Abstract
Background
Nerve-stimulated fade in muscle is generally accepted as a prejunctional phenomenon mediated by block of prejunctional acetylcholine receptors (AChRs) at the nerve terminal, while decrease of twitch tension is considered a postjunctional effect due to block of muscle AChRs. Using ligands with specific pre or postjunctional effects only, we tested the hypothesis that fade is not necessarily a prejunctional phenomenon.
Methods
Neuromuscular function in rats was evaluated after intramuscular (2.5U) or IV (12.0U) injection of botulinum toxin (Botx), or IV (250µg/kg) alpha-bungarotoxin (α-BTX) alone. The acute neuromuscular effects of IV 2mg/kg dihydro-β-erythroidine (DHβE), alone and in combination with α-BTX were also tested. Botx decreases vesicular release of acetylcholine (ACh), and α-BTX binds to postjunctional nicotinic AChRs only, while DHβE binds specifically to prejunctional α3β2 AChRs only. In view of the lack of acute effects of Botx even at 2 hours after IV injection, its neuromuscular effects were also evaluated at 24 hours after intramuscular injection (0.6 U) and compared to intramuscular injection of α-BTX (25 µg/kg) or saline also given 24 hours earlier. The sciatic nerve-tibialis muscle preparation, during train-of-four and tetanic stimulation, was used to test neuromuscular effects in vivo.
Results
Intravenous and intramuscular Botx had no observable neuromuscular effects at 2 hours. Intravenous α-BTX caused twitch depression within a few minutes, and significant fade (P=0.002) at 75% of baseline twitch tension; these effects persisted until the end of the observation period of 2 hours. Intravenous DHβE alone caused no significant change in single twitch (p=0.899) or train-of-four ratio (P=0.394), but significantly enhanced the fade of IV α-BTX (P=0.001 at 75% of baseline twitch tension). Intramuscular Botx or α-BTX, at 24 hours after their injection, resulted in a significant decrease of single twitch and tetanic tensions (p<0.0001), but Botx did not cause fade, while α-BTX caused significant (P<0.0001) fade at 24 hours. The tibialis muscle weights and protein expression of α1 subunit of AChR (Western blots) did not differ between Botx, α-BTX and saline-injected groups at 24 hours but increased in denervated muscle (positive control).
Conclusions
Botx-induced decreased ACh release in and of itself does not cause fade but does cause decrease of absolute tensions. Decrease of available (functional) postjunctional AChRs by α-BTX did induce fade. The prejunctional fade effects of DHβE on α3β2 AChRs become manifest only when the margin of safety was decreased by concomitant administration of α-BTX. Thus, fade during repetitive stimulation is not always a prejunctional phenomenon, and may also reflect the decreased margin of safety of neurotransmission, which can be due to a pure postjunctional AChRs block or to a combination of both pre and postjunctional AChRs block. Block of prejunctional α3β2 AChRs alone is not necessary and sufficient to cause fade.
Introduction
Neuromuscular blocking drugs are extensively used in the intra and postoperative period, including the intensive care unit. Assessment of neuromuscular recovery after the use of neuromuscular blockers is increasingly advocated.1–3 In the presence of neuromuscular block by muscle relaxants, train-of-four (TOF) fade, tetanic fade or Wedensky inhibition is observed.4 These neuromuscular changes, when present, reflect the inability of the muscle to maintain sustained (repetitive) contraction at the same initial level. Thus, for example, the response during 2Hz stimulation (the TOF ratio/fade) is used to evaluate the extent of neuromuscular block and adequacy of the recovery from relaxant-induced paralysis.1–3 It has been generally accepted that fade observed during repetitive nerve stimulation is a presynaptic phenomenon, while depression of twitch tension can be a prejunctional effect due to decreased release of acetylcholine (ACh) or a postjunctional effect related to decreased binding of ACh to the postjunctional acetylcholine receptors (AChRs) caused by occupation of these receptors by the muscle relaxant.5–7
Bowman has suggested that, during repetitive nerve stimulation, the prejunctional nicotinic AChRs enhance further mobilization of ACh by positive feedback mechanism to maintain twitch tension at the same level.4 He proposed that block of the prejunctional AChRs by the nondepolarizing muscle relaxant attenuates the enhanced mobilization of ACh that occurs during repetitive stimulation; attenuation of the enhanced mobilization by block of these prejuctional AChRs results in fade.8,9 Subsequently, Faria et al using the rat phrenic-diaphragm preparation (ex-vivo) showed that specific inhibition of the presynaptic nicotinic α3β2 AChRs, with dihydro-β-erythroidine (DHβE) or with α-conotoxin MII alone did not induce fade, although ACh release during repetitive stimulation was indeed decreased during block of α3β2.10 However, tetanic fade with inhibition of α3β2 AChRs by DHβE or α-conotoxin MII was noted only when the safety margin of neuromuscular transmission was decreased with high concentrations of magnesium (6–7 mM) ex-vivo in the bath.10,11 (Normal concentration of total plasma magnesium ranges from 0.7 – 1.0 mM.) Jonsson et al12 demonstrated that clinically used muscle relaxants inhibited some of the neuronal nicotinic AChRs including α3β2 expressed in oocytes, but the relationship of this inhibition to fade was not studied. Furthermore, almost all studies regarding contribution of pre and postjunctional AChRs to fade phenomenon used in vitro and ex-vivo models. Thus, the precise mechanism of the fade or even the specific AChRs causing fade has not been completely characterized, especially using an in vivo model.
This study in rats tested the hypothesis that the decreased release of ACh prejunctionally does not necessarily result in fade, while decreased availability of AChRs postjunctionally can lead to fade during repetitive nerve stimulation, even in the absence of any prejunctional block of AChRs. This hypothesis was tested using intramuscular or IV injections of two natural toxins, botulinum toxin (Botx) which has pure prejunctional effects decreasing the release of ACh, and alpha-bungarotoxin (α-BTX) which has exclusive postjunctional effects impairing binding of ACh to AChRs, respectively. In addition, the effect of DHβE, a specific antagonist of prejunctional α3β2 AChR,10,13 on fade was tested in vivo alone and in combination with IV α-BTX.
