Abstract
The purification of analytes is an important prerequisite for many analytical processes. Although automated infrastructure has dramatically increased throughput for many of these processes, the upstream analyte purification throughput has lagged behind, partially due to the complexity of conventional isolation processes. Here, we demonstrate automated operation of arrays of a new sample preparation technology—immiscible filtration assisted by surface tension (IFAST). IFAST uses surface tension to position an immiscible liquid barrier between a biological sample and downstream buffer. Paramagnetic particles are used to capture analytes of interest and draw them across the immiscible barrier, thus resulting in purification in a single step. Furthermore, the planarity of the IFAST design enables facile and simultaneous operation of multiple IFAST devices. To demonstrate the application of automation to IFAST, we successfully perform an array of 48 IFAST-based assays to detect the presence of a specific antibody. This assay array uses only a commercial automated liquid handler to load the devices and a custom-built magnet actuator to operate the assays. Automated operation of the IFAST devices resulted in more repeatable results relative to manual operation.
Keywords: sample preparation, immiscible phase, microfluidics, purification, high throughput
Introduction
The extraction of specific analytes (e.g., nucleic acids, proteins, metabolites, whole cells) from biological samples is a ubiquitous process spanning many areas within the life sciences, including diagnostics, biomedical research, agrosciences, drug discovery, forensics, biodefense, environmental monitoring, epigenetic analysis, and food safety screening. Furthermore, many recent advances have enabled high-throughput analysis of these analytes (e.g., high-throughput quantitative PCR [qPCR], DNA microarrays, multiplexed immunoassays, flow cytometry), but analogous advances in analyte purification (often termed sample preparation) have lagged behind, creating a potential processing bottleneck.1 Although some robotic sample preparation processes have been developed, they are typically automated versions of the traditional solid-phase extraction (SPE) process, in which an analyte is captured on solid phase, washed repeatedly, and then eluted. Unfortunately, the SPE process is inherently difficult to implement as a high-throughput system due to the large number of washing steps required by these processes. In this article, we attempt to address this shortcoming by demonstrating the compatibility of a fundamentally different sample preparation technique, immiscible phase filtration, with high-throughput infrastructure.
Immiscible phase filtration employs an immiscible phase (e.g., oil, wax) barrier to fluidically separate a sample from other, “clean” buffers.2–9 Functionalized paramagnetic particles (PMPs) are used to selectively capture an analyte from a sample, and then a magnetic force is used to draw the PMP-captured analyte through the oil phase and into a downstream buffer. In this manner, an analyte can be isolated in a single step, eliminating the need for multiple wash steps.
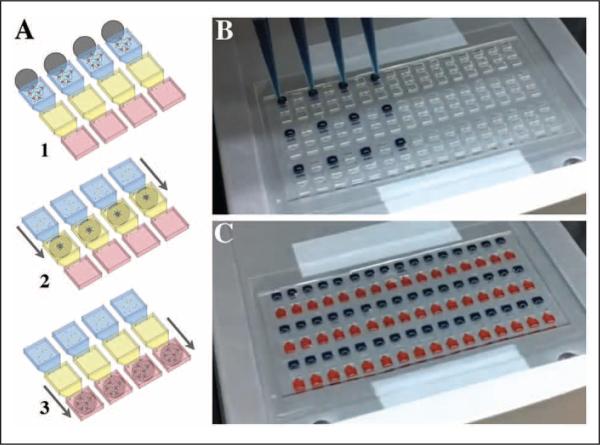
Immiscible filtration assisted by surface tension (IFAST) is one immiscible phase filtration configuration for isolation of nucleic acids, cells, and proteins. IFAST uses the principle of surface tension to “pin” multiple liquid phases in adjacent, connected compartments. Constriction points are added between these compartments, such that the phase interfaces are stabilized and relatively independent of gravitational effects (i.e., by making the actual interface between an aqueous and an oil phase small, the interface becomes stable in any orientation). In this manner, aqueous and oil phases can be positioned side by side, thus enabling a planar device (i.e., three wells in series—an input compartment to receive the sample, an oil compartment, and an output compartment from which to extract the purified analyte).6 This device, or an array of these devices, is operated by simply sliding a magnet beneath the IFAST in a single, linear motion (Fig. 1A). Although this planar configuration simplifies manufacturing, operation, and integration with other components, it also makes the IFAST device more amenable to automation and high-throughput operation. In this article, for the first time, we demonstrate the loading and operation of IFAST using automated infrastructure, such that many IFAST-based assays can be run in parallel with minimal manual handling of the samples or devices.
