Abstract
Background
Previous reports in adults have suggested that the effects experienced after cannabis use can be described in terms of positive and negative subtypes that are heritable and are associated with abuse and dependence. This study extends existing research by inclusion of adolescents and young adults in an offspring of twins design which makes it possible to take into account genetic and environmental risks for substance use disorder.
Methods
Data were collected from 725 twin members of the Vietnam Era Twin Registry, 839 of their 12–32 year old biological offspring and 427 mothers. Offspring who had ever used cannabis (n = 464) were asked the degree to which they typically experienced 13 subjective effects shortly after using cannabis. Latent class analysis (LCA) was used to derive subjective effect classes and logistic regression models were computed to test associations between subjective effect class and heavy cannabis use, abuse and dependence after adjusting for familial risk and psychopathology and sociodemographics.
Results
The best fitting LCA model included 4 classes of responders which were characterized as ‘high responders’ (39%), ‘positive responders’ (28%), ‘mixed/relaxed’ (22%), and ‘low responders’ (11%). Compared to low responders, members of other classes were heavier users (OR range 3.0–11.8). Compared to mixed/relaxed responders and positive responders, high responders were more likely to have cannabis abuse and dependence.
Conclusions
Subjective reactions to cannabis use are associated with use to heavy use, abuse and dependence in adolescents and young adults. This association exists above and beyond the genetic vulnerability for problem cannabis use.
Keywords: Cannabis, Twin, Offspring, Subjective effects
1. Introduction
Subjective responses to drugs have been shown to predict later heavy use and abuse. The majority of research in this field has focused on responses to alcohol and nicotine. There are considerable data suggesting that lower response to alcohol is correlated with familial risk and is predictive of long-term heavy drinking patterns and problem use (Schuckit et al., 1996, 2006). In contrast, meta-analyses of acute subjective reactions to nicotine intake support the hypothesis that the nicotine high may be the re-enforcing effect that partly explains continued smoking (Kalman and Smith, 2005). As compared to the abundant number of controlled experiments of subjective reactions to nicotine and alcohol, less is known about short-term subjective responses to cannabis in adolescent and young adults and their association with use and abuse/dependence.
In a comprehensive review, Iversen (2003) notes that the association of cannabis dependence and schizophrenic traits may be due to very extreme reactions to cannabis following ingestion of large amounts of the drug. This can result in auditory hallucinations, delusional thoughts, changed perception and blunted emotion. Yet it is unlikely that heavy cannabis use and risk of abuse and dependence are consequences of such marked reactions to cannabis. More likely, the rewarding effects of cannabis, such as euphoria, may explain continued use and progression to problem use, abuse and dependence. Rewarding properties may also partly explain the association between cannabis dependence and affective disorders in the rubric of self-medication (Iversen, 2003). In population based cohorts, increased risk of dependence has been associated with self-reported positive effects of cannabis use (Fergusson et al., 2003; Lyons et al., 1997). In a factor analysis of data from adult Vietnam Era Twin Registry (VETR) male twins, Lyons et al. (1997) derived a positive and a negative subjective effect factor. The positive factor was associated with longer duration of use, and the negative factor was correlated with shorter duration and lower frequency of cannabis use. Lyons also reported that these subjective effect factors were heritable in that 25% of the variance for each factor was due to genetic factors. We have recently reported results of a latent class analysis (LCA) of subjective responses to cannabis in the same sample of adult twins from the VETR (Grant et al., 2005), which indicated that both the negative and positive subjective effects were associated with cannabis abuse and dependence (Grant et al., 2005). While we identified classes of low and high responsiveness, our LCA results suggested that among twins who used cannabis five or more times, all had some level of subjective response to the drug. However, only twins using cannabis more than 5 times were asked about subjective effects; twins using less frequently were not queried about the effects they experienced. Therefore previous studies with the adult members of the VETR may be biased because twins that used very little cannabis may have done so because of adverse or negative reactions to the drug.
Though Grant et al. (2005) and Lyons et al. (1997) have provided empirical evidence for an association between reactions to cannabis and abuse and dependence, this existing literature was limited by the long recall period between use and reporting on subjective response, and could have been biased by omission of those who had only experimented with cannabis (i.e. users who took cannabis 5 or fewer times). The present analysis advances this line of research by utilizing a novel offspring of twins’ design which allowed us to adjust for the genetic and environmental contributions to cannabis use disorders. The present study used data from offspring of VETR twins with and without a history of drug abuse/dependence. Our current study had the following objectives: (1) determine if statistically significant and meaningful classes of subjective effects of cannabis use exist in adolescents and young adults who have ever used cannabis; (2) test if these classes are associated with the number of times cannabis was used and with lifetime DSM-IV cannabis abuse and dependence; (3) test if the associations between classes of subjective effects and cannabis outcomes remain after adjusting for genetic and family environmental influences, psychopathology and sociodemographic variables.
2. Methods
2.1. Subjects
2.1.1. Sample description
Participants were offspring of male twins who were members of the Vietnam Era Twin Registry (VETR). The VETR is a national registry of male, monozygotic (MZ) and dizygotic (DZ), like-sex twin pairs that served in the military during the Vietnam Era (1965–1975). Construction of the registry and method of determining zygosity have been previously reported (Eisen et al., 1987, 1989; Henderson et al., 1990). Additional information regarding the VETR can be obtained at http://www.eric.seattle.med.va.gov/vetr/home.html.
