Summary
In addition to the phosphoenolpyruvate:sugar phosphotransferase system (sugar PTS), most proteobacteria possess a paralogous system (nitrogen phosphotransferase system, PTSNtr). The first proteins in both pathways are enzymes (enzyme Isugar and enzyme INtr) that can be autophosphorylated by phosphoenolpyruvate. The most striking difference between enzyme Isugar and enzyme INtr is the presence of a GAF domain at the N-terminus of enzyme INtr. Since the PTSNtr was identified in 1995, it has been implicated in a variety of cellular processes in many proteobacteria and many of these regulations have been shown to be dependent on the phosphorylation state of PTSNtr components. However, there has been little evidence that any component of this so-called PTSNtr is directly involved in nitrogen metabolism. Moreover, a signal regulating the phosphorylation state of the PTSNtr had not been uncovered. Here, we demonstrate that glutamine and α-ketoglutarate, the canonical signals of nitrogen availability, reciprocally regulate the phosphorylation state of the PTSNtr by direct effects on enzyme INtr autophosphorylation and the GAF signal transduction domain is necessary for the regulation of enzyme INtr activity by the two signal molecules. Taken together, our results suggest that the PTSNtr senses nitrogen availability.
Keywords: GAF domain, glutamine, α–ketoglutarate, nitrogen PTS, phosphorylation-dependent mobility shift
Introduction
How cells respond to environmental (extracellular) signals is of fundamental importance in biology. Most organisms, including bacteria, use a signal transduction pathway to deliver an extracellular signal to some intracellular target. Many of the relay molecules in signal transduction pathways are protein kinases that create a “phosphorylation cascade”. The sugar phosphotransferase system (PTS) in bacteria is a group translocation system whose relay proceeds sequentially from PEP to Enzyme I, HPr, EIIA, EIIB, and finally to the incoming sugar that is transported through EIIC in the membrane and concomitantly phosphorylated (Deutscher et al., 2006). In addition to its primary functions in sugar uptake and phosphorylation, this complex protein system carries out numerous regulatory functions such as chemoreception (Lux et al., 1995), activation of glycogen phosphorylase by HPr (Seok et al., 1997), inhibition of non-PTS sugar permeases (Deutscher et al., 2006), activation of pyruvate decarboxylase (FrsA) by EIIAGlc (Koo et al., 2004; Lee et al., 2011), activation of adenylyl cyclase by phospho-EIIAGlc (Park et al., 2006) and regulation of Mlc activity to control the expression level of the carbohydrate PTS and related proteins (Lee et al., 2000; Nam et al., 2001; Tanaka et al., 2000).
Analysis of the Escherichia coli genome has revealed a parallel PTS that has been referred to as the nitrogen PTS (PTSNtr); it consists of EINtr encoded by ptsP, NPr encoded by ptsO, and EIIANtr encoded by ptsN which are paralogues of the carbohydrate PTS components EI, HPr, and EIIA, respectively (Peterkofsky et al., 2006; Pflüger-Grau and Görke, 2010; Powell et al., 1995). Since phosphoryl transfer to a specific substrate has not yet been demonstrated for the PTSNtr, it has been suggested that this system functions mainly in a regulatory capacity (Reizer et al., 1996). The PTSNtr has been implicated in poly-β-hydroxybutyrate accumulation and nitrogen fixation in Azotobacter vinelandii (Segura and Espin, 1998), virulence in Legionella pneumophila (Higa and Edelstein, 2001) and Salmonella enterica (Choi et al., 2010), melanin synthesis and nitrogen fixation in Rhizobium etli (Michiels et al., 1998), regulation of ATP-dependent transporters in Rhizobium leguminosarum (Prell et al., 2012) and pulmonary infection (Zhang et al., 2005) and carbon source-mediated inhibition of the σ54-dependent Pu promoter of the TOL plasmid in Pseudomonas species (Cases et al., 2001). In E. coli, EIIANtr was shown to be involved in the regulation of the essential GTPase, Era, which appears to function in cell cycle progression and the initiation of cell division (Powell et al., 1995), although the mechanism of this regulation has not yet been defined.
