Abstract
BACKGROUND
Randomized-controlled trials that examine the effects of Cholinesterase inhibitors (ChEI) and memantine on patient outcomes over long periods of time are difficult to conduct. Observational studies based on practice-based populations outside the context of controlled trials and open label extension studies that evaluate the effects of these medications over time are limited.
OBJECTIVES
To examine in an observational study (1) relationships between ChEI and memantine use and functional and cognitive endpoints and mortality in AD patients, (2) relationships between other patient characteristics on these clinical endpoints, and (3) whether effects of the predictors change across time.
DESIGN
Multicenter, natural history study.
SETTING
Three university-based AD centers in the US.
PARTICIPANTS
201 patients diagnosed with probable AD with modified Mini-Mental State Examination scores of 30 or higher at study entry followed annually for 6 years.
MEASUREMENTS
Discrete-time hazard analyses were used to examine relationships between ChEI and memantine use during the previous 6 months reported at each assessment and time to cognitive (Mini-Mental State Examination, MMSE≤10) and functional (Blessed Dementia Rating Scale, BDRS≥10) endpoints and mortality. Analyses controlled for clinical characteristics including baseline cognition, function, and comorbid conditions, and presence of extrapyramidal signs and psychiatric symptoms at each assessment interval. Demographic characteristics included baseline age, sex, education, and living arrangement at each assessment interval.
RESULTS
ChEI use was associated with delayed time in reaching functional endpoint and death. Memantine use was associated with delayed time to death. Different patient characteristics were associated with different clinical endpoints
CONCLUSION
Results suggest long term beneficial effects of ChEI and memantine on patient outcomes. As for all observational cohort study, observed relationships should not be interpreted as causal effects.
Keywords: Alzheimer’s disease, cholinesterase inhibitors, memantine, outcomes, longitudinal studies
INTRODUCTION
Since their introduction, cholinesterase inhibitors (ChEIs) and later the N-methyl-D aspartate receptor antagonist (memantine) have been shown in short-term clinical trials and longer-term open-label extension studies to stabilize or reduce the rate of decline in measures of cognitive function, activities of daily living, and behavior in some patients with Alzheimer’s disease (AD).[1-11] Most rigorous evidence of whether the effects of ChEIs and memantine are sustained over longer periods of time would come from long-duration, prospective, placebo-controlled trials. However, such trials are not only costly to conduct, there also are ethical concerns associated with exposing patients to placebo in trials of long duration because ChEIs and memantine have become standard of care for patients with AD. In the absence of these trials, observational studies based on practice-based populations may be one of the only ways to evaluate effects of these medications.[12]
Several studies have assessed the effects of ChEIs and/or memantine treatment in real-world clinic settings.[13-22] Results from these studies have been mixed. In one of the first observational studies on the effects of ChEI on patient outcomes, Doody and others found slowed decline in cognitive function after a year in patients treated with ChEIs compared to untreated patients.[13] Comparing patients treated with ChEI or ChEI+memantine combination therapy of to an untreated earlier cohort of patients, Atri and others also reported slower decline in cognition and function in the treated group.[17] Persistent treatment has been shown to be associated with slowed decline in cognition and function, but effects may be lost if treatment were disrupted.[3, 18] On the other hand, also comparing a cohort of patients treated with ChEI with an earlier cohort of untreated patients, Lopez and others reported no association between ChEI use and time to cognitive and functional decline or to death, but significant delays in nursing home admission.[14, 19]
In an earlier study using a large, multi-center cohort of patients with probable AD who were prospectively followed up to 6 years from early disease stages, we reported that patterns of ChEI and memantine use changed substantially over time and were consistent with practice guidelines of initiating ChEIs in mild to moderate AD and adding memantine in moderate to severe AD.[23] In the current study, we take advantage of the availability of important clinical characteristics (e.g., comorbid conditions, psychiatric symptoms) that were not controlled for before, the long follow up period, and more current data, and further investigated the following questions: (1) are ChEI or memantine use associated with length of time to reach cognitive and functional outcomes and death, and (2) are these associations stable over time?
METHODS
Sample
Data are drawn from the Predictors 2 cohort, consisting of patients recruited from Columbia University Medical Center, Johns Hopkins School of Medicine, and Massachusetts General Hospital. The study was approved by each local Institutional Review Board. The inclusion/exclusion criteria have been fully described elsewhere.[24-26] Briefly, subjects met DSM-III-R criteria for primary degenerative dementia of the Alzheimer type and NINDS-ADRDA criteria for probable AD. Enrollment required a modified Mini-Mental State Examination (mMMS) score ≥30, equivalent to approximately ≥16 on the Folstein Mini-Mental State Examination (MMSE).[27, 28] Clinical diagnosis of AD has been confirmed in 93% of those with postmortem evaluation.[26]
Study recruitment began in 1997, when widespread use of ChEIs began in the US, and is ongoing. After the baseline assessment, patients were followed annually. Those who missed a particular visit could respond at a subsequent visit. The cohort used in the current analysis included 201 patients who were followed for up to 6 years and provided data for 785 visits. Of these 201 patients, 13 had 6, 27 had 5, 37 had 4, 34 had 3, and 41 had 2 years of follow-up visits. 123 patients (61%) did not miss any visits, 15 (7%) missed 1, 19 (9%) missed 2, 22 (10%) missed 3, and the rest missed 4 or more visits. Median follow-up for the cohort was 4 years (mean=3.5, SD=2.0).