Data used to select the families studied in the present project were based on information reported by the twin fathers in 1987 and 1992. In 1987, a mailed questionnaire sent to the twins was used to obtain birth dates of their children, and in 1992 the Diagnostic Interview Schedule (DIS) (Robins et al., 1989) was administered by telephone to the twins to obtain their history of DSM-III-R drug dependence and other psychiatric diagnoses. In 2002, we began an offspring of twins study (OOT) focused on understanding genetic and environmental influences on the intergenerational transmission of illegal drug dependence. Criteria for selection of the twins into the OOT study included being a member of a twin pair where (i) both completed the 1987 questionnaire and the 1992 interview, (ii) at least one member of the pair had a child born between 1973 and 1987, and (iii) either one or both members of a twin pair met criteria for DSM-III-R lifetime drug dependence based on 1992 interview data, or both members of a pair were controls because neither met criteria for DSM-III-R lifetime drug dependence based on 1992 interview data.
2.2. Assessments—fathers, mothers, and offspring
Details of the contents of the paternal, maternal and offspring interview have been previously reported (Duncan et al., 2008; Scherrer et al., 2008). In brief, the 2003–04 telephone interviews with the father included family composition, sociodemographic information, smoking history and lifetime drinking history and a brief review of their drug use history. Permission to contact the biological mother of his offspring as well as the offspring themselves was also obtained during the father interview. The maternal interview, conducted by telephone in 2003–04, covered her own DSM-IV history of drug abuse/dependence and other psychiatric disorders via an adapted version of the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA) (Bucholz et al., 1994).
The offspring interviews, conducted between 2003 and 2004 were also based on a modification of the SSAGA (Bucholz et al., 1994 as adapted for use by the Midwest Alcoholism Research Center), and included demographic and family information as well as items that operationalized DSM-IV lifetime criteria for alcohol abuse and dependence, drug abuse and dependence, nicotine dependence, major depression, conduct disorder, generalized anxiety disorder, panic disorder, and social phobia.
Experienced staff from the Institute for Survey Research (ISR) at Temple University conducted data collection in 1987, 1992 and 2003–04. Interviewers were blind to subjects’ family history of substance use and gave equal effort to recruitment of all respondents. All participants gave verbal consent prior to being interviewed, as approved by the Institutional Review Board at the participating universities and the Seattle VA where the VETR is maintained. In addition, at least one parent provided written consent for their minor offspring to be interviewed.
2.2.1. Subjective effects to cannabis
As part of our survey on illegal substance use, subjective effects of cannabis were asked of all offspring who reported using cannabis one or more times. The subjective effect questions were based on items in the 1992 interview that were previously found to be useful in identifying subtypes of cannabis users in the twin fathers (Grant et al., 2005). For the present study, these items were improved by asking how the drug typically made them feel shortly after using cannabis as compared to whether the effect ever happened shortly after using the drug. The following 13 subjective effects were queried: (1) depressed/uninterested in things, (2) paranoid, (3) confused, (4) unable to concentrate, (5) so keyed up/overactive that it bothered you, (6) lazy, (7) drowsy, (8) energetic/ alert, (9) overconfident, (10) feeling very good/on top of the world, (11) creative/insightful; (12) sociable, and (13) relaxed/mellow/peaceful. The response to questions included ‘Almost Always, Quite a bit, A little and Not at all’. Because the response distribution was highly skewed, the responses were dichotomized, with: “almost always”, “quite a bit”, “a little” constituting one level and “not at all” the other.
2.3. Response rates and non-response bias
A total of 527 twin pairs were targeted for the study. After removing deceased individuals and those who had withdrawn from the Registry, the VETR released 455 pairs to the study (910 individuals). 15 of the 910 fathers targeted for data collection were incarcerated, too ill to participate or deceased. Eighty-one percent (n = 725) of the remaining 895 twins were interviewed. In the paternal interview, permission was obtained to contact the biological mother of his offspring as well as the offspring themselves. Of the 604 mothers identified, 444 completed the eligibility screen and 427 mothers (71%) were interviewed. Mothers did not have to be interviewed in order to recruit offspring, and only mothers who participated in rearing the offspring were included in the present analyses. Fathers gave consent to contact 950 offspring of whom 839 (88%) were interviewed. Of the 839 offspring interviewed, maternal interview data were available for 668 offspring (80%).
Analyses of non-response bias have been previously reported in detail (Duncan et al., 2008; Scherrer et al., 2008). Briefly, fathers with less than a high school education were significantly (p < 0.001) less likely to respond (56.4%) compared to those with only a high school education (69.8%) or more than a high school education (80.2%).We observed a significantly higher response rate (p < 0.05) for fathers with a history of any illicit drug dependence (79.1%) compared to those without such a history (73.6%). Mothers not currently married to the biological father and offspring whose fathers were not currently married to their biological mothers were significantly (p < 0.001) less likely to respond. In addition, women who were married to a twin father of an ethnic minority were also less likely to be interviewed (p < 0.001).
2.4. Data analysis
2.4.1. Latent class analysis (LCA)
LCA (McCutcheon, 1987) is a statistical method for finding mutually exclusive subsets (latent classes) of subjects based on their responses to a set of categorical or indicator items. In this sense, LCA may be thought of as a parametric clustering and data reduction technique. The underlying assumption is that the observed association among the indicators is due to an unobserved latent variable with a finite number of mutually exclusive classes. Parameters produced in an LCA model include the prevalence of each latent class, i.e. the probability that a randomly chosen individual is a member of a given class, and probabilities of endorsing individual items (item endorsement probabilities, or IEPs), given membership in a class. In the present analyses, subjects were assigned to the class with the highest posterior probability of membership based on their symptom profile. We used the Bayesian information criterion (BIC) to determine the best fitting model. Because LCA models are not nested within each other, parsimony indices such as the BIC are generally the preferred method for comparison of different models (Li and Nyholt, 2001). Models were computed using Latent Gold v.4.0 (Vermont and Magidson, 2005).
2.4.2. Regression analyses
2.4.2.1. Outcome variables
The outcome variable in these analyses was latent class assignment based on the patterns of reported subjective effects.
