automatic processing to more controlled processing of emotions via the recruitment of medial PFC (Williams et al., 2006).
In summary, age-related increases in frontal activity might reflect compensation, self-referential processing, or emotional regulation. It is important to note, however, that these accounts are not incompatible with each other. For example, emotional regulation can be seen as a form of compensation (cf. St Jacques et al., 2008), and self-referential processing could be seen as an emotional regulation strategy (cf. Kensinger & Leclerc, 2009). Yet, not all forms of emotional regulation are beneficial for performance (i.e., compensatory) and self-referential processing is not necessarily an effective regulation strategy. Thus, understanding the specific contributions of each of these processes and their interactions is a major challenge for future research.
CONCLUSIONS
Functional neuroimaging studies examining age-related differences in the perception and memory for emotional information converge on findings consistent with FADE. Whether the increased recruitment of the PFC and alterations in the amygdala reflect more general patterns of age-related differences or motivational differences in self-referential processing and emotional regulation will be an important avenue for future research. Furthermore, empirical testing of the FADE pattern is needed to determine whether this empirical regularity will hold more generally across different methodologies. Many of the complexities of emotion and age interactions in the brain such as valence versus arousal effects, automatic versus controlled processing of emotions, perception versus emotional experience, possible sex differences, and potential individual differences in brain volume have yet to be disentangled. In fact, we have only just begun to understand the neural bases of age-related effects in emotion on perception and memory, and extending these findings to other cognitive domains such as working memory and decision-making, as well as to understanding pathological aging such as Alzheimer’s disease, will be critical for a complete understanding of the emotional brain and aging.
Acknowledgments
The authors thank Jared Stokes for help with table preparation. This work was supported by grants AG19731 and NS41328.
References
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of Comparative Neurology. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: The medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–277. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R. Neuroimaging of healthy cognitive aging. In: Craik FIM, Salthouse TA, editors. The handbook of aging and cognition. 3. Mahwah, NJ: Lawrence Erlbaum Associates; 2008. pp. 1–54. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Erk S, Walter H, Abler B. Age-related physiological responses to emotion anticipation and exposure. Neuroreport. 2008;19:447–452. doi: 10.1097/WNR.0b013e3282f5d92f. [DOI] [PubMed] [Google Scholar]
- Fischer H, Sandblom J, Gavazzeni J, Fransson P, Wright CI, Backman L. Age-differential patterns of brain activation during perception of angry faces. Neuroscience Letters. 2005;386:99–104. doi: 10.1016/j.neulet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14(Pt 1):21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL. Cognitive neuroscience of aging. Annals of the New York Academy of Sciences. 2008;1124:127–144. doi: 10.1196/annals.1440.009. [DOI] [PubMed] [Google Scholar]
- Grady CL, Springer MV, Hongwanishkul D, McIntosh AR, Winocur G. Age-related changes in brain activity across the adult lifespan. Journal of Cognitive Neurosciences. 2006;18:227–241. doi: 10.1162/089892906775783705. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Clark CR, Williams LM, Peduto AJ, Gordon E. Preservation of limbic and paralimbic structures in aging. Human Brain Mapping. 2005;25:391–401. doi: 10.1002/hbm.20115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Gur RC, Perkins AC, Schroeder L, Turner T, Turetsky BI, et al. Age-related differences in brain activation during emotional face processing. Neurobiology of Aging. 2003;24:285–295. doi: 10.1016/s0197-4580(02)00099-4. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proceedings of the National Academy of Science of the United States of America. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Schacter DL. Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience. 2007a;2:117–133. doi: 10.1080/17470910701399029. [DOI] [PubMed] [Google Scholar]
- Gutchess AH, Kensinger EA, Yoon C, Schacter DL. Ageing and the self-reference effect in memory. Memory. 2007b;15:822–837. doi: 10.1080/09658210701701394. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Okada T, Murata T, Omori M, Kosaka H, Sadato N, et al. Age-related differences in the medial temporal lobe responses to emotional faces as revealed by fMRI. Hippocampus. 2002;12:352–362. doi: 10.1002/hipo.1113. [DOI] [PubMed] [Google Scholar]
