Abstract
We report a structure of a trimeric glutamate transporter homologue from Pyrococcus horikoshii with two protomers in an inward facing state, and the third in an intermediate conformation between the outward and inward facing states. The intermediate shows a cavity in the thinnest region of the transporter, which is potentially accessible to the extracellular and cytoplasmic solutions. Our findings suggest a structural principle by which transport intermediates may mediate uncoupled permeation of polar solutes.
Excitatory amino acid transporters (EAATs), or glutamate transporters, are trimeric pumps that couple the uptake of the neurotransmitter glutamate to the co-transport and counter-transport of cations 1. These transporters also mediate a bidirectional chloride flux, which is gated by glutamate and sodium binding, but is not required for transport 2–4, and may play an important role in regulating synaptic transmission 5. Previously reported crystal structures have captured the archaeal glutamate transporter homologue, GltPh, as a symmetric trimer with all protomers in the outward facing state (OFS) 6,7 and a mutant, GltPh-K55C/A364CHg, with protomers trapped in the inward facing state (IFS) by intra-molecular mercury (Hg2+)-mediated crosslinking 8. These structures showed that each protomer contains a transport domain, which binds substrate and ions and traverses ~15 Å of the membrane along the trimerization domain, moving its cargo between the extracellular milieu and the cytoplasm (Supplementary Figure 1).
To test whether GltPh-K55C/A364CHg structure captured the transporter in an energetic minimum, we undertook a crosslinking study, focusing on the possibility that the transport domain may move further inward (Supplementary Fig. 1 and Supplementary Methods). However, the analysis of Hg2+-binding stoichiometry to double cysteine mutants revealed that only positions adjacent in GltPh-K55C/A364CHg structure crosslinked (Supplementary Discussion, Supplementary Fig. 2, 3). For instance, GltPh-V216C/M385CHg was crosslinked and its crystal structure was identical to that of GltPh-K55C/A364CHg (Supplementary Fig. 4, Supplementary Table 1). In contrast, more distant cysteines did not crosslink and instead coordinated Hg2+ ions independently. We determined crystal structures of two of these mutants, Y195C/A364C and V198C/A380C (Supplementary Tables 1, 2). GltPh-Y195C/A364CHg showed all protomers in the OFS as in wild type GltPh (Supplementary Fig. 5, 6). However, the structure of GltPh-V198C/A380CHg pictured an asymmetric trimer with two protomers in the IFS and the third in an intermediate outward facing state (iOFS) (Figure 1, Supplementary Fig. 5, 7). We believe that the novel conformation observed for this mutant, which shows transport activity similar to the wild type (Supplementary Fig. 2, d), has been captured because the iOFS is a relatively low energy, accessible state, and due to stabilizing crystal packing contacts (Supplementary Fig. 8).
Figure 1.
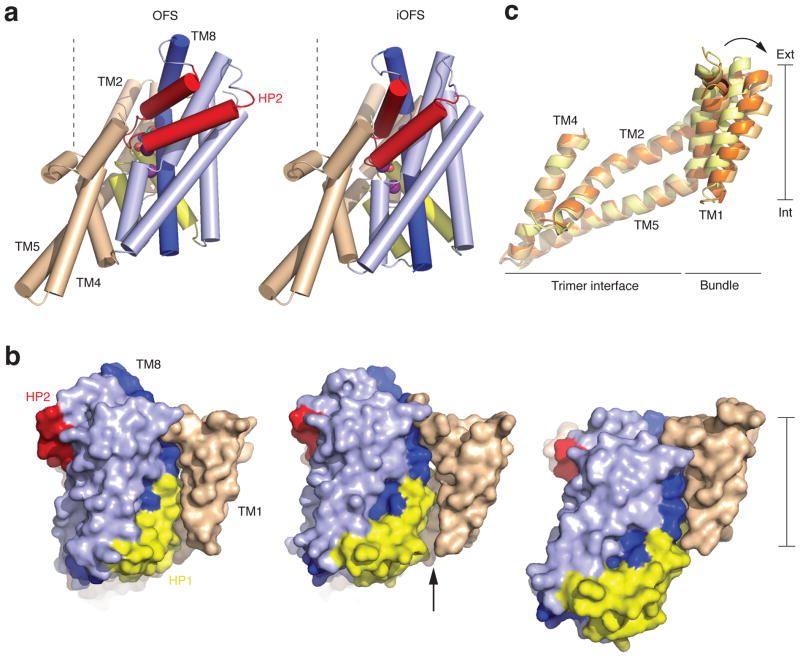
Crystal structure of the asymmetric trimer. a, b, Averaged 2fo-fc electron density maps at 2.5 σ (dark grey mesh) and anomalous difference Fourier maps at 5 σ (green mesh) for the IFS and the iOFS, respectively. c, Surface representations of the trimer viewed within the membrane plane. Trimerization and transport domains are colored wheat and blue, respectively. d, Cartoon representation of the protomers in the iOFS (left) and the IFS (right). The third protomer is omitted for clarity; HP1 and HP2 are yellow and red, respectively; and bound L-aspartate and sodium ions are shown as spheres.