2.4.2.2. Predictor variables
2.4.2.2.1. The 4-group paternal risk design variable
The 4-group paternal risk design variable, which reflected the sample ascertainment strategy based on the father’s and father’s co-twin’s lifetime drug dependence status, was included in all multivariate analyses, regardless of its significance. The 4-group design also accounted for degree of familial risk for cannabis abuse and dependence. Group 1 offspring had fathers with a lifetime diagnosis of DSM-III-R illicit drug dependence (not limited to cannabis), regardless of zygosity, and therefore, offspring in Group 1 were at high genetic (HG) risk because of their father’s history of drug dependence and at high environmental (HE) risk because they experienced the rearing environment associated with having a drug dependent father (i.e. HG–HE). Offspring in Group 2 had fathers who were not affected with drug dependence but had uncles (MZ co-twin of father) with drug dependence. Offspring in Group 3 had fathers without drug dependence but had uncles (DZ co-twin of father) with drug dependence. Therefore, offspring in Group 2 were at high genetic risk because they were the progeny of unaffected MZ twins whose identical co-twins were affected and these twin pairs share 100% of their genes. Offspring in Group 3 were at medium genetic (MG) risk because they were the progeny of unaffected DZ twins whose fraternal co-twin was affected and these twin pairs share, on average, 50% of their genes. Both Group 2 and Group 3 offspring were at low environmental (LE) risk because the offspring were not reared by a father who had a history of drug dependence. Thus Group 2 offspring were at HG–LE risk and offspring in Group 3 were at MG–LE risk. Offspring in Group 4 had fathers and uncles who were not affected with drug dependence. Therefore, these offspring were at low genetic (LG) and low environmental risk (LG–LE). This paternal 4-group design controlled for familial vulnerability previously found for cannabis abuse/dependence in the offspring cohort (Scherrer et al., 2008) and accounted for the sampling design.
2.4.2.2.2. Offspring cannabis use, abuse and dependence
In addition, offspring’s heaviness of cannabis use, and DSM-IV cannabis abuse and dependence diagnoses were included as predictor variables in multinomial logistic regression analyses. The inclusion of these variables allowed for the assessment of whether heaviness of use and/or problem use was associated with subjective effect profiles after adjustment for familial risk.
2.4.2.3. Covariates
Covariates were selected based on previous associations with cannabis use, abuse and dependence found in the literature and was retained in models if they were significantly associated with classes of subjective effects according to chi-square tests of association. Offspring covariates included age, gender, race, and paternal household income; conduct disorder, major depression, nicotine dependence, alcohol abuse, alcohol dependence and use of any illicit drug other than marijuana.
Maternal self-reported DSM-IV cannabis abuse/dependence was also included in the analyses to account for the genetic and environmental contribution from mothers to offspring cannabis use, abuse and dependence. In addition a dummy variable reflecting whether or not the mother was interviewed was included in the models to adjust for potential maternal non-response bias.
We computed multinomial regression models to test for an association between latent class and use, abuse and dependence of cannabis while adjusting for the genetic and environmental influences on cannabis use outcomes as well as for covariates that were significantly associated with the classes of subjective effects by chi-square tests of association adjusted for clustered data. When testing for associations between subjective effect class and heaviness of cannabis use the reference group was Class 4 (low responders). Because there were no cannabis dependent subjects in Class 4, we modeled the association between subjective effects and cannabis abuse and dependence in Classes 1 (high responders) and 2 (positive responders), using Class 3 (mixed/relaxed) as the reference group.
3. Results
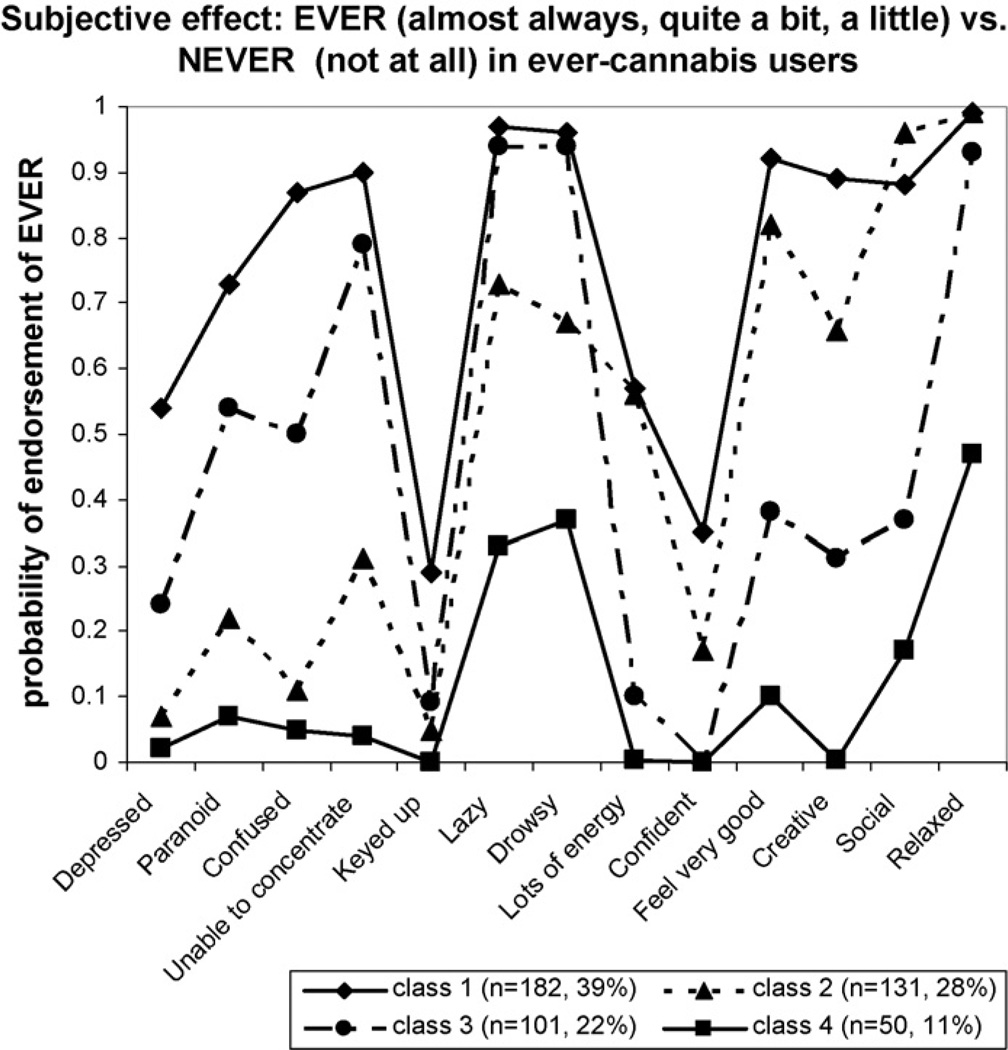
LCA indicated that a 4-class solution gave the best fit to the data, based on the smallest BIC value (6158.66) and greatest decrease in BIC value from the 1-class solution (ΔBIC = −719.7). Item endorsement probabilities from the 4-class solution are shown in Fig. 1 for each class. To aid in interpretation of the LCA, we used an endorsement probability of 0.50 or greater as a guide in determining which subjective effects characterized each class. As shown in Fig. 1, the symptom profiles provided evidence for both a severity dimension and qualitative distinctions (suggested by non-parallel profiles).
