Abstract
Concern for the impact of prenatal cocaine exposure (PCE) on human language development is based on observations of impaired performance on assessments of language skills in these children relative to non-exposed children. We investigated the effects of PCE on speech processing ability using event-related potentials (ERPs) among a sample of adolescents followed prospectively since birth. This study presents findings regarding cortical functioning in 107 prenatally cocaine-exposed (PCE) and 46 non-drug-exposed (NDE) 13-year-old adolescents. PCE and NDE groups differed in processing of auditorily presented non-words at very early sensory/phonemic processing components (N1/P2), in somewhat higher-level phonological processing components (N2), and in late high –level linguistic/memory components (P600). These findings suggest that children with PCE have atypical neural responses to spoken language stimuli during low-level phonological processing and at a later stage of processing of spoken stimuli.
Keywords: prenatal cocaine exposure, adolescence, risk, event-related potential
1. Introduction
Evidence from both human and animal research indicates that exposure to cocaine during gestation alters the development of neural systems, particularly the monoaminergic systems (dopamine, norepinepherine and serotonin) involved in cortical development (Bhide, 2009; Lester & Padbury, 2009; Mayes, 1994). In addition, a significant body of research has now identified impaired performance in PCE relative to NDE children across a number of cognitive tasks at multiple age points. Deficits have been identified in attentional processing, executive function, spatial learning and reaction times (Alessandri et al. 1993; Heffelinger et al. 1997; Savage et al. 2005; Schroder et al. 2004). One of the most consistent findings in the literature on prenatal cocaine exposure (PCE) is impairment across a variety of language tasks, suggesting that cocaine alters development of neurobiological systems responsible for language processing. Specific language deficits that have been linked to PCE include: deficits in speech processing, deficits in expressive language ability and semantic processing, and general language delay (e.g., Cone-Wesson, 2005; Delaney-Black et al. 2000; Malakoff et al. 1999; Singer et al. 2001;Bandstra et al. 2002; Bandstra, Vogel, Morrow, Xue, & Anthony, 2004). Despite this often-identified relationship between PCE and impaired language function, the specific locus of these deficits has not been identified. Moreover, not all studies find significant differences between exposed and unexposed children; Frank et al. (Frank, Augustyn, Grant Knight, Pell, & Zuckerman, 2001) performed a meta analysis of 36 studies of physical and cognitive development and did not find evidence of deficits in expressive or receptive language skills (or any cognitive skills measured) in children 6 years and younger after other variables such as SES and other substance exposure were taken into account. More recent studies, however, have found significant effects of cocaine exposure after controlling for these additional variables (e.g., Bandsta et al. 2004).
One limitation of the majority of the existing research on language function in PCE children and adolescents is that it is almost entirely limited to performance on standardized tests, which assess broadly defined cognitive skills but do not necessarily permit assessment of the underlying component processes supporting cognition. Reliance on standardized tests alone may be particularly problematic when evaluating individuals from low SES backgrounds (the majority of exposed children) because these tests are often normed on higher SES populations. Direct measures of neurocognitive functioning are likely to be more effective for identifying cognitive processes affected by disease or exposure to neurotoxins. Specifically, cognitive neuroscience methods such as fMRI and EEG/ERP can identify where and when (respectively) in the brain a specific cognitive skill is impacted. To date, no studies have examined language function in PCE children or adolescents at the level of neural activity. Behavioral findings may be mixed because they are not sensitive enough to underlying subtle differences in linguistic processing. To this end, we examined auditory non-word processing among adolescents exposed to cocaine prenatally with an event related potential (ERP) design.
Auditory non-word processing was chosen because it allows for examination of the sensitivity to the building blocks (phoneme combinations) of word learning and vocabulary development that are not already linked to semantic representations (e.g., Saffran et al. 1999). Moreover, examination of these relatively low-level non-word processing skills are not confounded with language experience, which may be impoverished in low SES children independent of PCE. Abnormality in processing of auditory non-words could indicate residual deficits in phonological processing, which may be responsible for the observed poorer performance in some speech and language tasks observed in PCE children. We also chose to use an old/new paradigm (presenting one series of non-words in the first block and then adding a second non-word to the second block, intermixing the two); this was chosen to allow for an examination of phonological memory processes, which are also critical in the formation of lexical representations.
ERPs have been widely used to study spoken language and printed language processing and have well defined components associated with particular aspects of speech and language processing. Early components including the N1/ P2 complex are sensitive to basic auditory processing (such as tone frequency and pitch) as well as phonemic and phonological processing (such as phoneme category and rhyme). Later components have been shown to be sensitive to higher level processing demands, such as semantics (N400) and syntax/ pragmatics (P600 ELAN), (see Kutas & Van Petten, 1994; Kutas, Van Petten, & Kluender, 2006 for an in-depth review of language processing components). In addition to these “classic” language components, additional task specific components that may be sensitive to speech and language processing depending on the study design have also been identified (e.g., the mismatch negativity or MMN).
In the current investigation we examined neural response to repeated and non-repeated spoken non-words using a task modeled after (Molfese, Morse, & Peters, 1990). Specifically, we auditorily presented two rhyming non-words (“bidu” and “gibu”) in an old/new design, presenting a block of bidu trials followed by a mixed block of randomly presented bidu and gibu trials (as described below). We were interested in both “lower-level” phonemic / phonological processing, associated with the N1/P2 complex and the P200 and N200 as well as “higher-level” components that would be sensitive to repetition of non-words (comparing old/new), such as the repetition sensitive P600-like component identified by Curran (1999a). We predicted that if PCE children have deficits in the lowest-level aspects of speech perception, then we would observe reduced N1/P2 complex amplitudes in this group compared to NDE children (for both old and new items). However, if exposed children do not have difficulty with the lowest-level perceptual aspects of speech processing, but instead have difficulty with somewhat higher level representation of linguistic units (phonology, rhyme), we would only expect altered responses to repeated relative to unrepeated items later in processing (possibly reflecting differences in phonological encoding or retrieval). A deficit in both high-level and low-level aspects of language processing in this task would be evidenced as abnormal early and late component response to stimuli, for both repeated and unrepeated items.
