Abstract
With increasing lifespan, therapeutic interventions for the treatment of disorders such as type 2 diabetes mellitus are in great demand. Despite billions of dollars invested to reduce the symptoms and complications due to diabetes mellitus, current treatments (e.g., insulin replacements, sensitization) remain inadequate, justifying the search for novel therapeutic approaches or alternative solutions, including dietary supplementation, for the treatment of diabetes mellitus in every age group. The involvement of the vanilloid system in the regulation of metabolism has been identified, and the emerging role of its receptors, the transient receptor potential vanilloid type 1 (TRPV1), in diabetes was recently demonstrated. Indeed, beneficial effects of dietary capsaicin, an agonist of TRPV1 receptors, were identified for improving glucose, insulin and glucagon-like peptide-1 levels. Recent findings regarding TRPV1 receptors in association with whole body metabolism including glucose homeostasis will be reviewed in this article.
Keywords: TRPV1, glucose, insulin, GLP-1, hypothalamus
Introduction
Obesity associated with insulin resistance is of increasing significance with aging as it leads to the development of type 2 diabetes mellitus. With increasing prevalence of type 2 diabetes mellitus, therapeutic interventions to reduce the symptoms and complications of the disease are in great demand; therefore, there is a continuous search for new compounds, receptors or modulators that raise the possibility of improving glycemic status. One of the potentially promising cation channel family is the transient receptor potential (TRP) superfamily, which has been associated with a variety of biological functions. The members of this superfamily are mainly permeable to cations, especially to Ca2+, which is necessary for many cellular processes including cell proliferation, gene transcription, and cell death. Furthermore, strong correlation has been discovered between the expression of TRP channels and certain pathophysiological symptoms (Nilius, Owsianik, Voets, Peters, 2007).
TRP channels can be divided into six subfamilies: 1) classical or canonical TRP family (TRPC); 2) melastatin-related family (TRPM); 3) polycystin-related family (TRPP); 4) ankyrin family (TRPA); 5) mucolipin-related family (TRPML); and 6) vanilloid family (TRPV) (Nilius, Owsianik, Voets, Peters, 2007). Studies of TRP channels in the last 15 years determined that they may play a role in the development of a variety of diseases including pulmonary diseases, cancer, renal diseases, cardiovascular diseases, brain disorders, obesity and diabetes mellitus (Nilius, Owsianik, Voets, Peters, 2007; Liu, Zhu, Tepel, 2008). TRPV family, which is one of the most studied TRP channels, received particular attention. TRPV family has six members: TRPV1–TRPV6. TRPV1–TRPV4 channels are temperature-activated channels, and they also could be activated by physical and chemical stimuli, including numerous endogenous and exogenous ligands as reviewed previously (Nilius, Owsianik, Voets, Peters, 2007; Clapham, Runnels, Strubing, 2001). Furthermore, one of the TRPV members, the transient receptor potential vanilloid type 1 (TRPV1) was shown to have beneficial effects on glucose homeostasis (Szallasi, Cortright, Blum, Eid, 2007; Starowicz, Nigam, DiMarzo, 2007), and this article will focus on the role of TRPV1 in whole body metabolism.
TRPV1
Transient receptor potential vanilloid type 1 (TRPV1) was identified and cloned in 1997 and it is the first identified member of the TRPV subfamily (Caterina, Schumacher, Tominaga, Rosen, Levine, Julius, 1997). TRPV1 is known to be the molecular integrator of inflammatory mediators; thus, antagonists of TRPV1 have been developed for the treatment of chronic inflammatory conditions (Szallasi, Cortright, Blum, Eid, 2007). Due to advances in research during the past decade, it became obvious that TRPV1 receptors also play a significant role in the regulation of glucose homeostasis, and that TRPV1 receptors could contribute to the development and progression of diabetes, including both type 1 diabetes mellitus (T1D) and type 2 diabetes mellitus (T2D) (Razavi, Chan, Afifiyan, Liu, Wan, Yantha, 2006; Suri and Szallasi, 2007; Tsui, Paltser, Chan, Dorfman, Dosch, 2011; Wang, Yan, Zhong, Chen, Ni, Li, et al., 2012). TRPV1 is a ligand-gated nonselective cation channel with high permeability to Ca2+. TRPV1 is widely expressed throughout the whole body, including sensory nerve fibers controlling insulin release from pancreatic beta cells (Razavi, Chan, Afifiyan, Liu, Wan, Yantha, 2006; Gram, Ahren, Nagy, Olsen, Brand, Sundler et al., 2007) and in brain areas governing liver function (Gao, Miyata, Bhaskaran, Derbenev, Zsombok, 2012). TRPV1 can be activated by physical, chemical stimulation (Tominaga and Tominaga, 2005; Starowicz, Nigam, DiMarzo, 2007) and by endovanilloids, the endogenous ligands of TRPV1 (van der Stelt, DiMarzo, 2004). Anandamide, an endogenous agonist of cannabinoid receptors, and the unsaturated long chain N-acylethanolamines were the first identified endogenous ligands of TRPV1 (Zygmunt, Petersson, Andersson, Chuang, Sorgard, Di Marzo, et al. 1999). Currently, the endogenous lipid families, the N-acyldopamines (e.g., NADA) and lipoxigenase metabolites of arachidonic acid (e.g., 12-(S)-HPETE, leukotriene B4), are known to activate TRPV1 receptors (Huang, Bisogno, Trevisani, Al-Hayani, De Petrocellis, Fezza, et al. 2002), and numerous articles have reviewed their synthesis, pharmacology and biochemistry in detail (Starowicz, Nigam, DiMarzo, 2007; Szallasi, Cortright, Blum, Eid, 2007). Among the various exogenous agonists, capsaicin, the pungent component of chili-pepper, has been shown to have beneficial effects on energy and glucose homeostasis; this will be reviewed in the following section.
Actions of dietary capsaicin
Capsaicin, an exogenous agonist of TRPV1 receptors, was shown to reduce food intake and increase energy expenditure in animals and humans; however, conflicting results have emerged from recent investigations, indicating the complexity of the TRPV1 system.