Materials and Methods
Animals
The study protocol was approved by the institutional animal care committee. Male Sprague-Dawley rats (Taconic, MA), weighing 155–250 g, were used. After at least one week of acclimatization at our animal facility, the animals were randomly allocated to different experimental groups.
In a group of animals (n=5), after establishment of steady twitch tension, the stability of the in vivo neuromuscular preparation was tested for 2 hours while no drug affecting neurotransmission was administered. The drugs used for our subsequent experiments included Botx, α-BTX and DHβE. In the initial experiments with Botx (Botox®; Allergan Inc., Irvine, CA), the rats received 2.5U (n=3) into tibialis muscle, or 12U IV (n=3) through the jugular vein during pentobarbital anesthesia (60 mg/kg) intraperitoneally. Another group of rats received IV injection of 250µg/kg α-BTX (α-Bungarotoxin from Bungarus multicinctus, EMD Chemicals, Inc. Gibbstown, NJ) alone (n = 4), or 2mg/kg DHβE (Tocris Bioscience, Ellisville, MO) IV alone (n=4). A third group of rats (n=4) received 250µg/kg α-BTX together with 2mg/kg DHβE IV.
Neuromuscular function studies at 24 hours after Botx injection were necessary since Botx caused no neuromuscular effects at the end of the observation period of 2 hours. Since IV administration of Botx causes systemic paralysis and death, the toxin was injected directly into the tibialis to produce a pure a local effect, and the neuromuscular effects of this injection tested 24 hours later. For comparative purposes, therefore, α-BTX or saline was also injected intramuscularly and studies performed at 24 hours after these injections. The first group of rats (n=8) received one injection of Botx 0.6U into the tibialis muscle of one side. Another group of rats (n=8) received intramuscular α-BTX (α-bungarotoxin from Bungarus multicinctus, Sigma-Aldrich, St. Louis, MO) 25µg/kg into the tibialis of one side. Time-matched control animals (n=9) received an equivalent volume of saline. For the intramuscular injection, the rats were anesthetized with pentobarbital (60 mg/kg intraperitoneally), and the limbs shaved and disinfected. The total volume (0.5 ml) of diluted toxin was aliquoted into two equal parts (0.25 ml), and then injected into the medial and lateral aspects of the middle of the tibialis muscle belly, where the neuromuscular junction is usually located. After injection of toxin or saline, the animals were monitored until recovery from anesthesia, and then returned to their cages. At 24 hours after the injection, neuromuscular function including TOF and tetanic fade was evaluated.
Anesthesia and Vital Parameters
The methods used were essentially the same as described previously.14,15 Anesthetized with pentobarbital, the animals were tracheostomized and their lungs ventilated using a Harvard ventilator (Harvard Apparatus, Holliston, MA). The right carotid artery was cannulated to measure arterial blood pressure and perform blood gas analyses. Heart rate and mean arterial blood pressure were continuously monitored to ensure stable hemodynamic conditions throughout the experiment. Arterial oxygen tension (PaO2), arterial carbon dioxide tension (PaCO2), and acid–base status were intermittently measured and ventilation corrected to normal range, if necessary. Body temperature was monitored with a rectal probe and maintained at 36°–38°C by a heat lamp. Anesthesia was maintained with repetitive doses of pentobarbital based on cardiovascular signs of inadequate anesthesia. A venous catheter was inserted into the right jugular vein for administration of the drugs and fluids.
Rats were excluded from the experiment if they were hemodynamically unstable (mean arterial blood pressure < 80 mmHg), or if their blood gas status throughout the experiment was not within the defined predefined ranges (PaO2 > 90 mmHg; pH 7.36–7.44; PaCO2 = 36–44 mmHg).
Neuromuscular Function Test
Neuromuscular transmission and function were monitored by evoked mechanomyography using a peripheral nerve stimulator (NS252; Fisher & Paykel Health Care, Irvine, CA) along with a Grass force transducer and software (Grass Technologies, West Warwick, RI). For this purpose, rats were placed in the dorsal recumbent position. The tendon of the insertion of tibialis muscle was surgically exposed on each side and individually attached to separate Grass FT03 force displacement transducers (Grass Technologies). Both sciatic nerves were exposed at the thigh and stimulation electrodes were attached for nerve-mediated stimulation of the tibialis muscle. The knees were stabilized rigidly with a clamp. A baseline tension of approximately 50 g was applied to the tendon of each tibialis muscle, which yielded optimal evoked tensions. The nerve-evoked tension of each muscle was recorded using the Grass Polyview Software.
Initially, during supramaximal stimuli, baseline mechanomyographic responses were stabilized over a period of 30 minutes using the TOF stimulation pattern, every 20 seconds. At the end of this period, the evoked muscle tension developed during TOF stimulation was recorded. For the experiments performed on the day of toxin injection, the effects of the drugs were evaluated using the TOF stimulation. In the experiments performed 24 hours after intramuscular injection, TOF stimulation was followed by tetanic stimulation at 50 Hz for 5 seconds to assess the maximal tetanic muscle tensions and the fade associated with tetanic stimulation. TOF fade was calculated as T4/T1 ratio, where T4 and T1 are the fourth and first twitch tensions in the same train. T1 height during TOF stimulation was termed single twitch tension. Tetanic fade was calculated as Te/Ti ratio, where Te and Ti are end and initial tetanic tensions during 50Hz tetanic stimulation. The peak tension during tetanic stimulation was termed tetanic tension. After neuromuscular function studies, the animals in the 24 hour study group were euthanized, and the tibialis muscles on both sides harvested, weighed, snap frozen in liquid nitrogen, and stored at −80°C for later biochemical analysis of protein expression of α1AChR subunit.