2. Material and Methods
Participants
Participants were a sub-sample of a larger sample of adolescents involved in a 16 year longitudinal study on the effects of fetal cocaine exposure on physical, cognitive, emotional, and social development. The full sample consists of 523 children, including both exposed children and non-exposed controls who were recruited at birth and returned for at least one visit in their first year, with children and families seen biannually thereafter. The two groups were initially selected to be of similar SES and racial background. Families were initially recruited when they sought prenatal care at the Yale-New Haven Hospital or when they were admitted to the postpartum ward in the case of no prenatal care. Prenatal cocaine exposure was determined by a combination of maternal report, urine toxicology in the prenatal or postpartum period, and meconium toxicology. We have maintained contact with 78% of the originally recruited cohort with no selective attrition between the cocaine-exposed (21.4% lost) and non-drug-exposed (24.4% lost).
The sub-sample that completed our ERP study consisted of 107 adolescents (51 females and 56 males) who were exposed to cocaine and other drugs prenatally (PCE group) and 46 non-drug exposed adolescents who were not exposed to cocaine or other drugs prenatally (NDE group, 19 females and 27 males). These participants were randomly selected from the longitudinal cohort, PCE participants were oversampled to ensure stable results given the potential for more subject to subject variability in many clinical populations (e.g., Dhar et al 2010). The PCE group includes 90.6% African American participants, 0.9% Latino participants, 2.8% Pacific Islander participants and 5.70% Caucasian participants. The NDE group includes 67.4 % African American participants 8.7% Pacific Islander participants and 23.9 % Caucasian participants. Most participants were right handed (N= 130) and a few participants were left–landed (N= 16) (with left handed participants distributed in equal proportion across the groups). All participants participated in the EEG experiment and took an IQ assessment within three months of their 13th birthdays. The two groups that participated in the ERP study did not differ on the verbal or performance IQ composites (measured by the Kaufman Brief Intelligence Test, KBIT), but they did differ on the mathematical reasoning subtest (t = 2.83, p <.01), with exposed children performing more poorly on this subtest. All participants had normal hearing (−20dB to +20dB) as measured by an audiometer. At the time of ERP testing, all children were fluent in English with no evidence of serious mental illness (e.g., psychosis), head injury (based on parental report, participant report and medical records if available) or substance use. See Appendix for additional birth variables available on this sample.
Maternal education: the number of mothers in the non-exposed group that completed high school was higher than in the PCE group X2 = 8.49, p < .005 (non using mothers who completed high school N = 40, non using mothers who did not complete high school N= 6; using mothers who completed high school N= 69, using moms who did not complete high school = 38).
Maternal drug use for PCE cohort: As mentioned above, prenatal cocaine exposure was determined by a combination of maternal report, urine toxicology in the prenatal or postpartum period, and meconium toxicology. In addition to cocaine use, which rarely occurs in isolation, 75% of mothers reported using tobacco (e.g., smoked cigarettes), 71% also reported some alcohol use, and 48% reported using marijuana intermittently.
Language assessment from earlier time points: In order to provide a more thorough characterization of the language profiles of the children in our longitudinal study, we present data from the Clinical Evaluation of Language Fundamentals – II (CELF- II), which measures both expressive and receptive language skills. Not all longitudinal participants were given the CELF, thus we present data for two large subsets of the current longitudinal cohort sample for which CELF data is available, one subset with data from age 11, and one partially overlapping sample with data from age 9. These subsets represent partially overlapping but not identical samples to the ERP study sample1. We also present CELF data for ages 9 and 11 from participants in the ERP study for whom this data was available. We note that because the initial focus of the project was on more general cognitive and executive function, and not language per se, not all subtests from the CELF were administered (See Table 1 for subtests administered).
Table 1.