Measures
Clinical Endpoints
We used MMSE to assess patients’ cognitive status and constructed a dichotomous variable indicating MMSE≤10 at each visit. We used Blessed Dementia Rating Scale (BDRS) Parts I and II (Instrumental and Basic Activities of Daily Living) to assess patients’ functional status and constructed a dichotomous variable indicating BDRS≥10 at each visit. We chose these cutoff points because similar scores have been used as outcomes in many studies. Exploratory analyses of neighboring end points (i.e., MMSE≤8, BDRS≥8) did not change estimation results substantively. Patient deaths were typically reported by family members when we attempted to complete follow-up visits. For patients who could not be contacted, information on death was obtained through the National Death Index.
Main Independent Variables: ChEI and Memantine Use
All prescription and over the counter medication use during the previous 6 months were reported at each visit by the patient and informant on a medication acquisition form. Information reported included name of medication, number of days taking the medication, dosage, and number of pills per day. Because ChEIs have been shown to have similar efficacy despite slightly different pharmacological properties, we combined all ChEIs into one group.
Because of the consistency of medication use reported in this sample, we constructed dichotomous variables indicating ChEI and memantine use during the 6 months prior to each assessment as our main independent variables instead of using more complex measures that take into account number of days of use or medication dosages.
Other Substantive Control Variables
To isolate the effects of ChEI and memantine use, we controlled for the following time-variant variables in the analysis. Columbia University Scale for Psychopathology in AD (CUSPAD), a semi-structured interview administered by physicians or trained research technicians, was used to measure psychotic symptoms.[29] A modified Unified Parkinson’s Disease Rating Scale (UPDRS) was used to measure presence of extrapyramidal signs (EPS).[30, 31] Information on patients’ living arrangements at each assessment was dichotomized as living at home or in a long-term care facility. The following time-invariant characteristics were included in the analysis: baseline cognition, function, number of comorbidities, baseline age, sex, education, and study site.[26, 32] Because ChEIs and memantine were approved by the FDA for treatment of AD at different times, year of study entry was included to control for availability of medications on the market and any differences that could be related to different entry times into the study. Because most of the sample was white (n=188, 94%), ethnicity was not included in the models.
Analysis
We used discrete time hazard models to examine the relationships between ChEI and memantine use and time to cognitive and functional endpoints and mortality. We used binary, time-specific event indicators for BDRS, MMSE and death to reflect the first year a patient reached a clinical endpoint. Right-censoring occurred when a patient did not reach a clinical endpoint by their most recent assessment. Such patients would have a 0 on the time-specific event indicator for all assessment intervals up to the most recent assessment. Observations with missing outcomes were dropped from the analysis.
The estimated discrete-time hazard is the conditional probability that a patient will reach a clinical endpoint in an assessment interval, given that they did not reach the endpoint in an earlier interval. Although multiple observations from each patient may be correlated in longitudinal data, this conditional probability can be treated as if it came from a distinct, independent observation, and therefore not necessary to account for clustering effects within the individual.[33] For each outcome, we first estimated a set of models that included only time effects (year=0, 1, 2... 5). This set of models describes for the entire sample the hazard profile for each clinical endpoint. If the estimated coefficients are approximately the same, the risk of reaching a clinical endpoint is unrelated to time and the hazard function is flat. If the estimated coefficient increases over time, the risk of reaching a clinical endpoint increases and the hazard function increases over time. We tested the constant hazard assumption using a likelihood-ratio test based on model deviance statistics.
Next, we examined bivariate relationship between each potential substantive covariate (e.g., ChEI use) and the clinical endpoints after controlling for time effects. These main effects models contain the underlying proportionality assumption that each covariate has the same effect on the outcome in every time period. We tested the proportionality assumption of each covariate using deviance statistics obtained from the unconstrained model that included the main effect of the covariate and an interaction term of the covariate and time in the constrained main effects model. If the unconstrained covariate model did not fit better than the main effects model, the covariate did not violate the proportionality assumption and only main effects for that covariate was included in subsequent analyses. If the unconstrained covariate model fits better than the main effects model, both the covariate and its interaction with time were included in subsequent analyses.[34]
Finally, we estimated a full, multivariate model that included (1) all 6 binary time-effects indicators, (2) main effects for covariates that did not violate the proportionality assumption, and (3) main effects and interaction with time effects for covariate that violated the proportionality assumption. To account for possible clustering, e.g., within sites, robust standard errors were reported. The results of this full model allowed interpretation for each individual covariate effect on our clinical endpoints after adjusting for all other covariates.
