Abstract
The genetic factor structure of a range of learning measures was explored in twin children, recruited in preschool and followed to Grade 2 (total N = 2084). Measures of orthographic learning and word reading were included in the analyses to determine how these patterned with the learning processes. An exploratory factor analysis of the genetic correlations among the variables indicated a three-factor model. Vocabulary tests loaded on the first factor, the Grade 2 measures of word reading and orthographic learning, plus preschool letter knowledge, loaded on the second, and the third was characterized by tests of verbal short-term memory. The three genetic factors correlated, with the second (print) factor showing the most specificity. We conclude that genetically-influenced learning processes underlying print-speech integration, foreshadowed by preschool letter knowledge, have a degree of independence from genetic factors affecting spoken language. We also argue that the psychology and genetics of associative learning be afforded a more central place in studies of reading (dis)ability, and suggest some links to molecular studies of the genetics of learning.
In this article we explore genetic and environmental influences on learning processes as they bear on early word identification, using data from a sample of young twins followed longitudinally from preschool to Grade 2. Learning processes have figured in research into reading ability from its early days, but this is the first exploration of them in young children using a genetically-sensitive design. We outline the basics of this methodology in the Method section.
Research on associative learning
In one of the earliest scientific descriptions of reading disability, Morgan (1896) described the case of a 14-year-old boy who had “no power of preserving and storing up the visual impression produced by words” (p. 1378), thus squarely characterizing the disorder as one of associative learning between orthographic word forms and their spoken forms. He also noted that the child (a) could read highly frequent words such as and, but, and of, (b) had had great difficulty learning letter names, and (c) had not experienced analogous problems learning and operating with numbers. The dissociation between dyslexia and number knowledge was quickly confirmed by Hinshelwood (1896), citing several cases similar to that described by Morgan, but adding that the two disabilities frequently went hand-in-hand. Thus, although there was agreement in this early set of reports that the problem was one of “storing up the visual impression” of words, questions were raised about the scope and specificity of the learning disability--does dyslexia include print-speech pairings of all levels (letters in addition to words), and does it extend to other domains?
In this article we consider those questions as they apply to normal-range variability in reading. There is evidence that at least as far as the genetics of reading ability is concerned, low-end scores, referred to in such terms as dyslexia and reading disability, are extensions of normal-range variability. In the words of Kovas, Haworth, Dale, and Plomin (2007), “the abnormal is normal.” This claim rests on the case for “generalist genes,” first made and defended by Plomin and Kovas in 2005. Part of this hypothesis holds that for learning disabilities known to be subject to genetic influence, as is the case for reading, the genes that affect normal-range variation are the same as those that are involved when ability is low enough to qualify as a disability (dyslexia in the case of reading)--the sufferer has inherited a high number of adverse alleles of the effective genes.
Another part of the generalist gene hypothesis is that within a single domain there are genes of broad effect, ones that influence all aspects of the phenotype--in the case of reading, this might include word identification and reading comprehension. But Plomin and Kovas (2005) also note that not all genetic effects are general — specific gene effects are also at work. In a sense, this is the “genetic” version of the specificity issue raised by Morgan (1896) and Hinshelwood (1896)—how general are the genetic underpinnings of this learning disability.
Modern conceptualizations of reading disability, and reading ability in general, primarily locate the source of variation within the language domain, particularly its phonological component (and certainly not within the visual domain, as implied by Morgan’s [1896] term, word blindness). But the processes of learning remain of interest to researchers, especially associative processes underlying the binding of print and speech in memory, or “unitization,” in Ehri’s (2005) terminology. For instance, in his wide-ranging review of dyslexia, Vellutino (1979) concluded that “poor readers consistently performed as well as normal readers on paired-associate learning involving material devoid of linguistic structure, presented both within and between various sense modalities. In contrast, poor readers did not perform at the level of the normals on learning tasks involving a verbal component” (p. 336). Vellutino has presented newer research that confirms his earlier conclusion. He showed, for example, that a visual-verbal paired associate learning task reliably discriminated between kindergarten children, identified by their teachers as experiencing reading difficulties, who made little versus substantial progress in reading following remedial tutoring. Notably, visuospatial tests, including visual memory, did not pick out children who were difficult to remediate from others (Vellutino et al., 1996). Snowling (1980) found that children with reading difficulties struggled with visual-verbal associative learning, though not with visual-visual or verbal-verbal, suggesting a degree of specificity narrower than just associative learning. There have been more recent correlational studies documenting the relationship between visual-verbal learning and reading ability. Wimmer, Mayringer, and Landerl (1998), Windfuhr and Snowling (2001), Hulme, Goetz, Gooch, and Adams (2007), and Warmington and Hulme (in press) have all found that this kind of cross-modal associative learning makes an independent contribution to predicting reading skill over other common correlates such as various phonological tasks. Reitsma (1983) demonstrated that children with reading difficulties require more exposures to printed words to fix them in memory than children who are reading normally for their age. Messabuer and de Jong (2003) reported that dyslexic children were impaired in a verbal paired-associate learning tasks involving both words and nonwords, but not in a nonverbal task. In imaging studies, Blau, van Atteveldt, Ekkebus, Goebel, & Blomert (2009) have shown that adult dyslexics have reduced integration of letters and speech sounds. Blomert and Froyen (2010) reviewed evidence that even after children “know” letter-sound associations in the sense of being able to say them reliably, it takes considerable reading experience to develop adult-like integration of letters and sounds, with even eleven-year-olds showing evidence of immaturity.
Across many studies, a variety of other language measures with a learning component have also been linked to reading ability, including vocabulary, short-term verbal memory for items from nonwords to stories, and working memory for verbal material (e.g., Hindson et al., 2005; Metsala & Walley, 1998; Shankweiler & Crain, 1986; Snowling, 2000; Snowling, Gallgher, & Frith, 2003). In the research that follows we have included measures that represent some of these processes.
Reading, genes, and learning
None of the research just summarized underpinning this focus on learning processes has been done using genetically-sensitive research designs. However, in our ongoing longitudinal study of twins’ early literacy development we have demonstrated high heritability for individual differences in word reading, with genetic variation accounting for between 70 and 80% of the variance in word identification, with similar rates for measures of reading comprehension (Byrne et al., 2007; 2009; Olson et al., in press; Samuelsson et al., 2008). Other groups using the twin design have also shown substantial heritability for reading. For example, in the Twins Early Development Study (TEDS) in the United Kingdom the authors report values for genetic influences on word reading of the order of .65 for boys and .67 for girls aged 7 years (Harlaar et al., 2005). Using a latent trait approach to measurement, Gayán and Olson (2003) estimated the genetic contribution to word recognition to be 85% in a Colorado sample ranging in age from 8 – 18 years. An Australian study of 18-year-old twins produced estimates of 73% and 71% for reading irregularly-spelled words and nonwords respectively (Castles, Bates, Coltheart, Luciano, & Martin, 2006).
In our study, titled the International Longitudinal Twin Study (ILTS), we elected to employ a variety of learning measures alongside those for reading and spelling. We did so because of the kind of evidence on learning in relation to reading reviewed above, and because of evidence from earlier work by members of our group that learning rate for literacy-relevant processes is a good predictor of later literacy (Byrne, Fielding-Barnsley, & Ashley, 2000; Hindson et al., 2005). In those studies we found that the rate at which preschool children progressed through lessons designed to develop phonemic awareness was an independent predictor of subsequent reading levels over and above the level of phonemic awareness that they achieved.