Recently, a direct role of the E. coli PTSNtr in regulation of K+ uptake was described. Dephosphorylated EIIANtr binds to and regulates the low-affinity K+ transporter TrkA (Lee et al., 2007) and the K+-dependent sensor kinase KdpD (Lüttmann et al., 2009; Pflüger-Grau and Görke, 2010). In the absence of dephosphorylated EIIANtr, K+ uptake through TrkA increases, resulting in unusually high intracellular K+ concentrations. K+ regulates global gene expression involving both σ70- and σS-dependent promoters (Lee et al., 2010). Furthermore, dephosphorylated EIIANtr was shown to modulate the phosphate starvation response through interaction with the sensor kinase PhoR (Lüttmann et al., 2012). A physiological role of NPr was also shown. The dephosphorylated form of NPr interacts with and inhibits LpxD, which catalyzes biosynthesis of lipid A of the lipopolysaccharide (LPS) layer (Kim et al., 2011).
Although several regulatory interactions involving proteins of the PTSNtr have been shown to be dependent on the phosphorylation state of PTSNtr components, the connection between them was obscure. The purpose of this study was to search for a signal regulating the phosphorylation state of the PTSNtr; this might serve as the central feature of the overall metabolic regulation by this system.
The mobility of many eukaryotic proteins is shifted on SDS-PAGE when they become phosphorylated (Zhou et al., 2000); we refer to this as a phosphorylation-dependent mobility shift (PDMS). This PDMS is also observed with some proteins in bacteria (Lee and Helmann, 2006; Nam et al., 2005). While EIIAGlc shows a significant PDMS on SDS-PAGE (Hogema et al., 1998), EIIANtr does not. By comparing amino acid sequences near the phosphorylation sites of EIIAGlc and EIIANtr, site-directed mutagenesis of EIIANtr produced a species exhibiting a PDMS; the PDMS of this form was used to identify the signals (glutamine and α-ketoglutarate) regulating the phosphorylation state of the PTSNtr. The primary site of the regulation was identified to be the GAF domain of EINtr.
Results
Engineering EIIANtr to exhibit a phosphorylation-dependent mobility shift (PDMS) on SDS-PAGE
EIIAGlc has previously been observed to change its mobility on SDS-PAGE upon phosphorylation of its active site histidine (Hogema et al., 1998), whereas EIIANtr does not exhibit such a PDMS (Fig. 1A). This PDMS property has been an important asset in studying the regulatory functions of EIIAGlc that depend on its state of phosphorylation. The focus of the current study was to define the mechanism for the various regulatory functions of EIIANtr. The first step in the study was to modify the structure of EIIANtr so that it exhibits a PDMS. A comparison of the amino acid sequences surrounding the phosphorylation site of EIIAGlc and EIIANtr revealed that, while EIIAGlc has two negatively charged amino acids (E86 and D94) situated on both sides of the phosphorylation site (H90), EIIANtr has three negatively charged amino acids clustered on one side of the phosphorylation site (H73) (Fig. S1A). To define the possible importance of the negatively charged amino acid E86 for the PDMS of EIIAGlc, the E86A mutant was generated. Fig. S1B demonstrates the requirement of E86 for the PDMS of EIIAGlc in spite of the fact that EIIAGlc(E86A) can still be phosphorylated (Fig. S1C). To further explore the importance of the distribution of the negatively charged amino acids around the phosphorylation site for the PDMS, we performed systematic site-directed mutagenesis in the region of residues 69 to 76 of EIIANtr by changing each residue, one at a time, to aspartate. Each mutant protein was expressed and tested for solubility and the PDMS on SDS-PAGE. Although all of the purified mutant proteins showed a measurable PDMS on SDS-PAGE (Fig. S2), only EIIANtr(K75D) was as soluble (did not make an inclusion body) and as phosphorylatable as the wild-type protein (Figs. 1A and S2). Therefore, we selected EIIANtr(K75D) as a good candidate to search for the signaling molecule(s) regulating the phosphorylation state of the PTSNtr.
Fig. 1.
