Abstract
Marijuana (MJ) use and HIV infection are both associated with neurocognitive deficits, yet there is little research to date examining their interactions, specifically how they pertain to procedural learning (PL). We examined a sample of 86 individuals with a history of dependence for multiple substances who underwent a comprehensive evaluation including measures of mental health, substance use history, and three measures of PL: the photoelectric Rotary Pursuit Task (RPT), the Star Mirror Tracing Task (SMT), and the Weather Prediction Task (WPT). We found that a positive HIV serostatus and a history of marijuana dependence were both independently associated with overall poorer performance on the SMT and RPT in this sample of individuals with a history of dependence for multiple substances. Rate of improvement across trial blocks did not differ as a function of HIV serostatus or history of marijuana dependence. Although we found no significant HIV × MJ interaction for any of the PL tasks, we did observe evidence of additive negative effects from HIV and a history of marijuana dependence on overall performance on the SMT and RPT, but not the WPT. The findings suggest that complex motor skills are adversely affected among abstinent polysubstance users with a history of marijuana dependence and that such deficits are compounded by HIV.
Keywords: HIV, Cannabis, Nondeclarative memory, Striatum, Motor skills
INTRODUCTION
Marijuana has been reported to alleviate neuropathic pain, nausea, mood problems, and appetite loss from HIV/AIDS (Abrams et al., 2007; Corless et al., 2009; Ellis et al., 2009; Haney et al., 2007; Prentiss, Power, Balmas, Tzuang, & Israelski, 2004), and over a dozen U.S. states have decriminalized marijuana for medical use, with most of them identifying HIV/AIDS as a condition that may benefit from medical marijuana (NORML, 2010). Not surprisingly, there is a high prevalence of medical and recreational marijuana use among individuals with HIV (Fairfield, Eisenberg, Davis, Libman, & Phillips, 1998; Prentiss et al., 2004; Sidney, 2001). Given the prevalence of marijuana use and its potential value to ameliorate symptoms of HIV/AIDS, it is important to understand possible negative interactive or additive effects that marijuana use may have on HIV-associated neurocognitive disorder.
Although marijuana use (Grant, Gonzalez, Carey, Natarajan, & Wolfson, 2003; Pope, Gruber, & Yurgelun-Todd, 1995) and a positive HIV serostatus (Grant et al., 1987; Heaton et al., 1995; Reger, Welsh, Razani, Martin, & Boone, 2002) have both been independently associated with neurocognitive deficits, there is no consensus on whether marijuana use by HIV+ individuals is detrimental to their neurocognitive functioning. This may stem from continued controversy in the scientific literature on the onset, magnitude, and duration of neurocognitive deficits from marijuana use. Specifically, there is substantial agreement on acute and residual negative effects from marijuana on neurocognitive functioning, but there is less agreement on permanent or more lasting effects from marijuana after abstinence (e.g., see review by Gonzalez, 2007). Some studies show that deficits in declarative memory, which are evident after recent abstinence, appear to dissipate after 28 days (Pope, Gruber, Hudson, Huestis, & Yurgelun-Todd, 2001). However, others have reported deficits in decision making that remain evident after 28 days of abstinence (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Bolla, Eldreth, Matochik, & Cadet, 2005; Verdejo-Garcia et al., 2007). A meta-analysis on the nonacute effects of marijuana found adverse neurocognitive effects from marijuana (of small effect size) on overall neuropsychological functioning that appeared to be driven primarily by deficits in memory function (Grant et al., 2003) in otherwise healthy subjects. Given the small magnitude of the observed effects, the authors concluded that the limited marijuana use that may be expected in a medical context may offer a tolerable margin of safety. However, it is conceivable that some populations may be more vulnerable to adverse effects of cannabis on neurocognition. For example, individuals with a history of dependence for multiple substances—herein referred to as substance-dependent individuals (SDIs) or polysubstance users—have often been shown to experience more severe HIV-associated neurocognitive impairments than their non-drug-using counterparts (reviewed in Gonzalez & Cherner, 2008). The additional neurocognitive burden of marijuana dependence may be more notable in such a sample. Furthermore, investigations of HIV and marijuana interactions among poly-substance users may offer greater ecological validity than those examining the effects of a single substance in isolation, since polydrug use is often the norm in substance-using populations (Darke & Hall, 1995; Leri, Bruneau, & Stewart, 2003), and polydrug use often co-occurs with HIV (Diaz et al., 1994; Mimiaga et al., 2008).
To date, only two published studies have specifically examined the neurocognitive effects of marijuana among HIV+ individuals and have reported mixed findings. Cristiani, Pukay-Martin, and Bornstein (2004) compared the neuropsychological performance of healthy controls and participants with HIV, stratified by history of marijuana use and HIV disease stage. They found that symptomatic HIV+ individuals who used marijuana frequently performed more poorly on a global measure of neuropsychological impairment than did those with history of minimal or no marijuana use. In contrast, Chang, Cloak, Yakupov, and Ernst (2006) found that use of marijuana did not affect neuropsychological performance among their HIV+ subjects, as they reported no significant interactions between HIV serostatus and history of marijuana use. However, they compared brain metabolite levels across groups using magnetic resonance spectroscopy (MRS) and found evidence of negative additive effects of marijuana use and HIV for some (but not all) metabolites in the basal ganglia and thalamus.
A common pathway by which marijuana use and HIV may adversely affect neurocognitive functioning is through their impact on the striatum. The striatum is rich in cannabinoid receptors (Herkenham, Lynn, de Costa, & Richfield, 1991; Herkenham et al., 1990), and several neuroimaging studies report abnormal striatal functioning among marijuana users (Chang et al., 2006; Kanayama, Rogowska, Pope, Gruber, & Yurgelun-Todd, 2004; Volkow et al., 1996), but these findings are not as consistent (Quickfall & Crockford, 2006) nor are they as well explored as with HIV. Damage or dysfunction of striatal structures have been reported among HIV+ individuals through a variety of research methodologies, which have revealed structural abnormalities (Aylward et al., 1993; Jernigan et al., 1993; Kieburtz et al., 1996; R. Paul, Cohen, Navia, & Tashima, 2002; Stout et al., 1998), functional abnormalities (Chang et al., 2001; Rottenberg et al., 1996; van Gorp et al., 1992; von Giesen et al., 2000), altered brain metabolites (Chang et al., 2004; Ernst, Itti, Itti, & Chang, 2000; Meyerhoff et al., 1999; R. H. Paul et al., 2007), and post mortem neuropathology (Masliah, Ge, Achim, DeTeresa, & Wiley, 1996; Nath et al., 2000; Navia, Cho, Petito, & Price, 1986; Wiley et al., 1998).
Despite an abundance of neuropsychological studies on effects of HIV and the evidence to suggest striatal dysfunction as a possible mechanisms for additive neurocognitive sequelae from HIV and marijuana use, there have been no studies published that specifically examined their association with measures of procedural learning (PL), which are thought to be particularly sensitive to striatal dysfunction. PL refers to an aspect of nondeclarative memory including gradual, incremental learning of associations, skills, and habits that can be demonstrated through improvements in behavioral performance, but do not require conscious memorization or recollection (e.g., riding a bike, tennis swing, driving). Caudate and putamen have been consistently reported as vital for PL (Packard & Knowlton, 2002; Salmon & Butters, 1995; Squire & Zola, 1996; Yin & Knowlton, 2006), and patients with basal ganglia abnormalities, particularly those with degenerative dementias affecting subcortical brain structures, show impairments on such tasks (Knowlton, Mangels, & Squire, 1996).
The reliance of PL on integrity of striatal structures makes it a promising construct to examine in the context of HIV and marijuana use. Furthermore, PL deficits among HIV+ individuals could conceivably contribute to impairments in important activities of daily living that may rely on PL, such as driving, medication management, or learning of other new habits and routines. Although the aforementioned evidence suggests that PL may be affected by HIV, it is worth noting that the few studies conducted to date show mixed results (Gonzalez et al., 2008; Martin, Heyes, Salazar, Law, & Williams, 1993). A prior investigation by our group (Gonzalez et al., 2008) based on the same sample as that presented in the current study found overall poorer complex motor skills among HIV+ than among HIV− SDIs, but did not find evidence of PL deficits, per se. That is, HIV+ participants performed more poorly overall than their matched controls, but groups showed no differences in their rates of improvement across trial blocks. To our knowledge, there are no published studies examining associations between history of marijuana use and PL. Basal ganglia structures critical for PL are dense in CB1 receptors, the primary targets of the psychoactive constituents of marijuana. This leads us to speculate that a history of marijuana dependence could have some effect on PL: a hypothesis that we test in this manuscript. It is possible that heavier marijuana use among SDIs may make prominent PL deficits among those with HIV or worsen even further the deficits in complex motor skills that we observed in our prior study.
Although we have made an argument for why a history of marijuana use may be detrimental for PL among those with HIV, there is evidence that would suggest an alternative hypothesis. That is, marijuana may also be neuroprotective in the context of HIV. CB1 agonists (including those naturally found in marijuana) are known to be potent antioxidants and can reduce glutamate mediated excitotoxic injury (Grundy, 2002; Guzman, Sanchez, & Galve-Roperh, 2001; Hampson et al., 2000; Marsicano, Moosmann, Hermann, Lutz, & Behl, 2002; Mechoulam, Spatz, & Shohami, 2002). Furthermore, they have been shown to inhibit proinflammatory cytokine production (Centonze, Finazzi-Agrò, Bernardi, & Maccarrone, 2007). Interestingly, some of the common mechanisms cited for HIV-associated brain injury include oxidative stress, microvascular accidents, inflammation, and excitotoxic injury through increased glutamate release (Anthony & Bell, 2008; Hult, Chana, Masliah, & Everall, 2008). Therefore, it is possible that marijuana may reduce neurotoxic injury from HIV through these mechanisms, but this has not been supported by existing studies on effects of marijuana on the brain functioning of HIV+ persons (Chang et al., 2006; Cristiani et al., 2004). However, it is worth noting that methamphetamine is thought to affect brain functioning through mechanisms similar to those of HIV (Gonzalez & Cherner, 2008; Nath et al., 2002; Rippeth et al., 2004), and a study examining neuropsychological effects of combined marijuana and methamphetamine use reported better neuropsychological performance among methamphetamine users who also had a history of a marijuana use disorder than among those who did not (Gonzalez et al., 2004).
