Abstract
Asthma and chronic obstructive pulmonary disease (COPD) are distinct but clinically overlapping airway disorders which often create diagnostic and therapeutic dilemmas. Current strategies to discriminate these diseases are limited by insensitivity and poor performance due to biologic variability. We tested the hypothesis that a gas chromatograph / differential mobility spectrometer (GC/DMS) sensor could distinguish between clinically well-defined groups with airway disorders based on the volatile organic compounds (VOCs) obtained from exhaled breath. After comparing VOC profiles obtained from 13 asthma, 5 COPD, and 13 healthy control subjects, we found that VOC profiles distinguished asthma from healthy controls and also a subgroup of asthmatics taking the drug omalizumab from healthy controls. The VOC profiles could not distinguish between COPD and any of the other groups. Our results show a potential application of the GC/DMS for non-invasive and bedside diagnostics of asthma and asthma therapy monitoring. Future studies will focus on larger sample sizes and patient cohorts.
Keywords: high-field asymmetric waveform ion mobility spectrometry, differential mobility spectroscopy, asthma, chronic obstructive pulmonary disease COPD, exhaled breath condensate, breath analysis, biomarkers, non-invasive diagnostics, volatile organic compounds (VOCs)
1. Introduction
Asthma and chronic obstructive pulmonary disease (COPD) are common and important respiratory disorders that lead to substantial morbidity and lung dysfunction. While the overt manifestation of these disorders is breathlessness, the underlying physiologic mechanisms and treatment paradigms are different. For example, asthma is often characterized by increased levels of allergen-mediated immunoglobulin E (IgE) production, which leads to mast cell release of bronchoconstrictive and pro-inflammatory mediators. For a subset of difficult-to-control asthmatics, treatment with an anti-IgE monoclonal antibody, omalizumab, may improve symptoms and reduce asthma flares over time (Rodrigo et al., 2011). In contrast, COPD is characterized by progressive breathlessness with exertion often due to dynamic hyperinflation and fixed airflow obstruction. Therefore, treatment with long-acting bronchodilators is a front-line therapy in COPD (Karner and Cates, 2012, Decramer et al., 2009, Qaseem et al., 2011). However, the reality is that approximately 50% of patient who present to primary care physicians and specialists have clinical features suggestive of both asthma and COPD (Zeki et al., 2011).
Current strategies to differentiate asthma from COPD include measurements of lung function, a prolonged smoking history in COPD, the presence of daily sputum production in COPD, and exhaled biomarkers including nitric oxide (eNO), which is classically elevated in asthma and normal in COPD (Barnes et al., 2010, Dweik et al., 2011). However, eNO may not correctly classify patients as evidenced by normal levels of eNO in nonallergic asthma (Dweik et al., 2010) and variable eNO levels in COPD (Gelb et al., 2012).
Similarities in clinical presentation, limited discriminatory power of conventional diagnostic tools, and a limited armamentarium of validated biomarkers create both diagnostic and therapeutic dilemmas. This confusion can result in inappropriate therapies such as monotherapy with long-acting bronchodilators in asthma leading to an excess of asthma-related deaths (Nelson et al., 2006) or monotherapy with inhaled steroids in COPD leading to an excess of pneumonias (Yang et al., 2012).
Complimentary diagnostic modalities to properly characterize asthma and COPD include the assessment of volatile organic compounds (VOCs) from exhaled breath. Several clinical studies have assessed VOCs in asthma and COPD, however, with certain limitations. Some clinical studies analyzing VOCs have used chemical sensors designed to detect but not identify VOCs (Fens et al., 2011, Fens et al., 2009, Montuschi et al., 2010, Dragonieri et al., 2007). The results led to acceptable disease classification, but no further analysis including the specific VOC identification and eventual biomarker discovery was possible. Alternative studies used gas chromatography / mass spectrometry (GC/MS) which is considered the gold standard for VOC identification but does not provide an instrumental platform for bedside or office use (Caldeira et al., 2012, Ibrahim et al., 2011, Van Berkel et al., 2010).
Additional studies used portable diagnostic platforms including ion mobility spectrometry methods with promising results. Westhoff et al. were able to differentiate 97 cases of COPD from 35 healthy control subjects using ion mobility spectrometry (IMS) coupled with a multi-capillary column (MCC) analysis of 10 mL exhaled breath (Westhoff et al., 2010, Westhoff et al., 2011). In this study the investigators identified a single compound, cyclohexanone, which classified COPD from healthy controls with 91% specificity and 95% predictive value. A similar study using IMS looked at 13 COPD patients and 33 healthy controls (Bessa et al., 2011). The investigators found 1 out of 98 peaks which significantly separated the clinical groups. Though they did not overtly identify this peak, the authors suggest the peak identification was benzofuran, phenol, 4-methylanisol or 1,2,4-trimethylbenzol. A third study looked at COPD patients with and without alpha-1-antitrypsin (A1AT), a protective enzyme found in the blood and lungs (Koczulla et al., 2011). This study identified 5 of 22 peaks which separated 17 A1AT-deficient COPD subjects from 8 non-A1AT-deficient COPD subjects.
Basanta et al. used differential mobility spectrometry (DMS) to analyze exhaled breath from 20 COPD subjects and 6 healthy controls who smoke cigarettes (Basanta et al., 2010). Using a validated exhaled breath collection method, the authors were able to separate the clinical groups based on partial least squares-discriminant analysis of the datasets with good reproducibility, a 19% Type I error rate (false-positive), and 12.4% Type II error rate (false-negative). One characteristic not addressed in this study was that all 6 healthy controls were active smokers while only 2 of the 20 COPD subjects were active smokers. This fact could have introduced confounders.
Given the difficulties in accurate diagnosis and the potential for harmful therapy, there remains an urgent need for improved diagnostic tools to classify asthma and COPD. Despite a number of illuminating studies, there is not a comprehensive understanding of the correlation of VOCs between asthma and COPD. Therefore, it is important to pursue further research efforts in these very common lung diseases with the ultimate goal of developing a protocol using a suitable analytical method that can be used for bed side diagnostics
As in the Basanta study, our lab has experience with DMS which is a highly miniaturized (i.e. micromachined), portable sensor for VOC detection. DMS (also known as high field asymmetric waveform ion mobility spectrometry [FAIMS]) (Shvartsburg et al., 2006, Guevremont, 2004, Eiceman et al., 2002, Borsdorf and Eiceman, 2006) is commonly applied for a wide variety of analytic purposes, including but not limited to: explosives detection (Perr et al., 2005, Guerra-Diaz et al., 2010, Buxton and Harrington Pde, 2003), drug detection (Gryniewicz et al., 2009, Dunn et al., 2011, Jafari et al., 2009), bacteria detection (Shnayderman et al., 2005), and VOC detection in human samples (Molina et al., 2008, Krebs et al., 2006).