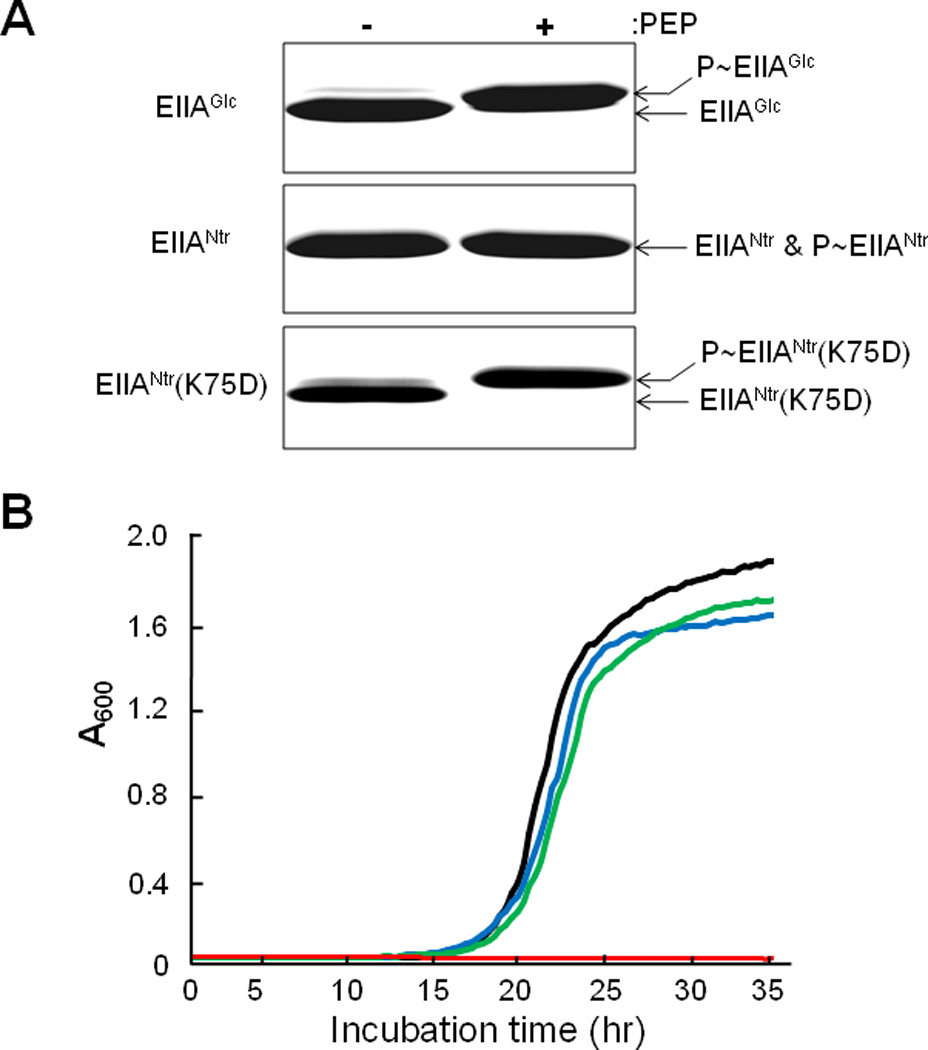
An EIIANtr mutant exhibits a PDMS on SDS-PAGE and neutralizes growth inhibition by Ala-Leu.
A. EIIAGlc, EIIANtr and EIIANtr(K75D) were incubated under phosphorylating conditions with and without 1 mM PEP (see Experimental Procedures) and then analyzed by SDS-PAGE.
B. Cells grown in LB medium overnight were washed with M9 medium, inoculated into M9 medium containing 0.5% glucose supplemented with 0.5 mM Ala-Leu, and growth at 37 °C was recorded by measuring the optical density at 600 nm: black line, MG1655; red, CR301(ΔptsN); blue, CR301/pCR3(IIANtr); and green, CR301/pCR3(K75D).
To validate the use of this mutant form of EIIANtr, we tested whether it behaves as does the wild type protein in an in vivo function test. It was previously demonstrated that deletion of ptsN (encoding EIIANtr) resulted in Ala-Leu dipeptide-dependent growth inhibition of E. coli in M9 medium containing glucose as a carbon source and that the growth inhibition was neutralized by ectopic expression of EIIANtr (Lee et al., 2005). Expression of EIIANtr(K75D) in the ptsN mutant also restored resistance to the Ala-Leu dipeptide-dependent growth inhibition to a level similar to the wild type protein (Fig. 1B), indicating that EIIANtr(K75D) can substitute for the wild type protein in vivo.
E. coli cells expressing EIIANtr(K75D) were tested for the PDMS under conditions known to influence the in vivo phosphorylation state of EIIANtr. Similar to previous observations (Bahr et al., 2011; Pflüger and de Lorenzo, 2007), only the dephosphorylated form of EIIANtr could be detected in an E. coli mutant deleted for the ptsP gene encoding EINtr, while EIIANtr exists mainly in a phosphorylated form in cells expressing the ptsP gene grown in LB medium (Fig. 2A). This provides further evidence for the adequacy of the K75D mutant of EIIANtr as a phosphorylation-state probe.
Fig. 2.