Protein expression of the α1 subunit of Acetylcholine Receptor
The α1AChR subunit protein expression in the tibialis muscles from Botx (n=5), α-BTX (n=5), time-matched and saline-injected rats at 24 hours (n=5), and denervated muscles at one week (n=5) were assayed using Western blotting. In brief, the tibialis muscle was homogenized in 4ml of ice-cold homogenization buffer [50mM HEPES-NaOH (pH 7.5), 100mM NaCl, 1%Triton X-100, 5mM EDTA, 8µg/ml Aprotinin, 8µM Leupeptin, 0.1mM phenylmethylsulfonyl fluoride, 25mM NaF, 2mM Na3VO4] with a POLYTRON PT 10/35 homogenizer (Brinkmann Instruments, Inc. Westbury, NY). The homogenized samples were centrifuged at 18,380g for 30 min. Aliquots of the supernatant containing equal amounts of protein, as determined using the CBQCA protein quantitation kit (Invitrogen Corporation, Carlsbad, CA), were subjected to SDS-PAGE on a 4–20% Tris-HCl Gel (Bio-Rad, Hercules, CA) after the addition of Laemmli sample buffer and boiling for 4 min. After electrophoretic transfer to polyvinylidene difluoride membrane (Bio-Rad), the membrane was blocked in 3% nonfat dry milk for 1 h at room temperature, followed by overnight incubation with primary antibodies at 4°C. Anti AChR α1 subunit antibody (BD Transduction Laboratories, San Jose, CA) and anti GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody (Abcam Inc., Cambridge, MA) were used as primary antibodies. This procedure was followed by another incubation with secondary antibodies conjugated with horseradish peroxidase for 1 h at room temperature. Western blotting chemiluminescence luminol reagent was used to visualize the blots. Bands of interest were scanned with the use of Epson Perfection 2450 Photo Scanner (Epson America, Inc) and quantified by ImageJ software (US. National Institutes of Health, Bethesda, MD, USA).
Data and statistical Analysis
Continuous variables are expressed as means ± SE. Prism 6 (GraphPAD Software, San Diego, CA) was used for the statistical analyses. Unpaired t-test was used to compare single twitch tension or TOF fade values from different animals at the same degree of depression of the first twitch (Figure 1), or at same time point (Figures 2 and 3). Paired t-test was used to compare the values from the same animal at the same time point (Figures 2 and 3). Repeated measures ANOVA followed by Dunnett’s multiple comparisons test was used to compare preadministration TOF neuromuscular responses to postdrug administration responses at 75%, 50%, and 25% of baseline twitch tension (Figure 1). One-way ANOVA followed by Tukey‘s multiple comparisons test was used to compare α1AChR subunit protein expression between denervated leg and drug-injected legs (Figure 4). Statistical significance was established as P < 0.05.
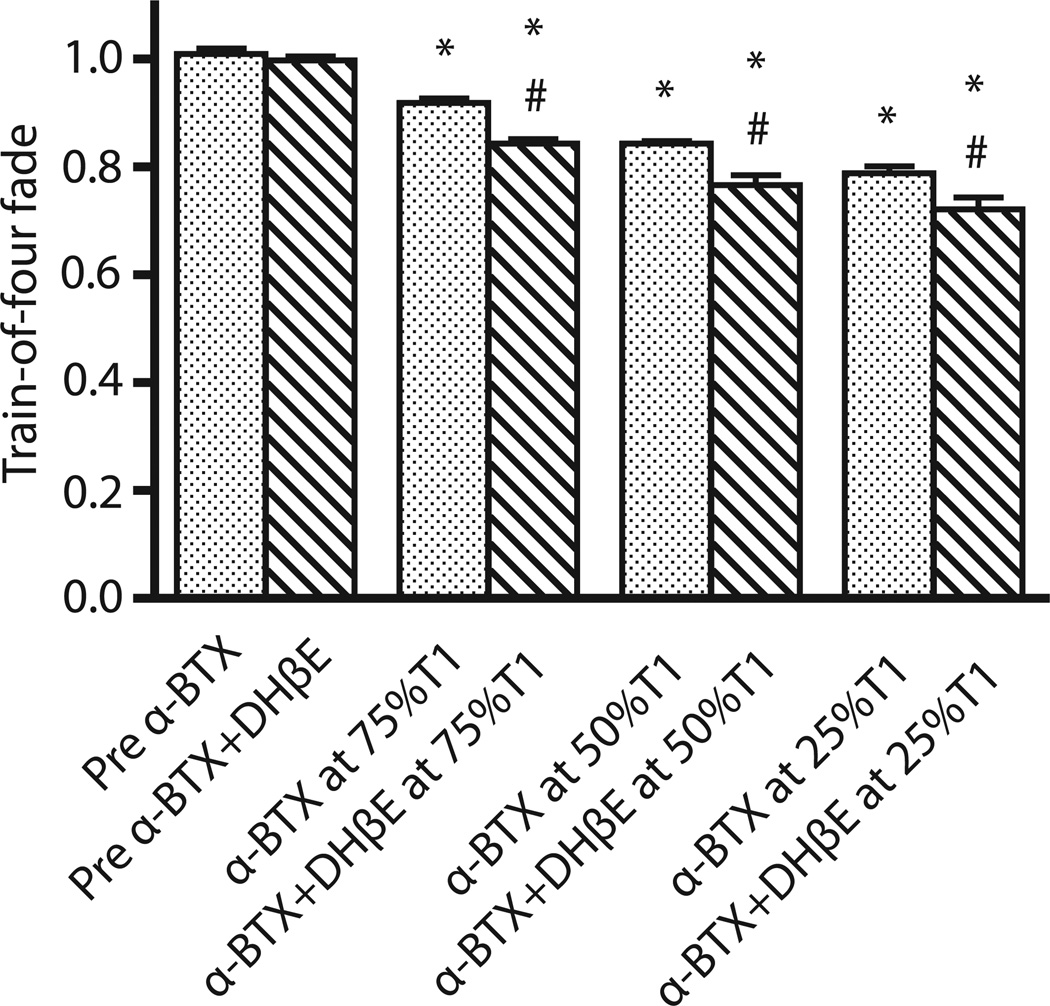
Figure 1. Train-of-four fade after IV administration of α-BTX with and without DHβE.