DMS may be used as an ion-filtering device in combination with mass spectrometry (MS) or liquid chromatography / mass spectrometry (LC/MS), or it can function as a stand-alone sensor. The latter renders DMS a suitable technology for VOC detection in breath diagnostics as it is small and portable, highly sensitive, and consumes little power. The analytical signal in DMS is generated from the differences in ion mobility at low and high electric field conditions. Each chemical species is characterized by a unique dependence of its mobility within an electric field; therefore, the differences in ion mobility may be used to identify and differentiate VOCs. An additional direct current (DC) voltage, called a “compensation voltage” (CV), is applied to compensate for ion drift at differential field conditions and to enable a particular chemical species to propagate through the device. The value of the CV is related to ion’s structure and, therefore, is specific for each ion species. In combination with gas chromatography (GC), DMS is highly suitable to profile complex VOC distributions.
In the present study, we performed proof-of-concept experiments to explore the feasibility of the gas chromatography / differential mobility spectrometry (GC/DMS)-based chemical sensing to discriminate between VOC profiles from subjects with asthma, COPD, and healthy controls using exhaled breath condensate. The DMS device provides orthogonal information to drift-time (DT) IMS methods in that in DMS the ion mobility-related parameter (CV) only weakly correlates with ion size (Aksenov et al., 2012), unlike in DT IMS where ion drift time is essentially linear with the ion cross-section (Borsdorf and Eiceman, 2006, Eiceman and Karpas, 2005). By this, DMS could differentiate two ions which are structurally different but have the same or similar mass and charge such as isomers and isobars. Though DT IMS is an excellent portable diagnostic instrument, we chose to use DMS because of our experience and for future studies where precise ion species identification is paramount. In addition to the differential mobility parameter, compensation voltage (CV), an additional dimension of separation can be achieved through a scan of radiofrequency waveform amplitude (RF) (Basanta et al., 2007). This may enable further expansion of information achievable in DMS measurements. The ultimate goal of this work is to identify VOC combinations which will improve diagnostic accuracy and differentiation of asthma and COPD, in addition to existing diagnostic tools. It is further anticipated that advanced VOC analysis may lead to specific identification of exhaled biomarkers of disease and inform future mechanistic studies.
2. Materials and Methods
2.1 Subject Selection
We performed a cross-sectional observational study in subjects with asthma and COPD, and in healthy control subjects. All subjects provided informed consent prior to enrollment. The UC Davis institutional review board approved all study activities including subject recruitment, methods, and informed consent documents. We used standard American Thoracic Society and Global Initiative for Chronic Obstructive Lung Diseases (GOLD) definitions to define asthma and COPD (1987, Rabe et al., 2007, Miller et al., 2005). The inclusion criteria for asthma were a subject having a clinical diagnosis of asthma by a lung specialist, compatible symptoms by history (e.g. episodic chest tightness, wheezing, and shortness of breath), a <10 pack-year smoking history, and partially or completely reversible airflow obstruction on spirometry or a positive response to a bronchoprovocation challenge (a reduction in the forced expiratory volume at 1 second [FEV1] by >20% after inhaling <8 mg/dL of methocholine). COPD subjects were defined as having a clinical diagnosis of COPD by a lung specialist, compatible symptoms by history (chronic shortness of breath with or without intermittent exacerbations), a smoking history of >10 pack-years, and predominantly fixed airflow obstruction on spirometry with an FEV1 to forced vital capacity ratio (FEV1/FVC) of <0.7. Healthy control subjects could not smoke, could not have any pulmonary disease, and required normal spirometry.
Medication use was not restricted, but subjects needed to be free of an asthma or COPD exacerbation for the preceding 28 days. All subjects were adults >18 years. Exclusion criteria were pregnancy, active asthma or COPD exacerbations, and inability to provide consent. Additionally, all subjects were asked to refrain from eating or drinking at least 4 hours prior to the study to avoid confounding factors.
2.2 Study Measurements
Anthropomorphic measurements were obtained as well as a brief history and physical exam. Subjects provided spirometry (if not done in the preceding 6 months) via an SDI Spirolab II spirometer (Easton, MA) and two exhaled nitric oxide (eNO) samples. The eNO samples were obtained by blowing into mylar bags per ATS standards (American Thoracic and European Respiratory, 2005). These were then analyzed on a Sievers 280i NO Analyzer (Boulder, CO).
2.3 Exhaled Breath Condensate Collection
Subjects rinsed their mouths with tap water and placed on noseclips to minimize oral and nasopharyngeal contamination. Exhaled breath condensate (EBC) was provided by breathing into a commercially available RTube™ EBC collector (Respiratory Research, Charlottesville, VA). We chose this modality at the time of study because of the RTube’s™ portability and ease of use. A sleeve cooled to between −40C to −50 °C by dry ice was placed over the RTube™ prior to collection. Subjects breathed between 10–15 minutes of tidal volume into the RTube™. The EBC was collected by sterile pipette and placed into an Eppendorf tube for storage at −80 °C.
2.4 GC/DMS Analysis
A 500 microliter to 1 mL aliquot of EBC was placed into a borosilicate vial capped with a silicon septum. The vial was placed in a water bath, heated to 90 °C, and the headspace over the EBC was sampled using a polyacrylate (PA) solid phase microextractionfiber (SPME, Supelco, Inc., Bellefonte, PA) to promote extraction of polar compounds from the sample head space. For extraction, the SPME fibre was inserted through septum and kept above the sample for 1 hour without EBC sample agitation. This was performed to optimize VOC collection on the SPME fibre according to previously published work in this area (Snow, 2000a, Davis et al., 2010). The sorbed chemicals were then analysed using a GC/DMS method described below.
The operational principles and design specifications the DMS sensor are described elsewhere (Kolakowski and Mester 2007) (Anderson, Markoski et al. 2008). A schematic representation of the system is shown in Fig. 1. For this study we utilized the microDMX sensor, manufactured by Sionex (Waltham, MA). The detailed design specifications and performance descriptions are given elsewhere (Petinarides et al., 2005, Coy et al., 2008, Miller et al., 2006) Specifically, the DMS device is comprised of two parallel electrodes with 0.5 mm gap. The GC column eluent in helium at 1 mL / min flow rate was mixed with ultra high purity nitrogen carrier gas (Airgas, Sacramento, CA) and passed at the flow rate of 300 mL / min through the DMS device. The analyte was ionized with 63Ni ionization source prior to entering the DMS device. The water content in the carrier gas was below 1 ppm, as reported by the manufacturer. The DMS unit was operated under the following conditions: asymmetric waveform amplitude 1000 V (in the gap of 0.5 mm, this corresponds to electric field of 20V/cm, ~80 Td); RF frequency 1.196 MHz with the asymmetric waveform about 34% high field and 66% low field; and CV scan range −43 to +15 V. The CV range of 60 V was scanned with 0.6 V step at 10 millisecond per step, corresponding to 100 steps and 1 second total CV scan time. The wave function generator employed in this study can produce waveform with maximum amplitude of 1500 V. However, due to the presence of helium in the carrier gas from the GC column eluent, we chose to operate at the lower dispersion voltage of 1000 V to avoid possible electrical breakdown..
Figure 1.