Fig. 1.
Item endorsement probabilities are shown for 13 subjective responses to cannabis among 464 adolescent and young adults who had used cannabis at least one time. Response options dichotomized into ever (almost always, quite a bit, a little) and never (not at all). Legend includes number of subjects and percent of total sample by class.
Members of Class 1 were characterized by high endorsement (>0.50) of 11 out of 13 subjective effects (Fig. 1). In contrast, subjects in Class 4 had very low endorsements for most subjective effects, with moderate endorsement of feeling relaxed, lazy and drowsy (0.33–0.47). Thus, Class 1 was labeled high responders and Class 4 low responders. Classes 2 and 3 differed on degree and type of subjective effect. Class 2 subjects had high item endorsements for the following subjective effects: feeling very good/on top of the world (0.82), creative/insightful (0.66), sociable (0.96), relaxed/mellow/peaceful, (0.99), lazy (0.73), drowsy (0.67) and energetic/alert (0.56), thereby indicating a positive subjective effect profile. Class 3 subjects reported higher endorsements for lazy (0.94), drowsy (0.94), and relaxed/peaceful/mellow (0.93), as well as high endorsements of the negative responses paranoid (0.54), confused (0.50) and unable to concentrate (0.79). Therefore, Class 2 was labeled positive responders and Class 3 as mixed/relaxed.
Associations between latent class assignment and offspring characteristics are shown in Table 1. Among sociodemographic variables, only male gender was associated with subjective response as evidenced by a lower prevalence of males in Mixed/Relaxed and Low Responder classes as compared to High and Positive Responder classes (p < 0.001). Though not statistically significant, the prevalence of conduct disorder, major depression, and alcohol dependence was highest in offspring who were members of the High Responder class, medium in the members of the Positive and Mixed/Relaxed Responder classes and lowest in the Low Responder class. The prevalence of anxiety disorders was low across all classes. Non-cannabis illegal drug use, heaviness of cannabis use, and DSM-IV cannabis abuse and dependence were all significantly associated with subjective response class (p < 0.0001), where the prevalence was highest in the members of the High Responder class, medium in the members of the Positive and Mixed/Relaxed Responder classes and lowest in the Low Responder class. Members of the High Responder class reported the greatest frequency of cannabis use (62% used more than 40 times), while members of the Low Responder class used infrequently (72% had used less than 6 times). In contrast to these classes, both Positive Responders and Mixed/Relaxed Responders were relatively equally distributed across lifetime levels of cannabis use.
Table 1.
Characteristics (%) of 464 offspring who have ever used cannabis within each latent class assignment.