We have already reported data exploring the relations between learning and literacy within a genetically-sensitive design. Using one of our learning tasks in the twin project, we showed that genetic factors that influence how well a child learns new print-speech pairings also influence prior learning of this kind (Byrne et al., 2008). Following a technique pioneered by Share (1995), second-grade twin children read short passages that included novel words, such as vade, with multiple possible spellings, and were then asked to spell the target words that they had seen. Prior learning was assessed with a standard test of spelling ability and by the speed and accuracy with which the children could read lists of words. All tasks were heritable, and most importantly were highly correlated genetically (with a range of .85 – .97). We postulated that a common genetic source influencing current learning had, historically, influenced prior learning; spelling and word identification were the “crystallized” products of the genes that support “on-line” learning.
The current study
If there are common genes influencing how well a child learns new words and the child’s existing orthographic knowledge, a major unanswered question is the scope of this genetically-influenced learning factor, an issue raised by Morgan (1896) and Hinshelwood (1896), as mentioned earlier. Does it affect just print-speech pairings, all visual-verbal associations, or indeed all kinds of learning relevant to reading? We probe its scope using the other learning measures incorporated in our study (see below) to investigate how genetic influences on learning tasks pattern, and how the genetics of reading fits into this pattern.
A project that was aimed at fully mapping out the genetic influences on learning capacities per se would include a very wide variety of tasks. In our study, the selection of learning tasks was constrained in two ways: The focus was on literacy development, and therefore tasks that lacked a prima facie link to reading were omitted (for instance, motor skill learning); our participants were young (preschool) children, and therefore we needed to limit the total testing time and the difficulty level of the procedures. Despite these constraints, to our knowledge this is the first study that has deliberately included a wide array of learning tasks within a genetically sensitive design with the aim of identifying how the tasks pattern and the genetic factors that can be derived from the pattern.
In terms of modality, the learning tasks that we selected can be classified as verbal and visuo-verbal. Verbal tests included vocabulary, nonword repetition, memory for sentences and memory for stories. The visuo-verbal measures were letter knowledge, learning to associate graphic forms and nonsense syllables, and learning spelling patterns for novel words (the orthographic learning task mentioned earlier). Each test could also be classified as historic (vocabulary, letter knowledge) or on-line (the remaining tests). All of these tasks except orthographic learning and one of the vocabulary tests were administered in the preschool assessment phase, with the other two assessed in the fourth year of this longitudinal project, when the children were in Grade 2. The reading measures used in this article, word and nonword identification speed and accuracy, were also gathered in Grade 2.
Method
Participants
Data were collected from 2084 preschool children, 520 monzygotic pairs (48% males) and 522 same-sex dizygotic pairs (52% males), with samples in the US, Sweden and Norway (Scandinavia) and Australia. In Grade 2, there were 433 MZ pairs (48% males) and 437 DZ pairs (52% males). Informed consent was obtained from all parents of the twins. The US sample was recruited from the Colorado Twin Registry, the Australian sample via the National Health and Medical Research Council’s Australian Twin Registry, and the Scandinavian sample from the Medical Birth Registries in Norway and Sweden. No payment was given for participation in Australia or Scandinavia, but the parents in the U.S. sample received a payment of $100 for participation. Parents of the Colorado twins were approached by mail or phone, and 88% of the 60% of families who could be contacted when the children were 4 agreed to participate. The twins’ parents in Australia and Scandinavia were all approached by mail, with a participation rate of 62% in Australia and 60% in Scandinavia. All twins were in their final preschool year at initial contact with a mean age of 59 months in the U.S., 58 in Australia, and 64 in Scandinavia. In Grade 2 the children were an average of 40 months older than in preschool. Zygosity was determined in most cases (81%) from DNA collected via cheek swabs, and in the other cases from selected items from the Nichols and Bilbro (1966) questionnaire concerning, for example, hair color and texture, eye color, facial appearance and complexion, and birth weight. The questionnaire has 95% accuracy when compared with blood samples.
Measures and Procedure
For a full description of all preschool tests administered to the twins, see Samuelsson et al. (2005), and for literacy tests administered in Grade 2, see Byrne et al. (2009). Here, we list measures for this article. Cronbach alpha values are based on the current US sample of up to 1000 children. Other reliability measures are from test manuals.
Preschool measures
Vocabulary
The Vocabulary subtest from the Wechsler Preschool and Primary Scale of Intelligence-Revised-WPPSI (Wechsler, 1989) asks children to provide definitions for pictured objects. The manual reports a test-retest reliability for 4.5-year-olds of .83. The Hundred Pictures Naming Test (Fisher & Glenister, 1992) is a picture-naming (recall) test. Cronbach alpha = .89.
Letter name recognition
In this test, children are shown a line of four letters and asked to point to the nominated one, e.g., “b.” All 26 letters are tested. Cronbach alpha = .92.
Nonword Repetition Test
In this test (Gathercole & Baddeley, 1996), children are presented with nonwords that obey normal English phonotactics and asked to repeat them. The words range from two to five syllables in length. Cronbach alpha = .84.
Sentence memory
This subtest from the WPPSI requires children to listen to and repeat verbatim sentences ranging upwards from two words in length. The manual reports a split-half reliability of .88 for 5-year-old children.
Story recall
This test is from the Wide Range Assessment of Memory and Learning (WRAML--Adams & Sheslow, 1990), and in it children listen to two short stories and are asked to recall as much of each as possible. Cronbach alpha = .87.
Sound-Symbol Learning
This test is also from the WRAML. Children are shown a meaningless line drawing and hear a nonsense syllable, and are asked to remember the association. There are eight items of this sort, and four trials of the set with feedback over which to learn the pairings. Cronbach alpha = .88.
Second-grade measures
Reading
We used the Sight Word Efficiency (SWE) and Phonemic Decoding Efficiency (PDE) tests of the Test of Word Reading Efficiency (TOWRE--Torgesen, Wagner, & Rashotte, 1999). Children are required to read as many real words (SWE) or nonwords (PDE) as they can in 45 seconds. Each test comes in two forms, and we administered both forms and averaged to increase reliability. The manual reports test-retest reliability for 6 – 9-year-olds of .97 for SWE and .90 for PDE.
Orthographic learning
This is a 15-item test modeled on a procedure devised by Share (1999). Children read short texts aloud, each of which contains a novel word that could be spelled at least two ways (e.g. vade), and were subsequently tested on the words’ spellings. Here is an example of an item:
The new word is vade. There is a hairy monster called a vade. The vade is very big. If you see a vade you should run away.
Cronbach alpha = .78.
Vocabulary
We selected the Boston Naming Test (Kaplan, Goodglass, & Weintraub, 2001), a confrontation naming measure in which the child is required to name pictures of 60 concrete objects, ranging from common ones like bed to rarer ones like abacus. We administered all 60 items. The test has options for recognition naming and phonological hints for items when recall fails, but we did not exercise those options. Thus score was simply number of items correctly named. Cronbach alpha = .84.
The preschool children were tested in their homes or preschools. The Grade 2 assessments took place in the children’s homes during the summer school break in the US and Scandinavia, and in the schools (occasionally at home) during the final two months of school in Australia (due to the shorter summer break in that country). A separate tester assessed each member of a pair at the same time in the US and Australia, though only one tester could be used in Scandinavia.