The un-crosslinked IFS protomers in GltPh-V198C/A380CHg are essentially indistinguishable from those in GltPh-K55C/A364CHg, suggesting that the observed conformation represents an energetic minimum within the transporter conformational space. In the novel iOFS, the transport domain has moved ~3.5 Å toward the cytoplasm compared to the OFS, and rotated ~15 ° (Figure 2, a and b, Supplementary Fig. 9, a). This re-arrangement corresponds to, respectively, one fourth and one half of the complete translation and rotation of the transport domain transitioning from the OFS to the IFS. We hypothesize that this early rotation of the domain prior to inward movement may be necessary to maintain the seal at the interface with the trimerization domain. Interestingly, the OFS to iOFS transition also involves conformational changes in the trimerization domain, encompassing TM1 and peripheral portions of TM2 and TM5 (Figure 2, c). In a concerted manner, these helices lean away from the trimer center by up to ~7.0 Å, to accommodate re-arrangement of TM5-TM6 loop, which connects trimerization and transport domains, and/or to facilitate passage of TM8 kink across the trimerization domain surface (Supplementary Fig. 9, b and c). As a result, a crevice between the transport and trimerization domains widens significantly, exposing part of the interface to the membrane core (Figure 2, b). We speculate that lipophilic molecules could enter such hydrophobic crevices, potentially stabilizing intermediate states, and note that EAATs are, indeed, modulated by polyunsaturated fatty acids 9–11.
Figure 2.
Conformational transition in the iOFS. a, Cylinder representation of GltPh viewed from the membrane plane in the OFS (left) and iOFS (right). The color scheme is as in Figure 1. TM8 is dark blue, and bound substrates are emphasized as spheres. Dashed lines, normal to the membrane plane, highlight the transport domain orientation differences. b, Superimposition of the trimerization domains in the OFS (yellow), iOFS (light orange) and IFS (orange). c, Surface representation of the protomers in the OFS (left), iOFS (center), and IFS (right) colored as in a. An arrow marks the enlarged crevice between the transport and trimerization domains.
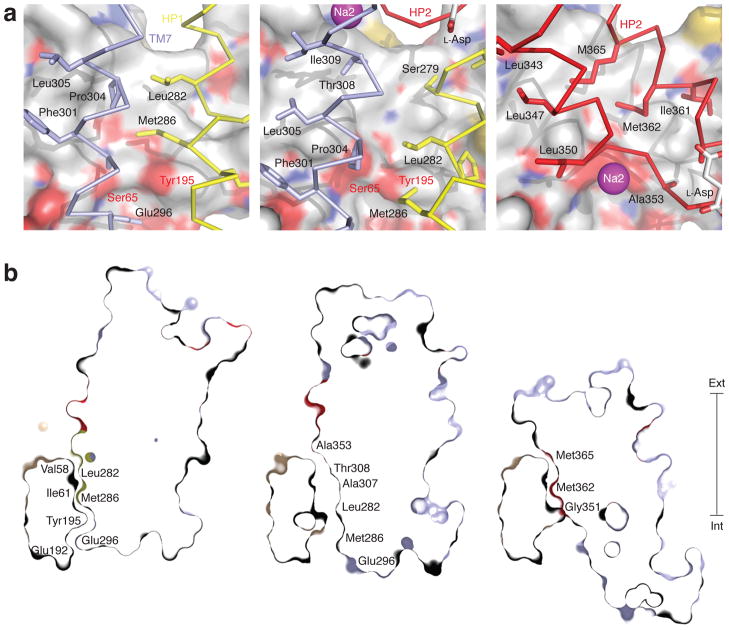
In the central region of the trimerization domain, TM2 and TM5 cross the membrane at an oblique angle and constitute the thinnest part of the transporter, separating the extracellular solution and the cytoplasm by ~15 Å (Supplementary Fig. 1). The structure of these helices, which participate in the inter-subunits interactions, is identical in the OFS, iOFS and IFS (Figure 2, c). In the OFS, the C-terminal arm of the helical hairpin (HP) 1 and the N-terminal half of TM7, pack against this region. In the IFS, two arms of HP2 take their place (Figure 3, a). Remarkably, even though different transport domain residues interact with the trimerization domain in the OFS and the IFS, they are similar in hydrophobicity and size. Transitioning from the OFS to the iOFS, the transport domain moves inward by about one turn of a helix, altering significantly the configuration of the interface. In particular, the non-helical regions at the tips of HP1 and HP2 and the unwound region of TM7 enter the interface (Figure 3, a). Furthermore, the transport domain interfacial residues are smaller in size and more polar. For example, positions occupied by Leu282, Met286 and Pro304 in the OFS, are taken by Ser279, Leu282 and Thr308 in the iOFS (Figure 3, a). These changes, accentuated by a slight distancing of TM7 from the trimerization domain, result in the formation of a cavity lined by conserved hydrophobic residues in TM2 and TM5 of the trimerization domain and flanked on the cytoplasmic side by two polar residues, Ser65 and Tyr195 (Figure 3, b, Supplementary Fig. 10). Ser65 and Tyr195 are completely occluded from the extracellular solution in the OFS and IFS, and are also occluded from the cytoplasm in the OFS. However, in iOFS they are exposed to the cytoplasm, and, via the interfacial cavity, may also become accessible to the extracellular solvent.