In this study, we examine the effects of HIV and a history of marijuana dependence on PL among a sample of individuals with a history of dependence for multiple substances. Given the often additive negative impact of HIV and substance dependence on neurocognitive functioning and the known adverse effects of both of these risk factors on striatum, we anticipate that our sample of SDIs may be particularly vulnerable to any adverse effects that a history of cannabis use may have on PL. However, the competing hypotheses on how marijuana may interact with HIV to affect neurocognitive functioning and the mixed results from extant literature on the topic may contribute to several possible outcomes. For example, finding evidence for better performance among those SDIs with a history of heavier marijuana use, after controlling for potential confounds, would support a neuroprotective role for marijuana in this context.
METHOD
Participants
Participants were 86 adults with history of substance use disorder recruited from the greater Chicago metropolitan area through flyers in the community, substance use treatment centers, health clinics, and word of mouth. The study was approved by the University of Illinois Chicago Institutional Review Board, and all participants provided informed consent. Participants met DSM–IV (Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition; American Psychiatric Association, 1994) criteria for lifetime dependence on cocaine and/or heroin; however, none met DSM–IV criteria for a current substance use disorder (with the possible exception of caffeine and nicotine use disorders, which were not assessed). Furthermore, no participant reported use of illicit substances for at least 10 days prior to the study visit. Alcohol breath test (AlcoMate Prestige, Model AL6000) and urine toxicology testing (DrugCheck NxStep OnSite) were used to confirm that all participants were not acutely intoxicated from alcohol and were abstinent from cocaine, heroin, other opioids, and other stimulants at the time of their visit. Participants with positive urine toxicology or breath test were not further evaluated and were rescheduled. Participants reported minimal recent drug use. Other exclusion criteria included a history of open head injury, closed head injury with loss of consciousness greater than 30 minutes, neurosyphilis, cerebrovascular accidents, other neurological illness, and schizophrenia. These participants have been described in detail in a prior study (Gonzalez et al., 2008).
Our sample consisted mostly of African-American men with approximately a high-school education. Almost half of the sample (46%, n = 40) met DSM–IV criteria for past marijuana dependence. Tables 1 and 2 present detailed information on participant demographics, indicators of mental health, and their substance use history stratified by both HIV serostatus (HIV+ or HIV−) and history of marijuana (MJ) dependence (MJ+ or MJ−). All participants in the study underwent blood tests for HIV, and approximately half of the sample (49%, n = 42) were seropositive (i.e., HIV+). Only 18% (n = 7) of HIV+ participants met immunological criteria for an AIDS diagnosis (CD4 counts: median = 366, IQR = 261 to 560, n = 401), and 48% had undetectable HIV RNA viral load in plasma (HIV RNA: median = 210, IQR = 75 to 1,644, n = 40). The vast majority (81%) of HIV+ participants were prescribed antiretroviral treatment, and almost half (48%) of the HIV+ group were on highly active antiretroviral therapy (HAART).
TABLE 1.
Demographic characteristics of sample
| MJ−/HIV− (n = 21)
|
MJ+/HIV− (n = 23)
|
MJ−/HIV+ (n = 25)
|
MJ+/HIV+ (n = 17)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | % | Mdn [IQR] | M (SD) | % | Mdn [IQR] | M (SD) | % | Mdn [IQR] | M (SD) | % | Mdn [IQR] | |
| Age (years) | 43.0 (6.1) | 45.5 (7.8) | 43.0 (5.9) | 44.4 (7.6) | ||||||||
| Education (years) | 12.3 (2.1) | 11.6 (1.4) | 12.0 (2.0) | 12.3 (1.7) | ||||||||
| AmNART estimated IQ | 101.1 (9.0) | 104.0 (10.4) | 101.1 (9.0) | 99.0 (5.5) | ||||||||
| % Male | 76 | 87 | 72 | 59 | ||||||||
| Ethnicity/race | ||||||||||||
| % Caucasian | 19 | 4 | 8 | 6 | ||||||||
| % Hispanic | 5 | 0 | 4 | 6 | ||||||||
| % African American | 76 | 96 | 88 | 82 | ||||||||
| % Other | 0 | 0 | 0 | 6 | ||||||||
| % Right-handed | 91 | 87 | 96 | 94 | ||||||||
| BDI–II * | 4 [1, 8] | 9 [5, 17] | 6 [3, 11] | 19 [12, 26] | ||||||||
| STAI–State* | 32.5 (14.0) | 37.7 (11.6) | 31.8 (10.1) | 45.8 (12.2) | ||||||||
| WURS* | 34.5 (24.7) | 34.4 (16.6) | 25.3 (11.6) | 40.7 (16.6) | ||||||||
| % Hepatitis C seropositive | 19 | 35 | 36 | 47 | ||||||||
Note. Mdn, median; IQR, interquartile range; MJ, marijuana; AmNART, American National Adult Reading Test; BDI–II, Beck Depression Inventory–Second Edition; STAI–State, State–Trait Anxiety Inventory–State portion; WURS, Wender–Utah Rating Scale.
p < .05; BDI–II: MJ+/HIV+ > MJ+/HIV−, MJ−/HIV+, MJ+/HIV+; STAI-State: MJ+/HIV+ > MJ−/HIV+, MJ−/HIV−; WURS: HIV+/MJ+ > HIV+/MJ−.
TABLE 2.
Substance use characteristics of sample
| MJ−/HIV− (n = 21)
|
MJ+/HIV− (n = 23)
|
MJ−/HIV+ (n = 25)
|
MJ+/HIV+ (n = 17)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | % | Mdn [IQR] | M (SD) | % | Mdn [IQR] | M (SD) | % | Mdn [IQR] | M (SD) | % | Mdn [IQR] | |
| Years of cocaine and/or heroin use | 20.8 (5.2) | 23.7 (9.0) | 21.0 (8.4) | 22.9 (9.7) | ||||||||
| Days since last use of cocaine and/or heroin | 229 [120, 1, 486] | 210 [90, 365] | 300 [135, 714] | 350 [187, 627] | ||||||||
| Days since last marijuana use | 2, 532 [158, 6, 844] | 2, 980 [240, 7, 200] | 2, 523 [350, 8, 121] | 365 [218, 2, 829] | ||||||||
| History of lifetime SCID-SAM dependence | ||||||||||||
| Alcohol | 86 | 83 | 84 | 88 | ||||||||
| Cocaine | 91 | 96 | 96 | 100 | ||||||||
| Heroin | 52 | 74 | 44 | 59 | ||||||||
| Stimulants | 0 | 9 | 12 | 24 | ||||||||
| KMSK peak | ||||||||||||
| Alcohol | 11 [8, 12] | 10 [8, 12] | 11 [9, 12] | 10 [7, 12] | ||||||||
| Cocaine | 13 [11, 16] | 13 [11, 16] | 15 [13, 15] | 15 [12, 15] | ||||||||
| Heroin | 2 [0, 11] | 9 [2, 11] | 2 [0, 12] | 8 [0, 12] | ||||||||
| Marijuana* | 7 [4, 12] | 12 [9, 15] | 6 [2, 11] | 13 [11, 15] | ||||||||
Note. Mdn, median; IQR, interquartile range; MJ, marijuana; SCID-SAM, Structured Clinical Interview for DSM–IV–Substance Abuse Module; DSM–IV, Diagnostic and Statistical Manual of Mental Disorders–Fourth Edition; KMSK, Kreek–McHugh–Schluger–Kellogg Scale.
p < .05; MJ+/HIV−, MJ+/HIV+ > MJ−/HIV−, MJ−/HIV+.
Assessment protocol and measures
A prior investigation (Gonzalez et al., 2008) described in detail the assessment protocol and measures employed in this study. Briefly, participants completed two separate visits lasting about 2 hours each and consisting of structured clinical interviews, self-report questionnaires, and three measures of PL. Information on participants’ substance use history was collected through clinical interview and self-report questionnaires. Substance use diagnoses were ascertained with the Substance Abuse Module of the Structured Clinical Interview for DSM–IV (SCID-SAM; First, Spitzer, Gibbon, & Williams, 2002). A modified version of the Kreek–McHugh–Schluger–Kellogg scale (KMSK; Kellogg et al., 2003) was employed to index severity of alcohol and drug use history. Additional questions were added to the KMSK in order to obtain information on history of marijuana use, as well as information on drug use during the prior month for several drug classes. The KMSK obtains information on frequency, amount, and duration of alcohol and drug use during an individual’s most intense period of drug consumption (i.e., their peak use) and arrives at a composite score for each substance to index the severity of their use. Higher KMSK scores indicate a more severe history of drug use, with the maximum score differing by substance (i.e., alcohol = 13; cocaine = 16; heroin = 13; marijuana = 16). For example, in order to obtain a score of 16 for marijuana, an individual would have to endorse peak use of marijuana that was “several times a day, every day or most days, or continuous use as long as drug is available” and that persisted for “more than a year,” and they spent more than $50 daily on marijuana. In contrast, the lowest minimum score for a marijuana user would be a 2, which would correspond to peak use of “fewer than 20 times in lifetime,” for “less than six months,” and expenditure of $10 or less on marijuana per day of use.
