Abstract
Several recent studies have demonstrated that innate immune NK cells exhibit memory-like properties with enhanced non-specific and specific recall responses. Cytokine activation alone of murine NK cells induces the differentiation of memory-like cells that are more likely to produce IFN-γ, a key NK cell cytokine important for activation of the immune response. Using an adoptive co-transfer system, we first show that cytokine-induced memory-like responses are NK intrinsic. However, engraftment of donor NK cells in NK-competent hosts is poor due to homeostatic control mechanisms. Therefore, we utilized alymphoid Rag- and common gamma chain (γc)-deficient mice as recipients and observed homeostatic expansion of co-transferred cytokine-activated and control donor NK cells. Despite proliferation of all cells, NK cells derived from those cells originally activated by cytokines retained an intrinsic enhanced capacity to produce IFN-γ when re-stimulated in vitro with cytokines or target cells. These NK cell memory-like responses persisted for at least 4 weeks in alymphoid hosts and 12 weeks in NK-competent hosts. These findings indicate that memory-like NK cells can readily self-renew and maintain enhanced function in a lymphopenic host for at least a month.
Introduction
Our immune response can be divided into two broad arms, innate and adaptive immunity. Until recently, a major distinction between these two arms was the exclusive ascription of immunologic memory to adaptive T and B lymphocytes. However, several recent reports have suggested memory-like responses by innate immune NK cells (1-4). NK cells are lymphocytes that express germline encoded receptors and are present in patients and mice with defects in proteins necessary for T and B cell receptor rearrangement (e.g. Rag-deficient) (5, 6). NK cells are important for the early control of infection, particularly viruses (7, 8). NK cells are also capable of killing tumor cells and may play a role in tumor surveillance and are currently being evaluated for cancer immunotherapy (9). NK cells mediate their effects via two primary mechanisms, production of cytokines and target cell killing.
Several studies have suggested that NK cells can retain a cellular memory of activation to both specific and non-specific stimuli (1-4, 10-12). Studies by von Andrian and colleagues demonstrated liver NK cell-mediated specific memory to multiple haptens and viral antigens using a contact hypersensitivity model (1, 4). In addition, a subset of previously sensitized liver NK cells exhibited specific killing of antigen pulsed cells and provided protection against systemic infection (4). Lanier and colleagues reported development of Ly49H+ memory splenic NK cells in vivo that was dependent on cytokines following infection with murine cytomegalovirus (MCMV), a virus that encodes a ligand recognized by the activating Ly49H receptor (3, 13). We established that cytokine-activation alone induces the differentiation of memory-like NK cells that are more likely to produce IFN-γ (2). The first model suggests that some liver NK cells might exhibit features of both cellular and immune memory, i.e. the ability to retain memory of prior activation as well as specificity to protect the host against infection with the same organism. Whereas cytokine-induced memory-like NK cells, including those induced by MCMV, likely represent cellular memory responses and are referred to as ‘memory-like’ here. Together, these studies suggest that NK cells can acquire memory-like, antigen-independent and dependent, phenotypes. In addition to these murine studies, we recently demonstrated that human NK cells pre-activated with cytokines acquire memory-like responses following prolonged in vitro culture (14). Additional studies have demonstrated possible human NK cell memory responses in vivo (15-17); although it is more challenging to accurately identify previously-activated human NK cells rather than primed cells responding to persistent viral stimulation (18).
The NK cell compartment comprises approximately 10% of human peripheral blood lymphocytes and 3-5% of murine splenocytes. This overall number of NK cells is tightly controlled and adoptive transfer of NK cells into NK-competent mice, including wild type (wt) and Rag-1−/− deficient hosts, results in low engraftment and little proliferation of donor NK cells due to limited availability of the survival and growth factor IL-15 (19, 20). However, adoptive transfer into NK-deficient hosts leads to robust proliferation and long-term engraftment of mature NK cells (21-23). Similarly, Miller et al. demonstrated that in patients, higher-dose lymphodepleting regimens prior to adoptive immunotherapy with allogeneic NK cells led to more successful engraftment and expansion of donor NK cells (24). Thus, homeostatic expansion has the potential to allow for proliferation of mature NK cells, long-term engraftment, and more successful immunotherapy.
We investigated whether cytokine-activated NK cells retain an intrinsic memory-like phenotype following expansion and proliferation in an alymphoid host. NK cells undergo rapid proliferation in alymphoid hosts and the long-lived pool of cells in reconstituted mice is renewed from dividing mature NK cells (19, 20). First, we definitively demonstrated that cytokine-induced NK cells memory-like responses are cell-intrinsic. Next, we found that despite extensive proliferation in alymphoid hosts, memory-like NK cells maintain the capacity to produce enhanced IFN-γ for at least one month. Finally, we found that unlike cells in alymphoid hosts, memory-like NK cells in NK-competent mice maintain an enhanced capacity for IFN-γ production for 3 months. These studies suggest that exogenous treatment of NK cells with cytokines for immunotherapy, or activation of NK cells in the context of an infection might lead to long-lived memory-like responses that persist with proliferation and self-renewal.
Materials and Methods
Mice
All mice were on the on the C57BL/6 background and bred and maintained at our facility. Rag-1 deficient (Rag-1−/−) mice expressing the C57BL/6 CD45.2 antigen were obtained from The Jackson Laboratory (Bar Harbor, ME). Mice deficient in Rag-2 (Rag-2−/−) and common gamma chain/IL2Rγ (γc−/−) were purchased from The Jackson Laboratory and bred to obtain Rag-2−/− γc−/− mice. Mice expressing the congenic CD45.1 receptor (CD45.1+) were obtained from The Jackson Laboratory and bred to Rag-1−/− animals to obtain CD45.1+Rag−/− mice. Animals were housed in specific pathogen free conditions and studies were approved by the Washington University Animal Studies Committee.