Analyses
Overview
We first present phenotypic analyses of the variables, including an exploratory factor analysis. Behavior-genetic analyses then follow; updated univariate estimates of genetic and environmental influences on the variables, genetic correlations derived from a Cholesky decomposition model, and an exploratory factor analysis of these genetic correlations. We next explain the behavior-genetic analyses in more detail.
Behavior-genetic analyses
Twin design
The twin design is based on a comparison of the correlations of identical (monozygotic, MZ) twins to those of fraternal (dizygotic, DZ) twins. Whereas MZ twins are genetically identical and share family environmental influences, DZ twins share half of their segregating genes, on average, as well as family environmental influences. Thus, assuming genetic influences are only additive, the correlation between MZ twins is a function of the heritability of the trait plus shared environmental influences, which are assumed to be no more highly correlated for MZ twins than for DZ twins; that for DZ twins is a function of one-half the heritability of the trait, plus shared environmental influences. Therefore, by analyzing these correlations, the contributions of genetic, shared environmental, and nonshared environmental influences can be estimated. See Plomin, DeFries, McClearn, & McGuffin (2008) for an introduction to twin methodology.
Cholesky decomposition and genetic and environmental correlations
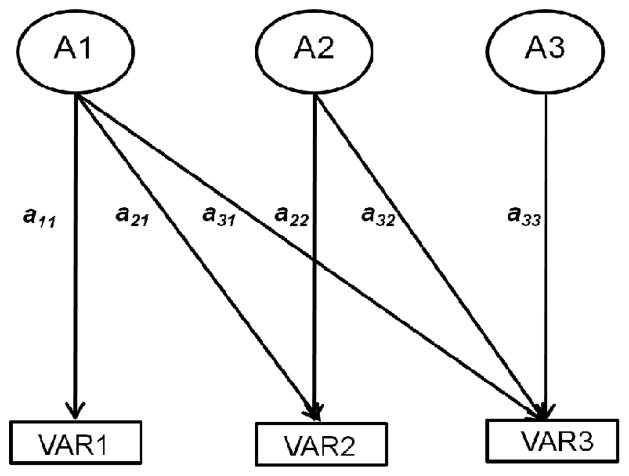
For the genetic analyses, the variances and covariances of the measures were collectively partitioned into their genetic, shared environmental and nonshared environmental components using a Cholesky decomposition model (Neale & Cardon, 1992). Cholesky decomposition is similar in principle to hierarchical regression in that the effects of an ‘independent’ variable on a ‘dependent’ variable are determined after the effects of a correlated independent variable have been removed. In this model the first latent factor contributes to the variance of all measures, represented as paths from this first latent factor to each measured variable. The second factor contributes to the variance of all measures except the first, represented as paths from this latent factor to the second and subsequent measured variables. In Figure 1 we present a 3-factor model of the genetic factors (A1, A2, A3) as an example.
Figure 1.
Example of a three-factor Cholesky decomposition of genetic factors. A1 = first latent genetic factor, VAR1 = first measured variable.
Estimates of the genetic, shared environmental and nonshared environmental correlations among the measures (i.e., the extent to which the same genes or environmental factors are influencing each measure, rA, rC and rE, respectively) are also obtained from the model (Plomin & DeFries, 1979). An intuitive example referring to Figure 1 is as follows; if most of the heritability of VAR2 is due to a21, the path from A1, and not to a22, the path from A2, the genetic correlation between the two variables is high. If path a21 is, say, zero, the genetic correlation between the two variables will also be zero.
Genetic factor analysis
A matrix of genetic correlations can be subjected to factor analysis in much the same manner as phenotypic correlations can be. The factors that emerge can probably best be thought of as resulting from genes or groups of genes acting in a pleiotropic manner to influence variability in two or more manifest variables (Crawford & DeFries, 1978). The pattern of genetic factors can differ from the phenotypic pattern, as when a particular variable loads with certain others in the phenotypic analysis but with different ones in the genetic analysis (Crawford & DeFries; see Heath & Martin, 1990, for an example from personality in which the multivariate genetic analysis resulted in a different picture from both the phenotypic factor analysis and from standard univariate genetic analyses). Genetic factor analysis has recently been employed to examine the genetic structure underlying 22 DSM-IV disorders, with four correlated genetic factors identified (Kendler et al., 2011).
Results
We have previously presented descriptive statistics for each of our measures (Byrne et al., 2007, 2009; Samuelsson et al., 2005, 2008), and in the interests of space do not repeat them here. We do make the point, however, that none of the variables that we used for this report were subject to floor or ceiling effects, an important consideration in a study that uses correlations and covariances. For our analyses we standardize all measures within samples because of some country differences in mean scores, noted in previous articles.
In Table 1 we present the intercorrelations among the variables, and in Table 2 the results of an exploratory factor analysis (EFA). We used an oblimin rotation to allow for correlations among factors and reported the pattern matrix to emphasize the unique contributions of factors to variables (discussed later). There were two factors with eigenvalues greater than 1, capturing 38.1 and 16.1% of the variance, to a total of 54.2%, a solution which optimized simple structure. The tests involving spoken language, letter knowledge and sound-symbol learning loaded most strongly on the first factor, and Grade 2 reading and orthographic learning on the second, Letter knowledge cross-loaded to some extent on both factors. The pattern is reasonably consistent with an earlier-published EFA of preschool measures in which language and a selection of preliteracy variables emerged separately (Samuelsson et al., 2005).
Table 1.
Correlations among Learning and Literacy Variables
| Measures | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. WRAML Story memory | |||||||||||
| 2. WRAML Sound-symbol | .26 | ||||||||||
| 3. Nonword repetition | .22 | .24 | |||||||||
| 4. WPPSI Sentence memory | .38 | .26 | .50 | ||||||||
| 5. Hundred pictures | .40 | .28 | .32 | .45 | |||||||
| 6. WPPSI Vocabulary | .45 | .25 | .35 | .46 | .48 | ||||||
| 7. Boston naming | .28 | .24 | .28 | .43 | .68 | .37 | |||||
| 8. Letter identification | .20 | .25 | .22 | .31 | .45 | .31 | .42 | ||||
| 9.Orthographic learning | .13 | .21 | .18 | .18 | .22 | .13 | .26 | .30 | |||
| 10. TOWRE SWE | .18 | .23 | .19 | .25 | .28 | .21 | .31 | .34 | .58 | ||
| 11. TOWRE PDE | .14 | .22 | .20 | .25 | .24 | .19 | .27 | .32 | .62 | .87 |
Note. WRAML = Wide Range Assessment of Memory and Learning; TOWRE SWE= Test of Word Reading Efficiency, Sight Word Efficiency; TOWRE PDE= Test of Word Reading Efficiency, Phonemic Decoding Efficiency; WPPSI = Wechsler Preschool and Primary Scale of Intelligence.
Table 2.
Factor Loadings for Exploratory Factor Analysis with Oblimin Rotation of Learning and Literacy Variables
| Test | Factor 1 | Factor 2 |
|---|---|---|
| WRAML Story memory | .66 | .12 |
| WRAML Sound-symbol | .42 | .14 |
| Nonword repetition | .59 | |
| WPPSI Sentence memory | .75 | |
| Hundred pictures | .79 | |
| WPPSI Vocabulary | .77 | |
| Boston Naming | .67 | .12 |
| Letter identification | .46 | .28 |
| Orthographic learning | .81 | |
| TOWRE SWE | .90 | |
| TOWRE PDE | .93 |
Note. Loadings < .10 are omitted. WRAML = Wide Range Assessment of Memory and Learning; TOWRE SWE= Test of Word Reading Efficiency, Sight Word Efficiency; TOWRE PDE= Test of Word Reading Efficiency, Phonemic Decoding Efficiency; WPPSI = Wechsler Preschool and Primary Scale of Intelligence.