Self-report questionnaires were also administered to assess mental health problems, including symptoms of depression (Beck Depression Inventory–2, BDI–II; Beck, Steer, Ball, & Ranieri, 1996), anxiety (“State” portion of the State–Trait Anxiety Inventory, STAI; Spielberger, Gorsuch, Lushene, Vagg, & Jacobs, 1983), and attention-deficit/hyperactivity disorder (ADHD; Wender–Utah Rating Scale, WURS; Stein et al., 1995; Ward, Wender, & Reimherr, 1993). Premorbid IQ was estimated with the American National Adult Reading Test (AmNART; Grober & Sliwinski, 1991).
Participants also completed three separate measures of PL: the photoelectric Rotary Pursuit Task (RPT), the Star Mirror Tracing Task (SMT), and the Weather Prediction Task (WPT). All three tasks assess performance across multiple trial blocks, thus allowing us to examine how performance changes over time—that is, with continued exposure and practice on the task. PL can be measured by examining improvements in performance across trial blocks. Thus, groups can be compared on their rate of learning in order to assess any differences in PL. We can also examine overall performance on the task (collapsed across trial blocks), as it is possible for groups to differ in their overall performance, but to show comparable rates of learning over trial blocks. For example, in a prior study (Gonzalez et al., 2008) we found overall poorer performance on SMT (and a trend for the RPT) among HIV+ compared to HIV− participants, but no differences in their rate of learning over trial blocks. Because of the strong complex motor demands of the RPT and SMT, we concluded that the HIV+ group showed evidence of deficits in complex motor skills, but no PL deficits per se.
Detailed procedures for these tasks can be found in Gonzalez et al. (2008). The RPT and the SMT are measures of motor skills learning that are performed abnormally by patients with disease that primarily affects the basal ganglia, such as Parkinson’s (e.g., Heindel, Salmon, Shults, Walicke, & Butters, 1989; Sarazin et al., 2002) and Huntington’s disease (e.g., Heindel, Butters, & Salmon, 1988; Heindel et al., 1989) compared to persons with brain disorders with relative sparing of the basal ganglia (for review see Salmon & Butters, 1995). Briefly, the RPT requires participants to hold a plastic stylus over a rotating disk, keeping the stylus directly over a patch of light that spins around the circumference of the disk at a set speed (set to 55 rpm for all trials). Eight trials lasting 20 seconds each were conducted. The seconds that the stylus was kept on the target during each trial was recorded. The SMT requires participants to trace within an outline of a six-point star on a flat metallic plate using a metal stylus. Participants do not see the actual star (or their hand) as they are tracing, but rather see only a mirror image. Eight trials were conducted, with the number of seconds required for the participant to trace the full outline of the star, one time, recorded for each trial. Participants also completed the WPT, a computer-administered, two-choice probabilistic classification task (Knowlton, Squire, & Gluck, 1994), also thought to depend on basal ganglia functioning (Poldrack et al., 2001; Poldrack, Prabhakaran, Seger, & Gabrieli, 1999); Shohamy, Myers, Onlaor, & Gluck, 2004).
On each trial, one, two, or three cards are displayed on the computer screen, each with a unique pattern. There are four different patterns that appear in various combinations on each trial. Each pattern is associated with a fixed probability of “sunshine” or “rain” that is unknown to participants (Experiment 2 of Gluck, Shohamy, & Myers, 2002). Participants are told that they may have to “guess at first” but that they should try “to get better at predicting the weather” as the task goes on. After each response, the computer provides participants immediate feedback on whether they are correct or incorrect. Performance was quantified by tabulating the number of correct selections made during each of four 50-trial blocks.
RESULTS
General statistical procedures
All statistical procedures where conducted with JMP 8.0 (SAS, Carey, NC) and SPSS 17.0 (Chicago, IL). Distributions of data for each variable and all statistical analyses were examined for outliers and violations of statistical assumptions (e.g., non-normal distribution, heterogeneity of variance). Non-normal data underwent transformation when appropriate. Square-root transformations were necessary for the BDI–II, CD4 counts, and SMT data. Nonparametric tests were conducted when examining HIV RNA viral load and KMSK scores, as they were not amenable to square-root or log transformation. Student’s t tests were used for between-group (HIV+ and HIV−) comparisons with one continuous dependent variable, whereas chi-square tests were employed when the single dependent variable was categorical. Analyses with more than two groups and a continuous dependent variable were conducted with analysis of variance (ANOVA), and all pairwise comparisons were examined using Tukey’s HSD (honestly significant difference). As is often the convention in the behavioral sciences, analyses were deemed statistically significant when p-values were less than or equal to .05. Standardized effect sizes were generated using Hedges’s g (Hedges, 1981; Hedges & Olkin, 1985), which is interpreted in the same manner as z scores and Cohen’s d. As we did in a prior study (Gonzalez et al., 2008), data reduction techniques were used for data from the RPT and the SMT. Specifically, performance on the eight trials of the task was reduced to four trial blocks for each of these two measures, such that each trial block represented the average performance across two successive trials for each individual (i.e., Trial Block 1 = average of Trial 1 and Trial 2; Trial Block 2 = average of Trial 3 and Trial 4; and so forth). This allowed us to reduce the number of dependent variables in analyses, reduce individual across-trial variability, and conduct analyses across all PL measures using the same number of dependent variables.
Group characteristics and control of confounds
Participants were stratified by HIV serostatus (HIV+ or HIV−) and/or lifetime history of marijuana dependence (MJ+ or MJ−). We used a history of marijuana dependence as a proxy for lifetime severity of cannabis use. All participants classified as MJ+ had a history of marijuana dependence; whereas none of those in the MJ− group ever met criteria for marijuana dependence. However, past cannabis use was common across our entire sample, and those individuals with only a history of past cannabis abuse were not excluded from the MJ− group. Indeed, 65% of participants classified as MJ− met criteria for a history of cannabis abuse. This presents a conservative bias in our ability to detect any “marijuana effect.”
We compared groups on various demographic, mental health, and substance use parameters in order to identify potential confounds. In order to maximize the possibility of identifying potential confounds, we conducted three sets of analyses to examine between-group differences among: (a) HIV+ (n = 42) and HIV− (n = 44) groups; (b) MJ+ (n = 40) and MJ− groups (n = 46); and (c) groups stratified based on both factors (MJ−/HIV−, n = 21; MJ+/HIV−, n = 23; MJ−/HIV+, n = 25; MJ+/HIV+ n = 17). Tables 1 and 2 present data on the variables we analyzed across the four groups. No significant between-group differences were observed on any of the demographic, mental health, and substance use variables when comparing HIV+ and HIV− participants. When stratified only by history of marijuana dependence, the MJ+ group showed statistically significant differences from the MJ− group on the BDI–II (MJ−: Mdn = 5, IQR = 2.75 to 8.25; MJ+: Mdn = 13, IQR = 8 to 24, p < .001) and STAI scores (MJ−: M = 32.1, SD = 11.9; MJ+: M = 41.1, SD = 12.4, p < .001). As expected, the MJ+ group also had significantly higher scores on KMSK peak marijuana use (MJ−: Mdn = 6, IQR = 3 to 11; MJ+: Mdn = 13, IQR = 10 to 15; p < .001), which substantiates our use of a marijuana dependence history as a proxy for severity of marijuana use. Importantly, there were no significant differences between the MJ+ and MJ− groups on KMSK scores for drugs of abuse other than marijuana. When stratified both by HIV serostatus and by history of marijuana dependence, statistically significant differences were observed on the BDI–II, WURS, and STAI (see Tables 1 and 2), indicating that MJ+ participants tended to endorse more symptoms of emotional distress. As expected, both MJ+ groups endorsed higher KMSK peak marijuana use scores than did both MJ− groups. Although the MJ−/HIV+ and MJ−/HIV− groups did not differ significantly on KMSK marijuana scores, there was a significantly higher prevalence of marijuana abuse history in the MJ−/HIV− groups (81% vs. 52%, respectively; p = .04). Of note, the MJ+/HIV+ group did not differ from the MJ−/HIV+ group in terms of CD4 counts (MJ+/HIV+: Mdn = 409, IQR = 250 to 683; MJ−/HIV+: Mdn = 351, IQR = 261 to 457; p = .46), percentage of participants on antiretroviral medications (MJ+/HIV+: 76%; MJ−/HIV+: 84%) or on HAART (MJ+/HIV+: 47%; MJ−/HIV+: 56%; p = .57), or detectable HIV RNA viral load in plasma (MJ+/HIV+: 63%; MJ−/HIV+: 46%; p = .30).
Because some group differences emerged on a few mental health variables, we conducted several analyses to ascertain whether any of those variables accounted for significant variance in performance on PL tasks. Three separate multivariable regressions were conducted, with BDI–II, WURS, and STAI scores as the independent variables and overall performance on one of the three PL task as the dependent variable (RPT, SMT, or WPT). None of the independent variables accounted for significant variance in any of the three PL tasks (all omnibus p-values > .10; all η2 < .07). Because of their lack of significant correlation with the dependent variables, they were not included in further analyses as covariates.