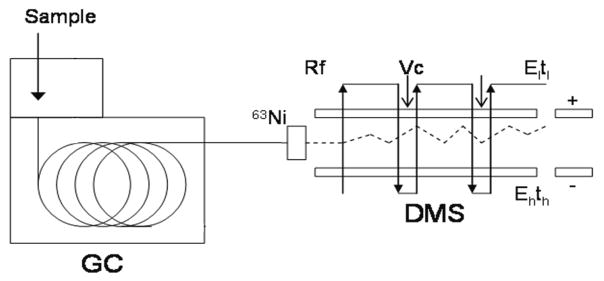
Schematic representation of the GC/DMS instrument setup. The volatile compounds collected from headspace of EBC are introduced into gas chromatograph (GC) column for chromatographic separation. The sample eluting from GC column is ionized with 63Ni ion source prior to entering the DMS sensor. The filtered ions are detected at their corresponding compensation voltage values. El:, low-field electric field strength, tl: duration of the low-field portion of the waveform ; Eh:, high-field electric field strength; th: duration of the high-field portion of the waveform Rf, radiofrequency waveform; Vc, compensation voltage
2.5 Statistical Analysis
All demographic patient characteristics were compared between clinical groups using the Student’s t-test or Wilson Rank Sum test where appropriate in STATISTICA (StatSoft, Tulsa, OK). DMS data analysis performed using MatLab R2011a (Mathworks, Torrence, CA) and PLS Toolbox 6.7.1 (Eigenvector Research Inc, Wenatchee, WA).
3. Results
3.1 Subjects
A total 31 subject samples were screened. The clinical groups differed significantly in their baseline characteristics including age, body mass index, pre-bronchodilator spirometry, and eNO levels (see Table 1). These differences were expected as patients with asthma tend to be younger, have better lung function, and a higher BMI than patients with COPD. There was an unanticipated similarity between eNO levels in the asthma and COPD groups, however, our subjects reflect patients often encountered in practice rather than an artificially-selected cohort.
Table 1.
Subject baseline characteristics.
| Healthy | Asthma | COPD | |
|---|---|---|---|
| n | 13 | 13 | 5 |
| Age in yrs (range)*‡§ | 33 (23–66) | 50 (18–69) | 70.5 (48–89) |
| Sex, M/F (n/n) | 11/12 | 7/14 | 13/5 |
| BMI (range)**§ | 24.2 (18.2–30.9) | 30.9 (20–53.4) | 25.2 (18.6–34.5) |
| Spirometry**ठ| |||
| FEV1 ±SD, prebronchodilator (L) | 3.53 ± 0.68 | 2.13 ± 0.93 | 1.21 ± 0.57 |
| FEV1/FVC ±SD (% predicted) | 82 ± 8.2 | 69 ± 13 | 49 ± 14 |
| NO ±SD (ppb)† | 16 ± 11 | 24 ± 22 | 25 ± 12 |
| Omalizumab use | 0 | 9 | 0 |
p<0.05;
p<0.001 Normal-Asthma
p<0.05;
p<0.001 Normal-COPD
p<0.01 Asthma-COPD
3.2 Algorithm for sample selection for analysis
Fig 2 delineates the algorithm for EBC VOC data sample selection used during multivariate data analysis.
Figure 2.
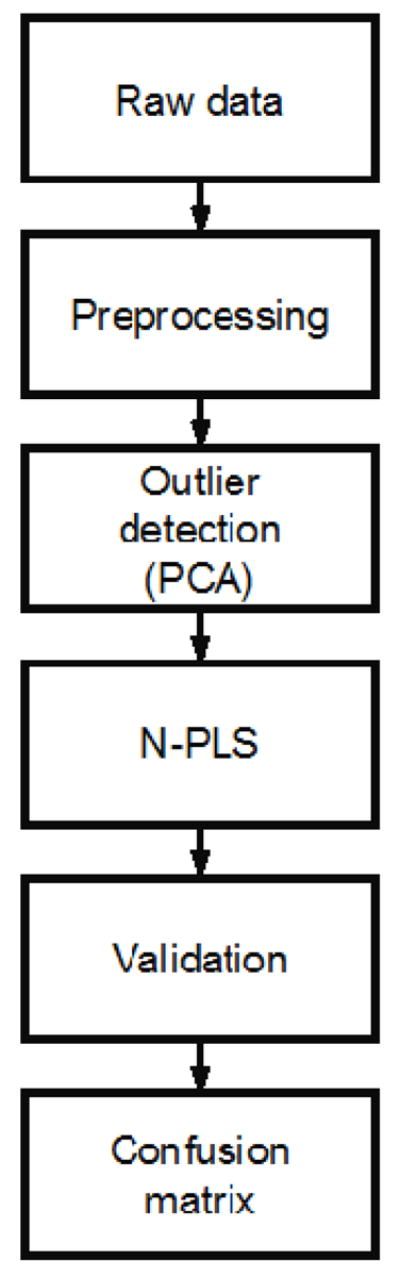
Algorithm for DMS dataset selection. The raw data is assessed for analytic suitability by applying principle component analysis (PCA); the prediction model is built after Partial Least Square analysis (N-PLS); the data is validated; and a confusion matrix is created
To select the DMS datasets which display high-quality information, the data was subject to several evaluation techniques. Initially, all datasets were preprocessed with baseline correction and smoothing (Savitzky and Golay, 1964) to facilitate data comparison. Principle component analysis (PCA) was used to reduce dimensionality (Wold et al., 1987). This enables composite data analysis, outlier detection, and selection of data fitting within an acceptable range for further analysis. Following the selection of datasets from PCA, we used multiway partial least square (N-PLS) regression to find the relation between the X (cube of data) and Y (labels) data spaces in order to model the covariance structures (Bro, 1996).
An N-PLS model attempts to determine the multidimensional direction in the X space that explains the maximum multidimensional variance direction in the Y space. A representative sample dataset from the asthma group is displayed in Figure 2a and 2b.
3.3 Group separation based on GC/DMS data
After processing the DMS datasets to select appropriate sets to analyze, we performed a validation based on previously published methods (Westerhuis et al., 2008). Briefly, the data cube was divided into a “test set” containing 10% of data and a model set containing 90% of data. The test set was then introduced into the model as quasi-unknown data resulting in a classification output. This output was compared to the apriori known classification of the datapoints (i.e. asthma, control or COPD) resulting in a correct classification (true positive, TP, or true negative, TN) or an incorrect classification (false positive, FP, or false negative, FN). This process was repeated several times in iterations called “loops” in order to identify the performance of the established model.
Figures 3a and 3b represent the confusion matrices produced from such multiple loops. The best levels of classification resulted from the asthma versus control groups and from the subjects taking omalizumab versus healthy patients not on this medication. The results show the mean percent classification for TP and FP for each group from all performed loops (20 for asthma vs. control, 40 for omalizumab vs. none), with each mean assigned a standard error.
Figure 3.
