Abstract
An efficient three component coupling (TCC) reaction toward a variety of 3-aminoindoline and 3-aminoindole derivatives has been developed. This cascade transformation proceeds via the copper-catalyzed coupling reaction between 2-aminobenzaldehyde, secondary amine, and alkyne leading to propargylamine intermediate, which, under the reaction conditions, undergoes cyclization into the indoline core. The latter, upon treatment with a base, smoothly isomerizes into indole. Alternatively, indole can directly be synthesized in a one-pot sequential reaction.
Keywords: three component coupling, 3-aminoidolines, 3-aminoindoles, copper, synthetic methods
Indole and indoline structural motifs are ubiquitously found in a wide range of natural products and pharmaceuticals.[1] In particular, 3-aminoindoles and indolines have found a broad application in medicinal chemistry as effective anticancer agents, compounds with analgesic properties, and agents for prevention of type II diabetes. However, the reported examples on synthesis of 3-aminoindoles[2] and 3-aminoindolines[3] are limited in scope and require multistep preparation of starting materials. Thus, the development of simple and general synthesis of 3-aminoindoles and 3-aminoindolines from easily available starting materials is warranted. One of the reasonable solutions to this problem would be the assembly of indole and indoline cores via multicomponent coupling reaction (MCR).[4]
Herein, we wish to report an efficient Cu-catalyzed three component coupling reaction of N-protected 2-aminobenzaldehydes 1 with secondary amines 2 and terminal acetylenes 3 into the 3-aminoindolines 5 and their subsequent isomerization into indoles 6 (Scheme 1). Multicomponent Mannich reaction between benzaldehydes, secondary amines and acetylenes is well-documented.[5] Recently, synthesis of benzofurans via three-component coupling reaction of o-hydroxybenzaldehydes, acetylenes and secondary amines catalyzed by copper salts has been reported.[6] However, analogous multicomponent reactions of 2-aminobenzaldehydes are unknown. We envisioned that a TCC reaction of N-protected 2-aminobenzaldehydes 1, secondary amine 2 and terminal alkyne 3 would lead to the formation of propargyl amine intermediate 4,[7] which, upon activation of triple bond with a π-philic metal, would undergo an intramolecular 5-exo-dig cyclization[8,9,10] into indoline 5. It was expected that under reaction conditions, a subsequent isomerization of 5 into 3-aminoindoles 6 would occur (Scheme 1).
Scheme 1.
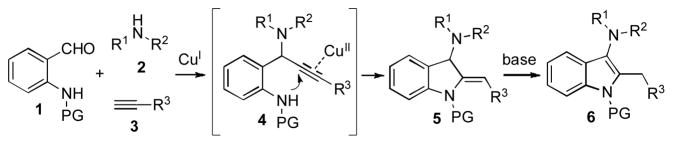
Proposed synthesis of 3-aminoindoles.
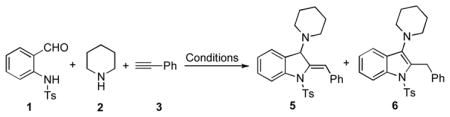
To test this hypothesis, the reaction of N-(2-formylphenyl)-4-methylbenzenesulfonamide, piperidine and phenylacetylene in the presence of different metal salts has been examined (Table 1). It was found that employment of gold (I) and (III), as well as silver and copper salts, was not effective resulting in formation of propargylamine intermediate 4 only (entires 1–6). The TCC reaction in the presence of CuCl and Cu(OTf)2 produced trace amounts of 5 (entry 7). Substantial improvement of the yields was achieved in the presence of stoichiometric amounts of Et3N and Cs2CO3 (entries 8 and 9). Furthermore, employment of DMAP led to nearly quantitative formation of indoline 5 (entry 10)! Employment of sole copper source in the presence of DMAP (entries 11–12) was less efficient as compared to use of CuI/CuII binary system.[11]
Table 1.