After IV administration of 250µg/kg alpha-bungarotoxin (α-BTX) with and without 2mg/kg dihydro-β-erythroidine (DHβE), train-of-four (TOF) fade was measured. Values are expressed mean ± SE. *P<0.01 versus predrug administration value of TOF fade. #P<0.05 versus TOF fade by α-BTX at the same degree of T1 depression. Administration of α-BTX alone caused significant fade at 75, 50 and 25% depression of baseline twitch height. The combined administration of α-BTX together with DHβE caused more significant fade (p<0.05) when compared with α-BTX alone.
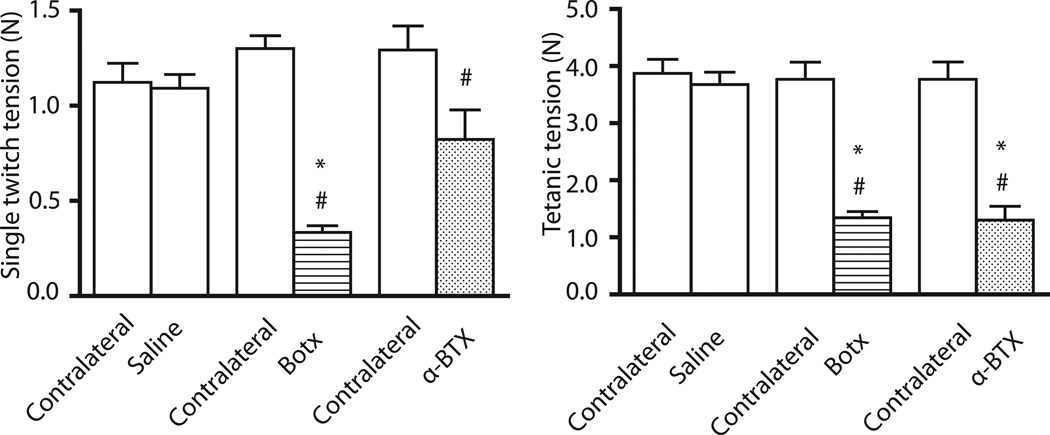
Figure 2. Single twitch and tetanic tensions after intramuscular administration of Btox, α-BTX or saline.
After intramuscular administration of 0.6U botulinum toxin (Botx), 25ug/kg alpha-bungarotoxin (α-BTX), or saline, muscle function was studied 24 hours later. Values are expressed mean ± SE. *P<0.001 versus time-matched saline-injected leg. #P<0.01 versus contralateral leg. Both toxins caused significant decrease in twitch and/or tetanic tensions compared to saline-injected controls and/or contralateral leg.
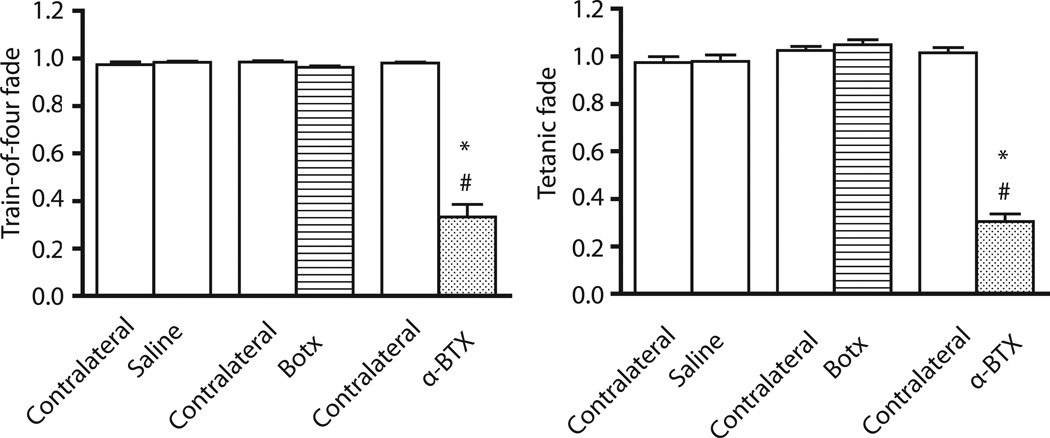
Figure 3. Train-of-four fade and tetanic fade after intramuscular administration of Btox, α-BTX, or saline.
After intramuscular administration of 0.6U botulinum toxin (Botx), 25ug/kg alpha-bungarotoxin (α-BTX), or saline, muscle train-of-four (TOF) fade or tetanic fade was evaluated 24 hours later. Values are expressed mean ± SE. *P<0.0001 versus time-matched saline-injected leg. #P<0.001 versus contralateral leg. Botx caused no fade during TOF or tetanic stimulation, while α-BTX caused significant fade during TOF and tetanic stimulation.