MJ, HIV, and performance on PL tasks
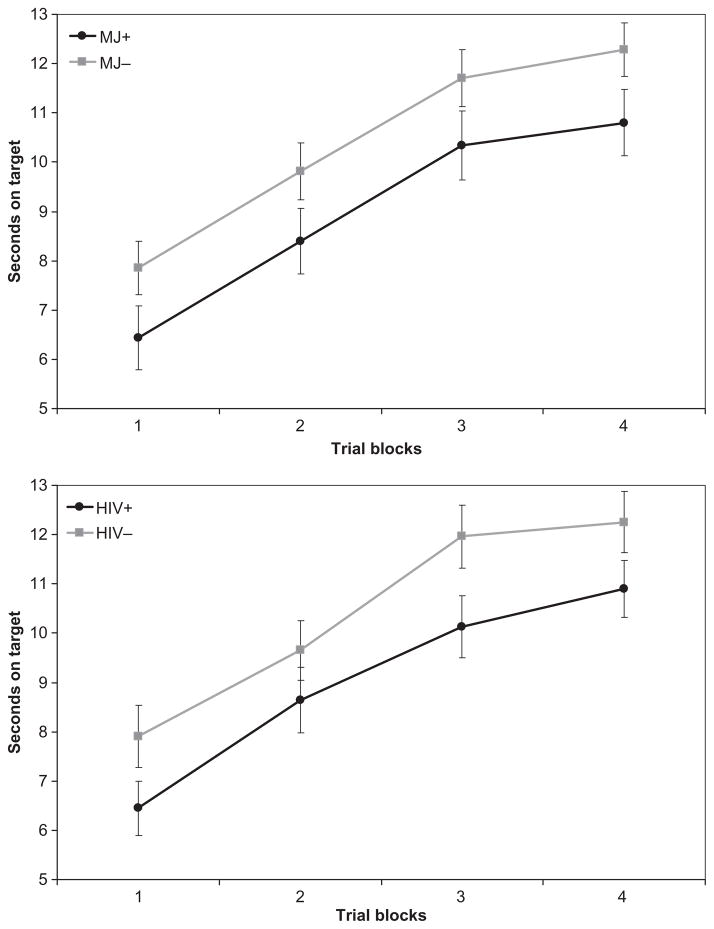
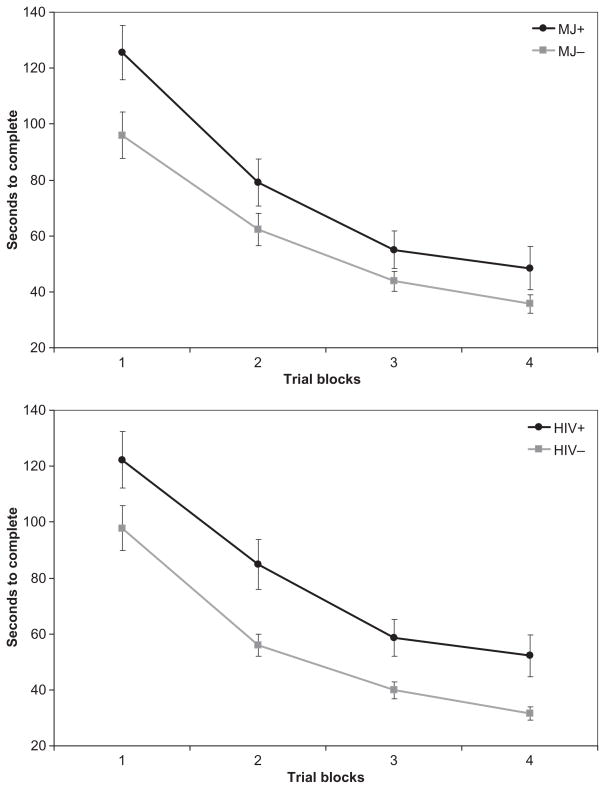
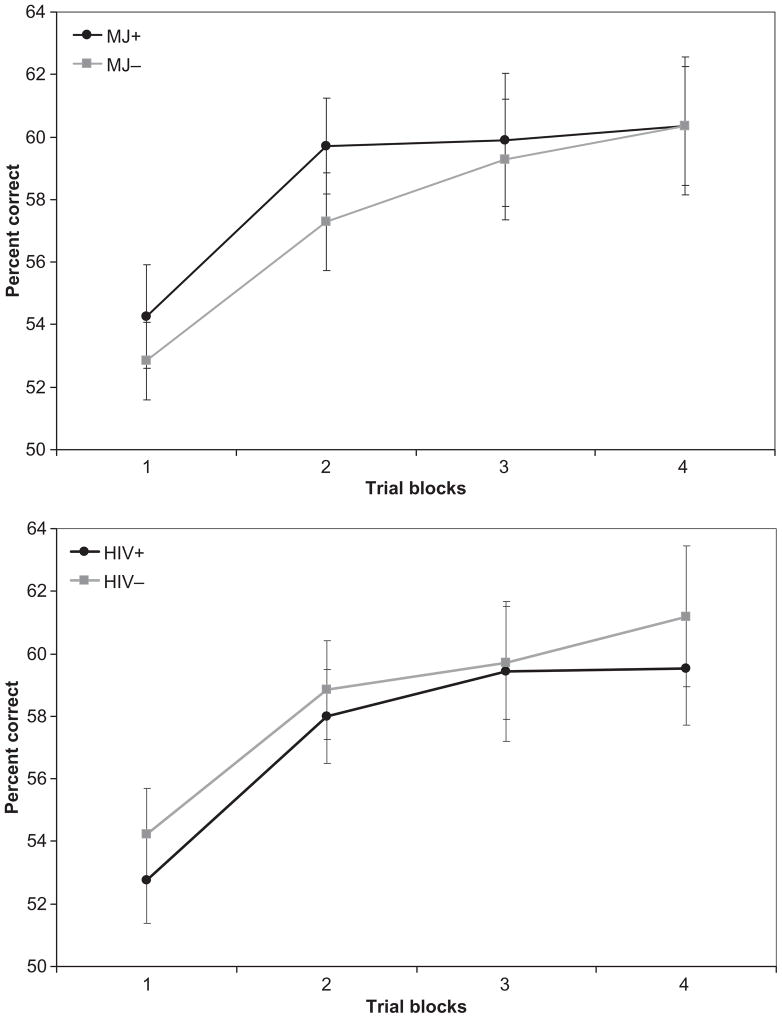
Performance on the PL tasks is presented in Table 3. Pearson product–moment correlations among the three PL tasks were statistically significant, but of small magnitude (SMT–RPT: r = −.37, p < .001; SMT–WPT: r = −.24, p = .03; WPT–RPT: r = .22, p = .04). Three separate 2 × 2 × 4 mixed-model ANOVAs with repeated measures were conducted (one for each PL task) using the Greenhouse–Geisser correction. Between-subjects factors were history of marijuana dependence (MJ+ or MJ−) and HIV serostatus (HIV+ or HIV−), with trial blocks on each task as the within-subjects repeated measure. All two-way and three-way interaction effects were examined. Across all three models, we observed a statistically significant within-subjects main effect for trial blocks, indicating that overall participant performance improved across trial blocks on all tasks [SMT: F(1.85, 151.72) = 171.2, p < .0001; RPT: F(2.58, 211.74) = 103.1, p < .0001; WPT: F(2.71, 216.89) = 9.39, p < .0001]. Thus, overall, participant performance improved with repeated exposure to the task. No significant MJ × Trial Block [SMT: F(1.85, 151.72) = 1.95, p = .15; RPT: F(2.58, 211.74) = 0.02, p = .99; WPT: F(2.71, 216.89) = 0.38, p = .74] or HIV × Trial Block interaction effects were observed [SMT: F(1.85, 151.72) = 0.35, p = .69; RPT: F(2.58, 211.74) = 0.82, p = .47; WPT: F(2.71, 216.89) = 0.14, p = .92], suggesting that rate of improvement across trial blocks did not differ as a consequence of HIV serostatus or history of marijuana dependence alone. That is, the rate of procedural learning did not differ as a consequence of HIV status or of MJ status. However, significant between-group main effects emerged for both HIV and MJ factors on the SMT [HIV: F(1, 82) = 10.67, p = .002; Hedges’s g = −0.60; MJ: F(1, 82) = 6.54, p = .012; Hedges’s g = −0.44] and RPT [HIV: F(1, 82) = 4.11, p = .046; Hedges’s g = −0.38; MJ: F(1, 82) = 4.09, p = .046; Hedges’s g = −0.38], but not the WPT [HIV: F(1, 80) = 0.41, p = .53; Hedges’s g = −0.13; MJ: F(1, 80) = 0.28, p = .60; Hedges’s g = 0.13]. On both the SMT and RPT, MJ+ participants showed overall poorer mean performance than MJ− participants, and HIV+ participants performed worse than HIV− participants (Figures 1, 2, and 3), when collapsing across all trial blocks. No significant MJ × HIV interaction effects were observed for any of the PL tasks [SMT: F(1, 82) = 1.22, p = .27; RPT: F(1, 82) = 0.09, p = .76; WPT: F(1, 80) = 2.39, p = .13]. In summary, participants evidenced significant improvements in performance across trial blocks on all PL tasks, and rates of improvement did not differ as a consequence of MJ or HIV status. That is, learning slopes across trial blocks were similar across groups. Overall, however, those with a history of MJ dependence (MJ+) performed more poorly than those without (MJ−) on the SMT and RPT, regardless of their HIV serostatus. Also, as we reported in our prior study with the same sample (Gonzalez et al., 2008), a positive HIV serostatus (HIV+) was also associated with poorer performance on the SMT and RPT, independently of MJ status. We found no evidence of HIV × MJ interactions.
TABLE 3.
