Abstract
Background
The prognosis of even early stage esophageal cancer is poor. Because there is not a consensus on how to manage T2N0 disease, we examined survival after resection of T2N0 esophageal cancer with or without radiation therapy.
Methods
Patients who underwent resection for T2N0 squamous cell carcinoma or adenocarcinoma of the mid or distal esophagus with or without radiation were identified using the Surveillance, Epidemiology and End Results cancer registry from 1998–2008. Five-year cancer specific survival (CSS) and overall survival (OS) after surgery alone and combined surgery with radiotherapy were compared using the Kaplan-Meier approach, risk-adjusted Cox proportional-hazard models, and competing risk models.
Results
The 5-year OS of 490 T2N0 patients was 40.3% (95% CI: 35.2–45.4%). Surgery alone was used in 267 (54%) patients and combined therapy in 223 (46%) patients. In patients undergoing surgery only, 5-year OS was 38.6% (CI: 31.7–45.5%) while it was 42.3% (CI: 34.7–49.6%) for combined therapy, p=0.48. No difference in OS was found even after risk-adjustment (HR: 1.14, CI: 0.87–1.48, p=0.35). However, in landmark studies with left truncation for 3 and 6 months, surgery only showed better OS than combined therapy (HR: 1.33, CI: 1.01–1.75, p=0.04; HR: 1.36, CI: 1.01–1.83, p=0.04, respectively). No such difference for CSS was detected even for the landmark study after 6 months (HR: 1.16, CI: 0.98–1.39, p=0.09).
Conclusion
Combining radiation with esophagectomy did not result in improved outcomes compared to esophagectomy alone for patients with T2N0 esophageal cancer in the Surveillance, Epidemiology and End Results database.
Keywords: Esophageal cancer, esophageal surgery, radiation therapy, tumor
Introduction
Although the incidence is increasing, esophageal cancer is relatively uncommon in the United States with 17,460 expected new cases in 2012 [1]. Surgical resection is generally considered the optimal therapy in early stage disease. However, the overall prognosis for patients with any stage of esophageal cancer remains disappointing at 19% despite improvements over the past few decades [1,2]. Desire to improve these outcomes has led to many trials investigating the use of surgery in combination with chemotherapy or radiation [3–6]. Inconsistent results from these trials have led guidelines for esophageal cancer treatment, such as those from the National Comprehensive Cancer Network, to allow a wide spectrum of possible treatments for tumors that are either not very early stage (stage I) or metastatic upon presentation (stage IV) [7].
One major difficulty in developing evidence to support therapeutic decision making is that most trials must include a heterogeneous group of stages in order to achieve adequate study accrual [6]. As a result, treatment decisions for the relatively more uncommon stages are often based on data in which those stages may not have been significantly represented. In particular, the optimal treatment strategy of T2N0 esophageal cancer is still subject of debate [8]. Review of single center data has led to conflicting recommendations for preoperative chemoradiation followed by surgery, as well as for surgery alone if after resection the tumor was not found to be initially understaged [8–10]. However, the maximum number of patients in these studies was only 53 [10]. Considering this lack of consensus, we aimed to analyze outcomes of a larger patient cohort with T2N0 esophageal cancer using the Surveillance Epidemiology and End Results (SEER) cancer registry and advanced statistical analyses including landmark studies and competing-risks regression in order to provide important additional evidence for guiding future therapy.
Material and Methods
Approval was obtained from the Duke University Institutional Review Board prior to conducting this retrospective cohort analysis using SEER data for patients from 1998 to 2008. SEER*Stat 7.0.5 was used to extract patients 18 years or older with cancer of the mid or lower esophagus. Patients were primarily identified through the “SEER Site Recode” using the term “esophagus”. The variable “Histologic Type ICD-O-3” (International classifications of Diseases for Oncology, 3rd edition) was used to restrict the study cohort to patients with either squamous cell cancer (codes 8050–8089) or adenocarcinomas (codes 8140–8389). To restrict the cohort to patients with T2 N0 M0 tumors, the tumor-node-metastasis (TNM) stage was either directly extracted from the SEER database or manually recoded using available SEER variables. The 6th edition of the AJCC Cancer Staging Manual served as basis for this recoding [11]. Patients with unknown or other TNM stages were excluded from the analysis.
