Abstract
Increasing evidence suggests a possible involvement of neuroinflammation in some psychiatric disorders, and also pharmacological reports indicate that anti-inflammatory effects are associated with therapeutic actions of psychoactive drugs, such as anti-depressants and antipsychotics. The purpose of this study was to explore whether clozapine, a widely used antipsychotic drugs, displays anti-inflammatory and neuroprotective effects. Using primary cortical and mesencephalic neuron-glia cultures, we found that clozapine was protective against inflammation-related neurodegeneration induced by lipopolysaccharide (LPS). Pretreatment of cortical or mesencephalic neuron–glia cultures with clozapine (0.1 or 1µM) for 24 hrs attenuated LPS-induced neurotoxicity. Clozapine also protected neurons against 1-methyl-4-phenylpyridinium+ (MPP+)-induced neurotoxicity, but only in cultures containing microglia, indicating an indispensable role of microglia in clozapine-afforded neuroprotection. Further observation revealed attenuated LPS-induced microglial activation in primary neuron-glia cultures and in HAPI microglial cell line with clozapine pretreatment. Clozapine ameliorated the production of microglia-derived superoxide and intracellular reactive oxygen species (ROS), as well as the production of nitric oxide and TNF-α following LPS. In addition, the protective effect of clozapine was not observed in neuron-glia cultures from mice lacking functional NADPH oxidase (PHOX), a key enzyme for superoxide production in immune cells. Further mechanistic studies demonstrated that clozapine pretreatment inhibited LPS-induced translocation of cytosolic subunit p47phox to the membrane in microglia, which was most likely though inhibiting the phosphoinositide 3-kinase (PI3K) pathway. Taken together, this study demonstrates that clozapine exerts neuroprotective effect via the attenuation of microglia activation through inhibition of PHOX-generated ROS production and suggests potential use of antipsychotic drugs for neuroprotection.
Keywords: clozapine, microglia, NADPH oxidase, neurodegeneration, neuroinflammation
Introduction
Neuroinflammation regulated by microglia, the primary resident immune cells (Kreutzberg, 1996), is increasingly accepted as a double-edged sword in regulating brain functions (Gao and Hong, 2008), Under normal conditions, microglial cells are quiescent and serve important immunosurveillance function of protecting neurons. Upon subtle changes in their micro-environment, or as a consequence of pathological insults, these immune cells rapidly transform into an activated state (Kreutzberg, 1996). Uncontrolled activation of microglia exerts cytotoxic effects by releasing inflammatory mediators and causes bystander injury to neurons (Block and Hong, 2007). Accumulating evidence indicates that neuroinflammation is closely associated with the pathogenesis of several neurodegenerative diseases like Alzheimer's and Parkinson’ diseases (McGeer and McGeer, 1998; Gao and Hong, 2008; Qian et al., 2010). Recent studies further suggested that neuroinflammation may also associated with several psychiatric disorders, such as schizophrenia (SCZ) and depression (Minghetti, 2005; Doorduin et al., 2009; Dobos et al., 2010). Progressive enlargement of ventricles and loss of gray matter among different brain regions have been observed in some SCZ patients by magnetic resonance imaging (Zipursky et al., 1992; Nair et al., 1997; Hulshoff Pol et al., 2002; van Haren et al., 2007) or postmortem studies (Altshuler et al., 1990; Byne et al., 2002). Although the mechanisms responsible for such degeneration are still largely unknown, inflammatory mechanisms and microglia dysfunction have gained more and more attention as an integrative theory of SCZ (Munn, 2000). Recent postmortem studies revealed an elevated number of HLA-DR-positive microglia in the brains of SCZ patients (Radewicz et al., 2000; Wierzba-Bobrowicz et al., 2005). Moreover, in vivo positron emission tomography (PET) study using the [11C] PK11195 radioligand demonstrated activated microglia in grey matter of patients with recent-onset of SCZ (van Berckel et al., 2008). These data supports the possibility that neuroinflammation plays a role in the pathogenesis of SCZ.
In recent years, some psychoactive drugs, such as antidepressants and antipsychotics, are found to be potent modulators of immune functions. Antidepressant drug bupropion interfered with lipopolysaccharide (LPS) - stimulated cytokine production in mice (Brustolim et al., 2006). Treatment with fluoxetine normalized the increased serum IL-6 in patients with acute depression. In animal study, antidepressant also significantly attenuated LPS-induced anorexia and body weight loss (Yirmiya et al., 2001). Similarly, tricyclic antidepressants were found to normalize the increased monocyte counts in depressed patients (Seidel et al., 1996), and attenuated LPS-elicited depressive-like behavioral syndrome in rats (Yirmiya et al., 2001). In a recent case report, a patient with arthritis and comorbid major depression was treated with antipsychotic drug quetiapine and achieved a significant decrease of systemic inflammation, which eventually led to a remission of pain and depression (Baune and Eyre, 2010). These evidence suggests that the therapeutic actions of some antipsychotic drugs may be associated with their anti-inflammatory properties.
In the course of developing anti-inflammatory drugs for therapeutic intervention of neurodegenerative diseases, we found that clozapine displayed potent neuroprotective effect. Since clozapine has been reported to modulate the production of inflammatory cytokines such as IL-6, IL-10 and IFN-γ in peripheral blood cells (Song et al., 2000; Paterson et al., 2006), and the serum concentrations of clozapine correlate inversely with the reactive oxygen species (ROS) production by monocyte from clozapine-treated SCZ patients (Gross et al., 2003), we postulated that these anti-inflammatory actions of clozapine maybe related to its neuroprotective action. In this study, we demonstrated that clozapine confers potent neuroprotection on dopaminergic neurons against inflammation-elicited neurodegeneration in primary cell cultures. We further elucidated mechanisms by which clozapine elicits anti-inflammatory effect and reported that this antipsychotic modulates brain immune function through the attenuation of microglia activation by inhibiting PHOX-generated ROS production and subsequent production of proinflammatory cytokines.
Materials and Methods
Materials and Reagents
Clozapine, MPP+, and Cytosine β-D-arabinofuranoside (Ara-C) were purchased from Sigma-Aldrich (St. Louis, MO). Lipopolysaccharide (LPS strain O111:B4) was purchased from Calbiochem (San Diego, CA). WST-1 was purchased from Dojindo Laboratories (Gaithersburg, MD). Cell culture ingredients were obtained from Invitrogen (Carlsbad, CA). [3H]DA was purchased from Perkin Elmer Life Sciences (Boston, MA). The polyclonal antibody against tyrosine hydroxylase (TH) was a kind gift from Dr. John Reinhard of GlaxoSmithKline (Research Triangle Park, NC). Polyclonal antibody against Iba1 was obtained from Wako Chemicals USA, Inc. (Richmond, VA).
Animals
Wild-type C57BL/6J (gp91phox+/+) and NADPH oxidase (PHOX)-deficient (gp91phox−/−) mice were obtained from the Jackson Laboratory (Bar Harbor, ME). Breeding of the mice was performed to obtain timed pregnancy with accuracy of 0.5 day. Timed-pregnant Fisher F344 rats were obtained from Charles River Laboratories (Raleigh, NC). Housing and breeding of animals were done in accordance with National Institutes of Health guidelines. All the animals were housed in a specific pathogen free facility in conditions of a constant temperature and relative humidity.
Mesencephalic and cortical neuron-glia cultures
Mesencephalic neuron-glia cultures were prepared from the ventral mesencephalic tissue of embryonic day 13–14 rats or day 12–13 mice. Cortical neuron-glia cultures were prepared from the cortical tissue of embryonic day 17–18 rats as described previously (Qin et al., 2002). Tissues were isolated and dissociated with gentle mechanical trituration. Cells were diluted to 1×106/ml and seeded in 24-well culture plates precoated with poly-D-lysine. Seven–day-old cultures were used for treatment.
Primary neuron-enriched cultures
Thirty-six hrs after seeding the cells, Ara-C was added to a final concentration of 6 µM to suppress glial proliferation. Two days later, cultures were changed back to maintenance medium and were used for treatment 7 days after initial seeding.
Primary microglia-enriched cultures
Primary microglia-enriched cultures were prepared from the whole brains of 1-day-old pups as described previously (Gao et al., 2003). Briefly, brain tissues were triturated after removing the meninges and blood vessels. Cells were seeded at 5×107 in a 150cm3 cultures flask. After a confluent monolayer of glia cells had been obtained (12–14 days after initial seeding), microglia were shaken off, collected and seeded.
Neuron-microglia reconstituted cultures
Enriched microglia (1×105/well) from 1-day-old rat were seeded to 6-day old neuron-enriched cultures. Reconstituted cultures were used for treatment the following day. Neurotoxicity was analyzed 7 days after treatment.
Microglial cell line
The rat microglia HAPI cell line was a gift from Dr. James R. Connor (Department of Neuroscience and Anatomy, M. S. Hershey Medical Center, Hershey, PA). Briefly, cells were maintained at 37°C in DMEM supplemented with 10% FBS, 50 U/ml penicillin, and 50 µg/ml streptomycin in a humidified incubator with 5% CO2 and 95% air.