In an early human study, red pepper was added to high-fat and high-carbohydrate meals; this was followed by measurement of energy and food intake in female subjects (Yoshioka, St-Pierre, Drapeau, Dionne, Doucet, Suzuki, Tremblay, 1999). The results indicated that addition of red pepper to the breakfast of the subjects significantly reduced protein and fat intake during lunch. Furthermore, red pepper, as an appetizer, decreased energy and carbohydrate intake in Caucasian male subjects, and this effect was observed to be associated with an increase in the activity of the sympathetic nervous system (Yoshioka, St-Pierre, Drapeau, Dionne, Doucet, Suzuki, Tremblay, 1999). Interestingly, both oral and gastrointestinal exposure to capsaicin resulted in increased satiety and decreased energy and fat intake, both in male and female subjects (Westerterp-Plantenga, Smeets, Lejeune, 2005); however, a greater reduction was observed to occur in energy and fat intake after oral exposure to capsaicin. Capsaicin, added as a supplement to the diet, reduced adipose tissue most likely due to alteration of catecholamine secretion from the adrenal medulla (Watanabe, Kawada, Yamamoto, Iwai, 1987; Kawada, Sakabe, Watanabe, Yamamoto, Iwai, 1988).
Despite the observed beneficial effects of capsaicin on satiety and food intake, it became increasingly apparent that the role of TRPV1 is much more complex than had been conceived previously, especially after Motter and Ahern reported that TRPV1 knockout mice are protected from diet induced obesity (Motter, Ahern, 2008). Wild-type mice sustained on a high-fat diet experienced greater body mass when compared to TRPV1 knockout mice, and this difference was observed both in male and female animals. The increase in body mass of wild-type mice was associated with enhanced adiposity, larger adipocytes and increased fat content of the liver as compared to TRPV1-null mice (Motter, Ahern, 2008). While the Motter and Ahern study (Motter, Ahern, 2008) revealed no difference in energy intake, it should be noted that the TRPV1 knockout mice possessed a greater thermogenic capacity than the wild-type animals. The observations made regarding TRPV1 knockout mice agreed with investigations following the destruction of sensory afferent neurons (Cui, Himms-Hagen, 1992), suggesting that TRPV1 signaling enhances fat accumulation, and thereby modulates energy and glucose homeostasis.
On the other hand, beneficial effects of TRPV1-activation on adipose tissue were also reported. The direct effect of capsaicin on lipolysis has been studied in vitro (Lee, Kim, Kim, Kim, 2011) and the study demonstrated that capsaicin treatment resulted in reduced intracellular lipid content in association with increased mRNA levels of hormone sensitive lipase, carnitine palmitoyl transferase-Iα and uncoupling protein 2 (UPC2). These data showed that genes expression of the lipid catabolic pathway and thermogenesis indicated by UPC2 might be modulated by capsaicin (Lee, Kim, Kim, Kim, 2011). Another investigation also demonstrated that TRPV1 activation modulates adipose tissue (Iwasaki, Tamura, Inayoshi, Narukawa, Kobata, et al., 2011). In this study, mice were kept on high-fat high-sucrose diet and monoacylglycerol, a TRPV1 agonist was included into their diet. TRPV1 activation by monoacylglycerol prevented hyperglycemia, hypercholesteremia, accumulation of white adipose tissue and increased UPC1 content of brown adipose tissue; however, there was no difference in total energy intake and fat digestibility. These data further support that orally administered TRPV1 agonists elevate UPC protein expression (Lee, Kim, Kim, Kim, 2011; Iwasaki, Tamura, Inayoshi, Narukawa, Kobata, et al., 2011), thereby influencing brown adipose tissue in addition to preventing accumulation of white adipose tissue.
Inflammatory adipocytokines, such as tumor necrosis factor-α (TNFα) or monocyte chemoattracttant protein-1 (MCP-1), are upregulated in obese and diabetic patients and animals, while adiponectin is downregulated (Xu, Barnes, Yang, Tan, Yang et al., 2003; Kadowaki, Yamauchi, Kubota, Hara, Ueki, Tobe, 2006). In vitro experiments revealed that capsaicin inhibited the expression of inflammatory adipocytokines and increased adiponectin levels (Kang, Kim, Han, Kawada, Yu, 2007), suggesting that the activation of TRPV1 receptors could have a beneficial effect by decreasing inflammation. This hypothesis was tested with pair-feeding experiments on obese mice with and without dietary capsaicin administration (Kang, Tsuyoshi, Han, Kawada, Kim, Yu, 2010). Dietary capsaicin decreased the body weight and adipose tissue of obese mice, and the levels of fasting glucose and insulin were lower when compared to non-treated mice (Kang, Tsuyoshi, Han, Kawada, Kim, Yu, 2010). The oral glucose tolerance test revealed that dietary capsaicin protects against obesity-induced glucose intolerance. The levels of free fatty acid and leptin were lower and those of adiponectin higher in the plasma of mice with capsaicin supplementation. Additionally, capsaicin treatment reduced adipose tissue inflammation (Kang, Tsuyoshi, Han, Kawada, Kim, Yu, 2010). The capsaicin treatment also decreased hepatic steatosis and inflammatory responses and caused upregulation and activation of PPARα, a key regulator of glucose and lipid metabolism. These observations indicate that dietary capsaicin can attenuate glucose intolerance in obese mice. This effect is associated with reduced inflammatory cytokines, increased TRPV1 expression in adipose tissue, reduced hepatic inflammation, and activation of the PPARα signaling. Similar findings were recently demonstrated by topical administration of capsaicin indicating that male mice on high-fat diet showed reduced weight gain and visceral fat (Lee, Shin, Yoon, Kim, Yu, et al., 2012)In addition, this study also revealed lower fasting glucose, total cholesterol, triglycerides and increased adiponectin, PPARα, PPARγ expression in combination with reduced inflammation. These findings strongly suggest that not only dietary, but also topical administration of capsaicin in pre-obese and post-obese conditions reduces fat accumulation, inflammation and insulin resistance (Lee, Shin, Yoon, Kim, Yu, et al., 2012).