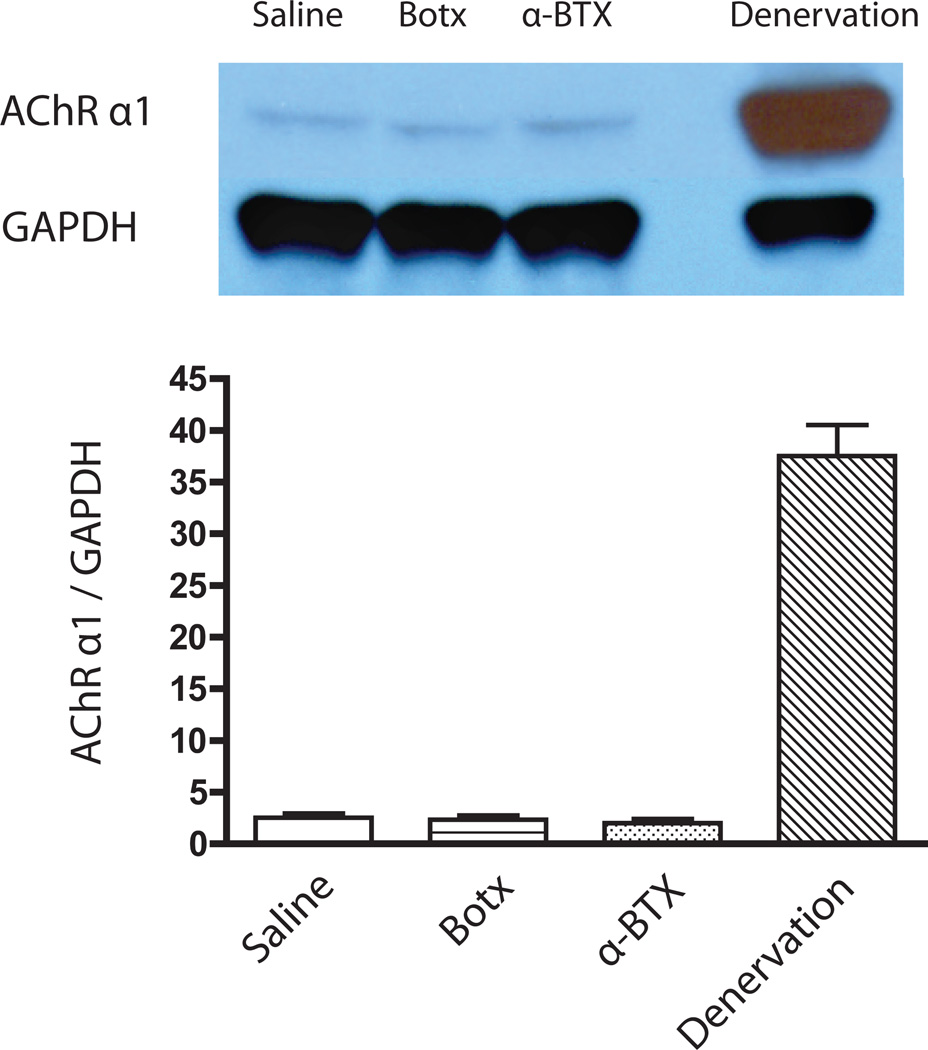
Figure 4. Protein expression of α1 subunit of AChR in the tibialis muscle.
Western blots of α1 subunit of acetylcholine receptor (AChR) and GAPDH at 24 hours after intramuscular administration of 0.6U botulinum toxin (Botx), 25ug/kg alpha-bungarotoxin (α-BTX), or saline. The muscle after denervation at one week was used as positive control. GAPDH was used as a loading control. Values are expressed mean ± SE (n=5/group). The protein expression of α1AChR subunit did not differ between Botx, α-BTX and saline groups at 24 hours after intramuscular injections. Denervation significantly (p<0.0001) increased α1 AChR expression at one week.
Results
Neuromuscular function test
In preliminary studies with no drug (toxin) injection, where the stability of the in vivo preparation was assessed, there were no significant changes in single twitch tensions (P=0.579) and TOF ratios (P=0.621) with time until the end of observation period of two hours. Consistent with previous studies,14,15 intramuscular injection of 2.5U Botx did not produce any significant neuromuscular effects up to 2 hours after its administration. Similarly, IV 12U Botx produced no significant changes in twitch responses (P=0.607) or in the TOF fade responses (P=0.451) during the 60 min of observation. In the next set of rats, IV administration of 2mg/kg DHβE showed no significant changes in single twitch tension (P=0.899) or TOF ratio (P=0.394) throughout 60 min after drug administration compared with predrug administration values. We next studied the neuromuscular effect of IV administration of α-BTX. After stabilization of twitch tension, IV administration of α-BTX (250µg/kg) caused depression of single twitch tension within 2–3 minutes. The times taken for decrease of twitch height to 75, 50, and 25% of baseline height were 13.5±0.25, 17.6±0.61, and 22.6±0.81 minutes, respectively. This dose of α-BTX caused 100% twitch depression within 60 minutes. The recovery of twitch or fade was not observed during the period of observation of 2 hours after IV administration of α-BTX. At 75, 50, and 25% of baseline twitch tension after α-BTX, the TOF fade associated with these twitch depressions were 0.92±0.01, 0.84±0.01, and 0.79±0.01, respectively, all of which were significantly different (P=0.002, P=0.002, and P=0.004, respectively) compared to TOF at predrug administration (Figure 1). At 75, 50, and 25% of baseline twitch tension the administration of 2mg/kg DHβE in combination with 250µg/kg α-BTX significantly accentuated the TOF fade produced by α-BTX alone P=0.001 at 75% of baseline twitch tension (T1), P=0.021 at 50% of T1, and P=0.038 at 25% of T1, Figure 1).
At 24 hours after intramuscular 0.6U Botx injection, the single twitch and tetanic tensions were significantly (P<0.0001) decreased compared with time-matched saline-injected leg as well as contralateral leg (Figure 2). Similarly, at 24 hours after intramuscular α-BTX, the tetanic tension was significantly (P<0.0001) decreased compared with time-matched saline-injected leg as well as contralateral leg (Figure 2). The single twitch tension was also significantly (P=0.004) decreased at 24 hours in the α-BTX leg compared with contralateral leg (Figure 2). During TOF and tetanic stimulations, the intramuscular Botx-injected leg showed no significant TOF fade and tetanic fade relative to contralateral leg (P=0.055, and P=0.267, respectively) as well as time-matched saline-injected leg (P=0.063, and P=0.068, respectively. Figure 3). Quite in contrast to Botx, the α-BTX-injected legs showed significant fade (P<0.0001) during both TOF and tetanic stimulations compared with contralateral leg as well as time-matched saline-injected leg at 24 hours (Figure 3).