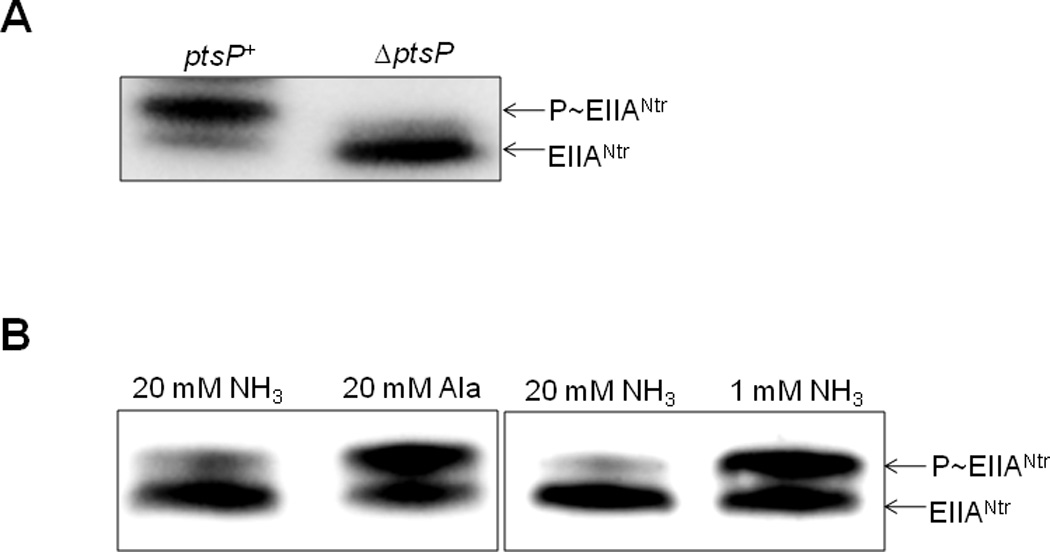
Measurement of the in vivo phosphorylation state of EIIANtr under various conditions. The phosphorylation state of EIIANtr was determined in cells harboring the pCR3(K75D) plasmid expressing EIIANtr(K75D) by Western blotting as described in Experimental Procedures.
A. Determination of the phosphorylation state of EIIANtr in CR301/pCR3(K75D) and CR103/pCR3(K75D) cells grown in LB medium to A600 = 2.0.
B. CR301/pCR3(K75D) cells were grown in W salts medium containing 20 mM (NH4)2SO4 and 0.2% glucose to mid-logarithmic phase, then centrifuged, washed and resuspended in W salts medium containing 0.2% glucose with 20 mM (NH4)2SO4, 20 mM alanine, or 1 mM (NH4)2SO4. When the A600 reached 0.8, intracellular phosphorylation states were determined.
It has previously been shown that the phosphorylation state of EIIANtr in Pseudomonas putida could be altered by nitrogen sources. The phosphorylated form of EIIANtr was more abundant than its dephosphorylated form in cells grown with nitrate as the sole nitrogen source, whereas the dephosphorylated form increased in cells grown in the presence of ammonium salts (Pflüger and de Lorenzo, 2007). Because E. coli cells cannot use nitrate as a nitrogen source, we employed alanine as a poor nitrogen source. The left panel of Fig. 2B shows that the dephosphorylated form of EIIANtr prevails in E. coli cells supplemented with 20 mM ammonium ion, whereas exposure of cells to the same concentration of the poorer nitrogen source favors the phosphorylated state, similar to the case in P. putida. These in vivo phosphorylation studies further validate the use of EIIANtr(K75D) as a probe to explore the regulatory aspects of the PTSNtr.
Reciprocal regulation by glutamine and α–ketoglutarate of autophosphorylation of EINtr; dependence on the GAF domain
Taking advantage of the unique PDMS exhibited by EIIANtr(K75D), numerous factors were screened for a signal(s) affecting the phosphorylation state of the nitrogen PTS. We have previously shown that the PTSNtr regulates the sensitivity to serine, isoleucine, leucine and leucine-containing peptides of an E. coli K-12 strain harboring a frameshift mutation in the ilvG gene (Lee et al., 2005) and that expression of several genes involved in amino acid metabolism was significantly influenced by the ptsN deletion (Lee et al., 2010). Therefore, we explored the possibility that an amino acid might regulate the phosphorylation state of the PTSNtr. Interestingly, out of the 20 amino acids tested, only glutamine showed an apparent inhibitory effect on the phosphorylation state of EIIANtr(K75D) (Fig. S3).