Figure 1.
(A) Immiscible filtration assisted by surface tension (IFAST) devices are operated by moving a magnet or array of magnets beneath the devices (1). As the magnets pass beneath the immiscible phase (shown in yellow), the paramagnetic particle (PMP)–bound analytes are separated from the bulk of the sample (shown in blue) (2). The purification is complete when the PMPs are drawn into the elution buffer (shown in red) and the devices are removed from the magnets (3). (B) An automated liquid handler is used to fill an array of 48 IFAST devices. (C) An array of 48 IFAST devices was successfully filled with the liquid handler.
Materials and Methods
Device Fabrication
IFAST devices were fabricated from polydimethylsiloxane (PDMS) (Sylgard 184; Dow Corning, Midland, MI) using a soft lithography process. Briefly, PDMS elastomer base and curing agent were mixed at a 10:1 ratio, and the mixture was degassed in a vacuum chamber for 30 min. Degassed elastomer was then poured on a mold and cured at 80 °C for 4 h. The mold was constructed by spinning on layers of photo-curable epoxy (SU-8 100; MicroChem, Newton, MA) on a silicon wafer, then patterning each layer via masked exposure to UV light. After curing, PDMS was peeled from the mold and pressed onto a sheet of cyclic olefin copolymer (COC; TOPAS, TOPAS Advanced Polymers, Florence, KY), which formed the bottom of the device. In this application, the inherent adhesiveness between the native PDMS and COC provided sufficient bond strength. Additional bond strength can be obtained by exposing the PDMS and COC to an oxygen plasma immediately prior to bonding.
IFAST devices used in this study were configured to replicate the footprint of a standard 384-well microplate (4.5-mm well-to-well spacing). Each IFAST device consisted of three wells interconnected by microfluidic conduits, and the volume of each well is approximately 8 μL. IFAST volumes are somewhat flexible if other configurations are desired; to date, IFAST devices have been fabricated with volumes ranging from 2 μL to 1 mL. Test arrays consisting of 48 devices (three rows of 16 devices) were fabricated for this study, although array size is also flexible. IFAST technology uses surface tension to sequester liquids in individual wells, such that aqueous and oil liquid phases can be positioned side by side. To facilitate this phenomenon, microfluidic constriction points are located between each well to provide a point of “pinning” of the liquid.10,11 In the devices used in this study, the constriction points were 0.5 mm wide by 0.25 mm high.
Device Loading
Devices were loaded using an automated liquid handler (Quad-Z 215; Gilson, Middleton, WI) (Fig. 1B,C and Suppl. Video S1). Device arrays were taped to the liquid handler stage such that the IFAST wells aligned with the well positions in a 384-well plate. Reagents (samples, oil, and elution buffer) were preloaded into a 96-well plate that was also placed on the liquid handler stage. Since IFAST uses surface tension to position liquids within the device, the oil must be loaded last due to its very low surface tension. Using a standard liquid-handling program, elution buffer was transferred into the output well of each IFAST device at a flow rate of approximately 10 μL/s. Next, samples (including premixed PMPs) were loaded into the IFAST input wells, with tip changes between each sample. Last, oil (FC-40 oil [3M, Maplewood, MN] or olive oil [Bertolli, Unilever, London, UK]) was loaded into the center wells of the IFAST devices. Due to increased viscosity, the oil was deposited at a slightly lower flow rate (~2 μL/s). Each well was loaded with 8.5 μL of the appropriate solution. Filled arrays were inspected visually to confirm correct placement of all reagents prior to operation. In initial demonstrations of loading, the aqueous phases were water with 0.1% Tween-20 (input) and pure water (output), each of which had been loaded with dye for improved visualization.
Device Operation
Once filled, IFAST arrays were transferred manually from the automated liquid handler to a custom-built magnetic actuator. This device consists of an array of magnets (D201-N52; K&J Magnetics, Jamison, PA) that moved beneath a thin (t = ~100 μm) stage constructed from a transparency (Transparency; 3M). The magnetic actuator can traverse the magnet array (1 × 16) beneath the IFAST array at an adjustable velocity. In these experiments, velocity was adjusted from approximately 0.5 to 5 mm/s. Although the magnetic array consisted of a single column of magnets, multiple arrays of IFAST devices were operated in series by sliding the magnet array across the entire IFAST array.