In Table 3 we present the intraclass MZ and DZ within-trait correlations (diagonals) and the cross-twin, cross-trait correlations (off-diagonals). We used the program Mx (Neale, Boker, Xie, & Maes, 2002) to compute updated univariate estimates of additive genetic, shared environment and nonshared environment influences on each variable, shown in Table 4. All variables were significantly heritable, with substantial levels of shared environment influence on letter knowledge and on vocabulary at both preschool and Grade 2 assessments. Note that the heritability of vocabulary increased from preschool (Hundred Pictures, .23, WPPSI, .16) to Grade 2 (Boston Naming, .45), a trend that we have reported previously (Byrne et al., 2009), and continues through grade 4 in the US sample (Olson et al., 2011).
Table 3.
Intraclass Correlations among Learning and Literacy Variables
|
A. Monozygotic twins
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Twin 1 | Twin 2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| 1. WRAML Story memory | .48 | |||||||||||
| 2. WRAML Sound-symbol | .23 | ..31 | ||||||||||
| 3. Nonword repetition | .22 | .26 | .56 | |||||||||
| 4. WPPSI Sentence memory | .33 | .27 | .45 | .71 | ||||||||
| 5. Hundred pictures | .41 | .27 | .27 | .44 | .76 | |||||||
| 6. WPPSI Vocabulary | .36 | .26 | .34 | .43 | .47 | .58 | ||||||
| 7. Boston naming | .30 | .18 | .23 | .43 | .66 | .38 | .82 | |||||
| 8. Letter identification | .18 | .26 | .22 | .31 | .38 | .26 | .37 | .76 | ||||
| 9. Orthographic learning | .09 | .17 | .18 | .13 | .13 | .10 | .17 | .22 | .61 | |||
| 10. TOWRE SWE | .16 | .20 | .17 | .21 | .20 | .17 | .26 | .30 | .50 | .81 | ||
| 11. TOWRE PDE | .09 | .15 | .15 | .23 | .15 | .12 | .23 | .28 | .53 | .76 | .80 | |
|
B. Dizygotic twins
| ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Twin 1 | Twin 2 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
| 1. WRAML Story memory | .27 | |||||||||||
| 2. WRAML Sound-symbol | .09 | .17 | ||||||||||
| 3. Nonword repetition | .13 | .11 | .34 | |||||||||
| 4. WPPSI Sentence memory | .26 | .13 | .27 | .47 | ||||||||
| 5. Hundred pictures | .27 | .18 | .24 | .34 | .62 | |||||||
| 6. WPPSI Vocabulary | .26 | .18 | .29 | .36 | .35 | .51 | ||||||
| 7. Boston naming | .16 | .17 | .24 | .29 | .52 | .28 | .59 | |||||
| 8. Letter identification | .14 | .16 | .19 | .26 | .36 | .27 | .34 | .68 | ||||
| 9. Orthographic learning | .02 | .09 | .10 | .11 | .13 | .09 | .18 | .19 | .32 | |||
| 10. TOWRE SWE | .07 | .12 | .14 | .14 | .22 | .18 | .22 | .23 | .30 | .43 | ||
| 11. TOWRE PDE | .05 | .12 | .15 | .14 | .20 | .18 | .21 | .21 | .30 | .41 | .43 | |
Note: Cross-twin, within trait correlations on diagonals in bold, cross-twin, cross-trait correlations off diagonals. WRAML = Wide Range Assessment of Memory and Learning; TOWRE SWE= Test of Word Reading Efficiency, Sight Word Efficiency; TOWRE PDE= Test of Word Reading Efficiency, Phonemic Decoding Efficiency; WPPSI = Wechsler Preschool and Primary Scale of Intelligence.
Table 4.
Mx Model Fitting Estimates for Learning and Literacy Measures (95% Confidence Intervals in Parentheses).
| Variable | a2 | c2 | e2 |
|---|---|---|---|
| WRAML Story Memory | ..40 (.21–.54) | .07 (.00–.24) | .54 (.46–.59) |
| WRAML Sound-symbol | .27 (.04–37) | .04 (.00–.22) | .70 (.63–.77) |
| Nonword repetition | .45 (.27–.61) | .12 (.00–.27) | .43 (.38–.49) |
| WPPSI Sentence memory | .49 (.35–.63) | .23 (.09–.35) | .28 (.25–.33) |
| Hundred Pictures | .23 (.12–.34) | .52 (.42–.61) | .25 (.22–.29) |
| WPPSI Vocabulary | .16 (.01–.31) | .42 (.29–.54) | .42 (.37–.47) |
| Boston naming | .45 (.34–.57) | .37 (.25–.57) | .19 (.16–.22) |
| Letter knowledge | .18 (.09–.28) | .58 (.49–.66) | .23 (.20–.27) |
| Orthographic learning | .58 (.39–.66) | .03 (.00–.20) | .39 (.34–.45) |
| TOWRE SWE | .83 (.70–.85) | .00 (.00–.13) | .17 (.15–.20) |
| TOWRE PDE | .81 (.67–.85) | .01 (.00–.14) | .18 (.15–.21) |
Note. WRAML = Wide Range Assessment of Memory and Learning; TOWRE SWE= Test of Word Reading Efficiency, Sight Word Efficiency; TOWRE PDE= Test of Word Reading Efficiency, Phonemic Decoding Efficiency; WPPSI =Wechsler Preschool and Primary Scale of Intelligence; a2 = additive genetic variance, c2 = shared environment variance, e2 = nonshared environment variance. Estimates with confidence intervals including .00 are not significantly greater than 0.
The 11 variables were then fitted to a genetic/environmental Cholesky decomposition analysis, and genetic correlations among the variables were computed from these results as described above. We present the genetic correlations in Table 5. (We omit the shared and nonshared environment correlation matrices in the interests of space, but full details are available from the first author.) The factor analysis of the genetic correlations was conducted using the package R Version 2.10.0 (The R Foundation for Statistical Computing, 2009), with an oblimin rotation. We focus just on the genetic correlations and their factor structure because of the high heritability of most measures and the primary question that we posed: how genetic influences on learning tasks pattern, and how the genetics of word reading fits into that pattern.
Table 5.
Genetic Correlations among Learning and Literacy Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. WRAML Story memory | |||||||||||
| 2. WRAML Sound-symbol | .50 | ||||||||||
| 3. Nonword repetition | .58 | .63 | |||||||||
| 4. WPPSI Sentence memory | .58 | .45 | .73 | ||||||||
| 5. Hundred pictures | .79 | .60 | .45 | .60 | |||||||
| 6. WPPSI Vocabulary | .97 | .47 | .52 | .68 | .84 | ||||||
| 7. Boston Naming | .56 | .35 | .29 | .55 | .81 | .64 | |||||
| 8. Letter identification | .18 | .58 | .21 | .18 | .54 | .17 | .65 | ||||
| 9. Orthographic learning | .33 | .63 | .23 | .22 | .54 | .31 | .37 | .80 | |||
| 10. TOWRE SWE | .36 | .55 | .16 | .37 | .37 | .38 | .32 | .59 | .78 | ||
| 11. TOWRE PDE | .27 | .44 | .19 | .41 | .38 | .30 | .36 | .67 | .84 | .94 |
Note. WRAML = Wide Range Assessment of Memory and Learning; TOWRE SWE= Test of Word Reading Efficiency, Sight Word Efficiency; TOWRE PDE= Test of Word Reading Efficiency, Phonemic Decoding Efficiency; WPPSI = Wechsler Preschool and Primary Scale of Intelligence.