Previous studies have established that cellular glutamine and α-ketoglutarate levels sense nitrogen availability in opposite directions and that they regulate the activity of glutamine synthetase antagonistically through GlnD and GlnE of the bona fide Ntr signal transduction system (Ninfa and Atkinson, 2000). Therefore, we also tested the effect of α-ketoglutarate on the phosphotransferase activity of the PTSNtr. The data of Figs. 3 and 4 demonstrate that glutamine (Gln) and α–ketoglutarate (α-KG) oppositely affect the in vitro phosphorylation of EIIANtr(K75D); glutamine inhibits and α–ketoglutarate stimulates. Both the inhibitory and stimulatory effects depend on the presence of the GAF domain of EINtr. The data in Fig. 4 indicate that both the inhibitory effect of glutamine and the stimulatory effect of α–ketoglutarate are concentration-dependent. Noteworthy was the finding that, while a form of EINtr deleted for the GAF domain supported phosphotransfer from PEP, there was no effect of either compound at any of the concentrations used. The requirement of the GAF domain for the activation or inhibition effects pointed to EINtr as the locus of the effect. The data in Fig. 5 validate this prediction. Autophosphorylation by [32P]PEP of EINtr is inhibited by glutamine (Fig. 5A) and stimulated by α–ketoglutarate (Fig. 5B). EINtr lacking the GAF domain was insensitive to the low molecular weight effectors.
Fig. 3.
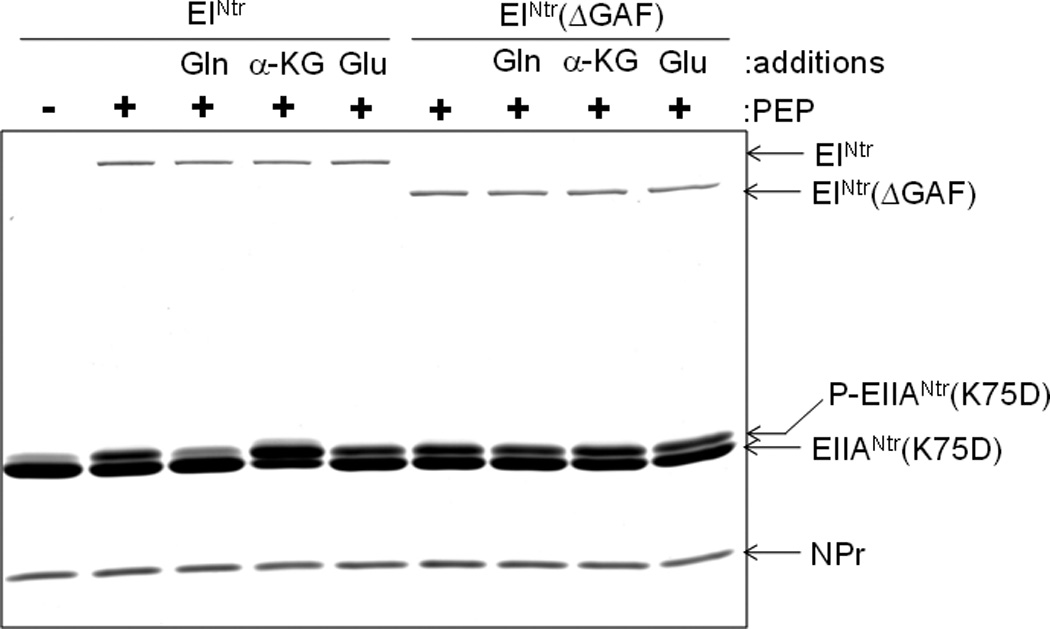
Effect of glutamine and α–ketoglutarate on the phosphorylation of EIIANtr(K75D). EIIANtr(K75D) (3 µg) was incubated with PEP (1 mM), EINtr or EINtr(ΔGAF) (0.1 µg) and NPr (0.3 µg) in the presence of glutamate (Glu), glutamine (Gln) or α-ketoglutarate (α-KG) at 5 mM. After incubation for 1 min at room temperature, reactions were stopped by the addition of SDS-PAGE sample buffer and analyzed by 14% SDS-PAGE. The proteins were stained with Coomassie Brilliant Blue.
Fig. 4.