Uptake assay
Uptake of [3H] dopamine (DA) was performed as previously described (Gao et al., 2003). Briefly, cultures were incubated for 20 minutes at 37 °C with 1 µM [3H] DA in Krebs-Ringer buffer (16 mM sodium phosphate, 119 mM NaCl, 4.7 mM KCl, 1.8 mM CaCl2, 1.2 mM MgSO4, 1.3 mM EDTA, and 5.6 mM glucose; pH 7.4). Nonspecific DA uptake was blocked with mazindole (10 µM). Cells were collected in 1N NaOH after washing with ice-cold Krebs-Ringer buffer. Radioactivity was determined by liquid scintillation counting. Specific [3H] DA uptake was calculated by subtracting the mazindole counts from the wells without the uptake inhibitor.
Immunostaining
Immunostaining was performed as described previously (Qin et al., 2002). Neurons were stained with anti-NeuN (1:1000). DAergic neurons were stained with the antibody against TH (1:5000). Microglia were stained with the antibody raised against Iba-1 (1:1000). Images were recorded with an inverted microscope (Nikon, Tokyo, Japan) connected to a charge-coupled device camera (DAGE-MTI, Michigan City, IN) operated with the MetaMorph software (Molecular Devices, Sunnyvale, CA). Nine representative areas per well of a 24-well plate were counted under the microscope at 100× magnification.
Extracellular Superoxide Assay
The extracellular superoxide production was determined by measuring the superoxide dismutase (SOD)-inhibitable reduction of tetrazolium salt, WST-1 as described before (Qin et al., 2002) with modifications. Microglial cells were plated at 1×105/well in 96-well plate for 12 hrs. The cells were washed twice and treated. Then 50 µl of WST-1(1mM) in HBSS, with or without SOD (600 units/ml), was added. The absorbance at 450 nm was read immediately with a Spectra Max Plus microtiter plate spectrophotometer (Molecular Devices, Sunnyvale, CA).
Assay of intracellular reactive oxygen species
Intracellular oxidative stress was measured by DCFH Oxidation. DCFH-DA enters cells passively and is deacetylated by esterase to nonfluorescent DCFH. DCFH reacts with ROS to form dichlorodifluorescein, the fluorescent product. DCFH-DA was dissolved in methanol at 10 mM and was diluted 500-fold in HBSS to give DCFH-DA at 20 µM. The cells were exposed to DCFH-DA for 1 hr and then treated for 2 hrs. The fluorescence was read immediately at wavelengths of 485 nm for excitation and 530 nm for emission using a SpectraMax Gemini XS fluorescence microplate reader (Molecular Devices, Sunnyvale, CA).
Confocal microscopy
HAPI cells were seeded in dishes at 5×104 cells/well and treated with LPS for 10 min. Cells were fixed with 3.7% paraformaldehyde in PBS for 10 min. After washing with PBS, cells were incubated with rabbit polyclonal antibody to p47phox. Cells were then washed and incubated with FITC-conjugated goat anti-rabbit antibody. Focal planes spaced at 0.4 µm intervals were imaged with a Zeiss 510 laser scanning confocal microscope (63× PlanApo 1.4 numerical aperture objective) equipped with LSM510 digital imaging software.
Cell Extracts
Whole cell lysates from neuron-glia cultures were prepared with lysis buffer (Cell Signaling, Danvers, MA). Subcellular fractionation was performed as described previously (Gao et al., 2008). For subcellular fractions, HAPI cells were lysed in hypotonic lysis buffer (1 mM EGTA, 1 mM EDTA, 10 mM β-glycerophosphate, 10 mM NaF, 1 mM sodium orthovanadate, 2 mM MgCl2, 10 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 10 µg/ml each of leupeptin, aprotinin, and pepstatin A), incubated on ice for 30 min, and then subjected to Dounce homogenization (20–25 stokes, tight pestle A). The lysates were loaded onto a sucrose gradient in lysis buffer (final 0.5 M) and centrifuged at 1,600 g for 15 min. The supernatant above the sucrose gradient was used as the cytosolic fraction after centrifugation at 150,000 g for 30 min. The pellets were solubilized in 1% Nonidet P-40 hypotonic lysis buffer and were used as the membranous fraction.
Western Blot Analysis
Equal amounts of protein were separated by 4–12% Bis-Tris-polyacrylamide electrophoresis gel and transferred to polyvinylidene difluoride membranes (Novex, San Diego, CA). The membranes were blocked with 5% nonfat milk and incubated with primary antibody (rabbit anti-p47phox antibody (1: 1000), rabbit anti-Iba1 antibody (1: 3000), rabbit anti-GAPDH (1:2000), mouse anti-gp91 (1:2000)) overnight at 4°C. The membrane was then incubated with horseradish peroxidase-linked anti-rabbit or mouse IgG (1:3000) for 1 hr at 25°C. ECL Plus reagents (GE Healthcare, Little Chalfont, Buckinghamshire, UK) were used as a detection system.
PMS/MTS cell viability assay
The 3,(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt (MTS) and phenazine methosulfate (PMS) was obtained from Promega (Madison, WI). MTS and PMS reagents were prepared according to manufacturer's instructions. Briefly, 20 µl of the MTS/PMS working solution were added to 100 µl of cell suspension in a flat-bottomed 96-well microtiter plate. The microplate was then incubated for 24 or 48 hrs at 37°C in the dark. The absorbance of formazan, the reduction product of MTS/PMS, was measured at 450 nm with a Spectra Max Plus microtiter plate spectrophotometer (Molecular Devices, Sunnyvale, CA).
Flow cytometry
HAPI microglial cells were seeded in 6-well plates (9.6 cm2) at 5×105 cells/well. Cells were pretreated with clozapine (0.1 or 1µM) for 24 hrs followed by stimulation with LPS for 24 hrs. Cells were dislodged in HBSS, pelleted (1,000 g for 5 min, 4°C) and resuspended in ice-cold blocking solution (HBSS containing 1% BSA) for 20 min. Cells were washed and stained for 0.5 hr on ice with a 1:200 dilution of PE-conjugated anti-rat OX6 (BD Pharmingen, San Diego, CA, USA). Isotype-matched controls were run in parallel. Cells were then washed three times and fixed. Analysis was performed on a FACScan flow cytometer (BD Biosciences) using FACSDiva software.
Statistical Analysis
The data were expressed as mean ± SEM. Statistical significance between two groups was assessed with an analysis of variance followed by Student’s t-test. Statistical significance between multiple groups was performed using an one-way analysis of variance (ANOVA). When ANOVA showed significant difference, (least significant difference) LSD multiple comparisons post-hoc test was performed. A value of P < 0.05 was considered statistically significant.
Results
Clozapine ameliorates LPS-induced neurodegeneration
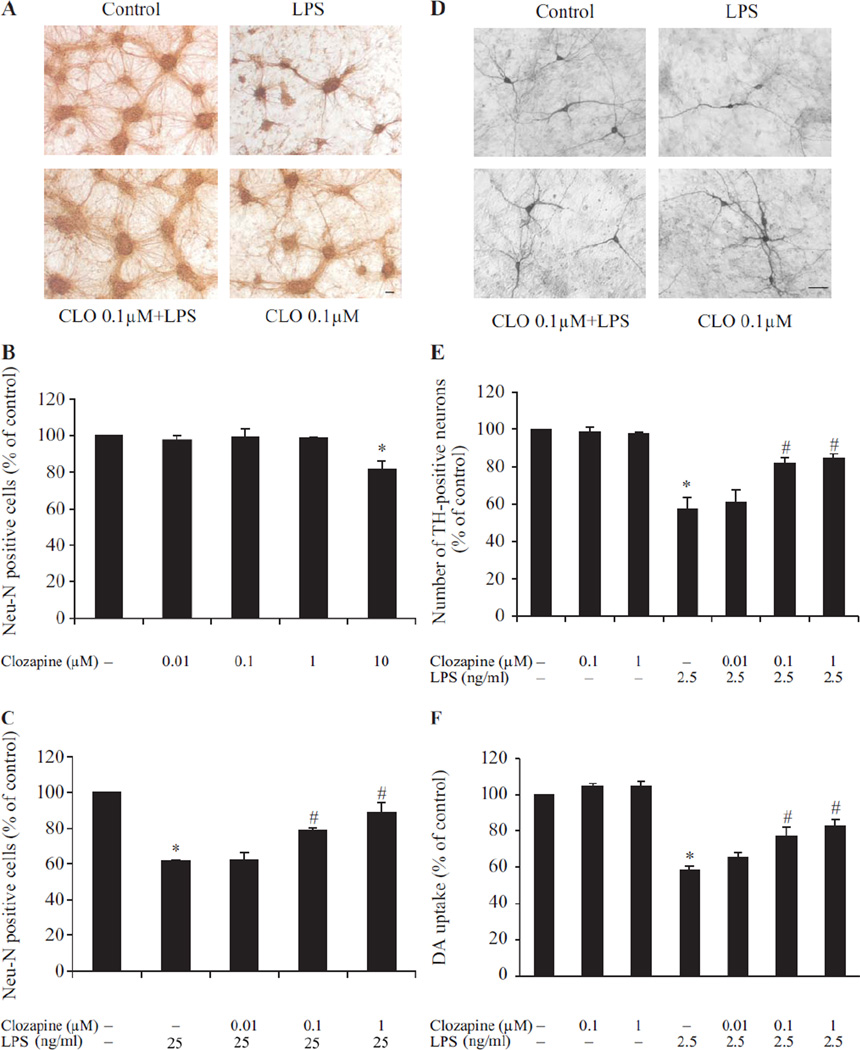
Neuroprotective effects of clozapine in inflammation-related neurodegeneration were investigated in primary neuron-glia cultures prepared from cortex or mesencephalon. LPS, an endotoxin from bacteria, was used as an immune insult to cause neurotoxicity. To examine the morphological changes of neurons in cortical cultures following LPS (25ng/ml), we performed microtubule-associated protein 2 (MAP)-2 immunostaining (Fig. 1A). In both cultures treated with vehicle or clozapine alone, strong MAP-2 immunoreactivity was detected both in soma and in neuronal processes, whereas in cultures treated with LPS the immunoreactivity in neuronal processes was interrupted. Clozapine pretreatment protected neurons from LPS-induced damage as shown by enhanced MAP-2 immunostaining and a dramatic increase in neurite outgrowth compare to LPS-treated cultures. The quantification of NeuN-positive cells (total neuronal counts) (Fig. 1C) confirmed the morphological observations. LPS reduced the number of NeuN-positive cells to about 60% of control. Clozapine at 0.1 µM and 1 µM concentrations effectively reduced LPS-induced neuronal loss. Neuron-glia cultures treated with clozapine (0.01 µM to 1 µM) alone showed no effect on the number of NeuN-positive cells, while there was a decline of NeuN-positive cells with 10 µM clozapine (Fig. 1B).