Muscle Mass and Acetylcholine Receptor α1 Subunit Expression
In experiments performed 24 hours after the injections, muscle weights on the toxin-injected side did not differ from the contralateral side and saline-injected side (data not shown). The protein expressions of α1 subunit of AChR in the tibialis muscle also showed no significant differences after Botx or α-BTX relative to time-matched saline-injected leg (P=0.956 and P=0.670, respectively, Figure 4). Equal protein loading was confirmed by the protein expression of GAPDH. Denervation for one week, in contrast, showed significant (P<0.0001) upregulation of α1AChR subunit compared with drug-injected legs.
Discussion
Our studies with Botx, αBTX and/or DHβE, or saline demonstrate that: 1. both IV and intramuscular administration of α-BTX causes fade; 2. acute intramuscular (2.5U) or higher IV dose (12U) of Botx has no significant neuromuscular effects for up to 2 hours, while a small 0.6U intramuscular dose of Botx causes significant twitch depression but no fade after 24 hours; 3. IV injection of presynaptic α3β2 antagonist, DHβE alone, does not induce twitch depression or fade; and 4. the effect of DHβE becomes manifest only when administrated with α-BTX, where the former enhances the fade produced by the latter when used alone. These results demonstrate that the block of α3β2 AChRs alone by DHβE is not necessary and sufficient to cause fade, but the functional effect of the block of α3β2 becomes manifest only when the margin of functional safety is diminished by decrease of functional postjunctional AChRs.
Botx binds with high specificity to the synaptosomal-associated protein of 25-kDa (SNAP-25) and prevents release of ACh at the nerve terminal release-sites.16 Thus, despite normal nerve conduction up to the nerve terminal, the ACh vesicles cannot be released, which results in decreased neuromuscular transmission and muscle tension, as evidenced in our present studies. Even with the decrease of the single twitch and/or tetanic tensions after Botx, no fade was observed at 24 hours. This observation suggests that decrease of ACh release by Botx in and of itself does not lead to fade. The lack of fade with Botx is consistent with previous observations that high concentrations of magnesium, which decreases ACh release, caused decrease of tension but no fade.10,17
Botx poisoning in the long-term leads to upregulation of AChRs evidenced as increased expression of AChR transcripts or protein (assessed by 125I-αBT).14,15,18 Thus, one may pose the question whether denervation-like changes could have modulated the neuromuscular effects of Botx studied at 24 hours. The muscle weights and α1AChR subunit expressions were used as surrogate markers of cellular and molecular changes at the junction at 24 hours. The lack of muscle weight changes and the unaltered expression of α1AChR subunit indicate that it is unlikely that neuromuscular responses to Botx were modulated by the prejunctional and postjunctional changes at 24 hours. Regardless, the lack of fade observed with Botx may be an expected finding; this toxin decreases nerve-induced release of ACh at the nerve terminal and does not necessarily block the prejunctional α3β2 receptors, implicated in the fade. Another possible explanation for the lack of fade with Botx is that despite decreased ACh release, the AChR number is normal. In other words, the decreased release of ACh activates only a small number of AChRs resulting in decreased twitch tension. However, the receptor reserve is abundant, so that ACh can bind to the available receptors without causing fade during repetitive stimulation. It is also tempting to speculate an alternative explanation for the lack of fade: The Botx-induced decreased release of ACh maintains larger quantities of vesicular ACh at the nerve terminal. The unreleased large quantities of readily available prejunctional ACh may also account for the lack of fade with Botx during repetitive stimulation.
The purified form of cobra venom, α-BTX, has been known to bind α1 subunit at the endplate of neuromuscular junction and neuronal AChR α7–10 subunit AChRs in the central nervous system, but has no binding to α3β2 subunit.10,19–21 In this study, fade was seen within a short time after IV dose of α-BTX. Fade was also observed after intramuscular injection at 24 hours, before changes in expression of α1 AChRs. Since α-BTX has no prejunctional effects,19 the fade observed acutely and at 24 hours was most likely due to decreased number of functional AChRs at the neuromuscular junction due to their binding to α-BTX. These observations indicate that fade can also be produced by a postjunctional effect and reflects the decreased margin of safety due to reduction of functional postjunctional AChRs. The fade during α-BTX is explicable: during α-BTX poisoning, because of the decrease in functional AChRs, the postjunctional receptor reserve is limited. The continued release of ACh with repetitive stimulation probably desensitizes some AChRs with little AChR reserve to respond to the next release of ACh; the end result is fade. The fade seen with α-BTX is reminiscent of the fade seen with myasthenia gravis, where the functional AChRs are decreased due to antibody-mediated downregulation of postjunctional AChRs.22
Some previous studies, however, have failed to show fade with α-BTX in vivo and ex vivo. For example, after IV administration of 100µg/kg α-BTX to cats, the effect developed slowly, did not attain a steady-state even at two hours after IV administration, and caused no fade at 50% block of compound muscle action potential.23 Application of α-BTX to rat isolated diaphragm also did not produce tetanic fade.10 Acute exposure of the mice phrenic nerve-diaphragm preparations to α-BTX did not produce fade initially, but fade was marked after prolonged incubation even with a lower concentration of toxin.24 These discrepancies of α-BTX to cause fade or not may be related to time points of measurement, different dosage, different species, and/or differences in vivo and ex vivo conditions.
DHβE is a competitive antagonist of neuronal nicotinic α4β2 and α3β2 AChRs.10,13 The α3β2 AChR is presumed to be the autoreceptor localized at the presynaptic nerve terminal and implicated in the mobilization and release of ACh into the synaptic cleft during high frequency stimulation.6 Although DHβE, a specific blocker of α3β2 AChRs, was capable of decreasing ACh release from motor nerve endings during high-frequency stimulation, fade was not seen.10 However, when the margin of safety was decreased with high magnesium concentration, fade was seen with DHβE.10 Consistently our results showed that DHβE alone had no effect on fade or twitch height, but the effect of DHβE became manifest when the margin of safety was reduced by α-BTX. These observations of others ex vivo10 and ours in vivo suggest that α3β2 AChR block alone does not necessarily cause fade; there should be other contributory factors such as a decrease in margin of safety being concomitantly present for the prejunctional α3β2 AChRs to manifest their effects. This explanation is consistent with the fade observed during administration of nondepolarizing relaxants, which has prejunctional plus postjunctional effects on the nerve and muscle AChRs, respectively, simultaneously.