Performance of groups on procedural learning measures
| MJ−/HIV− (n = 21) | MJ+/HIV− (n = 23) | MJ−/HIV+ (n = 25) | MJ+/HIV+ (n = 17) | ||
|---|---|---|---|---|---|
|
| |||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| SMT | |||||
| Trial Block 1 | 86.9 (55.0) | 107.8 (49.5) | 103.6 (57.4) | 149.6 (68.0) | |
| Trial Block 2 | 49.6 (21.1) | 61.8 (29.5) | 72.9 (46.2) | 102.4 (69.6) | |
| Trial Block 3 | 38.4 (16.0) | 41.2 (22.3) | 48.4 (28.6) | 73.5 (55.5) | |
| Trial Block 4 | 27.8 (10.2) | 35.0 (20.1) | 42.3 (26.5) | 66.7 (67.4) | |
| Overall mean performance | 50.7 (21.7) | 61.5 (24.7) | 66.8 (36.9) | 98.1 (59.6) | |
| RPT | |||||
| Trial Block 1 | 8.5 (4.3) | 7.4 (4.0) | 7.3 (3.0) | 5.2 (3.9) | |
| Trial Block 2 | 10.6 (3.9) | 8.8 (4.0) | 9.2 (4.0) | 7.9 (4.6) | |
| Trial Block 3 | 12.6 (4.3) | 11.3 (4.1) | 10.9 (3.5) | 9.0 (4.6) | |
| Trial Block 4 | 12.9 (4.0) | 11.6 (4.2) | 11.7 (3.3) | 9.7 (4.2) | |
| Overall mean performance | 11.2 (3.9) | 9.8 (3.7) | 9.8 (3.1) | 7.9 (4.1) | |
| WPTa | |||||
| Trial Block 1 | 53.7 (8.2) | 55.6 (10.3) | 52.2 (8.6) | 53.7 (9.9) | |
| Trial Block 2 | 56.3 (10.7) | 61.0 (10.1) | 58.1 (10.3) | 57.9 (9.3) | |
| Trial Block 3 | 56.2 (11.4) | 62.9 (11.1) | 61.8 (13.8) | 56.0 (15.1) | |
| Trial Block 4 | 59.6 (13.8) | 62.6 (15.4) | 61.0 (12.1) | 57.4 (11.1) | |
| Overall mean performance | 56.5 (8.8) | 60.5 (9.2) | 58.2 (8.6) | 56.2 (9.4) | |
Note. None of the data presented in this table have been transformed. MJ, marijuana; SMT, Star Mirror Tracing; RPT, Rotary Pursuit Task; WPT, Weather Prediction Task. Values for SMT are seconds to complete task (lower value is better); RPT values are seconds on target (higher value is better); WPT values are percentage of correct choices (higher value is better).
Sample sizes differ slightly for the WPT, as 1 person in the MJ−/HIV− group and 1 in the MJ+/HIV− group did not complete the task due to technical problems.
Figure 1.
Rotary Pursuit Task performance by marijuana (MJ) dependence and HIV serostatus. Data points represent mean performance and standard error for each trial block.
Figure 2.
Star Mirror Tracing performance by marijuana (MJ) dependence and HIV serostatus. Data points represent mean performance and standard error for each trial block.
Figure 3.
Weather Prediction Task performance by marijuana (MJ) dependence and HIV serostatus. Data points represent mean performance and standard error for each trial block.
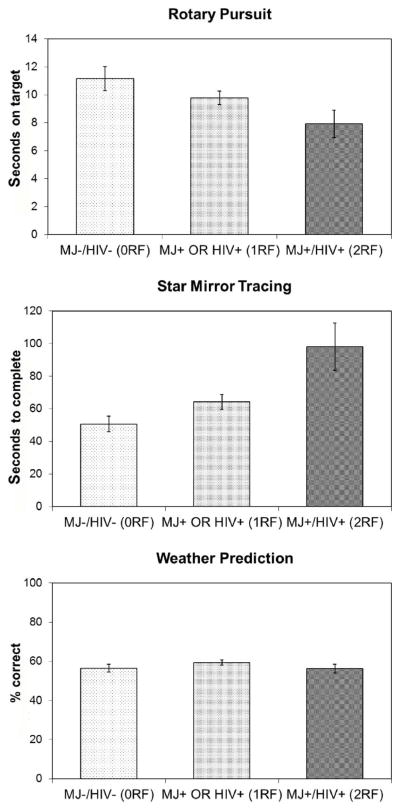
Additive effects of MJ and HIV on PL tasks
Because we found no significant interaction effects, we conducted further analyses to determine whether HIV serostatus and history of marijuana dependence served as additive risk factors for poorer PL performance in our sample of SDIs. Specifically, participants were stratified into three groups: (a) those with no risk factors (0RF; i.e., MJ− and HIV−; n = 21); (b) those with one risk factor (1RF; i.e., MJ+ or HIV+; n = 48); and (c) those with both risk factors (2RF; i.e., MJ+ and HIV+; n = 17). We used the Jonckheere–Terpstra test (Jonckheere, 1954) for ordered alternatives to examine the possibility of a monotonic change in PL performance with increasing risk factors. Performance on the RPT (p = .014) and SMT (p = .001) showed a monotonic worsening with increasing risk factors, whereas the WPT did not (p = .91; see Figure 4). Thus, performance showed a linear worsening trend in complex motor skills as groups increased in their number of risk factors.
Figure 4.
Performance on procedural learning (PL) measures by number of risk factors (marijuana, MJ, dependence and HIV serostatus). 0RF, no risk factors; 1RF, one risk factor; 2RF, two risk factors. Data points are means and standard errors. Statistically significant monotonic trend across number of risk factors were evidence for Rotary Pursuit Task (RPT; p = .014) and Star Mirror Tracing (SMT; p = .001), but not Weather Prediction Task (WPT; p = .91).
Relationship between immune function and PL
Finally, we conducted exploratory analyses to examine how immunological factors affected PL performance among our HIV+ participants when stratified by their history of marijuana dependence. Pearson correlations were computed for CD4 counts and overall performance on each of the three PL tasks, separately for the MJ+ and MJ− participants. No significant correlations were observed (all p-values > .10).
DISCUSSION
In this study, we examined how a history of marijuana dependence, independently or through an interaction with HIV, affected procedural learning (PL) in a sample of currently abstinent substance-dependent individuals (SDIs) with a history of polysubstance use. In a prior study with this sample (Gonzalez et al., 2008), we reported poorer overall performance in an HIV+ group than in an HIV− group on the Rotary Pursuit Task and the Star Mirror Tracing Task, but no evidence of differences in rate of improvement on either of these tasks, as well as no differences on the Weather Prediction Task. This suggested to us that HIV may negatively impact complex motor skills, but not specifically result in a PL deficit. As expected, these findings persist with the new analyses presented in this manuscript. However, of central focus to the current study are our results as they pertain to a history of marijuana dependence among this sample of past polysubstance users. Two lines of evidence prompted competing hypotheses, as prior literature suggested that a history of marijuana use could be beneficial or harmful to the neurocognitive functioning of HIV+ individuals. We found that a history of marijuana dependence, regardless of HIV serostatus, was associated with poorer performance on measures of PL in this sample. The deficits among those with a history of marijuana dependence were consistent with poorer complex motor skills, rather than a PL deficit per se, as rate of improvement across trial blocks did not differ between the MJ+ and MJ− groups, whereas the groups showed significant difference on overall performance of the SMT and RPT. Thus, the MJ+ participants show baseline differences in performance compared with performance of the MJ− participants on the SMT and RPT, which remained of similar magnitude even with additional practice. This result is similar to the effect of HIV on PL among SDIs, which we previously reported (Gonzalez et al., 2008).
Additionally, we found no evidence of an HIV and marijuana dependence interaction. The presence or absence of HIV did not affect differences in PL performance between the MJ+ and MJ− groups, and vice versa. However, further analyses revealed additive, rather than interactive, effects of HIV and MJ. Significant monotonic worsening in overall performance was observed on the SMT and RPT as participants increased from no risk factors to both risk factors (MJ+ and HIV+). When taken together with our finding of poorer overall performance on the RPT and SMT by the MJ+ group, our findings suggest that a history of marijuana dependence is associated with poorer performance of complex motor skills among HIV+ SDIs.
Our findings share similarities and differences with the few studies examining the impact of marijuana use on the neuropsychological performance of HIV+ individuals. Cristiani and colleagues (2004) found poorer overall neuropsychological functioning among HIV+ persons who used marijuana, which appeared to be driven primarily by a measure of delayed memory. Furthermore, the detrimental effects of marijuana on neuropsychological functioning were greater among those HIV+ persons with more severe disease. Unlike our study, they found no significant main effects for marijuana use. These differences may be due to the use of very different neuropsychological test batteries and/or differences in the participant samples. In contrast to Cristiani et al. and to our current results, Chang et al. (2006) found no evidence of interactive or additive effects of marijuana use and HIV on neuropsychological functioning. However, they found brain metabolite differences between groups, suggesting additive negative effects of HIV and marijuana use on brain metabolism. Specifically, HIV+ subjects with a history of marijuana use showed decreased glutamate in basal ganglia, whereas no other groups significantly differed. Similarly, in the thalamus, HIV+ subjects with a history of marijuana use showed the highest levels of creatine. The authors suggested that their results may indicate neuronal dysfunction and glial activation that appears to be worst among HIV+ subjects with a history of marijuana use. Amount of substance use other than marijuana likely differed across the two aforementioned studies and the current study; however, clear comparisons are difficult to make due to varying amounts of detail presented on the substance use history of participants across these studies. For example, Cristiani et al. reported that they excluded individuals with history of injection drug use, whereas Chang et al., (2006) excluded all individuals meeting criteria for other substance dependence or with positive urine toxicology testing; however, other detailed history of substance use among their participants was not provided. Participants in our study were also required to have negative urine toxicology test results and could not meet criteria for any current substance use disorder. Nonetheless, given the prevalence of past dependence for cocaine or heroin in our sample, it is likely that the participants in our study had a history of more coexisting substance use than those in the two prior studies. Despite these differences, collectively these results lend support to the idea that marijuana may be more detrimental to the neuropsychological functioning of those with HIV than to that of individuals without HIV. However, the issue of substance use other than marijuana in these samples complicates interpretation. It remains unclear whether these findings will generalize to HIV+ individuals who use marijuana but have no significant history of other drug or alcohol use. These studies await replication with samples of well-characterized marijuana users who have minimal use of other substances and comparable controls. This is a formidable challenge given the common co-occurrence of polysubstance use among individuals with substance use disorders (Darke & Hall, 1995; Leri et al., 2003).