We report the pattern matrix for the genetic EFA (Table 6). This solution contains coefficients which represent unique (and not common, as in the structure matrix) contributions of factors to variables (Pett, Lackey, & Sullivan, 2003), but in view of our goal, discussed below, of furnishing well-justified phenotypes for further molecular studies of reading (dis)ability, we consider this the preferable solution. This is because the best way to identify genetically “pure” phenotypes is by grouping manifest variables to include only ones that are clearly affected by a particular genetic factor and excluding ones that load only modestly on that factor but more heavily on another. In addition, the pattern matrix is preferred for determining factor scores of individuals, a necessary step in quantifying phenotypes.
Table 6.
Factor Loadings for the Genetic Exploratory Factor Analysis With Oblimin Rotation
| Test | Vocabulary | Print-speech Pairing | Verbal Short-term Memory |
|---|---|---|---|
| WRAML Story Memory | .60 | .13 | .49 |
| WRAML Sound-symbol | .51 | .57 | |
| Nonword repetition | .14 | .99 | |
| WPPSI Sentence memory | .19 | .74 | |
| Hundred Pictures | .84 | .10 | .12 |
| WPPSI Vocabulary | .69 | .14 | .44 |
| Boston Naming | .99 | .22 | |
| Letter knowledge | .34 | .80 | .30 |
| Orthographic learning | .93 | ||
| TOWRE SWE | .10 | .90 | .13 |
| TOWRE PDE | .95 |
Note. Loadings < .10 are omitted; WRAML = Wide Range Assessment of Memory and Learning; TOWRE SWE= Test of Word Reading Efficiency, Sight Word Efficiency; TOWRE PDE= Test of Word Reading Efficiency, Phonemic Decoding Efficiency; WPPSI = Wechsler Preschool and Primary Scale of Intelligence
In the EFA, we identified three genetic factors with eigenvalues greater than 1, accounting for 79% of the genetic variance. The measures of confrontation naming, Hundred Pictures and Boston Naming, load most heavily on Factor 1, with Story Memory and the WPPSI vocabulary measure also contributing. The second factor is characterized by the print variables; the reading tests, orthographic learning, and letter knowledge. The signature variable for Factor 3 is nonword repetition, with a smaller loading from Sentence Memory. The Sound-symbol test cross-loads on the second and third factors, with the vocabulary test that requires definitions, the WPPSI, also loading to some extent on Factor 3 as well as on Factor 1. We can assign the labels Vocabulary, Print-speech Pairings (Print as shorthand), and Verbal Short-term Memory (VSTM) to the three factors, respectively.
The three genetic factors are correlated as follow: Vocabulary and Print, .44 (95% confidence interval, .30 – .56); Vocabulary and VSTM, .71 (.55 – .85); Print and VSTM, .43 (.38 – .52). Thus there are genes that are shared across the factors and therefore the measured traits, in line with the idea that for many cognitive and academic skills there are generalist genes (Plomin & Kovas, 2005). The pattern also confirms that there is a degree of genetic specialization underlying the measured variables, as also postulated by Plomin and Kovas. The strongest correlation is between the genetic factors that underlie the tests of spoken language (Vocabulary and VSTM), with the print factor showing a higher degree of specificity.
Discussion
In this paper we have examined the genetic relations among a selection of learning tasks, chosen to be potentially relevant to early word reading development, and measures of word reading itself in young children. The central finding was that the learning tasks we employed did not form just a single genetic factor, but were best modeled with three factors. One was characterized by manifest variables assessing vocabulary at both preschool and Grade 2 levels. Another was characterized by orthographic learning and preschool letter name knowledge, and it was on this factor that word and nonword reading loaded. The measures that loaded most heavily on the third were nonword repetition and story memory, thus licensing a description of verbal short-term memory.
Thus the genes that influence individual differences in early word reading ability show a degree of separation from genes that influence other aspects of language processing such as nonword repetition and vocabulary, most of which have been shown to correlate with reading ability at the phenotypic level. But the results also support the view of Plomin and Kovas (2005) that there exist genes in common across the factors underlying these reading-relevant learning tasks in addition to specific genetic influences. The support comes in the form of the correlations among the genetic factors—they are real, thus confirming generalist genes, but well below unity, thus confirming specificity. The genes that specifically influence reading appear to underlie the ability to form bonds between the graphic forms of letters and the linguistic objects of phonemes and words and to use those bonds to interpret previously unencountered letter strings, as in the nonword reading task.
It is well documented that early letter knowledge is one of the most potent predictors of reading skill (Badian, 1995; Foulin, 2005; Gijsel, Bosman, & Verhoeven, 2009; Muter & Doethelm, 2001; Scarborough, 1998; Schatschneider, Fletchher, Francis, Carlson, & Foorman, 2004). Our results indicate that at least part of the reason for this relationship is that learning letter names and learning orthographic forms for words are based on a common set of genes (see also Byrne et al., 2007, 2009). This “third factor” explanation can also account for the observation that, despite the predictive power of letter knowledge, deliberate, early letter-name training does not flow on to improved reading in later years (Samuels, 1972).
The high shared-environment influence on letter knowledge that we have documented shows that most of the variance in actual levels prior to school entry is a function of the home and/or preschool environment. But the genetic influence that also holds for letter knowledge suggests that a child’s ability to learn letter names relative to opportunity (that is, considering the affordances of the environment) depends to a substantial degree on genetic endowment. If a test of this ability could be devised it could function as a predictor of risk for reading disability. The Sound-symbol test, a variant of the visual-verbal paired associate learning tasks used in some of the research reviewed earlier and which loaded equally on the print and VSTM genetic factors, may hold promise as a predictive tool, though its low MZ correlation of .30 suggests the need for improved reliability. It could be especially useful in social circumstances where a child has been deprived of letter learning opportunities. As such it would have a role as a “dynamic” test as these assessments were originally conceptualized—measures that assess learning prospects for children who have had limited opportunities for formal instruction (Coventry, Byrne, Olson, Corley, & Samuelsson, 2011; Grigorenko, 2009).
Phenotypically, preschool letter knowledge loads only modestly on the factor that is characterized by subsequent orthographic learning and reading (see EFA in Table 2), but at the genetic level it belongs squarely with those tasks (Table 6). This appears to be a case of phenotypic relations masking the etiological relations among variables—individual differences in letter knowledge are affected by genetic variation that become important for word reading even though those genes play only a modest role in determining variance in letter knowledge prior to the start of school. More generally, this aspect of the data suggests that multivariate analyses based solely on phenotypes may be of limited value in the search for latent factors that identify overlapping and independent processes of learning that are biologically informative—the genetic relation of letter knowledge and later word identification was not readily apparent from the loading of just .28 on the print factor in the phenotypic EFA. As pointed out previously, Crawford and DeFries (1978) noted that phenotypic and genetic factor analyses can produce different patterns of this kind. We return to this issue later.