CELF scores ages 9 & 11, for both the full sample and the ERP sample
| Age 9 (full sample) | ||||||
|---|---|---|---|---|---|---|
| Mean PCE (N= 116) |
Mean NDE (N= 99) |
t | df | p | ||
| Word Classes | 6.98 | 7.73 | 2.08 | 214 | 0.04 | |
| Semantic Relations | 7.41 | 8.47 | 2.62 | 214 | 0.01 | |
| Receptive language composite | 83 | 88 | 2.16 | 213 | 0.03 | |
| Forming sentences | 6.59 | 7.74 | 2.76 | 214 | 0.01 | |
| Sentence assembly | 8.06 | 8.88 | 2.19 | 213 | 0.03 | |
| Expressive language composite | 83.19 | 88.1 | 2.17 | 213 | 0.03 | |
| Total language composite | 81.78 | 88.12 | 2.18 | 212 | 0.03 | |
| Age 11 (full sample) | ||||||
| Mean PCE (N=218) |
Mean NDE (N= 67) |
t | df | p | ||
| Word Classes | 7.37 | 7.33 | −0.11 | 284 | 0.91 | |
| Semantic Relations | 8.63 | 8.02 | 1.37 | 282 | 0.17 | |
| Receptive language composite | 84.56 | 87.64 | 1.18 | 283 | 0.24 | |
| Forming sentences | 7.52 | 7.19 | −0.80 | 284 | 0.43 | |
| Sentence assembly | 9.12 | 9.51 | 0.83 | 283 | 0.41 | |
| Expressive language composite | 87.74 | 87.69 | −0.02 | 283 | 0.98 | |
| Total language composite | 85.13 | 86.69 | 0.63 | 283 | 0.53 | |
| Age 9 (ERP sample) | ||||||
| Mean PCE (N= 67) |
Mean NDE (N= 36) |
t | df | p | ||
| Word Classes | 6.64 | 7.44 | 2.50 | 102 | 0.12 | |
| Semantic Relations | 7.58 | 8.14 | 0.87 | 102 | 0.35 | |
| Receptive language composite | 82.31 | 87.14 | 2.09 | 102 | 0.15 | |
| Forming sentences | 6.7 | 7.28 | 0.86 | 102 | 0.35 | |
| Sentence assembly | 8.21 | 9.19 | 1.08 | 102 | 0.18 | |
| Expressive language composite | 83.04 | 87.25 | 1.20 | 102 | 0.28 | |
| Total language composite | 81.37 | 86.22 | 1.85 | 102 | 0.18 | |
| Age 11 (ERP sample) | ||||||
| Mean PCE (N=93) |
Mean NDE (N= 45) |
t | df | p | ||
| Word Classes | 7.13 | 7.20 | 0.01 | 143 | 0.90 | |
| Semantic Relations | 7.8 | 8.83 | 30.7 | 143 | 0.07 | |
| Receptive language composite | 82.75 | 87.39 | 2.04 | 143 | 0.16 | |
| Forming sentences | 7.24 | 7.51 | 0.06 | 143 | 0.81 | |
| Sentence assembly | 9.10 | 9.90 | 1.75 | 143 | 0.18 | |
| Expressive language composite | 85.29 | 88.22 | 0.99 | 143 | 0.33 | |
| Total language composite | 82.85 | 86.90 | 1.75 | 143 | 0.19 | |
ERP Experiment Procedures
After obtaining parental permission and child assent, each child was seated 1 m in front of a 17-inch Dell CRT monitor. Each child’s head was measured to determine the appropriate net size and to mark Cz at the juncture of the halfway point between nasion to inion and left and right preauricular points. Next, the electrode net, a high-density array of 128 Ag/AgCL electrodes, embedded in a sponge “elastomer” net (Geodesic Sensor Net, EGI Inc.) was soaked for 10 minutes in a warm potassium chloride solution (KCl) that served as a conductive electrolyte. The KCl solution enhances EEG collection through hair and eliminates the need for abrading the participant’s scalp. Finally, the net was placed on the child’s head using standard procedures outlined by EGI. Hardware filters were set at .1–100 Hz. Electrodes are kept in place by the elastomer net which fits tight to the head, thus no paste or gel is required. EEG data were recorded by Netstation v.4.2 (EGI, Inc.) with an EGI Net Amps 2.0 high impedance amplifier, sampling at 250hz. All impedances remained at or under 40kohms as indicated by impedance measures made immediately before and after the test session. At the end of the experiment participants were compensated with a payment of $70.
ERP Task
A passive pseudo word speech perception task modeled after Molfese, Morse, & Peters (1990) consisted of two consonant-vowel-consonant auditory stimuli, “bidu” and “gibu”, presented from an overhead speaker (RCA® 50-Watt, 2-Way Die-Cast Mini-Speaker) positioned above the participant (distance from the floor to the speaker was 190 cm). The task consisted of two blocks. During block 1, the sensitization block, one of the pseudo words was presented for 50 trials. Subsequently, during block 2, “the experimental block”, both stimuli were presented in random order and in equal proportion (100 trials total). In this manner, the participant was conditioned to one stimulus during block 1. During block 2, the previously presented stimulus served as the “old” stimulus and the second stimulus introduced in block 2 was the “new” stimulus. Which stimulus served as old vs. new (bidu or gibu) was counterbalanced across participants. Stimulus duration for each block was 1000 msec with a variable ITI of 1800 or 2800 msec. E-prime v.1.2 (PST, Inc) software package controlled the stimulus presentation and sent a trigger to Netstation for time locking of stimulus onset. Stimuli were presented at a comfortable listening volume between 70 and 80db (measured with a db meter); slight volume adjustments were made on a participant-by-participant basis to ensure participant comfort. Participants were instructed to passively listen to the stimuli and to look ahead at the center of a black display on a 14 inch LCD monitor (no overt response was required). Participants were also informed about eye blink artifact and asked to blink in between stimulus presentation when possible. Each child’s electroencephalogram (EEG) and behavior were continuously monitored across the session so that stimulus presentation occurred only when the child was sitting still and looking at the monitor. Participants were given a 20 second rest between the first “bidu or gibu only” block and the second “bidu/gibu” block.
ERP processing and Analysis
ERP Processing: ERP data were segmented into 1-second epochs including a 100 msec prestimulus baseline and a 900 msec post stimulus interval. All data were re-referenced offline after data collection from the vertex (Cz) to the average of all electrodes (Junghofer, Elbert, Tucker, & Braun, 1999). Next, artifact detection and rejection was carried out to eliminate ERPs contaminated by movement and eye artifacts from further analysis (bad channel > 200 µV, eye blink/movement > 150 µV). For every channel, 50% or greater bad segments was used as the criteria for marking the channel bad; for every segment, greater than 20 bad channels was used as a criterion for marking a segment bad. Missing data was estimated using spherical spline interpolation (Perrin, Pernier, Bertrand, & Echallier, 1989). The segmented data were then averaged individually, and baseline corrected (using the 100 ms prestimulus interval) for each participant. Ocular Artifact Correction (OAR) (Blink Slope Threshold = 14 µV/ms) (Gratton, Coles, & Donchin, 1983) was conducted on 22 out of 107 exposed and 8 out of 46 controls. The ratio of correction is comparable across groups (X2 = 0.21, p > .5). OAR was applied to participant data when there were less than 20 blink and other artifact free trials per category in the averaged file without using OAR. After applying OAR, if there were still less than 20 blink and other artifact free trials per condition, the participant was finally excluded from the EEG analysis. Thirteen out of 107 and 5 out of 46 participants were rejected because of too much artifact. The ratio of rejection is comparable across groups (X2 = 0.05, df = 1, p > .5). The number of accepted trials were comparable across stimulus conditions, F(1,133) = 1.72, ns and groups, F(1,133) = .27, ns; the Condition by Group interaction was also not significant, F(1,133) = 1.01, ns. The final analysis included 41 NDE and 94 PCE participants.