The above-mentioned experiments revealed the beneficial effect accruing from the administration of dietary capsaicin to obese mice sustained on a high-fat diet; however, the effect of capsaicin in severe metabolic conditions was not identified. To investigate this question, Yu’s group supplemented the diet of KKAy mice with capsaicin and measured various physiological parameters (Kang, Tsuyoshi, Ngoc, Kim, Tu, et al., 2011). KKAy mice, also known as Yellow KK obese mice, develop severe obesity, hyperglycemia, hyperinsulinemia and glucose intolerance, and thereby serve as good models for obesity and T2D (Sirinivasan, Ramarao, 2007). Levels of insulin, fasting glucose and triglyceride were reduced in the capsaicin-treated mice, and these changes were accompanied by reduced hepatic steatosis and decreased inflammation of hepatic and adipose tissues, while plasma adiponectin levels and adiponectin receptor 2 were upregulated (Kang, Tsuyoshi, Ngoc, Kim, Tu, et al., 2011). Furthermore, a more recent report using KKAy mice also observed that dietary supplementation of capsaicin reduced blood glucose levels; however, body weight, white and brown adipose tissue or total food intake was not altered by the diet (Okumura, Tsukui, Hosokawa, Miyashita, 2012). This study also investigated the synergistic effect of capsaicin and caffeine administration and demonstrated a similar beneficial effect on glucose levels. Insulin and leptin levels also showed reduction after dietary supplementation of capsaicin and caffeine; however, it did not reach significance (Okumura, Tsukui, Hosokawa, Miyashita, 2012). Together, these data further suggest that dietary capsaicin could have beneficial effects on metabolism both in diet induced obese and genetically obese-diabetic conditions. Furthermore, upregulation of adiponectin levels could be an attractive alternative solution for protecting against metabolic dysregulation. Capsaicin is a selective agonist for TRPV1 receptors; therefore, we can assume that TRPV1-receptor activation triggers cellular mechanisms leading to decreased inflammation, increased adiponectin levels and lower glucose and insulin; however, further investigation is required.
In addition to capsaicin, capsiate, a non-pungent capsaicin analogue has been shown to improve glucose metabolism even better than capsaicin (Kwon, Kim, Ryu, Cha, Yon, Yang, et al., 2012). Chronic administration of both capsaicin and capsiate lowered body weight without altering energy intake; decreased serum leptin and glucose concentrations in 90% pancreatectomized diabetic rats. The observations also demonstrated that the capsaicin- and capsiate-caused improvement of glucose homeostasis is due to enhanced glucose-stimulated insulin secretion and increased beta-cell mass; however, only capsiate increased insulin sensitivity (Kwon, Kim, Ryu, Cha, Yon, Yang, et al., 2012). The chronic dietary supplementation with capsaicin and capsiate also decreased hepatic PEPCK expression levels during hyperinsulinemic state indicating reduced hepatic glucose output. This suppression of PEPCK expression was greater in capsiate-treated mice. Furthermore, capsiate-induced stimulation of hepatic insulin signaling lead to enhanced glucose utilization resulting in increased glycogen accumulation (Kwon, Kim, Ryu, Cha, Yon, Yang, et al., 2012). These observations suggest that not only capsaicin, but also non-pungent analogues are promising anti-diabetic compounds via TRPV1 activation. On the other hand, it has to be noted that capsiate was observed to activate TRPA1, an another TRP channel; therefore further studies are necessary to reveal the mechanisms involved in modulating glucose metabolism (Shintaku, Uchida, Suzuki, Zhou, Fushiki, et al., 2012).
In contrast, conflicting observations indicated that dietary supplementation of capsaicin and dihydrocapsaicin (DHC) resulted in increased plasma glucose and free fatty acid levels in healthy rats, thus promoting energy metabolism and thermogenesis (Imaizumi, Sato, Kumazawa, Arai, Aritoshi, Akimoto, et al., 2011). On the other hand, DHC and capsaicin also decreased the body-weight gain of the rats compared to the control group. The authors suggest that the alteration of glucose, free fatty acid and glycerol levels could be due to sympathetic activation and catecholamine secretion or due to activation of glycogenolysis via β-adrenoreceptors of the liver; however, these possibilities were not elucidated. Furthermore, the difference observed in this study (Imaizumi, Sato, Kumazawa, Arai, Aritoshi, Akimoto, et al., 2011) as compared to the previous studies (Kang, Tsuyoshi, Han, Kawada, Kim, Yu, 2010; Kang, Tsuyoshi, Ngoc, Kim, Tu, et al., 2011) could also originate from the method of administration, subcutaneous versus dietary supplementation. A recent double-blind, placebo-controlled, single center, randomized, crossover study performed on male subjects did not detect changes in metabolic rate, fuel partitioning and blood pressure; however, body temperature increased after taking capsinoids, which have a similar structure to capsaicin (Galgani, Ryan, Ravussin, 2010).
In contrast, a crossover study performed on healthy male volunteers by using an oral glucose tolerance test revealed lower glucose levels and higher insulin after capsaicin ingestion as compared to the placebo group (Chaiyasit, Khovidhunkit, Wittayalertpanya, 2009). Importantly, this study also determined that capsaicin is rapidly absorbed, and it could be detected from the blood 10 min after ingestion. The capsaicin levels in the plasma were maintained up to 90 min and correlated well with the lower glucose levels and maintenance of the insulin levels (Chaiyasit, Khovidhunkit, Wittayalertpanya, 2009).
In summary, the above-mentioned studies suggest that dietary administration of capsaicin could have beneficial effects on whole body metabolism including glucose homeostasis. The conflicting results regarding dietary administration of capsaicin could be due to several factors, including dose, duration of the measurements, exposure time, the efficacy of capsaicin versus capsinoids or the conditions of the experimental animals, such as normoglycemic versus obese, diabetic; therefore, further elucidation of the capsaicin/TRPV1 effect is necessary to determine underlying mechanisms.