RESULTS
Baseline Characteristics
At baseline, patients’ average age was 76 (SD=8.1), 123 were female (61%), and 176 (88%) lived at home (Table 1). Patients were highly educated, with an average of 14 years of schooling (SD=3.1). Average MMSE was 22.0 (SD=3.4) and average BDRS was 3.6 (SD=2.1). 68 patients had psychotic symptoms (34%) and 29 had extrapyramidal signs (16%). Almost half of the patients (n=96, 48.4%) reported no comorbid conditions, a third (n=67, 34.1%) reported one, and the rest 38 patients (19%) reported two or more comorbid conditions.
Table 1. Patient Characteristics at Baseline (n=201).
| Variables | Mean (SD) | Range |
|---|---|---|
| Age | 76.3 (8.0) | [49, 95] |
| Female (%) | 61.2 | |
| Years of schooling (%) | ||
| ≤12 | 42.3 | |
| 13-15 | 15.9 | |
| ≥16 | 41.8 | |
| Living in a nursing home (%) | 12.4 | |
| Folstein Mini-Mental State Examination (MMSE) | 21.9 (3.4) | [12, 30] |
| Modified Mini-Mental State Examination (mMMS) | 37.2 (6.5) | [14, 52] |
| Blessed Dementia Rating Scale (BDRS) | 3.6 (2.1) | [0, 10.5] |
| Presence of psychiatric symptoms (%) | 33.8 | |
| Presence of extrapyramidal signs (%) | 15.9 | |
| Number of comorbidities | 0.8 (1.1) | [0, 8] |
| Site (%) | ||
| Columbia | 44.3 | |
| Johns Hopkins | 26.9 | |
| Mass General | 28.9 | |
| Year of study entry (%) | ||
| 1997-1998 | 13.2 | |
| 1999 | 7.7 | |
| 2000 | 25.8 | |
| 2001 | 25.3 | |
| 2002 | 13.2 | |
| 2003-2007 | 4.9 |
Table 2 presents data on the number patients who were available for observation at each assessment interval, and the number and proportion of those who were taking ChEI or memantine. Because only patients with mild AD were included at study entry, four-fifths of the 201 patients (n=161) reported using ChEI at baseline, only 2% (n=4) reported using memantine, all of whom used it in combination with ChEI. By year 6, the proportion of patients who reported using ChEIs slightly decreased to 74.4%, the proportion of patients who reported using memantine increased to 48.8%, and the proportion of patients who reported not taking either ChEIs or memantine remained relatively steady. Overall, 182 patients (91%) reported taking ChEIs at some point during the study, 19 (9.5%) were never treated with ChEI. 81 patients (40.2%) reported taking memantine at some point during the study, 120 (59.7%) were never treated with memantine. Patients who were treated with ChEIs at some point were followed for an average of 3.6 years (SD=2.0) compared to 2.1 years (SD=1.7) for those who were never treated with ChEIs. Patients who were treated with memantine at some point were followed for an average of 4.4 years (SD=1.5) compared to 2.9 years (SD=2.0) for those who were never treated with memantine.
Table 2. Proportion of Patients who Reported Taking ChEI or Memantine at Each Visit.
| Year | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | |
| Number of patients at each visit | 201 | 159 | 127 | 98 | 93 | 66 | 43 |
| Taking ChEI | |||||||
| Number of patients | 161 | 131 | 99 | 81 | 70 | 48 | 32 |
| Proportion of patients | 80.1 | 82.4 | 78.0 | 82.7 | 75.3 | 72.7 | 74.4 |
| Taking Memantine | |||||||
| Number of patients | 4 | 13 | 19 | 32 | 41 | 34 | 21 |
| Proportion of patients | 2.0 | 8.2 | 15.0 | 32.7 | 44.1 | 51.5 | 48.8 |
| Taking both ChEI and Memantine | |||||||
| Number of patients | 4 | 12 | 16 | 29 | 33 | 30 | 19 |
| Proportion of patients | 2.0 | 7.5 | 12.6 | 29.6 | 35.5 | 45.5 | 44.2 |
| Did not take either ChEI or Memantine | |||||||
| Number of patients | 40 | 27 | 25 | 14 | 15 | 14 | 9 |
| Proportion of patients | 19.9 | 17.0 | 19.7 | 14.3 | 16.1 | 21.2 | 20.9 |
Throughout the study period, the proportion of patients who used specific ChEIs remained relatively stable. During the visits in which patients reported taking ChEIs, 61.7% reported taking donepezil, 13.6% galantamine, and 4.8% rivastigmine. We examined in detail the extent and consistency of using individual medications among those who reported taking the medication. Table 3 presents baseline data on the proportion of patients who reported taking each medication every day (180+ days during the previous 6 months) and the proportion of those who took effective dosages for each medication. At baseline, 80.6% of patients who were taking donepezil reported taking it every day, and 98.3% of those who were taking donepezil reported taking it at effective doses. Over the course of the study period, in 93.3% of the visits in which patients reported taking donepezil, they also reported that they took it every day during the previous 6 months, and almost all reported taking it at effective doses.