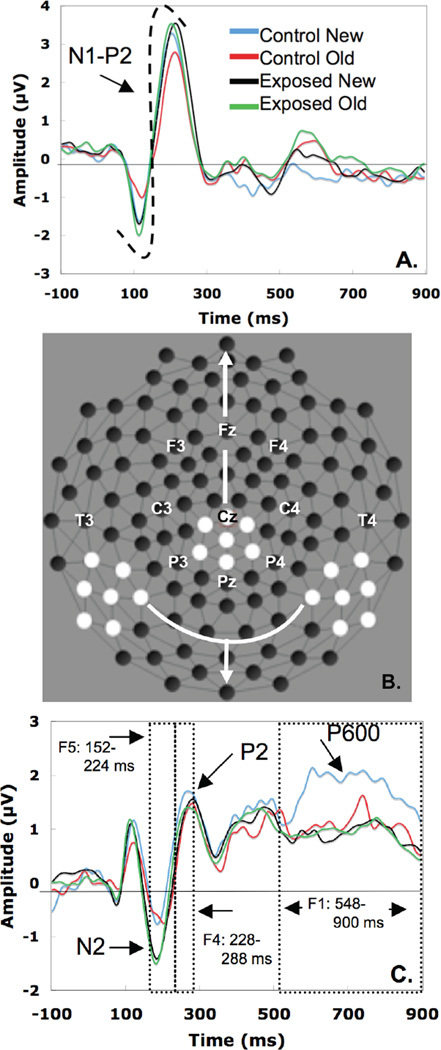
ERP Analysis method: Two sets of analyses were conducted. For the first set of analyses we used a traditional peak identification analysis. This approach was taken because this analysis focused on the N1/P2 complex, and the data driven approach (PCA) we used for our later components can split these two highly correlated components. The N1/P2 complex was identified visually in a cluster of channels in the medial-central cortical region (32 129 81 55 54 62 80, see Figure 1B for a map of electrode locations). Within this cluster, peaks were identified as the most negative peak occurring during the 50 to 200 ms post stimulus (N1), and the most positive going peak for the next 100 to 300 ms post stimulus (P2). The combined latency of the N1-P2 complex was identified by subtracting the latency of the N1 from the latency of the P2; the amplitude of N1/P2 was identified by subtracting the amplitude of the N1 from the amplitude of the P2. Then, the N1/P2 amplitude & latency difference effect for old relative to new were calculated by subtracting the N1/P2 amplitude and latency of the old condition from the new condition for each participant. Finally, independent samples t-tests comparing groups were conducted on the N1/P2 old-new amplitude and latency differences.
Figure 1.
ERP waveforms, and scalp topography for prenatally cocaine exposed (PCE) vs. non-drug exposed (NDE) groups and “New” and “Old” pseudo word stimuli. (A) ERPs at central electrodes with the N1/P2 complex noted. (B) EEG sensor layout with averaged channel clusters highlighted in white. (C) ERPs for averaged temporal-parietal electrodes with PCA windows and the N2, P2, and P600 components noted.
A second set of analyses involved matched pairs of channels in the left (50 51 57 58 59 63 64) and right (102 98 101 92 97 100 96) temporal-parietal regions (Figure 1B, EGI GSN 200 sensor layout), identified visually. We used temporal principal component analysis (PCA) following Dein et al.( Dien, Tucker, Potts, & Hartry-Speiser, 1997) to identify time windows of correlated neural activity in these temporal-parietal scalp regions during the presentation of the old and new stimuli (bidu/gibu). After identification of factors (and corresponding components), separate Group by Condition repeated measures ANOVAs were conducted for each factor.
3. Results
CELF
Age 11, full sample (N =219, 179 PCE children and 40 controls): At age 11 there were no significant differences in language function on any of the administered tests of expressive and receptive language. Age 9, full sample (N= 217, 118 PCE children and 99 controls): At age 9 there are significant differences in language performance between the two groups on many of the CELF subtests. PCE children performed more poorly on the following receptive language subtests: word classes, semantic relations, and the following expressive language subtests: forming sentences (see Table 1 for means and p values). At age 9, the groups also had significantly different scores on the receptive and expressive language composites (Table 1). When we examined CELF data for only those participants that completed the ERP experiment, we did not find significant differences at either age 9 or age 11 (Table 1); however, several of the individual subtest p values were marginal (.07ߝ .15). The lack of significant differences between the groups may be due to the smaller number of children who received the CELF and participated in the ERP experiment.
ERP
Central cortical analysis (N1/P2 complex): The N1/P2 complex amplitude analysis indicated a significant group difference in processing old relative to new tokens t(133) = 2.10, p < .05. Specifically, the N1/P2 amplitude change between old and new conditions was larger in the NDE group (M = .73 µV, SE = .41) than in the PCE group (M = −.24 µV, SE = .24), with a reduced amplitude for the old stimulus (for the N1/P2 complex) relative to the new stimulus for NDE but not PCE. No significant effect was found in the latency difference across groups, t = −.08, ns.
