Abstract
The amount of dopamine release from terminals in the forebrain following an electrical stimulus is variable. This dynamic regulation, both between and within trains of electrical stimuli, has fostered the notion that burst firing of dopamine neurons in vivo may be a determinant of dopamine release in projection areas. In the present study dendritic dopamine release was examined in the substantia nigra and ventral tegmental area in mouse brain slices using whole-cell recording of a dopamine-mediated inhibitory postsynaptic current (IPSC). Paired stimuli produced a depression of the IPSC that was not observed with paired pulses of exogenously applied dopamine. Increasing the number of electrical stimuli from one to five produced an increase in the amplitude the dopamine IPSC but the increase was less than additive, indicating a depression of transmission with each successive stimulus. Analysis with fast-scan cyclic voltammetry demonstrated that presynaptic D2-autoreceptors did not contribute to the depression. Facilitation of the IPSC was observed only after the probability of release was reduced. Thus the regulation of dopamine release in the cell body region was dependent on dopamine neuron impulse activity. Under circumstance where there was initially little activity the probability of dopamine release was high and repetitive activation resulted in depression of further release. With increased activity, the release probability decreased and a burst of activity caused a relative facilitation of dopamine release. This form of regulation would be expected to limit activity within the cell body region.
Keywords: burst, depression, mouse, paired-pulse, substantia nigra
Introduction
Dopamine neurons of the ventral midbrain play a crucial role in many physiological processes including learning and memory, initiation of movement, and signalling of natural rewards. In addition to terminal release, dopamine is also released from the cell bodies and dendrites of the substantia nigra and ventral tegmental area (Bjorklund & Lindvall, 1975; Geffen et al., 1976; Kalivas & Duffy, 1991; Rice et al., 1997; Jaffe et al., 1998; Hoffman & Gerhardt, 1999). Dopamine neurons make dendrodendritic synapses (Nirenberg et al., 1996; Wilson et al., 1977), and dopamine released from somatodendritic regions activates D2 receptors that can have a powerful feedback inhibitory effect on neuronal excitability (Lacey et al., 1987; Pucak & Grace, 1994; Beckstead et al., 2004). The activation of D2 receptors increases a G-protein-coupled potassium (GIRK) conductance and decreases a voltage-sensitive calcium conductance (Bigornia et al., 1990; Lacey et al., 1987), either of which could inhibit dopamine release. The time course and amplitude of the dopamine-dependent inhibitory postsynaptic current (IPSC) suggests that dendrodendritic dopamine transmission may play a role in terminating a burst of action potentials and creating the pause in firing typically observed after a burst (Beckstead et al., 2004).
In vivo, dopamine neurons exhibit two distinct discharge activity patterns: intermittent single spikes, or bursts of 2–6 consecutive action potentials commonly followed by a pause (Grace & Bunney, 1984). Bursting is thought to be a more efficient mode for increasing extracellular dopamine in terminals as it allows summation of the concentration for each pulse, as there is limited time for uptake between pulses (Chergui et al., 1994). In addition, there are adaptive processes in terminals that dynamically alter dopamine release during and between bursts of action potentials (Yavich & MacDonald, 2000; Cragg, 2003; Montague et al., 2004). However, the physiological consequences of multiple action potentials have not been investigated in areas that contain dopamine cell bodies.
In this report, dopamine-mediated IPSCs were recorded from dopamine neurons in horizontal mouse midbrain slices. Marked depression of IPSCs was observed with paired stimuli as well as pairs of burst-type stimuli, whereas facilitation was observed under circumstances where the probability of release was previously reduced. The results suggest that facilitation and depression of dopamine release in the cell body region are plastic and may be regulated by the moment-to-moment activity of afferent input. The identification of short-term presynaptic plasticity, together with the recent observation of long-term activity-dependent postsynaptic inhibition of the D2 autoreceptors (Beckstead & Williams, 2007), extends the known mechanisms that regulate dopamine-dependent transmission in the midbrain.
Materials and methods
Whole-cell patch-clamp electrophysiology
All animal protocols used in this study were approved by the animal care and use committee at Oregon Health & Science University and comply with NIH guidelines. Male and female C57Bl6J mice were obtained from Jackson Laboratories (Bar Harbour, ME, USA). Brain slices from their male and female first-generation offspring were obtained as described previously (Williams et al., 1984; Beckstead et al., 2004). Briefly, young adult mice (range 32–170 days old) were killed by decapitation under halothane anaesthesia. Brains were quickly removed and placed in a vibrating microtome filled with 0 °C physiological saline (containing, in mm: NaCl, 126; KCl, 2.5; MgCl2, 1.2; CaCl2, 2.4; NaH2PO4, 1.4; NaHCO3, 25; and d-glucose, 11). The ventral midbrain was cut horizontally in 220-μm-thick slices which were then incubated at 35 °C for at least 30 min. Slices were mounted in a recording chamber attached to an upright microscope (Carl Zeiss, Oberkochen, Germany) and superfused with 35 °C saline at a rate of 1.5 mL/min. Dopamine neurons were identified from known anatomical markers with the assistance of infrared illumination optics and a video camera. Cells were whole-cell patch-clamped with low resistance microelectrodes (1.5–2.0 MΩ) containing (in mm) K-methylsulphate, 115; NaCl, 20; MgCl2, 1.5; ATP, 2; GTP, 0.2; phosphocreatine, 10; and BAPTA, 10; pH 7.30–7.35, 274–280 mOsm. Cells were voltage-clamped at –60 mV to prevent spontaneous firing and permit measure of the GIRK conductance. Series resistance was compensated by 80% and monitored frequently to ensure stable electrical access to the inside of the neuron.
A bipolar platinum stimulating electrode was placed into the slice just caudal to the cell being monitored. Dopamine IPSCs were evoked by passing a train of 1–5 stimuli, spaced out at 80-ms intervals once every 50–60 s, in the presence of receptor blockers: prazosin (50 nm; α1 adrenergic), CGP 56999a (100 nm; GABAB), picrotoxin (100 μm; GABAA), MK-801 (10 μm; NMDA), hexamethonium (50 μm; nicotinic acetylcholine) and NBQX (5 μm) or DNQX (10 μm; AMPA). In two experiments dopamine IPSCs were evoked with five stimuli applied at shorter (10–25 ms) intervals. D2 receptor-mediated currents were also obtained by iontophoresis of dopamine. A thin-walled microelectrode was pulled to have a resistance of ~ 100 MΩ, filled with 1 m dopamine and placed within 10 μm of the cell being recorded. Dopamine was ejected as a cation with a single iontophoretic pulse of +20–150 nA for 20–100 ms. Leakage was prevented with constant application of a small negative back current (1.0–5.0 nA). Data were recorded through an Axopatch 1D amplifier (Axon Instruments, Foster City, CA, USA), stored on a personal computer running Axograph 4.5 (John Clements), and analysed off-line at a later time. One- and two-way anovas were used to compare experimental groups, and statistical significance was defined with α = 0.05.