| Class 1, high responder (n = 182) |
Class 2, positive responders (n = 131) |
Class 3, mixed/relaxed (n = 101) |
Class 4, low responder (n = 50) |
Test of significancea, p-value |
|
|---|---|---|---|---|---|
| 4-Group designb | |||||
| Group 1 HG–HE | 70.3 | 62.6 | 67.3 | 62.0 | |
| Group 2 HE–LE | 9.9 | 9.2 | 5.9 | 14.0 | 0.50 |
| Group 3 MG–LE | 6.0 | 13.0 | 10.9 | 6.0 | |
| Group 4 LG–LE | 13.7 | 15.3 | 15.8 | 18.0 | |
| Maternal cannabis abuse/dependence | 7.7 | 9.9 | 10.9 | 6.0 | 0.73 |
| Maternal data missing | 16.5 | 22.1 | 18.8 | 30.0 | 0.20 |
| Age (mean years ± S.D.) | 23.1 ± 3.8 | 23.8 ± 3.7 | 23.7 ± 3.7 | 24.3 ± 3.7 | 0.17 |
| Age first cannabis use (mean years ± S.D.)c | 15.4 ± 2.4 | 15.8 ± 2.7 | 15.5 ± 2.0 | 15.4 ± 0.84 | 0.66 |
| Male | 61.5 | 57.3 | 42.6 | 36.0 | 0.001 |
| White race | 81.6 | 69.8 | 76.8 | 77.6 | 0.12 |
| Paternal household income | |||||
| ≤20,999 | 4.4 | 8.4 | 4.0 | 2.0 | |
| 21,000–99,999 | 78.6 | 74.8 | 79.0 | 80.0 | 0.66 |
| >100,000 | 17.0 | 16.8 | 17.0 | 18.0 | |
| Offspring lifetime DSM-IV psychiatric disorders | |||||
| Conduct disorder | 17.0 | 13.0 | 11.9 | 4.0 | 0.11 |
| Major depression | 17.6 | 15.3 | 14.9 | 10.0 | 0.61 |
| Generalized anxiety disorder | 5.0 | 6.9 | 3.0 | 4.0 | 0.57 |
| Panic disorder | 2.8 | 3.8 | 5.0 | 4.0 | 0.84 |
| Social phobia | 2.8 | 1.5 | 0 | 2.0 | 0.39 |
| Offspring drug use and DSM-IV abuse/dependence: | |||||
| Nicotine dependence | 25.0 | 24.4 | 16.0 | 14.3 | 0.19 |
| Alcohol abuse/dep. | |||||
| No diagnosis | 39.6 | 51.9 | 47.5 | 52.0 | |
| Abuse | 26.4 | 28.2 | 30.7 | 26.0 | 0.10 |
| Dependence | 34.1 | 19.9 | 21.8 | 22.0 | |
| Other non-cannabis illegal drug use (ever) | 72.5 | 51.9 | 57.4 | 36.0 | <0.0001 |
| Cannabis use (lifetime) | |||||
| <6 times | 14.8 | 26.7 | 32.7 | 72.0 | |
| 6–40 times | 23.1 | 37.4 | 34.7 | 10.0 | <0.0001 |
| >40 times | 62.1 | 35.9 | 32.7 | 18.0 | |
| Cannabis abuse/dep. | |||||
| No diagnosis | 40.1 | 67.2 | 68.3 | 98.0 | |
| Abuse | 39.0 | 28.2 | 30.7 | 2.0 | <0.0001 |
| Dependence | 20.9 | 4.6 | 1.0 | 0 | |
Tests of significance are ANOVA for continuous variables and chi-square for categorical variables, robust variance estimate adjusted for clustered family data.
4-Group design: Group 1—offspring at high genetic (HG) and high environmental (HE) risk because fathers are MZ and DZ twins with DSM-III-R illicit drug dependence, Group 2—offspring at high genetic (HG) and low environmental (LE) risk because fathers are unaffected MZ twins with DSM-III-R illicit drug dependent co-twins, Group 3—offspring at medium genetic (MG) and LE risk because fathers are unaffected DZ with DSM-III-R illicit drug dependent co-twins, Group 4—offspring at low genetic and LE because fathers are unaffected MZ and DZ control pairs.
Age of first use available if cannabis used 5 or more times.
Results of multinomial regression models computed to test for associations between heaviness of cannabis use and subjective effect classes are shown in Table 2. As compared to members of the Low Responder class (Class 4), there was a trend for members of the High Responder class to be younger (OR = 0.93; 95%CI: 0.85–1.01). Offspring in the High Responder (OR = 2.30; 95%CI: 1.10–4.81) and Positive Responder (OR = 2.28; 95%CI: 1.05–4.98) classes were significantly more likely to be male. The High, Positive and Mixed/Relaxed Responder classes were all significantly more likely than the Low Responders to have used cannabis 6–40 times (OR range 6.99–10.71), or 41 or more times (OR range 3.02–11.78). In addition, post hoc contrasts indicated that the odds of using cannabis 41 or more times were significantly greater among High as compared to Positive and Mixed/Relaxed Responders (p < 0.05).
Table 2.
Multinomial logistic regression for the association (OR, 95%CI) between psychiatric disorders and heaviness of cannabis use and classesa of subjective effects to cannabis among 464 offspring who used cannabis at least once.
| Class 1 (high responder) vs. Class 4 (low responder) |
Class 2 (positive responder) vs. Class 4 (low responder) |
Class 3 (mixed/relaxed) vs. Class 4 (low responder) |
|
|---|---|---|---|
| Familial risk | |||
| 4-Group designb | |||
| Group 1 HG–HE | 0.87 (0.30–2.51) | 0.78 (0.28–2.18) | 0.84 (0.32–2.17) |
| Group 2 HE–LE | 0.55 (0.13–2.38) | 0.67 (0.14–3.17) | 0.38 (0.10–1.49) |
| Group 3 MG–LE | 1.00 (0.21–4.80) | 2.09 (0.48–9.13) | 1.43 (0.36–5.78) |
| Group 4 LG–LE | 1.0 | 1.0 | 1.0 |
| Maternal cannabis abuse/dependence | 0.59 (0.10–3.47) | 1.09 (0.19–6.28) | 1.22 (0.25–6.04) |
| Maternal data missing | 0.39 (0.16–0.95) | 0.58 (0.24–1.40) | 0.49 (0.19–1.24) |
| Sododemographics | |||
| Age | 0.93 (0.85–1.01) | 0.98 (0.90–1.07) | 0.97 (0.88–1.06) |
| Male gender | 2.30 (1.10–4.81)a | 2.28 (1.05–4.98)a | 1.12 (0.54–2.30)b |
| White | 1.26 (0.55–2.90) | 0.76 (0.33–1.75) | 0.94 (0.41–2.14) |
| Paternal household income | |||
| ≤20,000 | 1.84 (0.17–20.20) | 3.59 (0.42–30.58) | 1.54 (0.15–16.08) |
| 20,001–100,000 | 1.0 | 1.0 | 1.0 |
| >100,000 | 0.52 (0.21–1.33) | 0.61 (0.23–1.60) | 0.61 (0.23–1.57) |
| Used illegal drugs other than cannabis | 1.67 (0.65–4.33) | 0.98 (0.37–2.58) | 1.66 (0.64–4.35) |
| Heaviness of cannabis use | |||
| Number of times used cannabis | |||
| <6 times | 1.0 | 1.0 | 1.0 |
| 6–40 times | 10.71 (3.53–32.47) | 10.45 (3.44–31.71) | 6.99 (2.29–21.31) |
| >40 times | 11.78 (3.84–36.10)a | 5.00 (1.57–15.98)b | 3.02 (1.00–9.13)b |
a and b—post hoc contrasts: within rows, odds ratios with different letters (a and b) are significantly different, p < 0.05.