Temporal-parietal analyses (P600, N2, P200): For the temporal-parietal analyses, a 2-condition PCA was conducted (using a covariance matrix), followed by varimax rotation, with factor loadings obtained from the rescaled rotated matrix. The effective time of each factor was determined by non-overlap criteria such that the effective time of each different factor did not overlap with one another. The temporal PCA yielded five components accounting for 81.24% of the variance in the ERP signal. Factor 1 accounted for 57.90% of the variance and consisted of a slow wave apparent in time interval 540 – 900 ms (peak time 828 ms). Factor 2 accounted for 12.29% of the variance and appeared as a 292 – 436 ms time interval (peak time 352 ms). Factor 3 accounted for 4.85% and appeared as a 224 – 288 ms time interval (peak time 252 ms). Factor 4 accounted for 3.24% of the variance and appeared as a 464 – 536 ms time interval (peak time 516 ms). Factor 5 accounted for 2.97% of the variance and appeared 152 – 220 ms (peak time 188 ms).
We conducted follow-up analyses on the condition mean voltage values, separately for each of the PCA-derived windows. Repeated measures ANOVAs consisted of a condition (new vs. old) and hemisphere (left, right) as within subjects factor and Group (PCE vs. NDE) as the between subjects factor. We report only the significant effects here, and all F-tests are reported with Greenhouse-Geisser correction (Greenhouse & Geisser, 1959). Three significant factors that differentiated the NDE and PCE groups were identified corresponding to a P600, N2 and P200 differentiated the exposed and comparison groups (see Figure 1C).
For factor 1, the P600 effect (540 – 900 ms) there was a main effect of Condition, F(1, 133) = 5.04, p < .05, Partial Eta Squared = .04, Observed Power = .61, and a Condition × Group interaction, F(1, 133) = 5.72, p < .05, Partial Eta Squared = .04, Observed Power = .66. Followup paired sample t-tests were conducted for each group separately. The NDE group had a more positive P600 for the new condition relative to the old condition, M= 1.79 µV, SE = .31 vs. M = 1.02 µV, SE = .27) t(40) = 2.28, p < .05. The PCE group did not show a significant difference in response to old vs. new, t(93) = −.15, ns.
For factor 3, the P200 effect (224–288 ms), there was a significant Condition by Group interaction, F(1, 133) = 7,00, p <.05, Partial Eta Squared = .05, Observed Power = .75. Follow-up paired sample t-tests were conducted for each group separately. The NDE group had more positive amplitude for the new condition relative to the old condition, t(40) = 2.32, p < .05, (M= 1.34 µV, SE = .32 vs. M = .82 µV, SE = .30) indicating that during the P200 window, the NDE participants were able to discriminate the old and new conditions. The PCE group did not show a significant difference in response to the old vs. the new condition during this time window, t(93) = −1.31, ns.
For factor 5, the N200 (152–200 ms) we observed a main effect of Group, F(1, 133) = 4.84, p < .05, Partial Eta Squared = .04, Observed Power = .59. Follow-up pair-wise comparisons indicated that, during the 152 to 220 ms period, the NDE group had a less negative N2 component peak than did the PCE group (M = −.39 µV, SE = .22 vs. M = −.97 µV, SE = .15), p < .05.
Post-hoc SES covariate analysis: Because we observed significant differences in maternal ED in our ERP sample we ran all of our analyses with maternal ED as a covariate. Only one of our findings was altered by this analysis. Specifically, the Condition by Group interaction for the P600 component identified by factor 1 in the PCA became marginal, F(1,132) = 3.61, p = .059. No other main effects or interactions were significantly altered by the inclusion of the maternal ED covariate.
4. Discussion
Overall, our findings suggest that children with prenatal cocaine exposure have atypical responses to non-word speech stimuli, both in terms of early, low-level phonological processing and for later stage processing of repetition of spoken non-words. Specifically, we identified differential responses to repeated stimuli for PCE relative to NDE children in both early and late components. This finding is consistent with previous behavioral research documenting poorer speech processing and language skills in PCE children relative to matched controls (Malakoff, et al. 1999; Bandstra et al. 2002; 2004). Critically, this finding adds to the existing literature on academic and language skills in PCE children by providing direct neurophysiological evidence of altered processing of spoken non-word representations in PCE relative to NDE controls. Interestingly, our findings do not seem to be reflected in the higher-level language skills assessed by the CELF at age 11 or 9 for the cohort that participated in the ERP experiment (note however that CELF data was not available for all participants in this cohort thus limiting power). However, CELF assessments for age 9 but not age 11 for the full cohort indicate significant differences in multiple aspects of language processing. This suggests a possible interaction between development and language performance, possibly because language learning demands are greater for younger children. Given the lack of significant findings in the current experimental cohort at earlier time points we might conclude that the ERP signal is a more sensitive measure of speech processing and linguistic representations. Findings concerning each of the identified ERP components that differentiated the PCE and NDE groups will now be discussed in turn.
