INTRODUCTION
There is a strong association between infection-related cell-mediated immunity and autoimmune diseases such as diabetes, multiple sclerosis (MS), rheumatoid arthritis (RA) and lupus erythematosis (SLE)1. Infections have also been associated with unusual immunopathologies of unknown origin, such as Wegner granulomatosis, sarcoidosis, colitis, panniculitis, bronchiolitis obliterans and even chronic fatigue syndrome. Despite exhaustive efforts, a definitive link between one particular pathogen and any of one these pathologies has never been found. More often several pathogens have become associated with each of these conditions. For instance multiple sclerosis has been associated with Epstein Barr virus (EBV), measles virus, HHV-6, varicella-zoster virus, and Picornaviruses2-6. Panniculitis in the form of erythema nodosum and bronchiolitis obliterans have both been associated with unusual cell-mediated immune responses that occur following non-specified viral or intracellular bacterial infections 7-9. Erythema nodosum, which has also been associated with Crohn’s disease8, is a very painful condition, where nodules of inflamed subcutaneous fat often on the shins and forearms persist for months. There is no known therapy. Bronchiolitis obliterans is a lethal condition in humans where the bronchioles become occluded with immune cells and fibrinous material, with no known cause or treatment9.
Chronic fatigue syndrome (CFS) is another unusual multisystem disease which is thought to be associated with immune dysregulation. Over the past two decades millions of patients world wide have suffered from a clinical syndrome of disabling fatigue, myalgias, palpitations and cognitive dysfunction that lasts longer than 6 months. In 50% of cases it develops after a mild viral illness. Cases may appear sporadically or in clusters10,11. Many attempts have been made to define the syndrome on the basis of an etiologic agent. These agents have included Epstein-Barr virus10, Brucella12, Candida albicans13, Borrelia burgdorferi, and human herpesvirus-614,15. More recently it has been associated with enteroviruses and xenoretroviruses 16-18. The general conclusion has been that it is unlikely that the syndrome is caused by a single etiologic agent. The mechanisms mediating CFS are poorly understood, and there are few well designed studies examining its cause. The symptoms of CFS are similar to those experienced during viral infections such as infectious mononucleosis or influenza or in the setting of therapy with cytokines such as interferon or interleukin-2. It has been speculated that some or all the symptoms are reflective of an altered immune response to some pathogen with over production of one or more cytokines. An alternative hypothesis suggests that a number of infectious agents are involved and result in a regulatory imbalance of cytokines and the patient with CFS is unable to reestablish the appropriate balance of cytokines. These theories have been supported by reports of immune deficiency seen associated with CFS19.
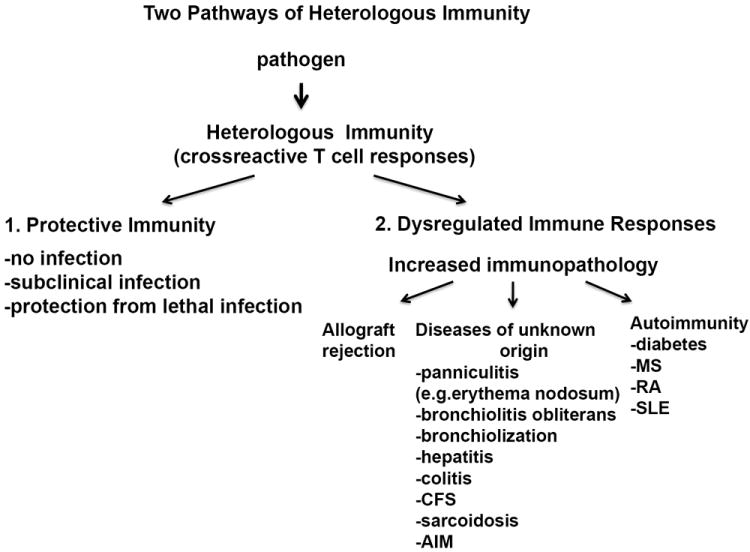
Much effort has been placed on trying to identify both T and B cell auto-antigen responses in autoimmune responses, but often with limited success. It seem that in the last 50 years we have made very slow progress in understanding the pathogenesis of autoimmune diseases and these syndromes of unknown origin, which for the purposes of this review I will call collectively autoimmune syndromes. In this review we would like to suggest a different perspective in trying to understand the mechanisms behind these diseases. Here, we will examine evidence to date arguing that it is not a specific pathogen and the immune response to it which leads to dysregulation of the immune system or to a specific autoantigen response in an autoimmune disease. Instead we would like to suggest that to better understand the pathogenesis of classic autoimmune diseases and these other unusual immunopathologies of unknown origin, which are often considered autoimmune-like syndromes it requires an evaluation of how T cells are regulated and evolve during multiple sequential infections under the influence of heterologous immunity. T cell responses to viral infections are extraordinarily dynamic and highly variable between individuals. The T cell response is impacted by previous infections with putatively unrelated viruses, and subsequent viral infections modulate the memory T cell pool created in response to previously encountered pathogens.20-23 The concept of “heterologous anti-viral T cell-mediated immunity,” takes into account the influence that T cell responses to one virus can have on that of another24,25. This review will by necessity focus predominantly on CD8 T cell responses, as more is generally known about epitope-specific CD8 than CD4 T cell responses to pathogens. The data presented here indicate that heterologous immunity can alter the outcome of a new infection and determine whether an infection is subclinical or progresses to severe immunopathology and death, even in genetically identical mice. Although superficially it may seem complex, it behooves us to have a better understanding of the general principles of heterologous immunity if we are ever going to understand the mechanisms behind these aberrant immune responses and how best to intervene. In addition to improving our understanding of aberrant immunopathological diseases there are important public health-relevant questions receiving considerable attention by viral immunologists: (1) can one design successful T cell vaccines that will give long term protective immunity without immunopathology, particularly in the elderly, and (2) under what circumstances do virus infections induce severe immunopathology, are there ways to circumvent it, and does this type of immunopathology contribute to the development of autoimmunity? (Figure 1).
Figure 1. Two alternative pathways for disease outcome during heterologous infections.
Because of the property of viruses to infect cells, peptide cleavage products from many of their encoded proteins get incorporated into nascent class I major histocompatibility complex (MHC) molecules and get presented at the cell surface to CD8 T cells bearing T cell receptors (TCR) specific for the peptide-MHC complex.26 As a result, viral infections frequently stimulate very potent class I-restricted CD8 T cell responses capable of perforin- or FasL-dependent cytotoxicity, and interferon (IFN)γ and TNF production. Indeed, CD8 T cells are essential regulators of viral infection, playing important roles in the clearance of virus-infected cells and in sometimes causing damaging immunopathology.27,28 the relative balances between protective immunity and immunopathology often determine the fate of the virus-infected host (Figure 2).
Figure 2. Disease outcome during a viral infection is dependent on a balance between antigen load and efficiency of effector function.
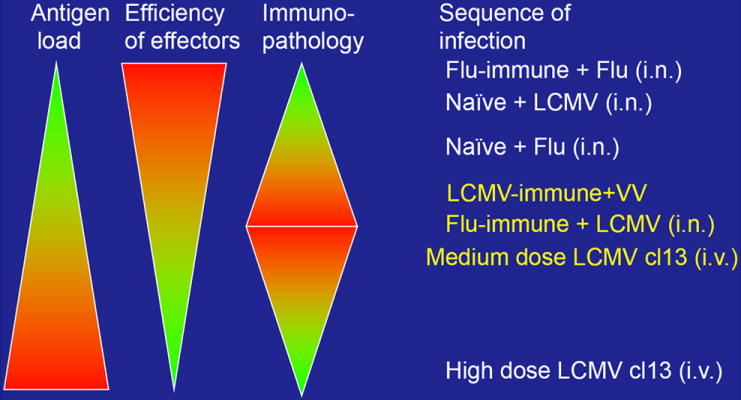
The increase in pathology observed during heterologous immunity may be due to dysregulation of the tight balance between the antigen load during the infection and the efficiency of the effector T cells to clear the infection. Efficient memory T and B cell responses clear virus rapidly without inducing immunopathology, as in a secondary infection of Flu-immune or LCMV-immune mice. During a primary Flu, LCMV or PV response virus is cleared more slowly and can lead to some immunopathology. If the efficiency of the T cell response is diminished, by low affinity cross-reactive memory T cells, the virus is cleared slower giving time for recruitment of many memory effector T cells, which can induce immunopathology and collateral damage by producing IFNγ, TNFα, and FasL, as seen in LCMV-immune mice challenge with VV. If the viral load becomes extremely high very rapidly it can result in clonal exhaustion of the T cell response and little immunopathology, as is seen in high dose LCMV clone13 infection.
Dendritic cells (DC) present viral antigen and co-stimulatory signals to T cells 26 causing the release of T cell growth factors (i.e. IL-2) which initiate a programmed expansion that does not require additional exposure to antigen29-31. However, the presence of antigen or factors produced from CD4 (T helper) cells may modulate this process32,33. After the contraction of this response, some virus-specific T cells are saved into memory, where they undergo steady state homeostatic proliferation34-37. During the early phase of most virus infections there is a dramatic reduction in lymphocyte number 38-42, which is associated with a high level of type I interferon (IFN)-dependent apoptosis,38,42-44 particularly in memory phenotype (CD44+) T cells. Following completion of a viral infection, frequencies of all previous memory cell populations not cross-reactive with the new pathogen decline22,23. In general, our studies have shown that an infection with an unrelated virus will induce the formation of new memory cells specific to the second virus and will delete memory cells specific to the previously encountered virus 21. This is a permanent change that remains for the lifetime of the mouse, though it has never been demonstrated or sufficiently studied in the human. Using the IMMSIM discrete model of a virtual immune system we proposed two models to explain this loss in memory T cell frequency: the passive attrition model, whereby old memory cells are lost simply by their competition with newly formed memory cells for survival niches in the immune system after immune response silencing, and the active attrition model, whereby there is a directed apoptosis of the pre-existing memory cells 45,46. Most of our in vivo data support the active attrition model 45,47
The great majority of the T cells responding will be directed against a discrete number of “immunodominant” peptides, which is dependent on a number of factors, including the frequency of available T cells with specificity for the peptide-MHC combination26,34,48. This T cell frequency is dependent on thymic selection, but is also a function of whether the host has experienced a T cell expansion to an identical or to a non-homologous cross-reactive epitope. This pattern of immunodominance in a virus infection can thus be greatly altered by previous exposures to other viruses that may encode cross-reactive epitopes20,21,49. The specificity of T cells can be very degenerate, and it has been calculated that a single TCR has the potential to react with as many as 106 peptide-MHC combinations50,51. It is now well-documented that many virus-specific T cells cross-react with epitopes encoded by other viruses and with uninfected targets displaying allogeneic MHC antigens52-56. A recent paper has elegantly analyzed the cross-reactive epitopes that we and others have defined and has concluded that seemingly distinct epitopes with low amino acid sequence identity can have biochemical similarity to the point where the authors could predict when two peptides may be cross-reactive, and the authors concluded that T cell cross-reactivity is more common than previously thought.57
The IMMSIM discrete model of a virtual immune system predicted that the early active attrition of memory cells may reduce the impact of a cross-reactive T cell response to another pathogen by leveling the playing field, thus allowing for a more diverse immune response42. Our computer modeling predicted that without the apoptosis of memory T cells at the early stages of infection, cross-reactive T cells might dominate the response to an infection 42,45. By selectively reducing the frequency of memory cells at the beginning of an immune response, there actually may develop a more diverse T cell response to a new pathogen, and that diversity may be beneficial to the host for control of the pathogen. We were able to test this prediction in vivo in elderly mice, as they undergo less IFN-driven T cell attrition than do young mice58. These elderly mice developed higher numbers of cross-reactive T cell responses in both influenza A (IAV)-immune mice challenged with lymphocytic chorimeningitis virus (LCMV) and the LCMV-immune mice challenged with Pichinde virus (PV)58.
The hierarchy of T cell responses to immunodominant epitopes in immunologically naive genetically identical mice is very consistent,59-62 but the amino acid sequences of the TCRs responding to these epitopes differ from mouse to mouse; these are sometimes called “private” specificities63-66. In a T cell repertoire, that which is common between individuals, be it TCR Vβ usage or a common amino acid sequence or “motif,” is a “public” specificity; that which is different between individuals, such as a CDR3 sequence, is a “private” specificity. Thus, genetically identical hosts such as identical twins have, as a consequence of random DNA recombination events, genetically different immune systems, and this diversity of TCR usage poses a challenge when one considers whether an epitope-specific T cell response may be cross-reactive with another epitope. This is because the expanded clones of virus-specific T cells in different individuals may have different private specificities, such that one individual may have a repertoire cross-reactive between two epitopes, whereas the other individual may not. This may help explain, for instance, why the concordance rate in twins for many autoimmune diseases such as diabetes and multiple sclerosis does not usually exceed 50%. We have shown that private specificity is an integral part of heterologous immunity by demonstrating that genetically identical mice use different cross-reactive T cell responses, resulting in tremendous variability in disease67-69. In order to demonstrate private specificity, memory cells from one LCMV-immune mouse were adoptively transferred into three naïve congenic recipients, and it was found that those three mice used similar cross-reactive T cell repertoires upon vaccinia (VV) or PV challenge. However, a different memory donor would generate a completely different cross-reactive response.
The question still remaining is exactly how do heterologous immune responses and, in particular, cross-reactive T cell responses alter the pathogenesis of viral diseases under conditions of sequential infections and persistent infections. There has been a strong association or correlation of cross-reactive T cells playing a role in heterologous immunity. Table 1 summarizes some of the mouse studies in this regard, and Table 2 summarizes human studies which demonstrate that heterologous immunity may be of considerable significance in IAV, EBV, dengue, and HCV viral infections in humans4,49,70-73. This review will examine these studies in more detail and also will touch on what role modeling of the immune system with discrete models such as IMMSIM may play in helping us understand these complex issues.