Optimization of TCC coupling.a)
| |||
|---|---|---|---|
| # | Conditions Catalyst (mol%) | Additive (equiv.) | 5, yield %b) |
| 1 | AuCl (5) | none | −(83) c) |
| 2 | AuCl3 (5) | none | −(75) c) |
| 3 | NaAuCl4•2H2O (5) | none | traces |
| 4 | AgOTf (5) | none | −(70) c) |
| 5 | CuCl (5) | none | −(60) c) |
| 6 | Cu(OTf)2 (5) | none | −(51) c) |
| 7 | CuCl (5), Cu(OTf)2 (5) | none | 7(80) c) |
| 8 | CuCl (5), Cu(OTf)2 (5) | NEt3(1.0) | 48 |
| 9 | CuCl (5), Cu(OTf)2 (5) | Cs2CO3(1.0) | 51 |
| 10 | CuCl (5), Cu(OTf)2 (5) | DMAP(1.0) | 98 |
| 11 | CuCl (5) | DMAP(1.0) | 70(10) |
| 12 | Cu(OTf)2 (5) | DMAP(1.0) | 47(21) |
All reactions were performed with 1 (0.3 mmol), 2 (0.3 mmol) and 3 (0.45 mmol) in MeCN at 80°C.
Yield of the isolated product after flash chromatography on silica gel.
Yield in parentheses given for formation of the corresponding propargylamine 4.
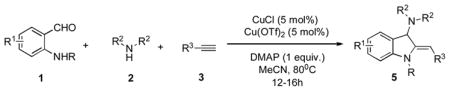
Surprisingly, no formation of indole 6 was observed under these reaction conditions. Next, under the optimized conditions, the scope of this new TCC reaction has been examined (Table 2). Gratifyingly, we found that this transformation is very general for a wide range of different acetylenes, aldehydes and secondary amines providing an easy access to densely-substituted indolines 5. Thus, employment of piperidine, morpholine, and pyrrolidine produced aminoindolines in good to excellent yields. Acyclic dibenzyl, diallyl, diethyl and diisobutyl amines were similarly effective. Different alkynes, bearing alkyl or aryl substituents, displayed high reactivity in this transformation. A variety of different groups at the aromatic moiety of aldehyde, such as chloro (entries 18–19), bromo (entries 20–23), methyl (entry 24), methoxy (entry 25), and fluoro (entry 26), were also perfectly tolerated. Additionally, we have shown that N-4-nitrobenzenesulfonyl group can be tolerated under reaction conditions resulting in formation of indoline 5aa in good yield (entry 27). Reaction of N-tosylamino-3-naphtalaldehyde resulted in formation of tricyclic indoline 5ab although in slightly decreased yield (entry 28). Employment of 1,2,3,4-tetrahydroquinoline resulted in formation of corresponding indoline 5ac in 68% isolated yield (entry 29). Furthermore, by utilizing trimethylsilylacetylene as acetylene surrogate,[11] an exomethylene moiety possessing indoline 5ad was obtained in 64% yield (entry 30).
Table 2.
TCC Synthesis of 3-Aminoindolinesa)
| ||
|---|---|---|
| entry | product | yieldb)% |
| 1 |
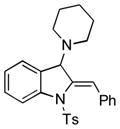 5a |
98 |
| 2 |
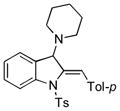 5b |
94 |
| 3 |
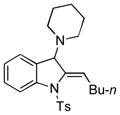 5c |
78 |
| 4 |
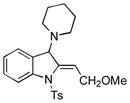 5d |
92 |
| 5 |
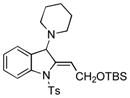 5e |
90 |
| 6 |
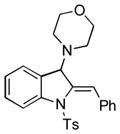 5f |
61 |
| 7 |
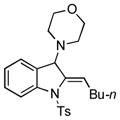 5g |
78 |
| 8 |
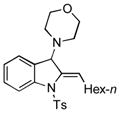 5h |
84 |
| 9 |
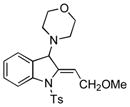 5i |
95 |
| 10 |
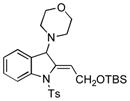 5j |
87 |
| 11 |
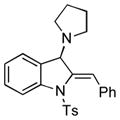 5k |
75 |
| 12 |
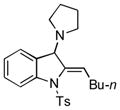 5l |
77 |
| 13 |
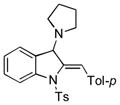 5m |
78 |
| 14 |
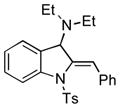 5n |
70 |
| 15 |
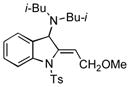 5o |
74 |
| 16 |
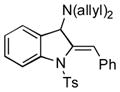 5p |
68 |
| 17 |
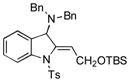 5q |
90 |
| 18 |
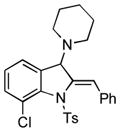 5r |
68 |
| 19 |
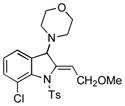 5s |
57 |
| 20 |
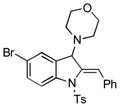 5t |
82 |
| 21 |
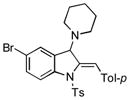 5u |
80 |
| 22 |
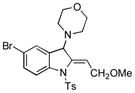 5v |
89 |
| 23 |
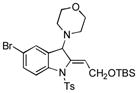 5w |
80 |
| 24 |
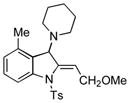 5x |
97 |
| 25 |
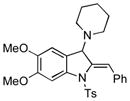 5y |
34 |
| 26 |
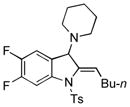 5z |
32 |
| 27 |
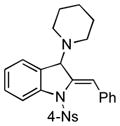 5aa |
73 |
| 28 |
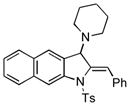 5ab |
45 |
| 29 |
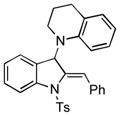 5ac |
68 |
| 30 |
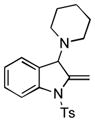 5ad |
64c) |
All reactions were performed with 1 (0.3 mmol), amine 2 (0.3 mmol), acetylene 3 (0.45 mmol) in MeCN (1M) at 80°C for 12–16h.