To quantify the effectiveness of the magnetic actuator, we had several users, both IFAST novices and IFAST experts, operate the device both manually (handheld magnet) and using the automated magnetic actuator. To enable better quantification of PMP transfer efficiency, the PMPs used in this study (Protein G Dynabeads; Life Technologies, Carlsbad, CA) were made fluorescent by incubating them with green fluorescent protein (GFP)–conjugated anti-IgG secondary antibody for 1 h at room temperature with shaking (PMP concentration = 10 mg/mL; Ab concentration = 13 μg/mL). After incubation, the beads were washed three times in phosphate-buffered saline (PBS) with 0.1% Triton X-100 (PBST). Fluorescently labeled PMPs were then loaded into the input well of IFAST devices as previously described. PBST was loaded into the output wells while olive oil was loaded into the center wells. IFAST users operate the IFAST devices either manually or using the automated actuator (n = 3 to 5 per user for each methodology). To quantify the effectiveness of PMP traverse, each IFAST was imaged with a fluorescent microscope (IX70; Olympus, Center Valley, PA) using MetaMorph software (Molecular Devices, Sunnyvale, CA). ImageJ software (National Institutes of Health, Bethesda, MD)12 was used to determine the proportion of PMPs that were drawn into the output buffer. Briefly, images were first thresholded to remove background signal. Next, regions of interest (ROIs) were drawn around each region of the IFAST devices (e.g., input, oil, and output), and the fluorescence in each region was measured and normalized to the total device fluorescence. Standard samples with known PMP concentrations were also run to confirm a linear relationship between PMP concentration and fluorescence.
Protein Assay
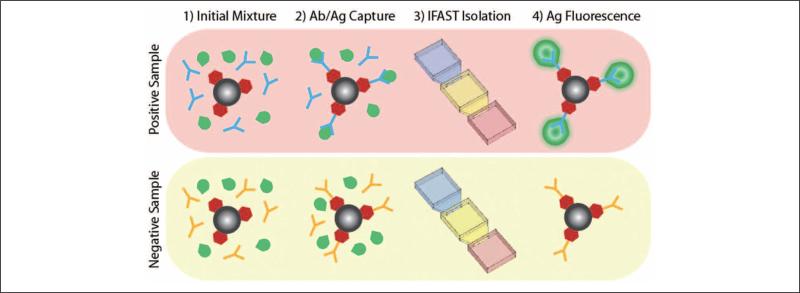
As previously demonstrated,6,7,9,13 IFAST can be used to extract multiple types of analytes from samples, including nucleic acids, proteins, and whole cells. Here, we demonstrate the ability of IFAST to quantify the presence of a specific antibody using the high-throughput infrastructure previously described. This experiment demonstrates proof-of-concept for using high-throughput IFAST to perform a series of mock seroconversion assays on samples with physiologically relevant antibody concentrations.14 In the experiment, samples were prepared that contained a fluorescently labeled epitope (GFP linked to an epitope derived from RNA polymerase15) and either an antibody specific to this epitope or an irrelevant antibody (Fig. 2). Specifically, the sample solutions contained 7.5 mg/mL protein G–conjugated PMPs (Dynabeads), 31 μg/mL antibody (~250 ng per assay), and approximately 12 μg/mL fluorescently tagged epitope (~100 ng per assay). Each sample was mixed with protein G PMPs (Protein G Dynabeads; Life Technologies) in a tube for 15 min at room temperature to facilitate formation of the PMP/antibody/GFP complex. In the presence of the epitope-specific antibody, the GFP was linked to the PMP, whereas the GFP remained unattached in the presence of the nonspecific antibody. Following incubation, the sample/PMP mixture was loaded into an IFAST array and actuated using the high-throughput infrastructure as described in the previous sections. The output wells of the IFAST devices were loaded with an eluting buffer containing 40% propylene glycol and 0.75M ammonium sulfate in a Tris solution. This buffer was previously shown to elute the fluorescently tagged epitope.15 Following actuation, the array was incubated for 5 min at room temperature without mixing to facilitate elution. The IFAST array was transferred from the magnetic actuator to a fluorescent scanner (Typhoon Trio; GE Healthcare, Piscataway, NJ). The array was scanned using an excitation wavelength of 492 nm, and the resulting image was quantified using ImageQuant software (GE Healthcare). Samples with no fluorescent epitope were used as blanks to determine background, and each sample was normalized to the total fluorescence of that sample.