Table 3. Extent and Consistency of ChEI and Memantine Use by Visit among Patients Who Reported Taking the Medication.
| Prevalence at Baseline Visit |
Study Period Prevalence From All Visits |
|||
|---|---|---|---|---|
|
| ||||
| Generic name | Percent taking medication for 180+ days |
Percent taking effective dosea |
Percent taking medication for 180+ days |
Percent taking effective dosea |
| Donepezil | 80.6 | 98.3 | 93.3 | 98.9 |
| Galantamine | 83.3 | 61.1 | 88.7 | 73.6 |
| Rivastigmine | 77.8 | 77.8 | 81.1 | 78.9 |
| Memantine | 88.9 | 88.9 | 86.1 | 96.4 |
Effective dose per day for Donepezil is defined to be 5mg or 10mg, for Galantamine 16-24mg, Rivastigmine 6-12mg, and for Memantine 10mg or 20mg. 201 patients were included at baseline and contributed to 785 observations for the longitudinal sample throughout the study period.
Unconditional Hazard Models
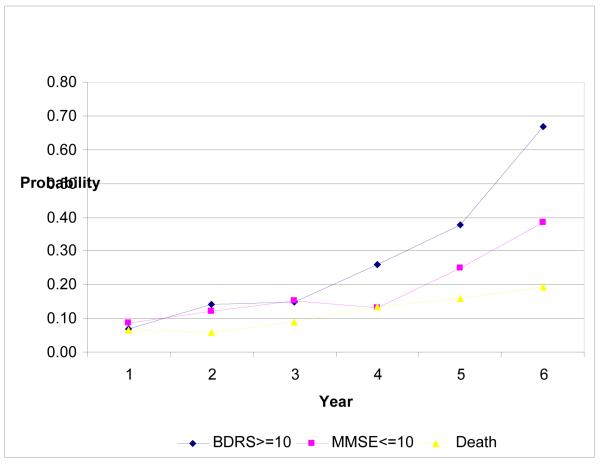
Figure 1 describes the hazard profile for the entire sample, i.e., the conditional probability that an individual would reach a clinical endpoint given that the individual did not reach the endpoint in an earlier interval. The hazard function for each outcome increased over time, and was most pronounced in patients’ function. Likelihood-ratio tests of the constant hazard assumption was rejected for all outcomes (all p<0.01).
Figure 1. Unconditional Hazard Model for Functional, Cognitive, and Mortality Endpoints.
Multivariate Discrete Time Hazard Analyses
Table 4 reports coefficient estimates of final multivariate models that examined time to reach functional and cognitive endpoints and mortality. Consistent with unconditional hazard models, estimated hazard for each outcome significantly increased over time. Year of study entry and site were not significantly associated with any outcome.
Table 4. Multivariate Results of Discrete-Time Hazard Models of Time to reach Functional and Cognitive Endpoints and Mortalitya.
| Functional Endpoint (BDRS≥10) |
Cognitive Endpoint (MMSE≤10) |
Death | |
|---|---|---|---|
| Coef. | Coef. | Coef. | |
| (SE) | (SE) | (SE) | |
| ChEI use | −1.282 *** | −0.379 | −0.894 ** |
| (0.433) | (0.573) | (0.387) | |
| Memantine use | −0.274 | 0.500 | −0.813 * |
| (0.388) | (0.498) | (0.450) | |
| Baseline MMSE | −0.095 * | −0.241 *** | −0.142 *** |
| (0.056) | (0.074) | (0.051) | |
| Baseline BDRS | 0.707 *** | 0.068 | 0.307 ** |
| (0.196) | (0.238) | (0.136) | |
| Baseline BDRS x year | −0.085 | −0.041 | −0.023 |
| (0.055) | (0.070) | (0.037) | |
| Presence of EPS | 1.727 *** | 0.836 | 0.832 ** |
| (0.483) | (0.552) | (0.405) | |
| Presence of psychiatric symptoms | 1.071 | 2.258 *** | 0.758 |
| (0.772) | (0.863) | (0.616) | |
| Presence of psychiatric symptoms x year | −0.072 | −0.526 ** | −0.109 |
| (0.209) | (0.249) | (0.170) | |
| Baseline comorbidities | −0.023 | −0.288 | −0.332 |
| (0.376) | (0.465) | (0.287) | |
| Baseline comorbidities × year | −0.036 | 0.140 | 0.099 |
| (0.107) | (0.139) | (0.070) | |
| Baseline age | −0.093 *** | −0.116 *** | 0.011 |
| (0.023) | (0.026) | (0.019) | |
| Female | −0.533 | 0.384 | −0.152 |
| (0.805) | (0.855) | (0.636) | |
| female × year | 0.357 | 0.191 | −0.031 |
| (0.217) | (0.248) | (0.173) | |
| Education | −0.066 | 0.148 | −0.074 |
| (0.108) | (0.134) | (0.087) | |
| Education × year | 0.030 | −0.021 | 0.022 |
| (0.030) | (0.040) | (0.025) | |
| Living in a nursing home | 0.609 * | 0.072 | 0.247 |
| (0.364) | (0.526) | (0.375) |
All models controlled for indicators of assessment interval, year of study entry, and site. Robust standard errors were reported.