For Print, the common characteristic of the tasks is that they all involve binding print and speech at either the letter or word level. We suggest that our analyses are consistent with the research into the neural integration of letters and speech sounds conducted by Blomert’s group that we reviewed earlier (Blau et al., 2009; Blau et al, 2010; Froyen, Bonte, van Atteveldt, & Blomert, 2008). These researchers did not assess integration of word-level print and speech, but we would predict that children and adults with reading difficulties would show a similar pattern of weak integration at that level as well. It also appears from our results that early signs of variability in the operation of this process can be seen in simple letter knowledge prior to the start of schooling.
We need to note that we have not explored the limits of the print factor. We cannot say, for example, that Print is limited to cross-modal associations between graphic forms and language, though we can say that it is not limited to actual letters and letter groups, as the loading of the Sound-symbol test on that factor demonstrated. We did not, however, test the limits of this factor by, for example, including a purely verbal association task, analogous to Sound-Symbol but using arbitrary sounds paired with words. We hope that this preliminary research will act as a spur to further work using a genetically-sensitive design to more clearly specify the scope of this factor.
At the level of genetic influence, it appears that there is a degree of specificity for Vocabulary and VSTM. There is also a degree of overlap, as evidenced by the genetic correlation (note also that in a two-factor solution to the genetic EFA, which accounted for 75% of genetic variance but which we have not presented here, all measures of spoken language loaded on a single factor, with Sound-symbol cross-loading on this and the second factor, encompassing the print variables). We suggest that the phonological loop, proposed by Baddeley and his colleagues (e.g., Baddeley, Gathercole, & Papagno, 1998) as underpinning aspects of language learning and use, including vocabulary acquisition and limited-term memory for linguistic input, and measured well by nonword repetition, is a plausible candidate for explaining the overlap—nonword repetition is the primary defining variable for VSTM, but implicated in vocabulary growth.
The specificity of the Vocabulary and VSTM factors is given some support from phenotypic and genetic analyses provided by Keenan et al. (2010). They showed that in both types of analysis vocabulary and a composite set of oral comprehension measures, including tests of story recall, separately influenced individual differences in reading comprehension, with both being independent of the phenotypic and genetic factor underlying word reading.
Possible links to molecular genetics
We suggest that multivariate genetic analyses of reading-related phenotypes, of which our genetic EFA is an example, can furnish genetically informative traits that in turn may contribute to molecular genetic studies of reading (dis)ability. Latent traits or composites formed on the basis of purely phenotypic analyses run the risk of aggregating measures that do not belong together as expressions of a genetic network; a composite created from letter knowledge and vocabulary, say, justified by the phenotypic EFA, may obscure the underlying genetics by combining measures that are the output of separate genetic influences. Better to combine letter knowledge with orthographic learning and the reading measures to form a composite to feed into the search for genes responsible for individual differences in reading. In general, our findings suggest that it could be informative to create separate groups of children characterized by very weak vocabulary growth, by very limited short-term verbal memory, and by weak letter knowledge and word/nonword identification skills as part of molecular genetic research.
There is, of course, already progress towards a picture of the genetics of dyslexia. A recent review by Poelmans, Buitelaar, Pauls, and Franke (2011) combines 10 replicated genes into a theoretical molecular network involved in neuronal migration and neurite outgrowth. Some of these genes are involved in synaptic plasticity (e.g. DIP2A, DOCK4) and are expressed strongly in the hippocampus (S100B). The hippocampus is thought to act as a network within which activity-dependent neural plasticity allows encoding of stimulus conjunctions so that different stimulus aspects of an event or object, such as paired graphemic forms and elements of language, are bound (Rolls & Kesner, 2006; Shams & Seitz, 2008). Other genes (e.g., CLSTN2) may have a role in verbal short-term and working memory. We also note that linkage of nonword repetition to regions of chromosomes 16 (Monaco, 2007), 4 and 12 (Brkanac et al., 2008) has been reported. Thus although much remains to be discovered about the genetics of reading (dis)ability, some of the available findings are consistent with the genetic factors we have identified in this report, and the search for others might usefully be guided by further multivariate genetic analyses using a wider range of manifest variables than we have included here.
Conclusions and implications
The study of literacy development and of dyslexia in recent years has focused on factors other than the kind of integrative learning that we have identified here: Reading disability at the level of word identification is often seen as stemming from deficits in phonological representation that undermine phonological awareness, the development of decoding, and, through consequent weakness in the self-teaching process, efficient sight-word vocabularies (Byrne, 2011.) We suggest that in addition to this important source of variance there exists genetically-influenced variability in binding print and speech. Although the phrase “word blindness” (Morgan, 1986) is too categorical and too extreme for describing children whose word identification skills are limited and slow to grow, it nevertheless captures something of the idea we are advocating here.
We suggest, too, that the genetic correlations among the vocabulary, print and verbal short-term memory factors offers a genetic basis for expecting a degree of dissociation between individual differences in word identification skill and reading comprehension, which in turn might show a degree of dissociation between vocabulary and short-term/working memory influences. These conclusions are supported by results from Keenan at al. (2006, 2010) with older children and Olson et al. (2011). As well as possible dissociations, some children might be compromised in all factors, either because of the operation of the correlated component of the factors, or because they have independently inherited less-than-favorable genes for all. Such children would be in need of very special support.
We believe that our results support renewed attention on the genetics and psychology of learning as they affect the development of literacy, from the earliest stages, such as learning the names and sound of alphabetic letters, through automatized integration of letters and their associated phonemes, to the latest, when rapid and automatic word recognition processes support efficient reading comprehension. This attention should spread to the design and delivery of instruction that can compensate children who are slower to form the necessary letter- and word-level associations than other children are. Presumably, this instruction will, at its core, present these learners with appropriate levels of repetition of letters and words and with abundant opportunities for practice. It will also take into account the motivational challenges that face children who struggle to lay down the thousands of automatically-accessible associations required for efficient word recognition.
Acknowledgments
Funding was provided by the Australian Research Council (DP0663498 and DP0770805), the National Institute for Child Health and Human Development (HD27802 and HD38526), the Swedish Research Council (345-2002-3701 and PDOKJ028/2006:1), and Riksbankens Jubileumsfond and The Knut and Alice Wallenberg Foundation (PDOKJ028/2006:1). We thank the Australian Twin Registry, our testers, and the twins and parents involved.
Contributor Information
Brian Byrne, Discipline of Psychology, School of Behavioural, Cognitive and Social Sciences, University of New England, Department of Behavioral Sciences, Linköping University.
Sally J. Wadsworth, Institute for Behavioral Genetics, University of Colorado at Boulder
Kristi Boehme, Discipline of Psychology, School of Behavioural, Cognitive and Social Sciences, University of New England.
Andrew C. Talk, Discipline of Psychology, School of Behavioural, Cognitive and Social Sciences, University of New England
William L Coventry, Discipline of Psychology, School of Behavioural, Cognitive and Social Sciences, University of New England.
Richard K. Olson, Institute for Behavioral Genetics, University of Colorado at Boulder, Department of Behavioral Sciences, Linköping University
Stefan Samuelsson, Department of Behavioral Sciences, Linköping University.
Robin Corley, Institute for Behavioral Genetics, University of Colorado at Boulder.