Chemicals and reagents were purchased from Sigma-Aldrich (St Louis, MO, USA) with few exceptions. K-methylsulphate was from Pfaltz & Bauer (Waterbury, CT, USA). CGP 56999a was a gift from Novartis Pharmaceuticals (Basel, Switzerland). Forskolin was purchased from Calbiochem (La Jolla, CA, USA).
Fast-scan cyclic voltammetry
Glass-encased carbon fibre electrodes were prepared from carbon fibers (7 μm diameter; 34-700; Goodfellow, PA, USA) and cut to an exposed final length of 30–100 μm. Carbon fibre electrodes were placed ~ 100 μm below the surface of the slice in the medial portion of the VTA. Voltammetric recordings were performed with a triangular waveform (–0.4 to –1.0 V vs. Ag/AgCl, 300 V/s) at 10 Hz while holding the electrode at –0.4 V between scans. Dopamine release was evoked by three pulses (0.7 ms duration, 200 ms separation) from a monopolar electrode placed ~ 150 μm away from the carbon fibre electrode. Background subtracted cyclic voltammogram currents were obtained by subtracting 10 cyclic voltammograms obtained before stimulation from voltammograms obtained after stimulation. After subtraction, two-dimensional voltammetric colour plots were used to examine the data. To determine the time course of voltammetrically detected dopamine, the current at the peak oxidation (~ +600 mV vs. Ag/Cl) was plotted against time. Peak oxidation and reduction peaks for exogenously iontophoresed dopamine (1 m) correlated with the signal obtained with the three-pulse stimulation protocol used (Fig. 3A, inset). Electrodes were not calibrated to [DA]o, rather values were left as voltammetric current responses.
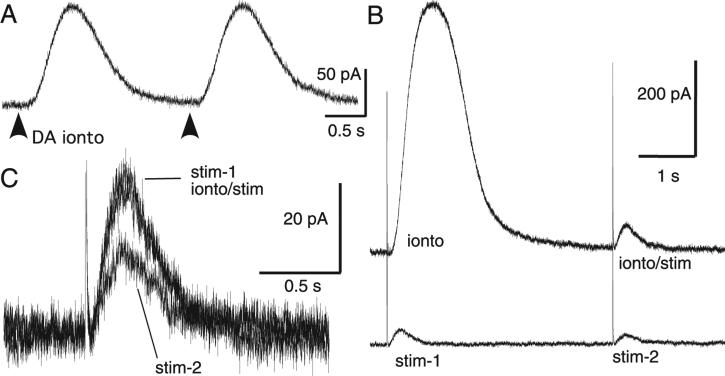
Fig. 3.
Paired-pulse depression was presynaptic. (A) Paired-pulse inhibition was not observed when dopamine (1 m) was applied exogenously through iontophoresis (dark arrows). Furthermore, a large iontophoretic pulse did not depress the dopamine IPSC. (B, bottom trace) Two IPSCs (stim-1 and stim-2) separated by 4 s exhibited paired-pulse depression. (B, top trace) In the same cell a large iontophoretic pulse (ionto) did not depress the dopamine IPSC evoked 4 s later (ionto/stim). (C) IPSC tracings from this experiment are overlaid, showing that paired stimuli produced depression while iontophoresis of dopamine did not.
Results
Presynaptic paired-pulse depression
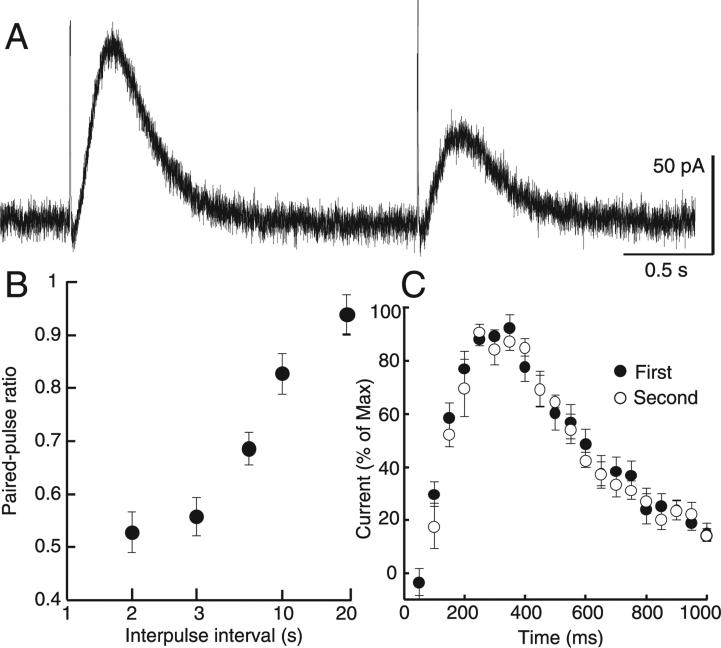
To investigate the probability of dopamine release, IPSCs were evoked using a paired-pulse protocol with an interstimulus interval that varied from 2 to 20 s. With an interval of 2 s the second IPSC was consistently smaller in amplitude, resulting in a paired-pulse ratio of 0.51 ± 0.022 (n = 9; Fig. 1A). This depression subsided when the interpulse interval was increased to 20 s (Fig. 1B), a time course similar to the decrease in dopamine release measured in the striatum using fast-scan cyclic voltammetry (Phillips et al., 2002). Although the second IPSC was always smaller, there was no difference in the kinetics from the first to the second IPSC (Fig. 1C).
Fig. 1.
Paired-pulse depression of dopamine IPSCs. (A) Using a pair of single pulses, the dopamine IPSC consistently exhibited substantial paired-pulse depression. (B) This depression subsided as the interpulse duration was extended from 2 to 20 s (n = 5). (C) Although the first pulse produced a larger amplitude current, there was no difference between the kinetics of the dopamine IPSC subsequent to the first and second pulse when the traces were normalized to their maxima (n = 10).