Fig. 1. Clozapine was neuroprotective in a LPS-induced neurotoxcity model.
Cortical or mesencephalic neuron–glia cultures were seeded in 24-well plates at 5 × 105 cells/well. (A) Representative images of control, LPS (25 ng/ml) without or with clozapine-treated cortical neuron-glia cultures stained by MAP2 antibody. (B) Bar graph showing the quantification of NeuN-positive neurons in cultures treated with different concentration of clozapine (0.01– 10 µM). (C) Quantification of NeuN-positive neurons in the cultures treated with clozapine (0.01–1 µM) for 24hrs prior to LPS administration. (D) Representative images of control, LPS (2.5 ng/ml) without or with clozapine-treated mesencephalic neuron-glia cultures stained by TH antibody. (E) Bar graph showing the quantification of TH-positive neurons in the mesencephalic neuron-glia cultures treated with clozapine (0.01–1 µM) and/or LPS. (F) Mesencephalic neuron-glia cultures were treated with clozapine (0.01–1 µM) for 24hrs before LPS (2.5 ng/ml) administration. The functional status of DA neurons was quantified by the [3H] DA uptake assay seven days after LPS stimulation. Results were expressed as a percentage of the vehicle-treated control cultures and were the mean ± SEM from three independent experiments in triplicate. *, P<0.05 compared with the vehicle-treated control cultures; #, P<0.05, versus LPS-treated cultures. Scale bar: 50 µm.
To further demonstrate the neuroprotective effect of clozapine, a well-established mesencephalic neuron-glia cultures which contain dopamine (DA) neurons were used. Since DA neurons are much more sensitive than other neurons to LPS challenge (Gao et al., 2002), a lower concentration of LPS (2.5 ng/ml) was used in mesencephalic neuron-glia cultures. Morphological change of DA neurons subjected to LPS treatment without or with different concentration of clozapine (0.01 µM to 1 µM) was determined by immunostaining of tyrosine hydroxylase (TH), a marker for DA neurons (Fig. 1D). LPS treatment caused a loss of TH-positive neuronal processes. With clozapine pretreatment, DA neurons were obviously less affected, displaying much longer and more elaborate TH-positive processes compared with those from LPS-treated cultures. When the number of TH-positive neurons was counted (Fig. 1E), clozapine at 0.1 µM and 1 µM concentration substantially reduced LPS-induced DA neuronal loss. DA homeostasis is maintained by the DA transporter (DAT), which functions to reuptake released DA from the synaptic cleft (Zhuang et al., 2001). Thus, a DA uptake assay, which measures the capacity of DAT to take up radiolabeled DA, was used to evaluate the function of DA neurons in mesencephalic cultures. In Fig. 1F, mesencephalic neuron-glia cultures were pretreated with clozapine or vehicle for 24 hrs and then stimulated with LPS for 6 days. The result showed that LPS reduced the uptake capacity of the cultures to approximately 60% of the vehicle control. LPS-induced reduction in DA uptake was ameliorated by pretreatment with 0.1 µM and 1 µM clozapine. The protective effect of clozapine was absent at concentrations below 0.01 µM (Fig. 1F), and high concentration of clozapine (10 µM) showed neurotoxicity (Fig.1B). Thus, the effective concentrations (0.1 µM and 1 µM) of clozapine were used for further analysis of neuroprotection and the associated mechanisms.
Clozapine-afforded neuroprotection is microglia-depended
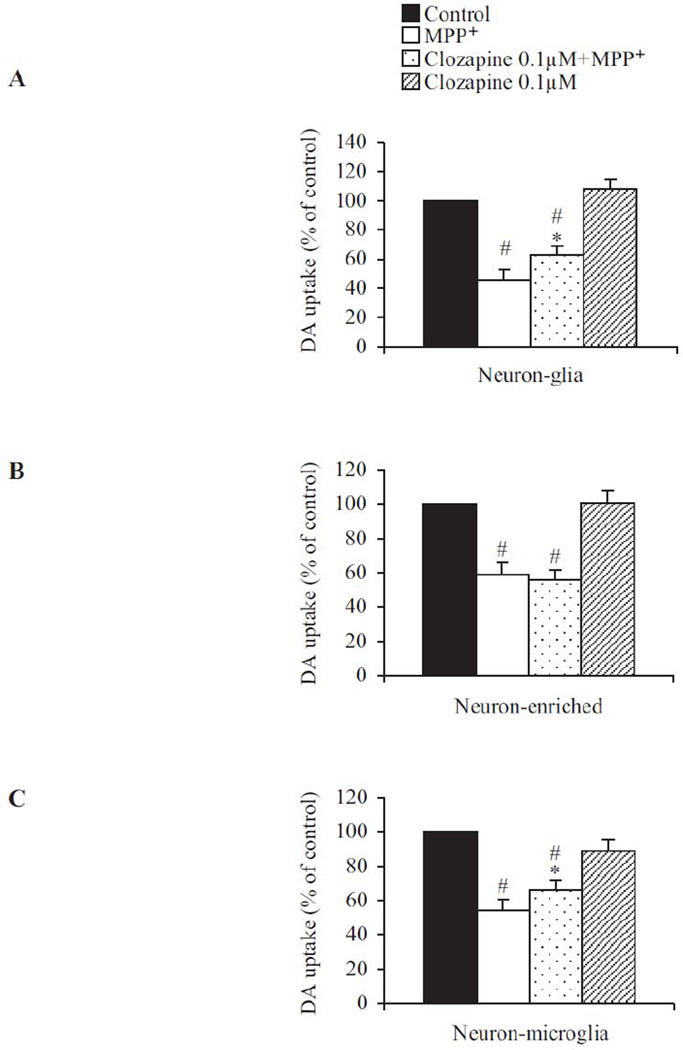
To investigate whether clozapine exerts its protective effect directly on neurons or indirectly through inhibiting inflammatory responses elicited by glial cells, we treated neuron-glial cultures with MPP+, the active metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). It has been previously reported (Gao et al., 2003) that microglia become activated in response to MPP+-induced direct DA neuronal damage and contribute to progressive neurotoxicity. Thus neuronal death induced by MPP+ can be attributed to its direct cytotoxic effects as well as neuroinflammatory insults due to reactivation of microglia, so called reactive microgliosis. Clozapine (0.1 µM) significantly but not completely protected neurons from MPP+-mediated neurotoxicity (Fig. 2A). This result is consistent with the view that clozapine protected MPP+-induced DA neuron toxicity by inhibiting reactive microgliosis, but failed in preventing the direct toxicity of MPP+ on neurons. To further confirm this possibility, we compared the ability of clozapine to reduce MPP+-induced neurotoxicity in neuron-enriched culture (Fig. 2B) and neuron-microglia co-cultures (Fig. 2C). DA uptake was reduced to about 60% of control in neuron-enriched cultures. Clozapine (0.1 µM) failed to show any protection in neuron-enriched cultures (Fig. 2B). Adding microglia back into the neuron-enriched cultures reinstated the protective effect of clozapine (Fig. 2C), supporting a microglia-dependent mechanism for its neuroprotective effect.
Fig. 2. The neuroprotective effect of clozapine was microglia-dependent.
Clozapine (0.1 µM) or vehicle was added to the following different cell cultures: (A) neuron-glia cultures, (B) neuron-enriched cultures, or (C) neuron-microglia co-cultures by adding 1×105/well of enriched microglia to the neuron-enriched cultures. MPP+ (0.25 µM) was added 24hrs after clozapine pretreatment. Neurotoxicity was quantified by the [3H] DA uptake assay 7 days after MPP+ treatment. Results were expressed as a percentage of corresponding control cultures and were the mean ± SEM from three to four independent experiments in triplicate. *, P < 0.05 compared with vehicle-treated control culture cultures; #, P<0.05, versus LPS-treated cultures.