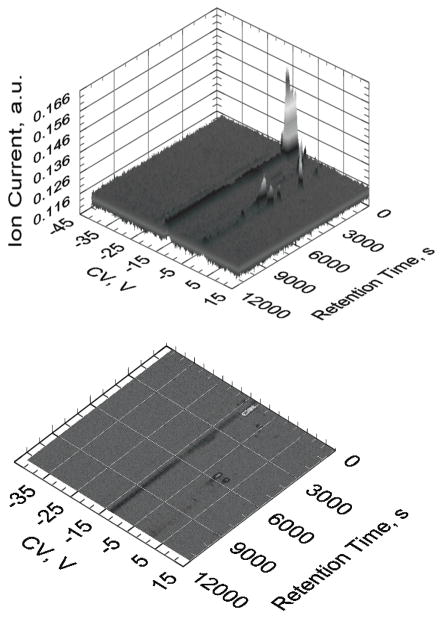
Figure 3a & 3b. Representations of quality DMS plots from our asthma data. Fig. 2a (top) shows a 3-dimensional plot with compensation voltage (CV) on the x-axis, retention time on the y-axis, and ion count (IC) on the z-axis. Each VOC has a unique RT & CV which can be used to ultimately identify the VOC. Fig 2b (bottom) shows a typical 2-dimensional output view of the 3-dimensional graph in 2a.
4. Discussion
4.1 Interpretation of the results
In the present study we demonstrated that clinically-relevant groups may in part be classified and identified using GC/DMS analysis of the VOCs from EBC and using appropriate multivariate data analysis strategies. After executing 20 classification optimization loops on the asthma-control groups, we were able to correctly classify asthma subjects 75% of the time. While this number is certainly lower than desired for a diagnostic test, the potential of the proposed analytical technique is readily demonstrated. With improvements in our small sample size, the classification may be further enhanced. Similarly, we were able to correctly discriminate subjects taking omalizumab from subjects not taking this medication 70% of the time after executing 40 loops.
Our study differs from previous efforts in the field of mobile high-dimensional breath diagnostics in several key ways. First, no study using DMS technology to discriminate between asthma and COPD populations has been conducted to date. Second, our study used EBC rather than single-breath capture. EBC theoretically contains a higher abundance of VOCs and non-volatile compounds, is easier to pre-concentrate, and may be easier to standardize, though data on this is lacking. Ultimate breath diagnostic methods will ideally use single breath capture, however, in our study we aimed to maximize the quantity of VOCs. Last, our study design included a mixed cohort of patients reflective of those commonly encountered in clinical practice. The intent was to present a potential real-world application of the DMS technology, though our groups may have been more similar biochemically than different (see Limitations below). Future studies of this nature will need to utilize highly-selected groups (i.e. COPD with advanced fixed airflow obstruction and radiographic emphysema).
The ability to classify asthma from non-asthmatic patients is of high clinical relevance. For example, a condition called vocal cord dysfunction (VCD) may mimic the symptoms of asthma, yet it is entirely different in clinical course and therapy (Benninger et al., 2011). Hence, correctly classifying a patient as not having asthma if confusing symptoms are present may prompt an appropriate evaluation and, consequently, effective therapy. Though the model established herein does not have sufficient discriminatory power for a diagnostic test to correctly differentiate asthma from non-asthma, the results are promising and future studies with larger numbers of subjects and more samples per case should provide significantly improved classification models.
The determination of significant differences in VOC profiles between subjects taking omalizumab and healthy controls not taking the medication is interesting. Asthmatics who are taking omalizumab often have moderate to severe disease, and they may represent a distinct biological group from those patients with mild asthma. Asthma severity may engender more robust VOC metabolites and, thus, severe asthmatics may be a good patient cohort to study with respect to noninvasive diagnosis. An alternative hypothesis is that omalizumab itself may yield a set of distinct VOCs after being metabolized, and this may contribute to the observed differences. Finally, omalizumab produces a host of pharmacological effects, which help bring down the inflammatory status in the omalizumab-treated patients, which, in turn, may ultimately manifest in alteration of VOC profile. While to date there is little knowledge on the secondary effects of medication on VOC production and presence in exhaled breath, a noteworthy contribution may be suspected and will be subject to future studies.
While the initial aim of this study was differentiating asthma from COPD as the clinically most interesting goal, the limitations of the currently available dataset did not support this classification. This is most likely a result of the limited sample size (see below); yet, the quality of the obtained data clearly indicates that with a larger group of subjects a separation between asthma and COPD is very likely. Also, in the course of the study it turned out that the asthma and COPD groups may have been biologically more related rather than different, which reflects in the elevated eNO levels of both groups when they should have been significantly lower in the COPD group. Elevated eNO levels in the COPD cohort suggest a significant amount of allergic disease, and future studies will need to identify and exclude such overlapping populations (Zeki et al., 2011) when trying to validate novel diagnostics. However, the DMS system was able to distinguish between the control and asthma groups, which had significantly different eNO levels after removing outliers.
4.2 Limitations
While this feasibility study clearly demonstrates the utility of GC/DMS for exhaled breath VOC analysis to discriminate clinically well-defined patient groups, several limitations of the current experimental design and analysis are recognized.
Most evident is that the small number of subjects within the clinical groups is insufficient for establishing robust predictive models, as reflected in the standard errors. In the investigated patient cohort, several of the COPD subjects were of the emphysema subtype, and thus, produced only small volumes of EBC. This resulted in inadequate EBC samples for analysis from many subjects and further limited the available sample size. In addition, the GC/DMS analysis and sampling methodologies could be further optimized. At present, there is little a priori knowledge of defined chemical constituents that are distinctive within the exhaled breath of asthma or COPD populations. Once such information is available, sorbent matrices may specifically be selected to enhance the recovery of specific VOCs of interest from the EBC headspace while simultaneously minimizing the matrix background. Finally, given the fact that this was a feasibility study, the recruitment numbers were expectedly low. Yet, demonstrating the potential of the method should significantly increase the number of subjects available to future extended studies. Nevertheless, it is evident that the between-group differences determined herein would likely be significantly more pronounced if the sample sizes per case were larger, thus advancing the discriminatory power of the developed predictive models.
During the present study, the RTube™ was used to collect EBC. Alternative breath collection devices may yield different quantities and qualities of EBC, although reliable data on this matter is disparate (Huttmann et al., 2011, Davidsson and Schmekel, 2010, Koczulla et al., 2009). Based on recent studies of our laboratory and others (Loyola et al., 2008, Czebe et al., 2008, Horvath et al., 2005, Liu et al., 2007), it is anticipated that conducting a similar clinical trial using continuously cooled EBC collection devices may yield more robust VOC data and further enhance the between-group differences.
The SPME headspace sample extraction procedure employed in the present work, though guided by the previous studies (Davis et al., 2010, Snow, 2000b) and directly utilizing protocols suggested by the SPME manufacturers, may not be specifically optimized for the cohort of subjects included in this study. We have conducted the extraction from the sample heated to 90 °C as suggested by the manufacturer as a possible mean to promote SPME headspace extraction (http://www.sigmaaldrich.com/analytical-chromatography/sample-preparation/spme/faq.html, 2012). This temperature is at the upper portion of the recommended temperature range, and we recognize that some VOC loss may occur into the septum using this protocol.