NI/P2
Over the central cortical region (Figure 1A, B), we observed a significant difference between the groups in the N1/P2 complex amplitude between the old and new condition (old – new amplitude difference score). Specifically, we observed greater N1-P2 amplitude (more negative N1 and more positive P2) for the new condition compared to the old condition for NDE participants but not the PCE participants. This finding suggests that the NDE group differentiated between the new and the old stimulus, whereas the PCE group demonstrated a much smaller reduction for old relative to new items. This reduction in amplitude within the N1/P2 complex for old relative to new stimuli for NDE participants may indicate better sensitivity to the phonemic structure of the two stimuli (i.e., bidu and gibu), in the NDE relative to the PCE group and thus greater ability to distinguish the two stimuli at this relatively early phonemic processing level. Another interpretation could be that the increased number of presentations of the old stimulus may have caused a form of habituation in the NDE group, but not for PCE children. Reduced habituation may result if the neurobiological structures responsible for auditory phonological processing in PCE do not show typical response to repetition, that is, reduced neuronal firing to repeated items or “repetition suppression” (see Haenschel, Vernon, Dwivedi, Gruzelier, & Baldeweg, 2005). This interpretation is also consistent withPotter et al. (2000), who found reduced habituation to novel stimuli in PCE relative to NDE neonates to auditory tokens in a head turning paradigm. Each of these possibilities (differences in phonological representation and hence discrimination or differences in habituation) is tenable. Further research will be needed to determine at what level and by what mechanism old and new auditory linguistic stimuli are affected by PCE. Repetition suppression is generally thought to occur at the level of sensory processing; thus, further examination of auditory sensory function in PCE children could help elucidate this finding.
N200
The N200 component showed a robust group difference (reduced N200 amplitude for the NDE group) that was not modulated by condition (old/new). The N200 has been associated with both general phonological processing and with deviance from context – either in the sense of sentence/semantic context or phonological context (e.g., a novel word in a stream of old words – and is related to the mismatch negativity or MMN). Several previous examinations of the N200 (Hagoort, 2008) indicate that this component is sensitive to the earliest aspects of phonological and semantic information encoded in words (i.e., pre-lexical access). Since current study relied on non-word stimuli, the N200 finding we observed likely represents a difference between the groups in processing abstract “pre-lexical” phonological representations that ultimately support lexical processing. If this is the case, this processing may require less effort for NDE participants, hence the reduced amplitude N2. This interpretation is consistent with Connolly et al. (Connolly, Stewart, & Phillips, 1990), who suggested that the N200 represents acoustical/phonological representations. Moreover, the reduction in amplitude for the N200 in the NDE controls relative to PCE participants is consistent with findings from comparisons of dyslexic and control participants (Bonte & Blomert, 2004) and SLI and control participants (Macarthur & Bishop, 2005); control participants showed less negative N200 responses relative to affected groups in both cases.
P600
In a window capturing the P600 we found that the NDE group had an increased positive response for new relative to old stimuli – the PCE group did not show a difference between conditions. We note that this finding became only marginally significant (p = .059) when we included a covariate for maternal education level; however, we believe further discussion of this effect is warranted given the proximity to accepted significance levels. We hypothesize that this effect indicates that NDE children may have stronger phonological representations of the stimuli such that they were able to discriminate old and new representations more easily than PCE children. Several studies have demonstrated old/new effects for word recognition associated with the P600 (e.g., Curran, 1999b; Paller, Kutas, & Mayes, 1987; Paller, Kutas, & McIsaac, 1995). Moreover,Paller et al. (1987) showed that these effects were recollection related (as they did not affect identification) and that they could be observed when no intentional retrieval was required. Consistent with the implicit nature of our task, this finding may have important implications for word and vocabulary learning in PCE (See Saffran, 1999). Specifically, if PCE children have more difficulty building up new non-word representations or if it takes them longer to build these representations, this would suggest an impaired mechanism for general vocabulary learning in PCE (as suggested by the work of Bandstra et al. 2002; 2004). This in turn could provide an explanation for the poorer receptive language measures observed both in our cohort at age 9 and in other studies of PCE.
We also acknowledge the possibility that our P600 effects reflect verbal or even more general memory processes (recollection), which may not be specific to phonological encoding. Most previous studies on the P600 and the old/new effect were designed to look at general memory processing but did not specifically test linguistic processing (though the stimuli used were typically words). Our experiment was designed to test speech processing, so we cannot address this issue directly. We suspect, however, that differences in the encoding of linguistic representations may underlie our findings in this study in part because our groups also differ in other lower–level language processing components.
P200
Finally, we observed a Group by Condition effect in the P200 component. The P200 showed a similar pattern to the P600 with a more positive deflection for new relative to old stimuli for the NDE group but not for the PCE group, though this effect was much smaller than it was for the P600. We believe that this component may be a somewhat earlier, more subtle marker of the old/new effect observed in the P600. We interpret this component cautiously at this point, as there is no existing literature on the P200 component response to phonological repetition effects.
Finally, although we did not perform source localization analyses, we can speculate about the underlying functional neural anomaly associated with poor phonological processing present in PCE based on a large body of research on phonological processing and associated deficits. Specifically, the posterior superior temporal gyrus (STG) and left inferior frontal gyrus (IFG) have been repeatedly implicated in auditory phonological processing in imaging studies (fMRI) and in patient work (e.g, Fiez et al. 1996; Price et al. 1996; Poldrack et al. 2001 Blumstein, 1994; Landi et al. 2010). Moreover, a recent study (Leff et al. 2009) found that the posterior portion of the left STG and superior temporal sulcus were uniquely associated with phonological short-term memory. This finding may be of particular relevance to the current study given our findings of differential processing for old vs. new auditory non-word tokens. Additional research using ERP source localization approaches is needed to confirm the precise neural mechanisms affected by prenatal cocaine exposure that lead to deviant ERP response to spoken non-word processing; however, given the large amount of existing research that points to a critical role for the left temporal and inferior frontal lobes, particularly the left STG/STS and left IFG in phonological processing, we suggest that altered neural development in these regions may underlie the observed deviant ERP response to spoken non-words.