Adoptive transfers
Splenocytes were obtained from Rag-1−/− donors (CD45.1 or CD45.2) and cultured overnight as described (2). For some experiments, NK cells were enriched prior to culture by negative selection via magnetic bead purification (Miltenyi Biotec, Germany) following the manufacturer’s recommendations. Briefly, cells were either activated with a combination of murine IL-12 (10ng/ml, PeproTech, Rocky Hill, NJ) plus murine IL-18 (50ng/ml, Medical and Biological Laboratories International, Woburn, MA), which stimulates >90% of cells to produce IFN-γ, or untreated (control cells) (2). Both groups of splenocytes were cultured in the presence of low-dose murine IL-15 (10ng/ml, Peprotech) to maintain NK cell survival as described (2, 20). After 12-16h of culture, non-adherent cells were washed at least 3 times, counted, and where indicated labeled with CFSE (2.5μM, Invitrogen, Grand Island, NY). For co-transfers, equal numbers of cells (~1-3 × 106) were adoptively transferred by intravenous injection into the indicated hosts (Rag-1−/−, CD45.1+Rag−/−, or Rag-2−/− γc−/−). For transfers into separate hosts, ~1-3 × 106 cells were adoptively transferred by intravenous injection into the indicated hosts. NK cell purity of donor cells was determined by staining for NK1.1 and was 50-90% for cells enriched by collection of non-adherent splenocytes, and >94% for enrichment with magnetic beads. Adoptive transfer experiments were performed with age and sex-matched mice between 6 and 14 weeks of age.
Antibodies and flow cytometry
Antibodies recognizing the following antigens were purchased from BD Pharmingen (San Diego, CA): NK1.1 (PK136), CD45.1 (A20), CD45.2 (104), IFN-γ (XMG1.2), CD69 (H1.2F3), CD11b (M1/70), CD107a/LAMP-1 (1D4B), and KLRG1 (2F1). Granzyme B antibody (GB12) was obtained from Life Technologies. A viability dye was used for some experiments to exclude dead cells (Live/Dead®, Invitrogen). For surface staining, cells were incubated in the presence of anti-FcγRII/III (2.4G2) to block non-specific binding followed by incubation with specific antibodies. For intracellular flow cytometry, cells were fixed and permeablized after surface staining according to manufacturer’s recommendations (BD Pharmingen, Cytofix/Cytoperm™) and then incubated with anti-IFN-γ or anti-granzyme B. Flow cytometry data was collected on FACSCanto (BD Biosciences, San Jose, CA) or Cytek-modified FACScan (BD Biosciences and Cytek Development Inc, Fremont, CA) instruments and analyzed with FlowJo software (TreeStar, Inc, Ashland, OR). NK cells were identified as lymphocytes by FSC/SSC and expression of NK1.1 and for some experiments CFSE, CD45.1, and/or CD45.2.
In vitro stimulation assays
Splenocytes were harvested from recipient mice and assayed for IFN-γ production or CD107a expression. Donor and host NK cells were identified as NK1.1+ lymphocytes expressing the appropriate congenic marker (CD45.1 or CD45.2) and/or CFSE. For cytokine stimulation, cells were cultured with IL-12 (10ng/ml) plus IL-15 (100ng/ml) or media alone for a total of 4 hours, with brefeldin A added after the first hour. Co-culture with Yac-1 (obtained from ATCC, Manassas, VA) targets was performed at a 10:1 ratio of splenocytes to targets with or without mIL-12 (10ng/ml) for 4 hours, with brefeldin A added after the first hour. For CD107a (LAMP-1) assays, splenocytes were cultured at a 10:1 ratio with Yac-1 targets for a total of 4 hours. CD107a antibody was added at the start of culture and monensin added after 1 hour to inhibit receptor internalization.
Poly(I:C) in vivo activation
One or 4 weeks after adoptive transfer, Rag-2−/− γc−/− recipients were given 75μg of the synthetic toll-like receptor-3 (TLR3) ligand polyinosine-polycytidylic acid [poly(I:C) HMW; InvivoGen, San Diego, CA] by intraperitoneal (i.p.) injection. Splenocytes were harvested 4-6h later and immediately stained ex vivo for surface markers and intracellular expression of IFN-γ.
Statistical analysis
Student’s t test was used for statistical analyses between 2 groups. For groups of 3 or more, a 1-way ANOVA test with Bonferroni’s Multiple Comparison Test was used. Statistical analysis was performed with GraphPad Prism software (La Jolla, CA) and p<0.05 considered significant.
Results
Cytokine stimulation results in initial NK cell priming followed by an NK-intrinsic memory-like response
We previously reported cytokine-induced memory-like NK cell responses following adoptive transfer of activated and control NK cells into separate hosts. To definitively determine whether memory-like responses are NK-intrinsic, we generated congenic Rag-1 deficient donor mice (CD45.1+Rag1−/−) and performed co-transfers of cytokine-activated and control NK cells into the same host (Figure 1A). Cytokine-activated (IL-12 plus IL-18 with low-dose IL-15) CD45.2+ or control treated (low-dose IL-15 alone for survival) CD45.1+ enriched NK cells were labeled with CFSE and adoptively transferred into CD45.1+Rag1−/− hosts (Figure 1A). Cytokine activation with IL-12 plus IL-18 stimulates IFN-γ production by >90% of NK cells, while low-dose IL-15 maintains survival without inducing IFN-γ production (2). Following adoptive transfer, donor and host splenic NK cells were identified as NK1.1+ lymphocytes expressing (Figure 1A): CD45.2−CFSE− (host); CD45.2+NK1.1+ (pre-activated donor); and CD45.2−CFSE+ (control donor). Control-treated NK cells exhibited minimal proliferation since Rag-deficient hosts have intact NK cell compartments (Figure 1C, D), allowing for reliable identification of these donor NK cells based on CFSE expression. There were slightly more control than previously-activated donor cells present at day 1, however despite proliferation of cytokine-activated cells there was no significant difference in the numbers of control versus activated donor cells present at days 3, 7, or 21 (Supplemental Fig. 1), perhaps reflecting the limitations of re-constituting cells in mice with an intact NK cell compartment. Splenocytes from recipients were harvested and stimulated for 4h with cytokines (IL-12 + IL-15) or media and IFN-γ production measured by intracellular flow cytometry. One day after adoptive transfer, a small percentage of previously-activated donor NK cells continued to produce IFN-γ spontaneously, and they had a primed phenotype with the majority of cells producing IFN-γ after in vitro cytokine re-stimulation (IL-12 + IL-15) (Figure 1B). By contrast, significantly fewer control-donor and host NK cells were positive for IFN-γ. At later time points, pre-activated donor NK cells remained more likely to produce IFN-γ as compared to control or host NK cells; however the percentage of IFN-γ positive pre-activated NK cells was less than that seen at Day 1. Production of IFN-γ by control donor NK cells was similar to endogenous host cells, with the exception of day 21, when there was a very small but statistically significant increased production of IFN-γ by control donor versus host NK cells (20.3 vs. 17.7%). The exaggerated IFN-γ response of pre-activated donor cells at Day 1 suggests a residual priming effect at this early timepoint, whereas the later responses, particularly day 21, are consistent with a persistent memory-like response. Similar results were obtained with NK cells enriched by magnetic bead purification (>94% purity) or harvest of non-adherent cells (50-90% purity), and independent of the source of cells (i.e. CD45.1 or CD45.2). These co-transfer experiments definitively demonstrate that memory-like effects are NK-intrinsic and not the result of transfer of other activated cells (e.g. macrophages and/or dendritic cells), since transfer of enriched cytokine-activated NK cells did not alter the phenotype of host or control-donor NK cells.