Table 1.
Sequence of Heterologous Virus Infections Influences Viral Clearance, Histopathology and cross-reactivity between viruses in mouse models.
| Mouse
| ||||
|---|---|---|---|---|
| Model | Virus titer | Pathology | Cross-reactivity | Sequence similarity |
| LCMV-immune + VV versus acute VV i.n. (ref. 75,76) |
|
|
VV a11r198 A I V N Y A N L | 3/8 aa |
| LCMV GP118 I S H N F C N L | 40% | |||
| VV a11r198 A I V N Y A N L | 3/8 aa | |||
| LCMV GP34 A V Y N F A T C | 40% | |||
| LCMV-immune + VV versus acute VV i.p. (ref. 24,69,75) |
|
|
VV a11r198 A I V N Y A N L | 4/8 aa |
| LCMV NP205 Y T V K Y P N L | 50% | |||
| MCMV-immune + VV versus acute VV i.n. (ref. 74) |
|
|
Not done | |
| Flu-immune + VV versus acute VV i.n. (ref. 74) |
|
|
Not done | |
| LCMV-immune + PV versus acute PV i.p. (ref. 21, 24, Chen A. et al.) |
|
|
LCMV NP205 Y T V K Y P N L | 6/8 aa |
| PV NP205 Y T V K F P N M | 75% | |||
| Flu-immune + LCMV versus acute LCMV i.n. (ref, 74, Wlodarczyk et al.) |
|
|
IAV PA224 S S L E N F R A Y V | 4/10 aa |
| LCMV GP276 S G V E N P G G Y C L | 40% | |||
| IAV PB1-703 S S Y R R P V G I | 1/9 aa | |||
| LCMV GP34 A V Y N F A T C | 10% | |||
| Flu-immune + MCMV versus acute MCMV i.n. (ref. 74) |
|
|
Not done | |
Table 2.
Sequence of Heterologous Virus Infections Influences Viral Clearance, Histopathology and cross-reactivity between viruses in humans.
| Human
| ||||
|---|---|---|---|---|
| Model | Virus titer | Pathology | Cross-reactivity | Sequence similarity |
| Flu-immune + EBV (ref. 49) | Not done |
|
Flu M158-66 G I L G F V F T L | 3/9 aa |
| EBV BMLF1280 G L C T L V A M L | 30% | |||
| Flu M158-66 G I L G F V F T L | 1/9 aa | |||
| EBV BRLF1109 Y V L D H L I V V | 10% | |||
| Flu-immune + HCV (ref 70,92) | Not done |
|
HCV NS31073 C I N G V C W T V | 6/9 aa |
| Flu NA231 C V N G S C F T V | 70% | |||
| Coronavirus + HPV (ref. 56) | Not done |
|
HPV 16E7 Y M L D L Q P E T | 6/9 aa |
| NS252-60 T M L D I Q P E D | 70% | |||
| Different dengue serotypes (ref. 53,142,166) |
|
|
High variety in cross-reactivity between NS4b111-119, NS4b181-189, NS4a56-64 and E211-219 within the four serotypes. With sequence similarity between 3/9 (30%) to 8/9 (90%) depending on the serotype. | |
| Flu-immune + HIV (ref 55) | Not done | Not done | HIV-1 p17 Gag77-85 S L Y N T I A V L | 1/9 aa |
| Flu M158-66 G I L G F V F T L | 10% | |||
HETEROLOGOUS IMMUNITY AND THE FINE BALANCE BETWEEN PROTECTION AND PATHOLOGY DURING VIRAL INFECTIONS
The term “heterologous immunity” was used when describing differences in protective immunity and immunopathology in C57BL/6 mice immunized with one virus and later challenged with either of five viruses, IAV, LCMV, PV, VV, and murine cytomegalovirus (MCMV)24,74(Table 1). Only recently has direct evidence been published that heterologous immunity is mediated by cross-reactive T cells, and that cross-reactive T cells may cause dramatic differences in T cell-dependent protection and immunopathology in the fat and lung69,75(Wlodarczyk,MF & Selin LK unpublished). Heterologous immunity can provide partial protective immunity and, in experimental models, can provide the difference between life and death in the infected individual24,76,77. For example, LCMV-immune mice control PV infection, due to the cross-reactivity of T cells specific for the subdominant NP205 epitope78,79(Chen A, Welsh RM & Selin LK unpublished). LCMV-immune mice also manifest strong protective immunity against infections with the large DNA poxvirus, VV, compared to naïve mice24. This heterologous immunity prevented mortality to an otherwise lethal dose of VV76,77. Adoptive transfer studies demonstrated that CD4 and CD8 T cells from LCMV-immune mice were required to transfer protective immunity to naive mice challenged with PV or VV24. Selective expansion of LCMV-specific memory CD8 T cells upon VV infection suggested the possibility of cross-reactive CD8 T cell responses between these two viruses 76,78. In fact, VV-specific CD8 T cell epitopes were identified in mice by searching for sequence similarity to a potentially cross-reactive LCMV epitope 54,80. These cross-reactive CD8 T cells from LCMV-immune mice mediate protective immunity against VV as demonstrated by adoptive transfer of T cells lines 75.
However, T cells can be mediators not only of protective immunity, but also of substantial immunopathology27,75,81-85 Classic examples are that of LCMV, where the same clone of T cells responsible for viral clearance can mediate a severe leptomeningitis if the virus is replicating in the brain27,81. The pathology that is induced by T cells during an acute infection most likely results from the inflammatory conditions brought about by the presence of high numbers of T cells lysing infected tissues via perforin and FasL, producing pro-inflammatory cytokines including TNF and chemokines which recruit even more cells. An important factor in how rapidly the virus is cleared is the efficiency of the activated T cells. The avidity of the TCR interaction with its ligand is one of the factors which lead to the induction of high potency T cells. Functional studies with altered peptide ligands (APL) show for both CD4 and CD8 T cell clones that high- and low- potency ligands differ in the length of time the TCR interacts with MHC/ligand, often referred to as the “affinity” of the TCR86-89. High avidity or agonist TCR interactions for T cell clones have been shown to result in strong signaling and activation of the full functional potential of the cells, including cytokine production, cytotoxicity, and proliferation. Low avidity or weak agonists induce CD4 T cell clones to produce cytokines, but not proliferate, while in CD8 T cell clones they induce all functions, but it requires 10- to 100-fold more ligand. One can easily imagine that a low avidity TCR interaction with a cross-reactive ligand may produce different cytokines, different amounts of cytokines, or be less efficient at killing and proliferating than the high avidity interaction with the original inducing ligand. Recent work using mutations in the H2Kb-restricted SIINFEKL epitope of ovalbumin and ovalbumin-specific transgenic T cells indicates that low affinity naïve T cells initially expand with kinetics similar to that of high affinity naive T cells, but leave the lymph node earlier and do not have the sustained expansion of higher affinity T cell clones, which eventually out compete the low affinity clones and dominate the response90. Although the exact mechanisms of ligand binding and transmission of this extracellular interaction into a productive intracellular signaling sequence remains incomplete, it has been known for many years that the immunoreceptor tyrosine activation motifs (ITAMs) of the T-cell receptor (TCR):CD3 complex are required for initiation of this signaling cascade. It remains unclear why the TCR:CD3 complex requires 10 ITAMs, while many other ITAM-containing immune receptors, such as Fc receptors (FcRs) and the B cell receptor (BCR), contain far fewer ITAMs. Vignali and his colleagues have recently demonstrated that various parameters of T cell development and activation are influenced by the number, as well as location and type, of ITAMs within the TCR:CD3 complex and hence have proposed that the TCR is capable of ‘scalable signaling’ that facilitates the initiation and orchestration of diverse T-cell functions91. Their work would suggest that rather than simply signal initiation, individual ITAMs may also be responsible for the differential recruitment of signaling and regulatory molecules and that this ultimately affects T-cell development, activation, and differentiation.
Not unexpectedly, heterologous immunity is not as protective as homologous immunity, which elicits high avidity T cell and antibody responses against a previously encountered pathogen. Recruitment of a large number of lower avidity cross-reactive memory T cells early in infection by a heterologous virus might be more conducive to amplifying a potent pro-inflammatory response in the presence of on going viral replication, due to the inability to efficiently clear the virus, thus leading to enhanced immunopathology. Due to the competition between cells that gives rise to immunodominance, low avidity cross-reactive memory cells may also prevent the development of more effective high-avidity T cells responding to the normally immunodominant epitopes. Disease outcome is ultimately based on a fine balance between the number of memory T cells recruited to sites of viral replication, the efficiency of these T cells to clear the virus, and the length of time these T cells are present and amplifying the pro-inflammatory responses before they are able to clear the virus (Figure 2).
Some of the examples that this review will focus on to demonstrate the fine balance that exists within heterologous immunity will include VV infection of LCMV-immune mice, where the price for partial protective T cell immunity is altered immunopathology. After an intraperitoneal inoculation, LCMV-immune mice challenged with VV develop necrosis of visceral fat, termed acute fatty necrosis or panniculitis24. This form of panniculitis is analogous to human erythema nodosum and Weber-Christian disease. Also, in a respiratory infection model, reduced mortality of LCMV-immune mice infected with VV is accompanied by altered lung pathology76. Their lungs are significantly infiltrated by LCMV-specific T cells, which contributed to obstruction of bronchioles by fibrin and inflammatory cells (bronchiolitis obliterans). As mentioned above, in humans, erythema nodosum and bronchiolitis obliterans are of unknown etiology, but can be seen in some viral and bacterial infections and are also associated with autoimmune diseases7-9. Erythema nodosum has been observed after vaccination for smallpox or hepatitis B. The development of bronchiolitis obliterans in lung allografts is associated with transplant rejection9. Human T cell cross-reactivity exists between the major immunodominant HLA-A2-restricted epitopes of IAV virus and EBV, and a substantial part of the acute EBV-specific CD8 T cell response during infectious mononucleosis can be mediated by T cells cross-reactive with IAV contributing to the induction of infectious mononucleosis49. Other studies have correlated fulminant hepatitis with cross-reactive CD8 T cell responses between IAV and HCV72,92,93 and have suggested a role for cross-reactive T cells in severe dengue virus infections71,94. Heterologous immunity and cross-reactive T cell responses between pathogens may be the basis for greatly dysregulated immune responses and enhanced immunopathology that can contribute to the induction of autoimmune syndromes.
LCMV AND VV
LCMV-immune mice infected with VV, is one of the first mouse models of heterologous immunity to demonstrate significant protective immunity and significant immunopathologies that are very similar to human diseases of unknown origin, falling into to the nebulous category of potentially autoimmune disease processes.
Panniculitis
The type of pathology that these mice develop is dependent on the route of infection. When LCMV-immune mice are infected with VV using the traditional intraperitoneal (i.p.) route some of these mice develop severe panniculitis, in the form of inflammation and necrosis of visceral fat tissue in the presence of a lower virus titer than naïve mice infected with VV24,95. This type of abdominal fat pathology is seen in human syndromes of unknown etiology such as Weber-Christian disease or lupus erythematosis, while erythema nodosum, a more benign and more common form of panniculitis involves inflammation of subcutaneous fat tissue 24,95. Interestingly, this pathology is not directly associated with viral load (Figure 3), and there is tremendous variation between these genetically identical individual mice, with some mice having no pathology and others having very severe pathology that sometimes leads to death69.
Figure 3. Independent of VV load, variable levels of panniculitis were found in genetically identical LCMV-immune mice during VV.
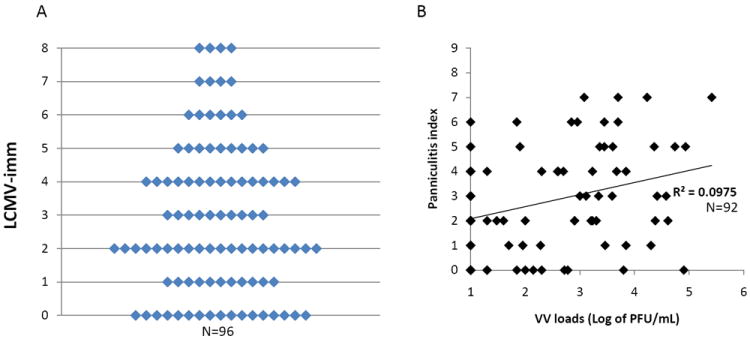
LCMV-immune (A and B) mice were challenged with VV i.p. At day 6 of infection, the levels of panniculitis were recorded, and VV loads in fat-pads were assayed. A. Level of panniculitis varied between individual mice after VV challenge. No correlation between VV loads and levels of panniculitis were found in either LCMV-immune (B) mice (adapted from Nie et al. 2010.)
Role of cross-reactive T cells
VV infection activates LCMV-specific memory T cell populations, and we sought to clarify whether and how cross-reactive T cells could be mediating either the protective immunity and/or the pathology 69,75,77,96. If VV were cross-reactive with an LCMV epitope, then a VV infection of an LCMV-immune mouse should expand LCMV-specific T cells with the cross-reactive specificity 20. VV sometimes, but not all the time would expand populations of LCMV NP205-specific T cells 76, so we searched the VV proteome for potentially cross-reactive epitopes based on sequence similarity to NP205 and found two, within proteins e7r and a11r, to be recognized by VV-specific T cells 54,75,80. Both these epitope-specific responses are protective against VV infection 80,97. Of these two epitopes with sequence similarity to LCMV NP205, only the a11r response turned out to be cross-reactive with LCMV.