Interaction between TRPV1 and GLP-1
Glucagon-like peptide-1 (GLP-1) is a gut hormone directly binding to GLP-1 receptors (Thorens, 1995). GLP-1 plays a major role in the regulation of glucose metabolism through modulating insulin secretion and activating the gut-brain-periphery axis (Burcelin, Serino, Cabou, 2009). GLP-1 is rendered inactive within minutes by the enzyme dipeptidyl peptidase-4 (DPP-4). Some of the currently used anti-diabetic therapies based on the inhibition of DPP-4 (e.g., sitagliptin, saxagliptin, linagliptin); therefore, selective activation of GLP-1 with dietary interventions could be used as an alternative solution for the treatment of diabetes. A human, single blind, randomized crossover study performed on male and female subjects revealed that a lunch containing capsaicin increased GLP-1 levels and tended to decrease ghrelin levels (Smeets, Westerterp-Plantenga, 2009), suggesting a possible interaction between TRPV1 and GLP-1. Moreover, in a new study, it was revealed that TRPV1 receptors are present in the GLP-1-expressing intestinal cells, and activation of TRPV1 stimulates GLP-1 release through a Ca2+-dependent mechanism (Wang, Yan, Zhong, Chen, Ni, Li, et al., 2012). A glucose challenge that was performed on fasting mice detected enhancement of GLP-1 secretion in the mice with intragastric administration of capsaicin; this effect was inhibited by TRPV1 antagonists and was absent in TRPV1 knockout mice. These observations suggest that acute capsaicin administration enhances GLP-1 secretion in vivo via activation of TRPV1 receptors (Wang, Yan, Zhong, Chen, Ni, Li, et al., 2012). While GLP-1 secretion also increased in the absence of a glucose challenge, nevertheless, plasma GLP-1 levels were observed to be lower than in mice faced with a glucose challenge (Wang, Yan, Zhong, Chen, Ni, Li, et al., 2012). A similar increase in insulin levels was also observed in mice with capsaicin administration. Inhibition of the GLP-1 signaling partially prevented the capsaicin-caused increase of insulin, indicating that TRPV1 activation might cause a reduction in blood glucose levels through GLP-1-dependent insulin secretion and through direct action on the pancreas. TRPV1 activation by chronic dietary capsaicin improved glucose tolerance, and the mean 24-h blood glucose level was lower in mice administered with chronic dietary capsaicin (Wang, Yan, Zhong, Chen, Ni, Li, et al., 2012). Even more interestingly, a chronic capsaicin diet improved insulin sensitivity and decreased fasting blood glucose levels in the type 2 diabetic db/db mice, indicating that dietary capsaicin has the potential to improve the dysregulated glucose homeostasis in db/db mice via a TRPV1-dependent mechanism involving GLP-1 secretion and could have important implications for therapeutic drug development.
TRPV1 and insulin secretion
Activation of TRPV1 receptors influences insulin secretion in human subjects (Chaiyasit, Khovidhunkit, Wittayalertpanya, 2009) and experimental animals (Tolan, Ragoobirsingh, Morrison, 2001). TRPV1 receptors are expressed in rat islet β cells and β cell lines (Akiba, Kato, Katsube, Nakamura, Takeuchi, Ishii, Hibi, 2004). Capsaicin dose-dependently increased insulin secretion in vitro, and this effect was inhibited by pretreatment with a TRPV1 antagonist. Furthermore, subcutaneous administration of capsaicin increased plasma insulin levels in vivo in rats indicating a systemic effect (Akiba, Kato, Katsube, Nakamura, Takeuchi, Ishii, Hibi, 2004). TRPV1 receptors are also present in the afferent sensory nerves innervating the islets; therefore, TRPV1 activation might modulate insulin secretion (a) directly, by altering secretion of β cells, or (b) indirectly, by modulating the sensory nerves. The sensory nerves innervating the pancreas were identified as major players in the development of pancreatitis, islet inflammation, islet destruction and T1D (Razavi, Chan, Afifiyan, Liu, Wan, Yantha, 2006; Tsui, Razavi, Chan, Yantha, Dosch, 2007). Razavi et al. (Razavi, Chan, Afifiyan, Liu, Wan, Yantha, 2006) reported that TRPV1-expressing sensory neurons govern pancreatic islet inflammation and insulin resistance (Razavi, Chan, Afifiyan, Liu, Wan, Yantha, 2006). The authors used the spontaneous T1D non-obese diabetic (NOD) mouse model to reveal that the action of eliminating the capsaicin-expressing fibers significantly reduces islet infiltration, thus delaying the onset, and reducing the incidence of T1D. Furthermore, the authors determined TRPV1 polymorphism in NOD mice, which was associated with hypofunctional TRPV1, lead to a decrease in substance P release. Restoration of normal substance P levels resulted in reduced islet infiltration and T cell proliferation, indicating that neural dysregulation of pancreatic islets contributes to the development and progression of T1D (Razavi, Chan, Afifiyan, Liu, Wan, Yantha, 2006). These data support the existence of a powerful connection between the autonomic nervous system and pancreatic functions.
Islet inflammation plays a major role in the progression of T1D; moreover, T2D is also associated with a low-grade of inflammation (Festa, D’Agostino, Howard, Mykkanen, Tracy, Haffner, 2000), and the threshold of TRPV1 activation could be lowered during inflammation. Moreover, the nerve fibers innervating the islets of Langerhans release the calcitonin gene-related peptide (CGRP) that inhibits insulin secretion (Ahren, Martensson, Nobin, 1987; Kogire, Ishizuka, Thompson, Greeley, 1991). These CGRP-expressing fibers contain TRPV1 receptors, thus raising the question of whether these fibers could contribute to the development of reduced insulin secretion in diabetic conditions. Experiments on Zucker fatty diabetic rats revealed that selective chemodenervation of capsaicin-sensitive structures prevented the decline of metabolism and glucose homeostasis and increased glucose–dependent insulin secretion; moreover, these changes were associated with the absence of TRPV1-CGRP-coexpressing nerve fibers in the islets (Gram, Ahren, Nagy, Olsen, Brand, Sundler, et al, 2007). Similar results were obtained following oral administration of BCTC, a TRPV1 antagonist in ob/ob mice (Tanaka, Shimaya, Kiso, Kuramochi, Shimokawa, Shibasaki, 2011). Four-week treatment with the TRPV1 inhibitor reduced fasting glucose, triglyceride and insulin levels in the ob/ob mice and blocking TRPV1 resulted in enhanced insulin secretion during oral glucose tolerance test indicating that TRPV1 plays a role in controlling insulin secretion (Tanaka, Shimaya, Kiso, Kuramochi, Shimokawa, Shibasaki, 2011).