Yield of the isolated product after flash chromatography on silica gel.
Reaction was performed with trimethylsilylacetylene(1.5equiv.).
Next, conversion of indolines 5 into their more stable aromatic isomers, indoles 6, was explored. We reasoned that this isomerization reaction should occur in the presence of a base.[12] Indeed, it was found that heating indolines 5 with cesium carbonate in THF/MeOH mixture at 65°C resulted in formation of the corresponding indoles 6a–c in excellent yields (Eq 1).
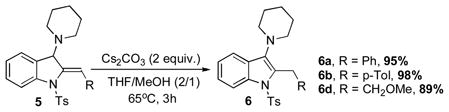 |
(eq 1) |
Encouraged by these results, we attempted a three-component one-pot synthesis of 3-aminoindoles 6 (Scheme 2).
Scheme 2.
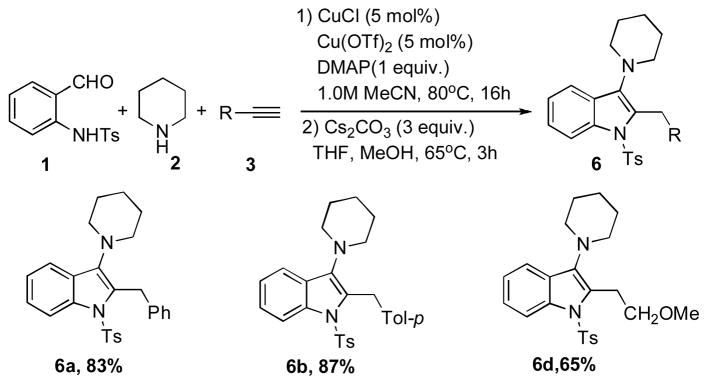
One-pot TCC synthesis of indoles 6.
Thus, the Cu-catalyzed TCC reaction of N-(2-formylphenyl)-4-methylbenzenesulfonamide 1, piperidine 2, and acetelynes 3 produced indolines 5. Subsequent base-assisted one-pot isomerization of the latter produced indoles 6a,b,d in good to high yields (Scheme 2). It deserves mentioning that N-tosylindoles 6 upon treatment with magnesium powder[13] could smoothly be detosylated into the corresponding N-H indoles 7a,c (Eq 2). Interestingly, treatment of N-tosylindolines 5a,c with Mg not only caused N-detosylation, but also highly efficient isomerization into indoles 7a,c (Eq 3).