Figure 1. Memory-like NK cell responses are cell-intrinsic.
Congenic NK cells were enriched from Rag1−/− mice and CFSE-labeled cytokine-activated and control cells adoptively transferred into Rag1−/− hosts. (A) Host, control, and previously-activated (Pre-act) NK cells were identified by CFSE and/or CD45.2 as shown. Following re-stimulation for 4h with IL-12 + IL-15 significantly more pre-activated than control cells produce IFN-γ. Representative flow cytometry data from 7 day after adoptive transfer is shown, gated on NK1.1+ cells. (B) Splenic NK cells from recipient mice were harvested 1, 3, 7, and 21 days after adoptive transfer and assayed for IFN-γ production following culture with IL-12 + IL-15 (black bars) or media (gray bars) in vitro. Early after adoptive transfer, pre-activated NK cells were primed and produced very high levels of IFN-γ (Days 1 and 3). At later timepoints, pre-activated cells acquired a memory-like phenotype (Days 7 and 21) and were more likely to produce IFN-γ than control-transferred or host NK cells. (C) Donor pre-activated NK cells proliferate in vivo as shown by CFSE dilution (host NK cells shaded histogram; pre-activated NK cells solid line; control NK cells dashed line). (D) Most pre-activated NK cell (black bars) proliferation occurred within the first 3 days of transfer and very few control NK cells (open bars) proliferated in vivo (n.d., proliferation not detected). ****p<0.0001; ***p≤0.0001; **p≤0.001; *p<0.05. Graphed data represents mean +/− SEM of 3-7 independent experiments.
NK cells homeostatically proliferate in NK-deficient hosts and maintain functional capacity
It remains possible that enhanced memory-like NK responses are due to in vivo proliferation and not related to a prior activation event. Therefore we next examined naïve and cytokine-activated NK cell responses following enforced proliferation of both subsets using an alymphoid host. NK cells were enriched from congenic (CD45.1 and CD45.2) Rag-1−/− mice, cytokine activated or control treated, CFSE labeled, and adoptively co-transferred into Rag-2−/− γc−/− hosts (Figure 2A), which lack endogenous T, B, and NK cells. Prior reports have demonstrated that NK cells undergo homeostatic proliferation in alymphoid hosts with maintenance of effector functions (19, 21, 23). NK cells are absent in Rag-2−/− γc−/− mice due to a defect in IL-15 signaling, which is required for NK cell development (25). However, Sun et al. (26) observed IL-12-induced development of NK cells in the setting of a viral infection in mice lacking IL-15. To ensure that we were measuring donor NK cells, and not endogenous NK cells that differentiated in response to cytokine stimulation, we injected Rag-2−/− γc−/− mice (CD45.2+) with activated CD45.1+ NK cells alone (Supplemental Figure 2). As expected, there was no development of endogenous CD45.1-negative NK cells as late as 4 months following transfer.
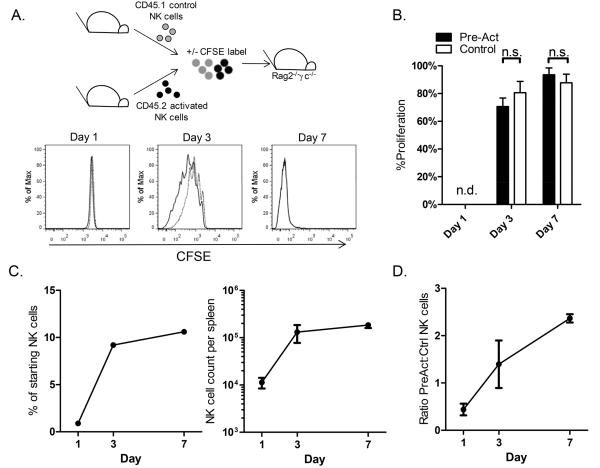
Figure 2. Homeostatic expansion of activated and control NK cells in alymphoid hosts.
(A) Cytokine-activated or control treated congenic NK cells from Rag1−/− mice were CFSE labeled and adoptively transferred into Rag-2−/− γc−/− hosts. Nearly all splenic NK cells proliferated within 7d of adoptive transfer as shown by dilution of CFSE of NK1.1+ pre-activated (solid line) and control (dashed line) NK cells and quantitated in panel B (mean +/− SEM of 4 independent experiments; n.s., not significant). (C) Percentage and absolute number of NK cells per spleen following adoptive transfer. (D) Ratio of pre-activated to control NK cells 1, 3, and 7 days after adoptive transfer.
Pre-activated and control NK cells both proliferated extensively in vivo, and CFSE was not detectable in either subset after 7 days, with the majority of NK cells dividing by day 3 (Figure 2A and B). Relatively few NK cells were recovered from the spleens of hosts 1d after transfer (Figure 2C). However, total NK cell counts increased over 7d (Figure 2C), and were similar to those observed previously (23). The ratio of pre-activated to control cells was initially low, and most splenic NK cells at day 1 were control cells with numbers of pre-activated cells increasing over 7 days (Figure 2D). It is unclear whether this is due to engraftment or homing of pre-activated NK cells, although at early timepoints we observed similar ratios of transferred cells in the liver and lungs, the other major organs housing NK cells (Supplemental Figure 3).