References
- Adams W, Sheslow D. Wide range assessment of memory and learning. Wilmington, Delaware: Jastak Associates; 1990. [Google Scholar]
- Baddeley AD, Gathercole SE, Papagno C. The phonological loop as a language learning device. Psychological Review. 1998;105:158–173. doi: 10.1037/0033-295x.105.1.158. [DOI] [PubMed] [Google Scholar]
- Badian NA. Predicting reading ability over the long term: The changing roles of letter naming, phonological awareness and orthographic processing. Annals of Dyslexia. 1995;45:79–96. doi: 10.1007/BF02648213. [DOI] [PubMed] [Google Scholar]
- Blau V, Reithler J, van Atteveldt N, Seitz J, Gerretsen P, Goebel R, Blomert L. Deviant processing of letters and speech sounds as proximate cause of reading failure: A functional magnetic resonance imaging study of dyslexic children. Brain. 2010;133:868–879. doi: 10.1093/brain/awp308. [DOI] [PubMed] [Google Scholar]
- Blau V, van Atteveldt N, Ekkebus M, Goebel R, Blomert L. Reduced neural integration of letters and speech sounds links phonological and reading deficits in adult dyslexia. Current Biology. 2009;19:503–508. doi: 10.1016/j.cub.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Blomert L, Froyen D. Multi-sensory learning and learning to read. International Journal of Psychophysiology. 2010;77:195–204. doi: 10.1016/j.ijpsycho.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Brkanac Z, Chapman NH, Igo RP, Matsushita MM, Nielsen K, Berninger VW, Raskind WH. Genome scan of a nonword repetition phenotype in families with dyslexia: Evidence for multiple loci. Behavior Genetics. 2008;38:462–475. doi: 10.1007/s10519-008-9215-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B. Evaluating the role of phonological factors in early literacy development: Insights from experimental and behavior-genetic studies. In: Brady S, Braze D, Fowler C, editors. Explaining individual differences in reading: Theory and evidence. New York: Taylor and Francis; 2011. pp. 175–195. [Google Scholar]
- Byrne B, Coventry WL, Olson RK, Hulslander J, Wadsworth S, DeFries JC, …Samuelsson S. A behavioral-genetic analysis of orthographic learning, spelling, and decoding. Journal of Research in Reading. 2008;31:8–21. [Google Scholar]
- Byrne B, Coventry WL, Olson RK, Samuelsson S, Corley R, Willcutt EG, …DeFries JC. Genetic and environmental influences on aspects of literacy and language in early childhood: Continuity and change from preschool to Grade 2. Journal of Neurolinguistics. 2009;22:219–236. doi: 10.1016/j.jneuroling.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne B, Delaland C, Fielding-Barnsley R, Quain P, Samuelsson S, Hoien T, …Olson RK. Longitudinal twin study of early reading development in three countries: Preliminary results. Annals of Dyslexia. 2002;52:49–73. [Google Scholar]
- Byrne B, Fielding-Barnsley R, Ashley L. Effects of preschool phoneme identity training after six years: Outcome level distinguished from rate of response. Journal of Educational Psychology. 2000;92:659–667. [Google Scholar]
- Byrne B, Samuelsson S, Wadsworth S, Hulslander J, Corley R, DeFries JC, …Olson RK. Longitudinal twin study of early literacy development: Preschool through Grade 1. Reading and Writing: An Interdisciplinary Journal. 2007;20:77–102. [Google Scholar]
- Castles A, Bates T, Coltheart M, Luciano M, Martin NG. Cognitive modelling and the behaviour genetics of reading. Journal of Research in Reading. 2006;29:92–103. [Google Scholar]
- Coventry WL, Byrne B, Olson RK, Corley R, Samuelsson S. Dynamic and static assessment of phonological awareness in preschool: A behavior-genetic study. Journal of Learning Disabilities. 2011;44:322–329. doi: 10.1177/0022219411407862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CB, DeFries JC. Factor analysis of genetic and environmental correlation matrices. Multivariate Behavioral Research. 1978;13:297–318. doi: 10.1207/s15327906mbr1303_3. [DOI] [PubMed] [Google Scholar]
- Ehri LC. Development of sight word reading: Phases and findings. In: Snowling MJ, Hulme C, editors. The science of reading: A handbook. Oxford, UK: Blackwell; pp. 135–154. [Google Scholar]
- Fisher JP, Glennister JM. The hundred pictures naming test. Hawthorn, Australia: Australian Council for Educational Research; 1992. [Google Scholar]
- Froyen D, van Atteveldt N, Bonte M, Blomert L. Cross-modal enhancement of the MMN to speech sounds indicates early and automatic integration of letters and speech sounds. Neuroscience Letters. 2008;430:23–28. doi: 10.1016/j.neulet.2007.10.014. [DOI] [PubMed] [Google Scholar]
- Foulin JN. Why is letter-name knowledge such a good predictor of learning to read? Reading& Writing: An Interdisciplinary Journal. 2005;18:129–155. [Google Scholar]
- Gathercole SE, Baddeley AD. Children’s Test of Nonword Repetition. London: Pearson Assessment; 1996. [Google Scholar]
- Gayan J, Olson RK. Genetic and environmental influences on individual differences in printed word recognition. Journal of Experimental Child Psychology. 2003;84:97–123. doi: 10.1016/s0022-0965(02)00181-9. [DOI] [PubMed] [Google Scholar]
- Gijsel MAR, Bosman AMT, Verhoeven L. Kindergarten risk factors, cognitive factors, and teacher judgments as predictors of early reading in Dutch. Journal of Learning Disabilities. 2009;42:483–493. doi: 10.1177/00222194060390060701. [DOI] [PubMed] [Google Scholar]
- Grigorenko EL. Dynamic assessment and response to intervention: Two sides of one coin. Journal of Learning Disabilities. 2009;42:111–132. doi: 10.1177/0022219408326207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlaar N, Spinath FM, Dale PS, Plomin R. Genetic influences on early word recognition abilities and disabilities: A study of 7-year-old twins. Journal of Child Psychology and Psychiatry. 2005;46:373–384. doi: 10.1111/j.1469-7610.2004.00358.x. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG. Psychoticism as a dimension of personality: A multivariate genetic test of Eysenck and Eysenck’s psychoticism construct. Journal of Personality and Social Psychology. 1990;58:111–121. doi: 10.1037//0022-3514.58.1.111. [DOI] [PubMed] [Google Scholar]
- Hindson BA, Byrne B, Fielding-Barnsley R, Newman C, Hine D, Shankweiler D. Assessment and early instruction of preschool children at risk for reading disability. Journal of Educational Psychology. 2005;94:687–704. [Google Scholar]
- Hinshelwood J. The visual memory for words and figures. The British Medical Journal. 1896;2:1543–1544. 1873. [Google Scholar]
- Hulme C, Goetz K, Gooch D, Adams J, Snowling MJ. Paired-associate learning, phoneme awareness and learning to read. Journal of Experimental Child Psychology. 2007;96:150–166. doi: 10.1016/j.jecp.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. 2. Baltimore, MD: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Keenan JM, Betjemann R, Wadsworth SJ, DeFries JC, Olson RK. Genetic and environmental influences on reading and listening comprehension. Journal of Research in Reading. 2006;29:75–91. [Google Scholar]