The significance is the OR and 95%CI. Readers will use the CI to interpret significance.
Class 1—high responder, Class 2—positive responders, Class 3—mixed/relaxed, Class 4—low responder.
4-Group design: Group 1—offspring at high genetic (HG) and high environmental (HE) risk because fathers are MZ and DZ twins with DSM-III-R illicit drug dependence; Group 2—offspring at high genetic (HG) and low environmental (LE) risk because fathers are unaffected MZ twins with DSM-III-R illicit drug dependent co-twins: Group 3—offspring at medium genetic (MG) and LE risk because fathers are unaffected DZ with DSM-III-R illicit drug dependent co-twins: Group 4—offspring at low genetic and LE because fathers are unaffected MZ and DZ control pairs.
Results of the multinomial regression model computed for the High, Positive, and Mixed/Relaxed Responder classes only to test the association between cannabis abuse, cannabis dependence and class assignment, are shown in Table 3. The Low Responder class was not included in this model because of the low prevalence of DSM-IV cannabis diagnosis: none of those in the Low Responder class met DSM-IV cannabis dependence criteria, and only one had DSM-IV cannabis abuse. Thus, models were run only among the other 3 classes, using as the reference group the Mixed/Relaxed Responder class. High and Positive Responders were significantly more likely to be male than the Mixed/Relaxed Responders (OR = 2.08, 95%CI: 1.24–3.48 and OR= 2.03, 95%CI: 1.17–3.50, respectively). In addition, High Responders had significantly higher rates of DSM-IV cannabis abuse and DSM-IV cannabis dependence than either the Mixed/Relaxed Responders or the Positive Responders.
Table 3.
Multinomial logistic regression for the association (OR, 95%CI) between psychiatric disorders and DSM-IV cannabis abuse/dependence and three classesa of subjective effects to cannabis among 414 offspring who used cannabis at least once.
| Class 1 (high responder) vs. Class 3 (mixed/relaxed) |
Class 2 (positive responder) vs. Class 3 (mixed/relaxed) |
|
|---|---|---|
| Familial risk | ||
| 4-Group designb | ||
| Group 1 HG–HE | 0.91 (0.38–2.19) | 0.95 (0.42–2.17) |
| Group 2 HE–LE | 1.37 (0.43–4.41) | 1.84 (0.49–6.93) |
| Group 3 MG–LE | 0.73 (0.24–2.27) | 1.53 (0.55–4.28) |
| Group 4 LG–LE | 1.0 | 1.0 |
| Maternal cannabis abuse/dependence | 0.49 (0.18–1.32) | 0.94 (0.37–2.38) |
| Maternal data missing | 0.75 (0.36–1.53) | 1.21 (0.60–2.46) |
| Sociodemographics | ||
| Age | 0.96 (0.89–1.03) | 1.01 (0.95–1.09) |
| Male gender | 2.08 (1.24–3.48) | 2.03 (1.17–3.50) |
| White | 1.67 (0.82–3.40) | 0.83 (0.45–1.54) |
| Paternal household income | ||
| ≤20,000 | 1.80 (0.55–5.90) | 2.61 (0.81–8.36) |
| 20,001–100,000 | 1.0 | 1.0 |
| ≥100,000 | 0.91 (0.44–1.87) | 1.04(0.46–2.38) |
| Used illegal drugs other than cannabis | 1.04 (0.58–1.88) | 0.64 (0.34–1.18) |
| Cannabis use disorder | ||
| No diagnosis | 1.0 | 1.0 |
| Abuse | 2.05 (1.13–3.73)a | 1.03 (0.55–1.92)b |
| Dependence | 37.13 (4.60–299.78)a | 5.76 (0.63–52.43)b |
a and b—post hoc contrasts: within rows, odds ratios with ditterent letters (a and b) are significantly different, p < 0.01.
The significance is the OR and 95%CI. Readers will use the CI to interpret significance.
Class 1—high responder, Class 2—positive responders, Class 3—mixed/relaxed.
4-Group design: Group 1—offspring at highgenetic (HG) and high environmental (HE) risk because fathers are MZ and DZ twins with DSM-III-R illicit drug dependence: Group 2—offspring at high genetic (HG) and low environmental (LE) risk because fathers are unaffected MZ twins with DSM-III-R illicit drug dependent co-twins: Group 3—offspring at medium genetic (MG) and LE risk because fathers are unaffected DZ with DSM-III-R illicit drug dependent co-twins: Group 4—offspring at low genetic and LE because fathers are unaffected MZ and DZ control pairs.
4. Discussion
In an offspring of drug dependent twin design, we used latent class analysis to characterize patterns of subjective responses to cannabis use in 464 adolescent and young adult offspring who had ever used cannabis. We identified 4 latent classes that best characterized offspring’s typical response to cannabis. Based on item endorsement probabilities, we categorized offspring into High (39% of ever users), Positive (28%), Mixed/Relaxed (22%) and Low Responders (11%). High responders had large endorsement probabilities for 11 of 13 subjective effects including positive (e.g. feeling very good/on top of the world) and negative response (e.g. paranoid) reactions. Positive Responders had very high endorsement of positive effects (relaxed/mellow/peaceful, sociable), with low endorsement of negative reactions, and differed from mixed/relaxed responders in that the latter group had high endorsement of both sedation (e.g. relaxed, lazy, drowsy) and negative subjective effects such as confusion and paranoia. Low Responders had low item endorsement probabilities on all 13 subjective effects. Subjective effect classes were also differentiated by the number of times cannabis was used.