- Kensinger EA. Emotional memory across the adult lifespan. New York: Psychology Press; 2008. [Google Scholar]
- Kensinger EA, Leclerc CM. Age-related changes in the neural mechanisms supporting emotion processing and emotional memory. European Journal of Cognitive Psychology. 2009;21:192–215. [Google Scholar]
- Kensinger EA, Schacter DL. Neural processes supporting young and older adults’ emotional memories. Journal of Cognitive Neurosciences. 2008;20:1161–1173. doi: 10.1162/jocn.2008.20080. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS) Gainesville, FL: University of Florida; 1997. (Producer) [Google Scholar]
- Leclerc CM, Kensinger EA. Age-related differences in medial prefrontal activation in response to emotional images. Cognitive, Affective & Behavioral Neuroscience. 2008a;8:153–164. doi: 10.3758/cabn.8.2.153. [DOI] [PubMed] [Google Scholar]
- Leclerc CM, Kensinger EA. Effects of age on detection of emotional information. Psychology and Aging. 2008b;23:209–215. doi: 10.1037/0882-7974.23.1.209. [DOI] [PubMed] [Google Scholar]
- Mather M, Canli T, English T, Whitfield S, Wais P, Ochsner K, et al. Amygdala responses to emotionally valenced stimuli in older and younger adults. Psychological Science. 2004;15:259–263. doi: 10.1111/j.0956-7976.2004.00662.x. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and motivated cognition: The positivity effect in attention and memory. Trends in Cognitive Sciences. 2005;9:496–502. doi: 10.1016/j.tics.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: A proposed model of depression. The Journal of Neuropsychiatry and Clinical Neurosciences. 1997;9:471–481. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of pre-frontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan H, Callicott JH, Goldberg TE, et al. Age-related alterations in simple declarative memory and the effect of negative stimulus valence. Journal of Cognitive Neuroscience. 2008 doi: 10.1162/jocn.2009.21130. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Petit-Taboue MC, Landeau B, Desson JF, Desgranges B, Baron JC. Effects of healthy aging on the regional cerebral metabolic rate of glucose assessed with statistical parametric mapping. Neuroimage. 1998;7:176–184. doi: 10.1006/nimg.1997.0318. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: A meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Selective preservation and degeneration within the prefrontal cortex in aging and Alzheimer disease. Archives of Neurology. 2001;58:1403–1408. doi: 10.1001/archneur.58.9.1403. [DOI] [PubMed] [Google Scholar]
- Samanez-Larkin GR, Gibbs SE, Khanna K, Nielsen L, Carstensen LL, Knutson B. Anticipation of monetary gain but not loss in healthy older adults. Nature Neuroscience. 2007;10:787–791. doi: 10.1038/nn1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Price JL, Rundle MM, Vaishnavi SN, Snyder AZ, et al. The default mode network and self-referential processes in depression. Proceedings of the National Academy of Science of the United States of America. 2009;106:1942–1947. doi: 10.1073/pnas.0812686106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala during negative evaluation: A network analysis of fMRI data. Neurobiology of Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.03.012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Jacques PL, Dolcos F, Cabeza R. Effects of aging on functional connectivity of the amygdala for subsequent memory of negative pictures: A network analysis of fMRI data. Psychological Science. 2009;20:74–84. doi: 10.1111/j.1467-9280.2008.02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessitore A, Hariri AR, Fera F, Smith WG, Das S, Weinberger DR, et al. Functional changes in the activity of brain regions underlying emotion processing in the elderly. Psychiatry Research. 2005;139:9–18. doi: 10.1016/j.pscychresns.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Brown KJ, Palmer D, Liddell BJ, Kemp AH, Olivieri G, et al. The mellow years? Neural basis of improving emotional stability over age. Journal of Neuroscience. 2006;26:6422–6430. doi: 10.1523/JNEUROSCI.0022-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Dickerson BC, Feczko E, Negeira A, Williams D. A functional magnetic resonance imaging study of amygdala responses to human faces in aging and mild Alzheimer’s disease. Biological Psychiatry. 2007;62:1388–1395. doi: 10.1016/j.biopsych.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Wright CI, Negreira A, Gold AL, Britton JC, Williams D, Barrett LF. Neural correlates of novelty and face-age effects in young and elderly adults. Neuroimage. 2008;42:956–968. doi: 10.1016/j.neuroimage.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Wedig MM, Williams D, Rauch SL, Albert MS. Novel fearful faces activate the amygdala in healthy young and elderly adults. Neurobiology of Aging. 2006;27:361–374. doi: 10.1016/j.neurobiolaging.2005.01.014. [DOI] [PubMed] [Google Scholar]