We offer some speculations on mechanisms by which marijuana may exert potentially negative effects on the complex motor skills of individuals with HIV that are worth considering. However, some of these mechanisms are based on studies examining effects of cannabis alone, which may limit their direct relevance to our findings among polydrug users. First, as we describe in the introduction, HIV and marijuana may negatively impact striatal functioning, which is important for PL and motor skills. Although we did not find evidence of impaired PL, per se, we did find evidence of poorer complex motor skills. The findings of Chang et al. (2006) also support the hypothesis that the marijuana effects may be due to its impact on striatal function, as they found significant correlations between some of the metabolic abnormalities in basal ganglia and amount of past marijuana use. However, another possible mechanism for marijuana use to worsen HIV-associated neurocognitive disorders is through suppression of immune functioning (Cabral, 2006; McPartland & Pruitt, 1997; Pandey, Mousawy, Nagarkatti, & Nagarkatti, 2009). In our study, HIV+ groups were well matched on markers of immunological status for the primary analyses. Exploratory analyses revealed no significant associations between CD4 counts and PL task performance among our HIV+ participants when stratified by history of marijuana dependence. However, it is important to note that our sample sizes for these analyses were not sufficiently large to detect small effect sizes. In comparison, Cristiani et al. (2004) only found evidence of a marijuana effect among the HIV+ individuals with more severe HIV disease, but Chang et al. (2006) found no relationships between marijuana use and HIV disease severity. Neither study examined associations between specific biomarkers of immune functioning and neuropsychological performance. Thus, although current evidence suggests that marijuana use may be detrimental to the neuropsychological functioning of HIV+ persons, the exact mechanisms that underlie such impairments may be varied. Future studies attempting to delineate mechanisms through which cannabis may affect neurocognition should include more detailed analyses of immunological biomarkers and their relationships to marijuana use, as well as explore the impact of past versus ongoing cannabis use.
To our knowledge, the current study is the first to report on the impact of marijuana dependence history on PL. Although we did not find evidence of a PL deficit, our results suggest that a history of marijuana dependence is associated with poorer complex motor skills, regardless of HIV serostatus, in our sample of individuals with a history of polydrug use. Prior studies suggest that learning and memory are the primary neurocognitive abilities that remain affected by abstinent marijuana users (Gonzalez, 2007; Grant et al., 2003); however, they appear to improve with prolonged abstinence (Pope et al., 2001). The absence of studies on PL in the context of marijuana use prevents us from comparing our findings with similar investigations. We do note, however, that both Cristiani et al. (2004) and Chang et al. (2006) included measures of motor skills in their battery of tests (i.e., the Grooved Pegboard and Trail Making tests) but neither found evidence of a marijuana effect after controlling for confounds. Future studies will need to include a more varied array of motor tests to better understand how marijuana is likely to affect motor functioning in individuals with or without HIV.
Several limitations of our study should be considered, and caution is warranted in interpreting and generalizing our findings. First, although substance use disorders are known to commonly co-occur with HIV (e.g., Diaz et al., 1994; Mimiaga et al., 2008; Schulden, Thomas, & Compton, 2009), it is not clear how many HIV+ marijuana users in the general population use marijuana alone compared to use of multiple substances. However, some studies suggest that, at a minimum, alcohol use is common among HIV+ individuals who use marijuana (Prentiss et al., 2004). Like many studies including samples of substance-dependent individuals, participants in our study had a history of using multiple substances. Among drug users, this is often the norm (Darke & Hall, 1995; Leri et al., 2003). Clearly, this information has a bearing on the generalizability of our findings. Our results are most likely to generalize to those individuals with a history of dependence for multiple substances, including cocaine. All individuals in our marijuana dependence group also met criteria for other substance use disorders, and thus definitively isolating “pure” effects of marijuana use was not possible. We attempted to deal with this issue by stratifying individuals based on their history of marijuana dependence and then comparing their recency and overall severity of their drug use for several substance classes. We found no significant differences on these variables between groups, with the exception of those in the marijuana using groups reporting greater severity of marijuana use, as expected. Thus, this approach allowed us to indirectly infer how a history of significant marijuana use (i.e., those that met dependence criteria) may have affected performance, as participants were well matched on other potential confounds. Nonetheless, our study design limits our ability to generalize beyond individuals with a history of dependence for multiple substances, and because it is cross-sectional, causal inferences about the effects of marijuana cannot be concluded. It is also important to note that most participants in the nonmarijuana dependence group (MJ−) also had a history of marijuana use and abuse, but never met criteria for marijuana dependence and reported less severe history of marijuana use. This may have presented a conservative bias by attenuating differences in performance attributable to marijuana between the MJ+ and MJ− groups. It is also important to note that although there is no gold standard for assessing substance use severity, the approach we employed in this study has its limitations. Specifically, we classified individuals based on their history of marijuana dependence, which served as our benchmark for differentiating heavy, regular cannabis users from those with less significant use of the substance. However, this approach relies on a measure of problems from marijuana use rather than actual exposure to marijuana use. Our finding of significantly higher KMSK marijuana scores among the MJ+ group suggests that these individuals did indeed engage in significantly more cannabis use than those in the MJ− group. Nonetheless, this cannot be definitely ascertained, and future studies should incorporate more fine-grained measures of amount, frequency, duration, and recency of marijuana use to more directly examine relationships between exposure to marijuana and its impact on the neurocognitive functioning of HIV+ individuals. Finally, we also note that HIV+ participants in our sample were relatively healthy compared to those in other studies examining HIV effects, with only a small percentage of our sample with an immunological AIDS diagnosis. It is possible, as suggested by the results from Cristiani et al. (2004), that effects of marijuana may be more pronounced with a more severely ill sample.
Future investigations may benefit from examining potentially important variables that were not within the scope of the current study. For example, a more robust examination on the role of mental health factors in the complex relationships among PL, substance use, and HIV may yield interesting results. In this study, we found some statistically significant between-group differences in self-reported symptoms of depression (BDI–II), anxiety (STAI–State), and ADHD (WURS). Moreover, the group with both HIV and history of marijuana dependence as risk factors tended to endorse more of these symptoms, along with higher rates of hepatitis C (although not statistically significant), which may have had some impact on their neurocognitive performance. However, none of these variables were found to be correlated with PL performance in our sample and thus were not further explored. In contrast, Kalechstein, Hinkin, van Gorp, Castellon, and Satz (1998) found relationships between symptoms of depression and PL in a sample of predominantly white, nondrug using, gay and bisexual men. Specifically, they found that affective/cognitive symptoms of depression were associated with poorer PL, but not with episodic memory or immunosuppression. Conversely, somatic symptoms of depression were found to correlate with levels of immunosuppression, but not PL or episodic memory. Such a nuanced examination of interactions between mental health factors and PL may prove to be a line of fruitful research in examining PL and HIV among substance users. Another interesting area of exploration that has received attention in recent years pertains to interactions between nicotine and marijuana on neurocognitive functioning (Viveros, Marco, & File, 2006). Early studies suggest that cannabis use may exacerbate the adverse neurocognitive effects of nicotine withdrawal (Jacobsen, Pugh, Constable, Westerveld, & Mencl, 2007). In our study, we did not collect detailed data on nicotine use; however, we do not think that nicotine withdrawal effects may have affected our findings since participants were not required to make any changes to their smoking habits. Nonetheless, the absence of systematic analyses on the effects of nicotine in this sample remains a limitation of this study.
In summary, our results suggest that a history of marijuana dependence is associated with poorer complex motor skills in a sample with a history of substance dependence and polysubstance use. More importantly, a history of marijuana dependence appears to have additive detrimental effects on the complex motor skills of HIV+ individuals with a history of dependence on multiple substances, including cocaine. When taken together with the few clinical studies published in this area, our current findings lend further support to the idea that marijuana use may be harmful to the neurocognitive functioning of at least a subset of HIV+ individuals. HIV+ individuals are already vulnerable to neurocognitive deficits and motor abnormalities—our results suggest that a history of heavy marijuana use may worsen complex motor skills even further among SDIs. This is of some concern given the national trend to facilitate access to marijuana for individuals with HIV. It is unclear how well our results would generalize to a sample with circumscribed and controlled use of marijuana, as might be the case when marijuana is consumed specifically for medical reasons. Indeed, our findings require replication with samples of HIV+ individuals who have a minimal history of other drug use and who predominantly use marijuana alone. Our study and those of others have examined samples with a history of heavy marijuana use without differentiating whether use was recreational or medicinal. Medical marijuana use might be specifically contraindicated from such individuals, who may suffer from marijuana abuse or dependence. Neurocognitive data from clinical trials examining medicinal effects of marijuana are clearly needed to help identify and weigh the possible benefits and harm that marijuana may have among individuals living with HIV. On the other hand, such studies are not likely to fully capture the impact of daily marijuana use over a longer period of time. Given the lengthening lifespan for individuals with HIV, marijuana use for treatment of symptoms would likely be daily and chronic. Thus, observation and longitudinal studies will be critical to understand how daily marijuana use over long periods of time affects the neurocognitive functioning of those with HIV, especially considering the growing trend for allowing access to legal marijuana use for medicinal purposes in the United States.
Acknowledgments
The authors extend their gratitude to Joanna Jacobus for her efforts as a research assistant on this project and to Anup Amatya for statistical consultation. This work was supported by K23DA023560 and F32DA018522 to Raul Gonzalez and R01DA12828 to Eileen Martin.
Footnotes
Information on immunological markers of HIV disease severity was not available for 2 HIV+ participants.