During future studies, several adaptations may further enhance the discriminatory power with the most substantial impact possibly achieved via concerted mass spectrometry studies for reliably establishing the chemical identities of asthma and COPD VOC biomarkers. Apart from the optimization of sampling methods, DMS measurements may also be improved. The ionization of analyte occurs according to the basicity distributions in proton transfer reactions or according to dissociation energies in charge transfer reactions. Thus, they differ in efficiency for each individual chemical moiety. Therefore, it is possible that the analytes of interest are suppressed by matrix interferences or are not efficiently ionized. Both scenarios lead to suppressed useful analytical signals, and thus, to impaired diagnostic results. For example, if the analytes of interest include aliphatic hydrocarbons, the 63Ni source used in the present study would not efficiently ionizes these species. However, it has been reported that alkanes are highly relevant biomarkers indicative of oxidative stress associated with inflammation processes (Phillips et al., 2010). Further advancements may be achieved by modification of the carrier gas. In the present study, predominantly (dry) nitrogen was used. Addition of up to 50% of He would lead to a significant increase of DMS separation space (McCooeye et al., 2001), and may thus enable better resolving signals from biomarkers species that are clinically important. While the DMS device used herein was interfaced to a conventional bench-top GC system, the potential of GC/DMS technology for bed-side monitoring could be fully unlocked upon further miniaturization of the GC component.
Last, an analyte diagnostic library does not currently exist for the DMS system in spite of the parameters which could be used for species identification. In this study, comparison of the GC/DMS data to GC/MS data would have greatly helped with species identification. Future studies may need to obtain both GC/DMS and GC/MS data from the same subjects until a suitable DMS library exists.
Despite these shortcomings, this proof-of-principle study shows the potential of DMS sensing in combination with gas chromatography providing potentially valuable diagnostic information on VOC biomarker panels in exhaled breath for non-invasive disease diagnostics and disease discrimination.
5. Conclusion
Asthma and COPD represent two airway disorders which are difficult to accurately diagnose and treat. Consequently, improved diagnostic strategies are needed which are sensitive, portable, and complementary to available diagnostic tools. DMS is such a platform, and this study demonstrates that analysis of DMS VOC spectra can recognize differences between clinically well-defined groups. Though this study discriminated asthma from healthy controls as well as the subgroup of asthmatics taking omalizumab from healthy controls, the DMS technology can be applied to many other respiratory disorders. DMS has implications for both office diagnostics and as a research tool to ultimately identify specific VOCs which may function in the pathobiology of respiratory diseases. Further clinical studies should focus on larger subject recruitment numbers, improved EBC collection, and improved DMS (or other FAIMS technology) analyte measurements.
Figure 4.
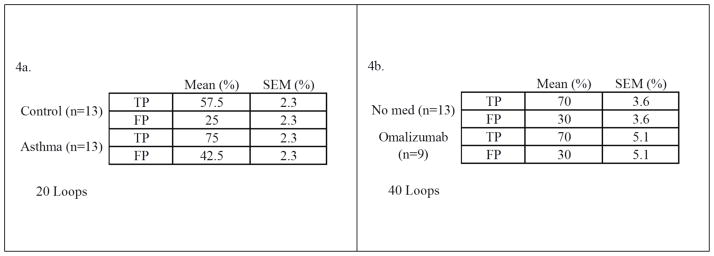
Figure 4a & 4b. Confusion matrices describing the performance of the models to classify asthma from controls (Fig. 3a, left) and subjects taking omalizumab from those not taking this medication (Fig 3b, right). The left graph shows the mean results of 20 executed loops of the dataset. Right, the results of 40 loops are shown.
Figure 5.
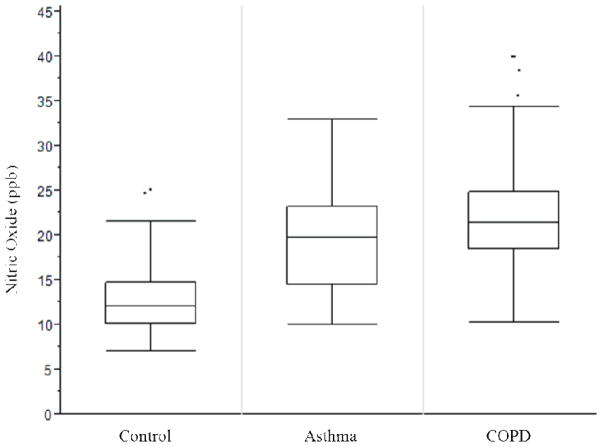
Box-whisker plot of exhaled nitric oxide (eNO) levels by group. Though there were no statistical eNO differences between the asthma and COPD groups, there is a clear separation between the control and asthma groups after appropriate removal of 3 outliers from each group (p<0.001, unpaired Student’s t-test). This shows that the selected control group was biologically different than the asthma group, as detected by the DMS system. The similar eNO levels between the asthma and COPD groups indicates a biologic similarity between these groups.
Acknowledgments
This work was generously and partially supported by several funding agencies. The content of this work is solely the responsibility of the authors and does not necessarily represent the official view of these agencies. Partial support is acknowledged from: UL1 RR024146 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research [CED, NJK]; NIH #HL 105573 [NJK]; Gilead Sciences, Inc. [CED]; the Defense Advanced Research Projects Agency (DARPA) [CED]; Department of the Army [CED], The Hartwell Foundation [CED, NJK]; NIH #T32-HL007013 and #T32-ES007059 [MS]; UC Davis School of Medicine and NIH #8KL2TR000134-07 K12 mentored training award [MS]; the German Academic Exchange Services (DAAD) [FS]; US Department of Veterans Affairs, Post-9/11 GI-Bill [DJP].