p<.10
p<.05
p<.01
After controlling for patient characteristics, ChEI use was associated with longer time to reaching functional endpoint (estimated coefficient=-1.282, SE=0.433, computed odds ratio [OR]=0.278) and mortality (OR=0.409). Memantine use was associated with longer time to mortality (OR=0.443). (Full computations of odds ratios are available upon request.) Better baseline cognition was associated with longer time to reaching all endpoints. For each point improvement in baseline MMSE, estimated odds of reaching functional endpoint was 11% lower, of reaching cognitive endpoint was 21% lower, and of dying was 13% lower. Better baseline function also was associated with longer time to reaching functional endpoint and mortality. A one-point worsening in baseline BDRS almost doubled the estimated odds of reaching functional endpoint (OR=2.0). Similarly, a one-point worsening in baseline BDRS was associated with 36% increased odds of dying (OR= 1.36). Over time, increased risk of reaching functional endpoint and mortality from a one-point increase in baseline BDRS attenuated, but the effect was not statistically significant. Compared to patients without extrapyramidal signs, the odds of reaching functional endpoints for those with extrapyramidal signs was 5.6 times higher and the odds of dying was 2.3 times higher. At each assessment interval, compared to patients without psychiatric symptoms, the odds of reaching functional endpoints for those with psychiatric symptoms were significantly higher, with attenuating effects over time.
Secondary Sensitivity Analyses: Exploring Potential Association between Clinical Endpoints and Irregular ChEI Use
A small subsample of patients (n=19) in this study reported never been treated with ChEIs. Compared to patients who were treated with ChEIs at some point during the study, these patients were older, more likely to be female, with lower education, had shorter years of follow up, and had worse baseline MMSE scores. To test whether our results were sensitive to including patients who were never treated, we re-estimated our models using only patients who were treated with ChEIs at some point during the study. Results were substantively similar.
During the long course of the illness, it is likely that patients may change from treated to non-treated status or vice versa. The following treatment changes were observed in 56 patients: (1) 26 reported taking ChEIs at study entry and later discontinued; (2) 14 reported either taking ChEIs at study entry, discontinued at some point, and resumed treatment later, or were not taking ChEIs at study entry, initiated treatment at some point, and discontinued later; (3) 16 initiated and continued ChEI treatment after study enrollment. Because persistent treatment has been associated with slowed decline in cognition and function,[3, 18] we explored patterns of discontinuation as related to reaching clinical outcomes in these 56 patients. Bivariate distributions between medication use and clinical endpoints did not show patients were taken off ChEIs as their conditions deteriorated, although power to detect differences was limited. Among the 81 patients who used memantine at some point during the study, the vast majority continued treatment once started, only 6 discontinued after one or two assessments, one of whom reported resuming at a later time.
DISCUSSION
In this study, we assessed long term effects of ChEI and memantine on the natural history of AD outside the context of controlled trials and open label extension studies in order to provide a more ‘real world’ perspective. A large cohort of patients from early stages of AD was prospectively followed over 6 years. After controlling for important clinical characteristics, we found ChEI use was associated with longer time to reaching functional endpoints and death and memantine use was associated with delayed mortality. As for all observational cohort study, common caution in interpreting results is needed. In particular, the effects of ChEI and memantine use observed in this study should not be interpreted as causal effects.
A small number of studies have examined the relationship between ChEI use and mortality. An early randomized trial on tacrine use reported a trend toward lower mortality among those who received higher doses (>120mg/day) in open label follow up.[5] Another study reported significantly lower 3-year mortality in tacrine users than nonusers.[20] However, a more recent observational study comparing patients on ChEIs to a matched, historical sample of patients who were never exposed to ChEIs reported no association between ChEI use and cognitive and functional status at 1-year follow up or 3-year mortality.[14] Another study reported ChEI use alone, or ChEI+memantine use were not associated with time to death.[19]
Direct comparison of our results to these studies is difficult due to differences in study sample and methodology. Perhaps the closest study to ours is Lopez 2009, in which 443 patients with probable AD with at least one annual follow-up in a large urban ADRC were followed since 1997.[19] Several differences in patient characteristics between these two studies should be noted. First, compared to the Lopez study, patients in our sample were on average 3 years older (mean age = 76 vs. 73), but with higher MMSE at study entry (mean MMSE=21.9 vs. 18.2). It is possible that patients in our sample had an earlier start in ChEI therapy, similar to those who participate in clinical trials, and that this may have led to stronger mortality effects observed in our study.[35-37] Compared to the Lopez study, in which only 3% of subjects were never treated with ChEIs, 10% of our patients reported never been treated with ChEIs. Interestingly, compared to the treated patients, the never treated group in our study was older while they were significantly younger in the Lopez study.