References
- Abrams D, Jay C, Shade S, Vizoso H, Reda H, Press S, et al. Cannabis in painful HIV-associated sensory neuropathy: A randomized placebo-controlled trial. Neurology. 2007;68(7):515. doi: 10.1212/01.wnl.0000253187.66183.9c. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: Author; 1994. [Google Scholar]
- Anthony IC, Bell JE. The neuropathology of HIV/AIDS. International Review of Psychiatry. 2008;20(1):15–24. doi: 10.1080/09540260701862037. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Henderer JD, McArthur JC, Brettschneider PD, Harris GJ, Barta PE, et al. Reduced basal ganglia volume in HIV-1-associated dementia: Results from quantitative neuroimaging. Neurology. 1993;43(10):2099–2104. doi: 10.1212/wnl.43.10.2099. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories–IA and –II in psychiatric outpatients. Journal of Personality Assessment. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59(9):1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26(2):480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Cabral G. Drugs of abuse, immune modulation, and AIDS. Journal of Neuroimmune Pharmacology. 2006;1(3):280–295. doi: 10.1007/s11481-006-9023-5. [DOI] [PubMed] [Google Scholar]
- Centonze D, Finazzi-Agrò A, Bernardi G, Maccarrone M. The endocannabinoid system in targeting inflammatory neurodegenerative diseases. Trends in Pharmacological Sciences. 2007;28(4):180–187. doi: 10.1016/j.tips.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Yakupov R, Ernst T. Combined and independent effects of chronic marijuana use and HIV on brain metabolites. Journal of Neuroimmune Pharmacology. 2006;1(1):65–76. doi: 10.1007/s11481-005-9005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Lee PL, Yiannoutsos CT, Ernst T, Marra CM, Richards T, et al. A multicenter in vivo proton-MRS study of HIV-associated dementia and its relationship to age. NeuroImage. 2004;23(4):1336–1347. doi: 10.1016/j.neuroimage.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Chang L, Speck O, Miller EN, Braun J, Jovicich J, Koch C, et al. Neural correlates of attention and working memory deficits in HIV patients. Neurology. 2001;57(6):1001–1007. doi: 10.1212/wnl.57.6.1001. [DOI] [PubMed] [Google Scholar]
- Corless I, Lindgren T, Holzemer W, Robinson L, Moezzi S, Kirksey K, et al. Marijuana effectiveness as an HIV self-care strategy. Clinical Nursing Research. 2009;18(2):172. doi: 10.1177/1054773809334958. [DOI] [PubMed] [Google Scholar]
- Cristiani SA, Pukay-Martin ND, Bornstein RA. Marijuana use and cognitive function in HIV-infected people. Journal of Neuropsychiatry and Clinical Neuroscience. 2004;16(3):330–335. doi: 10.1176/jnp.16.3.330. [DOI] [PubMed] [Google Scholar]
- Darke S, Hall W. Levels and correlates of poly-drug use among heroin users and regular amphetamine users. Drug and Alcohol Dependence. 1995;39(3):231–235. doi: 10.1016/0376-8716(95)01171-9. [DOI] [PubMed] [Google Scholar]
- Diaz T, Chu SY, Byers RH, Jr, Hersh BS, Conti L, Rietmeijer CA, et al. The types of drugs used by HIV-infected injection drug users in a multistate surveillance project: Implications for intervention. American Journal of Public Health. 1994;84(12):1971–1975. doi: 10.2105/ajph.84.12.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R, Toperoff W, Vaida F, van den Brande G, Gonzales J, Gouaux B, et al. Smoked medicinal cannabis for neuropathic pain in HIV: A randomized, crossover clinical trial. Neuropsychopharmacology. 2009;34(3):672–680. doi: 10.1038/npp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst T, Itti E, Itti L, Chang L. Changes in cerebral metabolism are detected prior to perfusion changes in early HIV-CMC: A coregistered (1)H MRS and SPECT study. Journal of Magnetic Resonance Imaging. 2000;12(6):859–865. doi: 10.1002/1522-2586(200012)12:6<859::aid-jmri8>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Fairfield K, Eisenberg D, Davis R, Libman H, Phillips R. Patterns of use, expenditures, and perceived efficacy of complementary and alternative therapies in HIV-infected patients. Archives of Internal Medicine. 1998;158(20):2257. doi: 10.1001/archinte.158.20.2257. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV–TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). Version 2.0 [Questionnaire] New York, NY: New York State Psychiatric Institute, Biometrics Research; 2002. [Google Scholar]
- Gluck MA, Shohamy D, Myers C. How do people solve the “weather prediction” task?: Individual variability in strategies for probabilistic category learning. Learning and Memory. 2002;9(6):408–418. doi: 10.1101/lm.45202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychology Review. 2007;17(3):347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Cherner M. Co-factors in HIV neurobehavioural disturbances: Substance abuse, hepatitis C and aging. International Review of Psychiatry. 2008;20(1):49–60. doi: 10.1080/09540260701872028. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Jacobus J, Amatya AK, Quartana PJ, Vassileva J, Martin EM. Deficits in complex motor functions, despite no evidence of procedural learning deficits, among HIV plus individuals with history of substance dependence. Neuropsychology. 2008;22(6):776–786. doi: 10.1037/a0013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R, Rippeth JD, Carey CL, Heaton RK, Moore DJ, Schweinsburg BC, et al. Neurocognitive performance of methamphetamine users discordant for history of marijuana exposure. Drug and Alcohol Dependence. 2004;76(2):181–190. doi: 10.1016/j.drugalcdep.2004.04.014. [DOI] [PubMed] [Google Scholar]
- Grant I, Atkinson JH, Hesselink JR, Kennedy CJ, Richman DD, Spector SA, et al. Evidence for early central nervous system involvement in the acquired immunodeficiency syndrome (AIDS) and other human immunodeficiency virus (HIV) infections. Annals of Internal Medicine. 1987;107:828–836. doi: 10.7326/0003-4819-107-6-828. [DOI] [PubMed] [Google Scholar]
- Grant I, Gonzalez R, Carey CL, Natarajan L, Wolfson T. Non-acute (residual) neurocognitive effects of cannabis use: A meta-analytic study. Journal of the International Neuropsychological Society. 2003;9(5):679–689. doi: 10.1017/S1355617703950016. [DOI] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Journal of Clinical and Experimental Neuropsychology. 1991;13(6):933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Grundy RI. The therapeutic potential of the cannabinoids in neuroprotection. Expert Opinions on Investigational Drugs. 2002;11(10):1365–1374. doi: 10.1517/13543784.11.10.1365. [DOI] [PubMed] [Google Scholar]
- Guzman M, Sanchez C, Galve-Roperh I. Control of the cell survival/death decision by cannabinoids. Journal of Molecular Medicine. 2001;78(11):613–625. doi: 10.1007/s001090000177. [DOI] [PubMed] [Google Scholar]
- Hampson AJ, Grimaldi M, Lolic M, Wink D, Rosenthal R, Axelrod J. Neuroprotective antioxidants from marijuana. Annals of the New York Academy of Sciences. 2000;899:274–282. [PubMed] [Google Scholar]
- Haney M, Gunderson E, Rabkin J, Hart C, Vosburg S, Comer S, et al. Dronabinol and marijuana in HIV-positive marijuana smokers: Caloric intake, mood, and sleep. Journal of Acquired Immune Deficiency Syndromes. 2007;45(5):545. doi: 10.1097/QAI.0b013e31811ed205. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, et al. The HNRC 500—neuropsychology of HIV infection at different disease stages. HIV Neurobehavioral Research Center. Journal of the International Neuropsychological Society. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Hedges L. Distribution theory for Glass’s estimator of effect size and related estimators. Journal of Educational and Behavioral Statistics. 1981;6(2):107. [Google Scholar]
- Hedges LV, Olkin I. Statistical methods for meta-analysis. Orlando, FL: Academic Press; 1985. [Google Scholar]
- Heindel WC, Butters N, Salmon DP. Impaired learning of a motor skill in patients with Huntington’s disease. Behavioral Neurosciences. 1988;102(1):141–147. doi: 10.1037//0735-7044.102.1.141. [DOI] [PubMed] [Google Scholar]
- Heindel WC, Salmon DP, Shults CW, Walicke PA, Butters N. Neuropsychological evidence for multiple implicit memory systems: A comparison of Alzheimer’s, Huntington’s, and Parkinson’s disease patients. Journal of Neuroscience. 1989;9(2):582–587. doi: 10.1523/JNEUROSCI.09-02-00582.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Research. 1991;547(2):267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, et al. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences. 1990;87(5):1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult B, Chana G, Masliah E, Everall I. Neurobiology of HIV. International Review of Psychiatry. 2008;20(1):3–13. doi: 10.1080/09540260701862086. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Pugh KR, Constable RT, Westerveld M, Mencl WE. Functional correlates of verbal memory deficits emerging during nicotine withdrawal in abstinent adolescent cannabis users. Biological Psychiatry. 2007;61(1):31–40. doi: 10.1016/j.biopsych.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald S, Hesselink JR, Atkinson JH, Velin RA, McCutchan JA, et al. Magnetic resonance imaging morphometric analysis of cerebral volume loss in human immunodeficiency virus infection. The HNRC Group. Archives of Neurology. 1993;50(3):250–255. doi: 10.1001/archneur.1993.00540030016007. [DOI] [PubMed] [Google Scholar]
- Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometricka. 1954;41:133–145. [Google Scholar]
- Kalechstein AD, Hinkin CH, van Gorp WG, Castellon SA, Satz P. Depression predicts procedural but not episodic memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 1998;20(4):529–535. doi: 10.1076/jcen.20.4.529.1473. [DOI] [PubMed] [Google Scholar]