About half the time VV infection expands populations of NP205-specific T cells, but sometimes it expands populations of LCMV GP34- or GP118-specific T cells upon adoptive transfers of LCMV-immune splenocyte populations into naïve mice 68. This variability in responses is not due to random stochastic events, but instead reflects the private specificity of the LCMV-immune T cell repertoire in individual mice. Adoptive transfer into three recipients from the same donor generates the same specificity of outgrowth of LCMV epitope-specific T cells, but recipients from a different donor can stimulate a different specificity. These results suggest that the private specificities of the LCMV memory population dictate which cross-reactive epitope would be recognized. Interestingly, it turned out that T cell responses to the Kb-restricted VV-a11r epitope can cross-react with either LCMV-encoded NP205, GP34, or GP118, all of which are Kb-restricted, though no T cell recognized all epitopes 68,75. In fact, we found a network of cross-reactive epitopes encoded by VV, LCMV, and PV (Figure 4). Also, even though the e7r-specific T cell population is not cross-reactive with the LCMV-encoded epitopes, a part of the e7r T cell population is cross-reactive with a11r, which engages some T cells specific to each of the three LCMV epitopes or to the PV-encoded NP205 epitope. These experiments indicate that the epitope specificity of a T cell response in genetically identical individuals with the same histories of infection can be influenced by the private specificity of the individual.
Figure 4. Summary of networks of cross-reactive patterns of in vitro and in vivo generated T cells stained with tetramers or tested in ICS assays in a large number of studies.
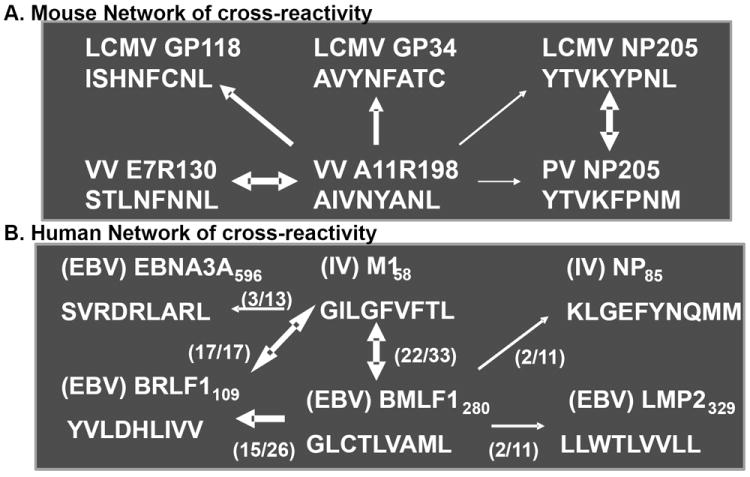
A.) represents H2Kb-restricted responses in B6 mice. B.) shows HLA-A2-restricted responses in humans. Numbers in parentheses represents number of positive responses per individual (adapted from Cornberg et al. 2010).
What is the evidence that these cross-reactive responses mediate protective immunity or altered immunopathology? A history of an LCMV infection, as well as infections with BCG, PV, IAV, and MCMV, all provide a level of protective immunity to VV, and VV titers at day 3-4 after infection of LCMV-immune mice are generally 1-2 logs lower than those in naïve mice 24,74,76,98. Adoptive transfer of CD8 and CD4 splenocytes from LCMV-immune mice can provide protective immunity to VV 24, or induce panniculitis 69. Adoptive transfer of cross-reactive T cell lines generated by stimulating LCMV-immune T cell populations with the VV-encoded and cross-reactive a11r epitope also can mediate protective immunity to VV 75. Interestingly, the presence of cross-reactivity does not necessarily lead to protective immunity, as some of the VV-a11r-specific cross-reactive lines were not protective against VV. Furthermore, the variation in the presence or level of panniculitis in VV-infected LCMV-immune recipients is a product of the private specificity of the T cells in the LCMV-immune host, as demonstrated by adoptive transfer of LCMV-immune T cells into paired naïve congenic recipients 69. It still needs to be determined whether there are certain cross-reactive responses that lead to protective immunity versus those that lead to immunopathology.
Potential therapeutics with IFNγ and TNF blockers
Both protective immunity and the induction of immunopathology in this system seem to be heavily dependent on IFNγ, as neither protective immunity nor panniculitis occur in mice treated with antibody to IFNγ or in mice lacking IFNγ receptors 24,76. The panniculitis, but not the protective immunity, was also dependent on TNF, as shown in TNF-deficient mice and by blocking TNF with soluble TNF receptors (Enbrel) (Nie and Selin, unpublished). In fact, whereas the control of VV infection was partially dependent on TNF in naïve mice, the presence of heterologous protective immunity made resistance to VV less dependent on TNF77. Perhaps heterologous immunity may help explain why TNF blockers such as Enbrel and Remicade (anti-TNF antibody) used in the treatment of human autoimmune diseases such as rheumatoid arthritis and colitis are tolerated with relatively few infectious complications77.
Protection not reciprocal
Heterologous immunity is not necessarily reciprocal, as a history of a VV infection will not confer any protection of mice to LCMV, PV or MCMV 24. Consistent with this observation, CFSE-labeled LCMV-specific immune cells transferred into mice which then get infected with VV, proliferate substantially (CFSE-loss), but VV-immune cells transferred into mice that are then infected with LCMV, show little to no T cell expansion 78. The reason for this is unclear, but there are far more potentially cross-reactive NP205-, GP34-, and GP118-specific T cells in the LCMV-immune memory pool than there are A11R-specific cells in the VV-immune memory pool. We also have not resolved all the potential patterns of cross-reactivity between these two viruses, and it may be that one of the many thousand of potential epitopes encoded by the very large VV, which encodes >200 proteins, could engage a sufficient number of memory cells to have a biologically meaningful effect. In contrast, LCMV encodes only four proteins, reducing the likelihood that they could successfully engage the VV-specific memory pool. It is interesting that large viruses like herpes simplex virus (HSV) and CMV encode many proteins that interfere with class 1 antigen presentation99 and thus perhaps escape the protective effects of heterologous immunity. There remains the possibility that VV-specific memory cells may be qualitatively different than LCMV-specific memory cells. For instance, different cytokines play a role in their T cell maturation, where VV-specific T cells develop in the presence of IL-12, but not IFN1 and LCMV-specific T cells develop under the opposite conditions, high IFN1 and low IL-12100,101 The long term effect of these cytokines on the responsiveness of these memory cells is uncertain. That being said, VV infection will readily expand populations of VV-specific memory cells, indicating that they are capable of excellent recall responses.
Bronchiolitis Obliterans and Enhanced BALT
Heterologous immunity between LCMV and VV has also been studied in respiratory infections, and the most common route of entry of both viruses in nature is the respiratory route 74,76. After intranasal (i.n.) infection there was protective immunity and aberrant immunopathology. Acute VV infection of naïve mice was characterized by high virus loads, necrotizing bronchiolitis, acute inflammatory neutrophilic infiltrates, pulmonary edema, and severe respiratory distress of the host. In contrast, in LCMV-immune mice the VV infection was characterized by reduced virus loads, little pulmonary edema, areas of massive chronic lymphocytic infiltration, and enhanced bronchus-associated lymphoid tissue (BALT). As with panniculitis, the severity of the pathology could vary from insignificant to very severe and some mice presented with an unusual pathology, bronchiolitis obliterans, which is an obstruction of the airways with plugs of fibrin and inflammatory cells. This pathology in humans is highly lethal and is thought to be induced by cell-mediated immunity. It is of unknown etiology, but is sometimes seen in humans following viral infections and is strongly associated with lung transplant rejection. Despite these pathologies, the LCMV-immune VV-infected mice experienced less respiratory distress, due to areas of the lung being reasonably intact rather than edematous. In fact, LCMV-immune mice were able to survive doses of VV that were lethal to naïve mice. This is yet another example where models of heterologous immunity can reveal new diseases with pathologies resembling poorly defined autoimmune syndromes in humans.
Another interesting feature of heterologous immunity between LCMV and VV in the respiratory infection model is that there is a profound deviation in virus-induced cytokine production. Acute VV infection of naïve mice is associated with high levels of the proinflammatory cytokine IL-6 and decreased levels of the immune response cytokine IFNγ. Presumably because of the presence of IFNγ-producing cross-reactive memory T cells, the cytokine levels in VV-infected LCMV-immune mice are high in IFNγ and low in IL-674,76. This is consistent with the concept that heterologous immunity is influenced by the cytokine-producing capacity of cross-reactive memory cells which may have the capacity to skew Th1 to Th2 responses during any new infection.
There is always a question of how much of heterologous immunity is mediated exclusively by cross-reactive mechanisms and how much by non-specific bystander mechanisms. Some studies have concluded that memory T cells can be subjected to bystander activation by cytokines independent of TCR signaling and that these non-specifically activated cells may contribute to heterologous immunity102-105. IL-12 and IL-18 have been reported to non-specifically stimulate the production of IFNγ by memory T cells106, and VV infection does induce IL-12 in mice 74. Thus is the IFNγ-dependent protection against VV entirely a consequence of T cell cross-reactivity or is it in part due to non-specific liberation of IFNγ from memory cells. In our studies, LCMV-specific memory cells need TCR engagement to produce elevated IFNγ levels in the presence of IL-12, but this does not rule out a non-specific effect under certain conditions. It is also possible that up-regulation of host MHC by IFNs and other cytokines provides some low level TCR signaling to memory cells, making them more receptive to activation by cytokines without engagement of viral peptides 107,108. This area needs more study, but it should be noted that BCG immunization can provide protective immunity to VV by a CD4 T cell-dependent mechanism 98. Using an in vivo IFNγ assay 109, VV infection preferentially induced IFNγ from CD4 T cells in BCG-immune mice, but not from CD8 T cells in LCMV-immune mice 98, although both primary infections resulted in memory phenotype CD4 and CD8 T cell populations in the peritoneal cavity and visceral fat pads. This preferential bias of T cell subtype would argue against non-specific stimulation of cytokines from memory cells under conditions of the natural infection in vivo even though the cross-reactive CD4 epitopes have not yet been identified.
Interestingly, to date there are few examples of cross-reactive CD4 T cells in models of heterologous immunity although CD4 T cells, due to their low affinities and longer peptide targets, could be more cross-reactive than CD8 T cells. A problem may lie in the possibility that there may be broad-based CD4 T cell cross-reactivity between the two pathogens, coupled with a rather low proliferative index that would prevent a CD8 T cell-like expansion that can aid in the identification of the cross-reactive epitope. As it stands, this is a system that clearly shows T cell-dependent heterologous immunity between different classes of pathogens which needs further investigation.
LCMV AND PV
LCMV-immune mice produce about 10-fold lower PV titers compared to naïve mice 24,79, and we assume this is due to the cross-reactive T cell response, in part because LCMV-immune splenocytes transferred into naïve mice can partially protect against PV infection. Since LCMV-immune mice are relatively resistant to infections with VV and MCMV, it could be argued that nonspecific activation of memory cells might mediate this protective immunity and that some non-specific mechanisms are at work. However, the NP205 epitope-escape variant67 of LCMV had reduced ability to protect mice against PV (A. Chen, R.M. Welsh, & LK Selin, unpublished). This correlation of protective immunity and cross-reactivity adds to the argument that protective immunity is not a non-specific phenomenon, but is instead a consequence of MHC-restricted T cell cross-reactivity.
Panniculitis
Although LCMV-immune challenged with PV do not develop significant pathology associated with heterologous immunity we have also observed panniculitis in some mice that are both PV and then LCMV immune and then are rechallenged with PV (A. Chen,R.M. Welsh & LK Selin, unpublished). In this circumstance we would predict that the T cell repertoire to the NP205 cross-reactive response, which varies between mice due to private specificity, would be skewed towards LCMV-specific during the second infection and would not be optimized to protect against PV leading. This might lead to immunopathology.
Role of T cell cross-reactivity
LCMV and PV encode cross-reactive nucleoprotein epitopes at positions 205-212 that have 6 of 8 amino acids in common and differing in amino acids only in their anchoring sites to the Kb MHC 79. This might suggest that these PV and LCMV epitopes should be similarly recognized by T cells. Most T cells generated in response to one virus will recognize the alternative peptide epitope 79, but with different affinities, as suggested by tetramer binding patterns. The LCMV-induced repertoire is almost exclusively oriented to Vβ16 while the PV-induced repertoire also includes Vβ5-specific T cells, and uses a different CDR3 motif within the Vβ16 T cells 67. Sequential infections with these viruses could lead to the generation of highly variable and skewed narrow oligoclonal TCR repertoires that differed between hosts, frequently not focused to Vβ16 or Vβ5, reflecting the private specificity of the T cell response. These variations in repertoire reflected private specificity patterns rather than random stochastic patterns produced by a programmed expansion random encounters of cross-reactive T cell clones with its cognate ligand. Transfer of splenocytes from one immune mouse into three recipient mice infected with the heterologous virus generated similar T cell repertoires, but recipients from other donors all generated a different set of repertoires 67. This indicates that the repertoire available for cross-reactive expansions was established during the first virus infection and was unique for each immune mouse.
Narrowing of TCR repertoire and predictive modeling
Narrowing of the diversity of TCR repertoires for a viral epitope may occur by evolution for the most perfect fit during persistent virus infections, such as those with HIV, HCV, CMV, and EBV, or with repeated antigenic challenges, such as with influenza virus. There are many examples of T cell cross-reactive peptides encoded by different viruses 53,54, and another major cause of TCR repertoire restriction could be due to cross-reactive T cell responses. A rather narrow subset of an epitope-specific T cell memory pool is selectively stimulated to proliferate on exposure to a cross-reactive pathogen 67,110. On infection with a heterologous virus, these high-frequency, but not-very-diverse set of clones may immunodominate an emerging T cell response from naïve precursors and cause a further restriction of the repertoire, as we have demonstrated in LCMV-immune mice infected with PV 67. Furthermore, our results using the IMMSIM model, where we incorporated both adoptive transfer and private specificity, would suggest that TCR affinity plays an important role in driving this phenomenon 67.