Figure 3.
Cavity at the domain interface in the iOFS. a, The interface is viewed from within the transport domain in the OFS (left), iOFS (center) and IFS (right). The trimerization domain is in surface representation and colored by atom type. Interacting transport domain elements are in ribbon representation and colored light blue (TM7), yellow (HP1) and red (HP2). The rest of the transport domain is omitted for clarity. The side chains making contact with the trimerization domain and bound L-aspartate are emphasized as sticks, and sodium ion in Na2 site is shown as a sphere. b, Thin cross-sections of the protomers approximately through Ser65 and Tyr195 and normal to the membrane plane. Residues lining the domain interface are labeled.
Interestingly, Ser65 has been implicated in anion permeation in EAATs and GltPh 12,13. Moreover, it is thought to be part of the selectivity filter in EAATs because mutations at this position alter anion selectivity 12. Also, mutations of other residues at or near the domain interface modulate uncoupled anion fluxes through the transporter (Supplementary Fig. 11) 12,14. Based on these data, we hypothesize that during the transition from the OFS to the IFS, a cavity at the domain interface, like the one observed in our iOFS structure, may provide a transient permeation pathway for anions. If so, modifications, which prevent the progression of the transporter to the IFS, but allow it to reach conducting intermediates, should abrogate substrate transport but retain anion conductance. Indeed, such mutations have been reported for EAATs (Supplementary Fig. 11). Specifically, modifications of cysteines introduced into HP2 and the extracellular end of TM6 with bulky charged reagents prevent coupled transport, but preserve or even increase the substrate-gated anion conductance 15–18. In the OFS and the iOFS, these residues are solvent-exposed, but in the IFS, the residues in HP2 are buried at the domain interface and the residue in TM6 descends into the hydrophobic core of the membrane. Modifications of these residues may render the IFS inaccessible and favor the OFS and the intermediate states. We also note that the transport domain movement trajectory, deduced from iOFS structure, suggests that the sodium ion in Na2 site will enter the domain interface moving toward Ser65 (Figure 3, a), perhaps contributing to the anionic selectivity of the pathway. Consistently, while coupled transport by EAAT3 is driven by both sodium and lithium ions, the latter do not support anion conductance 19.
In conclusion, the iOFS structure demonstrates how potentially solvent accessible cavities may form in glutamate transporters during transport, possibly accounting for their reported sodium an substrate-gated permeability to a range of polar solutes, including water, urea and anions 20. Finally, we hypothesize that such cavities may either yield a conducting channel or harbor binding sites with alternate accessibility to the extracellular and intracellular solutions due to movements of the transport domain. In the latter case, multiple transitions between the OFS and IFS before substrate release could also account for non-stoichiometric transport of these solutes.