Clozapine inhibits microglial activation
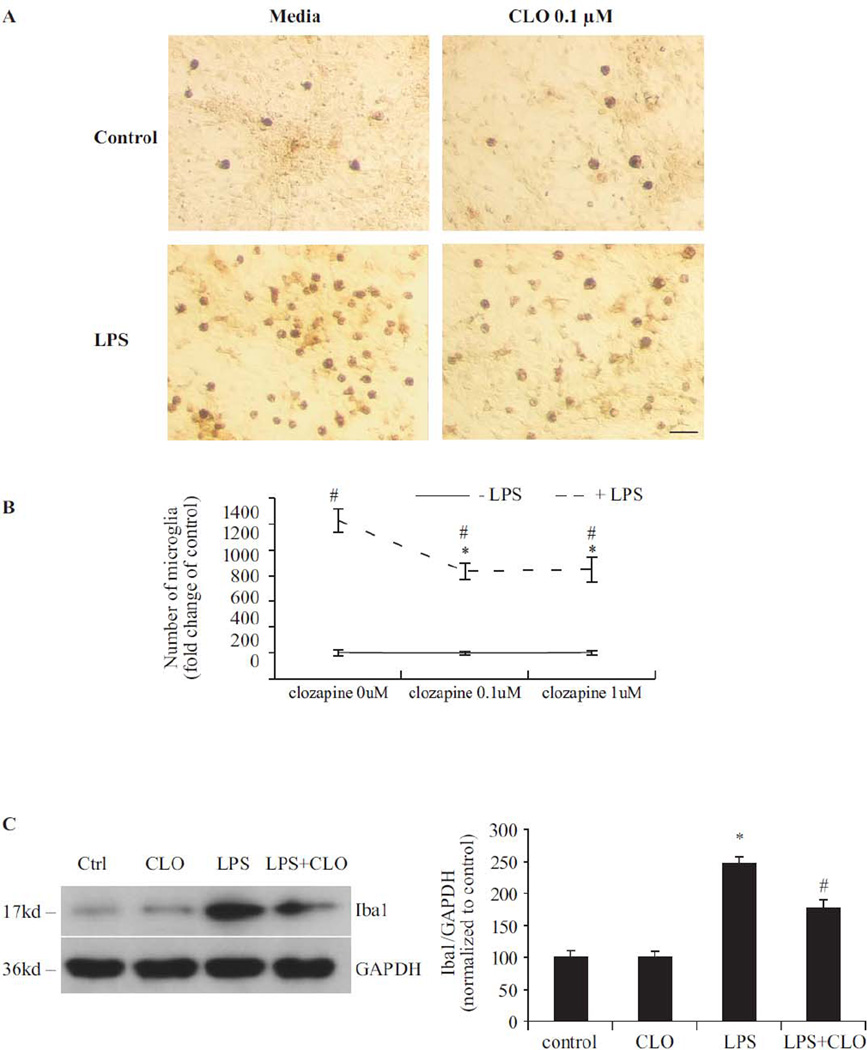
LPS-induced microglial activation and the consequent production of pro-inflammatory factors have been linked to its neurotoxicity (Gao et al., 2002). To discern the effect of clozapine on microglia, the morphological changes that accompany microglial activation were examined by immunostaining of Iba1, a specific microglial marker. Seven days after LPS exposure, numerous activated microglia, characterized by intensified Iba1 staining and enlarged cell size, were observed (Fig. 3A). LPS-induced activation of microglia was less pronounced in the cultures pretreated with clozapine (0.1 µM and 1 µM), exhibiting reduced Iba1 expression and minimal morphological changes. Enumeration of microglia showed that LPS treatment significantly increased the number of activated microglia; while clozapine pretreatment significantly reduced the number of activated microglia compare to LPS-treated group (Fig. 3B). Clozapine treatment alone had no effect on microglial activation. Western blot analysis of Iba1 was then performed to provide another quantitative estimation of microglial activation. Seven days after LPS treatment, Iba1 expression increased significantly. Clozapine pretreatment inhibited the LPS-induced increase of Iba1 expression (Fig. 3C).
Fig. 3. Clozapine inhibited LPS-induced microglial activation without affecting microglial viability.
(A) Mesencephalic neuron-glia cultures were treated with LPS and/or clozapine (0.1 and 1 µM). Cultures were fixed at 7 days after treatments. Activation of microglia was visualized by immunostaining of the Iba1 antigen, a microglia marker. The images presented are representative of three independent experiments. Scale bar: 50 µm. (B) Quantification of Iba1-positive microglia in panel A. Results were the mean ± SEM from three independent experiments in triplicate. (C) Western blot analysis of microglial activation. Cell lysates of mesencephalic neuron-glia cultures were prepared 7 days after LPS and/or clozapine (0.1 µM) treatment. Immunoblot analysis was performed to assess Iba1 antigen. GAPDH was used as loading control. The ratio of densitometry values of Iba1 and GAPDH was analyzed and normalized to control. The experiment has been performed three times. Results were presented as the mean ± SEM. (D, E) HAPI cells were pretreated with vehicle or clozapine (0.1 or 1µM) for 24 hrs followed by stimulation with LPS (2.5 ng/ml) for 24 hrs. Expression of OX6 (MHCII) was monitored by flow cytometry. Percentage of OX-6 positive cells was analyzed on a FACSCalibur. Values are mean ± SEM from three experiments. (F) Microglia-enriched culture was treated with clozapine (0.1 and 1 µM) for 24h or 48h. Cell viability was measured by PMS/MTS assay. Results were expressed as a percentage of control and were the mean ± SEM. *, P < 0.05 compared with vehicle-treated control culture cultures; #, P<0.05, versus LPS-treated cultures.
To further confirm the effect of clozapine on LPS-induced microglial activation, we used HAPI cells (microglial cell line from rats) to determine the effect of clozapine (0.1 µM and 1 µM) on the LPS-induced expression of major histocompatibility antigen class II (MHC-II, OX-6). MHC-II was chosen as a marker for microglial activation here because MHC-II-positive microglia have been suggested to be involved in progressive neurodegeneration (Yasuda et al., 2007). MHC-II (OX-6) was measured by flow cytometry analysis 24 hrs after the LPS (2.5ng/ml) treatment (Fig. 3D, E). The OX-6 expression on untreated HAPI cells is very low (2.5%); LPS treatment markedly increased the percentage of OX-6-positive cells (11.37%). Treatment with clozapine (0.1 µM and 1 µM) 24 hrs prior to LPS significantly decreased the percentage of OX-6-positive cells (7.3% and 5.5%, respectively) when compared with LPS-treated cultures.
PMS/MTS analysis revealed that clozapine at 0.1 µM and 1 µM did not change the viability of microglia (Fig. 3F) in enriched microglia cultures, suggesting that clozapine affects microglial activation via a functional inhibitory mechanism other than inducing microglial death.
Clozapine inhibits LPS-induced production of reactive oxygen species (ROS) and pro-inflammatory factors
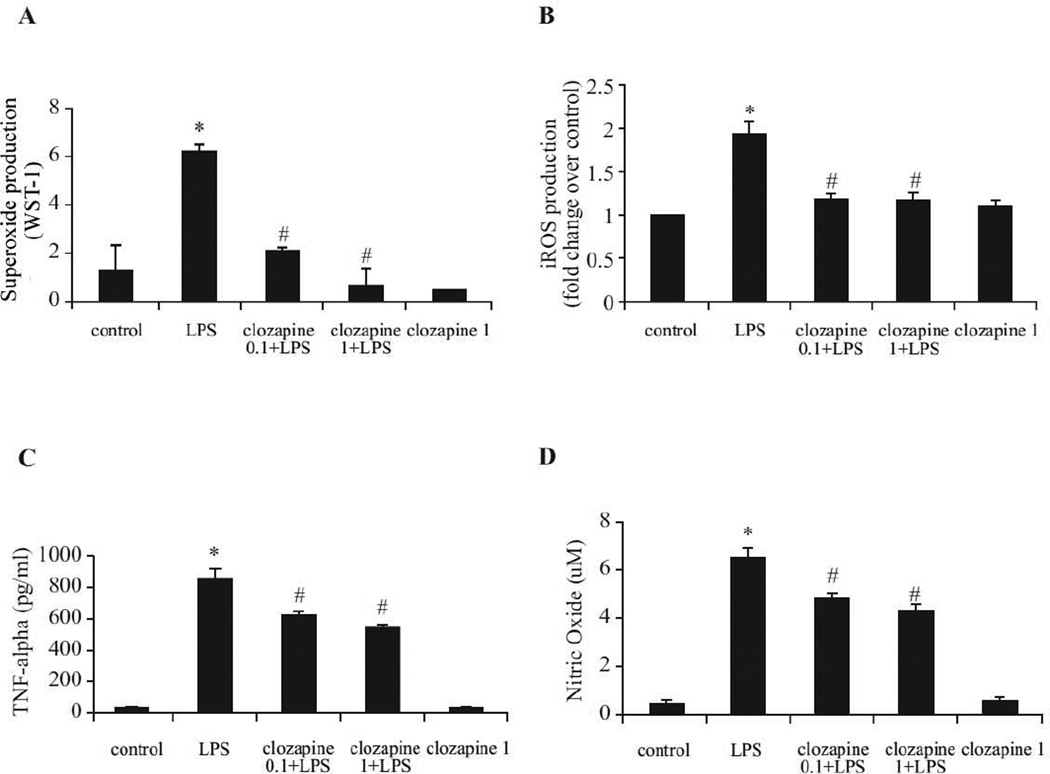
To test the ability of clozapine to attenuate the production of microglial extracellular superoxide, microglia-enriched cultures were pretreated with clozapine (0.1 µM and 1 µM) for 24 hrs, and then exposed to LPS. Clozapine significantly reduced LPS-induced extracellular superoxide production to nearly control levels (Fig. 4A). Intracellular ROS are critical for the microglial inflammatory response and are components of a signaling pathway regulating proinflammatory gene expression in multiple cell types, including microglia (Gorlach et al., 2002). As shown in Fig. 4B, clozapine significantly reduced LPS-induced intracellular ROS production in microglia-enriched cultures. Clozapine alone has no effect on extracellular superoxide and intracellular ROS. Release of nitric oxide (NO) and TNF-α from neuron-glia cultures was also measured after LPS stimulation. As shown in Fig. 4C and 4D, pretreatment with 0.1 µM and 1 µM clozapine decreased the production of NO and TNF-α compared with cultures treated with LPS alone.