The primary outcome was 5-year cancer specific (CSS) and overall survival (OS), measured in months. Patients alive at the last available follow-up date in SEER were right censored at this date in the survival analysis. The following additional patient characteristics were extracted from the dataset: age, gender, race (White, Black, other/unknown), marital status (married, other/unknown), and cause of death (alive, esophagus, other cause of death). In addition, data on tumor grade (well/moderate, poor/undifferentiated, unknown), tumor location (mid or distal esophagus), and histology (adenocarcinoma, squamous cell) were collected. Based on treatment information on surgery and radiotherapy available in SEER, we defined two distinct treatment groups: esophagectomy only and radiation with esophagectomy. All other patients were excluded from the analysis.
Statistical analysis
Comparisons of patient characteristics among the two treatment groups were performed using chi-square test for categorical (count, percentages) and t-test for continuous variables (mean, standard deviations). Cancer specific survival (CSS) was defined by a cause of death from esophageal etiology while patients dying from another cause and patients alive were right censored. Meanwhile, overall survival (OS) included all deaths from any cause in the follow-up period while only patients alive were right censored.
To compare CSS and OS among the two treatment groups, survival curves were initially constructed according to the Kaplan-Meier approach and compared using the log-rank test. Subsequently, unadjusted and multivariable adjusted Cox proportional hazard models for OS and competing-risks regression models for CSS were calculated. Results are presented as Hazard ratios (HR) and 95% confidence intervals (CI). Adjustment in the survival analyses was performed for the following covariates: gender, age (grouped as ≤65 and >65 years), race, marital status, tumor histology, tumor grade, tumor location, and year of diagnosis (five groups). To account for immortal time bias in regard of receipt of esophagectomy and radiation therapy after diagnosis, we performed two sets of landmark studies in the survival analyses by left truncating patients who survived less than 3 or 6 months [12]. Immortal time bias does reflect the fact that patients dying early after receiving one treatment are not eligible for any additional treatment finally favoring the combined treatment regimen. Performing landmark studies by excluding patients with short-term adverse perioperative outcomes and also those who did not survive long enough to complete both, radiation therapy and esophagectomy, does exclude the patients who are potentially responsible for this bias. The selection of the landmarks – the preselected time-points for left truncation – do allow comparing survival of patients between the two treatment groups conditional on survival for at least 3 and 6 months in our case.
To assess the trend in use of surgery with and without radiation therapy from 1998 to 2008, multivariable adjusted logistic regression analyses were performed having year (in three groups) as the outcome variable, type of treatment as main predictor, while the aforementioned variables served as covariates. All statistical analyses were performed using STATA/SE version 11.2 (Stata Corporation, College Station, TX, USA), the significance level alpha was set at 0.05 and two-sided p-values were calculated for all analyses.
Results
A total of 490 patients with T2N0 esophageal cancer of the mid and lower esophagus were identified in the SEER cancer registry during the study period from 1998 to 2008: 267 (54%) were treated with surgery only and 223 (46%) had both esophagectomy and radiation therapy. Detailed patient and tumor characteristics stratified by treatment group are presented in table 1. Patients undergoing surgery and radiation therapy were younger, more often male, and had more often cancers in the lower esophagus compared to patients treated with surgery alone. Of the patients who had radiation therapy and esophagectomy, most (189 patients, 85%) had radiation in the preoperative setting. The distribution of treatment regimens did not change significantly from 1998 to 2008 (Table 2). Use of surgery alone varied between 53.1% and 55.5% over the time periods examined, while radiation and esophagectomy conversely varied between 44.5% and 46.9%.
Table 1.