Supplementary Material
Acknowledgments
We thank Dr. Nicolas Reyes (Pasteur Institute, Paris, France) for assistance with the design of ITC experiments and helpful discussions. Dr. Vincent Chaptal (Institute of Biology and Chemistry of Proteins, Lyon, France) is acknowledged for his help with the anisotropy correction procedure, and Dr. Michael Sawaya (Institute for Genomics and Proteomics, University of California, Los Angeles, USA) for providing the suite of programs for performing the anisotropy correction. We are thankful for the access to beamlines X25 and X29 at the National Synchrotron Light Source (Brookhaven National Laboratory, USA). This work was supported by NINDS NS064357 grant to OB.
Footnotes
Author contributions. GV and OB designed the experiments, analyzed data and wrote the manuscript. GV conducted the experiments and collected X-ray data.
Accession codes. Data and coordinates for the GltPh-V216C/M385CHg and GltPh-V198C/A380CHg structures have been deposited at the Protein Data Bank with the accession codes YXXX and AXXX, respectively.
REFRENCES
- 1.Danbolt NC. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 2.Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- 3.Wadiche JI, Arriza JL, Amara SG, Kavanaugh MP. Neuron. 1995;14:1019–27. doi: 10.1016/0896-6273(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 4.Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Proc Natl Acad Sci U S A. 1997;94:4155–60. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Veruki ML, Morkve SH, Hartveit E. Nat Neurosci. 2006;9:1388–96. doi: 10.1038/nn1793. [DOI] [PubMed] [Google Scholar]
- 6.Yernool D, Boudker O, Jin Y, Gouaux E. Nature. 2004;431:811–8. doi: 10.1038/nature03018. [DOI] [PubMed] [Google Scholar]
- 7.Boudker O, Ryan RM, Yernool D, Shimamoto K, Gouaux E. Nature. 2007;445:387–93. doi: 10.1038/nature05455. [DOI] [PubMed] [Google Scholar]
- 8.Reyes N, Ginter C, Boudker O. Nature. 2009;462:880–5. doi: 10.1038/nature08616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulsen MV, Vandenberg RJ. J Physiol. 2001;534:159–67. doi: 10.1111/j.1469-7793.2001.00159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zerangue N, Arriza JL, Amara SG, Kavanaugh MP. J Biol Chem. 1995;270:6433–5. doi: 10.1074/jbc.270.12.6433. [DOI] [PubMed] [Google Scholar]
- 11.Fairman WA, Sonders MS, Murdoch GH, Amara SG. Nat Neurosci. 1998;1:105–13. doi: 10.1038/355. [DOI] [PubMed] [Google Scholar]
- 12.Ryan RM, Mitrovic AD, Vandenberg RJ. J Biol Chem. 2004;279:20742–51. doi: 10.1074/jbc.M304433200. [DOI] [PubMed] [Google Scholar]
- 13.Ryan RM, Mindell JA. Nat Struct Mol Biol. 2007;14:365–71. doi: 10.1038/nsmb1230. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Vandenberg RJ. Biochemistry. 2007;46:9685–92. doi: 10.1021/bi700647f. [DOI] [PubMed] [Google Scholar]
- 15.Seal RP, Shigeri Y, Eliasof S, Leighton BH, Amara SG. Proc Natl Acad Sci U S A. 2001;98:15324–9. doi: 10.1073/pnas.011400198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borre L, Kavanaugh MP, Kanner BI. J Biol Chem. 2002;277:13501–7. doi: 10.1074/jbc.M110861200. [DOI] [PubMed] [Google Scholar]
- 17.Ryan RM, Vandenberg RJ. J Biol Chem. 2002;277:13494–500. doi: 10.1074/jbc.M109970200. [DOI] [PubMed] [Google Scholar]
- 18.Shachnai L, Shimamoto K, Kanner BI. Neuropharmacology. 2005;49:862–71. doi: 10.1016/j.neuropharm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 19.Borre L, Kanner BI. J Biol Chem. 2001;276:40396–401. doi: 10.1074/jbc.M104926200. [DOI] [PubMed] [Google Scholar]
- 20.Vandenberg RJ, Huang S, Ryan RM. Channels (Austin) 2008;2:51–8. doi: 10.4161/chan.2.1.6047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.