It is also important to note methodological differences between these studies. For example, while we included all recruited patients in our analyses, analysis in the Lopez et al study was restricted to patients with at least one follow-up. Differential rates of decline or differential effectiveness of medications in patients who did not have any follow-up data may have contributed to differences in study results. In several earlier studies, patients who were not treated with ChEI were derived from historical cohorts that may have confounding effects on the results.[14, 17] It is likely that patients in the treated, more recent cohorts were diagnosed earlier than those in the untreated earlier cohorts. Improvements in medical care in more recent cohorts may have contributed to the relative slowing of disease progression in treated groups. These possibilities should be tested empirically in future studies.
While estimated associations between ChEI and memantine use and clinical endpoints are independent of patient characteristics that we controlled for in the models, unobserved characteristics that may differ between groups may remain and lead to possible biases in our results. It is possible that patients who took these drugs have naturally slower disease progression and their milder disease course was attributed to drug treatment. As in all observational studies, we cannot rule out the possibility of effects influencing observed relationships between treatment and outcomes. It is possible that from frequent, consistent contact with AD center staff, medical management of our patients, particularly those who were actively treated with medications, were better than those without such access or were untreated, and may have biased our results.
Several studies that examined the effects of ChEI+memantine combination therapy reported additional beneficial effects of combination therapy in slowed cognitive and functional decline and delayed nursing home placement.[17, 19] In this study, the vast majority of patients who used memantine used it in combination with ChEI, with memantine monotherapy reported in only 20 visits from 14 patients. We therefore could not distinguish the effects of memantine monotherapy versus ChEI+memantine combination therapy. However, the statistically significant effects of both ChEI and memantine suggest beneficial effect of ChEI+memantine combination therapy in delaying mortality.
Our ability to estimate duration of medication use was limited by our data collection method. At each visit, patients were asked about medication use in the previous 6 months, however, follow-up was performed annually. Therefore, medication use was known for half of the interval between visits. This loss of information adds noise to estimation models. However, unless there were systematic biases in medication use or discontinuation at 6 month intervals that were not captured in the data, this loss of information should not bias our estimation results.
There are several important strengths of this study. First, there is the large number of well characterized AD patients who were prospectively followed from early disease stages and examined by the same measures, in the same memory disorders units, and by the same group of clinicians over multiple years with good follow-up rates. In contrast to AD clinical trial and open-label extension studies that typically included data of at most one or two years, duration of our study is substantially longer. Second, our study avoids the limitations of most clinical trials which often have stringent inclusion and exclusion criteria. The sample of patients included in this study and results obtained from these patients therefore reflect more real-world clinical practice. Third, this study addresses several methodological issues in longitudinal analyses. As data on clinical outcomes are often collected at discrete-time intervals (e.g., annually), the commonly used continuous-time methods (e.g., Cox models) are limited. We used the more appropriate discrete-time survival models, included both time-invariant and time-variant covariates, and relaxed the often violated proportional hazards assumption when appropriate.[33, 34, 38] Therefore, our results go beyond findings from short-term clinical trials and provide evidence of the effects of cholinesterase-inhibitors and memantine that is fuller and more nuanced and have real-world clinical relevance in the treatment of patients with AD.
Systematic review
Randomized-controlled trials that examine the effects of ChEI and memantine on patient outcomes over long periods of time are difficult to conduct. Observational studies are limited with mixed results.
Interpretation
Our results extend beyond the context of controlled trials and open label extension studies, as our sample has less stringent inclusion/exclusion criteria, is more current with substantially longer study duration. Our methodology allows a more nuanced analysis so results have more ‘real-world’ relevance in the treatment of patients with AD.
Future directions
We will consider instrumental variables or propensity scores approaches to address some study limitations, such as the possible that patients who took ChEI and memantine have naturally slower disease progression and their milder disease course was attributed to drug treatment, or the possibility of other variables influencing observed relationships between treatment and outcomes. We will extend our study from our current clinical sample to community samples.