IAV AND LCMV
A history of an intranasal IAV infection of mice can generate a level of protective immunity against VV, possibly through a similar mechanism as that between LCMV and VV described above. However, a history of IAV infection can lead to enhanced titers of LCMV and MCMV, with altered immunopathology 74. It has long been known that IAV infection can break down structural barriers to bacterial infection in the lung, and bacterial invasion of the lung is often responsible for the mortality in human influenza patients. Here, however, mice are receiving LCMV and MCMV long after the IAV infection has resolved and when the lung no longer shows signs of structural damage. This suggests that dysregulated immunity can occur as a consequence of heterologous immunity.
Bronchiolization and Consolidating Mononuclear Pneumonia
Heterologous immunity between IAV and LCMV is quite complex. IAV-immune mice developed enhanced replication of LCMV and enhanced immune pathology in the lung, characterized by massive consolidating mononuclear pneumonia with bronchiolization instead of the mild lymphocytic pneumonitis observed during LCMV infection of naïve mice 74. Bronchiolization is another novel pathology observed in humans and is of unknown etiology thought perhaps to be associated with lung repair processes111. Memory T cell depletion studies indicated that the enhanced LCMV titers seem to be dependent on IAV-specific memory CD4 T cells (perhaps by regulating CD8 T cells), and the enhanced immune pathology is caused by cross-reactive IAV-specific CD8 T cell responses (Wlodarczyk MF & Selin LK, unpublished).
Therapeutics using peptide tolerization, mutant vaccines and IFNγ blockers
Cross-reactive epitopes for IAV and LCMV have been defined in these studies in C57BL/6 mice (Table 1). The importance of these epitopes in these patterns of heterologous immunity was shown by using variants of IAV and LCMV with deletions in these epitopes, and those deletions inhibited the immunopathology associated with the heterologous immunity. In addition, epitope-specific T cells were deleted or functionally inactivated by intravenous infusion of the peptides into mice. This resulted in selective depletion of the epitope-specific memory T cells and decreased lung pathology induced by the heterologous immunity. We were also able to inhibit immunopathology by treating the IAV-immune mice with anti-IFNγ prior to challenge with LCMV. We took 4 different novel approaches that help explain the increased severity in lung pathology during “dysregulated” heterologous immunity, 1. identification of cross-reactive epitopes, 2. correlation of cross-reactive epitope-specific memory responses with disease severity, 3. ablation of inappropriate cross-reactive T cell responses or 4. temporary attenuation of T cell effector cytokines such as IFNγ (Wlodarczyk MF & Selin LK, unpublished). These four approaches suggest for the first time that therapeutic interventions may be possible to circumvent severe immunopathology induced by heterologous immunity112.
EOSINOPHILIA, IMMUNE DEVIATION AND HETEROLOGOUS IMMUNITY
It seems highly possible that, if memory T cell pools are skewed in a Th1, Th2, or Th17 direction, infection with a cross-reactive pathogen could activate and induce cytokine secretion by some of those T cells, which could then alter the differentiation of the immune response against the pathogen. There have not been studies to unambiguously show this, though there are hints with infection of BALB/c mice with RSV. RSV causes severe respiratory infections in children and adults, and inactive vaccines against RSV in the past have resulted in increased morbidity and mortality in children when the children became naturally exposed to the virus 82. These children presented with severe lung pathology associated with intense eosinophilia, presumably brought above by a type 2 cytokine response involving IL-5 and other Th2 cell-produced cytokines. This phenomenon can be mimicked in mice immunized with RSV G protein and later challenged with live RSV 113,114. These mice develop a narrowly focused Vβ14-expressing CD4 Th2 response associated with high levels of IL-5 production and severe eosinophilia. These T cells are directed against a single G-encoded epitope and are rather similar in CDR3 sequences between mice 113. Priming with RSV G can be done in the context of a VV-G recombinant virus, but if a host first has an infection with IAV, VV-G will no longer prime for a Th2 response 115. IAV provides a modest level of heterologous immunity to VV, but, importantly, there is a different cytokine milieu in VV-infected IAV-immune mice in comparison to VV-infected naïve mice 74. This milieu is deviated in a type 1 cytokine, IFNγ-oriented direction. Thus, it is possible that the IAV infection caused an immune deviation of the VV-G immunization, such that on infection with the third virus, RSV, severe Th2-dependent pathology did not develop. These results would suggest that heterologous immunity could play a role in the development of allergies and asthma following viral infections.
HUMAN EXAMPLES OF HETEROLOGOUS IMMUNITY
In the setting of human disease it is more difficult to test whether heterologous immunity can play a role in protective immunity. At this time the only direct evidence for this comes from epidemiological vaccine studies in very young children in the third world, where it has been found that BCG or the combined live attenuated measles, mumps and rubella (MMR) vaccine can protect in some unknown manner against mortality from other infectious diseases such as rotavirus-induced diarrhea and pneumonias116-119. Interestingly, a subsequent vaccination with the killed combined diphtheria, tetanus and pertussis (DTP) vaccine can reverse these beneficial effects and in fact increase mortality117,120,121. We also have data demonstrating that 3 middle-aged EBV sero-negative adults who are constantly being exposed to EBV in their laboratory environment have strong HLA-A2 restricted cross-reactive CD8 T cell responses between IAV M158 and EBV BMLF1280, with unique repertoires that are able to produce IFNγ and lyse EBV-infected targets (Watkin et al., unpublished). There are cohorts of sex workers in Kenya which appear to have some form of protective immunity to HIV as long as they continue to work122,123. Elderly individuals may also be completely dependent on cross-reactive memory responses when they encounter any new pathogen. The problem with studying the protective effects of heterologous immunity is that reduced sickness would normally go unnoticed, except in a large scale epidemiological study.
Identification of a role of heterologous immunity in the induction of human immunopathology is more approachable. CD8 cross-reactive responses have been identified between IAV and HCV or EBV, and these cross-reactive T cells have been associated with the induction of either fulminant hepatitis or infectious mononucleosis, respectively49,70,75,124. There are increasing reports of significant side effects to HPV vaccines in young women125-130, and a new controversy is arising as more and more parents refuse to vaccinate their children for fear of side effects. This even led to a television documentary recently aired on the Public Broadcasting Network, PBS, called the “Vaccine Wars”. In a time of complacency when many childhood infections have almost disappeared due to vaccines, parents are focusing on the side effects of vaccines and demanding demonstrations that giving as many as 6 vaccines in one day and a total of 18 vaccines before 2 years of age is safe. Considering our preliminary studies concerning dramatic changes in T cell responses in mice co-infected with LCMV and PV together compared to singly, where immunodominance hierarchies to the two viruses are completed altered with tremendous variability between individual mice, perhaps the issue should be examined more closely (Kenney L & Selin LK, unpublished). These issues all suggest that it behooves us as viral immunologists to better understand when, in what sequence and how vaccines are given, or we will have ever increasing serious issues with compliance. These issues were recently discussed at a Workshop in Copenhagen on “Nonspecific effects and sex differences in vaccines”, attended by an international group of vaccinologists, where we formed a Consortium to begin to address these types of issues, and a summary of the meeting will be published117.
Heterologous immunity is, of course, more difficult to study in human systems, but there is evidence that it occurs. Some studies have been done on what has been called “heterotypic” immunity in the IAV system, where many viral strains and variants are found. T cells cross-reactive between these variants are easy to detect, and an unresolved question is how important these cross-reactive T cells are in immunity or immune pathology associated with human IAV infections 131,132. Two epidemiological studies have indicated that exposure to one strain of IAV (H1N1) apparently provided some level of protection against another strain of IAV (H2N2) under conditions of very little serological cross-reactivity among the IAV targets of neutralizing antibody133,134. Different IAV strains can also encode some extremely conserved epitopes, such as the HLA-A2-restricted M1 epitope, which generates an immunodominant CD8 T cell response 135,136. Whether that specific response provides any resistance to infection is unclear.
IAV and HCV and Fulminant Hepatitis
A more concrete example of heterologous immunity between unrelated human viruses occurs between IAV and HCV. A T cell response to a defined HCV-encoded HLA-A2-restricted epitope NS31073-1081 was found to stimulate a T cell response in non-HCV-immune individuals and ended up being strongly cross-reactive and sharing 7 of 9 amino acids with an IAV-encoded NA231-239 epitope 92. In a remarkable study, the breadth of immune responses to the HCV proteome was addressed in a number of HCV-infected patients by ELISPOT analyses of their T cells stimulated against HCV-encoded peptides. Most patients presented with a broadly reactive response with signals seen among a large number of the HCV peptides. Two patients, however, had an extremely focused response against a peptide spanning the HCV and IAV cross-reactive epitopes. These two patients had an unusual presentation for HCV infection, with severe fulminant necrotizing hepatitis 70. Hence, many of the parameters of heterologous immunity addressed above in mouse models are in play in this study, including a 1. narrowly focused cross-reactive response associated with 2. severe pathology and reflective of 3. private specificity, as all of the patients had likely been exposed to the IAV strains encoding the epitope, but only a subset mounted this immunodominant and narrow response.
IAV and EBV and Infectious Mononucleosis
An unresolved phenomenon of viral infections in humans is that infections with a number of viruses, such as Epstein-Barr (EBV), varicella-zoster virus, measles, or mumps are more severe in young adults than in children137,138. Usually there is more immune pathology associated with the young adult infections, and we propose that one reason for that phenomenon may be heterologous immunity. As the memory T cell repertoire of children becomes more diversified due to the acquisition of a sequence of infections, the probability of having memory cells cross-reactive with another pathogen increases, and these may not be the best cells to rapidly clear the virus. Acute infectious mononucleosis (AIM) associated with EBV infections is one of the best examples of such a phenomenon. Children usually have subclinical infections, but teenagers of college age and young adults can have a much more severe and longer lasting infection. The characteristic pathology of AIM is the appearance of “atypical lymphocytes,” which are, in fact, cytotoxic granule-containing activated CD8 T cells responding to EBV-infected B cells and epithelial tissue. There is no evidence that the EBV load is any higher in AIM patients than in the subclinically infected 139. The major pathological feature of AIM, then, is that of an overzealous CD8 T cell response that is not very effective at controlling the virus. Many HLA-A2+ AIM patients have an increase in the frequency of their IAV-M158 –specific CD8 T cell responses. We have demonstrated human T cell cross-reactivity between the major immunodominant HLA-A2-restricted epitopes of IAV and EBV and find that a substantial part of the acute EBV-specific CD8 T cell response during AIM is mediated by T cells cross-reactive with IAV (Table 2) 49. We now have data that demonstrates that the frequency of the IAV M158-specific cells directly correlates with the severity of infectious mononucleosis symptoms (Aslan N & Selin LK, unpublished).
Broad crossreactive T cell repertoire and predictive modeling
We examined cross-reactive responses in vitro between two of these cross-reactive epitopes, IAV-M1 and EBV BMLF1, which have little sequence similarity, and found that the cross-reactive repertoire was broad rather than narrow and devoid of highly dominant clonotypes compared to the non-cross-reactive repertoire for each epitope. The breadth of the cross-reactive T cell repertoire may depend on various factors, such as the level of similarity between the epitopes, a hypothesis which was supported by our computer simulation (Clute SC & Selin LK, unpublished). Based on our earlier observations, we hypothesized that if there are a few high frequency memory clones to the initial immunogen with high affinity to the cross-reactive epitope (which may occur when epitopes are more similar i.e. “near” cross-reactivity), this type of clone will dominate the TCR repertoire to the cross-reactive epitope and lead to a narrower repertoire. However, if this type of clone does not exist in the memory pool (which may occur when epitopes are more dissimilar i.e. “distant” cross-reactivity) the repertoire will actually become broader without any dominant pre-existing cross-reactive memory clones. Cross-reactive TCR repertoires were generated from the two different memory populations to the first antigen. The “near” cross-reacting clonotypes were found in high frequency positions, as their dominance is assured by competition with the development of new clones, thus causing restriction of the repertoire. On the other hand, the ”distant” cross-reacting clonotypes were present at low frequencies, and the absence of high frequencies of high affinity clones allow ample space for development of repertoire diversity124.
Our continued analyses of the CD8 T cell response to EBV in AIM patients and immune controls has revealed a whole network of HLA-A2-restricted cross-reactivities between EBV-encoded and IAV-encoded epitopes75, much like the Kb-restricted network of cross-reactivities discussed between LCMV-, VV-, and PV- encoded epitopes (Figure 3). Of note is that these cross-reactive T cells were found in some subjects, but not others, likely revealing a strong role for private specificity in this process.
Dengue Viruses and Hemorrhagic Shock Syndrome
Dengue viruses are closely related viruses that are found in four serotypes 140,141. Other than by neutralization assay, these viruses serologically cross-react and encode cross-reactive T cell epitopes. The most severe manifestation of dengue disease is dengue hemorrhagic fever and shock syndrome, and this most commonly presents when individuals immune to one strain (serotype) of dengue virus become infected with another strain. This has long been thought due to cross-reactive non-neutralizing antibody that binds to viruses without inactivating them and instead enhances the infection of cells like macrophages that bear Fc receptors, in a process known as immune enhancement 141. However, this may be only part of the mechanism, as extensive T cell cross-reactivity occurs between these viruses (Table 2)53,142. One report shows that a substantial part of the T cell response to the second dengue virus infection consisted of CD8 T cells having higher affinity to the previously encountered dengue virus than to the one causing the current infection 71. Thus, a combination of enhanced viral load due to antibody-dependent immune enhancement plus cross-reactive low affinity T cell responses may help contribute to the severity of the disease.