Patient characteristics
| Esophagectomy only | RT + Esophagectomy | p-value | |
|---|---|---|---|
|
| |||
| Number of patients | 267 | 223 | |
|
| |||
| Age (mean, SD), years | 67.8 (10.7) | 61.4 (10.2) | <0.001 |
| ≤65 | 90 (33.7) | 140 (62.8) | <0.001 |
| >65 | 177 (66.3) | 83 (37.2) | |
|
| |||
| Female | 69 (25.8) | 41 (18.4) | 0.05 |
|
| |||
| Race | |||
| White | 244 (91.4) | 200 (89.7) | 0.55 |
| Black | 11 (4.1) | 14 (6.3) | |
| Other/Unknown | 12 (4.5) | 9 (4.0) | |
|
| |||
| Marital Status | |||
|
| |||
| Married | 180 (67.4) | 159 (71.3) | 0.35 |
| Other/Unknown | 87 (32.6) | 64 (28.7) | |
|
| |||
| Tumor location | |||
|
| |||
| Mid esophagus | 70 (26.2) | 41 (18.4) | 0.04 |
| Lower esophagus | 197 (73.8) | 182 (81.6) | |
|
| |||
| Tumor grade | |||
|
| |||
| G1/2 (well/moderate) | 155 (58.1) | 117 (52.5) | 0.008 |
| G3/4 (poor/undifferentiated) | 105 (39.3) | 86 (38.6) | |
| Unknown | 7 (2.6) | 20 (9.0) | |
|
| |||
| Histology | |||
|
| |||
| Squamous cell carcinoma | 84 (31.5) | 67 (30.0) | 0.74 |
| Adenocarcinoma | 183 (68.5) | 156 (70.0) | |
|
| |||
| Cause of death | |||
|
| |||
| Alive | 121 (45.3) | 107 (48.0) | 0.80 |
| Esophagus | 101 (37.8) | 78 (35.0) | |
| Other cause of death | 45 (16.9) | 38 (17.0) | |
|
| |||
| Preoperative RT | 189 (84.8) | ||
| Postoperative RT | 34 (15.2) | ||
Values are counts and % if not otherwise indicated. RT=radiotherapy
Table 2.
Multivariable adjusted trend of treatment use over time
| Surgery only | HR (95% CI) | Surgery plus radiation therapy | HR (95% CI) | |
|---|---|---|---|---|
|
| ||||
| 1998–2001 | 86 (55.5) | Ref. | 69 (44.5) | Ref. |
| 2002–2005 | 104 (54.7) | 0.84 (0.53–1.34) | 86 (45.3) | 1.19 (0.75–1.88) |
| 2006–2008 | 77 (53.1) | 0.88 (0.54–1.43)* | 68 (46.9) | 1.14 (0.70–1.86)* |
P-value for trend:
p≥0.05. Adjusted for: gender, age, race, marital status, tumor grade, tumor location, and cancer histology. CI=confidence interval; HR=hazard ratio
Overall 5-year survival was 40.3% (95% CI: 35.2–45.4) and CSS was 53.5% (CI: 47.8–58.9). Both CSS and OS after surgery alone and radiation with esophagectomy were not significantly different (Table 2, Figures 1 and 2). To address potential immortal bias in the survival analyses, landmark studies for CSS and OS were performed by applying 3 and 6 month left truncation to survival times. In these landmark studies, surgery only showed better OS than radiation with esophagectomy (HR: 1.33, CI: 1.01–1.75, p=0.04; HR: 1.36, CI: 1.01–1.83, p=0.04, respectively) (Table 3). No such difference for CSS was detectable even for the landmark study after 6 months (HR: 1.16, CI: 0.98–1.39, p=0.09).
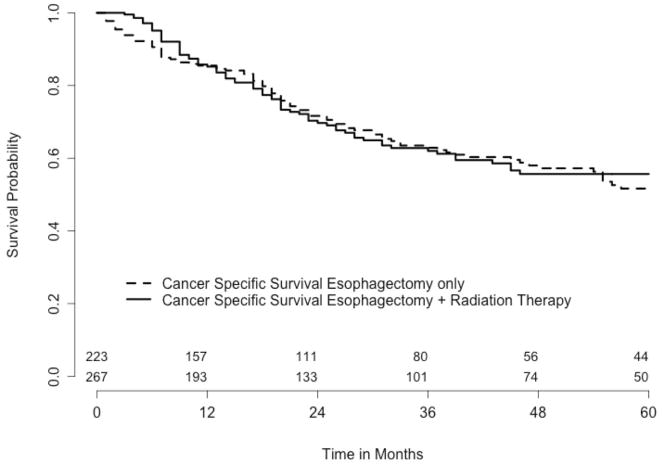
Figure 1.
Cancer specific survival comparing esophagectomy only versus esophagectomy + radiation therapy. The number of patients at risk at time 0 is 267 for esophagectomy only and 223 for esophagectomy and radiation therapy. The p-value of the logrank test is 0.76.