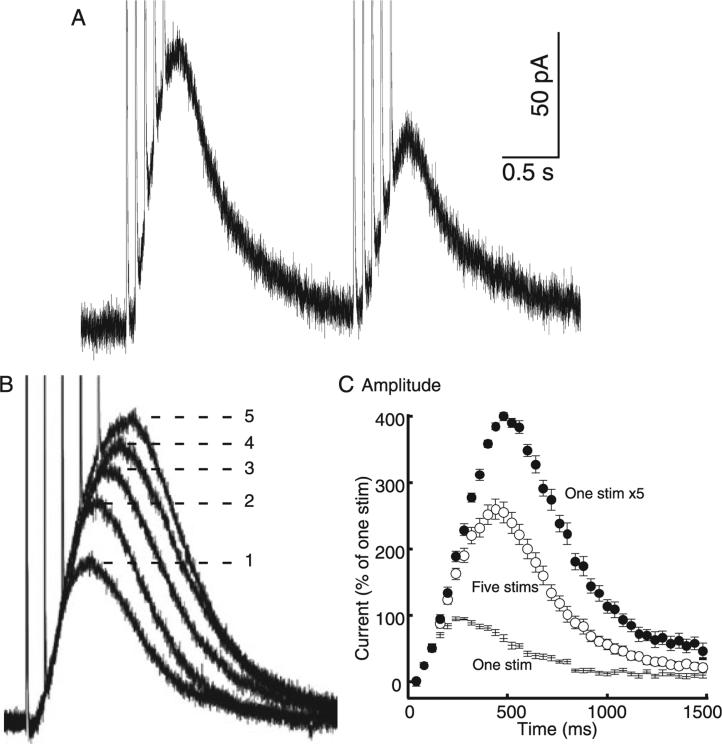
In vivo, excitatory input on to dopamine neurons can produce a burst of several action potentials with an interspike interval of ~60 ms. Burst stimulation (five stimuli at 12.5 Hz) was used to simulate burst firing of dopamine cells and the resultant dopamine IPSC was analysed. This train of stimuli produced an IPSC with a greater amplitude than that produced by a single pulse. Paired-stimulus depression was maintained, although the depression of the second IPSC was not as pronounced as that seen with pairs of single stimuli (a ratio of 0.68 ± 0.018, n = 13; Fig. 2A). To further investigate the effect of burst-type stimulation on the dopamine IPSC, the number of stimuli was varied from one to five. The first stimulus contributed the greatest to the amplitude and area of the IPSC, with subsequent stimuli producing less and less added current (Fig. 2B and C).
Fig. 2.
Depression was observed when multiple stimuli were used to mimic bursting. (A) The paired-pulse depression produced by a pair of single pulses was maintained but somewhat blunted by using a pair of stimulus trains. (B) However, as the number of stimuli was increased from one to five (at 12.5 Hz) each successive pulse produced less postsynaptic current. (C) The convolution can be observed by mathematically simulating perfect temporal summation of five single IPSCs. The measured time course of 11 normalized single pulse IPSCs is plotted as ‘One stim’. Perfect additivity of five pulses (at 12.5 Hz) would produce the simulated time course predicted with the closed circles labelled ‘One stim × 5’. The measured IPSC evoked by a train of five stimuli is considerably smaller (‘Five stims’; F1,20 = 23.5, P < 0.0001, n = 11), illustrating the extent of subadditivity in the IPSC produced by a train of five stimuli.
One possible mechanism to explain the depression could be a postsynaptic depression of the D2-receptor-dependent current. To test this possibility, dopamine was applied using paired iontophoretic applications. The amplitude of the two resulting currents was the same, suggesting that the paired-pulse depression of IPSCs was presynaptic in origin (Fig. 3A). When the IPSC was preceded by an iontophoretic application of dopamine there was no change in the amplitude relative to an IPSC that was evoked without prior application of dopamine (Fig. 3B and C).
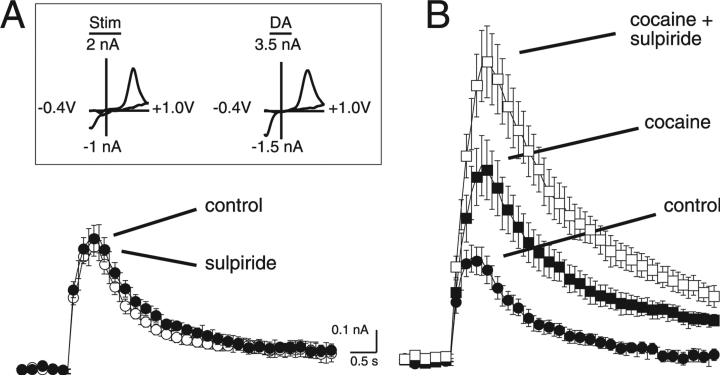
Another possibility is that synaptically released dopamine can activate presynaptic D2-autoreceptors, resulting in presynaptic inhibition that underlies the paired-pulse depression. Fast-scan cyclic voltammetry was used to determine the potential role of D2-autoreceptor activation on the somatodendritic release of dopamine. Electrical stimulation of the VTA (three pulses, 200 ms separation) evoked the release of dopamine. This was detected as an increase in current at the oxidation potential for dopamine (600 mV; Fig. 4A and B). The voltammagrams produced with electrical stimulation matched the voltammagrams produced by the exogenous application of dopamine (Fig. 4A, inset), thus confirming the identity of synaptically released transmitter as dopamine (r2 = 0.87 ± 0.01, P < 0.05; n = 7 stimulation vs. n = 3 dopamine; data not shown). To determine the role of autoreceptors in regulating dopamine release, the D2 antagonist sulpiride (200 nm) was applied. In control conditions, sulpiride did not affect the release of dopamine (control, 0.58 ± 0.07; sulpiride, 0.55 ± 0.05 nA; P > 0.05, n = 7; Fig. 4A). Blocking the re-uptake of dopamine with cocaine (1 μm) produced an increase in the peak (to 0.88 ± 0.11 from 0.46 ± 0.06 nA; P < 0.05, n = 9) and duration of the dopamine signal (Fig. 4B). Once dopamine reuptake was blocked, sulpiride (200 nm) induced an increase in the amplitude of the voltammetric dopamine signal (1.4 ± 0.16 nA; P < 0.05, n = 9; Fig. 4B). This result suggests that D2 receptor activation can regulate somatodendritic release of dopamine but is only detectable when the tonic level of dopamine in the slice is increased.
Fig. 4.