T REGULATORY CELLS, VIRUSES AND IMMUNOPATHOLOGY
There is another component of the immune system which plays an important role in modulating viral load and immunopathology, regulatory T cells. If regulatory CD4+ T cells (Treg) are generated in the thymus they are referred to as natural Treg cells, but if they differentiate from naïve T cells into Treg cells in peripheral tissue they are referred to as induced or adaptive Treg cells. Treg cells can suppress the function of many types of immune cells including CD8+ and CD4+ T cells, B cells, DC, NK and NKT cells 143. Based on factors such as where these cells are generated (thymus or periphery) and which cytokines they release (IL10 and TGFbeta1) Treg cells are divided into different subtypes. The most studied type is the natural Treg cell, CD4+CD25+Foxp3+, which make up to 5-10% of the peripheral CD4+ T cell population 144,145 and play an important role in protecting the host against autoimmune diseases like colitis, gastritis and type 1 diabetes 144,146-149. They have unique features such as expression of Foxp3 (“forkhead/winged-helix family of transcription factors”), a nuclear transcription factor which plays a critical role in their development and function. They are also highly dependent on exogenous IL-2 for their survival in the periphery 144 and express a variety of accessory molecules including CTLA-4 and GITR which are involved in Treg cell activation, expansion, and suppression. These markers assist in differentiating Tregs from other effector T cells.
Treg cells have been intensively studied in autoimmunity and in the establishment of persistent pathogen infections 145,150-152. The lack or dysfunction of Treg cells resulted in autoimmune diseases with severe pathology. Increased numbers of Treg cells and a loss of functional virus-specific effector T cells have been reported in persistent, but not in acute virus infection (e.g. HCV, HIV/FV and HSV). Depletion of the suppressive Treg cells during a persistent retroviral infection resulted in enhanced effector T cell function and reduced viral load153,154. On the other hand Treg cells can prevent extensive immunopathology during viral infection. Depletion of natural Treg cells, using anti-CD25 prior to infection enhanced anti-viral responses without any evidence of enhanced immunopathology if HSV-1 was injected into the footpad155. But, Treg depletion prior to corneal HSV-1 infection resulted in severe T cell-mediated tissue lesions156. These results suggest that Treg cells influence disease outcome during viral infection differently depending on the virus and on the site of infection. Do virus-induced Treg cells play any role modulating the balance between viral load and quality of T cell responses during heterologous immunity?
Heterologous immunity between IAV and LCMV is quite complicated. IAV-immune mice developed enhanced replication of LCMV and enhanced immune pathology in the lung, characterized by massive consolidating mononuclear pneumonia with bronchiolization instead of the mild lymphocytic pneumonitis observed during LCMV infection of naïve mice 74. Memory T cell depletion studies indicated that the enhanced LCMV titers seem to be dependent on IAV-specific memory CD4 T cells (perhaps by regulating CD8 T cells), and the enhanced immune pathology is caused by cross-reactive IAV-specific CD8 T cell responses (Wlodarczyk M and Selin LK, unpublished). In this system T regulatory cells stimulated by IAV infection might be influencing the response to LCMV. Enhanced numbers of FoxP3+ CD25+ Treg phenotype CD4 cells are found in the lung and draining lymph nodes after IAV infection, and depletion of these cells with mAb to CD25 decreases the lung pathology induced by LCMV infection (Kraft A and Selin LK, unpublished).
PRECIPITATION OF AUTOIMMUNITY BY HETEROLOGOUS IMMUNITY
Autoimmune diseases can be initiated by infection of animals with viruses that encode T cell epitopes cross-reactive with self antigens, or infected with recombinant viruses that encode engineered self-epitopes. These have been reviewed elsewhere 4. For instance, in an HSV-1-induced murine model of autoimmune keratitis T cells specific to an epitope expressed by the UL6 protein of HSV-1 cross-react with a corneal antigen157. In the mouse model of MS, both class I and II epitopes in myelin basic protein have been described to induce autoimmune encephalitis (EAE)4. In humans HLA-DR3 predisposes to autoimmune diabetes, targeting the autoantigen glutamic acid decarboxylase. An HLA-DR3-restricted CD4 T cell response has been found to cross-react against epitopes with sequence similarity between an autoantigen and a CMV-encoded antigen158.
The presence of cross-reactive responses that recognize self and pathogen antigens does not necessarily lead to autoimmunity. There needs to be a precipitating event such as a viral infection. Transgenic mice that express viral proteins as self antigens have been used to study the ability of viral infections to break tolerance159-162. LCMV infection induces a transient pancreatitis or encephalitis depending on the model in mice that express LCMV nucleoprotein in the pancreas or brain. A subsequent heterologous infection such as PV or VV, which have a cross-reactive epitope with LCMV, NP205, can induce a second wave of inflammation. In the insulitis model the LCMV infection could break tolerance to NP205, and induce insulitis, but the subsequent PV infection via activation of the cross-reactive T cells pushed the mouse into diabetes162. A similar phenomenon may be occurring in the induction of MS as mice infected with VV encoding the CNS protein PLP cleared the virus, but when challenged subsequently with murine CMV they developed CNS white matter lesions163,164. In humans, several viruses encode polyarginine sequences that can be recognized by CD4 T cells that have been isolated from the CNS of patients with MS165. Thus, heterologous immunity may play an important role in induction of autoimmune diseases such as diabetes or MS. The first virus that encodes a self-like epitope breaks tolerance and initiates an autoimmune process. The subsequent second virus amplifies the cross-reactive response pushing the self reactive T cell response above a certain threshold in the presence of a strong inflammatory response, shifting a controlled response into an overt autoimmune disease.
CONCLUSIONS
Heterologous immunity may be a common event in human infections and help explain the variability in disease outcome from asymptomatic to death in different individuals exposed to the same pathogens. It may occur (1) with diseases such as EBV-associated mononucleosis, where immunopathological features are more pronounced in young adults than in children49,124; (2) with diseases associated with marked variations in pathogenesis, such as dengue and HCV70,94,166; (3) with viruses that exist as related serotypes (e.g. dengue-, entero-, papillomaviruses (HPV)) or quasi-species with variations in T cell epitopes (e.g. HIV, HCV)70,71,166; and (4) with persistent infections, where constant stimulation by antigens may alter immunity to other pathogens167. Heterologous immunity may also be a factor in unusual responses to vaccination, particularly with vaccines designed to induce strong T cell responses in adults, who have highly developed memory pools, and in young children who frequently get multicomponent vaccines. Human vaccinations can sometimes lead to erythema nodosum, a form of panniculitis (inflammation of fat tissue), which we see as acute fatty necrosis (AFN) of abdominal fat pads under experimental conditions of heterologous immunity in mice 25,168. The recent adenovirus vector-based vaccine for HIV is another example, where vaccinees with higher initial antibody titers to adenovirus responded poorly with less protective immunity to HIV, and it has been suggested that heterologous immunity may have affected this outcome169. Peptide vaccines for HCV currently being constructed include an epitope cross-reactive between HCV and IAV, and there is no telling how this will affect the immune response after live virus challenge. Data are now showing complications in some individuals vaccinated with HPV including panniculitis, where there are many closely related strains125-130,170,171. Many parents are now refusing to vaccinate their children due to perceived severe pathological side effects of vaccines. Some of the best anti-viral vaccines are with attenuated viruses, which can stimulate CD8 T cell responses172, but insights on heterologous immunity are necessary for the intelligent design of effective modern vaccines without unwanted side effects. We contend that heterologous immunity is the norm rather than the exception in human viral infections, and that manifestations of heterologous immunity may vary with the virus sequence and the host. We would predict that there are general principles of heterologous immunity which can be elucidated and ultimately manipulated. A better understanding of heterologous immunity and how it influences viral immunopathogenesis could lead to potential therapeutic interventions to circumvent severe disease that may contribute to the development of autoimmune syndromes and also to the intelligent design of vaccines.
Acknowledgments
This research was supported by US National Institute of Health (NIH) grants AI-49320, AI-42845, AI-054455, AI17672, AI46629, AI46578, AI49320, AR35506 DR-32520, an immunology training grant 5 T32 AI-07349-16 and DFG fellowship CO310/1-1. The contents of this publication are solely the responsibility of the authors and do not represent the official view of the NIH.
References
- 1.Posnett DN. Herpesviruses and autoimmunity. Curr Opin Investig Drugs. 2008 May;9(5):505–14. [PubMed] [Google Scholar]
- 2.Sips GJ, Chesik D, Glazenburg L, Wilschut J, De KJ, Wilczak N. Involvement of morbilliviruses in the pathogenesis of demyelinating disease. Rev Med Virol. 2007 Jul;17(4):223–44. doi: 10.1002/rmv.526. [DOI] [PubMed] [Google Scholar]
- 3.Libbey JE, McCoy LL, Fujinami RS. Molecular mimicry in multiple sclerosis. Int Rev Neurobiol. 2007;79:127–47. doi: 10.1016/S0074-7742(07)79006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welsh RM, Fujinami RS. Pathogenic epitopes, heterologous immunity and vaccine design. Nat Rev Microbiol. 2007 Jul;5(7):555–63. doi: 10.1038/nrmicro1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sotelo J, Martinez-Palomo A, Ordonez G, Pineda B. Varicella-zoster virus in cerebrospinal fluid at relapses of multiple sclerosis. Ann Neurol. 2008 Mar;63(3):303–11. doi: 10.1002/ana.21316. [DOI] [PubMed] [Google Scholar]
- 6.Giraudon P, Bernard A. Chronic viral infections of the central nervous system: Aspects specific to multiple sclerosis. Rev Neurol (Paris) 2009 Oct;165(10):789–95. doi: 10.1016/j.neurol.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Requena L, Requena C. Erythema nodosum. Dermatol Online. 2002;8:4. [PubMed] [Google Scholar]
- 8.Smoller BR, Weishar M, Gray MH. An unusual cutaneous manifestation in Crohn’s disease. Arch Pathol Lab Med. 1990;6:609–10. [PubMed] [Google Scholar]
- 9.Schlesinger C, Meyer CA, Veeraraghavan S, Koss MN. Constrictive (obliterative) bronchiolitis: diagnosis, etiology, and a critical review of the literature. Ann Diagn Pathol. 1998;2:321–34. doi: 10.1016/s1092-9134(98)80026-9. [DOI] [PubMed] [Google Scholar]
- 10.Jones JF, Ray CG, Minnich LL, et al. Evidence fo reactive Epstein-Barr virus infection inpatients with persistent, unexplained illnesses:Elevated anti-early antigen antibodies. Ann Intern Med. 1985;102:7–16. doi: 10.7326/0003-4819-102-1-. [DOI] [PubMed] [Google Scholar]
- 11.Ho-Yen DO, McNamara I. The epidemiology of post viral fatigue syndrome. Scott Med J. 1988;33:368–9. doi: 10.1177/003693308803300607. [DOI] [PubMed] [Google Scholar]
- 12.Evans AC. Brucellosis in the United States. Am J Public Health. 1947;37:139–51. [PMC free article] [PubMed] [Google Scholar]
- 13.Truss CO. The role of Candida Albicans in human illness. J Orthomol Psychiatry. 1981;10:228–38. [Google Scholar]
- 14.Daugherty SA, Henry BE, Peterson DL, et al. Chronic fatigue syndrome in northern Nevada. Rev Infect Dis. 1991;13(suppl 1):S39–S44. doi: 10.1093/clinids/13.supplement_1.s39. [DOI] [PubMed] [Google Scholar]
- 15.Buchwald D, Cheney PR, Peterson DL, et al. A chronic illness characterized by fatigue, neurologic and immunologic disorders, and active herpesvirus type 6 infection. Ann Intern Med. 1992;116:103–13. doi: 10.7326/0003-4819-116-2-103. [DOI] [PubMed] [Google Scholar]
- 16.Chia JK, Chia AY. Chronic fatigue syndrome is associated with chronic enterovirus infection of the stomach. J Clin Pathol. 2008 Jan;61(1):43–8. doi: 10.1136/jcp.2007.050054. [DOI] [PubMed] [Google Scholar]