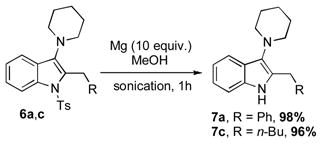 |
(eq 2) |
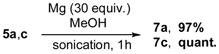 |
(eq 3) |
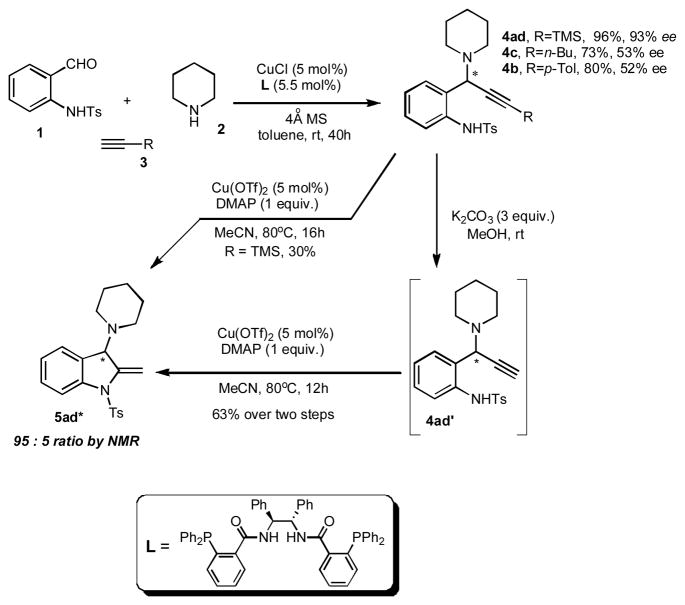
With the new efficient methodology for assembly of the 3-aminoindoline core in hand, we performed initial studies toward enantioselective version of this transformation (Scheme 3). It was found that under modified Knochel’s protocol for enantioselective TCC assembly of propargyl amines[14, 15] (with Trost’s C2-symmetrical ligand[16]), N-(2-formylphenyl)-4-methylbenzenesulfonamide 1, piperidine 2, and trimethylsilylacetelyne 3 underwent smooth coupling reaction to produce 4ad in high yield and 93% enantioselectivity. Employment of alkyl- and arylacetylenes 3 in the synthesis of propargyl amines (4c and 4b) resulted in good chemical yields, but moderate enantioselectivity (53 and 52% ee, respectively). Direct cycloisomerization of 4ad gave poor yield of desired indoline 5ad* (30%). However, desilylation of 4ad into 4ad′ followed by its cyclization under standard conditions produced enantioenriched indoline 5ad* in good yield with virtually complete preservation of enantioselectivity (Scheme 3).
Scheme 3.
Enantioselective TCC Synthesis of Indoline 5*
In summary, we have developed a novel highly efficient and general copper-catalyzed three component coupling reaction of N-protected 2-aminobenzaldehydes with secondary amines and terminal acetylenes into 3-aminoindolines. It was shown that the 3-aminoindolines, under basic conditions, could highly efficiently be transformed into the isomeric 3-aminoindoles. Alternatively, the latter can be obtained via a one-pot TCC procedure. In addition, we have demonstrated that the optically active indoline could be synthesized via a stepwise enantioselective version of this novel TCC protocol.
Experimental Section
General Procedure: In a dry and argon flushed Wheaton 1mL V-vial, equipped with a magnetic stirring bar and a screw cap, CuCl (0.015 mmol, 5 mol%), Cu(OTf)2 (0.015 mmol, 5 mol%), DMAP (0.3 mmol, 1 equiv.) and aldehyde (0.3 mmol, 1 equiv.) were suspended in dry acetonitrile (0.3 mL). Secondary amine (0.3 mmol, 1 equiv.) and alkyne (0.45 mmol, 1.5 equiv.) were added and the reaction mixture was stirred at 80°C until TLC analysis showed full conversion of an aldehyde. Reaction mixture then was filtered through Celite and washed with dichloromethane. The crude product was concentrated in vacuo and purified by column chromatography on silica gel.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support of the National Institute of Health (1P50 GM-086145).
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/adsc.200######.