Despite proliferation of all control and pre-activated NK cells by day 7, progeny of pre-activated NK cells, never before exposed to cytokines, maintained an enhanced capacity to produce IFN-γ while control NK cell progeny maintained a naïve phenotype (Figure 3). Donor-derived NK cells were easily identified based on cell surface expression of NK1.1 and CD45.1 or CD45.2 (Figure 3A). Upon stimulation with IL-12 plus IL-15, significantly more NK cells derived from pre-activated cells produced IFN-γ than control NK cells (Figure 3). Similar to co-transfer studies in Rag-deficient hosts, 1 day after transfer a very high percentage of pre-activated NK cells produced IFN-γ, likely due to priming of those cells. At 3d and 7d after transfer, overall IFN-γ production was lower, and NK cells that originated from pre-activated cells continued to produce significantly more IFN-γ upon cytokine stimulation than control cells from the same host. These enhanced responses could represent a residual priming effect, rather than a memory-like response, although it is interesting that this enhanced capacity for IFN-γ production was passed on to daughter cells. It was reported that murine cytomegalovirus (MCMV)-induced memory NK cells express high levels of KLRG1 and we measured expression of this antigen as well as CD11b and the activation marker CD69 (Supplemental Figure 4). We saw high expression of KLRG1 on cytokine-activated and control NK cells 1 and 4 weeks after co-transfer into Rag-2−/− γc−/− hosts. Expression of KLRG1 on both donor subsets was much higher than that seen on unmanipulated Rag-1−/− NK cells, suggesting that expression of this antigen is related to in vivo proliferation (Supplemental Figure 4). Cytokine-activated and control cells also had similar expression of CD69 and CD11b, both of which were higher than seen on unmanipulated Rag-1−/− NK cells. These results demonstrate that cytokine-activated NK cells maintain an enhanced capacity for IFN-γ production early following homeostatic expansion and proliferation upregulates multiple markers of NK cell activation/maturation.
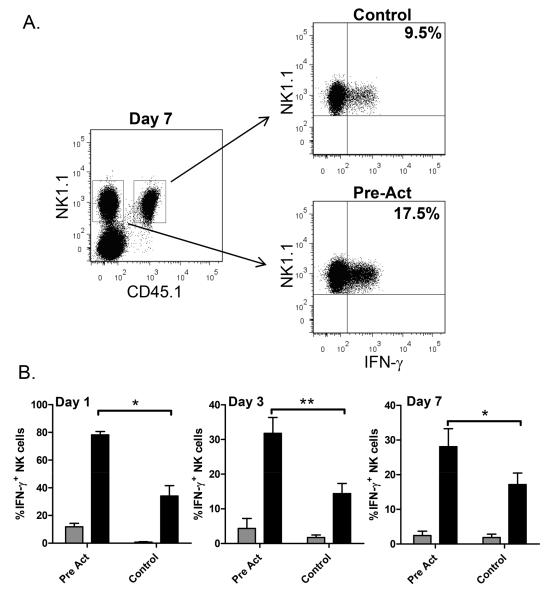
Figure 3. Maintenance of a memory-like phenotype after NK cell expansion.
Cytokine-activated or control treated congenic NK cells enriched from Rag1−/− mice were adoptively transferred into Rag-2−/− γc−/− hosts. (A) Seven days after adoptive transfer, pre-activated NK cells were more likely to produce IFN-γ following a 4 hour in vitro stimulation with IL-12 + IL-15 as shown in a representative intracellular flow plot gating on NK1.1+ CD45.1+ control or CD45.1− pre-activated NK cells. (B) Adoptively transferred cells were re-stimulated in vitro with IL-12 + IL-15 (black bars) or cultured with media alone (grey bars). Pre-activated NK cells had a primed phenotype with very high IFN-γ production 1d after adoptive transfer and spontaneous production of low levels of IFN-γ. By 7d, pre-activated NK cells exhibited a memory-like phenotype and were more likely to produce IFN-γ than control cells. Results represent the mean +/− SEM of 3-5 independent experiments. *p=0.01; **p=0.007.
Expanded memory-like and control NK cells respond more robustly to activation with target cells, while responses to in vivo TLR activation are dependent on early priming
We next determined NK cell IFN-γ production in response to tumor targets in vitro and TLR3 activation in vivo. Cytokine-activated and control treated NK cells were adoptively transferred into separate Rag-2−/− γc−/− hosts. After 7d (Figure 4A) or 4 weeks (Figure 4B) of expansion in vivo, NK cells were assayed for production of IFN-γ by culturing in vitro for 4h with Yac-1 tumor cells alone or with IL-12, which enhances IFN-γ production via co-stimulation. Significantly more donor NK cells derived from cytokine-activated cells produced IFN-γ as compared to control cells at both timepoints. While responses at 1 week could represent NK cell priming, enhanced NK cell responses at 4 weeks are consistent with a memory-like phenotype in response to tumor cell stimulation.
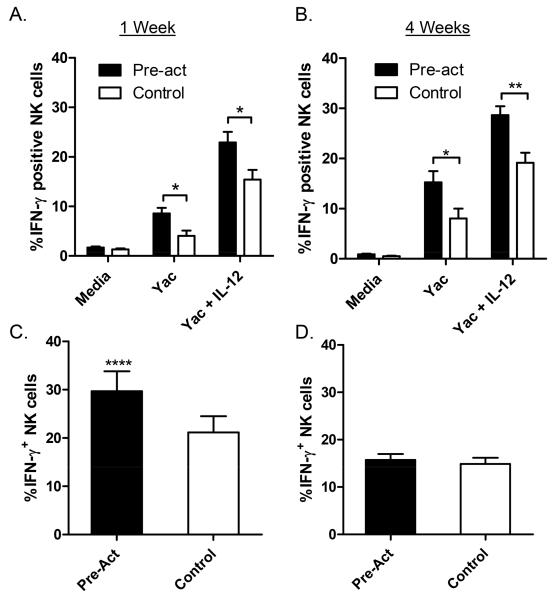
Figure 4. Expanded memory-like NK cells production of IFN-γ in response to tumor targets and in vivo Poly(I:C).
(A & B) Cytokine-activated (Pre-act) and control-treated NK cells were adoptively transferred into separate Rag-2−/− γc−/− hosts. Transferred splenic NK cells were re-stimulated (A) 7d and (B) 4 weeks later in vitro with tumor targets (Yac-1 cells) alone or with IL-12. Significantly more memory-like (pre-act) than control cells produced IFN-γ at both timepoints. Data represent mean +/− SEM of 7 and 4 independent experiments, *p<0.05; **p<0.005). (C & D) Cytokine-activated and control-treated NK cells were adoptively co-transferred into Rag-2−/− γc−/− hosts and injected (C) 7d or (D) 4 weeks later with poly(I:C) and NK cell IFN-γ production measured (mean +/− SEM of 4 independent experiments, ****p<0.0001; no significant difference at 4 weeks).