Fig. 4. Clozapine inhibited LPS-induced production of reactive oxygen species (ROS) and pro-inflammatory factors.
(A, B) Microglia-enriched cultures seeded at 5 × 104/well were pretreated with clozapine (0.1 and 1 µM) for 24hrs before LPS (2.5 ng/ml) administration. (A) Production of extracellular superoxide was measured as SOD-inhibitable reduction of WST-1. (B) Intracellular ROS (iROS) production was determined by a fluorescence probe DCFH-DA. Results were normalized to fold change of control. (C, D) Rat primary midbrain neuron-glia cultures were pretreated with vehicle or clozapine (0.1 and 1 µM) for 24 hrs before the LPS (2.5 ng/ml) stimulation. (C) The level of TNF-α in medium was determined at 3 hrs after LPS challenge. (D) Nitric oxide was measured at 24 hrs after LPS treatment.. Results were expressed as mean ± SEM. from three to four independent experiments in triplicate. *, P<0.05 compared with the vehicle-treated control cultures; #, P<0.05, versus LPS-treated cultures.
PHOX plays a critical role in clozapine-mediated protection against LPS-induced neurodegeneration
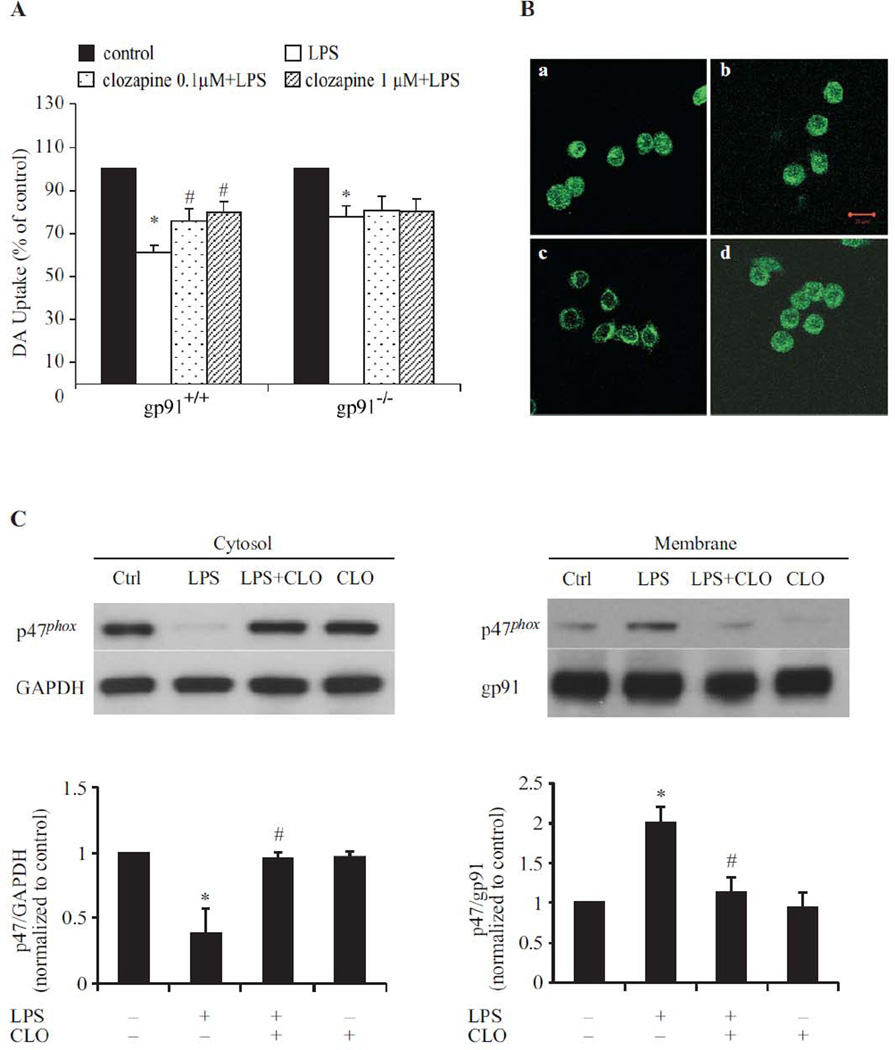
Phagocyte NADPH oxidase (PHOX), is the major enzyme for the production of extracellular superoxide in phagocytes (Babior, 1999). Considering the notable decrease of superoxide production by clozapine, mutant mice deficient in gp91phox, the catalytic subunit of PHOX, were used to determine if microglial PHOX plays a role in clozapine-afforded neuroprotection. Neuron-glia cultures from wild type (gp91phox+/+) mice and mutant (gp91phox−/−) mice were treated with LPS with/without clozapine (0.1µM and 1µM) pretreatment. DA neurotoxicity was assessed by [3H] DA uptake assay (Fig. 5A). In cultures prepared from gp91phox+/+ mice, LPS markedly decreased DA uptake capacity by 40 %, which was greatly attenuated by clozapine (0.1µM and 1µM) pretreatment. In contrast, although LPS also induced a significant albeit smaller reduction (20%) in [3H] DA uptake capacity in gp91phox−/− mice, clozapine failed to show any protection on DA neurons from these mice.
Fig. 5. Microglia PHOX was the target of clozapine (CLO)-induced neuroprotection in LPS-induced neurotoxicity.
(A) Gp91phox+/+ and gp91phox–/– mice neuron-glia cultures were pretreated with vehicle or clozapine for 24hrs followed by 2.5 ng/ml LPS treatment. Neurotoxicity was assessed by the [3H] DA uptake assay. Results were expressed as a percentage of the control culture, and were the means ± SEM of six individual experiments in triplicate in each experiment. *, P < 0.05 compared with vehicle-treated control culture cultures; #, P<0.05, versus LPS-treated cultures. (B) HAPI cells seeded in a dish at 5 × 104 cells/well were treated with LPS for 10 min in the absence or presence of clozapine pretreatment for 24 hrs. Cells were incubated with a rabbit polyclonal antibody against p47phox and then with a FITC-conjugated goat anti-rabbit antibody. Focal planes spaced at 0.4 µm intervals were imaged. The signal of p47phox is shown. Scale bar: 20 µm. (C) Western blot assays for p47phox levels in membrane and cytoslic fractions of HAPI cells 10 min after treatment. Densitometry analysis was performed with values of p47phox normalized to each respective loading control (GAPDH for cytosolic fraction, gp91 for membrane fraction) and further normalized to vehicle-treated controls. Experiments were performed at least three times.
It is known that the activation of PHOX requires the translocation of cytoplasmic regulators (p47phox, p67phox, p40phox, and Rac1) and subsequent interaction with the membrane-spanning catalytic subunit flavocytochrome b558, in order to commence activation of superoxide production (Babior,1999). To determine whether clozapine modulates the translocation of cytosolic components of PHOX, levels of p47phox in cytosolic and membrane fractions were measured following LPS and/or clozapine treatment in HAPI cells. Confocal microscopy analysis showed that LPS increased the translocation of p47phox from the cytosol to the membrane (Fig. 5B-c). The translocation of p47phox was absent when cells were treated with clozapine for 24 hrs prior to LPS (Fig. 5B-d). Likewise, western blot analyses showed a decrease in p47phox levels in the cytosolic fraction, but an increase in membrane fraction, in HAPI cells 10 min after LPS exposure (Fig. 5C). The translocation of p47phox was much less prominent with clozapine treatment 24 hrs before LPS exposure, suggesting that clozapine may inhibit superoxide production in microglia through the inhibition of p47phox translocation to the cell membrane.