References
- 1.a) Takayama H, Kitajima M, Kogure N. Curr Org Chem. 2005;9:1445. [Google Scholar]; b) Lewis SE. Tetrahedron. 2006;62:8655. [Google Scholar]; c) Sánchez C, Méndez C, Salas JA. Nat Prod Rep. 2006;23:1007. doi: 10.1039/b601930g. [DOI] [PubMed] [Google Scholar]; d) Higuchi K, Kawasaki T. Nat Prod Rep. 2007;24:843. doi: 10.1039/b516351j. [DOI] [PubMed] [Google Scholar]; e) Gupta L, Talwar A, Chauchan PMS. Curr Med Chem. 2007;14:1789. doi: 10.2174/092986707781058904. [DOI] [PubMed] [Google Scholar]; f) Patil SA, Patil R, Miller DD. Curr Org Chem. 2008;12:691. [Google Scholar]
- 2.a) Przheval’skii NM, Skvortsova NS, Magedov IV. Chem Het Comp. 2002;38:1055. [Google Scholar]; b) Przheval’skii NM, Skvortsova NS, Magedov IV. Chem Het Comp. 2004;40:1435. [Google Scholar]; c) Seong CM, Park CM, Choi J, Park NS. Tetrahedron Lett. 2009;50:1029. [Google Scholar]; d) Couture A, Deniau E, Gimbert Y, Grandclaudon P. Tetrahedron. 1993;49:1431. [Google Scholar]
- 3.a) Gan Z, Reddy PT, Quevillon S, Couve-Bonnaire S, Arya P. Angew Chem Int Ed. 2005;44:1366. doi: 10.1002/anie.200462298. [DOI] [PubMed] [Google Scholar]; b) Sunazuka T, Hirose T, Omura S. Acc Chem Res. 2008;41:302. doi: 10.1021/ar6000044. [DOI] [PubMed] [Google Scholar]; c) Marsden SP, Watson EL, Raw SA. Org Lett. 2008;10:2905. doi: 10.1021/ol801028e. [DOI] [PubMed] [Google Scholar]
- 4.For reviews on MCR, see: Posner G. Chem Rev. 1986;86:831.Armstrong RW, Combs AP, Tempest PA, Brown SD, Keating TA. Acc Chem Res. 1996;29:123.Bienaymé H, Hulme C, Oddon G, Schmitt P. Chem–Eur J. 2000;6:3321. doi: 10.1002/1521-3765(20000915)6:18<3321::aid-chem3321>3.0.co;2-a.Tietze LF. Chem Rev. 1996;96:115. doi: 10.1021/cr950027e.Dömling A, Ugi I. Angew Chem Int Ed. 2000;39:3168. doi: 10.1002/1521-3773(20000915)39:18<3168::aid-anie3168>3.0.co;2-u.
- 5.a) Yan B, Liu Y. Org Lett. 2007;9:4323. doi: 10.1021/ol701886e. [DOI] [PubMed] [Google Scholar]; b) Nguyen RV, Li CJ. Synlett. 2008:1897. [Google Scholar]; c) Salter MM, Kobayashi J, Shimizu Y, Kobayashi S. Org Lett. 2006;8:3533. doi: 10.1021/ol0613012. [DOI] [PubMed] [Google Scholar]; d) Azizi N, Torkiyan L, Saidi MR. Org Lett. 2006;8:2079. doi: 10.1021/ol060498v. [DOI] [PubMed] [Google Scholar]; e) Black DA, Arndtsen BA. J Org Chem. 2005;70:5133. doi: 10.1021/jo0503557. [DOI] [PubMed] [Google Scholar]; f) Ollevier T, Nadeau E. J Org Chem. 2004;69:9292. doi: 10.1021/jo048617c. [DOI] [PubMed] [Google Scholar]; g) Loh TP, Chen SL. Org Lett. 2002;4:3647. doi: 10.1021/ol0265968. [DOI] [PubMed] [Google Scholar]; h) Loh TP, Liung SBKW, Tan KL, Wei LL. Tetrahedron. 2000;56:3227. [Google Scholar]; i) Kobayashi S, Busujima T, Nagayama S. Synlett. 1999:545. [Google Scholar]; j) Loh TP, Wei LL. Tetrahedron Lett. 1998;39:323. [Google Scholar]
- 6.a) Sakai N, Uchida N, Konakahara T. Tetrahedron Lett. 2008;49:3437. [Google Scholar]; b) Li H, Liu J, Yan B, Li Y. Tetrahedron Lett. 2009;50:2353. [Google Scholar]
- 7.For selected examples of Cu-catalyzed syntheses of propargylamines via TCC, see: Patil MK, Keller M, Reddy BM, Pale P, Sommer J. Eur J Org Chem. 2008:4440.Kantam ML, Laha S, Yadav J, Bhargava S. Tetrahedron Lett. 2008;49:3083.Niu M, Yin Z, Fu H, Jiang Y, Zhao Y. J Org Chem. 2008;73:3961. doi: 10.1021/jo800279j.Park SB, Alper H. Chem Commun. 2005:1315. doi: 10.1039/b416268d.Bieber LW, da Silva MF. Tetrahedron Lett. 2004;45:8281.Lee ASY, Chen GA, Chang YT, Chu SF. Synlett. 2009:441.For gold catalyzed, see: Loa VKY, Kung KKY, Wong MK, Che CM. J Organomet Chem. 2009;694:583.Zhang X, Corma A. Angew Chem Int Ed. 2008;47:4358. doi: 10.1002/anie.200800098.Kidwai M, Bansal V, Kumarb A, Mozumdar S. Green Chem. 2007;9:742.Huang B, Yao X, Lib CJ. Adv Synth Catal. 2006;348:1528.Lo VKY, Liu Y, Wong MK, Che CM. Org Lett. 2006;8:1529. doi: 10.1021/ol0528641.Elie BT, Levine C, Ubarretxena-Belandia I, Varela-Ramírez A, Aguilera RJ, Ovalle R, Contel M. Eur J Inorg Chem. 2009:3421. doi: 10.1002/ejic.200900279.For other transition metal catalyzed, see: Zhang Y, Li P, Wang M, Wang L. J Org Chem. 2009;74:4364. doi: 10.1021/jo900507v.Li P, Zhang Y, Wang L. Chem–Eur J. 2009;15:2045. doi: 10.1002/chem.200802643.