Fast-scan cyclic voltammetry determination of the regulation of dopamine release from the VTA by D2 autoreceptors. (A) Summarized voltammetric current vs. time responses were generated by three pulses at 5 Hz (n = 7) under control conditions and in the presence of sulpiride (200 nm). (Inset) Cyclic voltammagrams recorded at the peak of the stimulated dopamine response and after the exogenous application of dopamine with iontophoresis (100 nA, 50 ms). Peak oxidation and reduction peaks were +637 and –352 mV for the electrically evoked signal and +609 and –352 mV for the exogenous dopamine signal. Voltammagrams were generated by a voltage waveform from –0.4 to +1.0 V at 300 V/s. (B) Summarized voltammetric current vs. time responses were generated by three pulses at 5 Hz (n = 9) under control conditions, in the presence of cocaine (1 μm) and cocaine (1 μm) + sulpiride (200 nm).
Does increasing the amount of dopamine release affect short-term plasticity?
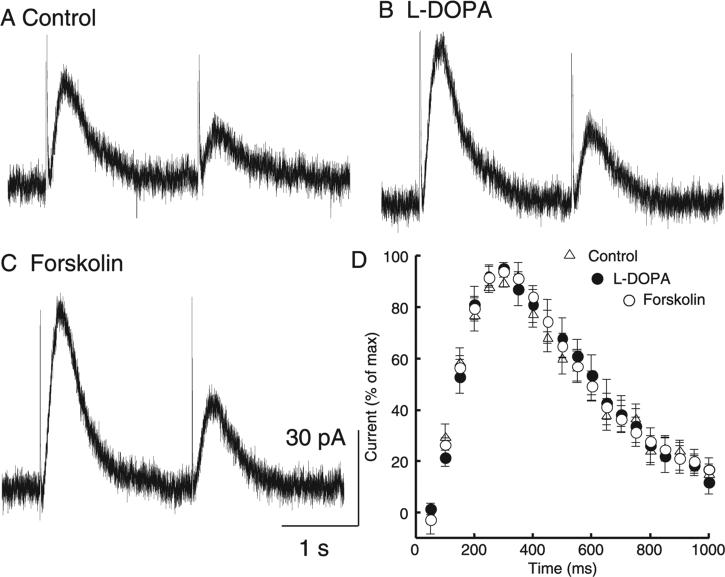
To investigate potential mechanisms of paired-pulse depression the actions of L-DOPA and forskolin were examined. L-DOPA is a dopamine precursor that is rapidly converted to dopamine to increase vesicular content and increase the amplitude of the dopamine IPSC (Pothos et al., 1998; Pothos, 2002; Beckstead et al., 2004). Forskolin activates adenylyl cyclase and is known to increase the probability of transmitter release at many synapses. As expected, treatment with L-DOPA and forskolin both increased the amplitude of the dopamine IPSC. There was, however, no effect on the paired-pulse ratio (Fig. 5A–C; F2,27 = 0.372, P = 0.69, n = 9–11) or kinetics of the synaptic current (Fig. 5D; F2,21 = 0.0367, P = 0.96, n = 6–11), suggesting that increasing vesicular content or increasing the probability of release did not affect the paired-pulse depression measured with this protocol.
Fig. 5.
Increasing the amplitude of the IPSC did not change paired-pulse depression or IPSC kinetics. A pair of single pulses 2 s apart (A) always yielded paired-pulse depression that (B) did not change when vesicular content was increased with L-DOPA (10 μm) or (C) release probability was increased with forskolin (10 μm). These manipulations did yield larger amplitude IPSCs (control, 26.2 ± 3.0 pA; L-DOPA, 36.6 ± 4.7 pA; forskolin, 64.2 ± 14.3 pA; n = 9–11). (D) Normalizing the traces to their maxima revealed that the increase in size was not accompanied by a change in time course of the IPSC (n = 6–11).
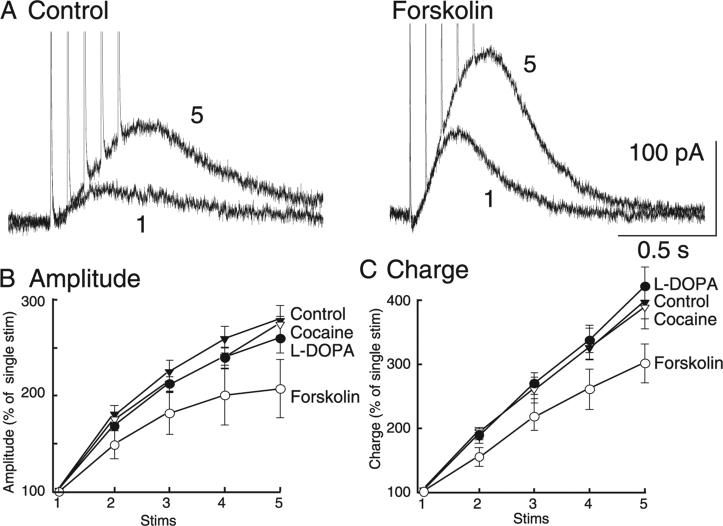
Although forskolin had no effect on the paired-pulse ratio, the application of forskolin did produce a significant effect on the relative contribution of the first stimulus of a train (Fig. 6A). The relative effect of forskolin on the IPSC were revealed when the amplitude and charge were normalized to the amplitude of the IPSC evoked by a single stimulus (Fig. 6B and C). The slope of the amplitude versus stimulus number for forskolin was significantly different from those for L-DOPA, cocaine and control (Fig. 6B; two-way anova interaction, F12,124 = 2.99, P = 0.001). The effect of forskolin was significantly different from all other manipulations, suggesting that increasing the probability of release via the activation of adenylyl cyclase results in an increase in the depression of dopamine release during a burst of stimuli. This was not merely an effect of increasing the amplitude of the IPSC, as application of neither L-DOPA nor cocaine increased the relative contribution of the first pulse.
Fig. 6.
When multiple stimuli were applied, forskolin increased the relative contribution of the first pulse. (A) Increasing release probability with forskolin (10 μm) produced IPSCs with larger amplitudes whether one or five stimuli were applied. (B) The IPSC peak amplitude and (C) total charge were subsequently plotted as a function of number of stimuli and normalized to the first pulse. Pharmacological manipulations (cocaine, L-DOPA and forskolin) did not change the general observation of a decreasing contribution of each successive pulse (n = 6–11). The difference in the forskolin curve demonstrates an increased relative contribution of the first pulse. This was not observed subsequent to cocaine or L-DOPA application, both of which also increased the amplitude of the IPSC.
Does decreasing dopamine release affect short-term plasticity?