Clozapine impairs activation of PHOX through inhibiting PI3K pathway
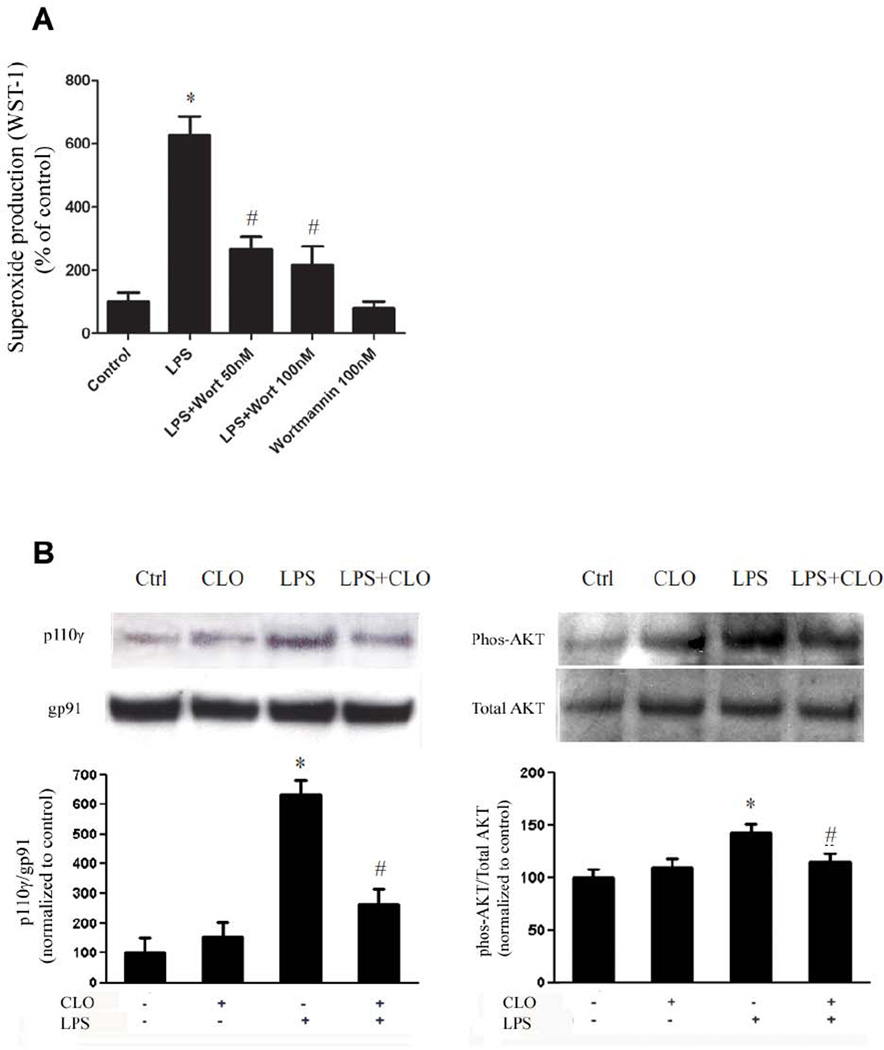
Previous studies suggested that the phosphatidylinositol 3-kinases (PI3K) is an important upstream signal protein in the process of activation of PHOX (Anderson et al., 2008; Zhang et al., 2011). Our study in primary microglial cultures confirmed that PI3K is critical in LPS-induced PHOX activation. Pretreatment with wortmannin (50nM and 100 nM), a commonly used PI3K inhibitor, for 10 min significantly mitigated LPS-induced superoxide production (Fig. 6A). Further studies were then conducted to evaluate the effect of clozapine on the activation of PI3K. PI3K is a heterodimeric complex composed of an 85 kD regulatory subunit and a 110 kD catalytic subunit. In the resting condition, subunits of PI3K were mainly located in the cytoplasm in an inactive form. Upon activation, p110 was translocated to the plasma membrane. Our western blot analysis showed that LPS treatment resulted in increased PI3K p110 in the membrane fraction. Pretreatment of clozapine (1 µM) significantly reduced LPS-induced PI3K activation in primary microglia cultures as shown by the decrease in the recruitment of p110γ to membrane and decreased phosphorylation of AKT, a key downstream kinase of PI3K (Fig. 6B). These data indicates that clozapine reduced the activation of PHOX through inhibiting PI3K signaling pathway.
Fig. 6. Clozapine impairs activation of PHOX through inhibiting PI3K pathway.
(A) Microglia-enriched cultures seeded at 5 × 104/well were pretreated with wortmannin (50nM, 100 nM) for 10 min before LPS (10 ng/ml) administration. Production of extracellular superoxide was measured as SOD-inhibitable reduction of WST-1. Results were expressed as a percentage of the control. *, P < 0.05 compared with vehicle-treated control. (B) Microglia-enriched cultures were pretreated with clozapine (1 µM) for 30 min before LPS (10 ng/ml) administration. After 10 min LPS administration, cells were collected for western blot for the determination of levels of membrane p110γ and cytosolic phosphorylated AKT. Densitometry analysis was performed with values normalized to each respective loading control (gp91phox as a membrane marker, total AKT for its phosphorylated level) and further normalized to vehicle-treated controls. Experiments were performed at least three times. *, P < 0.05 compared with vehicle-treated control; #, P<0.05, versus LPS-treated cultures.
Discussion
In this study the neuroprotective effect of clozapine was demonstrated by the findings that clozapine greatly attenuated neurodegeneration induced by LPS or MPP+ in a microglia-depended manner. Mechanistic studies revealed that clozapine-afforded neuroprotection was related to its anti-inflammatory effect, which was mainly mediated through the inhibition of microglial PHOX.
For decades, neurotransmitters disturbance has been considered to be an important feature to explain many symptoms of SCZ and a common target for the basis of pharmacological therapy of SCZ patients. For example, superior therapeutic effects of clozapine over typical antipsychotics have been attributed to its relatively lower affinity for DA D2 receptor and higher affinity to 5HT2A receptor (Kapur et al., 1999). However, several inadequacies of the neurotransmitter theory explaining the pharmacological basis of antipsychotics became evident. For instance, antipsychotics block receptors of neurotransmitters instantly while antipsychotic effect is not evident until weeks after medication. Thus, additional mechanisms might partake in the therapeutic effect of antipsychotics.
The neuroprotective effect of clozapine has been postulated with the observation that clozapine can attenuate the loss of grey matter over time in SCZ patients (van Haren et al., 2007). In the present study, we used mesencephalic and cortical neuron-glia mixed cultures as an in vitro model to investigate the neuroprotective effect of clozapine on neurons and to elucidate the underlying mechanisms. We showed that clozapine treatment attenuated the LPS-induced neuronal damage in cortical cultures. Furthermore, the survival of DA neurons, the neurons most sensitive to LPS-induced inflammatory damage in mesencephalic culture system, was enhanced by clozapine pretreatment. The DA uptake capacity was also preserved; suggesting that these spared DA neurons has preserved transporter activity to properly regulate the uptake and inactivation of DA.
Clozapine has been documented to directly protect PC12 cells from death induced by a variety of stimuli (Bai et al., 2002; Qing et al., 2003). The effective concentrations of clozapine used (25–50 µM) were, however, much higher than the concentration (10 µM), which showed neurotoxicity in our primary culture studies (Fig. 1B). In addition, clozapine at effective concentration (0.1 µM) in neuron-glia cultures failed to show any protection in neuron-enriched cultures (Fig. 2). Therefore, it is likely that clozapine-elicited neuroprotection is mediated through an indirect mechanism. This study confirmed this possibility showing that clozapine-afforded neuroprotection is microglia-dependent since it ameliorated MPP+-induced neurotoxicity only in the presence of microglia. This microglia-dependent neuroprotective mechanism of clozapine could be associated with its delayed onset of antipsychotic effect since microglia contributed to the neuronal damage in a delayed and progressive manner. The possible contribution of other glial cells such as astroglia in clozapine-elicited neuroprotection was not evaluated in this study. In view of previously reported evidence that clozapine stimulate GDNF release from C6 glioma cells (Shao et al., 2006), further work is warranted to determine roles of astroglia in clozapine-related neruoprotection.
Although microglia activation originally aims to protect CNS, the uncontrolled microglial hyperactivity may lead to neuronal damage by releasing inflammatory mediators, such as free radicals and pro-inflammatory factors (Liu and Hong, 2003). Microglia-dependent neuroinflammation has been increasingly accepted to be responsible for the progression of neurodegenerative diseases (Gao and Hong, 2008; Zarifkar et al., 2010). Thus, microglia-suppressing agents have been considered promising therapy to slow the progress of neurodegenerative diseases (Zhang et al., 2006; Adams et al., 2007; Zhang et al., 2008). In the present study, we showed that the microglia-dependent neuroprotective effect of clozapine could also be explained by its ability to inhibit microglial activation and subsequent release of inflammatory mediators, including ROS (Fig. 4A and B), NO (Fig. 4C) and TNF-α (Fig. 4D). We further showed that the reduction of these proinflammatory factors by clozapine reflected functional inhibition of microglial activation, since the viability of microglia was not affected.