References
- 1.Standardization of spirometry-- update. Official statement of American Thoracic Society. Respir Care. 1987;32:1039–60. [PubMed] [Google Scholar]
- 2.AKSENOV AA, KAPRON J, DAVIS CE. Predicting Compensation Voltage for Singly-charged Ions in High-Field Asymmetric Waveform Ion Mobility Spectrometry (FAIMS) J Am Soc Mass Spectrom. 2012;23:1794–1798. doi: 10.1007/s13361-012-0427-6. [DOI] [PubMed] [Google Scholar]
- 3.AMERICAN THORACIC, S. & EUROPEAN RESPIRATORY, S. . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 4.BARNES PJ, DWEIK RA, GELB AF, GIBSON PG, GEORGE SC, GRASEMANN H, PAVORD ID, RATJEN F, SILKOFF PE, TAYLOR DR, ZAMEL N. Exhaled nitric oxide in pulmonary diseases: a comprehensive review. Chest. 2010;138:682–92. doi: 10.1378/chest.09-2090. [DOI] [PubMed] [Google Scholar]
- 5.BASANTA M, JARVIS RM, XU Y, BLACKBURN G, TAL-SINGER R, WOODCOCK A, SINGH D, GOODACRE R, THOMAS CL, FOWLER SJ. Non-invasive metabolomic analysis of breath using differential mobility spectrometry in patients with chronic obstructive pulmonary disease and healthy smokers. Analyst. 2010;135:315–20. doi: 10.1039/b916374c. [DOI] [PubMed] [Google Scholar]
- 6.BASANTA M, SINGH D, FOWLER S, WILSON I, DENNIS R, THOMAS CL. Increasing analytical space in gas chromatography-differential mobility spectrometry with dispersion field amplitude programming. J Chromatogr A. 2007;1173:129–38. doi: 10.1016/j.chroma.2007.09.082. [DOI] [PubMed] [Google Scholar]
- 7.BENNINGER C, PARSONS JP, MASTRONARDE JG. Vocal cord dysfunction and asthma. Curr Opin Pulm Med. 2011;17:45–9. doi: 10.1097/MCP.0b013e32834130ee. [DOI] [PubMed] [Google Scholar]
- 8.BESSA V, DARWICHE K, TESCHLER H, SOMMERWERCK U, RABIS T, BAUMBACH JI, FREITAG L. Detection of volatile organic compounds (VOCs) in exhaled breath of patients with chronic obstructive pulmonary disease (COPD) by ion mobility spectrometry. Int J Ion Mobility Spectrom. 2011;14:7–13. [Google Scholar]
- 9.BORSDORF H, EICEMAN GA. Ion mobility spectrometry: principles and applications. Appl Spectrosc Rev. 2006;41:323–375. [Google Scholar]
- 10.BRO R. Multiway calibration. Multilinear PLS. Journal of Chemometrics. 1996;10:47–61. [Google Scholar]
- 11.BUXTON TL, DE HARRINGTON PB. Trace explosive detection in aqueous samples by solidphase extraction ion mobility spectrometry (SPE-IMS) Appl Spectrosc. 2003;57:223–32. doi: 10.1366/000370203321535150. [DOI] [PubMed] [Google Scholar]
- 12.CALDEIRA M, PERESTRELO R, BARROS AS, BILELO MJ, MORETE A, CAMARA JS, ROCHA SM. Allergic asthma exhaled breath metabolome: A challenge for comprehensive twodimensional gas chromatography. J Chromatogr A. 2012;1254:87–97. doi: 10.1016/j.chroma.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 13.COY SL, KRYLOV EV, NAZAROV EG. DMS-prefiltered mass spectrometry for the detection of biomarkers. Proc SPIE. 2008;6954:695411/1–695411/10. [Google Scholar]
- 14.CZEBE K, BARTA I, ANTUS B, VALYON M, HORVATH I, KULLMANN T. Influence of condensing equipment and temperature on exhaled breath condensate pH, total protein and leukotriene concentrations. Respir Med. 2008;102:720–5. doi: 10.1016/j.rmed.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 15.DAVIDSSON A, SCHMEKEL B. Efficacy of two breath condensers. J Clin Lab Anal. 2010;24:219–23. doi: 10.1002/jcla.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DAVIS CE, BOGAN MJ, SANKARAN S, MOLINA MA, LOYOLA BR, ZHAO W, BENNER WH, SCHIVO M, FARQUAR GR, KENYON NJ, FRANK M. Analysis of volatile and non-volatile biomarkers in human breath using differential mobility spectrometry (DMS) IEEE Sens J. 2010;10:114–122. [Google Scholar]
- 17.DECRAMER M, CELLI B, KESTEN S, LYSTIG T, MEHRA S, TASHKIN DP, INVESTIGATORS U. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): a prespecified subgroup analysis of a randomised controlled trial. Lancet. 2009;374:1171–8. doi: 10.1016/S0140-6736(09)61298-8. [DOI] [PubMed] [Google Scholar]
- 18.DRAGONIERI S, SCHOT R, MERTENS BJ, LE CESSIE S, GAUW SA, SPANEVELLO A, RESTA O, WILLARD NP, VINK TJ, RABE KF, BEL EH, STERK PJ. An electronic nose in the discrimination of patients with asthma and controls. J Allergy Clin Immunol. 2007;120:856–62. doi: 10.1016/j.jaci.2007.05.043. [DOI] [PubMed] [Google Scholar]
- 19.DUNN JD, GRYNIEWICZ-RUZICKA CM, KAUFFMAN JF, WESTENBERGER BJ, BUHSE LF. Using a portable ion mobility spectrometer to screen dietary supplements for sibutramine. J Pharm Biomed Anal. 2011;54:469–74. doi: 10.1016/j.jpba.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 20.DWEIK RA, BOGGS PB, ERZURUM SC, IRVIN CG, LEIGH MW, LUNDBERG JO, OLIN AC, PLUMMER AL, TAYLOR DR AMERICAN THORACIC SOCIETY COMMITTEE ON INTERPRETATION OF EXHALED NITRIC OXIDE LEVELS FOR CLINICAL A. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med. 2011;184:602–15. doi: 10.1164/rccm.9120-11ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DWEIK RA, SORKNESS RL, WENZEL S, HAMMEL J, CURRAN-EVERETT D, COMHAIR SA, BLEECKER E, BUSSE W, CALHOUN WJ, CASTRO M, CHUNG KF, ISRAEL E, JARJOUR N, MOORE W, PETERS S, TEAGUE G, GASTON B, ERZURUM SC NATIONAL HEART L & BLOOD INSTITUTE SEVERE ASTHMA RESEARCH P. Use of exhaled nitric oxide measurement to identify a reactive, at-risk phenotype among patients with asthma. Am J Respir Crit Care Med. 2010;181:1033–41. doi: 10.1164/rccm.200905-0695OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.EICEMAN GA, KARPAS Z. Ion Mobility Spectrometry. 2. CRC Press; 2005. [Google Scholar]
- 23.EICEMAN GA, NAZAROV EG, MILLER RA, KRYLOV EV, ZAPATA AM. Micro-machined planar field asymmetric ion mobility spectrometer as a gas chromatographic detector. Analyst. 2002;127:466–71. doi: 10.1039/b111547m. [DOI] [PubMed] [Google Scholar]