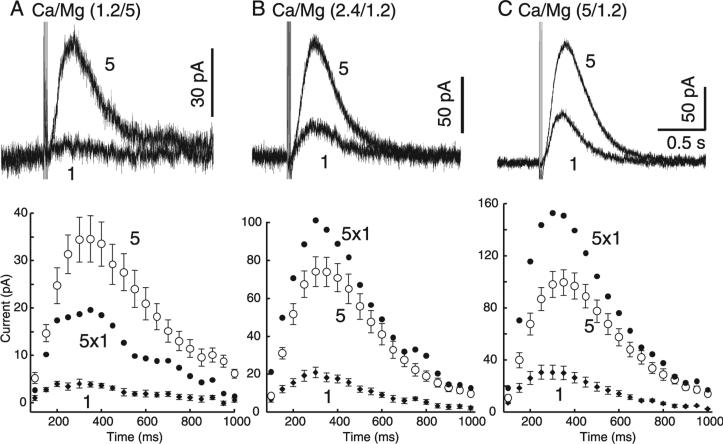
One manipulation that is well known to affect the release probability and thus the paired-pulse ratio is alteration of the extracellular content of calcium. When the ratio of calcium to magnesium was lowered the paired-pulse ratio was not changed using an interpulse interval of 2 s (change, –3.8 ± 4.6%, n = 3; not shown). When the interpulse interval was decreased to 10 ms, however, a change in the regulation of dopamine release could be detected. In order to measure facilitation or depression using such a short interstimulus interval the amplitude of the IPSC induced by a single stimulus was compared to that induced by a train of five stimuli applied at 100 Hz. In control (Ca, 2.4; Mg, 1.2 mm), the amplitude of the IPSC resulting from a single stimulus averaged ~20 pA but the amplitude of the IPSC induced by five stimuli applied at 100 Hz was only ~80 pA rather than the arithmetic sum of five individual IPSCs (Fig. 7B). When the calcium and magnesium concentrations were changed to 1.2 and 5 mm, respectively, the amplitude of the IPSC evoked by a single stimulus was dramatically decreased and the relative amplitude of the IPSC induced by five stimuli was larger than predicted (Fig. 7A). This experiment suggests that, under conditions of low release probability, there is substantial facilitation of dopamine release with a train of stimuli. When the calcium to magnesium concentrations were changed to 5 and 1.2 mm, respectively, the amplitude of the IPSC evoked by a single stimulus increased substantially but the relative increase in IPSC evoked by five stimuli was reduced relative to that predicted by the sum of each individual IPSC (Fig. 7C). Thus the combination of reduced extracellular calcium and short interstimulus intervals affected the regulation of dopamine release in a predictable fashion.
Fig. 7.
Facilitation of dopamine release was demonstrated by lowering release probability. Extracellular calcium and magnesium concentrations were altered to manipulate release probability (PR): (A) low PR (1.2 mm Ca, 5 mm Mg), (B) control PR (2.4 mm Ca, 1.2 mm Mg) and (C) high PR (5 mm Ca, 1.2 mm Mg). Dopamine IPSCs were evoked with single stimuli or trains of five rapid (10 ms interval) stimuli. (A, top) When PR was low, one stimulus produced very little current while five stimuli still produced a substantial IPSC. If the current produced by a single stimulus (‘1’) was constant over the next four stimuli, the IPSC would be the amplitude and time course predicted by the closed circles labelled ‘5 × 1’. (A, bottom, ‘5’) This predicted IPSC is smaller than the measured IPSC evoked by five stimuli. (B and C, bottom) When PR was increased the predicted IPSC ‘5 × 1’ was larger than the measured IPSC evoked by five stimuli, suggesting that facilitation of dopamine release occurred only when PR was low (n = 7–9). Total charged passed by one vs. five stimuli: low PR, 8.8 ± 1.6%; normal PR, 27.6 ± 2.3%; high PR, 28.8 ± 2.9%; n = 7–9.
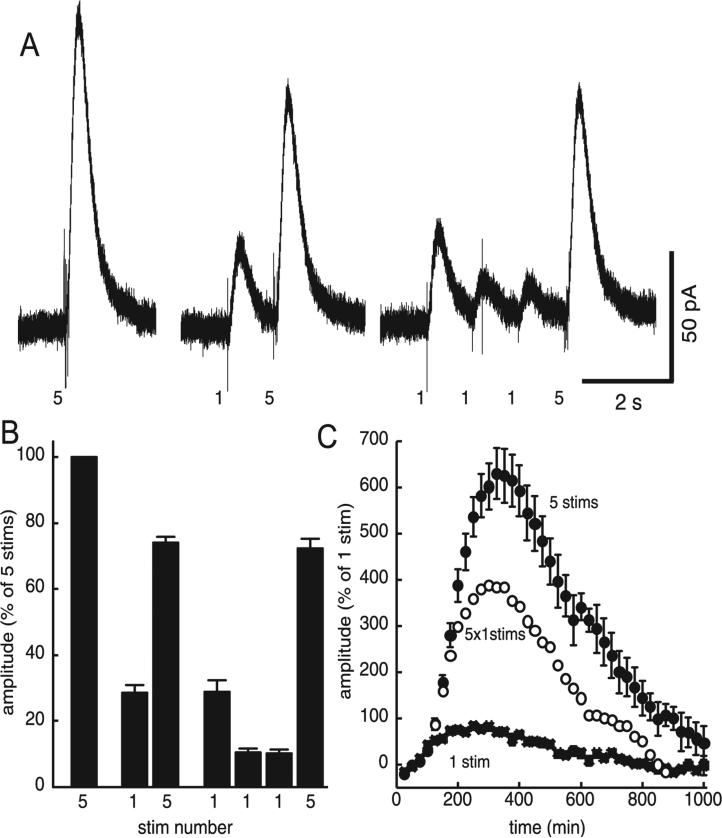
A second manipulation that was used to test for the facilitation of dopamine release was to apply repeated stimulation at a low frequency to induce the depression of release and follow that depression with a train of stimuli. The application of a single prepulse 1 s before a train of five stimuli was sufficient to significantly depress the IPSC produced by the train (Fig. 8A and B). When three prepulses were applied at 1-s intervals the amplitude of the IPSC evoked by the second and third single pulse was depressed relative to the first stimulus, but the IPSC evoked by a train of stimuli was not different from that which followed a single pulse (Fig. 8C). With that protocol, the arithmetic sum of five single-pulse IPSCs was smaller than the IPSC induced by a train of five high frequency stimuli. Thus, once the IPSC was depressed, a train of high frequency stimuli resulted in facilitation (Fig. 8C).
Fig. 8.