The levels of CGRP also increase with age and could contribute to the development of insulin resistance and obesity. Furthermore, deletion of TRPV1 was shown to prevent aging-related obesity and insulin resistance (Melnyk, Himms-Hagen, 1995). Based on observations that TRPV1 could be activated by inflammatory agents and that the T2D condition is associated with a low-grade of inflammation, it is possible that TRPV1-expressing nerves are continuously activated, leading to CGRP release, which likely endorses insulin resistance, as mentioned by Suri and Szallasi (2007).
The above summarized reports strongly suggest that TRPV1 has an essential role in the regulation of pancreatic functions and most likely not the existence or lack of TRPV1 receptors, but an abnormal neural regulation of the pancreas play crucial role during the development of diabetes.
TRPV1 and autonomic control
Claude Bernard in the 19th century introduced the idea that the brain controls systemic glucose levels (Bernard, 1854); however, with the discovery of insulin and the working mechanism of insulin, the focus of normalizing glucose homeostasis shifted to the end organs (e.g., insulin replacement, insulin sensitizers) and the role of the central nervous system regarding glucose homeostasis was neglected. However, during the last two decades with the availability of precise, new experimental approaches the original idea of the regulation of glucose homeostasis by the brain was rediscovered and the hypothalamus was shown to respond to glucose, insulin, leptin, fatty acid, GLP-1 and many more metabolically important factors (Sandoval, Obici, Seeley, 2009). One of the main discoveries was for instances that insulin receptors located in the hypothalamus, especially in the arcuate nucleus play crucial role in the regulation of hepatic glucose production (Obici, Feng, Karkanias, Baskin, Rossetti, 2002; Obici, Zhang, Karkanias, Rossetti, 2002). Then, the role of leptin and ATP-dependent potassium channel was determined leading to altered neuronal activity in the hypothalamus, thus results in changes in glucose homeostasis (Pocai, Morgan, Buettner, Gutierrez-Juarez, Obici, Rossetti, 2005; Pocai, Lam, Gutierez-Juarez, Obici, Schwartz, Bryan et al., 2005). All of these studies and many more highlighted the importance of the autonomic nervous system and the brain-liver axis regarding glucose homoeostasis.
The autonomic nervous system plays a significant role in the regulation of glucose homeostasis via the activity of preautonomic neurons located in the autonomic centers of the brain. Indeed, a prospective cohort study revealed the increased prevalence of T2D if autonomic dysfunction is present (Carnethon, Golden, Folsom, Haskell, Liao, 2003), indicating the importance of autonomic regulation in glucose homeostasis. The activity of preautonomic neurons is controlled by hormones and nutrients, which in turn modulates the autonomic output in order to adapt to the needs of the body. The paraventricular nucleus (PVN) of the hypothalamus is considered a critical command component of the autonomic pathway and integrates signals from many different brain areas, including hypothalamic nuclei, with well established roles in glucose and energy homeostasis (e.g., dorso-medial hypothalamic nucleus, arcuate nucleus). The PVN is in a unique position, due to its function, to integrate neuronal and humoral metabolic signals in order to organize the autonomic and neuroendocrine outflow (Swanson, Sawchencko, 1980; Yi, laFleur, Fliers, Kalsbeek, 2010). Moreover, preautonomic neurons in the PVN belong either to the sympathetic nervous system or to the parasympathetic nervous system (Buijs, la Fleur, Wortel, Van Heyningen, Zuiddam, Mettenleiter, et al, 2003) and have opposite effects on the function of the organs. This is an intriguingly important detail because it suggests that nutrients, peptides or hormones could be effective on one of the autonomic pathways and ineffective on the other. Therefore, identification of new pharmacological targets is a continuous challenge and hope is presented for the manipulation of the autonomic nervous system in order to normalize glucose homeostasis in diabetic patients through improving autonomic control.
TRPV1 receptors have been identified in various brain areas, including the hypothalamus (Cristino, de Petrocelli, Pryce, Baker, Guglielmotti, Di Marzo, 2006; Cavanaugh, Chesler, Jackson, Sigal, Yamanaka, Grant, et al. 2010; Zsombok, Gao, Miyata, Issa, Derbenev, 2011). Indeed, our unpublished data indicate that activation of TRPV1 receptors in the PVN lowers blood glucose levels (O’Hare, Zsombok, unpublished); however, the underlying mechanism is currently under investigation. With the retrograde viral tracer technique (e.g., pseudorabies virus), the identification of neuronal populations directly or indirectly connected to an organ became possible. Using this approach, liver-related PVN neurons were identified, and Friedman’s group revealed that a subpopulation of liver-related PVN neurons expressed corticotrophin-releasing hormone and oxytocin (Stanley, Pinto, Segal, Perez, Viale, DeFalco, et al, 2010). Using the same methods, our group determined that liver-related PVN neurons express TRPV1 receptors (Zsombok, Gao, Miyata, Issa, Derbenev, 2011). Furthermore, we demonstrated that insulin receptor substrate 2, a functional component of the insulin signaling system also expressed in liver-related PVN neurons, provided anatomical background for a possible functional interaction between TRPV1 and insulin signaling. To reveal this functional connection, we identified liver-related PVN neurons and used patch-clamp electrophysiology to determine mechanisms controlling the activity of liver-related PVN neurons. Our data revealed that TRPV1 receptors contribute to the regulation of liver-related PVN neurons (Gao, Miyata, Bhaskaran, Derbenev, Zsombok, 2012). Furthermore, the TRPV1-dependent excitation of liver-related PVN neurons was absent in streptozotocin-treated type 1 diabetic mice and this TRPV1-dependent neuronal activity was recovered with insulin treatment. Interestingly, both in vitro and in vivo insulin treatment was observed to have the same restoring effect on TRPV1 activity likely in a PI3-kinase/PKC-dependent manner and/or through enhancing TRPV1 trafficking to the plasma membrane (Gao, Miyata, Bhaskaran, Derbenev, Zsombok, 2012). The data also suggested that not all liver-related PVN neurons responded to TRPV1, leading to the speculation that TRPV1 might stimulate only the sympathetic or the parasympathetic nervous system; however, this requires further investigation.