- 17.Chia JK. The role of enterovirus in chronic fatigue syndrome. J Clin Pathol. 2005 Nov;58(11):1126–32. doi: 10.1136/jcp.2004.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lombardi VC, Ruscetti FW, Das GJ, Pfost MA, Hagen KS, Peterson DL, Ruscetti SK, Bagni RK, Petrow-Sadowski C, Gold B, Dean M, Silverman RH, Mikovits JA. Detection of an infectious retrovirus, XMRV, in blood cells of patients with chronic fatigue syndrome. Science. 2009 Oct;326(5952):585–9. doi: 10.1126/science.1179052. [DOI] [PubMed] [Google Scholar]
- 19.Jones JF. Serologic and immunologic responses in chronic fatigue syndrome with emphasis on the Epstein-Barr virus. Rev Infect Dis. 1991;13(suppl 1):S26–S31. doi: 10.1093/clinids/13.supplement_1.s26. [DOI] [PubMed] [Google Scholar]
- 20.Selin LK, Nahill SR, Welsh RM. Cross-reactivities in memory cytotoxic T lymphocyte recognition of heterologous viruses. J Exp Med. 1994;179:1933–43. doi: 10.1084/jem.179.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002 Jul;3(7):627–34. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 22.Selin LK, Vergilis K, Welsh RM, Nahill SR. Reduction of otherwise remarkably stable virus-specific cytotoxic T lymphocyte memory by heterologous viral infections. J Exp Med. 1996;183:2489–99. doi: 10.1084/jem.183.6.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selin LK, Lin MY, Kraemer KA, Schneck JP, Pardoll D, Varga SM, Santolucito PA, Pinto AK, Welsh RM. Attrition of T cell memory:selective loss of lymphocytic choriomeningitis virus (LCMV) epitope-specific memory CD8 T cells following infections with heterologous viruses. Immunity. 1999;11:733–42. doi: 10.1016/s1074-7613(00)80147-8. [DOI] [PubMed] [Google Scholar]
- 24.Selin LK, Varga SM, Wong IC, Welsh RM. Protective heterologous antiviral immunity and enhanced immunopathogenesis mediated by memory T cell populations. J Exp Med. 1998;188:1705–15. doi: 10.1084/jem.188.9.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Welsh RM, Selin LK. No one is naive: the significance of heterologous T-cell immunity. Nat Rev Immunol. 2002 Jun;2(6):417–26. doi: 10.1038/nri820. [DOI] [PubMed] [Google Scholar]
- 26.Rock KL, York IA, Saric T, Goldberg AL. Protein degradation and the generation of MHC class I-presented peptides. Adv Immunol. 1903 Feb;80:1–70. doi: 10.1016/s0065-2776(02)80012-8. JID - 0370425. [DOI] [PubMed] [Google Scholar]
- 27.Doherty PC, Zinkernagel RM. T-cell-mediated immunopathology in viral infections. Transplant Rev. 1974;19:89–120. doi: 10.1111/j.1600-065x.1974.tb00129.x. [DOI] [PubMed] [Google Scholar]
- 28.Marshall DR, Turner SJ, Belz GT, Wingo S, Andreansky S, Sangster Riberdy JM, Liu T, Tan M, Doherty PC. Measuring the diaspora for virus-specific CD8+ T cells. Proc Natl Acad Sci USA. 2001;98:6313–8. doi: 10.1073/pnas.101132698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat Immunol. 2001;2:415–22. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mercado R, Vijh S, Allen SE, Kerksiek K, Pilip IM, Pamer EG. Early programming of T cell populations responding to bacterial infection. J Immunol. 2000;165:6833–9. doi: 10.4049/jimmunol.165.12.6833. [DOI] [PubMed] [Google Scholar]
- 31.van Stipdonk MJ, Lemmens EE, Schoenberger SP. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat Immunol. 2001;2:423–9. doi: 10.1038/87730. [DOI] [PubMed] [Google Scholar]
- 32.Borrow P, Tishon A, Lee S, Xu J, Grewal IS, Oldstone MB, Flavell RA. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–42. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006 Jun;441(7095):890–3. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.York IA, Brehm MA, Zendzian S, Towne CF, Rock KL. Endoplasmic reticulum aminopeptidase 1 (ERAP1) trims MHC class I-presented peptides in vivo and plays an important role in immunodominance. Proc Natl Acad Sci U S A. 2006 Jun;103(24):9202–7. doi: 10.1073/pnas.0603095103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Razvi ES, Welsh RM, McFarland HI. In vivo state of antiviral CTL precursors: characterization of a cycling population containing CTL precursors in immune mice. J Immunol. 1995;154:620–32. [PubMed] [Google Scholar]
- 36.Selin LK, Welsh RM. Cytolytically active memory CTL present in lymphocytic choriomeningitis virus (LCMV)-immune mice after clearance of virus infection. J Immunol. 1997;158:5366–73. [PubMed] [Google Scholar]
- 37.Zimmermann C, Brduscha-Riem K, Blaser C, Zinkernagel RM, Pircher H. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J Exp Med. 1996;183:1367–75. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McNally JM, Zarozinski CC, Lin MY, Brehm MA, Chen HD, Welsh RM. Attrition of bystander CD8 T cells during virus-induced T cell and interferon responses. J Virol. 2001;75:5965–76. doi: 10.1128/JVI.75.13.5965-5976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tumpey TM, Lu X, Morken T, Zaki SR, Katz JM. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000 Jul;74(13):6105–16. doi: 10.1128/jvi.74.13.6105-6116.2000. JID - 0113724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geisbert TW, Hensley LE, Gibb TR, Steele KE, Jaax NK, Jahrling PB. Apoptosis induced in vitro and in vivo during infection by Ebola and Marburg viruses. Lab Invest. 2000 Feb;80(2):171–86. doi: 10.1038/labinvest.3780021. JID - 0376617. [DOI] [PubMed] [Google Scholar]
- 41.Dietz WHJ, Peralta PH, Johnson KM. Ten clinical cases of human infection with venezuelan equine encephalomyelitis virus, subtype I-D. Am J Trop Med Hyg. 1979 Mar;28(2):329–34. doi: 10.4269/ajtmh.1979.28.329. JID - 0370507. [DOI] [PubMed] [Google Scholar]
- 42.Bahl K, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Selin LK, Welsh RM. IFN-induced attrition of CD8 T cells in the presence or absence of cognate antigen during the early stages of viral infections. J Immunol. 2006 Apr;176(7):4284–95. doi: 10.4049/jimmunol.176.7.4284. [DOI] [PubMed] [Google Scholar]
- 43.Jiang J, Gross D, Nogusa S, Elbaum P, Murasko DM. Depletion of T cells by type I interferon: differences between young and aged mice. J Immunol. 2005 Aug;175(3):1820–6. doi: 10.4049/jimmunol.175.3.1820. [DOI] [PubMed] [Google Scholar]
- 44.Jiang J, Lau LL, Shen H. Selective depletion of nonspecific T cells during the early stage of immune responses to infection. J Immunol. 2003 Oct;171(8):4352–8. doi: 10.4049/jimmunol.171.8.4352. [DOI] [PubMed] [Google Scholar]
- 45.Selin LK, Cornberg M, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM. CD8 memory T cells: cross-reactivity and heterologous immunity. Semin Immunol. 2004 Oct;16(5):335–47. doi: 10.1016/j.smim.2004.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Selin LK, Welsh RM. Plasticity of T cell memory responses to viruses. Immunity. 2004 Jan;20(1):5–16. doi: 10.1016/S1074-7613(03)00356-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim SK, Welsh RM. Comprehensive early and lasting loss of memory CD8 T cells and functional memory during acute and persistent viral infections. J Immunol. 2004 Mar;172(5):3139–50. doi: 10.4049/jimmunol.172.5.3139. [DOI] [PubMed] [Google Scholar]
- 48.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 49.Clute SC, Watkin LB, Cornberg M, Naumov YN, Sullivan JL, Luzuriaga K, Welsh RM, Selin LK. Cross-reactive influenza virus-specific CD8+ T cells contribute to lymphoproliferation in Epstein-Barr virus-associated infectious mononucleosis. J Clin Invest. 2005 Dec;115(12):3602–12. doi: 10.1172/JCI25078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mason D. A very high level of crossreactivity is an essential feature of the T cell repertoire. Immunol Today. 1998;19:395–404. doi: 10.1016/s0167-5699(98)01299-7. [DOI] [PubMed] [Google Scholar]
- 51.Wilson DB, Wilson DH, Schroder K, Pinilla C, Blondelle S, Houghten RA, Garcia KC. Specificity and degeneracy of T cells. Mol Immunol. 2004 Feb;40(14-15):1047–55. doi: 10.1016/j.molimm.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 52.Selin LK, Brehm MA. Frontiers in nephrology: heterologous immunity, T cell cross-reactivity, and alloreactivity. J Am Soc Nephrol. 2007 Aug;18(8):2268–77. doi: 10.1681/ASN.2007030295. [DOI] [PubMed] [Google Scholar]
- 53.Welsh RM, Che JW, Brehm MA, Selin LK. Heterologous immunity between viruses. Immunol Rev. 2010 May;235(1):244–66. doi: 10.1111/j.0105-2896.2010.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Welsh RM, Selin LK, Szomolanyi-Tsuda E. Immunological memory to viral infections. Annu Rev Immunol. 2004;22:711–43. doi: 10.1146/annurev.immunol.22.012703.104527. [DOI] [PubMed] [Google Scholar]
- 55.Acierno PM, Newton DA, Brown EA, Maes LA, Baatz JE, Gattoni-Celli S. Cross-reactivity between HLA-A2-restricted FLU-M1:58-66 and HIV p17 GAG:77-85 epitopes in HIV-infected and uninfected individuals. J Transl Med. 2003;1:3–7. doi: 10.1186/1479-5876-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nilges K, Hohn H, Pilch H, Neukirch C, Freitag K, Talbot PJ, Maeurer MJ. Human papillomavirus type 16 E7 peptide-directed CD8+ T cells from patients with cervical cancer are cross-reactive with the coronavirus NS2 protein. J Virol. 2003 May;77(9):5464–74. doi: 10.1128/JVI.77.9.5464-5474.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frankild S, De Boer RJ, Lund O, Nielsen M, Kesmir C. Amino acid similarity accounts for T cell cross-reactivity and for “holes” in the T cell repertoire. PLoS ONE. 2008;3(3):e1831. doi: 10.1371/journal.pone.0001831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bahl K, Huebner A, Davis RJ, Welsh RM. Analysis of apoptosis of memory T cells and dendritic cells during the early stages of viral infection or exposure to toll-like receptor agonists. J Virol. 2010 May;84(10):4866–77. doi: 10.1128/JVI.02571-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van der Most RG, Sette A, Oseroff C, Alexander J, Murali-Krishna K, Lau LL, Southwood S, Sidney J, Chesnut RW, Matloubian M, Ahmed R. Analysis of cytotoxic T cell responses to dominant and subdominant epitopes during acute and chronic lymphocytic choriomeningitis virus infection. J Immunol. 1996;157(12):5543–54. [PubMed] [Google Scholar]
- 60.Whitton JL, Southern PJ, Oldstone MBA. Analyses of the cytotoxic T lymphocyte responses to glycoprotein and nucleoprotein components of lymphocytic choriomeningitis virus. Virol. 1988;162:321–7. doi: 10.1016/0042-6822(88)90471-0. [DOI] [PubMed] [Google Scholar]
- 61.Chen W, Anton LC, Bennink JR, Yewdell JW. Dissecting the multifactorial causes of immunodominace in class I-restricted T cell responses to viruses. Immunity. 2000;12:83–93. doi: 10.1016/s1074-7613(00)80161-2. [DOI] [PubMed] [Google Scholar]
- 62.Vitiello A, Yuan L, Chesnut RW, Sidney J, Southwood S, Farness P, Jackson MR, Peterson PA, Sette A. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two dominant and subdominant Kb-restricted epitopes. J Immunol. 1996;157:5555–62. [PubMed] [Google Scholar]
- 63.Maryanski JL, Attuil V, Hamrouni A, Mutin M, Rossi M, Aublin A, Bucher P. Individuality of Ag-selected and preimmune TCR repertoires. Immunol Res. 2001;23(1):75–84. doi: 10.1385/IR:23:1:75. [DOI] [PubMed] [Google Scholar]
- 64.Lin MY, Welsh RM. Stability and diversity of T cell receptor (TCR) repertoire usage during lymphocytic choriomeningitis virus infection of mice. J Exp Med. 1998;188:1993–2005. doi: 10.1084/jem.188.11.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Blattman JN, Sourdive DJ, Murali-Krishna K, Ahmed R, Altman JD. Evolution of the T cell repertoire during primary, memory, and recall responses to viral infection. J Immunol. 2000;165:6081–90. doi: 10.4049/jimmunol.165.11.6081. [DOI] [PubMed] [Google Scholar]
- 66.Turner SJ, Kedzierska K, Komodromou H, La Gruta NL, Dunstone MA, Webb AI, Webby R, Walden H, Xie W, McCluskey J, Purcell AW, Rossjohn J, Doherty PC. Lack of prominent peptide-major histocompatibility complex features limits repertoire diversity in virus-specific CD8+ T cell populations. Nat Immunol. 2005 Apr;6(4):382–9. doi: 10.1038/ni1175. [DOI] [PubMed] [Google Scholar]
- 67.Cornberg M, Chen AT, Wilkinson LA, Brehm MA, Kim SK, Calcagno C, Ghersi D, Puzone R, Celada F, Welsh RM, Selin LK. Narrowed TCR repertoire and viral escape as a consequence of heterologous immunity. J Clin Invest. 2006 May;116(5):1443–56. doi: 10.1172/JCI27804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim SK, Cornberg M, Wang XZ, Chen HD, Selin LK, Welsh RM. Private specificities of CD8 T cell responses control patterns of heterologous immunity. J Exp Med. 2005 Feb;201(4):523–33. doi: 10.1084/jem.20041337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nie S, Lin SJ, Kim SK, Welsh RM, Selin LK. Pathological Features of Heterologous Immunity Are Regulated by the Private Specificities of the Immune Repertoire. Am J Pathol. 2010 Mar;176(5):2107–12. doi: 10.2353/ajpath.2010.090656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urbani S, Amadei B, Fisicaro P, Pilli M, Missale G, Bertoletti A, Ferrari C. Heterologous T cell immunity in severe hepatitis C virus infection. J Exp Med. 2005;201:675–80. doi: 10.1084/jem.20041058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A, Sawasdivorn S, Duangchinda T, Dong T, Rowland-Jones S, Yenchitsomanus PT, McMichael A, Malasit P, Screaton G. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med. 2003 Jul;9(7):921–7. doi: 10.1038/nm887. [DOI] [PubMed] [Google Scholar]