Presynaptic depression of release unmasked facilitation. (A) Dopamine IPSCs were measured subsequent to three patterns of discharge activity: a train of five stimuli alone (5), a train preceded by one prepulse (1, 5) and a train preceded by three prepulses (1, 1, 1, 5). A single prepulse was sufficient to depress release and the amplitude of the IPSC. (B) Summarized data suggest that, subsequent to a prepulse, the IPSC produced by a single pulse was much more depressed than the IPSC produced by a train. (C) As this IPSC (‘5 stims’) is more than the arithmetic summation of five single-pulse IPSCs (‘5 × 1 stim’), presynaptic depression may be in effect unmasking the facilitation of release.
Discussion
The results describe presynaptic regulation of dendrodendritic dopamine transmission in the midbrain. Paired-pulse protocols resulted in depression of dopamine release similar in time course to that observed in the striatum (Benoit-Marand et al., 2001; Phillips et al., 2002). Burst stimuli resulted in a smaller IPSC relative to that predicted by summing the amplitude an IPSC evoked by a single stimulus. This was somewhat surprising considering that facilitation of dopamine release results from burst-type stimuli in limbic terminal regions (Gonon, 1988; Chergui et al., 1994; Cragg, 2003). The present results also show, however, that decreasing the probability of release resulted in facilitated release. There are two potential interpretations. It could be that the regulation of somatodendritic and terminal release of dopamine is very different. It is also possible that the elimination of afferent input in brain slices increases the probability of dopamine release such that paired stimuli will always result in depression. The lack of intermittent excitatory drive may therefore account for the difficulty in observing the temporal summation that has been reported in terminal regions.
Time course of dopamine release
Electrochemical and anatomical studies have suggested that dopamine transmission in the midbrain proceeds in a manner consistent with volume transmission, i.e. without a point-to-point structural arrangement like a classical synapse (Chen & Rice, 2001; Fuxe & Agnati, 1991; Rice, 2000; Cragg et al., 2001). Indeed, dopamine is not the only neurotransmitter capable of spilling over out of the synapse and activating extrasynaptic receptors (e.g. Isaacson et al., 1993; Matsui & Jahr, 2003). Previous work suggests that under physiological conditions the time that midbrain D2 receptors are exposed to a concentration of dopamine capable of initiating a postsynaptic current is insignificant compared to the kinetics of the G-protein coupling and subsequent hyperpolarization (Beckstead et al., 2004). Those observations are confirmed here; enhancing release with forskolin or L-DOPA did not prolong the time course of the postsynaptic current in spite of the fact that the amplitude of the IPSC was increased. If super-threshold concentrations of dopamine were typically traveling micrometers to reach target D2 receptors then increasing release would have prolonged the time course of the synaptic current. Inhibition of uptake through the application of cocaine did prolong the time course of the IPSC. This confirms the crucial role of uptake transporters in limiting the time that dopamine is in the extracellular space and producing a physiological time course almost completely dependent on steps occurring subsequent to agonist binding.
Physiological role of the dopamine IPSC
In vivo, dopamine neurons exhibit two distinct types of discharge activity (Grace & Bunney, 1984). During times of low excitation, single action potentials occur in either a pacemaker or a random pattern at ~4 Hz. Excitatory input results in bursts of 2–6 more closely spaced (~ 15 Hz) action potentials followed by a pause that may be partially due to the dopamine-mediated IPSC. In this study, stimulation protocols were applied that mimicked the firing observed in vivo using both single stimuli and ‘bursts’ of stimuli at 12.5 Hz. While both stimulation patterns resulted in paired-pulse depression, this depression was substantially greater when pairs of single stimuli were applied. In vivo this would limit the ability of single action potentials to produce dopamine release even during times of low excitability. When pairs of trains were applied the IPSC was only modestly suppressed, suggesting that presynaptic inhibition of release does not prevent bursts from producing dopamine IPSCs even during times of high excitability. This evidence was strengthened by the observations made subsequent to single prepulses; tonic depression of release decreased the amplitude of IPSCs produced by a single stimulus while preserving those produced by burst-type stimuli. Thus, presynaptic depression of release provides a high pass filter for dendrodendritic dopamine transmission, reducing dopamine signalling when firing is low while leaving burst-induced dopamine release intact.
Mechanism of presynaptic depression
Although the source of paired-pulse depression is presynaptic, the precise mechanisms remain to be determined. It has been proposed that dopamine neurons release via a flickering fusion pore mechanism, which may be subject to regulation by processes that differ from allor-none vesicular fusion (Staal et al., 2004). Increasing vesicular content with L-DOPA did not change the paired-pulse ratio, suggesting that the paired-pulse depression probably did not result from a decrease in dopamine within vesicles. Increasing release with forskolin did not decrease the paired-pulse ratio when single stimuli were applied but increased the depression observed with burst-type stimulation.
In terminal regions, depression of release results from two independent mechanisms. First, activation of D2 autoreceptors inhibits the release of dopamine in the striatum with a time course very similar to the time course of the GIRK conductance in the midbrain (Benoit-Marand et al., 2001; Phillips et al., 2002; Beckstead et al., 2004). Second, an undetermined mechanism also inhibits release, but with a much slower time course that is similar to the depression observed in the present study (Phillips et al., 2002; Montague et al., 2004). In the midbrain, presynaptic and postsynaptic dopamine receptors are both of the D2 type. Thus, directly testing of the role of presynaptic autoreceptors using the D2 receptor antagonist sulpiride was not possible because this manipulation also blocks the postsynaptic receptors responsible for the IPSC (Beckstead et al., 2004; Ford et al., 2007). However, measuring dopamine release with fast-scan cyclic voltammetry is not dependent on D2 receptors. The results from those experiments suggest that D2 dopamine autoreceptors do not contribute to presynaptic depression observed on a subsecond time scale. Autoreceptor-mediated inhibition of release was only observed in the midbrain after application of the dopamine uptake blocker cocaine, presumably by increasing the tonic extracellular dopamine concentration sufficiently to activate presynaptic autoreceptors. Iontophoresis provided evidence that activating autoreceptors did not inhibit dopamine release after the cessation of the GIRK conductance, further suggesting that the observed paired-pulse depression was not a consequence of autoreceptor activation at any time point.