It is also important to note that presynaptic positive feedback to mobilize ACh may not exclusively rely on α3β2 AChRs. Motor nerve terminals are endowed with other nicotinic, muscarinic, purinergic P2Y, adenosine receptors, which can cooperatively modulate the facilitatory release of nerve terminal ACh.25–27 Our study did not explore the roles of these other receptors and channels in the modulation of neurotransmission and/or fade.
In summary, our results demonstrate that, 1. decreased release of ACh by Botx, or DHβE alone acting on α3β2 AChRs, does not necessarily cause fade; 2. block of postjunctional AChRs by α-BTX does cause fade; 3. the role of α3β2 AChRs in fade becomes manifest only when the margin of safety is decreased by α-BTX. These observations suggest that fade could reflect the decreased margin of safety of neurotransmission, which can be due to a postjunctional effect alone or result from a pre and postjunctional effect in combination.
Acknowledgment
The authors gratefully acknowledge Hui Zheng, Ph.D. (Assistant Professor in Medicine, Harvard Medical School, Massachusetts General Hospital Biostatistics Center) for his assistance with statistical analysis, and Christiane G Frick, Hyung-yul Lee, Hiroshi Iwasaki and Stefan Schaller for scientific discussions.
This manuscript was handled by: Marcel E. Durieux, MD, PhD
Funding: This research was supported in part by National Institutes of Health grant number RO1 GM 05882 and GM P50-2500, and by SHC Research Philanthropy, Tampa, FL (to JAJM)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
DISCLOSURES:
Name: Michio Nagashima, MD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Dr. Nagashima has seen the original study data, reviewed the analysis of the data, approved the final manuscript, and is the author responsible for archiving the study files
Name: Shingo Yasuhara, MD, PhD
Contribution: This author helped with the biochemical experiments
Attestation: Dr. Yasuhara approved the final manuscript
Name: J. A. Jeevendra Martyn, MD, FRCA, FCCM
Contribution: This author helped design the study, supervised the work, analyzed the data, and edited the manuscript
Attestation: Dr. Martyn has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Contributor Information
Michio Nagashima, Department of Anesthesiology, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts; Department of Anesthesiology, Critical Care and Pain Medicine, Shriners Hospitals for Children, Boston, Massachusetts; Department of Anesthesiology, Critical Care and Pain Medicine, Harvard Medical School, Boston, Massachusetts.
Shingo Yasuhara, Department of Anesthesiology, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts; Department of Anesthesiology, Critical Care and Pain Medicine, Shriners Hospitals for Children, Boston, Massachusetts; Department of Anesthesiology, Critical Care and Pain Medicine, Harvard Medical School, Boston, Massachusetts.
J. A. Jeevendra Martyn, Department of Anesthesiology, Critical Care and Pain Medicine, Massachusetts General Hospital, Boston, Massachusetts; Department of Anesthesiology, Critical Care and Pain Medicine, Shriners Hospitals for Children, Boston, Massachusetts; Department of Anesthesiology, Critical Care and Pain Medicine, Harvard Medical School, Boston, Massachusetts.
References
- 1.Brull SJ, Murphy GS. Residual neuromuscular block: lessons unlearned. Part II: methods to reduce the risk of residual weakness. Anesth Analg. 2010;111:129–140. doi: 10.1213/ANE.0b013e3181da8312. [DOI] [PubMed] [Google Scholar]
- 2.Murray MJ, Cowen J, DeBlock H, Erstad B, Gray AW, Jr, Tescher AN, McGee WT, Prielipp RC, Susla G, Jacobi J, Nasraway SA, Jr, Lumb PD. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med. 2002;30:142–156. doi: 10.1097/00003246-200201000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Brull SJ, Naguib M. What we know: precise measurement leads to patient comfort and safety. Anesthesiology. 2011;115:918–920. doi: 10.1097/ALN.0b013e318234367d. [DOI] [PubMed] [Google Scholar]
- 4.Bowman WC. Prejunctional and postjunctional cholinoceptors at the neuromuscular junction. Anesth Analg. 1980;59:935–943. [PubMed] [Google Scholar]
- 5.Naguib M, Lien C. In: Pharmacology of muscle relaxants and their antagonist, Miller's Anesthesia. 7th edition. Miller RD, Eriksson LI, Fleisher LA, Wiener-Kronish JP, editors. Philadelphia: Churchill Livingstone; 2010. pp. 859–911. [Google Scholar]
- 6.Fagerlund MJ, Eriksson LI. Current concepts in neuromuscular transmission. Br J Anaesth. 2009;103:108–114. doi: 10.1093/bja/aep150. [DOI] [PubMed] [Google Scholar]
- 7.Vizi ES, Lendvai B. Side effects of nondepolarizing muscle relaxants: relationship to their antinicotinic and antimuscarinic actions. Pharmacol Ther. 1997;73:75–89. doi: 10.1016/s0163-7258(96)00139-8. [DOI] [PubMed] [Google Scholar]
- 8.Bowman WC, Marshall IG, Gibb AJ, Harborne AJ. Feedback control of transmitter release at the neuromuscular junction. Trends Pharmacol Sci. 1988;9:16–20. doi: 10.1016/0165-6147(88)90236-2. [DOI] [PubMed] [Google Scholar]
- 9.Bowman WC, Prior C, Marshall IG. Presynaptic receptors in the neuromuscular junction. Ann N Y Acad Sci. 1990;604:69–81. doi: 10.1111/j.1749-6632.1990.tb31983.x. [DOI] [PubMed] [Google Scholar]