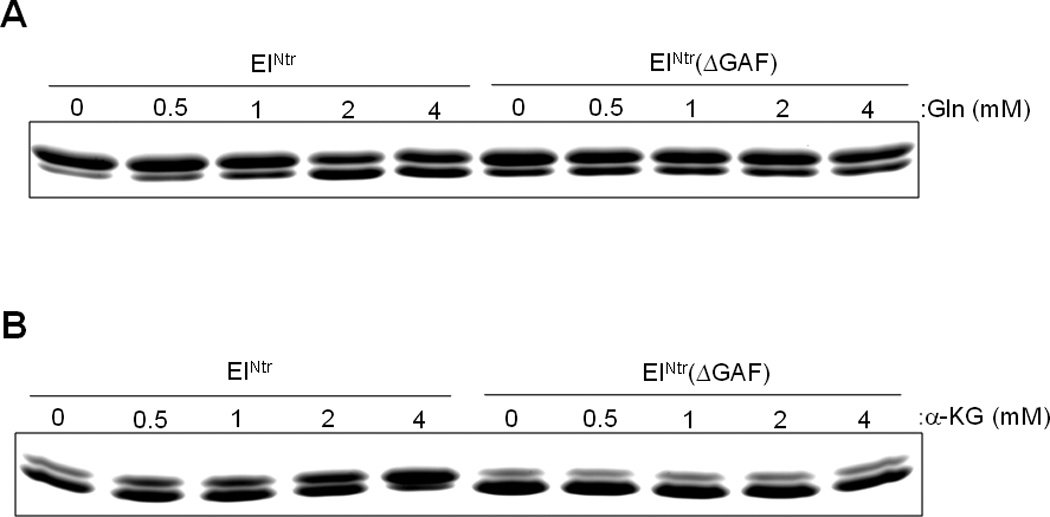
Glutamine and α–ketoglutarate affect the phosphorylation of EIIANtr(K75D) in a dose-dependent manner. In vitro phosphorylation reactions were carried out as described in Figure 3 in the presence of purified EINtr or EINtr(ΔGAF), NPr and EIIANtr(K75D) with PEP (1 mM) and the indicated concentrations of glutamine (A) or α–ketoglutarate (B). After incubation at room temperature for 2 min (A) or 0.5 min (B), reactions were stopped by the addition of SDS-PAGE sample buffer and then run on a 14% polyacrylamide gel under denaturing conditions.
Fig. 5.
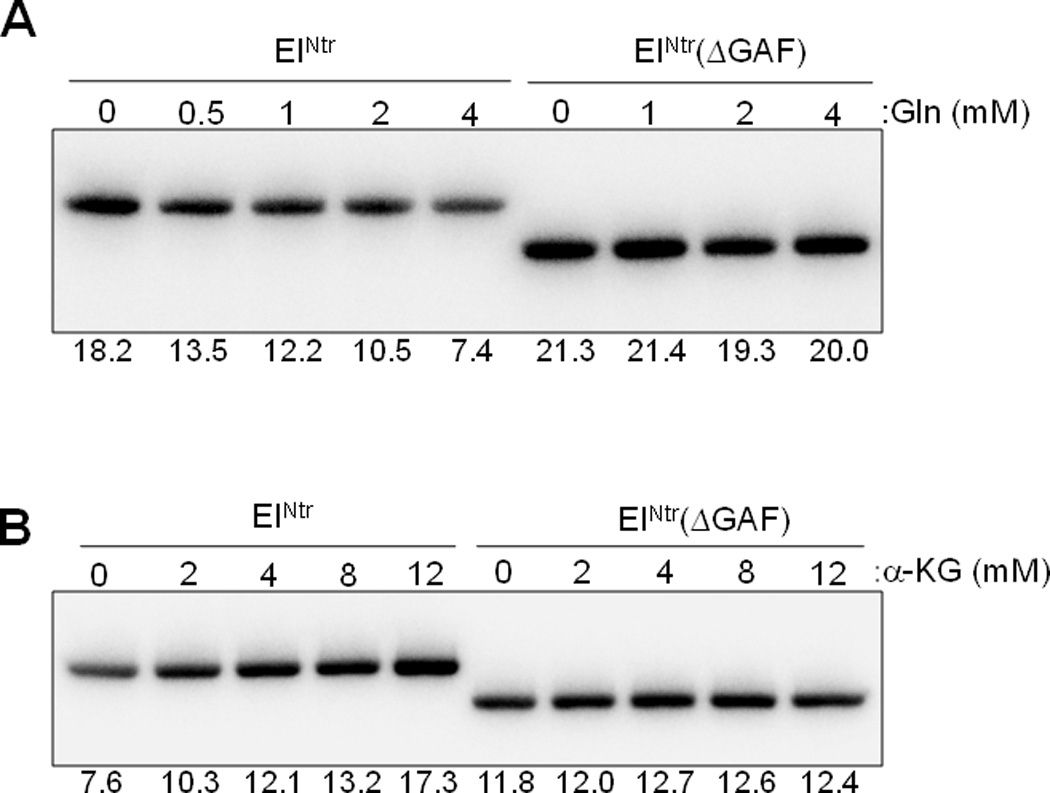
The autophosphorylation of EINtr is affected by glutamine and α–ketoglutarate. In vitro phosphorylation reactions were carried out in the presence of purified EINtr or EINtr(ΔGAF) and [32P]PEP (20 µM) with the indicated concentrations of glutamine (A) or α–ketoglutarate (B) at room temperature for 2 min (A) or 1 min (B) and run on a 14% polyacrylamide gel under denaturing conditions. The labeled proteins were visualized by autoradiography. Band intensities were quantified using Multi Gauge V3.0 software and relative band intensities are shown under each panel.