Multinomial logistic regression results suggest members in High, Positive and Mixed/Relaxed Responder classes were all significantly more likely than the Low Responders to be heavier cannabis users. Over 60% of the High Responders had used more than 40 times, roughly 1/3 of Positive and Mixed/Relaxed Responders had used cannabis <6 times, 6–40 times, and >40 times, and 72% of Low Responders had used cannabis less than 6 times. High Responders also had significantly larger odds of DSM-IV cannabis abuse and dependence than either the Mixed/Relaxed Responders or the Positive Responders. Thus exposure to cannabis may account for some of the characteristics of High and Low Responders, but appears unlikely to explain differences in subjective response patterns of Positive and Mixed/Relaxed users.
Class assignment was not associated with familial risk for illicit drug abuse and dependence in univariate analyses. However this does not rule out that there is common genetic vulnerability to response to cannabis and abuse/dependence. Indeed,Lyons et al. (1997) estimated 25% of the variance in positive and negative subjective effects to cannabis in VETR twins (i.e. the source of fathers of the current offspring study) was due to genetic factors. Individual differences in the distribution of cannabis receptors may be the mechanism for variation in subjective effects. Zuurman et al.’s (2009) recent review of the literature suggests the mechanisms underlying subjective effects may be associated with two cannabis receptors (CB1 and CB2). Because the class profiles in the present study are similar to those previously found in the twin fathers (Grant et al., 2005), these classes may be useful biomarkers and endophenotypes. Subjective effect profiles could be used as biomarkers when studying the pharmacological benefit of cannabis and profiles could be used as endophenotypes to refine our ability to link the underlying mechanisms of cannabis dependence with specific genes.
Because alcohol dependence and other illicit drug use were associated with membership in the High Responder class, we speculate that this is evidence for a general liability to respond to substances. In our earlier work (Grant et al., 2005) we found cannabis dependence and cocaine dependence were strongly associated with high reactivity to both substances. Taken together, the present and past results indicate a liability for drug abuse and dependence that may be associated with an overall high subjective response. However we are unable to conclude the temporal direction of this effect and the association may be explained by common genetic vulnerability as previously discussed.
Our observation of a higher proportion of males in the High and Positive Responder classes suggests that there might be gender differences in reactivity. Animal studies suggest greater reactivity in females as compared to males after exposure to cannabinoids characterized by greater hypothermia, increased motor activity and higher pain tolerance in female rats as compared to male rats (Craft, 2005). However, data from the human studies literature are inconsistent. There is some evidence that women have a stronger physiological response after smoking marijuana, as indicated by a greater drop in arterial pressure and more dizziness, but there are a number of studies suggesting no gender differences when assessing level of intoxication. If “response” is comparable to “reactivity”, our data would suggest that men, not women, are more reactive. However, because we did not assess some of the reactions that were part of the previous research, we are unable to resolve this issue at this time. The mechanisms underlying gender differences and subjective effects warrant further research in a controlled laboratory paradigm.
The classes that were derived from the self-reported items are similar to responses to cannabis that have been reported in controlled laboratory settings (Hart et al., 2002). The present results are consistent with two previous studies in adult male twins (the fathers of the offspring in the current study). While cross-sectional data do not resolve temporal relationships, our current evidence that high responders are more likely to have cannabis dependence is consistent with Fergusson et al.’s (2003) observation in the longitudinal Christchurch Health and Development Study that a greater number of positive subjective effects at ages 14–16 was associated with increased risk of dependence by age 21.
4.1. Strengths
We have strengthened previous analyses of subjective effects in adult twins in several ways. By collecting data from a younger cohort with more recent cannabis use we have limited recall bias. The VETR twins were only asked for their subjective response if they had used cannabis a minimum of 5 times, thus limiting the range of profiles that could be detected if initial adverse reactions limited continued cannabis use. The present study did not require a minimum number of times used as a threshold to query subjective effects. Our data suggest that the lack of response to cannabis, rather than an aversive reaction, is most closely associated with less use. Offspring who had only positive responses were not at significantly greater risk of abuse or dependence and had a similar number of lifetime cannabis exposures as compared to those who had mixed/relaxed responses (which included negative effects).
4.2. Limitations
The present results should be interpreted in light of several limitations.
First, we did not measure the amount of tetrahydrocannabinol (THC) consumed. Because some evidence suggests that THC concentrations in cannabis have increased over time in the U.S. (Newell, 2003), the subjective reactions experienced by this cohort may vary in unknown ways from those who used cannabis 10–20 years ago. Second, not all offspring in the current study were past the age of risk for cannabis abuse and dependence. However, we did not observe differences across subjective effect classes in either age at interview or age at initiation, which suggests that age is unlikely to bias the associations that we have reported. In addition, a typical response to cannabis in the present young cohort may not be typical for much older, middle aged, cohorts who have used cannabis for decades. Because the sample is predominantly White, with married parents who have relatively high educational attainment, results may not generalize to non-White populations or offspring of divorced fathers with lower educational attainment. Missing data from maternal non-response is a potential limitation, but the association between subjective effect and heaviness of use, abuse and dependence remained after controlling for missing maternal data. Last, as with any research, unmeasured variables may contribute to the association between subjective effects and problem cannabis use.
4.3. Conclusions and public health implications
The subjective response to cannabis has previously been shown to be heritable and may partly explain why some persons progress beyond occasional use to heavy use, abuse and dependence (Lyons et al., 1997). We can conclude that cannabis use, abuse and dependence are associated with the type of subjective response even after adjusting for genetic influence, environmental factors, sociodemographics and psychiatric covariates. Longitudinal data currently being collected will help determine if subjective response to cannabis predicts new onset of abuse and dependence. Such evidence will help resolve the temporal relationships between subjective effects and use, abuse and dependence and greatly inform where public health efforts ought to be focused.