In conclusion, the investigations summarized in this review suggest that TRPV1 receptors play a significant role in the regulation of metabolism through modulating the function of different organs and also through the effect they have on the hypothalamus, thus contributing to the autonomic regulation of the liver. These data together indicate that pharmacological manipulation of TRPV1 receptors might have a beneficial effect in diabetic and obese conditions; however, further clinical and experimental investigations are necessary before this can be fully determined.
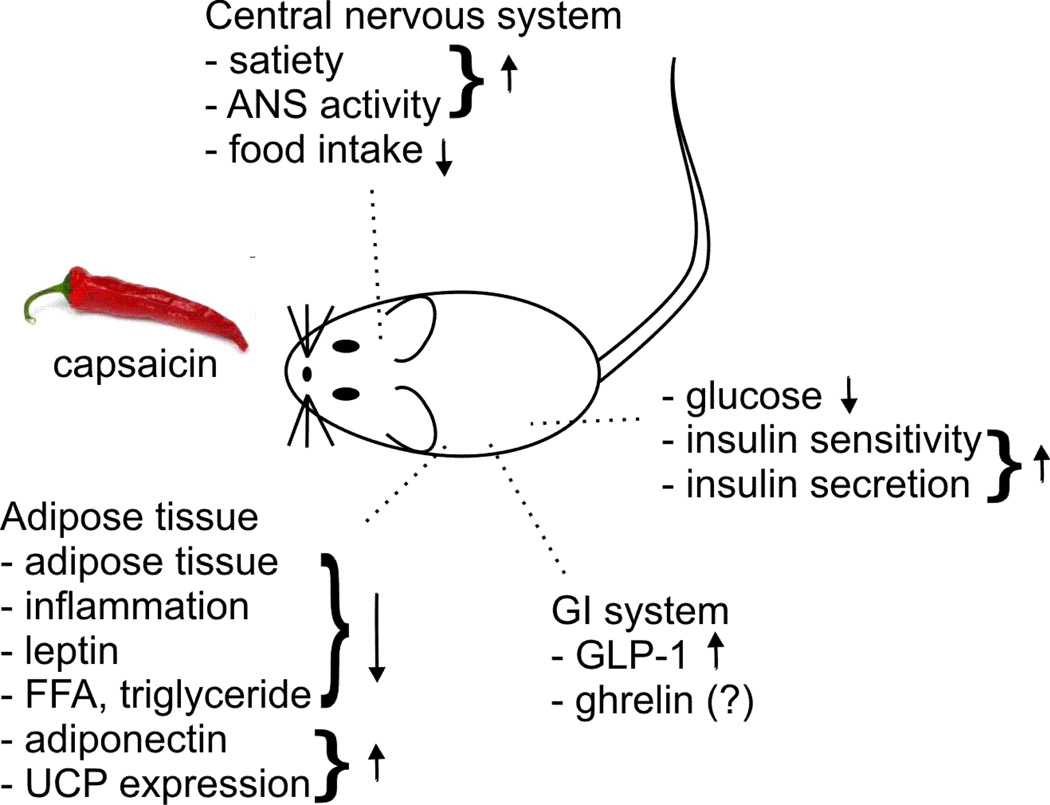
Figure 1.
Dietary capsaicin through TRPV1-dependent mechanisms was shown to have beneficial effects on glucose homoeostasis. Up to date the adipose tissue, GI system, liver, pancreas and the central nervous system were determined as site of action. ANS: autonomic nervous system; FFA: free fatty acid; GLP-1: glucagon-like peptide-1; UPC: uncoupling protein.
Acknowledgement
This work was supported by grants from the NIH (Tulane BIRCWH Program NIH 2K12HD043451 and Tulane Aging COBRE P20GM103629).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahren B, Martensson H, Nobin A. Effects of calcitonin gene-related peptide (CGRP) on islet hormone secretion in the pig. Diabetologia. 1987;30:354–359. doi: 10.1007/BF00299030. [DOI] [PubMed] [Google Scholar]
- Akiba Y, Kato S, Katsube K, Nakamura M, Takeuchi K, Ishii H, Hibi T. Transient receptor potential vanilloid subfamily 1 expressed in pancreatic islet β cells modulates insulin secretion in rats. Biochemical and Biophysical Research Communications. 2004;321:219–225. doi: 10.1016/j.bbrc.2004.06.149. [DOI] [PubMed] [Google Scholar]
- Bernard C. Eds Baillere et Fils. Paris: 1854. Lecons de physiologie experimentale appliqués a la mededine. [Google Scholar]
- Burcelin R, Serino M, Cabou C. A role for the gut-to brain GLP-1-dependent axis in the control of metabolism. Current Opinion in Pharmacology. 2009;9:744–752. doi: 10.1016/j.coph.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Buijs RM, la Fleur SE, Wortel J, Van Heyningen C, Zuiddam L, Mettenleiter TC, et al. The suprachiasmatic nucleus balances sympathetic and parasympathetic output to peripheral organs through separate preautonomic neurons. Journal of Comparative Neurology. 2003;464:36–48. doi: 10.1002/cne.10765. [DOI] [PubMed] [Google Scholar]
- Cavanaugh DJ, Chesler AT, Jackson AC, Sigal YM, Yamanaka H, Grant R, et al. TRPV1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. Journal of Neuroscience. 2010;31:5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterina M, Schumacher M, Tominaga M, Rosen T, Levine J, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Chaiyasit K, Khovidhunkit W, Wittayalertpanya S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. Journal of Medical Association Thailand. 2009;92:108–113. [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nature Reviews Neuroscience. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Cristino L, de Petrocelli L, Pryce G, Baker D, Guglielmotti V, Di Marzo V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience. 2006;139:1405–1415. doi: 10.1016/j.neuroscience.2006.02.074. [DOI] [PubMed] [Google Scholar]
- Cui J, Himms-Hagen J. Long-term decrease in body fat and in brown adipose tissue in capsaicin-desensitized rats. American Journal of Physiology. 1992;262:R568–R573. doi: 10.1152/ajpregu.1992.262.4.R568. [DOI] [PubMed] [Google Scholar]
- Festa A, D’Agostino R, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–47. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- Galgani JE, Ryan DH, Ravussin E. Effect of capsinoids on energy metabolism in human subjects. British Journal of Nutrition. 2010;103:38–42. doi: 10.1017/S0007114509991358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Miyata K, Bhaskaran MD, Derbenev AV, Zsombok A. Transient receptor potential vanilloid type 1-dependnet regulation of liver-related neurons in the paraventricular nucleus of the hypothalamus diminished in the type 1 diabetic mouse. Diabetes. 2012;61:1381–1390. doi: 10.2337/db11-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gram DX, Ahren B, Nagy I, Olsen UB, Brand CL, Sundler F, et al. Capsaicin-sensitive sensory fibers in the islets of Langerhans contribute to defective insulin secretion in Zucker diabetic rat, an animal model for some aspects of human type 2 diabetes. European Journal of Neuroscience. 2007;25:213–223. doi: 10.1111/j.1460-9568.2006.05261.x. [DOI] [PubMed] [Google Scholar]