The specificity for the interaction of glutamine and α–ketoglutarate with EINtr was studied by quenching of tryptophan fluorescence (Fig. 6). While there was a concentration-dependent decrease in the fluorescence by either glutamine or α–ketoglutarate, the fluorescence spectrum was unaffected by glutamic acid. The calculated Kd for glutamine was 0.7 mM and that for α–ketoglutarate was 3.3 mM. A similar study carried out with EINtr lacking the GAF domain showed no effect on the fluorescence spectrum of any of the three low molecular weight compounds (Fig. S4). Physiological concentrations of α-ketoglutarate were reported to be between 0.14 and 0.91 mM in E. coli cells grown in the presence of excess ammonium salt (Senior, 1975) but were higher than 10 mM in the presence of poor nitrogen sources (Doucette et al., 2011). While the concentration of glutamine is very low in cells under nitrogen-limiting conditions, it abruptly increases to higher than 10 mM with a concomitant decrease in the α-ketoglutarate pool to below 1 mM when cells were subjected to ammonium upshift (Doucette et al., 2011). Therefore, the Kd values for binding of α-ketoglutarate and glutamine to EINtr determined in this study suggest that the observed effects of these two metabolites on the phosphorylation state of the PTSNtr are physiologically relevant. Then we compared the effect of glutamine and α-ketoglutarate at 10 mM on the velocity of the phosphotransferase reaction (Fig. 7). When we measured the phosphorylation level of EIIANtr in the presence of trace amounts of EINtr and NPr as a function of reaction time, about 1/3 of EIIANtr was phosphorylated in 1.5 min in the reaction without any effector (control) while it took 4 min to get the same level of EIIANtr phosphorylation in the reaction containing 10 mM glutamine. In contrast, in the presence of 10 mM α-ketoglutarate, EIIANtr was essentially completely phosphorylated in 2 min. Because intracellular concentrations of glutamine and α-ketoglutarate are reciprocally regulated in wild-type E. coli cells, the data in Fig. 7 imply that the reciprocal regulation of the autophosphorylation of EINtr by α-ketoglutarate and glutamine can result in significant difference (more than 5 times) in the phosphotransferase reaction velocity of the PTSNtr in response to nitrogen availability in vivo. To obtain more accurate steady-state kinetics associated with the regulation of the phosphorylation state of the PTSNtr, the mechanism of PTSNtr dephosphorylation needs to be identified.
Fig. 6.
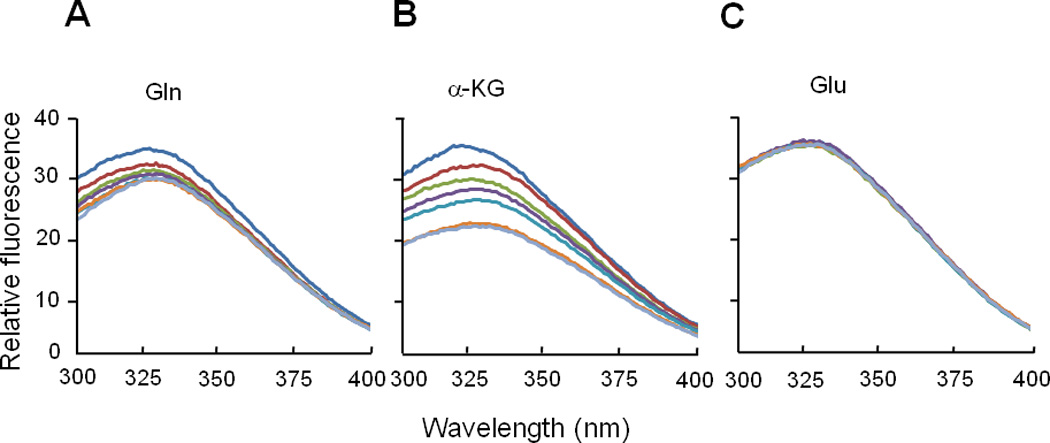
Effect of glutamine, α-ketoglutarate and glutamate on tryptophan fluorescence of EINtr. Tryptophan fluorescence was measured as described in “Experimental Procedures”. EINtr was present at 95 µg/ml in 50 mM Tris, pH 7.5, 20 mM NaCl. For determination of the respective Kd values, a series of ligand concentrations were examined for the degree of quenching of fluorescence: blue, control; brown, 0.5 mM; yellow-green, 1 mM; purple, 2 mM; sky-blue, 4 mM; orange, 16 mM; gray, 32 mM.