Acknowledgments
The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin Registry (VETR). Numerous organizations have provided invaluable assistance in the conduct of this study, including: Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; National Opinion Research Center; National Research Council, National Academy of Sciences; the Institute for Survey Research, Temple University. Most importantly, the authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Role of funding source
Funding for this study was provided by NIH grants DA14363, AA11998 and AA13717 and by the Department of Veterans Affairs Health Services Research and Development Service and the Cooperative Studies Program (Study 992). Dr. Scherrer is also supported by a Career Development Award-2 from the Department of Veterans Affairs Health Services Research and Development Service. The NIDA, NIAAA and VA HSR&D had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Contributors
Authors Scherrer, Grant, Duncan, Haber, Jacob and Bucholz designed the study and wrote the protocol. Author Scherrer managed the literature searches and summaries of previous related work Authors Grant, Scherrer and Bucholz undertook the statistical analysis. Authors Scherrer, Grant, Duncan, Sartor, Haber, Jacob and Bucholz wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J. Stud. Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in behavioral effects of cannabinoids. Life Sci. 2005;77:2471–2478. doi: 10.1016/j.lfs.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Duncan AE, Sartor CE, Scherrer JF, Grant JD, Heath AC, Nelson EC, Jacob T, Bucholz KK. The association between cannabis abuse and dependence and childhood physical and sexual abuse: evidence from an offspring of twins design. Addiction. 2008;103:990–997. doi: 10.1111/j.1360-0443.2008.02210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen S, Neuman R, Goldberg J, Rice J, True W. Determining zygosity in the Vietnam Era Twin Registry: an approach using questionnaires. Clin. Genet. 1989;35:423–432. doi: 10.1111/j.1399-0004.1989.tb02967.x. [DOI] [PubMed] [Google Scholar]
- Eisen S, True W, Goldberg J, Henderson W, Robinette CD. The Vietnam Era Twin (VET) Registry: method of construction. Acta Genet. Med. Gemellol. (Roma) 1987;36:61–66. doi: 10.1017/s0001566000004591. [DOI] [PubMed] [Google Scholar]
- Fergusson DM, Horwood LJ, Lynskey MT, Madden PAF. Early reactions to cannabis predict later dependence. Arch. Gen. Psychiatry. 2003;60:1033–1039. doi: 10.1001/archpsyc.60.10.1033. [DOI] [PubMed] [Google Scholar]
- Grant JD, Scherrer JF, Lyons MJ, Tsuang M, True WR, Bucholz KK. Subjective reactions to cocaine and marijuana are associated with abuse and dependence. Addict. Behav. 2005;30:1574–1586. doi: 10.1016/j.addbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Hart CL, Haney M, Ward AS, Fischman MW, Foltin RW. Effects of oral THC maintenance on smoked marijuana self-administration. Drug Alcohol Depend. 2002;67:301–309. doi: 10.1016/s0376-8716(02)00084-4. [DOI] [PubMed] [Google Scholar]
- Henderson WG, Eisen S, Goldberg J, True WR, Barnes JE, Vitek ME. The Vietnam Era Twin Registry: a resource for medical research. Public Health Rep. 1990;105:368–373. [PMC free article] [PubMed] [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Kalman D, Smith SS. Does nicotine do what we think it does? A meta-analytic review of the subjective effects of nicotine in nasal spray and intravenous studies with smokers and nonsmokers. Nicotine Tob. Res. 2005;7:317–333. doi: 10.1080/14622200500125385. [DOI] [PubMed] [Google Scholar]
- Li W, Nyholt DR. Marker selection by Akaike information criterion and Bayesian information criterion. Genet. Epidemiol. 2001;21(Suppl. 1):S272–S277. doi: 10.1002/gepi.2001.21.s1.s272. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Toomey R, Meyer JM, Green AI, Eisen SA, Goldberg J, True WR, Tsuang MT. How do genes influence marijuana use? The role of subjective effects. Addiction. 1997;92:409–417. [PubMed] [Google Scholar]
- McCutcheon AL. Latent Class Analysis. Newbury Park, CA: SAGE Publications; 1987. [Google Scholar]
- Newell CJ. Potency of cannabis seized in central Florida during June 2002. Microgram J. 2003;1:37–39. [Google Scholar]
- Robins LN, Helzer JE, Cottler LB, Goldring E. The Diagnostic Interview Schedule, Version III-R. St. Louis, MO: Washington University; 1989. [Google Scholar]
- Scherrer JF, Grant JD, Duncan AE, Pan H, Waterman B, Jacob T, Haber JR, True WR, Heath AC, Bucholz KK. Measured environmental contributions to cannabis abuse/dependence in an offspring of twins design. Addict. Behav. 2008;33:1255–1266. doi: 10.1016/j.addbeh.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tsuang JW, Anthenelli RM, Tipp JE, Nurnberger JI., Jr Alcohol challenges in young men from alcoholic pedigrees and control families: a report from the COGA project. J. Stud. Alcohol. 1996;57:368–377. doi: 10.15288/jsa.1996.57.368. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Pierson J, Danko GP, Beltran IA. Relationships among the level of response to alcohol and the number of alcoholic relatives in predicting alcohol-related outcomes. Alcohol Clin. Exp. Res. 2006;30:1308–1314. doi: 10.1111/j.1530-0277.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Vermont JK, Magidson J. Latent Gold 4.0 User’s Guide. Belmont, MA: Statistical Innovations, Inc.; 2005. [Google Scholar]
- Zuurman L, Ippel AE, Moin E, van Gerven JMA. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br. J. Clin. Pharmacol. 2009;67:5–21. doi: 10.1111/j.1365-2125.2008.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]