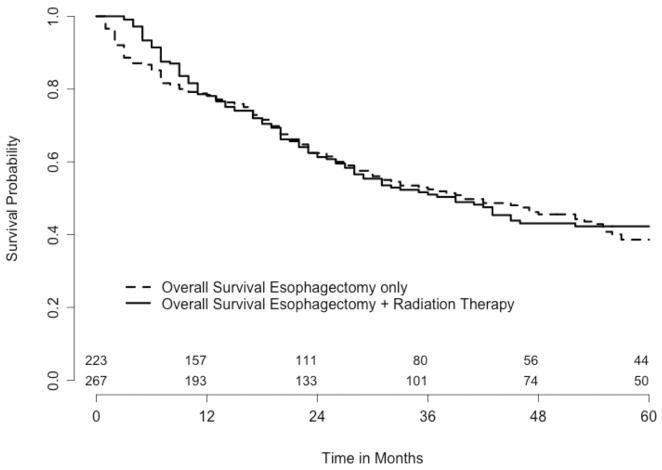
Figure 2.
Overall survival comparing esophagectomy only versus esophagectomy + radiation therapy. The number of patients at risk at time 0 is 267 for esophagectomy only and 223 for esophagectomy and radiation therapy. The p-value of the logrank test is 0.66.
Table 3.
Cancer specific and overall survival
| CCS | OS | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 5-year survival (95% CI) | Multivariable adjusted HR (95% CI) | p-value | 5-year survival (95% CI) | Multivariable adjusted HR (95% CI) | p-value | |
|
| ||||||
| Overall | 53.5 (47.8–58.9) | 40.3 (35.2–45.4) | ||||
| Treatment | ||||||
| Esophagectomy only | 51.7 (43.7–59.1) | Ref. | 0.40 | 38.6 (31.7–45.5) | Ref. | 0.35 |
| Surgery plus radiation therapy | 55.7 (47.3–63.2) | 1.07 (0.91–1.26) | 42.3 (34.7–49.6) | 1.14 (0.87–1.48) | ||
Adjusted for: gender, age, race, marital status, tumor grade, tumor location, cancer histology, and year (5 groups). CCS=cancer specific survival; OS=overall survival
Comment
In this study using the SEER database, which is the largest United States population based cancer registry, we demonstrate that approximately half of the patients treated with surgery for T2N0M0 esophageal cancer from 1998–2008 also were treated with radiation therapy. Radiation was given in the preoperative setting in the majority of cases. The percentages of patients treated with both, surgery and radiation, did not change significantly over time. However, patients treated with both radiation and surgery did not have improved survival compared to patients treated with surgery alone. The lack of a benefit to adding radiation to surgery held true even after taking potential immortal bias of six months into account to improve the chance of only considering patients having finished first-line treatment regimen including surgery and radiotherapy.
The treatment of patients with T2N0 esophageal cancer has previously been the subject of debate [8,9]. Whether or not there is a favorable risk/benefit ratio with multimodality therapy in these patients is contradictory and existing reports are based on small and highly selected single-center experiences [8–10]. Given it’s uncommon incidence, the population of patients with T2N0 esophageal cancer is typically underrepresented in studies of multimodality therapy in which T2N0 is included [6]. Using a population-based database that allows evaluation of a relatively large number of patients with an uncommon disease seems particularly ideal for this stage of esophageal cancer. Our results suggest that adding radiation to surgery does not improve outcomes for patients with T2N0 esophageal cancer.
However, these results can not necessarily be used to argue against the use of radiation therapy and esophagectomy in this setting. Treatment decisions for esophageal cancer must be made based on clinical staging. Tumor stage in SEER is defined as clinical tumor stage if neoadjuvant therapy including radiotherapy, chemotherapy, hormone therapy, or immunotherapy was performed while in patients without neo-adjuvant therapy, the pathological tumor stage is recorded. As many as 55% of patients with clinically staged T2N0 esophageal cancers have been reported as having nodal disease after resection [9,10]. Therefore, it is possible that the patients treated with radiation followed by surgery in our study, which was the case in 85% of patients treated with surgery and radiation, were understaged. This understaging could clearly negatively bias the results seen in the therapy group with surgery and radiation therapy, and potentially even explain the lack of benefit seen. Unfortunately, any study of patients with this stage of esophageal cancer face the same challenge and limits of having to assign treatment based on clinical and not pathologic stage.