Strong evidence indicates that oxygen free radicals play an important role in the pathophysiology of SCZ (Reddy and Yao, 1996), as indicated by altered antioxidant enzyme activities and increased level of lipid peroxidation (Mahadik et al., 1998; Herken et al., 2001; Zhang et al., 2003). Superoxide is one of the prominent factors released by activated microglia (Block et al., 2007), which may serve as a major source for increased oxidative stress in SCZ patients. The potent inhibitory effect of clozapine on microglia-derived superoxide and intracellular ROS (Fig. 4A and 4B) can be a critical mechanism related to its therapeutic effects. This is consistent with previous reports that clozapine reduces the ROS production (Gross et al., 2003) and lipid peroxidation (Kropp et al., 2005) from monocytes of SCZ patients. We further showed that PHOX is critical for the inhibitory action of clozapine on microglial activation. Genetic knockout of the catalytic subunit gp91phox of PHOX completely abolished clozapine-afforded DA neuroprotection (Fig. 5A). The critical involvement of PHOX in clozapine-mediated neuroprotection is further supported by our results demonstrating that translocation of p47phox from cytosol to cell membrane in microglia after LPS treatment was attenuated by clozapine pretreatment (Fig. 5B and 5C).
Previous studies indicate that PI3K plays an important role in regulating signal transduction leading to PHOX activation (Anderson et al., 2008). Class I PI3K is necessary for the formation of phagosomes (Araki et al., 1996; Vieira et al., 2001), and Class III PI3K contributes to phagosome maturation (Ellson et al., 2001; Tian et al., 2008), which is the important process followed by PHOX activation in neutrophils. Importantly, PtdIns(3)P generated by PI3K can regulate PHOX assembly and activation through binding to the cytosolic subunit p40phox (Ellson et al., 2001; Tian et al., 2008). Our study demonstrated that inhibition on microglial PHOX activation by clozapine was most likely though impairing the PI3K signaling pathway. This is consistent with the study in glioblastoma cells showing that clozapine inhibited PI3K/Akt pathway (Shin et al., 2006). Interestingly, previous observations in PC12 cells (Lu and Dwyer, 2005) and SH-SY5Y neuroblastoma cells (Kang et al, 2004) indicated that clozapine increases the phosphorylation of Akt and the cell survival. It is possible that clozapine exerts differential regulation on PI3K signaling in different cell types. The mechanism of clozapine’s inhibition on PI3K pathway is still not clear. It has been suggested in glioblastoma cells that clozapine inhibits PI3K signaling and the downstream phosphorylation of Akt possibly through inhibiting Ca2+/CaM signaling (Shin et al., 2006). Calcium signaling is also involved in microglia activation (Moller, 2002); therefore, further studies are warranted to investigate the effect of clozapine on calcium signaling in microglia.
Taken together, this study illustrates alternative anti-inflammatory function and neuroprotective mechanisms for therapeutic effects of clozapine. In addition to the traditional view of clozapine as a neurotransmitter modulator, it can also afford neuroprotection by inhibiting microglial over-activation and subsequent release of ROS and cytokines during inflammation. This study further supports the notion that anti-inflammatory effect maybe associated with the actions of psychoactive drugs, and also suggests a possible use of antipsychotic drug for treatment of neurodegenerative diseases.
Acknowledgements
We would like to thank Anthony Lockhart for assistance with animal colony management and maintenance of the timed pregnant mice. This research was supported by the Intramural Research Program of the National Institute of Health, the National Institute of Environmental Health Sciences.
Footnotes
Conflict of interest:
The authors declare that they have nocompeting interests or conflicts of interest.
References
- Adams RA, Bauer J, Flick MJ, Sikorski SL, Nuriel T, Lassmann H, Degen JL, Akassoglou K. The fibrin-derived gamma377-395 peptide inhibits microglia activation and suppresses relapsing paralysis in central nervous system autoimmune disease. J Exp Med. 2007;204:571–582. doi: 10.1084/jem.20061931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altshuler LL, Casanova MF, Goldberg TE, Kleinman JE. The hippocampus and parahippocampus in schizophrenia, suicide, and control brains. Arch Gen Psychiatry. 1990;47:1029–1034. doi: 10.1001/archpsyc.1990.01810230045008. [DOI] [PubMed] [Google Scholar]
- Anderson KE, Boyle KB, Davidson K, Chessa TA, Kulkarni S, Jarvis GE, Sindrilaru A, Scharffetter-Kochanek K, Rausch O, Stephens LR, Hawkins PT. CD18-dependent activation of the neutrophil NADPH oxidase during phagocytosis of Escherichia coli or Staphylococcus aureus is regulated by class III but not class I or II PI3Ks. Blood. 2008;112:5202–5211. doi: 10.1182/blood-2008-04-149450. [DOI] [PubMed] [Google Scholar]
- Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior BM. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- Bai O, Wei Z, Lu W, Bowen R, Keegan D, Li XM. Protective effects of atypical antipsychotic drugs on PC12 cells after serum withdrawal. J Neurosci Res. 2002;69:278–283. doi: 10.1002/jnr.10290. [DOI] [PubMed] [Google Scholar]
- Baune BT, Eyre H. Anti-inflammatory effects of antidepressant and atypical antipsychotic medication for the treatment of major depression and comorbid arthritis: a case report. J Med Case Reports. 2010;4:6. doi: 10.1186/1752-1947-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–1132. doi: 10.1042/BST0351127. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB. A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-alpha and interferon-gamma in mice. Int Immunopharmacol. 2006;6:903–907. doi: 10.1016/j.intimp.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L. Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry. 2002;159:59–65. doi: 10.1176/appi.ajp.159.1.59. [DOI] [PubMed] [Google Scholar]
- Dobos N, Korf J, Luiten PG, Eisel UL. Neuroinflammation in Alzheimer's disease and major depression. Biol Psychiatry. 2010;67:503–504. doi: 10.1016/j.biopsych.2010.01.023. [DOI] [PubMed] [Google Scholar]
- Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. doi: 10.2967/jnumed.109.066647. [DOI] [PubMed] [Google Scholar]
- Dos Santos S, Delattre AI, De Longueville F, Bult H, Raes M. Gene expression profiling of LPS-stimulated murine macrophages and role of the NF-kappaB and PI3K/mTOR signaling pathways. Ann N Y Acad Sci. 2007;1096:70–77. doi: 10.1196/annals.1397.071. [DOI] [PubMed] [Google Scholar]
- Ellson CD, Gobert-Gosse S, Anderson KE, Davidson K, Erdjument-Bromage H, Tempst P, Thuring JW, Cooper MA, Lim ZY, Holmes AB, Gaffney PR, Coadwell J, Chilvers ER, Hawkins PT, Stephens LR. PtdIns(3)P regulates the neutrophil oxidase complex by binding to the PX domain of p40(phox) Nat Cell Biol. 2001;3:679–682. doi: 10.1038/35083076. [DOI] [PubMed] [Google Scholar]
- Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao HM, Liu B, Zhang W, Hong JS. Critical role of microglial NADPH oxidase-derived free radicals in the in vitro MPTP model of Parkinson's disease. Faseb J. 2003;17:1954–1956. doi: 10.1096/fj.03-0109fje. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson's disease. J Neurochem. 2002;81:1285–1297. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Gao X, Hu X, Qian L, Yang S, Zhang W, Zhang D, Wu X, Fraser A, Wilson B, Flood PM, Block M, Hong JS. Formyl-methionyl-leucyl-phenylalanine-induced dopaminergic neurotoxicity via microglial activation: a mediator between peripheral infection and neurodegeneration? Environ Health Perspect. 2008;116:593–598. doi: 10.1289/ehp.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach A, Kietzmann T, Hess J. Redox signaling through NADPH oxidases: involvement in vascular proliferation and coagulation. Ann N Y Acad Sci. 2002;973:505–507. doi: 10.1111/j.1749-6632.2002.tb04691.x. [DOI] [PubMed] [Google Scholar]
- Gross A, Joffe G, Joutsiniemi SL, Nyberg P, Rimon R, Appelberg B. Decreased production of reactive oxygen species by blood monocytes caused by clozapine correlates with EEG slowing in schizophrenic patients. Neuropsychobiology. 2003;47:73–77. doi: 10.1159/000070012. [DOI] [PubMed] [Google Scholar]
- Herken H, Uz E, Ozyurt H, Sogut S, Virit O, Akyol O. Evidence that the activities of erythrocyte free radical scavenging enzymes and the products of lipid peroxidation are increased in different forms of schizophrenia. Mol Psychiatry. 2001;6:66–73. doi: 10.1038/sj.mp.4000789. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Bertens MG, van Haren NE, van der Tweel I, Staal WG, Baare WF, Kahn RS. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002;159:244–250. doi: 10.1176/appi.ajp.159.2.244. [DOI] [PubMed] [Google Scholar]