- Huang SM, Bisogno T, Trevisani M, Al-Hayani A, De Petrocellis L, Fezza F, et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proceedings of the National Academy of Sciences USA. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imaizumi K, Sato S, Kumazawa M, Arai N, Aritoshi S, Akimoto S, et al. Capsaicinoids-induced changes of plasma glucose, free fatty acid and glycerol concentrations in rats. The Journal of Toxicological Sciences. 2011;36:109–116. doi: 10.2131/jts.36.109. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Tamura Y, Inayoshi K, Narukawa M, Kobata K, Chiba H, Muraki E, Tsunoda N, Watanabe T. TRPV1 agonist monoacylglycerol increases UCP1 content in brown adipose tissue and suppresses accumulation of visceral fat in mice fed a high-fat and high-sucrose diet. Bioscience Biotechnology Biochemistry. 2011;75:904–909. doi: 10.1271/bbb.100850. [DOI] [PubMed] [Google Scholar]
- Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. Journal of Clinical Investigations. 2006;116:1784–1792. doi: 10.1172/JCI29126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Kim CS, Han IS, Kawada T, Yu R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Letters. 2007;581:4389–4396. doi: 10.1016/j.febslet.2007.07.082. [DOI] [PubMed] [Google Scholar]
- Kang JH, Tsuyoshi G, Han IS, Kawada T, Kim YM, Yu R. Dietary capsaicin reduces obesity-induced insulin resistance and hepatic steatosis in obese mice fed a high-fat diet. Obesity. 2010;18:780–787. doi: 10.1038/oby.2009.301. [DOI] [PubMed] [Google Scholar]
- Kang JH, Tsuyoshi G, Ngoc HL, Kim HM, Tu TH, et al. Dietary capsaicin attenuates metabolic dysregulation in genetically obese diabetic mice. Journal of Medicinal Food. 2011;14:310–315. doi: 10.1089/jmf.2010.1367. [DOI] [PubMed] [Google Scholar]
- Kawada T, Sakabe S, Watanabe T, Yamamoto M, Iwai K. Some pungent principles of spices cause the adrenal medulla to secrete catchecholamine in anesthetized rats. Proceedings of the Society for Experimental Biology and Medicine. 1988;188:229–233. doi: 10.3181/00379727-188-2-rc1. [DOI] [PubMed] [Google Scholar]
- Kogire M, Ishizuka J, Thompson JC, Greeley GH. Inhibitory action of islet amyloid polypeptide and calcitonin gene-related peptide on release of insulin from the isolated perfused rat pancreas. Pancreas. 1991;6:459–463. doi: 10.1097/00006676-199107000-00013. [DOI] [PubMed] [Google Scholar]
- Kwon DY, Kim YS, Ryu AY, Cha MR, Yon GH, Yang HJ, Kim MJ, Kang S, Park S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. Journal of Nutritional Biochemistry. 2012 doi: 10.1016/j.jnutbio.2012.08.006. E-published. [DOI] [PubMed] [Google Scholar]
- Lee MS, Kim CT, Kim IH, Kim Y. Effects of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytotherapy Research. 2011;25:935–939. doi: 10.1002/ptr.3339. [DOI] [PubMed] [Google Scholar]
- Lee GR, Shin MK, Yoon DY, Kim AR, Yu R, Park NH, Han IS. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity. 2012 doi: 10.1002/oby.20246. E-published. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhu Z, Tepel M. The role of transient receptor potential channels in metabolic syndrome. Hypertension Research. 2008;31:1989–1995. doi: 10.1291/hypres.31.1989. [DOI] [PubMed] [Google Scholar]
- Melnyk A, Himms-Hagen J. Resistance to aging-associated obesity in capsaicin-desensitized rats one year after treatment. Obesity Research. 1995;3:337–344. doi: 10.1002/j.1550-8528.1995.tb00159.x. [DOI] [PubMed] [Google Scholar]
- Motter AL, Ahern GP. TRPV1-null mice are protected from diet-induced obesity. FEBS Letters. 2008;582:2257–2262. doi: 10.1016/j.febslet.2008.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilius B, Owsianik G, Voets T, Peters JA. Transient receptor potential cation channels in disease. Physiology Reviews. 2007;87:165–217. doi: 10.1152/physrev.00021.2006. [DOI] [PubMed] [Google Scholar]
- Obici S, Feng Z, Karkanias G, Baskin DG, Rossetti L. Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nature Neuroscience. 2002;5:566–572. doi: 10.1038/nn0602-861. [DOI] [PubMed] [Google Scholar]
- Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nature Medicine. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- Okumura T, Tsukui T, Hosokawa M, Miyashita K. Effect of caffeine and capsaicin on the blood glucose levels of obese/diabetic KK-Ay mice. Journal of Oleo Science. 2012;61:515–523. doi: 10.5650/jos.61.515. [DOI] [PubMed] [Google Scholar]
- Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–1031. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes. 2005;54:3182–3189. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- Razavi R, Chan Y, Afifiyan FN, Liu XJ, Wan X, Yantha J, et al. TRPV1+ sensory neurons control β cell stress and islet inflammation in autoimmune diabetes. Cell. 2006;127:1123–1135. doi: 10.1016/j.cell.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Sandoval DA, Obici S, Seeley RJ. Targeting the CNS to treat type 2 diabetes. Nature Reviews Drug Discovery. 2009;8:386–398. doi: 10.1038/nrd2874. [DOI] [PubMed] [Google Scholar]