- 8.For example on the Pt-catalyzed 5-exo-dig/aza cope rearrangement cascade toward indoles, see: Cariou K, Ronan B, Mignani S, Fensterbank L, Malacria M. Angew Chem Int Ed. 2007;46:1881. doi: 10.1002/anie.200604026.
- 9.For selected reviews on palladium catalyzed 5-endo-dig cyclizations into indoles, see: Cacchi S, Fabrizi G. Chem Rev. 2005;105:2873. doi: 10.1021/cr040639b.Lautens M, Alberico D, Bressy C. Pure Appl Chem. 2006;78:351.
- 10.Yu L, Ukhin, Komissarov VN, Orlova GI, Borodkin GS, Lindeman SV, Khrustalev VN, Struchkov Yu T. Izv Akad Nauk, Ser Khim. 1995:2236. [Google Scholar]
- 11.Apparently, CuCl is more efficient catalyst for the assembly of propargylamine 4 from 2-aminobenzaldehyde, secondary amine, and terminal alkyne; while Cu(OTf)2 is more active catalyst for the following 5-exo-dig cyclization of 4 into indoline 5 (Scheme 1).
- 12.For desilylation of TMS-alkynes during cycloisomerizations, see for example: Larock RC, Yum EK, Refvic MD. J Org Chem. 1998;63:7652.Crewley ML, Goljer I, Jenkin DJ, Mehlmann JF, Nogle L, Dooley R, Mahaney PE. Org Lett. 2006;8:5837. doi: 10.1021/ol062424p. See also ref. 6a.
- 13.For analogous base-assisted isomerization of dihydrofuranes into furanes, see: Tiecco M, Testaferri L, Tingoli M, Marini F. J Org Chem. 1993;58:1349.
- 14.Nyasse B, Grehn L, Ragnarsson U. Chem Commum. 1997:1017. [Google Scholar]
- 15.For synthesis of propargylamines via enantioselective TCC reaction, see: Li CJ, Wei C. Chem Comm. 2002:268. doi: 10.1039/b108851n.Gommermann N, Koradin C, Polborn K, Knochel P. Angew Chem Int Ed. 2003;42:5763. doi: 10.1002/anie.200352578.Knöpfel TF, Aschwanden P, Ichikawa T, Watanabe T, Carreira EM. Angew Chem Int Ed. 2004;116:6097. doi: 10.1002/anie.200461286.Bisai A, Singh VK. Org Lett. 2006;8:2405. doi: 10.1021/ol060793f.
- 16.For a recent review on enatioselective addition of alkynes to carbonyl compounds, see: Trost BM, Weiss AH. Adv Synth Catal. 2009;351:963. doi: 10.1002/adsc.200800776.
- 17.a) Trost BM, Van Vranken DL, Bingel C. J Am Chem Soc. 1992;114:9327. [Google Scholar]; b) Trost BM, Machacek MR, Aponick A. Acc Chem Res. 2006;39:747. doi: 10.1021/ar040063c. [DOI] [PubMed] [Google Scholar]; c) Trost BM, Crawley ML. Chem Rev. 2003;103:2921. doi: 10.1021/cr020027w. [DOI] [PubMed] [Google Scholar]; d) Lu Z, Ma S. Angew Chem Int Ed. 2008;47:258. doi: 10.1002/anie.200605113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.