- 72.Kasprowicz V, Ward SM, Turner A, Grammatikos A, Nolan BE, Lewis-Ximines L, Sharp C, Woodfruff J, Fleming VM, Sims S, Walker BD, Sewell AK, Lauer GM, Klenerman P. Defining the directionality and quality of influenza virus-specific CD8 T cell cross-reactivity in individuals infected with hepatitis C virus. J Clin Invest. 2008 Feb; doi: 10.1172/JCI33082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Page KR, Scott AL, Manabe YC. The expanding realm of heterologous immunity: friend or foe? Cell Microbiol. 2006 Feb;8(2):185–96. doi: 10.1111/j.1462-5822.2005.00653.x. [DOI] [PubMed] [Google Scholar]
- 74.Chen HD, Fraire AE, Joris I, Welsh RM, Selin LK. Specific history of heterologous virus infections determines anti-viral immunity and immunopathology in the lung. Am J Pathol. 2003 Oct;163(4):1341–55. doi: 10.1016/S0002-9440(10)63493-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cornberg M, Clute SC, Watkin LB, Saccoccio FM, Kim SK, Naumov YN, Brehm MA, Aslan N, Welsh RM, Selin LK. CD8 T cell cross-reactivity networks mediate heterologous immunity in human EBV and murine vaccinia virus infections. J Immunol. 2010 Mar;184(6):2825–38. doi: 10.4049/jimmunol.0902168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen HD, Fraire AE, Joris I, Brehm MA, Welsh RM, Selin LK. Memory CD8+ T cells in heterologous antiviral immunity and immunopathology in the lung. Nat Immunol. 2001;2:1067–76. doi: 10.1038/ni727. [DOI] [PubMed] [Google Scholar]
- 77.Nie S, Cornberg M, Selin LK. Resistance to Vaccinia Virus Is Less Dependent on TNF under Conditions of Heterologous Immunity. J Immunol. 2009 Oct;183(10):6554–60. doi: 10.4049/jimmunol.0902156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kim SK, Brehm MA, Welsh RM, Selin LK. Dynamics of memory T cell proliferation under conditions of heterologous immunity and bystander stimulation. J Immunol. 2002 Jul;169(1):90–8. doi: 10.4049/jimmunol.169.1.90. [DOI] [PubMed] [Google Scholar]
- 79.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3:627–34. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 80.Cornberg M, Sheridan BS, Saccoccio FM, Brehm MA, Selin LK. Protection against vaccinia virus challenge by CD8 memory T cells resolved by molecular mimicry. J Virol. 2007 Jan;81(2):934–44. doi: 10.1128/JVI.01280-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cole GA, Nathanson N, Prendergast RA. Requirement for θ-bearing cells in lymphocytic choriomeningitis virus-induced central nervous system disease. Nature. 1972;238:335–7. doi: 10.1038/238335a0. [DOI] [PubMed] [Google Scholar]
- 82.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiological study of altered clinical reactivity to respiratory syncitial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405–21. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 83.Cannon MJ, Openshaw PJM, Askonas BA. Cytotoxic T cells clear virus but augment lung pathology in mice infected with respiratory syncytial virus. J Exp Med. 1988;168:1163–8. doi: 10.1084/jem.168.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88:1026–33. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moskophidis D, Kioussis D. Contribution of virus-specific CD8+ cytotoxic T cells to virus clearance or pathologic manifestations of influenza virus infection in a T cell receptor transgenic mouse model. J Exp Med. 1998;188:223–32. doi: 10.1084/jem.188.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sloan-Lancaster J, Allen PM. Altered peptide ligand-induced partial T cell activation: Molecular mechanisms and role in T cell biology. Annu Rev Immunol. 1996;14:1–27. doi: 10.1146/annurev.immunol.14.1.1. [DOI] [PubMed] [Google Scholar]
- 87.Kersh GJ, Kersh EN, Fremont DH, Allen PM. High- and low-potency ligands with similar affinities for the TCR: the importance of kinetics in TCR signaling. Immunity. 1998;9:817–26. doi: 10.1016/s1074-7613(00)80647-0. [DOI] [PubMed] [Google Scholar]
- 88.Ding Y-H, Baker BM, Garboczi DN, Biddison WE, Wiley DC. Four A6-TCR/peptide/HLA-A2 structures that generate very different T cell signals are nearly identical. Immunity. 1999;11:45–56. doi: 10.1016/s1074-7613(00)80080-1. [DOI] [PubMed] [Google Scholar]
- 89.Hemmer B, Stefanova I, Vergelli M, Germain RN, Martin R. Relationships among TCR ligand potency, thresholds for effector function elicitation, and the quality of early signalling events in human T cells. J Immunol. 1998;160:5807–14. [PubMed] [Google Scholar]
- 90.Zehn D, Lee SY, Bevan MJ. Complete but curtailed T-cell response to very low-affinity antigen. Nature. 2009 Mar;458(7235):211–4. doi: 10.1038/nature07657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guy CS, Vignali DA. Organization of proximal signal initiation at the TCR:CD3 complex. Immunol Rev. 2009 Nov;232(1):7–21. doi: 10.1111/j.1600-065X.2009.00843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wedemeyer H, Mizukoshi E, Davis AR, Bennink JR, Rehermann B. Cross-reactivity between hepatitis C virus and influenza A virus determinant-specific cytotoxic T cells. J Virol. 2001;75:11392–400. doi: 10.1128/JVI.75.23.11392-11400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rehermann B, Shin EC. Private aspects of heterologous immunity. J Exp Med. 2005 Mar;201(5):667–70. doi: 10.1084/jem.20050220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zivny J, DeFronzo M, Jarry W, Jameson J, Cruz J, Ennis FA, Rothman AL. Partial agonist effect influences the CTL response to a heterologous dengue virus serotype. J Immunol. 1999 Sep;163(5):2754–60. [PubMed] [Google Scholar]
- 95.Yang H, Joris I, Majno G, Welsh RM. Necrosis of adipose tissue induced by sequential infections with unrelated viruses. Am J Pathol. 1985;120:173–7. [PMC free article] [PubMed] [Google Scholar]
- 96.Yang H, Dundon PL, Nahill SR, Welsh RM. Virus-induced polyclonal cytotoxic T lymphocyte stimulation. J Immunol. 1989;142:1710–8. [PubMed] [Google Scholar]
- 97.Moutaftsi M, Salek-Ardakani S, Croft M, Peters B, Sidney J, Grey H, Sette A. Correlates of protection efficacy induced by vaccinia virus-specific CD8+ T-cell epitopes in the murine intranasal challenge model. Eur J Immunol. 2009 Mar;39(3):717–22. doi: 10.1002/eji.200838815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mathurin KS, Martens GW, Kornfeld H, Welsh RM. CD4 T-cell-mediated heterologous immunity between mycobacteria and poxviruses. J Virol. 2009 Apr;83(8):3528–39. doi: 10.1128/JVI.02393-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ploegh HL. Viral strategies of immune evasion. Science. 1998;280:248–53. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 100.Xiao Z, Casey KA, Jameson SC, Curtsinger JM, Mescher MF. Programming for CD8 T cell memory development requires IL-12 or type I IFN. J Immunol. 2009 Mar;182(5):2786–94. doi: 10.4049/jimmunol.0803484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005 Sep;202(5):637–50. doi: 10.1084/jem.20050821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gilbertson B, Germano S, Steele P, Turner S, Fazekas de St GB, Cheers C. Bystander activation of CD8+ T lymphocytes during experimental mycobacterial infection. Infect Immun. 2004 Dec;72(12):6884–91. doi: 10.1128/IAI.72.12.6884-6891.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003 Nov;198(10):1583–93. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bangs SC, Baban D, Cattan HJ, Li CK, McMichael AJ, Xu XN. Human CD4+ memory T cells are preferential targets for bystander activation and apoptosis. J Immunol. 2009 Feb;182(4):1962–71. doi: 10.4049/jimmunol.0802596. [DOI] [PubMed] [Google Scholar]
- 105.Polley R, Sanos SL, Prickett S, Haque A, Kaye PM. Chronic Leishmania donovani infection promotes bystander CD8+-T-cell expansion and heterologous immunity. Infect Immun. 2005 Dec;73(12):7996–8001. doi: 10.1128/IAI.73.12.7996-8001.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raue HP, Brien JD, Hammarlund E, Slifka MK. Activation of virus-specific CD8+ T cells by lipopolysaccharide-induced IL-12 and IL-18. J Immunol. 2004 Dec;173(11):6873–81. doi: 10.4049/jimmunol.173.11.6873. [DOI] [PubMed] [Google Scholar]
- 107.Bukowski JF, Welsh RM. Enhanced susceptibility to cytotoxic T lymphocytes of target cells isolated from virus-infected or interferon-treated mice. J Virol. 1986;59:735–9. doi: 10.1128/jvi.59.3.735-739.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bukowski JF, Welsh RM. Interferon enhances the susceptibility of virus-infected fibroblasts to cytotoxic T cells. J Exp Med. 1985;161:257–62. doi: 10.1084/jem.161.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu F, Whitton JL. Cutting edge: re-evaluating the in vivo cytokine responses of CD8+ T cells during primary and secondary viral infections. J Immunol. 2005 May;174(10):5936–40. doi: 10.4049/jimmunol.174.10.5936. [DOI] [PubMed] [Google Scholar]
- 110.Haanen JB, Wolkers MC, Kruisbeek AM, Schumacher TN. Selective expansion of cross-reactive CD8(+) memory T cells by viral variants. J Exp Med. 1999 Nov;190(9):1319–28. doi: 10.1084/jem.190.9.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nettesheim P, Szakal AK. Morphogenesis of alveolar bronchiolization. Lab Invest. 1972;26:210–9. [PubMed] [Google Scholar]
- 112.Wlodarczyk MF, Kraft AR, Chen HD, Kenney LL, Selin LK. Pathological consequences of heterologous immunity prevented by inhibition of cross-reactive influenza A specific memory CD8 T-cells. 2010 manuscript submitted. [Google Scholar]
- 113.Varga SM, Wang X, Welsh RM, Braciale TJ. Immunopathology in RSV infection is mediated by a discrete oligoclonal subset of antigen-specific CD4+ T cells. Immunity. 2001;15:637–46. doi: 10.1016/s1074-7613(01)00209-6. [DOI] [PubMed] [Google Scholar]
- 114.Johnson TR, Graham BS. Secreted respiratory syncytial virus G glcoprotein induces interleukin-5 (IL-5), IL-13, and eosinophilia by an IL-4-dependent mechanism. J Virol. 1999;73:8485–95. doi: 10.1128/jvi.73.10.8485-8495.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Walzl G, Tafuro S, Moss P, Openshaw PJ, Hussell T. Influenza virus lung infection protects from respiratory syncitial virus-induced immunopathology. J Exp Med. 2000;192:1317–26. doi: 10.1084/jem.192.9.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aaby P, Samb B, Simondon F, Seck AM, Knudsen K, Whittle H. Non-specific beneficial effect of measles immunisation:analysis of mortality studies from developing countries. Brit Med J. 1995;311:481–5. doi: 10.1136/bmj.311.7003.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Flanagan KL, Klein S, Skakkebaek NE, Marriott I, Marcha A, Selin LK, Fish E, Prentice A, Whittle H, Benn C, Aaby P on behalf of the Optimize Immunization Network (Optimmunize) Sex-specific and non-specific effects of vaccines: do they exist and what should be done? 2010 submitted. [Google Scholar]
- 118.Stensballe LG, Nante E, Jensen IP, Kofoed PE, Poulsen A, Jensen H, Newport M, Marchant A, Aaby P. Acute lower respiratory tract infections and respiratory syncytial virus in infants in Guinea-Bissau: a beneficial effect of BCG vaccination for girls community based case-control study. Vaccine. 2005 Jan;23(10):1251–7. doi: 10.1016/j.vaccine.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 119.Farrington CP, Firth MJ, Moulton LH, Ravn H, Andersen PK, Evans S. Epidemiological studies of the non-specific effects of vaccines: II--methodological issues in the design and analysis of cohort studies. Trop Med Int Health. 2009 Sep;14(9):977–85. doi: 10.1111/j.1365-3156.2009.02302.x. [DOI] [PubMed] [Google Scholar]
- 120.Benn CS, Rodrigues A, Yazdanbakhsh M, Fisker AB, Ravn H, Whittle H, Aaby P. The effect of high-dose vitamin A supplementation administered with BCG vaccine at birth may be modified by subsequent DTP vaccination. Vaccine. 2009 May;27(21):2891–8. doi: 10.1016/j.vaccine.2009.02.080. [DOI] [PubMed] [Google Scholar]
- 121.Aaby P, Biai S, Veirum JE, Sodemann M, Lisse I, Garly ML, Ravn H, Benn CS, Rodrigues A. DTP with or after measles vaccination is associated with increased in-hospital mortality in Guinea-Bissau. Vaccine. 2007 Jan;25(7):1265–9. doi: 10.1016/j.vaccine.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 122.Jennes W, Verheyden S, Demanet C, Adje-Toure CA, Vuylsteke B, Nkengasong JN, Kestens L. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J Immunol. 2006 Nov;177(10):6588–92. doi: 10.4049/jimmunol.177.10.6588. [DOI] [PubMed] [Google Scholar]