We next evaluated in vivo memory-like responses using the TLR3 ligand poly(I:C). One or 4 weeks after adoptive co-transfer of cytokine-activated and control NK cells into Rag-2−/− γc−/−, mice were injected i.p. with poly(I:C) and NK cell IFN-γ production measured directly ex vivo. We observed that the progeny of previously-activated NK cells had increased IFN-γ production at 1 week (Figure 4C). However, this effect was not observed at 4 weeks, suggesting that the earlier response could be related to residual NK cell priming and not memory.
Cytotoxic potential of memory-like and control NK cells
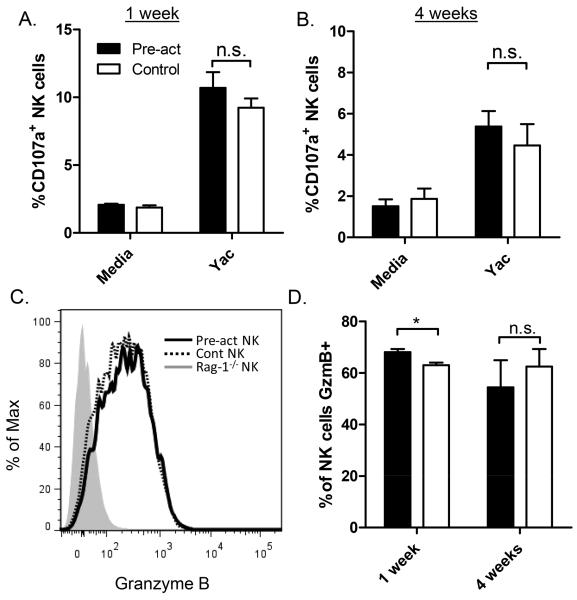
To determine whether homeostatic proliferation has any effect on memory-like NK cell killing, we assessed cell-surface expression of CD107a following target cell engagement as a surrogate for degranulation and the ability to kill targets. One or 4 weeks after adoptive transfer into alymphoid hosts, cytokine-activated and control NK cells exhibited similar levels of cell-surface CD107a in response to Yac-1 cells (Figure 5 A & B), suggesting that there is no difference in their ability to kill target cells, similar to our previous studies. Interestingly, both cytokine-activated and control NK cells expressed very high levels of granzyme B protein 1 and 4 weeks after adoptive transfer (Figure 4 C & D). As previously reported, naïve NK cells from Rag-1−/− mice did not express appreciable granzyme B protein (Figure 4C, shaded) (27).
Figure 5. Memory-like and control NK cells exhibit similar degranulation with tumor targets and expression of granzyme B.
(A & B) Cytokine-activated (Pre-act, filled bars) and control-treated (white bars) NK cells were adoptively transferred into separate Rag-2−/− γc−/− hosts. Seven days after adoptive transfer, NK cell degranulation in response to co-culture with a tumor target (Yac-1 cells) was measured by assaying for cell surface CD107a. Pre-activated memory-like and control NK cells had similar degranulation with Yac-1 targets at (A) 1 and (B) 4 weeks after adoptive transfer. Results represent the mean +/− SEM of 3-4 independent experiments at each timepoint. (C) Intracellular expression of granzyme B was measured in pre-activated (solid line) and control NK cells (dotted line) 4 weeks after adoptive transfer co-transfer into Rag2−/− γc−/− hosts. Granzyme B expression of freshly isolated Rag-1−/− NK cells is also shown (grey filled histogram). (D) Pre-activated and control NK cells expressed high levels of granzyme B (GzmB) 1 and 4 weeks after adoptive transfer. Results represent mean +/− SEM of 3 independent experiments at each timepoint, *p=0.047; n.s., not significant.
Long-term maintenance of memory-like NK cell phenotype
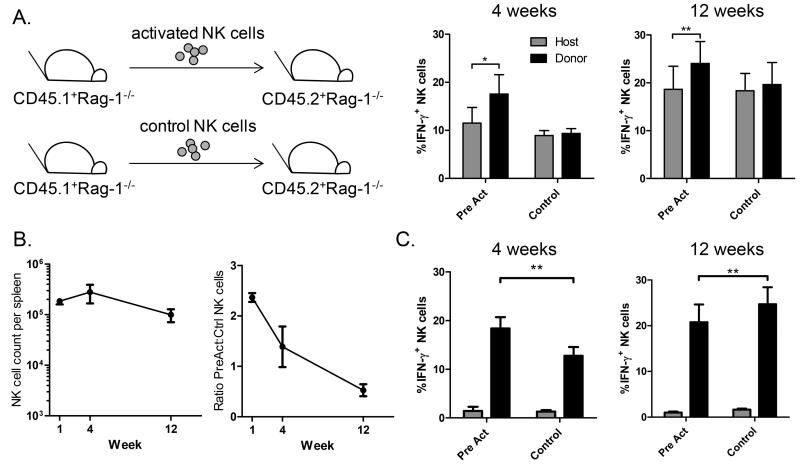
Having determined that NK cell memory-like responses are intrinsic and maintained in alymphoid hosts, we next sought to determine how long memory-like responses are preserved. First, we examined the longevity of memory-like responses in NK-competent hosts (Rag-1−/−). Cytokine-activated NK cells adoptively transferred into separate NK-sufficient hosts (Rag-1−/−) do not undergo homeostatic proliferation, and engraft in low numbers but maintained a memory-like phenotype for 12 weeks with enhanced production of IFN-γ following cytokine re-stimulation (Figure 6A). To determine if expanded and proliferating NK cells also maintain this phenotype, cytokine-activated and control NK cells were co-transferred into Rag-2−/− γc−/− hosts and analyzed 4 and 12 weeks later. NK cell counts remained relatively stable 1-12 weeks after transfer (Figure 6B), however the ratio of previously-activated to control cells decreased by 12 weeks, when the majority of NK cells were derived from control donors. Following activation with IL-12 + IL-15 in vitro, previously-activated NK cells maintained a memory-like phenotype at 4 weeks (Figure 6C), similar to results seen with tumor cell stimulation (Figure 4). At this timepoint memory-like NK cells constitute the majority of the NK compartment in the host (Figure 6B). However, by 12 weeks, when control cells outnumber previously-activated cells (Figure 6B), control-treated NK cells actually produced slightly more IFN-γ (Figure 6C). Interestingly, the levels of IFN-γ were higher at 12 weeks in both Rag-1−/− and control NK cells from Rag-2−/− γc−/− mice (6A & 6C). This could experimental variability or a gain of function in NK cells due to aging or proliferation (in Rag-2−/− γc−/− hosts).