ACKNOWLEDGMENTS
The Predictors Study is supported by Federal grant AG07370, with additional support from federal grants, RR00645, and U01AG010483. Dr. Sano is supported by the Department of Veterans Affairs, Veterans Health Administration. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Sponsor has no role in the design, methods, subject recruitment, data collections, analysis and preparation of paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: Study concept and design: Zhu, Livote, Scarmeas, Stern. Acquisition of data: Scarmeas, Albert, Brandt, Blacker, Sano, Stern. Analysis and interpretation of data: Zhu, Livote, Scarmeas, Stern. Drafting of manuscript: Zhu. Critical revision of manuscript for important intellectual content: Zhu, Livote, Scarmeas, Brandt, Blacker, Sano, Stern. Statistical analysis: Zhu, Livote.
The authors all certify that they have no conflict of interest to report in this manuscript.
REFERENCES
- 1.Grimmer T, Kurz A. Effects of cholinesterase inhibitors on behavioural disturbances in Alzheimer’s disease: a systematic review. Drugs Aging. 2006;23(12):957–967. doi: 10.2165/00002512-200623120-00003. [DOI] [PubMed] [Google Scholar]
- 2.McShane R, Areosa S, Minakaran N. Memantine for dementia. Cochrane Database Syst Rev. 2006;2(CD003154) doi: 10.1002/14651858.CD003154.pub5. [DOI] [PubMed] [Google Scholar]
- 3.Burns A, Gauthier S, Perdomo C. Efficacy and safety of donepezil over 3 years: an open-label, multicentre study in patients with Alzheimer’s disease. Int J Geriatr Psychiatry. 2007;22(8):806–812. doi: 10.1002/gps.1746. [DOI] [PubMed] [Google Scholar]
- 4.Birks J. Cholinesterase inhibitors for Alzheimer’s disease. Cochrane Database Syst Rev. 2009;(1):CD005593. doi: 10.1002/14651858.CD005593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Knopman D, Schneider L, Davis K, et al. Long-term tacrine (Cognex) treatment: effects on nursing home placement and mortality, Tacrine Study Group. Neurology. 1996;47(1):166–177. doi: 10.1212/wnl.47.1.166. [DOI] [PubMed] [Google Scholar]
- 6.Winblad B, Engedal K, Soininen H, et al. A 1-year, randomized, placebo-controlled study of donepezil in patients with mild to moderate AD. Neurology. 2001;57(3):489–495. doi: 10.1212/wnl.57.3.489. [DOI] [PubMed] [Google Scholar]
- 7.Winblad B, Poritis N. Memantine in severe dementia: results of the 9M-Best Study (Benefit and efficacy in severely demented patients during treatment with memantine) Int J Geriatr Psychiatry. 1999;14(2):135–146. doi: 10.1002/(sici)1099-1166(199902)14:2<135::aid-gps906>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med. 2003;348(14):1333–1341. doi: 10.1056/NEJMoa013128. [DOI] [PubMed] [Google Scholar]
- 9.Reisberg B, Doody R, Stoffler A, et al. A 24-week open-label extension study of memantine in moderate to severe Alzheimer disease. Arch Neurol. 2006;63(1):49–54. doi: 10.1001/archneur.63.1.49. [DOI] [PubMed] [Google Scholar]
- 10.Farlow M, Anand R, Messina J, Jr, et al. A 52-week study of the efficacy of rivastigmine in patients with mild to moderately severe Alzheimer’s disease. Eur Neurol. 2000;44(4):236–241. doi: 10.1159/000008243. [DOI] [PubMed] [Google Scholar]
- 11.Mohs RC, Doody RS, Morris JC, et al. A 1-year, placebo-controlled preservation of function survival study of donepezil in AD patients. Neurology. 2001;57(3):481–488. doi: 10.1212/wnl.57.3.481. [DOI] [PubMed] [Google Scholar]
- 12.Karlawish JH, Whitehouse PJ. Is the placebo control obsolete in a world after donepezil and vitamin E? Arch Neurol. 1998;55(11):1420–1424. doi: 10.1001/archneur.55.11.1420. [DOI] [PubMed] [Google Scholar]
- 13.Doody RS, Dunn JK, Clark CM, et al. Chronic donepezil treatment is associated with slowed cognitive decline in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2001;12(4):295–300. doi: 10.1159/000051272. [DOI] [PubMed] [Google Scholar]
- 14.Lopez OL, Becker JT, Wisniewski S, et al. Cholinesterase inhibitor treatment alters the natural history of Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;72(3):310–314. doi: 10.1136/jnnp.72.3.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brodaty H, Woodward M, Boundy K, et al. Naturalistic treatment of Alzheimer’s disease with galantamine: 12-month follow-up from the NATURE study. CNS Drugs. 2007;21(4):335–336. doi: 10.2165/00023210-200721040-00006. [DOI] [PubMed] [Google Scholar]
- 16.Hansen RA, Gartlehner G, Lohr KN, et al. Functional outcomes of drug treatment in Alzheimer’s disease: A systematic review and meta-analysis. Drugs Aging. 2007;24(2):155–167. doi: 10.2165/00002512-200724020-00007. [DOI] [PubMed] [Google Scholar]