Based on our finding that radiotherapy added to esophagectomy does not improve CSS and OS in patients with T2N0 disease, one could argue that in patients with pathologically confirmed node negative disease, radiotherapy could be omitted when preoperative clinical staging revealed accurate lymph node status. It has been shown that pathologic N0 status is associated with improved survival. Rizk et al. analyzed world esophageal cancer collaboration data and demonstrated the importance of a complete lymphadenectomy at the time of resection. N0 status is associated with decreased likelihood of systemic disease [13]. The prognosis for patients with N0 esophageal cancer is modulated by T status and histologic grade [13,14]. Therefore in those patients with preoperative N0 status, the benefit of induction therapy is likely to be minimal given their better prognosis, and indeed may be harmful. However, in case of upstaging in the pathological report to N1 disease, multimodality therapy could still be implemented using adjuvant radiotherapy and chemotherapy. This strategy is supported by others based on gastroesophageal adenocarcinomas, while others advocate for neoadjuvant treatment in cT2N0 esophageal cancer patients [8,15]. In addition, a metaanalysis comparing neoadjuvant chemoradiotherapy and chemotherapy to surgery alone in patients with resectable esophageal cancer showed, that multimodality treatment was superior to surgery alone [16]. However, this metaanalysis did include patients with node negative and positive tumors. In contrast, Rice and colleagues found that induction therapy resulted in worse outcomes in cT2N0 patients compared to adjuvant therapy [9]. This study is very small, though, as their results are based on only 8 patients undergoing neoadjuvant treatment while 5 of them experienced early cancer recurrence leading to death.
The use of the SEER-cancer registry is most beneficial to investigate uncommon tumors while its population based nature provides enough power to even perform subgroup analysis. However, SEER does also have some inherent limitations. First, data regarding chemotherapy administration are lacking. Many if not most of the patients in our study population who received radiation were likely to also have had chemotherapy, given that relatively early randomized trials failed to show a survival advantage for preoperative or postoperative radiation alone and definitive chemoradiation has been shown to be superior to radiation alone [17–20]. The combination of chemotherapy with surgery alone may be less likely, which could have negatively biased the results seen when surgery was used without radiation. However, the exact influence of chemotherapy in this patient cohort cannot be evaluated. Second, there is a lack of data regarding patient comorbidities which could be important in predicting both survival and treatment, particularly multimodality therapy. Even though multivariable adjusted analysis can correct for measured covariates, unmeasured confounding cannot be ruled out. Third, information regarding radiation doses and fields are not contained in the database. Although we only included patients who received beam radiation in this study, our observed survival results could be biased if inadequate radiation treatment was performed.
In conclusion, treatment of T2N0 esophageal cancer is highly variable, as approximately half of patients in the SEER database who were treated with surgery also received radiation therapy. In this study, we did not find that combining radiation therapy with surgery resulted in improved outcomes compared to surgery alone. The lack of benefit seen in this population-based analysis does not support the clinical practice of treating this stage of esophageal cancer with combined therapy, especially if the cost and potential morbidity of radiation therapy are considered. The use of radiation therapy likely should be confined to carefully controlled studies designed to better assess treatment of this disease entity, though it is extremely unlikely that any clinical trial will be able to assemble the number of patients that were considered in this study. Given the relatively uncommon nature of T2N0 esophageal cancer, clinicians should strongly consider including patients with this stage of disease in multi-institutional registries to allow further evaluation of different treatment strategies and outcomes.
Table 4.