Figure 6. Long-term engraftment and production of IFN-γ by memory-like NK cells.
Cytokine-activated (Pre-act) and control-treated NK cells were adoptively transferred into separate Rag-1−/− hosts (A) or co-transferred into Rag-2−/− γc−/− hosts (B&C). NK cell IFN-γ responses following in vitro culture with IL-12 + IL-15 were measured 4 and 12 weeks later. (A) Following transfer into separate NK-competent hosts, pre-activated NK cells were more likely to produce IFN-γ than naïve host NK cells (*p=0.03 at 4 weeks and **p=0.007 at 12 weeks, mean +/− SEM of 3-5 independent experiments). There was no significant difference between the percentage of control-treated donor and host IFN-γ+ NK cells. (B) Following co-transfer of cells into alymphoid Rag-2−/− γc−/− hosts (as schematized in Figure 2, without CFSE labeling), the absolute number of splenic NK cells remained stable, while the ratio of pre-activated to control cells decreased between 1 and 12 weeks after adoptive transfer (mean +/− SEM of 3-8 mice per timepoint; data shown for 1 week is also shown in Figure 2C and D). (C) Four weeks after adoptive transfer, significantly more pre-activated than control NK cells produced IFN-γ. Twelve weeks after co-transfer into alymphoid hosts, slightly more control donor NK cells produce IFN-γ. (Mean +/− SEM of 4-5 independent experiments; black bars represent IL-12 + IL-15 stimulation, grey bars media; **p<0.004).
Discussion
While immunologic memory has traditionally been the hallmark of adaptive immunity, several recent studies have demonstrated that innate immune NK cells have the capacity for memory-like responses (1-4). Here, we found that cytokine-induced memory-like NK cells expand in alymphoid hosts via homeostatic proliferation and maintain their phenotype for at least a month despite extensive proliferation of all NK cells. As expected, activated NK cells made very high amounts of IFN-γ shortly after adoptive transfer in to alymphoid hosts, likely due to a primed phenotype. However, both cytokine-activated and control NK cells quickly proliferated, and by day 7 nearly all cells present in the hosts had never before been primed in vitro. Memory-like NK cells had enhanced IFN-γ responses to re-stimulation with cytokines and tumor cells in vitro up to 4 weeks after adoptive transfer. In vivo TLR-activation showed enhanced responses by pre-activated NK cells at early (7d) but not later (4 weeks) timepoints. No difference in expression of granzyme B or the ability to degranulate in response to tumor cells was observed. Interestingly, all NK cells, regardless of prior activation, expressed high levels of granzyme B after transfer into alymphoid hosts, perhaps due to proliferation or other host factors.
Our data demonstrate that only a portion of daughter cells from previously-activated NK cells retain a capacity for enhanced IFN-γ production, since the majority of cells did not produce IFN-γ, and identification of a cell surface marker to identify memory-like NK cells will be important for future studies. As shown here, and based on expression profiling (M.A.C. unpublished data) we have not found a cell-surface marker that distinguishes memory-like from naïve splenic NK cells. KLRG1, an inhibitory cell-surface receptor (28), has been proposed as a marker of splenic NK cell memory and was elevated on the cell surface of MCMV-induced memory NK cells (3). Here, we found that KLRG1 was highly expressed by both cytokine-induced memory-like and naïve expanded NK cells (supplemental Figure 4), suggesting that KLRG1 may be a marker of mature NK cell proliferation, as was previously suggested (28, 29), rather than memory.
NK cell numbers are normally tightly regulated in healthy hosts. Homeostatic expansion occurs in the setting of lymphopenia, and mature NK cells can proliferate and expand to “fill” the empty NK compartment (21-23). In patients, this can occur as the result of infection or chemotherapy-induced lymphopenia. Following adoptive transfer into alymphoid hosts, both cytokine-activated and control NK cells proliferated extensively within a week of adoptive transfer. Cytokine-activated NK cells maintained a memory-like phenotype for 12 weeks in NK-competent Rag-1−/− hosts, while memory-like cells lost this phenotype by 3 months in alymphoid hosts. The observed differences may be related to NK cell homeostasis in the two models. In alymphoid, Rag-2−/− γc−/− hosts, mature NK cells undergo homeostatic proliferation driven by a lack of endogenous lymphocytes. Whereas in NK-competent, Rag-1−/− hosts, only a subset of memory-like cells proliferate 3-7d after adoptive transfer. Thus, one explanation for the loss of memory in Rag-2−/− γ c−/− hosts at 12 weeks is that multiple rounds of proliferation ultimately alters the memory-like phenotype of NK cells, while NK cells with low turnover in NK-competent hosts maintain a memory-like phenotype. An alternative hypothesis is that there is a gain of function in all (or only control) NK cells related to homeostatic proliferation, analogous to the memory-phenotype observed in T cells following homeostatic proliferation in a lymphopenic host (30). It will be interesting to determine whether NK cells exhibit a similar type of proliferation-driven memory-phenotype differentiation at late timepoints.
Regardless, the maintenance of clear memory-like effects after 4 weeks of proliferation demonstrates that cytokine-induced memory is stable and hereditary. From a therapeutic standpoint, clinically-relevant lymphopenia in patients (i.e., infection or chemotherapy-induced) and inhibition of NK cell development is likely temporary, lasting from a few days to months (31). Thus, transient lymphopenia in patients might allow for the expansion of adoptively transferred or infection-induced memory-like NK cells that, with resumption of normal NK cell differentiation and resolution of lymphopenia, are subsequently maintained in a quiescent state. Based on the model here, such non-dividing memory-like NK cells would be predicted to maintain their phenotype long-term.