Figure 2.
Seroconversion assay samples consisted of protein G paramagnetic particles (PMPs), green fluorescent protein (GFP)–tagged epitope (Ag), and either an antibody specific to the tagged epitope or an irrelevant antibody (Ab) (1). Following incubation (2), samples are processed through immiscible filtration assisted by surface tension (IFAST) devices (3). If the epitope-specific antibody is present, the fluorescent epitope will be affixed to the PMPs (4) and drawn across the IFAST oil barrier.
Results and Discussion
Loading and Operation
IFAST arrays were successfully loaded with the automated liquid handler without any device failure (e.g., the breakdown of virtual walls, trapping of air bubbles in the device). However, mixing of the samples prior to IFAST loading was required to mitigate the settling of the dense PMPs during setup of the liquid handler. In addition, attempts to deposit the oil phase more rapidly (>5 μL/s) resulted in the trapping of air bubbles between the oil and aqueous phases. In preliminary experiments, the presence of bubbles has been correlated with incomplete transfer of PMPs across the IFAST devices.
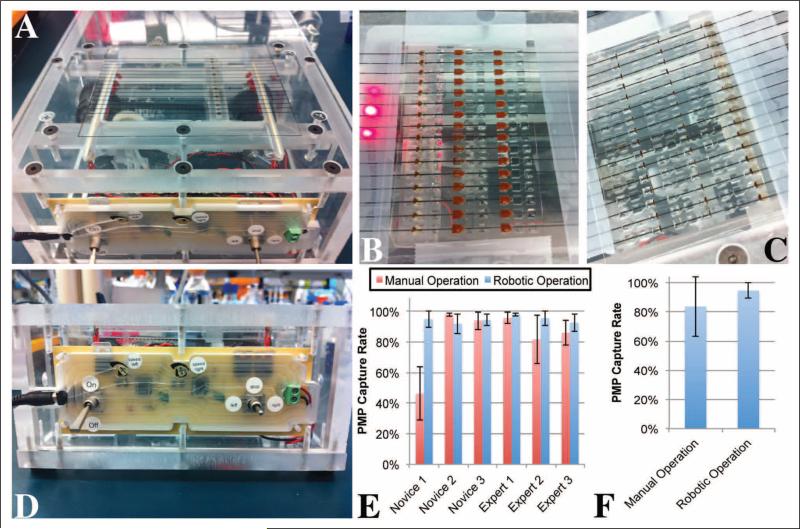
Six users (three novices and three experts) operated arrays of IFAST devices both manually and using the automated magnetic actuator (Fig. 3A). Using the automated actuator, an average of 94.6% of the PMPs were successfully drawn across the IFAST devices (Fig. 3B–D and Suppl. Video S2). This value dropped slightly to 84.5% when the devices were operated manually. However, the major difference between the two techniques was the overall repeatability of the process. On average, the standard deviations of the data for a single user were 4.6% for the automated actuator and 8.7% for the manual technique (Fig. 3E). An analysis of variance (ANOVA) was performed on these data to determine if particular users have statistically significant effects on PMP capture rate. Using the manual data, the ANOVA analysis concluded that the means of the data collected by each user were not equal (p < 0.0001). Much of this variation is due to novice 1, who performed very poorly with the manual technique and could be considered an outlier. However, when the novice 1 data set is omitted, the difference between the means remained significant but by a smaller margin (p = 0.0008). Some significant user-to-user effects were seen with the automated system, perhaps due to alignment differences on the magnetic actuator stage, but this effect was much less significant (p = 0.02). Indeed, when the data from all users were pooled, the overall standard deviation for the manual technique climbed to 20.4%, whereas the pooled standard deviation for the automated actuator increased to only 5.2% (Fig. 3F). Overall, these data suggest that user-to-user variability is substantially greater with the manual technique. This finding implies that an IFAST-based assay run by several technicians will have higher intrinsic variability if the devices are operated manually versus using the automated actuator.
Figure 3.