- 24.FENS N, ROLDAAN AC, VAN DER SCHEE MP, BOKSEM RJ, ZWINDERMAN AH, BEL EH, STERK PJ. External validation of exhaled breath profiling using an electronic nose in the discrimination of asthma with fixed airways obstruction and chronic obstructive pulmonary disease. Clin Exp Allergy. 2011;41:1371–8. doi: 10.1111/j.1365-2222.2011.03800.x. [DOI] [PubMed] [Google Scholar]
- 25.FENS N, ZWINDERMAN AH, VAN DER SCHEE MP, DE NIJS SB, DIJKERS E, ROLDAAN AC, CHEUNG D, BEL EH, STERK PJ. Exhaled breath profiling enables discrimination of chronic obstructive pulmonary disease and asthma. Am J Respir Crit Care Med. 2009;180:1076–82. doi: 10.1164/rccm.200906-0939OC. [DOI] [PubMed] [Google Scholar]
- 26.GELB AF, BARNES PJ, GEORGE SC, RICCIARDOLO FL, DIMARIA G, ZAMEL N. Review of exhaled nitric oxide in chronic obstructive pulmonary disease. J Breath Res. 2012;6:047101. doi: 10.1088/1752-7155/6/4/047101. [DOI] [PubMed] [Google Scholar]
- 27.GRYNIEWICZ CM, REEPMEYER JC, KAUFFMAN JF, BUHSE LF. Detection of undeclared erectile dysfunction drugs and analogues in dietary supplements by ion mobility spectrometry. J Pharm Biomed Anal. 2009;49:601–6. doi: 10.1016/j.jpba.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 28.GUERRA-DIAZ P, GURA S, ALMIRALL JR. Dynamic planar solid phase microextraction-ion mobility spectrometry for rapid field air sampling and analysis of illicit drugs and explosives. Anal Chem. 2010;82:2826–35. doi: 10.1021/ac902785y. [DOI] [PubMed] [Google Scholar]
- 29.GUEVREMONT R. High-field asymmetric waveform ion mobility spectrometry: a new tool for mass spectrometry. J Chromatogr A. 2004;1058:3–19. [PubMed] [Google Scholar]
- 30.HORVATH I, HUNT J, BARNES PJ, ALVING K, ANTCZAK A, BARALDI E, BECHER G, VAN BEURDEN WJ, CORRADI M, DEKHUIJZEN R, DWEIK RA, DWYER T, EFFROS R, ERZURUM S, GASTON B, GESSNER C, GREENING A, HO LP, HOHLFELD J, JOBSIS Q, LASKOWSKI D, LOUKIDES S, MARLIN D, MONTUSCHI P, OLIN AC, REDINGTON AE, REINHOLD P, VAN RENSEN EL, RUBINSTEIN I, SILKOFF P, TOREN K, VASS G, VOGELBERG C, WIRTZ H, CONDENSATE AETFOEB. Exhaled breath condensate: methodological recommendations and unresolved questions. Eur Respir J. 2005;26:523–48. doi: 10.1183/09031936.05.00029705. [DOI] [PubMed] [Google Scholar]
- 31. [Accessed 10/15/2012 2012];Solid Phase Microextraction (SPME): Sample Preparation and Purification. 2012 HTTP://WWW.SIGMAALDRICH.COM/ANALYTICAL-CHROMATOGRAPHY/SAMPLEPREPARATION/SPME/FAQ.HTML. [Online]. On line: Sigma Aldrich, LLC.
- 32.HUTTMANN EM, GREULICH T, HATTESOHL A, SCHMID S, NOESKE S, HERR C, JOHN G, JORRES RA, MULLER B, VOGELMEIER C, KOCZULLA AR. Comparison of two devices and two breathing patterns for exhaled breath condensate sampling. PLoS One. 2011;6:e27467. doi: 10.1371/journal.pone.0027467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.IBRAHIM B, BASANTA M, CADDEN P, SINGH D, DOUCE D, WOODCOCK A, FOWLER SJ. Non-invasive phenotyping using exhaled volatile organic compounds in asthma. Thorax. 2011;66:804–9. doi: 10.1136/thx.2010.156695. [DOI] [PubMed] [Google Scholar]
- 34.JAFARI MT, REZAEI B, ZAKER B. Ion mobility spectrometry as a detector for molecular imprinted polymer separation and metronidazole determination in pharmaceutical and human serum samples. Anal Chem. 2009;81:3585–91. doi: 10.1021/ac802557t. [DOI] [PubMed] [Google Scholar]
- 35.KARNER C, CATES CJ. Long-acting beta(2)-agonist in addition to tiotropium versus either tiotropium or long-acting beta(2)-agonist alone for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;4:CD008989. doi: 10.1002/14651858.CD008989.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.KIKOWATZ A, BECHER G, DIETZE S, STEINHAUSSER W, BECK E. Differential ion mobility spectroscopy: non-invasive real-time diagnostics and therapy control in metabolic diseases. Eur J Med Res. 2009;14(Suppl 4):121–5. doi: 10.1186/2047-783X-14-S4-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.KOCZULLA R, DRAGONIERI S, SCHOT R, BALS R, GAUW SA, VOGELMEIER C, RABE KF, STERK PJ, HIEMSTRA PS. Comparison of exhaled breath condensate pH using two commercially available devices in healthy controls, asthma and COPD patients. Respir Res. 2009;10:78. doi: 10.1186/1465-9921-10-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.KOCZULLA R, HATTESOHL A, SCHMID S, BOEDEKER B, MADDULA S, BAUMBACH JI. MCC/IMS as potential noninvasive technique in the diagnosis of patients with COPD with and without alpha 1-antitrypsin deficiency. Int J Ion Mobility Spectrom. 2011;14:177–185. [Google Scholar]
- 39.KREBS MD, MANSFIELD B, YIP P, COHEN SJ, SONENSHEIN AL, HITT BA, DAVIS CE. Novel technology for rapid species-specific detection of Bacillus spores. Biomol Eng. 2006;23:119–27. doi: 10.1016/j.bioeng.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 40.LIU J, CONRAD DH, CHOW S, TRAN VH, YATES DH, THOMAS PS. Collection devices influence the constituents of exhaled breath condensate. Eur Respir J. 2007;30:807–8. doi: 10.1183/09031936.00080207. [DOI] [PubMed] [Google Scholar]
- 41.LOYOLA BR, BHUSHAN A, SCHIVO M, KENYON NJ, DAVIS CE. Temperature changes in exhaled breath condensate collection devices affect observed acetone concentrations. J Breath Res. 2008;2:037005. doi: 10.1088/1752-7155/2/3/037005. [DOI] [PubMed] [Google Scholar]
- 42.MCCOOEYE MA, ELLS B, BARNETT DA, PURVES RW, GUEVREMONT R. Quantitation of morphine and codeine in human urine using high-field asymmetric waveform ion mobility spectrometry (FAIMS) with mass spectrometric detection. J Anal Toxicol. 2001;25:81–87. doi: 10.1093/jat/25.2.81. [DOI] [PubMed] [Google Scholar]