- Kang UG, Seo MS, Roh MS, Kim Y, Yoon SC, Kim YS. The effects of clozapine on the GSK-3-mediated signaling pathway. FEBS Lett. 2004;560:115–119. doi: 10.1016/S0014-5793(04)00082-1. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Kropp S, Kern V, Lange K, Degner D, Hajak G, Kornhuber J, Ruther E, Emrich HM, Schneider U, Bleich S. Oxidative stress during treatment with first- and second-generation antipsychotics. J Neuropsychiatry Clin Neurosci. 2005;17:227–231. doi: 10.1176/jnp.17.2.227. [DOI] [PubMed] [Google Scholar]
- Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304:1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Lu XH, Dwyer DS. Second-generation antipsychotic drugs, olanzapine, quetiapine, and clozapine enhance neurite outgrowth in PC12 cells via PI3K/AKT, ERK, and pertussis toxin-sensitive pathways. J Mol Neurosci. 2005;27:43–64. doi: 10.1385/jmn:27:1:043. [DOI] [PubMed] [Google Scholar]
- Mahadik SP, Mukherjee S, Scheffer R, Correnti EE, Mahadik JS. Elevated plasma lipid peroxides at the onset of nonaffective psychosis. Biol Psychiatry. 1998;43:674–679. doi: 10.1016/s0006-3223(97)00282-5. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Exp Gerontol. 1998;33:371–378. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Minghetti L. Role of inflammation in neurodegenerative diseases. Curr Opin Neurol. 2005;18:315–321. doi: 10.1097/01.wco.0000169752.54191.97. [DOI] [PubMed] [Google Scholar]
- Moller T. Calcium signaling in microglial cells. Glia. 2002;40:184–194. doi: 10.1002/glia.10152. [DOI] [PubMed] [Google Scholar]
- Munn NA. Microglia dysfunction in schizophrenia: an integrative theory. Med Hypotheses. 2000;54:198–202. doi: 10.1054/mehy.1999.0018. [DOI] [PubMed] [Google Scholar]
- Nair TR, Christensen JD, Kingsbury SJ, Kumar NG, Terry WM, Garver DL. Progression of cerebroventricular enlargement and the subtyping of schizophrenia. Psychiatry Res. 1997;74:141–150. doi: 10.1016/s0925-4927(97)00013-9. [DOI] [PubMed] [Google Scholar]
- Paterson GJ, Ohashi Y, Reynolds GP, Pratt JA, Morris BJ. Selective increases in the cytokine, TNFalpha, in the prefrontal cortex of PCP-treated rats and human schizophrenic subjects: influence of antipsychotic drugs. J Psychopharmacol. 2006;20:636–642. doi: 10.1177/0269881106062025. [DOI] [PubMed] [Google Scholar]
- Pei Z, Pang H, Qian L, Yang S, Wang T, Zhang W, Wu X, Dallas S, Wilson B, Reece JM, Miller DS, Hong JS, Block ML. MAC1 mediates LPS-induced production of superoxide by microglia: the role of pattern recognition receptors in dopaminergic neurotoxicity. Glia. 2007;55:1362–1373. doi: 10.1002/glia.20545. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson's disease and a prime target for therapy. J Neural Transm. 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin L, Liu Y, Cooper C, Liu B, Wilson B, Hong JS. Microglia enhance beta-amyloid peptide-induced toxicity in cortical and mesencephalic neurons by producing reactive oxygen species. J Neurochem. 2002;83:973–983. doi: 10.1046/j.1471-4159.2002.01210.x. [DOI] [PubMed] [Google Scholar]
- Qing H, Xu H, Wei Z, Gibson K, Li XM. The ability of atypical antipsychotic drugs vs. haloperidol to protect PC12 cells against MPP+-induced apoptosis. Eur J Neurosci. 2003;17:1563–1570. doi: 10.1046/j.1460-9568.2003.02590.x. [DOI] [PubMed] [Google Scholar]
- Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. doi: 10.1093/jnen/59.2.137. [DOI] [PubMed] [Google Scholar]
- Reddy RD, Yao JK. Free radical pathology in schizophrenia: a review. Prostaglandins Leukot Essent Fatty Acids. 1996;55:33–43. doi: 10.1016/s0952-3278(96)90143-x. [DOI] [PubMed] [Google Scholar]
- Seidel A, Arolt V, Hunstiger M, Rink L, Behnisch A, Kirchner H. Major depressive disorder is associated with elevated monocyte counts. Acta Psychiatr Scand. 1996;94:198–204. doi: 10.1111/j.1600-0447.1996.tb09849.x. [DOI] [PubMed] [Google Scholar]
- Shao Z, Dyck LE, Wang H, Li XM. Antipsychotic drugs cause glial cell line-derived neurotrophic factor secretion from C6 glioma cells. J Psychiatry Neurosci. 2006;31:32–37. [PMC free article] [PubMed] [Google Scholar]
- Shin SY, Choi BH, Ko J, Kim SH, Kim YS, Lee YH. Clozapine, a neuroleptic agent, inhibits Akt by counteracting Ca2+/calmodulin in PTEN-negative U-87MG human glioblastoma cells. Cell Signal. 2006;18:1876–1886. doi: 10.1016/j.cellsig.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Smith MV, Lee MJ, Islam AS, Rohrer JL, Goldberg VM, Beidelschies MA, Greenfield EM. Inhibition of the PI3K-Akt signaling pathway reduces tumor necrosis factor-alpha production in response to titanium particles in vitro. J Bone Joint Surg Am. 2007;89:1019–1027. doi: 10.2106/JBJS.F.00615. [DOI] [PubMed] [Google Scholar]
- Song C, Lin A, Kenis G, Bosmans E, Maes M. Immunosuppressive effects of clozapine and haloperidol: enhanced production of the interleukin-1 receptor antagonist. Schizophr Res. 2000;42:157–164. doi: 10.1016/s0920-9964(99)00116-4. [DOI] [PubMed] [Google Scholar]
- Tian W, Li XJ, Stull ND, Ming W, Suh CI, Bissonnette SA, Yaffe MB, Grinstein S, Atkinson SJ, Dinauer MC. Fc gamma R-stimulated activation of the NADPH oxidase: phosphoinositide-binding protein p40phox regulates NADPH oxidase activity after enzyme assembly on the phagosome. Blood. 2008;112:3867–3877. doi: 10.1182/blood-2007-11-126029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD, Cahn W, Lammertsma AA, Kahn RS. Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822. doi: 10.1016/j.biopsych.2008.04.025. [DOI] [PubMed] [Google Scholar]
- van Haren NE, Hulshoff Pol HE, Schnack HG, Cahn W, Mandl RC, Collins DL, Evans AC, Kahn RS. Focal gray matter changes in schizophrenia across the course of the illness: a 5-year follow-up study. Neuropsychopharmacology. 2007;32:2057–2066. doi: 10.1038/sj.npp.1301347. [DOI] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Rameh L, Brachmann SM, Matsuo T, Davidson HW, Schreiber A, Backer JM, Cantley LC, Grinstein S. Distinct roles of class I and class III phosphatidylinositol 3-kinases in phagosome formation and maturation. J Cell Biol. 2001;155:19–25. doi: 10.1083/jcb.200107069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepien T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol. 2005;43:81–89. [PubMed] [Google Scholar]
- Yasuda Y, Shinagawa R, Yamada M, Mori T, Tateishi N, Fujita S. Long-lasting reactive changes observed in microglia in the striatal and substantia nigral of mice after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Brain Res. 2007;1138:196–202. doi: 10.1016/j.brainres.2006.12.054. [DOI] [PubMed] [Google Scholar]
- Yirmiya R, Pollak Y, Barak O, Avitsur R, Ovadia H, Bette M, Weihe E, Weidenfeld J. Effects of antidepressant drugs on the behavioral and physiological responses to lipopolysaccharide (LPS) in rodents. Neuropsychopharmacology. 2001;24:531–544. doi: 10.1016/S0893-133X(00)00226-8. [DOI] [PubMed] [Google Scholar]
- Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N, Rastegar K, Moosavi M. Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur J Pharmacol. 2010;634:84–88. doi: 10.1016/j.ejphar.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Zhang D, Hu X, Qian L, Chen SH, Zhou H, Wilson B, Miller DS, Hong JS. Microglial MAC1 receptor and PI3K are essential in mediating beta-amyloid peptide-induced microglial activation and subsequent neurotoxicity. J Neuroinflammation. 2011;8:3. doi: 10.1186/1742-2094-8-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Hu X, Wei SJ, Liu J, Gao H, Qian L, Wilson B, Liu G, Hong JS. Squamosamide derivative FLZ protects dopaminergic neurons against inflammation-mediated neurodegeneration through the inhibition of NADPH oxidase activity. J Neuroinflammation. 2008;5:21. doi: 10.1186/1742-2094-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Shin EJ, Wang T, Lee PH, Pang H, Wie MB, Kim WK, Kim SJ, Huang WH, Wang Y, Zhang W, Hong JS, Kim HC. 3-Hydroxymorphinan, a metabolite of dextromethorphan, protects nigrostriatal pathway against MPTP-elicited damage both in vivo and in vitro. Faseb J. 2006;20:2496–2511. doi: 10.1096/fj.06-6006com. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Zhou DF, Cao LY, Zhang PY, Wu GY. Elevated blood superoxide dismutase in neuroleptic-free schizophrenia: association with positive symptoms. Psychiatry Res. 2003;117:85–88. doi: 10.1016/s0165-1781(02)00303-7. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Oosting RS, Jones SR, Gainetdinov RR, Miller GW, Caron MG, Hen R. Hyperactivity and impaired response habituation in hyperdopaminergic mice. Proc Natl Acad Sci U S A. 2001;98:1982–1987. doi: 10.1073/pnas.98.4.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky RB, Lim KO, Sullivan EV, Brown BW, Pfefferbaum A. Widespread cerebral gray matter volume deficits in schizophrenia. Arch Gen Psychiatry. 1992;49:195–205. doi: 10.1001/archpsyc.1992.01820030027004. [DOI] [PubMed] [Google Scholar]