(A) The magnetic actuator consists of 16 magnets mounted on a slide that moves underneath the device stage. (B) An array of immiscible filtration assisted by surface tension (IFAST) devices is loaded on the automated magnetic actuator. (C) Following actuation, paramagnetic particles (PMPs) in all of the IFAST devices are transferred into the IFAST output wells. (D) The magnetic actuator control panel is used to control the direction and speed of the magnetic slide. (E) Six users (three with experience with IFAST, three without experience) operated IFAST devices manually and using the automated actuator. In general, more user-to-user variation was seen during manual operation. (F) The data from panel C were compiled into a single data series, further illustrating the reduction in variation seen with automated operation.
Protein Assay
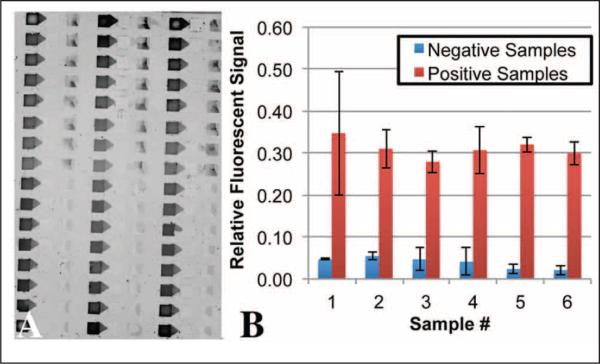
Samples with and without the fluorescent epitope-specific antibody were loaded onto an array of 48 IFAST devices using the automated liquid handler. In total, eight positive and eight negative samples were loaded in triplicate. The device was actuated with the automated actuator and imaged using a fluorescent scanner. Following passive elution (i.e., no mixing was performed following IFAST operation), it was observed that fluorescence was transferred to the IFAST output wells only in the cases of the epitope-specific antibody (Fig. 4A). After background subtraction, output well intensities were determined and all positive samples generated significantly more signal than all negative samples (p < 0.01 by unpaired t-test) (Fig. 4B). The variability of the positive samples in Figure 4B is possibly due to the nonuniform dispersion of the PMPs in the wells as the IFAST array is removed from the magnetic actuator. Mixing each well following actuation but prior to scanning could likely reduce this variation. However, this would add additional steps to the assay.
Figure 4.
(A) A fluorescent scan of an array of mock seroconversion assays following automated loading and operation. The top eight rows contain epitope-specific antibody (positive samples), whereas the bottom eight rows contain irrelevant antibody (negative samples) in triplicate. Note the presence of fluorescence in the output wells of the positive samples. (B) Quantification of fluorescence in the immiscible filtration assisted by surface tension (IFAST) output wells following actuation.
In this study, we have demonstrated the ability to operate IFAST assays in a high-throughput manner, using a mock seroconversion assay to validate the functionality of the technology. Furthermore, we believe that the processes developed in this article could be applied to a wide range of clinical and biomedical research applications. In past studies,6,7,9,13 we have used IFAST in low-throughput settings to isolate nucleic acids, whole cells, proteins, and protein complexes. This flexibility, coupled with the advantages gained through automation—specifically, lower assay variance and higher throughput—make the IFAST potentially very powerful as a tool for diagnostics or research.
In conclusion, automated liquid handling and magnetic actuation were employed to load and operate arrays of IFAST devices. These devices were previously used in low-throughput settings to isolate many different types of analytes, including nucleic acids, cells, proteins, and protein complexes. Here, automation is used to improve throughput and reduce operator-to-operator variation (3.9-fold reduction in standard deviation). This improvement in repeatability potentially improves the utility of IFAST-based assays since lower variance will result in improvements to sensitivity and specificity. In addition, we used the automated IFAST platform to perform an array of seroconversion assays, in which the presence of a specific antibody was successfully detected in all positive samples.
Supplementary Material
Acknowledgments
The authors would like to thank Richard Burgess and Nancy Thompson of the University of Wisconsin for donating the antibodies and fluorescent reagents used in this manuscript.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funded by a grant from the Bill & Melinda Gates Foundation through the Grand Challenges in Global Health initiative, a DOD PCRP Idea grant (W81XWH-09-1-0192), and an NIH NCI R33 grant (CA137673).
Footnotes
Supplementary material for this article is available on the Journal of Laboratory Automation Web site at http://jla.sagepub.com/supplemental.
Declaration of Conflicting Interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S. M. Berry and D. J. Beebe are co-founders of Elaion Biosciences.