In sum, our findings suggest a broad effect of PCE on spoken nonword processing and repetition. We observed differences between the groups in processing auditorily presented non-words in very early phonemic processing components (N1/P2 complex), in somewhat higher - level phonological processing components (the N200), and in late high-level linguistic/memory components (P600). In several of the components (N1/P2; P600; P200), we observed Group by Condition (old/new) effects suggesting the role of memory (most likely at the level of encoding linguistic features) though further investigations of more general memory processing in individuals exposed to cocaine is needed. For the N200, however, we observed group effects that were independent of condition suggesting a pure phonological processing difference between the groups. Based on existing research on the localization of phonological processing and phonological memory we speculate that altered neural circuitry in the left hemisphere STG/STS and IFG may underlie the patterns of ERP response observed in our study. This work represents a first attempt at characterizing neurobiological profiles of speech and language processing in children exposed to cocaine in utero.
The current study, while providing critical neurobiological data on language function in PCE adolescents does not provide a full account of language or speech processing in this population. Further examination of low-level auditory skills, lexical semantic skills, and syntactic skills will be necessary to understand the language and speech processing profiles of these children. Additionally, more extensive behavioral testing is needed to determine the relationship between EEG signal, behavioral language profiles, and speech processing skills. Our existing behavioral data are from earlier time points in development and do not test the same skills measured in our ERP experiment. Tests of both overlapping and distinct linguistic skills measured at the same time points when neuroimaging (ERP or fMRI) data is aquired will be necessary to fully understand the language and speech processing profile in PCE children and the relationship between their behavioral performance on language assessments and their neurobiological profiles. While groups in our full longitudinal cohort were matched on socioeconomic status, participants in the current experiment were not matched; specifically the non-exposed groups’ mothers attained higher levels of education on average, thus we feel further investigation of socioeconomic status is warranted. A recent study (Stevens et al. 2009) demonstrated that children whose mothers had lower levels of education (no college) showed reduced ability to filter out irrelevant auditory information relative to children whose mothers had attained higher levels (some college). Moreover, as in most existing studies of cocaine exposure, other drug and alcohol use was present in our sample, thus it is difficult to determine what portion of the observed effects are due to cocaine alone and what is due to cocaine in combination with other drugs and alcohol. Future work that focuses on understanding the relationship between multiple different risk factors (e.g, multiple drug exposure and SES) is critical for understanding the full nature of our observed differences in speech processing.
• Prenatal cocaine exposure (PCE) can have adverse effects on language development. •We examine the effects of PCE on speech processing using ERP in adolescents. • PCE participants had abnormal ERPs to speech relative to controls. • We conclude that PCE alters the neural systems responsible for language development.
Acknowledgements
This research was supported by a NARSAD Young Investigator Award (MJC); NIDA grants RO1-DA-06025 (LCM), DA-017863 (LCM) and KO5 (LCM), and a grant from the Gustavus and Louise Pfeiffer Research Foundation (LCM). Thank you to Eric Langlois for his work on data collection for this project, and to Dr. Dennis Molfese for use of his paradigm.
Abbreviations
- ERP
Event Related Potential
- PCE
prenatally cocaine-exposed
- NDE
non-drug-exposed
Appendix
Birth measures: cocaine exposed participants had significantly smaller head circumference (PCE= 32.70 cm; NDE = 33.73 cm), t (151) = 3.019, p < .01 and lower birth weight (PCE= 2767.19 grams; NDE = 3336.87 grams), t (151) = 5.659, p <.001.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Note that there was no systematic bias with respect to which participants completed the CELF (i.e., individuals with CELF data are not more likely to be impaired) those who did not receive it simply missed the visit when CELF data was obtained.
References
- Bandstra ES, Morrow CE, Vogel AL, Fifer RC, Ofir AY, Dausa AT, et al. Longitudinal influence of prenatal cocaine exposure on child language functioning. Neurotoxicology & Teratology. 2002;24:297–308. doi: 10.1016/s0892-0362(02)00192-7. [DOI] [PubMed] [Google Scholar]
- Bandstra ES, Vogel AL, Morrow CE, Xue L, Anthony JC. Severity of prenatal cocaine exposure and child language functioning through age seven years: a longitudinal latent growth curve analysis. Substance Use & Misuse. 2004;39(1):25–59. doi: 10.1081/JA-120027765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumstein SE. Impairments of speech production and speech perception in aphasia. Philosophical Transactions of the Royal Society of London, Series B; Biological Sciences. 1994;346:29–36. doi: 10.1098/rstb.1994.0125. [DOI] [PubMed] [Google Scholar]
- Bonte ML, Blomert L. Developmental dyslexia: ERP correlates of anomalous phonological processing during spoken word recognition. Cognitive brain research. 2004;21:360–373. doi: 10.1016/j.cogbrainres.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Connolly JF, Stewart SH, Phillips NA. The effects of processing requirements on neurophysiological responses to spoken sentences. Brain and Language. 1990;39:302–318. doi: 10.1016/0093-934x(90)90016-a. [DOI] [PubMed] [Google Scholar]