Landmark analysis with left truncation for 3 and for 6 months - cancer specific and overall survival
| Left truncation for 3 months | Left truncation for 6 months | |||
|---|---|---|---|---|
|
| ||||
| Multivariable adjusted HR (95% CI) | p-value | Multivariable adjusted HR (95% CI) | p-value | |
|
| ||||
| Cancer specific survival | ||||
|
| ||||
| Surgery only | Ref. | Ref. | ||
| Surgery plus radiation therapy | 1.11 (0.94–1.31) | 0.22 | 1.16 (0.98–1.39) | 0.09 |
|
| ||||
| Overall survival | ||||
|
| ||||
| Surgery only | Ref. | Ref. | ||
| Surgery plus radiation therapy | 1.33 (1.01–1.75) | 0.04 | 1.36 (1.01–1.83) | 0.04 |
Adjusted for: gender, age, race, marital status, tumor grade, tumor location, cancer histology, and year (5 groups). CI=confidence interval
Acknowledgments
This work was in part supported by the NIH funded Cardiothoracic Surgery Trials Network (M.F.B).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Dubecz A, Gall I, Solymosi N, et al. Temporal trends in long-term survival and cure rates in esophageal cancer: a SEER database analysis. J Thorac Oncol. 2012;7:443–7. doi: 10.1097/JTO.0b013e3182397751. [DOI] [PubMed] [Google Scholar]
- 3.Tepper J, Krasna MJ, Niedzwiecki, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92. doi: 10.1200/JCO.2007.12.9593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followed by surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med. 1997;337:161–7. doi: 10.1056/NEJM199707173370304. [DOI] [PubMed] [Google Scholar]
- 5.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–13. doi: 10.1200/JCO.2001.19.2.305. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 7.Ajani JA, Barthel JS, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers. J Natl Compr Canc Netw. 2011;9:830–87. doi: 10.6004/jnccn.2011.0072. [DOI] [PubMed] [Google Scholar]
- 8.Kountourakis P, Correa AM, Hofstetter WL, et al. Combined modality therapy of cT2N0M0 esophageal cancer: the University of Texas M. D. Anderson Cancer Center experience. Cancer. 2011;117:925–30. doi: 10.1002/cncr.25651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rice TW, Mason DP, Murthy SC, et al. T2N0M0 esophageal cancer. J Thorac Cardiovasc Surg. 2007;133:317–24. doi: 10.1016/j.jtcvs.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Stiles BM, Mirza F, Coppolino A, et al. Clinical T2-T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg. 2011;92:491–6. doi: 10.1016/j.athoracsur.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging manual. 6. Springer-Verlag; 2003. [Google Scholar]
- 12.Park HS, Gross CP, Makarov DV, Yu JB. Immortal Time Bias: A Frequently Unrecognized Threat to Validity in the Evaluation of Postoperative Radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:1365–73. doi: 10.1016/j.ijrobp.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46–50. doi: 10.1097/SLA.0b013e3181b2f6ee. [DOI] [PubMed] [Google Scholar]
- 14.Rice TW, Rusch VW, Ishwaran H, Blackstone EH. Cancer of the esophagus and esophagogastric junction: data-driven staging for the seventh edition of the American Joint Committee on Cancer/International Union Against Cancer Cancer Staging Manuals. Cancer. 2010;116:3763–73. doi: 10.1002/cncr.25146. [DOI] [PubMed] [Google Scholar]
- 15.Kofoed SC, Muhic A, Baeksgaard L, et al. Survival after adjuvant chemoradiotherapy or surgery alone in resectable adenocarcinoma at the gastro-esophageal junction. Scand J Surg. 2012;101:26–31. doi: 10.1177/145749691210100106. [DOI] [PubMed] [Google Scholar]
- 16.Gebski V, Burmeister B, Smithers BM, Foo K, Zalcberg J, Simes J. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol. 2007;8:226–34. doi: 10.1016/S1470-2045(07)70039-6. [DOI] [PubMed] [Google Scholar]
- 17.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group. Jama. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 18.Teniere P, Hay JM, Fingerhut A, Fagniez PL. Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle and lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet. 1991;173:123–30. [PubMed] [Google Scholar]
- 19.Arnott SJ, Duncan W, Kerr GR, et al. Low dose preoperative radiotherapy for carcinoma of the oesophagus: results of a randomized clinical trial. Radiother Oncol. 1992;24:108–13. doi: 10.1016/0167-8140(92)90287-5. [DOI] [PubMed] [Google Scholar]
- 20.Wang M, Gu XZ, Yin WB, Huang GJ, Wang LJ, Zhang DW. Randomized clinical trial on the combination of preoperative irradiation and surgery in the treatment of esophageal carcinoma: report on 206 patients. Int J Radiat Oncol Biol Phys. 1989;16:325–7. [PubMed] [Google Scholar]