- Keenan J, Priebe S, Miller A, Meenan C, Hua A. Speaking up for listening comprehension. Paper presented at the annual meeting of the Society for the Scientific Study of Reading; Berlin, Germany. 2010. Jul, [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud MD. The structure of genetic and environmental risk factors for syndromal and subsyndromal DSM-IV Axis I and all Axis II disorders. American Journal of Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovas Y, Haworth CMA, Dale PS, Plomin R. The genetic and environmental origins of learning abilities and disabilities in the early school years. Monographs of the Society for Research in Child Development. 2007;72:1–144. doi: 10.1111/j.1540-5834.2007.00439.x. whole number. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messbauer VC, de Jong PF. Words, nonwords, and visual paired associate learning in Dutch dyslexic children. Journal of Experimental Child Psychology. 2003;80:77–96. doi: 10.1016/s0022-0965(02)00179-0. [DOI] [PubMed] [Google Scholar]
- Metsala JL, Walley AC. Spoken vocabulary growth and the segmental restructuring of lexical representations: Precursors to phonemic awareness and early reading ability. In: Metsala JL, Ehri LC, editors. Word recognition and beginning lliteracy. Hillsdale, NJ: Erlbaum; 1998. pp. 89–120. [Google Scholar]
- Monaco AP. Multivariate linkage analysis of Specific Language Impairment (SLI) Annals of Human Genetics. 2007;71:1–14. doi: 10.1111/j.1469-1809.2007.00361.x. [DOI] [PubMed] [Google Scholar]
- Morgan WP. A case of congenital word blindness. The British Medical Journal. 1896;2(1871):1378. doi: 10.1136/bmj.2.1871.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muter V, Diethelm K. The contribution of phonological skills and letter knowledge to early reading development in a multilingual population. Language Learning. 2001;51:187–219. [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical Modeling. 6 VCU Box 900126, Richmond, VA 23298: Department of Psychiatry; 2002. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. 1992. [Google Scholar]
- NATO ASI series. Dordrecht, The Netherlands: Kluwer Academic Press; [Google Scholar]
- Nichols RC, Bilbro WC. The diagnosis of twin zygosity. Acta Genetica. 1966;16:265–275. doi: 10.1159/000151973. [DOI] [PubMed] [Google Scholar]
- Olson RK, Keenan JM, Byrne B, Samuelsson S, Coventry WL, Corley R, Hulslander J. Genetic and environmental influences on vocabulary and reading development from pre-kindergarten through grade 4. Scientific Studies of Reading. 2011;15:26–46. doi: 10.1007/s11145-006-9018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pett MA, Lackey NR, Sullivan JJ. Making sense of factor analysis: The use of factor analysis for instrument development in health care research. Thousand Oaks: Sage; 2003. [Google Scholar]
- Poelmans G, Buitelaar JK, Pauuls DK, Franke B. A theoretical molecular network for dyslexia: Integrating available genetic findings. Molecular Psychiatry. 2011;16:365–382. doi: 10.1038/mp.2010.105. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC. Multivariate behavioral genetic analysis of twin data on scholastic abilities. Behavior Genetics. 1979;9:505–517. doi: 10.1007/BF01067347. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, McClearn GE, McGuffin P. Behavioral genetics. 5. New York: Worth Publishers; 2008. [Google Scholar]
- Plomin R, Kovas Y. Generalist genes and learning disabilities. Psychological Bulletin. 2005;131:592–617. doi: 10.1037/0033-2909.131.4.592. [DOI] [PubMed] [Google Scholar]
- Reitsma P. Printed word learning in beginning readers. Journal of Experimental Child Psychology. 1983;75:321–339. [Google Scholar]
- Rolls ET, Kesner RP. A computational theory of hippocampal function, and empirical tests of the theory. Progress in Neurobiology. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Samuels SJ. The effect of letter-name knowledge on learning to read. American Educational Research Journal. 1972;9:65–74. [Google Scholar]
- Samuelsson S, Byrne B, Olson RK, Hulslander J, Wadsworth S, Corley R, …DeFries JC. Response to early literacy instruction in the United States, Australia, and Scandinavia: A behavioral-genetic analysis. Learning and Individual Differences. 2008;18:289–295. doi: 10.1016/j.lindif.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson S, Byrne B, Quain P, Wadsworth S, Corley R, DeFries JC, Olson RK. Environmental and genetic influences on prereading skills in Australia, Scandinavia, and the United States. Journal of Educational Psychology. 2005;97:705–722. [Google Scholar]
- Scarborough HS. Early detection of children at risk for reading disabilities: Phonological awareness and other promising predictors. In: Shapiro BK, Accardo PJ, Capute AJ, editors. Specific reading disability: A view of the spectrum. Timonium, MD: York Press; 1998. pp. 75–119. [Google Scholar]
- Schatschneider C, Felthcher JM, Francis DJ, Carlson CD, Foorman BR. Kindergarten predictors of reading skills: A longitudinal comparative analysis. Journal of Educational Psychology. 2004;96:265–282. [Google Scholar]
- Shams L, Seitz AR. Benefits of multisensory learning. Trends in Cognitive Science. 2008;12:411–417. doi: 10.1016/j.tics.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Shankweiler D, Crain S. Language mechanisms and reading disorder: A modular approach. Cognition. 1986;24:139–168. doi: 10.1016/0010-0277(86)90008-9. [DOI] [PubMed] [Google Scholar]
- Share DL. Phonological recoding and self-teaching: Sine qua non of reading acquisition. Cognition. 1995;55:151–218. doi: 10.1016/0010-0277(94)00645-2. [DOI] [PubMed] [Google Scholar]
- Share DL. Phonological recoding and orthographic learning: A direct test of the self-teaching hypothesis. Journal of Experimental Child Psychology. 1999;72:95–129. doi: 10.1006/jecp.1998.2481. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. The development of grapheme-phoneme correspondence in normal and dyslexic readers. Journal of Experimental Child Psychology. 1980;29:294–305. doi: 10.1016/0022-0965(80)90021-1. [DOI] [PubMed] [Google Scholar]
- Snowling MJ. Dyslexia. 2. Oxford: Blackwell; 2000. [Google Scholar]
- Snowling MJ, Gallagher A, Frith U. Family risk of dyslexia is continuous: Individual differences in the precursors of reading skill. Child Development. 2003;74:358–373. doi: 10.1111/1467-8624.7402003. [DOI] [PubMed] [Google Scholar]
- The R Foundation for Statistical Computing. 2009 Retrieved from http://www.r-project.org/
- Torgesen JK, Wagner RK, Rashotte CA. Test of word reading efficiency. Austin, TX: Pro-ed; 1999. [Google Scholar]
- Vellutino FR. Dyslexia: Theory and Research. Cambridge, MA: MIT Press; 1979. [Google Scholar]
- Vellutino FR, Scanlon DM, Sipay ER, Small SG, Pratt A, Chen R, Denckla MB. Cognitive profiles of difficult-to-remediate and readily remediated poor readers: Early intervention as a vehicle for distinguishing between cognitive and experiential deficits as basic causes of specific reading disability. Journal of Educational Psychology. 1996;88:601–638. [Google Scholar]
- Warminton M, Hulme C. Phoneme awareness, visual-verbal paired-associate learning, and rapid automatized naming as predictors of individual differences in reading ability. Scientific Studies of Reading (in press) [Google Scholar]
- Wechlser D. Manual for the Wechsler Preschool and Primary Scale of Intelligence-Revised. New York: Psychological Corporation; 1989. [Google Scholar]
- Wimmer H, Mayringer H, Landerl K. The double-deficit hypothesis and difficulties learning to read a regular orthography. Journal of Educational Psychology. 2000;92:668–680. [Google Scholar]
- Windfuhr KL, Snowling MJ. The relationship between paired associate learning and phonological skills in normally developing readers. Journal of Experimental Child Psychology. 2001;80:160–173. doi: 10.1006/jecp.2000.2625. [DOI] [PubMed] [Google Scholar]