- 43.MILLER MR, HANKINSON J, BRUSASCO V, BURGOS F, CASABURI R, COATES A, CRAPO R, ENRIGHT P, VAN DER GRINTEN CP, GUSTAFSSON P, JENSEN R, JOHNSON DC, MACINTYRE N, MCKAY R, NAVAJAS D, PEDERSEN OF, PELLEGRINO R, VIEGI G, WANGER J, FORCE AET. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 44.MILLER RA, NAZAROV E, COY SL, KRYLOV E. Miniature differential mobility spectrometer as a pre-filter for atmospheric-pressure mass spectrometry. Int J Ion Mobility Spectrom. 2006;9:35–39. doi: 10.1016/j.ijms.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.MOLINA MA, ZHAO W, SANKARAN S, SCHIVO M, KENYON NJ, DAVIS CE. Design-of-experiment optimization of exhaled breath condensate analysis using a miniature differential mobility spectrometer (DMS) Anal Chim Acta. 2008;628:155–61. doi: 10.1016/j.aca.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MONTUSCHI P, SANTONICO M, MONDINO C, PENNAZZA G, MANTINI G, MARTINELLI E, CAPUANO R, CIABATTONI G, PAOLESSE R, DI NATALE C, BARNES PJ, D’AMICO A. Diagnostic performance of an electronic nose, fractional exhaled nitric oxide, and lung function testing in asthma. Chest. 2010;137:790–6. doi: 10.1378/chest.09-1836. [DOI] [PubMed] [Google Scholar]
- 47.NELSON HS, WEISS ST, BLEECKER ER, YANCEY SW, DORINSKY PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- 48.PERR JM, FURTON KG, ALMIRALL JR. Solid phase microextraction ion mobility spectrometer interface for explosive and taggant detection. J Sep Sci. 2005;28:177–83. doi: 10.1002/jssc.200401893. [DOI] [PubMed] [Google Scholar]
- 49.PETINARIDES J, GRIFFIN MT, MILLER RA, NAZAROV EG, BASHALL AD. Implementation of a new technology for point detection. Proc SPIE-Int Soc Opt Eng. 2005;5795:65–74. [Google Scholar]
- 50.PHILLIPS M, CATANEO RN, CHATURVEDI A, DANAHER PJ, DEVADIGA A, LEGENDRE DA, NAIL KL, SCHMITT P, WAI J. Effect of influenza vaccination on oxidative stress products in breath. J Breath Res. 2010;4:026001. doi: 10.1088/1752-7155/4/2/026001. [DOI] [PubMed] [Google Scholar]
- 51.QASEEM A, WILT TJ, WEINBERGER SE, HANANIA NA, CRINER G, VAN DER MOLEN T, MARCINIUK DD, DENBERG T, SCHUNEMANN H, WEDZICHA W, MACDONALD R, SHEKELLE P AMERICAN COLLEGE OF, P., AMERICAN COLLEGE OF CHEST, P., AMERICAN THORACIC, S. & EUROPEAN RESPIRATORY, S. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155:179–91. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 52.RABE KF, HURD S, ANZUETO A, BARNES PJ, BUIST SA, CALVERLEY P, FUKUCHI Y, JENKINS C, RODRIGUEZ-ROISIN R, VAN WEEL C, ZIELINSKI J GLOBAL INITIATIVE FOR CHRONIC OBSTRUCTIVE LUNG D. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–55. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 53.RODRIGO GJ, NEFFEN H, CASTRO-RODRIGUEZ JA. Efficacy and safety of subcutaneous omalizumab vs placebo as add-on therapy to corticosteroids for children and adults with asthma: a systematic review. Chest. 2011;139:28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 54.SAVITZKY A, GOLAY MJE. Smoothing + Differentiation of Data by Simplified Least Squares Procedures. Analytical Chemistry. 1964;36:1627. [Google Scholar]
- 55.SHNAYDERMAN M, MANSFIELD B, YIP P, CLARK HA, KREBS MD, COHEN SJ, ZESKIND JE, RYAN ET, DORKIN HL, CALLAHAN MV, STAIR TO, GELFAND JA, GILL CJ, HITT B, DAVIS CE. Species-specific bacteria identification using differential mobility spectrometry and bioinformatics pattern recognition. Anal Chem. 2005;77:5930–7. doi: 10.1021/ac050348i. [DOI] [PubMed] [Google Scholar]
- 56.SHVARTSBURG AA, MASHKEVICH SV, SMITH RD. Feasibility of higher-order differential ion mobility separations using new asymmetric waveforms. J Phys Chem A. 2006;110:2663–73. doi: 10.1021/jp055349t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.SNOW NH. Solid-phase micro-extraction of drugs from biological matrices. J Chromatogr A. 2000a;885:445–55. doi: 10.1016/s0021-9673(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 58.SNOW NH. Solid-phase micro-extraction of drugs from biological matrices. J Chromatogr A. 2000b;885:445–455. doi: 10.1016/s0021-9673(00)00192-8. [DOI] [PubMed] [Google Scholar]
- 59.VAN BERKEL JJBN, DALLINGA JW, MOLLER GM, GODSCHALK RWL, MOONEN EJ, WOUTERS EFM, VAN SCHOOTEN FJ. A profile of volatile organic compounds in breath discriminates COPD patients from controls. Respiratory Medicine. 2010;104:557–563. doi: 10.1016/j.rmed.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 60.WESTERHUIS JA, HOEFSLOOT HCJ, SMIT S, VIS DJ, SMILDE AK, VAN VELZEN EJJ, VAN DUIJNHOVEN JPM, VAN DORSTEN FA. Assessment of PLSDA cross validation. Metabolomics. 2008;4:81–89. [Google Scholar]
- 61.WESTHOFF M, LITTERST P, FREITAG L, BAUMBACH JI. Ion mobility spectrometry in the diagnosis of sarcoidosis: results of a feasibility study. J Physiol Pharmacol. 2007;58(Suppl 5):739–51. [PubMed] [Google Scholar]
- 62.WESTHOFF M, LITTERST P, FREITAG L, URFER W, BADER S, BAUMBACH JI. Ion mobility spectrometry for the detection of volatile organic compounds in exhaled breath of patients with lung cancer: results of a pilot study. Thorax. 2009;64:744–8. doi: 10.1136/thx.2008.099465. [DOI] [PubMed] [Google Scholar]
- 63.WESTHOFF M, LITTERST P, MADDULA S, BOEDEKER B, BAUMBACH JI. Statistical and bioinformatical methods to differentiate chronic obstructive pulmonary disease (COPD) including lung cancer from healthy control by breath analysis using ion mobility spectrometry. Int J Ion Mobility Spectrom. 2011;14:139–149. [Google Scholar]
- 64.WESTHOFF M, LITTERST P, MADDULA S, BOEDEKER B, RAHMANN S, DAVIES AN, BAUMBACH JI. Differentiation of chronic obstructive pulmonary disease (COPD) including lung cancer from healthy control group by breath analysis using ion mobility spectrometry. Int J Ion Mobility Spectrom. 2010;13:131–139. [Google Scholar]
- 65.WOLD S, ESBENSEN K, GELADI P. Principal Component Analysis. Chemometrics and Intelligent Laboratory Systems. 1987;2:37–52. [Google Scholar]
- 66.YANG IA, CLARKE MS, SIM EH, FONG KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;7:CD002991. doi: 10.1002/14651858.CD002991.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.ZEKI AA, SCHIVO M, CHAN A, ALBERTSON TE, LOUIE S. The Asthma-COPD Overlap Syndrome: A Common Clinical Problem in the Elderly. J Allergy (Cairo) 2011;2011:861926. doi: 10.1155/2011/861926. [DOI] [PMC free article] [PubMed] [Google Scholar]