- Kanayama G, Rogowska J, Pope HG, Gruber SA, Yurgelun-Todd DA. Spatial working memory in heavy cannabis users: A functional magnetic resonance imaging study. Psychopharmacology. 2004;176(3–4):239–247. doi: 10.1007/s00213-004-1885-8. [DOI] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, et al. The Kreek–McHugh–Schluger–Kellogg scale: A new, rapid method for quantifying substance abuse and its possible applications. Drug and Alcohol Dependence. 2003;69(2):137–150. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, Ketonen L, Cox C, Grossman H, Holloway R, Booth H, et al. Cognitive performance and regional brain volume in human immunodeficiency virus type 1 infection. Archives of Neurology. 1996;53(2):155–158. doi: 10.1001/archneur.1996.00550020059016. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273(5280):1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Squire LR, Gluck MA. Probabilistic classification learning in amnesia. Learning and Memory. 1994;1(2):106–120. [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding poly-drug use: Review of heroin and cocaine co-use. Addiction. 2003;98(1):7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C. Neuroprotective properties of cannabinoids against oxidative stress: Role of the cannabinoid receptor CB1. Journal of Neurochemistry. 2002;80(3):448–456. doi: 10.1046/j.0022-3042.2001.00716.x. [DOI] [PubMed] [Google Scholar]
- Martin A, Heyes MP, Salazar AM, Law WA, Williams J. Impaired motor-skill learning, slowed reaction time, and elevated cerebrospinal fluid quinolinic acid in a subgroup of HIV-infected individuals. Neuropsychology. 1993;7(2):149–157. [Google Scholar]
- Masliah E, Ge N, Achim CL, DeTeresa R, Wiley CA. Patterns of neurodegeneration in HIV encephalitis. Journal on NeuroAIDS. 1996;1(1):161–173. doi: 10.1300/j128v01n01_08. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Pruitt PL. Medical marijuana and its use by the immunocompromised. Alternative Therapies in Health and Medicine. 1997;3(3):39–45. [PubMed] [Google Scholar]
- Mechoulam R, Spatz M, Shohami E. Endocannabinoids and neuroprotection. Science. 2002;2002(129):RE5. doi: 10.1126/stke.2002.129.re5. [DOI] [PubMed] [Google Scholar]
- Meyerhoff DJ, Bloomer C, Cardenas V, Norman D, Weiner MW, Fein G. Elevated subcortical choline metabolites in cognitively and clinically asymptomatic HIV+ patients. Neurology. 1999;52(5):995–1003. doi: 10.1212/wnl.52.5.995. [DOI] [PubMed] [Google Scholar]
- Mimiaga MJ, Reisner SL, Vanderwarker R, Gaucher MJ, O’Connor CA, Medeiros MS, et al. Polysubstance use and HIV/STD risk behavior among Massachusetts men who have sex with men accessing Department of Public Health mobile van services: Implications for intervention development. AIDS Patient Care STDS. 2008;22(9):745–751. doi: 10.1089/apc.2007.0243. [DOI] [PubMed] [Google Scholar]
- Nath A, Anderson C, Jones M, Maragos W, Booze R, Mactutus C, et al. Neurotoxicity and dysfunction of dopaminergic systems associated with AIDS dementia. Journal of Psychopharmacology. 2000;14(3):222–227. doi: 10.1177/026988110001400305. [DOI] [PubMed] [Google Scholar]
- Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, et al. Molecular basis for interactions of HIV and drugs of abuse. Journal of Acquired Immune Deficiency Syndrome. 2002;31(Suppl 2):S62–S69. doi: 10.1097/00126334-200210012-00006. [DOI] [PubMed] [Google Scholar]
- Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Annals of Neurology. 1986;19(6):525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- NORML. Active State Medical Marijuana Programs. 2010 Retrieved February 17, 2010, from http://www.norml.org/index.cfm?Group_ID=3391.
- Packard MG, Knowlton BJ. Learning and memory functions of the basal ganglia. Annual Review of Neuroscience. 2002;25:563–593. doi: 10.1146/annurev.neuro.25.112701.142937. [DOI] [PubMed] [Google Scholar]
- Pandey R, Mousawy K, Nagarkatti M, Nagarkatti P. Endocannabinoids and immune regulation. Pharmacological Research. 2009;60(2):85–92. doi: 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Cohen R, Navia B, Tashima K. Relationships between cognition and structural neuroimaging findings in adults with human immunodeficiency virus type-1. Neuroscience and Biobehavioral Review. 2002;26(3):353–359. doi: 10.1016/s0149-7634(02)00006-4. [DOI] [PubMed] [Google Scholar]
- Paul RH, Yiannoutsos CT, Miller EN, Chang L, Marra CM, Schifitto G, et al. Proton MRS and neuropsychological correlates in AIDS dementia complex: Evidence of subcortical specificity. Journal of Neuropsychiatry and Clinical Neuroscience. 2007;19(3):283–292. doi: 10.1176/jnp.2007.19.3.283. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare-Blagoev EJ, Shohamy D, Creso MJ, Myers C, et al. Interactive memory systems in the human brain. Nature. 2001;414(6863):546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Prabhakaran V, Seger CA, Gabrieli JD. Striatal activation during acquisition of a cognitive skill. Neuropsychology. 1999;13(4):564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis MA, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Archives of General Psychiatry. 2001;58(10):909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Jr, Gruber AJ, Yurgelun-Todd D. The residual neuropsychological effects of cannabis: The current status of research. Drug and Alcohol Dependence. 1995;38(1):25–34. doi: 10.1016/0376-8716(95)01097-i. [DOI] [PubMed] [Google Scholar]
- Prentiss D, Power R, Balmas G, Tzuang G, Israelski DM. Patterns of marijuana use among patients with HIV/AIDS followed in a public health care setting. Journal of Acquired Immune Deficiency Syndromes. 2004;35(1):38–45. doi: 10.1097/00126334-200401010-00005. [DOI] [PubMed] [Google Scholar]
- Quickfall J, Crockford D. Brain neuroimaging in cannabis use: A review. Journal of Neuropsychiatry and Clinical Neuroscience. 2006;18(3):318–332. doi: 10.1176/jnp.2006.18.3.318. [DOI] [PubMed] [Google Scholar]
- Reger M, Welsh R, Razani J, Martin DJ, Boone KB. A meta-analysis of the neuropsychological sequelae of HIV infection. Journal of the International Neuropsychological Society. 2002;8(3):410–424. doi: 10.1017/s1355617702813212. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, et al. Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, et al. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. Journal of Nuclear Medicine. 1996;37(7):1133–1141. [PubMed] [Google Scholar]
- Salmon DP, Butters N. Neurobiology of skill and habit learning. Current Opinion in Neurobiology. 1995;5(2):184–190. doi: 10.1016/0959-4388(95)80025-5. [DOI] [PubMed] [Google Scholar]
- Sarazin M, Deweer B, Merkl A, Von PN, Pillon B, Dubois B. Procedural learning and striatofrontal dysfunction in Parkinson’s disease. Movement Disorders. 2002;17(2):265–273. doi: 10.1002/mds.10018. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. JMP 8 User Guide. 2. Cary, NC: SAS Institute Inc; 2009. [Google Scholar]
- Schulden J, Thomas Y, Compton W. Substance abuse in the United States: Findings from recent epidemiologic studies. Current Psychiatry Reports. 2009;11(5):353–359. doi: 10.1007/s11920-009-0053-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Myers CE, Onlaor S, Gluck MA. Role of the basal ganglia in category learning: How do patients with Parkinson’s disease learn? Behavioral Neuroscience. 2004;118(4):676–686. doi: 10.1037/0735-7044.118.4.676. [DOI] [PubMed] [Google Scholar]
- Sidney S. Marijuana use in HIV-positive and AIDS patients: Results of an anonymous mail survey. Cannabis Therapeutics in HIV/AIDS. 2001:35–41. [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State–Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Squire LR, Zola SM. Structure and function of declarative and nondeclarative memory systems. Proceedings of the National Academy of Sciences. 1996;93(24):13515–13522. doi: 10.1073/pnas.93.24.13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MA, Sandoval R, Szumowski E, Roizen N, Reinecke MA, Blondis TA, et al. Psychometric characteristics of the Wender Utah Rating Scale (WURS): Reliability and factor structure for men and women. Psychopharmacology Bulletin. 1995;31(2):425–433. [PubMed] [Google Scholar]
- Stout JC, Ellis RJ, Jernigan TL, Archibald SL, Abramson I, Wolfson T, et al. Progressive cerebral volume loss in human immunodeficiency virus infection: A longitudinal volumetric magnetic resonance imaging study. HIV Neurobehavioral Research Center Group. Archives of Neurology. 1998;55(2):161–168. doi: 10.1001/archneur.55.2.161. [DOI] [PubMed] [Google Scholar]
- van Gorp WG, Mandelkern MA, Gee M, Hinkin CH, Stern CE, Paz DK, et al. Cerebral metabolic dysfunction in AIDS: Findings in a sample with and without dementia. Journal of Neuropsychiatry and Clinical Neurosciences. 1992;4(3):280–287. doi: 10.1176/jnp.4.3.280. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Benbrook A, Funderburk F, David P, Cadet JL, Bolla KI. The differential relationship between cocaine use and marijuana use on decision-making performance over repeat testing with the Iowa Gambling Task. Drug and Alcohol Dependence. 2007;90(1):2–11. doi: 10.1016/j.drugalcdep.2007.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Nicotine and cannabinoids: Parallels, contrasts and interactions. Neuroscience and Biobehavial Reviews. 2006;30(8):1161–1181. doi: 10.1016/j.neubiorev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Gillespie H, Mullani N, Tancredi L, Grant C, Valentine A, et al. Brain glucose metabolism in chronic marijuana users at baseline and during marijuana intoxication. Psychiatry Research: Neuroimaging. 1996;67(1):29–38. doi: 10.1016/0925-4927(96)02817-x. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: Electrophysiologic and positron emission tomography findings. Archives of Neurology. 2000;57(11):1601–1607. doi: 10.1001/archneur.57.11.1601. [DOI] [PubMed] [Google Scholar]
- Ward MF, Wender PH, Reimherr FW. The Wender Utah Rating Scale: An aid in the retrospective diagnosis of childhood attention deficit hyperactivity disorder. American Journal of Psychiatry. 1993;150(6):885–890. doi: 10.1176/ajp.150.6.885. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Soontornniyomkij V, Radhakrishnan L, Masliah E, Mellors J, Hermann SA, et al. Distribution of brain HIV load in AIDS. Brain Pathology. 1998;8(2):277–284. doi: 10.1111/j.1750-3639.1998.tb00153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]