- Shintaku K, Uchida K, Suzuki Y, Zhou Y, Fushiki T, Watanabe T, Yazawa S, Tominaga M. Activation of transient receptor potential A1 by a non-pungent capsaicin-like compound, capsiate. British Journal of Pharmacology. 2012;165:1476–1486. doi: 10.1111/j.1476-5381.2011.01634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri A, Szallasi A. The emerging role of TRPV1 in diabetes and obesity. TRENDS in Pharmacological Sciences. 2007;29:29–36. doi: 10.1016/j.tips.2007.10.016. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Cortright DN, Blum CA, Eid SR. The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of concept. Nature Reviews Drug Discovery. 2007;6:357–372. doi: 10.1038/nrd2280. [DOI] [PubMed] [Google Scholar]
- Sirinivasan K, Ramarao P. Animal models in type 2 diabetes research: An overview. Indian Journal of Medical Research. 2007;125:451–472. [PubMed] [Google Scholar]
- Smeets AJ, Westerterp-Plantenga MS. The acute effects of a lunch containing capsaicin on energy and substrate utilization, hormones, and satiety. European Journal of Nutrition. 2009;48:229–234. doi: 10.1007/s00394-009-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley S, Pinto S, Segal J, Perez CA, Viale A, DeFalco J, et al. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proceedings of the National Academy of Sciences USA. 2010;107:7024–7029. doi: 10.1073/pnas.1002790107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Nigam S, DiMarzo V. Biochemistry and pharmacology of endovanilloids. Pharmacology and Therapeutics. 2007;114:13–33. doi: 10.1016/j.pharmthera.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchencko PE. Paraventricular nucleus: a site for the integration of neuroendocrine and autonomic mechanisms. Neuroendocrinology. 1980;31:410–417. doi: 10.1159/000123111. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Shimaya A, Kiso T, Kuramochi T, Shimokawa T, Shibasaki M. Enhanced insulin secretion and sensitization in diabetic mice on chronic treatment with a transient receptor potential vanilloid 1 antagonist. Life Sciences. 2011;88:559–563. doi: 10.1016/j.lfs.2011.01.016. [DOI] [PubMed] [Google Scholar]
- Thorens B. Glucagon-like peptide-1 and control of insulin secretion. Diabetes Metabolism. 1995;21:311–318. [PubMed] [Google Scholar]
- Tolan I, Ragoobirsingh D, Morrison EY. Isolation and purification of the hypoglycemic principle present in Capsicum frutescens. Phytotherapeutic Research. 2001;18:95–96. doi: 10.1002/ptr.1328. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Tominaga T. Structure and function of TRPV1. Pflugers Archives European Journal of Physiology. 2005;451:143–150. doi: 10.1007/s00424-005-1457-8. [DOI] [PubMed] [Google Scholar]
- Tsui H, Paltser G, Chan Y, Dorfman R, Dosch HM. ‘Sensing’ the link between type 1 and type 2 diabetes. Diabetes Metabolism Research Reviews. 2011;27:913–918. doi: 10.1002/dmrr.1279. [DOI] [PubMed] [Google Scholar]
- Tsui H, Razavi R, Chan Y, Yantha J, Dosch HM. ‘sensing’ autoimmunity in type 1 diabetes. TRENDS in Molecular Medicine. 2007;13:405–413. doi: 10.1016/j.molmed.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan, Yang, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. Journal of Clinical Investigations. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi CX, laFleur SE, Fliers E, Kalsbeek A. The role of the autonomic nervous liver innervation in the control of energy metabolism. Biochimica et Biophysica Acta. 2010;1802:416–431. doi: 10.1016/j.bbadis.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, St-Pierre S, Drapeau V, Dionne I, Doucet E, Suzuki M, Tremblay A. Effects of red pepper on appetite and energy intake. British Journal of Nutrition. 1999;82:115–123. [PubMed] [Google Scholar]
- Van der Stelt M, DiMarzo V. Endovanilloids putative endogenous ligands of transient receptor potential vanilloid 1 channels. European Journal of Biochemistry. 2004;271:1827–1834. doi: 10.1111/j.1432-1033.2004.04081.x. [DOI] [PubMed] [Google Scholar]
- Wang P, Yan Z, Zhong J, Chen J, Ni Y, Li L, et al. Transient receptor potential vanilloid 1 activation enhances gut glucagon-like peptode-1 secretion and improves glucose homeostasis. Diabetes. 2012;61:2155–2165. doi: 10.2337/db11-1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Kawada T, Yamomoto M, Iwai K. Capsaicin, a pungent principle of hot pepper, evokes catchecolamine secretion from the adrenal medulla of anesthetized rats. Biochemical and Biophysical Research Communications. 1987;142:259–264. doi: 10.1016/0006-291x(87)90479-7. [DOI] [PubMed] [Google Scholar]
- Westerterp-Plantenga MS, Smeets A, Lejeune MPG. Sensory and gastrointestinal effects of capsaicin on food intake. International Journal of Obesity. 2004;29:682–688. doi: 10.1038/sj.ijo.0802862. [DOI] [PubMed] [Google Scholar]
- Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- Zsombok A, Gao H, Miyata K, Issa A, Derbenev AV. Immunohistochemical localization of transient receptor potential vanilloid type 1 and insulin receptor substrate 2 and their co-localization with liver-related neurons in the hypothalamus and brainstem. Brain Research. 2011;1398:30–39. doi: 10.1016/j.brainres.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]