- 17.Atri A, Shaughnessy LW, Locascio JJ, et al. Long-term course and effectiveness of combination therapy in Alzheimer disease. Alzheimer Dis Assoc Disord. 2008;22(3):209–221. doi: 10.1097/WAD.0b013e31816653bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rountree SD, Chan W, Pavlik VN, et al. Persistent treatment with cholinesterase inhibitors and/or memantine slows clinical progression of Alzheimer disease. Alzheimers Res Ther. 2009;1(2):7. doi: 10.1186/alzrt7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez OL, Becker JT, Wahed AS, et al. Long-term effects of the concomitant use of memantine with cholinesterase inhibition in Alzheimer disease. J Neurol Neurosurg Psychiatry. 2009;80(6):600–607. doi: 10.1136/jnnp.2008.158964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ott BR, Lapane KL. Tacrine therapy is associated with reduced mortality in nursing home residents with dementia. J Am Geriatr Soc. 2002;50(1):35–40. doi: 10.1046/j.1532-5415.2002.50005.x. [DOI] [PubMed] [Google Scholar]
- 21.Lopez OL, Becker JT, Saxton J, et al. Alteration of a clinically meaningful outcome in the natural history of Alzheimer’s disease by cholinesterase inhibition. J Am Geriatr Soc. 2005;53(1):83–87. doi: 10.1111/j.1532-5415.2005.53015.x. [DOI] [PubMed] [Google Scholar]
- 22.Geldmacher DS Donepezil (Aricept) for treatment of Alzheimer’s disease and other dementing conditions. Expert Rev Neurother. 2004;4(1):5–16. doi: 10.1586/14737175.4.1.5. [DOI] [PubMed] [Google Scholar]
- 23.Zhu CW, Livote EE, Kahle-Wrobleski K, et al. Longitudinal Medication Usage in Alzheimer Disease Patients. Alzheimer Dis Assoc Disord. 2010 doi: 10.1097/WAD.0b013e3181e6a17a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “Predictors Study”). I. Study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord. 1993;7(1):3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- 25.Richards M, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the “Predictors Study”). II. Neurological, psychiatric, and demographic influences on baseline measures of disease severity. Alzheimer Dis Assoc Disord. 1993;7(1):22–32. doi: 10.1097/00002093-199307010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, et al. Motor signs during the course of Alzheimer disease. Neurology. 2004;63(6):975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Stern Y, Sano M, Paulson J, et al. Modified mini-mental state examination: validity and reliability. Neurology. 1987;37(suppl 1):179. [Google Scholar]
- 29.Devanand DP, Miller L, Richards M, et al. The Columbia University Scale for Psychopathology in Alzheimer’s disease. Arch Neurol. 1992;49(4):371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- 30.Stern MB, Hurting HI. The Comprehensive Management of Parkinson’s Disease. PMA Corp; New York: 1978. The clinical characteristics of Parkinson’s Disease and parkinsonian syndromes: diagnosis and assessment; pp. 3–50. [Google Scholar]
- 31.Richards M, Marder K, Bell K, et al. Interrater reliability of extrapyramidal signs in a group assessed for dementia. Arch Neurol. 1991;48(11):1147–1149. doi: 10.1001/archneur.1991.00530230055021. [DOI] [PubMed] [Google Scholar]
- 32.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.Allison P. Event history analysis: Regression for longitudinal event data, in Sage University paper series on quantitative applications in the social sciences. Sage; Beverly Hills, CA: 1984. Series No. 07-046. [Google Scholar]
- 34.Singer J, Willet J. Applied Longitudinal Data Analysis: Modeling Change and Event Occurrence. Oxford University Press; New York: 2003. [Google Scholar]
- 35.Doody RS, Geldmacher DS, Gordon B, et al. Open-label, multicenter, phase 3 extension study of the safety and efficacy of donepezil in patients with Alzheimer disease. Arch Neurol. 2001;58(3):427–433. doi: 10.1001/archneur.58.3.427. [DOI] [PubMed] [Google Scholar]
- 36.Raskind MA, Peskind ER, Truyen L, et al. The cognitive benefits of galantamine are sustained for at least 36 months: a long-term extension trial. Arch Neurol. 2004;61(2):252–256. doi: 10.1001/archneur.61.2.252. [DOI] [PubMed] [Google Scholar]
- 37.Farlow MR, Lilly ML. Rivastigmine: an open-label, observational study of safety and effectiveness in treating patients with Alzheimer’s disease for up to 5 years. BMC Geriatr. 2005;5:3. doi: 10.1186/1471-2318-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Agostino RB, Lee ML, Belanger AJ, et al. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med. 1990;9(12):1501–1515. doi: 10.1002/sim.4780091214. [DOI] [PubMed] [Google Scholar]