References
- 1.Zhang C, Xu J, Ma W, Zheng W. PCR Microfluidic Devices for DNA Amplification. Biotechnol. Adv. 2006;24(3):243–284. doi: 10.1016/j.biotechadv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Shikida M, Takayanagi K, Honda H, Ito H, Sato KJ. Development of an Enzymatic Reaction Device Using Magnetic Bead-Cluster Handling. J. Micromech. Microeng. 2006;16:1875–1883. [Google Scholar]
- 3.Shikida M, Takayanagi K, Inouchi K, Honda H, Sato K. Using Wettability and Interfacial Tension to Handle Droplets of Magnetic Beads in a Micro-Chemical-Analysis System. Sensors Actuators B. 2006;113:563–569. [Google Scholar]
- 4.Sur K, McFall SM, Yeh ET, Jangam SR, Hayden MA, Stroupe SD, Kelso DM. Immiscible Phase Nucleic Acid Purification Eliminates PCR Inhibitors with a Single Pass of Paramagnetic Particles through a Hydrophobic Liquid. J. Mol. Diagn. 2010;12:620–628. doi: 10.2353/jmoldx.2010.090190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Abolmatty A, Faghri M. Microfluidic Inverse Phase ELISA via Manipulation of Magnetic Beads. Microfluid. Nanofluid. 2010;10:593–605. [Google Scholar]
- 6.Berry SM, Alarid ET, Beebe DJ. One-Step Purification of Nucleic Acid for Gene Expression Analysis via Immiscible Filtration Assisted by Surface Tension (IFAST). Lab Chip. 2011;11:1747–1753. doi: 10.1039/c1lc00004g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry SM, Strotman LN, Kueck JD, Alarid ET, Beebe DJ. Purification of Cell Subpopulations via Immiscible Filtration Assisted by Surface Tension (IFAST). Biomed. Microdevices. 2011;13:1033–1042. doi: 10.1007/s10544-011-9573-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bordelon H, Adams NM, Klemm AS, Russ PK, Williams JV, Keipe HK, Wright DW, Haselton FR. Development of a Low Resource RNA Extraction Cassette Based on Surface Tension Valves. Appl. Mater. Interfaces. 2011;3:2161–2168. doi: 10.1021/am2004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berry SM, Maccoux LJ, Beebe DJ. Streamlining Immunoassays with Immiscible Filtrations Assisted by Surface Tension. Anal. Chem. 2012;84:5518–5523. doi: 10.1021/ac300085m. [DOI] [PubMed] [Google Scholar]
- 10.Atencia J, Beebe DJ. Controlled Microfluidic Interfaces. Nature. 2005;437:648–655. doi: 10.1038/nature04163. [DOI] [PubMed] [Google Scholar]
- 11.Zhao B, Moore JS, Beebe DJ. Surface-Directed Liquid Flow inside Microchannels. Science. 2001;291:1023–1026. doi: 10.1126/science.291.5506.1023. [DOI] [PubMed] [Google Scholar]
- 12.Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics Int. 2004;11:36e42. [Google Scholar]
- 13.Goel S, Chin EN, Fakhraldeen SA, Berry SM, Beebe DJ, Alexander CM. Both LRP5 and LRP6 Receptors Are Required to Respond to Physiological Wnt Ligands in Mam-mary Epithelial Cells and Fibroblasts. J. Biol. Chem. 2012;287:16454–16466. doi: 10.1074/jbc.M112.362137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wright PF, Kozlowski PA, Rybczyk GK, Goepfert P, Staats HF, Vancott TC, Trabattoni D, Sannella E, Mestecky J. Detection of Mucosal Antibodies in HIV Type-1-Infected Individuals. AIDS Res. Hum. Retroviruses. 2002;18:1291–1300. doi: 10.1089/088922202320886334. [DOI] [PubMed] [Google Scholar]
- 15.Stalder ES, Nagy LH, Batalla P, Arthur TM, Thompson NE, Burgess RR. The Epitope for the Polyol-Responsive Monoclonal Antibody 8RB13 Is in the Flap-Domain of the Beta-Subunit of Bacterial RNA Polymerase and Can Be Used as an Epitope Tag for Immunoaffinity Chromatography. Protein Expression Purific. 2011;77:26–33. doi: 10.1016/j.pep.2010.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.