- Curran T. The electrophysiology of incidental and intentional retrieval: ERP old/new effects in lexical decision and recognition memory. Neuropsychologia. 1999a;37:771–785. doi: 10.1016/s0028-3932(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Curran T. The electrophysiology of incidental and intentional retrieval: ERP old/new effects in lexical decision and recognition memory. Neuropsychologia. 1999b;37:771–785. doi: 10.1016/s0028-3932(98)00133-x. [DOI] [PubMed] [Google Scholar]
- Dhar M, Been PH, Minderaa RB, Althaus M. Information processing differences and similarities in adults with dyslexia and adults with Attention Deficit Hyperactivity Disorder during a Continuous Performance Test: a study of cortical potentials. Neuropsychologia. 2010;10:3045–3056. doi: 10.1016/j.neuropsychologia.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Dien J, Tucker DM, Potts G, Hartry-Speiser A. Localization of auditory evoked potentials related to selective intermodal attention. Journal of Cognitive Neuroscience. 1997;9:799–823. doi: 10.1162/jocn.1997.9.6.799. [DOI] [PubMed] [Google Scholar]
- Fiez JA, Raichle MR, Balota DA, Tallal P, Peterson SE. PET activation of posterior temporal regions during auditory word presentation and verb generation. Cerebral Cortex. 1996;6:1–10. doi: 10.1093/cercor/6.1.1. [DOI] [PubMed] [Google Scholar]
- Frank DA, Augustyn M, Grant Knight W, Pell T, Zuckerman B. Growth, development, and behavior in early childhood following prenatal cocaine exposure. Journal of the American Medical Association. 2001;285:1613–1625. doi: 10.1001/jama.285.12.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Haenschel C, Vernon DJ, Dwivedi P, Gruzelier JH, Baldeweg T. Event-related brain potential correlates of human auditory sensory memory-trace formation. Journal of Neuroscience. 2005;25:10494–10501. doi: 10.1523/JNEUROSCI.1227-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. The fractionation of spoken language understanding by measuring electrical and magnetic brain signals. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2008;363:1055–1069. doi: 10.1098/rstb.2007.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghofer M, Elbert T, Tucker DM, Braun C. The polar average reference effect: a bias in estimating the head surface integral in EEG recording. Clinical Neurophysiology. 1999;110:1149–1155. doi: 10.1016/s1388-2457(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Kutas M, Van Petten C. Psycholinguistics Electrified: Event-related potential investigations. In: Gernsbacher MA, editor. Handbook of Psycholinguistics. New York: Academic Press; 1994. pp. 83–143. [Google Scholar]
- Kutas M, Van Petten C, Kluender R. Psycholinguistics electrified II: 1994–2005. In: Traxler M, Gernsbacher MA, editors. Handbook of Psycholinguistics. 2nd Edition. New York: Elsevier; 2006. pp. 659–724. [Google Scholar]
- LaGasse LL, Seifer R, Lester BM. Interpreting research on prenatal substance exposure in the context of multiple confounding factors. Clinical Perinatology. 1999;26:39–54. [PubMed] [Google Scholar]
- Landi N, Mencl WE, Frost SJ, Sandak R, Chen H, Pugh KR. An fMRI comparison of semantic and phonological processing in non-impaired and reading disabled adolescents. Annals of Dyslexia. 2010;60:102–121. doi: 10.1007/s11881-009-0029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff A, Schofield T, Crinion J, Seghier M, Grogan A, Green D, Price C. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132:3401–3410. doi: 10.1093/brain/awp273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macarthur GM, Bishop DV. Speech and non-speech processing in people with specific language impairment: A behavioural and electrophysiological study. Brain and Langauge. 2005;94:260–273. doi: 10.1016/j.bandl.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Mayes LC, Bornstein MH, Chawarska K, Haynes O, et al. Impaired regulation of arousal in 3-month-old infants exposed prenatally to cocaine and other drugs. Development and Psychopathology. 1996;8:29–42. [Google Scholar]
- Mayes LC, Molfese DL, Key AP, Hunter NC. Event-related potentials in cocaine-exposed children during a Stroop task. Neurotoxicology Teratology. 2005;27:797–813. doi: 10.1016/j.ntt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- Molfese DL, Morse PA, Peters CJ. Auditory evoked responses to names for different objects: Cross-modal processing as a basis for infant language acquisition. Developmental Psychology. 1990;26:780–795. [Google Scholar]
- Paller KA, Kutas M, Mayes AR. Neural correlates of encoding in an incidental learning paradigm. Electroencephalography and Clinical Neurophysiology. 1987;67:360–371. doi: 10.1016/0013-4694(87)90124-6. [DOI] [PubMed] [Google Scholar]
- Paller KA, Kutas M, McIsaac HK. Monitoring conscious recollection via the electrical activity of the brain. Psychological Science. 1995;6:107–111. [Google Scholar]
- Paul R, Murray C, Clancy K, Andrews D. Reading and metaphonological outcomes in late talkers. Journal of Speech, Language, and Hearing Research. 1997;40:1037–1047. doi: 10.1044/jslhr.4005.1037. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr, Miller GA, Ritter W, Ruchkin DS, Rugg MD, Taylor MJ. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Poldrack RA, Temple E, Protopapas A, Nagarajan S, Tallal P, Merzenich M, Gabrielli JD. Relations between the neural bases of dynamic auditory processing and phonological processing: Evidence from fMRI. Journal of Cognitive Neuroscience. 2001;13:687–697. doi: 10.1162/089892901750363235. [DOI] [PubMed] [Google Scholar]
- Potter S, Zelazo PR, Stack D, Papageorgio N. Adverse Effects of Fetal Cocaine Exposure on Neonatal Auditory Information Processing. Pediatrics. 2000;105:e40. doi: 10.1542/peds.105.3.e40. [DOI] [PubMed] [Google Scholar]
- Price CJ, Wise RJS, Warrburton EA, Moore CJ, Howard D, Peterson K, Fracowiak RSJ, Friston KJ. Hearing and saying; The functional neuroanatomy of auditory word processing. Brain. 1996;119:919–931. doi: 10.1093/brain/119.3.919. [DOI] [PubMed] [Google Scholar]
- Stevens C, Lauinger B, Neville H. Differences in the neural mechanisms of selective attention in children from different socioeconomic backgrounds: an event-related brain potential study. Developmental Science. 2009;12:634–646. doi: 10.1111/j.1467-7687.2009.00807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]