Facilitation and depression of slow synaptic transmission
Short-term plasticity at glutamate and GABA synapses has been studied intensely for more than a decade and it is suggested that the type of plasticity can vary with the frequency of stimulation, the transmitter released, the presence of presynaptic receptors and even the resting level of modulators such as adenosine (Salin et al., 1996; Moore et al., 2003; Kukley et al., 2005). Even the slowest examples of short-term facilitation of release typically recover to control within < 400 ms (Cragg, 2003; Salin et al., 1996; Thomson, 2000). The temporal resolution of studies examining short-term plasticity of slow synaptic transmission are limited by the slow kinetics of the various synaptic events. A long-lasting depression is by far the most common form of short-term plasticity found at slow inhibitory potentials that include GABA-B (Otis et al., 1993) and 5-HT (Bobker & Williams, 1990; Morikawa et al., 2000) IPSCs. In each of those studies, pairs of single stimuli were used to evoke slow IPSCs; the paired-pulse depression lasted for 15–30 s and was thought to result from the activation of presynaptic autoreceptors. The only completely convincing demonstration of an autoreceptor-dependent mechanism used 5-HT-1B receptor-knockout animals to show that the paired-pulse depression of the 5-HT-1A receptor-dependent IPSC was largely eliminated (Morikawa et al., 2000). None of these studies examined depression induced by trains of stimuli, or the effect of stimulus number on the amplitude of the synaptic potential.
Although presynaptic depression of release was observed under most recording conditions, two experiments suggested that facilitation of release occurs when the probability of release is decreased. First, a low calcium, high magnesium solution in combination with high frequency stimulation was able to produce facilitation. Second, a single prepulse decreased release probability sufficiently to permit the observation of facilitation. During times of spontaneous activity in vivo it is possible that presynaptic dopamine release is tonically depressed such that a bust of activity results in facilitated release of dopamine. Thus, it may be the combination of facilitation of release unmasked by regular presynaptic depression that dictates the primary form of short-term plasticity under physiological conditions.
Conclusions
Three decades ago, anatomical studies in dopamine neuron cell body regions led to the hypothesis of dendrodendritic transmission between dopamine neurons (Bjorklund & Lindvall, 1975; Groves et al., 1975). This evidence was bolstered by the identification of dopamine D2 receptor-mediated activation of a potassium conductance (Lacey et al., 1987) and later by measurement of dopamine overflow with microdialysis (Bradberry & Roth, 1989; Kalivas & Duffy, 1991; Robertson et al., 1991) and fast-scan cyclic voltammetry (Rice et al., 1997). The identification and direct measurement of the dopamine IPSC (Beckstead et al., 2004) has recently permitted an analysis of the factors contributing to the short- and long-term regulation of dendrodendritic dopamine transmission. Repeated stimulation produces long-term depression (LTD; Beckstead & Williams, 2007), indicating that dopamine synapses can undergo activity-dependent changes in strength. This form of plasticity resembles some forms of depression at glutamate synapses, as it is persistent and blocked by postsynaptic chelation of calcium. The present study examined presynaptic regulation of dendrodendritic dopamine transmission, as calcium-dependent postsynaptic LTD was blocked throughout by inclusion of BAPTA in the recording pipette. The results indicate that dendritic dopamine release is subject to short-term depression and facilitation that is dependent on the pattern of stimulation. Both presynaptic and postsynaptic forms of plasticity would serve to limit dendrodendritic dopamine transmission during times of high activity. However, the time course of presynaptic action is considerably shorter in onset and duration than the previously reported postsynaptic LTD (Beckstead & Williams, 2007). The time course of presynaptic depression and facilitation suggests that, despite decades of debate concerning the irregularity of vesicular release of dendritic dopamine, this form of release exhibits many similarities to the release of GABA and glutamate.
Acknowledgements
We would like to thank Dr Shane Hentges for critical suggestions. Supported by NIDA grants F32 DA16467 and K01 DA21699 (M.J.B.), R03 17155 (P.E.M.P.), and R01 DA4523 and the NARSAD Ritter Foundation (J.T.W.).
Abbreviations
- GIRK
G-protein-coupled inwardly rectifying potassium (conductance)
- IPSC
inhibitory postsynaptic current
References
- Beckstead MJ, Grandy DK, Wickman K, Williams JT. Vesicular dopamine release elicits an inhibitory postsynaptic current in midbrain dopamine neurons. Neuron. 2004;42:939–946. doi: 10.1016/j.neuron.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Beckstead MJ, Williams JT. Long-term depression of a dopamine IPSC. J. Neurosci. 2007;27:2074–2080. doi: 10.1523/JNEUROSCI.3251-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigornia L, Allen CN, Jan CR, Lyon RA, Titeler M, Schneider AS. D2 dopamine receptors modulate calcium channel currents and catecholamine secretion in bovine adrenal chromaffin cells. J. Pharmacol. Exp. Ther. 1990;252:586–592. [PubMed] [Google Scholar]
- Bjorklund A, Lindvall O. Dopamine in dendrites of substantia nigra neurons: suggestions for a role in dendritic terminals. Brain Res. 1975;83:531–537. doi: 10.1016/0006-8993(75)90849-5. [DOI] [PubMed] [Google Scholar]
- Bobker DH, Williams JT. Serotonin-mediated inhibitory postsynaptic potential in guinea-pig prepositus hypoglossi and feedback inhibition by serotonin. J. Physiol. (Lond.) 1990;422:447–462. doi: 10.1113/jphysiol.1990.sp017994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Roth RH. Cocaine increases extracellular dopamine in rat nucleus accumbens and ventral tegmental area as shown by in vivo microdialysis. Neurosci. Lett. 1989;103:97–102. doi: 10.1016/0304-3940(89)90492-8. [DOI] [PubMed] [Google Scholar]
- Chen BT, Rice ME. Novel Ca2+ dependence and time course of somatodendritic dopamine release: substantia nigra versus striatum. J. Neurosci. 2001;21:7841–7847. doi: 10.1523/JNEUROSCI.21-19-07841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chergui K, Suaud-Chagny MF, Gonon F. Nonlinear relationship between impulse flow, dopamine release and dopamine elimination in the rat brain in vivo. Neuroscience. 1994;62:641–645. doi: 10.1016/0306-4522(94)90465-0. [DOI] [PubMed] [Google Scholar]