There are currently three experimental models of murine NK cell memory: 1) cytokine-induced splenic NK cell memory-like function (2), as discussed here, 2) splenic NK memory conferred via stimulation with cytokines and a germline-encoded NK cell receptor following recognition of a pathogen-encoded ligand (3, 13), and 3) antigen-specific liver NK cell memory not mediated by a known germline-encoded receptor, which was the first type to be described (1, 4). Together, these models are complementary, rather than mutually exclusive, and definitively demonstrate that NK cells have the capacity for recall responses. Our model suggests that after an initial cytokine activation event and production of IFN-γ, NK cells retain the capacity for enhanced IFN-γ responses to multiple stimuli. Thus, cytokine-induced NK cell memory responses are predicted to provide non-specific protection. This is physiologically relevant, since NK cells express only a limited number of activation receptors and often rely upon cytokine signals to mediate effector function (6).
Studies from the Lanier group using an adoptive transfer system demonstrated that MCMV-induced expansion of long-lived Ly49H+ NK cells with enhanced cytokine production and the ability to provide superior protection against MCMV infection in neonatal mice (3). Similar to our system, this model was also dependent on cytokines, in particular IL-12 (13). Additional studies by Schlub et al. in an intact host found that the kinetics of Ly49H+ NK cell expansion and contraction are different from T cells and there may not be a physiologically significant pool of memory-like NK cells that can expand in a secondary response, perhaps more consistent with the idea of enhanced cellular function rather than long-lived protective immunologic memory (32). It is not known whether Ly49H+ MCMV-induced memory NK cells are virus-specific, however since they respond more robustly to non-viral stimuli (i.e., anti-NK1.1) (3), it is possible that they might also provide enhanced protection against other infections. Indeed, it may be the inherent non-specific nature of cellular memory-like responses in NK cells that could allow them to better protect the host, rather than specific immunologic memory to infections.
Studies from von Andrian’s group were the first to demonstrate the capacity for liver NK cell immunologic memory with the finding that NK cells can mediate hapten-specific contact hypersensitivity (CHS) responses in the absence of T cells (1). Subsequent studies demonstrated a small subset of liver lymphocytes from Rag-deficient mice expressing NK1.1, CXCR6, Thy1, and other markers had the capacity for antigen-specific intrinsic memory, but not splenic cells expressing the same markers (4). This type of memory appears more distinct from the two previous models based on its antigen specificity and limitation to lymphocytes from the liver. Memory NK1.1+ liver cells were present in genetically diverse strains of mice (BALB/c and C57BL/6); capable of recognizing haptens and viral like particles from HIV, influenza, and vesicular stomatitis virus; and conferred specific protection against viral infection in vivo (4). The recognition of such a diverse pool of antigens, and the fact that no antigen failed to induce NK cell memory responses (4), is reminiscent of T and B lymphocyte diversity and suggests that these NK1.1+ cells have the capacity for receptor editing, either via post-transcriptional modification or non Rag-mediated recombination events. A study from another group demonstrated that the character and quality of CHS responses mediated by NK cells may be distinct from T cell CHS, suggesting a unique mechanism for liver NK1.1+ cell memory (33). It will be interesting to determine the mechanism of specific antigen recognition in these NK cells.
Collectively, these studies demonstrate that NK cells “learn” from prior experiences. In all of the experimental systems the memory phenotype appears to be heritable, and persists longer than the estimated 7-17 day half-life of NK cells (34, 35). In the case of cytokine and MCMV-induced memory, NK cells were more responsive not only to the original stimuli but also other activation signals (e.g. cytokines, receptor ligation, tumor target cells), suggesting a generalized enhanced state of activation that could be exploited for immunotherapy (2, 3). Indeed, a recent study by the Cerwenka laboratory demonstrated that IL-12, -15, -18 pre-activation of NK cells prior to adoptive transfer into sub-lethally irradiated tumor-bearing hosts led to significant reduction in established tumors (36). Here, we discovered that cytokine-induced memory-like NK cells proliferate and expand in alymphoid hosts. There are currently multiple studies investigating NK cell adoptive therapy for cancer (9), and homeostatic expansion may be critical for treatment success as evidenced by the finding that patients treated with more intense conditioning regimens have enhanced donor NK cell engraftment (24). A recent study from Gill et al. found that murine NK cells adoptively transferred into Rag-2−/− γc−/− hosts completely lost the ability to produce IFN-γ within 5 days. They concluded that these NK cells become exhausted due to homeostatic proliferation and that caution should be used with NK cell adoptive therapy (37). However, we and others (19, 21, 23) clearly demonstrates that adoptively transferred NK cells retain the capacity to produce IFN-γ following proliferation in alymphoid hosts. The cause for discrepancy between these studies is unclear; however the murine NK cells used by Gill et al. were expanded with IL-2 prior to adoptive transfer, which may result in NK exhaustion, whereas we demonstrate that control (low-dose IL-15) or IL-12 + IL-18 activated NK cells retain the capacity to produce IFN-γ following in vitro and in vivo stimulation. This suggests that thorough investigation of growth factors that support optimal NK cell function, such as IL-12 and IL-18, is critical for successful NK cell immunotherapy.
Many questions regarding the nature of NK cell memory responses remain. Can memory-like NK cells provide enhanced protection to a variety of infections and tumors? What are the mechanisms of heritable memory-like responses and are they present in humans? NK cell memory has only recently been recognized, and as we gain a better understanding of these and other fundamental questions it will be interesting to see how innate immune memory can be exploited for NK cell vaccination and adoptive therapy strategies.
Supplementary Material
Acknowledgments
We thank W. Yokoyama and T. Fehniger for thoughtful discussion and review of the manuscript.
Footnotes
This work was supported by the NIH-NIAID grant K08AI085030, The Child Health Research Center of Excellence at Washington University (K12-HD01487), and The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital.