- 10.Faria M, Oliveira L, Timoteo MA, Lobo MG, Correia-De-Sa P. Blockade of neuronal facilitatory nicotinic receptors containing alpha 3 beta 2 subunits contribute to tetanic fade in the rat isolated diaphragm. Synapse. 2003;49:77–88. doi: 10.1002/syn.10211. [DOI] [PubMed] [Google Scholar]
- 11.Lu Z, Yu B. Role of presynaptic acetylcholine autoreceptors at motor nerve endings on tetanic and train-of-four fade seen during a nondepolarizing neuromuscular block. Anesthesiology. 2007;106:1243. doi: 10.1097/01.anes.0000265457.47797.4c. author reply 1243–4. [DOI] [PubMed] [Google Scholar]
- 12.Jonsson M, Gurley D, Dabrowski M, Larsson O, Johnson EC, Eriksson LI. Distinct pharmacologic properties of neuromuscular blocking agents on human neuronal nicotinic acetylcholine receptors: a possible explanation for the train-of-four fade. Anesthesiology. 2006;105:521–533. doi: 10.1097/00000542-200609000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Chavez-Noriega LE, Crona JH, Washburn MS, Urrutia A, Elliott KJ, Johnson EC. Pharmacological characterization of recombinant human neuronal nicotinic acetylcholine receptors h alpha 2 beta 2, h alpha 2 beta 4, h alpha 3 beta 2, h alpha 3 beta 4, h alpha 4 beta 2, h alpha 4 beta 4 and h alpha 7 expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1997;280:346–356. [PubMed] [Google Scholar]
- 14.Frick CG, Richtsfeld M, Sahani ND, Kaneki M, Blobner M, Martyn JA. Long-term effects of botulinum toxin on neuromuscular function. Anesthesiology. 2007;106:1139–1146. doi: 10.1097/01.anes.0000267597.65120.16. [DOI] [PubMed] [Google Scholar]
- 15.Frick CG, Fink H, Blobner M, Martyn J. A single injection of botulinum toxin decreases the margin of safety of neurotransmission at local and distant sites. Anesth Analg. 2012;114:102–109. doi: 10.1213/ANE.0b013e31823526bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma J, Shen J, Lee CA, Elsaidi GA, Smith TL, Walker FO, Rushing JT, Tan KH, Koman LA, Smith BP. Gene expression of nAChR, SNAP-25 and GAP-43 in skeletal muscles following botulinum toxin A injection: a study in rats. J Orthop Res. 2005;23:302–309. doi: 10.1016/j.orthres.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Correia-de-Sa P, Ribeiro JA. Tonic adenosine A2A receptor activation modulates nicotinic autoreceptor function at the rat neuromuscular junction. Eur J Pharmacol. 1994;271:349–355. doi: 10.1016/0014-2999(94)90793-5. [DOI] [PubMed] [Google Scholar]
- 18.Witzemann V, Brenner HR, Sakmann B. Neural factors regulate AChR subunit mRNAs at rat neuromuscular synapses. J Cell Biol. 1991;114:125–141. doi: 10.1083/jcb.114.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apel C, Ricny J, Wagner G, Wessler I. alpha-Bungarotoxin, kappa-bungarotoxin, alpha-cobratoxin and erabutoxin-b do not affect [3H]acetylcholine release from the rat isolated left hemidiaphragm. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:646–652. doi: 10.1007/BF00171324. [DOI] [PubMed] [Google Scholar]
- 20.Lindstrom JM. Nicotinic acetylcholine receptors of muscles and nerves: comparison of their structures, functional roles, and vulnerability to pathology. Ann N Y Acad Sci. 2003;998:41–52. doi: 10.1196/annals.1254.007. [DOI] [PubMed] [Google Scholar]
- 21.Boulter J, Connolly J, Deneris E, Goldman D, Heinemann S, Patrick J. Functional expression of two neuronal nicotinic acetylcholine receptors from cDNA clones identifies a gene family. Proc Natl Acad Sci U S A. 1987;84:7763–7767. doi: 10.1073/pnas.84.21.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitahara K, Sugi Y, Higa K, Shono S, Hamada T. Neuromuscular effects of sevoflurane in myasthenia gravis patients. Br J Anaesth. 2007;98:337–341. doi: 10.1093/bja/ael368. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Nagai H, Katsumata N, Ogawa S, Suzuki H. Investigation of fading responses induced by non-depolarising muscle relaxants in the evoked EMG of the gastrocnemius muscle of the cat. Acta Anaesthesiol Scand. 1999;43:658–662. doi: 10.1034/j.1399-6576.1999.430611.x. [DOI] [PubMed] [Google Scholar]
- 24.Chang CC, Hong SJ. Dissociation of the end-plate potential run-down and the tetanic fade from the postsynaptic inhibition of acetylcholine receptor by alpha-neurotoxins. Exp Neurol. 1987;98:509–517. doi: 10.1016/0014-4886(87)90260-3. [DOI] [PubMed] [Google Scholar]
- 25.De Lorenzo S, Veggetti M, Muchnik S, Losavio A. Presynaptic inhibition of spontaneous acetylcholine release mediated by P2Y receptors at the mouse neuromuscular junction. Neuroscience. 2006;142:71–85. doi: 10.1016/j.neuroscience.2006.05.062. [DOI] [PubMed] [Google Scholar]
- 26.Santafe MM, Salon I, Garcia N, Lanuza MA, Uchitel OD, Tomas J. Modulation of ACh release by presynaptic muscarinic autoreceptors in the neuromuscular junction of the newborn and adult rat. Eur J Neurosci. 2003;17:119–127. doi: 10.1046/j.1460-9568.2003.02428.x. [DOI] [PubMed] [Google Scholar]
- 27.Wessler I. Control of transmitter release from the motor nerve by presynaptic nicotinic and muscarinic autoreceptors. Trends Pharmacol Sci. 1989;10:110–114. doi: 10.1016/0165-6147(89)90208-3. [DOI] [PubMed] [Google Scholar]