- Cragg SJ. Variable dopamine release probability and short-term plasticity between functional domains of the primate striatum. J. Neurosci. 2003;23:4378–4385. doi: 10.1523/JNEUROSCI.23-10-04378.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, Nicholson C, Kume-Kick J, Tao L, Rice ME. Dopamine-mediated Volume transmission in midbrain is regulated by distinct extracellular geometry and uptake. J. Neurophysiol. 2001;85:1761–1771. doi: 10.1152/jn.2001.85.4.1761. [DOI] [PubMed] [Google Scholar]
- Ford CP, Beckstead MJ, Williams JT. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J. Neurophysiol. 2007;97:883–891. doi: 10.1152/jn.00963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K, Agnati L. Volume Transmission in the Brain: New Aspects for Electrical and Chemical Communications. Raven Press; New York: 1991. [Google Scholar]
- Geffen LB, Jessell TM, Cuello AC, Iversen LL. Release of dopamine from dendrites in rat substantia nigra. Nature. 1976;260:258–260. doi: 10.1038/260258a0. [DOI] [PubMed] [Google Scholar]
- Gonon FG. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- Grace AA, Bunney BS. The control of firing pattern in nigral dopamine neurons: burst firing. J. Neurosci. 1984;4:2877–2890. doi: 10.1523/JNEUROSCI.04-11-02877.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves PM, Wilson CJ, Young SJ, Rebec GV. Self-inhibition by dopaminergic neurons. Science. 1975;190:522–528. doi: 10.1126/science.242074. [DOI] [PubMed] [Google Scholar]
- Hoffman AF, Gerhardt GA. Differences in pharmacological properties of dopamine release between the substantia nigra and striatum: an in vivo electrochemical study. J. Pharmacol. Exp. Ther. 1999;289:455–463. [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Jaffe EH, Marty A, Schulte A, Chow RH. Extrasynaptic vesicular transmitter release from the somata of substantia nigra neurons in rat midbrain slices. J. Neurosci. 1998;18:3548–3553. doi: 10.1523/JNEUROSCI.18-10-03548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P. A comparison of axonal and somatodendritic dopamine release using in vivo dialysis. J. Neurochem. 1991;56:961–967. doi: 10.1111/j.1471-4159.1991.tb02015.x. [DOI] [PubMed] [Google Scholar]
- Kukley M, Schwan M, Fredholm BH, Dietrich D. The role of extracellular adenosine in regulating mossy fiber synaptic plasticity. J. Neurosci. 2005;25:2832–2837. doi: 10.1523/JNEUROSCI.4260-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey MG, Mercuri NB, North RA. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J. Physiol. (Lond.) 1987;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K, Jahr CE. Ectopic release of synaptic vesicles. Neuron. 2003;40:1173–1183. doi: 10.1016/s0896-6273(03)00788-8. [DOI] [PubMed] [Google Scholar]
- Montague PR, McClure SM, Baldwin PR, Phillips PEM, Budygin EA, Stuber GD, Kilpatrick MR, Wightman RM. Dynamic gain control of dopamine delivery in freely moving animals. J. Neurosci. 2004;24:1754–1759. doi: 10.1523/JNEUROSCI.4279-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KA, Nicoll RA, Schmitz D. Adenosine gates synaptic plasticity at hippocampal mossy fiber synapses. Proc. Natl. Acad. Sci. USA. 2003;100:14397–14402. doi: 10.1073/pnas.1835831100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Manzoni OJ, Crabbe JC, Williams JT. Regulation of central synaptic transmission by 5-HT-1B auto- and heteroreceptors. Mol. Pharmacol. 2000;58:1271–1278. doi: 10.1124/mol.58.6.1271. [DOI] [PubMed] [Google Scholar]
- Nirenberg MJ, Chan J, Liu Y, Edwards RH, Pickel VM. Ultrastructural localization of the vesicular monoamine transporter-2 in midbrain dopaminergic neurons: potential sites for somatodendritic storage and release of dopamine. J. Neurosci. 1996;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis TS, DeKoninck Y, Mody I. Characterization of synaptically elicited GABA-B responses using patch-clamp recording in rat hippocampus. J. Physiol. (Lond.) 1993;463:391–407. doi: 10.1113/jphysiol.1993.sp019600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PE, Hancock PJ, Stamford JA. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse. 2002;44:15–22. doi: 10.1002/syn.10049. [DOI] [PubMed] [Google Scholar]
- Pothos EN. Regulation of dopamine quantal size in midbrain and hippocampal neurons. Behav. Brain. Res. 2002;130:203–207. doi: 10.1016/s0166-4328(01)00419-3. [DOI] [PubMed] [Google Scholar]
- Pothos EN, Davila V, Sulzer D. Presynaptic recording of quanta from midbrain dopamine neurons and modulation of quantal size. J. Neurosci. 1998;18:4106–4118. doi: 10.1523/JNEUROSCI.18-11-04106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucak ML, Grace AA. Evidence that systemically administered dopamine antagonists activate dopamine neuron firing primarily by blockade of somatodendritic autoreceptors. J. Pharmacol. Exp. Ther. 1994;271:1181–1192. [PubMed] [Google Scholar]
- Rice ME. Distinct regional differences in dopamine-mediated Volume transmission. Prog. Brain Res. 2000;125:277–290. doi: 10.1016/S0079-6123(00)25017-6. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, Greenfield SA. Characteristics of electrically evoked somatodendritic dopamine release in substantia nigra and ventral tegmental area in vitro. J. Neurophysiol. 1997;77:853–862. doi: 10.1152/jn.1997.77.2.853. [DOI] [PubMed] [Google Scholar]
- Robertson GS, Damsma G, Fibiger HC. Characterization of dopamine release in the substantia nigra by in vivo microdialysis in freely moving rats. J. Neurosci. 1991;11:2209–2216. doi: 10.1523/JNEUROSCI.11-07-02209.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salin PA, Scanziani M, Malenka RC, Nicoll RA. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc. Natl. Acad. Sci. USA. 1996;93:13304–13390. doi: 10.1073/pnas.93.23.13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal RG, Mosharov EV, Sulzer D. Dopamine neurons release transmitter via a flickering fusion pore. Nat. Neurosci. 2004;7:341–346. doi: 10.1038/nn1205. [DOI] [PubMed] [Google Scholar]
- Thomson AM. Facilitation, augmentation and potentiation at central synapses. Trends Neurosci. 2000;23:305–312. doi: 10.1016/s0166-2236(00)01580-0. [DOI] [PubMed] [Google Scholar]
- Williams JT, North RA, Shefner SA, Nishi S, Egan TM. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984;13:137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM, Fifkova E. Monoaminergic synapses, including dendro-dendritic synapses in the rat substantia nigra. Exp. Brain Res. 1977;30:161–174. doi: 10.1007/BF00237248. [DOI] [PubMed] [Google Scholar]
- Yavich L, MacDonald E. Dopamine release from pharmacologically distinct storage pools in rat striatum following stimulation at frequency of neuronal bursting. Brain Res. 2000;870:73–79. doi: 10.1016/s0006-8993(00)02403-3. [DOI] [PubMed] [Google Scholar]