References
- 1.O’Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell-and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol. 2006;7:507–516. doi: 10.1038/ni1332. [DOI] [PubMed] [Google Scholar]
- 2.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci U S A. 2009;106:1915–1919. doi: 10.1073/pnas.0813192106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW, von Andrian UH. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol. 2010;11:1127–1135. doi: 10.1038/ni.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yokoyama WM. Mistaken notions about natural killer cells. Nat Immunol. 2008;9:481–485. doi: 10.1038/ni1583. [DOI] [PubMed] [Google Scholar]
- 6.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron CA, Brossay L. NK cells and NKT cells in innate defense against viral infections. Curr Opin Immunol. 2001;13:458–464. doi: 10.1016/s0952-7915(00)00241-7. [DOI] [PubMed] [Google Scholar]
- 8.French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- 9.Cooley S, Weisdorf DS. Natural killer cells and tumor control. Curr Opin Hematol. 2010;17:514–521. doi: 10.1097/MOH.0b013e32833f10f1. [DOI] [PubMed] [Google Scholar]
- 10.Cooper MA, Yokoyama WM. Memory-like responses of natural killer cells. Immunol Rev. 2010;235:297–305. doi: 10.1111/j.0105-2896.2010.00891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paust S, von Andrian UH. Natural killer cell memory. Nat Immunol. 2011;131:500–508. doi: 10.1038/ni.2032. [DOI] [PubMed] [Google Scholar]
- 12.Sun JC, Lopez-Verges S, Kim CC, DeRisi JL, Lanier LL. NK cells and immune “memory”. J Immunol. 2011;186:1891–1897. doi: 10.4049/jimmunol.1003035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med. 2012;209:947–954. doi: 10.1084/jem.20111760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012 doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ, Nixon DF, Lanier LL. Expansion of a unique CD57NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–14732. doi: 10.1073/pnas.1110900108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjorkstrom NK, Lindgren T, Stoltz M, Fauriat C, Braun M, Evander M, Michaelsson J, Malmberg KJ, Klingstrom J, Ahlm C, Ljunggren HG. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J Exp Med. 2011;208:13–21. doi: 10.1084/jem.20100762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol. 2012;189:5082–5088. doi: 10.4049/jimmunol.1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.White DW, Keppel CR, Schneider SE, Reese TA, Coder J, Payton JE, Ley TJ, Virgin HW, Fehniger TA. Latent herpesvirus infection arms NK cells. Blood. 2010;115:4377–4383. doi: 10.1182/blood-2009-09-245464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Muller W, Di Santo JP. IL-15 is an essential mediator of peripheral NK-cell homeostasis. Blood. 2003;101:4887–4893. doi: 10.1182/blood-2002-11-3392. [DOI] [PubMed] [Google Scholar]
- 20.Cooper MA, Bush JE, Fehniger TA, VanDeusen JB, Waite RE, Liu Y, Aguila HL, Caligiuri MA. In vivo evidence for a dependence on interleukin 15 for survival of natural killer cells. Blood. 2002;100:3633–3638. doi: 10.1182/blood-2001-12-0293. [DOI] [PubMed] [Google Scholar]
- 21.Prlic M, Blazar BR, Farrar MA, Jameson SC. In vivo survival and homeostatic proliferation of natural killer cells. J Exp Med. 2003;197:967–976. doi: 10.1084/jem.20021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranson T, Vosshenrich CA, Corcuff E, Richard O, Laloux V, Lehuen A, Di Santo JP. IL-15 availability conditions homeostasis of peripheral natural killer T cells. Proc Natl Acad Sci U S A. 2003;100:2663–2668. doi: 10.1073/pnas.0535482100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med. 2011;208:357–368. doi: 10.1084/jem.20100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–3057. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 25.Fehniger TA, Caligiuri MA. Interleukin 15: biology and relevance to human disease. Blood. 2001;97:14–32. doi: 10.1182/blood.v97.1.14. [DOI] [PubMed] [Google Scholar]
- 26.Sun JC, Ma A, Lanier LL. Cutting edge: IL-15-independent NK cell response to mouse cytomegalovirus infection. J Immunol. 2009;183:2911–2914. doi: 10.4049/jimmunol.0901872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fehniger TA, Cai SF, Cao X, Bredemeyer AJ, Presti RM, French AR, Ley TJ. Acquisition of murine NK cell cytotoxicity requires the translation of a pre-existing pool of granzyme B and perforin mRNAs. Immunity. 2007;26:798–811. doi: 10.1016/j.immuni.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Robbins SH, Nguyen KB, Takahashi N, Mikayama T, Biron CA, Brossay L. Cutting edge: inhibitory functions of the killer cell lectin-like receptor G1 molecule during the activation of mouse NK cells. J Immunol. 2002;168:2585–2589. doi: 10.4049/jimmunol.168.6.2585. [DOI] [PubMed] [Google Scholar]
- 29.Huntington ND, Tabarias H, Fairfax K, Brady J, Hayakawa Y, Degli-Esposti MA, Smyth MJ, Tarlinton DM, Nutt SL. NK cell maturation and peripheral homeostasis is associated with KLRG1 up-regulation. J Immunol. 2007;178:4764–4770. doi: 10.4049/jimmunol.178.8.4764. [DOI] [PubMed] [Google Scholar]
- 30.Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 31.Castelino DJ, McNair P, Kay TW. Lymphocytopenia in a hospital population--what does it signify? Aust N Z J Med. 1997;27:170–174. doi: 10.1111/j.1445-5994.1997.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 32.Schlub TE, Sun JC, Walton SM, Robbins SH, Pinto AK, Munks MW, Hill AB, Brossay L, Oxenius A, Davenport MP. Comparing the kinetics of NK cells, CD4, and CD8 T cells in murine cytomegalovirus infection. J Immunol. 2011;187:1385–1392. doi: 10.4049/jimmunol.1100416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rouzaire P, Luci C, Blasco E, Bienvenu J, Walzer T, Nicolas JF, Hennino A. Natural Killer cells and T cells induce different types of skin reactions during recall responses to haptens. Eur J Immunol. 2011;42:80–88. doi: 10.1002/eji.201141820. [DOI] [PubMed] [Google Scholar]
- 34.Jamieson AM, Isnard P, Dorfman JR, Coles MC, Raulet DH. Turnover and proliferation of NK cells in steady state and lymphopenic conditions. J Immunol. 2004;172:864–870. doi: 10.4049/jimmunol.172.2.864. [DOI] [PubMed] [Google Scholar]
- 35.Koka R, Burkett PR, Chien M, Chai S, Chan F, Lodolce JP, Boone DL, Ma A. Interleukin (IL)-15R[alpha]-deficient natural killer cells survive in normal but not IL-15R[alpha]-deficient mice. J Exp Med. 2003;197:977–984. doi: 10.1084/jem.20021836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med. 2012 doi: 10.1084/jem.20120944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gill S, Vasey AE, De Souza A, Baker J, Smith AT, Kohrt HE, Florek M, Gibbs KD, Jr., Tate K, Ritchie DS, Negrin RS. Rapid development of exhaustion and down-regulation of eomesodermin limit the antitumor activity of adoptively transferred murine natural killer cells. Blood. 2012;119:5758–5768. doi: 10.1182/blood-2012-03-415364. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.