- 123.Tillmann HL, Heiken H, Knapik-Botor A, Heringlake S, Ockenga J, Wilber JC, Goergen B, Detmer J, McMorrow M, Stoll M, Schmidt RE, Manns MP. Infection with GB virus C and reduced mortality among HIV-infected patients. N Engl J Med. 2001;345:715–24. doi: 10.1056/NEJMoa010398. [DOI] [PubMed] [Google Scholar]
- 124.Clute SC, Naumov YN, Watkin LB, Aslan N, Sullivan JL, Luzuriaga K, Welsh RM, Puzone R, Celada F, Selin LK. Broad cross-reactive T cell receptor repertoires recognizing dissimilar Epstein-Barr and influenza A virus epitopes. J Immunol. 2010 doi: 10.4049/jimmunol.1000812. under re-vision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Borja-Hart NL, Benavides S, Christensen C. Human papillomavirus vaccine safety in pediatric patients: an evaluation of the Vaccine Adverse Event Reporting System. Ann Pharmacother. 2009 Feb;43(2):356–9. doi: 10.1345/aph.1L492. [DOI] [PubMed] [Google Scholar]
- 126.Debeer P, De MP, Bruyninckx F, Devlieger R. Brachial plexus neuritis following HPV vaccination. Vaccine. 2008 Aug;26(35):4417–9. doi: 10.1016/j.vaccine.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 127.Debold V, Hurwitz E. Adverse events and quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009 Dec;302(24):2657–8. doi: 10.1001/jama.2009.1880. [DOI] [PubMed] [Google Scholar]
- 128.Ojaimi S, Buttery JP, Korman TM. Quadrivalent Human Papillomavirus recombinant vaccine associated lipoatrophy. Vaccine. 2009 Aug;27(36):4876–8. doi: 10.1016/j.vaccine.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 129.Khalifa YM, Monahan PM, Acharya NR. Ampiginous choroiditis following quadrivalent human papilloma virus vaccine. Br J Ophthalmol. 2010 Jan;94(1):137–9. doi: 10.1136/bjo.2009.159293. [DOI] [PubMed] [Google Scholar]
- 130.Slade BA, Leidel L, Vellozzi C, Woo EJ, Hua W, Sutherland A, Izurieta HS, Ball R, Miller N, Braun MM, Markowitz LE, Iskander J. Postlicensure safety surveillance for quadrivalent human papillomavirus recombinant vaccine. JAMA. 2009 Aug;302(7):750–7. doi: 10.1001/jama.2009.1201. [DOI] [PubMed] [Google Scholar]
- 131.Effros RB, Doherty PC, Gerhard W, Bennink J. Generation of both cross-reactive and virus-specific T-cell populations after immunization with serologically distinct influenza A viruses. J Exp Med. 1977 Mar;145(3):557–68. doi: 10.1084/jem.145.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee LY, Ha do LA, Simmons C, de Jong MD, Chau NV, Schumacher R, Peng YC, McMichael AJ, Farrar JJ, Smith GL, Townsend AR, Askonas BA, Rowland-Jones S, Dong T. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008 Oct;118(10):3478–90. doi: 10.1172/JCI32460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Epstein SL. Prior H1N1 influenza infection and susceptibility of Cleveland Family Study participants during the H2N2 pandemic of 1957: an experiment of nature. J Infect Dis. 2006 Jan;193(1):49–53. doi: 10.1086/498980. [DOI] [PubMed] [Google Scholar]
- 134.SLEPUSHKIN AN. The effect of a previous attack of A1 influenza on susceptibility to A2 virus during the 1957 outbreak. Bull World Health Organ. 1959;20(2-3):297–301. [PMC free article] [PubMed] [Google Scholar]
- 135.Moss PAH, Moots RJ, Rosenberg WMC, Rowland-Jones SJ, Bodmer HC, McMichael AJ, Bell JI. Extensive conservation of α and β chains of the human T-cell antigen receptor recognizing HLA-A2 and influenza A matrix peptide. Proc Natl Acad Sci. 1991;88:8987–90. doi: 10.1073/pnas.88.20.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Naumov YN, Naumova EN, Yassai MB, Kota K, Welsh RM, Selin LK. Multiple glycines in TCR alpha-chains determine clonally diverse nature of human T cell memory to influenza A virus. J Immunol. 2008 Nov;181(10):7407–19. doi: 10.4049/jimmunol.181.10.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Weinstein L, Meade RH. Respiratory manifestations of chickenpox. Arch Intern Med. 1956;98:91–9. doi: 10.1001/archinte.1956.00250250097013. [DOI] [PubMed] [Google Scholar]
- 138.Rickinson AB, Kieff E. Epstein-Barr virus. In: Fields BN, Knipe DM, Howley PM, Chanock RM, Melnick JL, Monath TP, Roizman B, Straus SS, editors. Virology. Vol. 2. Philadelphia: Lippincott-Raven Publishers; 1996. pp. 2397–446. [Google Scholar]
- 139.Silins SL, Sherritt MA, Silleri JM, Cross SM, Elliott SL, Bharadwaj M, Le TT, Morrison LE, Khanna R, Moss DJ, Suhrbier A, Misko IS. Asymptomatic primary Epstein-Barr virus infection occurs in the absence of blood T-cell repertoire perturbations despite high levels of systemic viral load. Blood. 2001 Dec;98(13):3739–44. doi: 10.1182/blood.v98.13.3739. [DOI] [PubMed] [Google Scholar]
- 140.Morens DM. Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin Infect Dis. 1994 Sep;19(3):500–12. doi: 10.1093/clinids/19.3.500. [DOI] [PubMed] [Google Scholar]
- 141.Halstead SB. Antibody, macrophages, dengue virus infection, shock and hemorrhage: a pathogenetic cascade. Rev Infect Dis. 1989;11:S830–S839. doi: 10.1093/clinids/11.supplement_4.s830. [DOI] [PubMed] [Google Scholar]
- 142.Matthew A, Kurane I, Green S, Stephens HAF, Vaughn DW, Kalayanarooj S, Suntayakorn S, Chandanayingyong D, Ennis FA, Rothman AL. Predominance of HLA-restricted cytotoxic T-lymphocyte responses to serotype-cross-reactive epitopes on nonstructural proteins following natural secondary dengue virus infection. J Virol. 1998;72:3999–4004. doi: 10.1128/jvi.72.5.3999-4004.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suri-Payer E, Fritzsching B. Regulatory T cells in experimental autoimmune disease. Springer Semin Immunopathol. 2006 Aug;28(1):3–16. doi: 10.1007/s00281-006-0021-8. [DOI] [PubMed] [Google Scholar]
- 144.Sakaguchi S, Ono M, Setoguchi R, Yagi H, Hori S, Fehervari Z, Shimizu J, Takahashi T, Nomura T. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006 Aug;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 145.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008 May;133(5):775–87. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 146.Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol. 2009;27:313–38. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- 147.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003 Apr;170(8):3939–43. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 148.Morgan ME, Sutmuller RP, Witteveen HJ, van Duivenvoorde LM, Zanelli E, Melief CJ, Snijders A, Offringa R, de Vries RR, Toes RE. CD25+ cell depletion hastens the onset of severe disease in collagen-induced arthritis. Arthritis Rheum. 2003 May;48(5):1452–60. doi: 10.1002/art.11063. [DOI] [PubMed] [Google Scholar]
- 149.Lindley S, Dayan CM, Bishop A, Roep BO, Peakman M, Tree TI. Defective suppressor function in CD4(+)CD25(+) T-cells from patients with type 1 diabetes. Diabetes. 2005 Jan;54(1):92–9. doi: 10.2337/diabetes.54.1.92. [DOI] [PubMed] [Google Scholar]
- 150.Mittrucker HW, Kaufmann SH. Mini-review: regulatory T cells and infection: suppression revisited. Eur J Immunol. 2004 Feb;34(2):306–12. doi: 10.1002/eji.200324578. [DOI] [PubMed] [Google Scholar]
- 151.Rouse BT, Sarangi PP, Suvas S. Regulatory T cells in virus infections. Immunol Rev. 2006 Aug;212:272–86. doi: 10.1111/j.0105-2896.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- 152.Belkaid Y, Tarbell K. Regulatory T cells in the control of host-microorganism interactions (*) Annu Rev Immunol. 2009;27:551–89. doi: 10.1146/annurev.immunol.021908.132723. [DOI] [PubMed] [Google Scholar]
- 153.Zelinskyy G, Dietze KK, Husecken YP, Schimmer S, Nair S, Werner T, Gibbert K, Kershaw O, Gruber AD, Sparwasser T, Dittmer U. The regulatory T-cell response during acute retroviral infection is locally defined and controls the magnitude and duration of the virus-specific cytotoxic T-cell response. Blood. 2009 Oct;114(15):3199–207. doi: 10.1182/blood-2009-03-208736. [DOI] [PubMed] [Google Scholar]
- 154.Zelinskyy G, Kraft AR, Schimmer S, Arndt T, Dittmer U. Kinetics of CD8+ effector T cell responses and induced CD4+ regulatory T cell responses during Friend retrovirus infection. Eur J Immunol. 2006 Oct;36(10):2658–70. doi: 10.1002/eji.200636059. [DOI] [PubMed] [Google Scholar]
- 155.Suvas S, Kumaraguru U, Pack CD, Lee S, Rouse BT. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J Exp Med. 2003 Sep;198(6):889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Suvas S, Azkur AK, Kim BS, Kumaraguru U, Rouse BT. CD4+CD25+ regulatory T cells control the severity of viral immunoinflammatory lesions. J Immunol. 2004 Apr;172(7):4123–32. doi: 10.4049/jimmunol.172.7.4123. [DOI] [PubMed] [Google Scholar]
- 157.Zhao Z-S, Granucci F, Yeh L, Schaffer PA, Cantor H. Molecular mimicry by herpes simplex virus-type 1: autoimmune disease after viral infection. Science. 1998;279:1344–7. doi: 10.1126/science.279.5355.1344. [DOI] [PubMed] [Google Scholar]
- 158.Hiemstra HS, Schloot NC, van Veelen PA, Willemen SJ, Franken KL, van Rood JJ, de Vries RR, Chaudhuri A, Behan PO, Drijfhout JW, Roep BO. Cytomegalovirus in autoimmunity: T cell crossreactivity to viral antigen and autoantigen glutamic acid decarboxylase. Proc Natl Acad Sci U S A. 2001 Mar;98(7):3988–91. doi: 10.1073/pnas.071050898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Oldstone MB, Nerenberg M, Southern P, Price J, Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991;65(2):319–31. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 160.Evans CF, Horwitz MS, Hobbs MV, Oldstone MBA. Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease. J Exp Med. 1996;184:2371–84. doi: 10.1084/jem.184.6.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kagi D, Odermatt B, Ohashi PS, Zinkernagel RM, Hengartner H. Development of insulitis without diabetes in transgenic mice lacking perforin-dependent cytotoxicity. J Exp Med. 1996;183(5):2143–52. doi: 10.1084/jem.183.5.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Christen U, Edelmann KH, McGavern DB, Wolfe T, Coon B, Teague MK, Miller SD, Oldstone MB, von Herrath MG. A viral epitope that mimics a self antigen can accelerate but not initiate autoimmune diabetes. J Clin Invest. 2004;114:1290–8. doi: 10.1172/JCI22557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.McCoy L, Tsunoda I, Fujinami RS. Multiple sclerosis and virus induced immune responses: autoimmunity can be primed by molecular mimicry and augmented by bystander activation. Autoimmunity. 2006 Feb;39(1):9–19. doi: 10.1080/08916930500484799. [DOI] [PubMed] [Google Scholar]
- 164.Theil DJ, Tsunoda I, Rodriguez F, Whitton JL, Fujinami RS. Viruses can silently prime for and trigger central nervous system autoimmune disease. J Neurovirol. 2001 Jun;7(3):220–7. doi: 10.1080/13550280152403263. [DOI] [PubMed] [Google Scholar]
- 165.Sospedra M, Zhao Y, zur HH, Muraro PA, Hamashin C, de Villiers EM, Pinilla C, Martin R. Recognition of conserved amino acid motifs of common viruses and its role in autoimmunity. PLoS Pathog. 2005 Dec;1(4):e41. doi: 10.1371/journal.ppat.0010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Welsh RM, Rothman AL. Dengue immune response: low affinity, high febrility. Nature Med. 2003;9:820–2. doi: 10.1038/nm0703-820. [DOI] [PubMed] [Google Scholar]
- 167.Barton ES, White DW, Cathelyn JS, Brett-McClellan KA, Engle M, Diamond MS, Miller VL, Virgin HW. Herpesvirus latency confers symbiotic protection from bacterial infection. Nature. 2007 May;447(7142):326–9. doi: 10.1038/nature05762. [DOI] [PubMed] [Google Scholar]
- 168.Di Giusto CA, Bernhard JD. Erythema nodosum provoked by hepatitis B vaccine. Lancet. 1986 Nov;2(8514):1042. doi: 10.1016/s0140-6736(86)92654-1. [DOI] [PubMed] [Google Scholar]
- 169.Cheng C, Gall JG, Nason M, King CR, Koup RA, Roederer M, McElrath MJ, Morgan CA, Churchyard G, Baden LR, Duerr AC, Keefer MC, Graham BS, Nabel GJ. Differential specificity and immunogenicity of adenovirus type 5 neutralizing antibodies elicited by natural infection or immunization. J Virol. 2010 Jan;84(1):630–8. doi: 10.1128/JVI.00866-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.DiMario FJ, Jr, Hajjar M, Ciesielski T. A 16-year-old girl with bilateral visual loss and left hemiparesis following an immunization against human papilloma virus. J Child Neurol. 2010 Mar;25(3):321–7. doi: 10.1177/0883073809349322. [DOI] [PubMed] [Google Scholar]
- 171.Williams OM, Hart KW, Wang EC, Gelder CM. Analysis of CD4(+) T-cell responses to human papillomavirus (HPV) type 11 L1 in healthy adults reveals a high degree of responsiveness and cross-reactivity with other HPV types. J Virol. 2002 Aug;76(15):7418–29. doi: 10.1128/JVI.76.15.7418-7429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. doi: 10.1126/science.272.5258.54. [DOI] [PubMed] [Google Scholar]