Fig. 7.
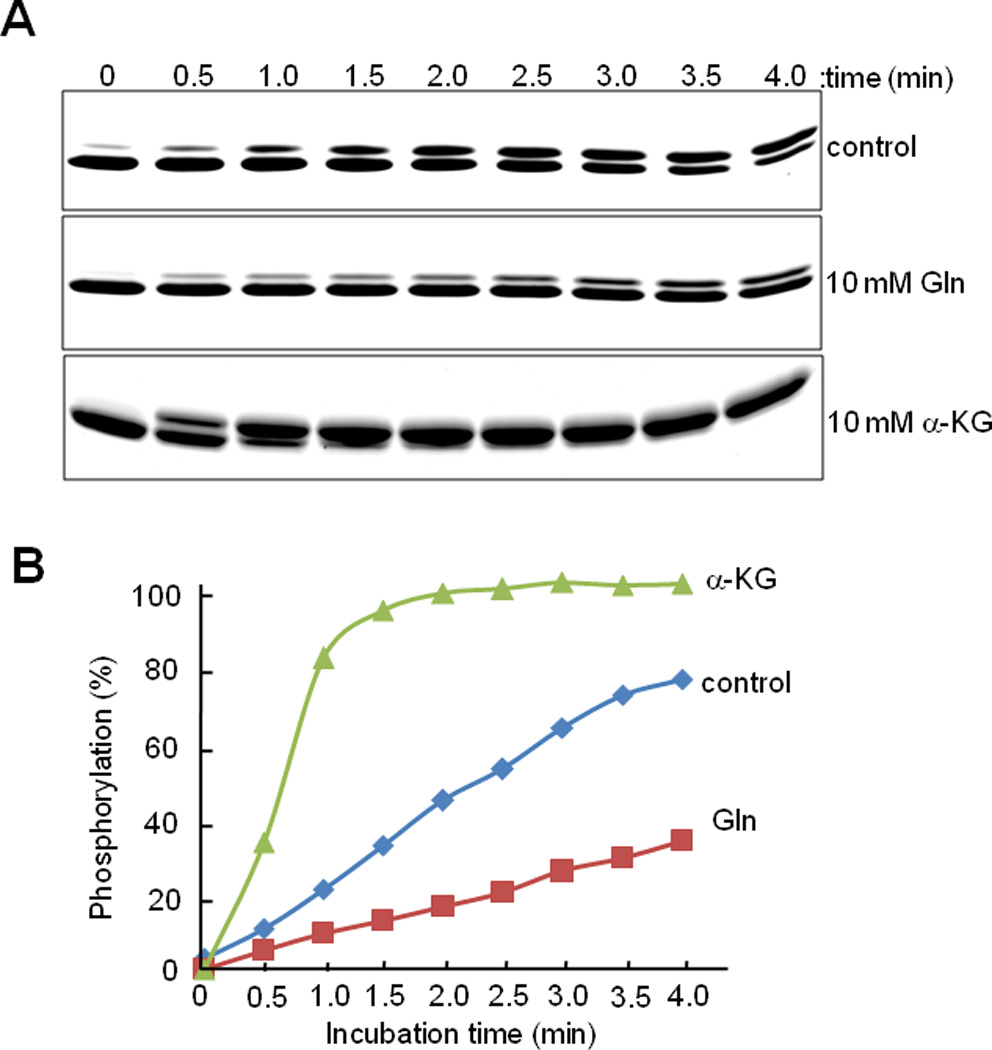
Effect of glutamine and α–ketoglutarate on the phosphorylation of EIIANtr(K75D) as a function of time.
A. EIIANtr(K75D) (3 µg) was incubated with PEP (1 mM), EINtr (0.05 µg) and NPr (0.2 µg) in the presence of glutamine (Gln) or α-ketoglutarate (α-KG) at 10 mM. After incubation for the indicated time periods at room temperature, reactions were stopped by the addition of SDS-PAGE sample buffer and analyzed by 14% SDS-PAGE. The proteins were stained with Coomassie Brilliant Blue.
B. The band intensities in panel A were quantified using the Multi Gauge V3.0 software and the levels of the phosphorylated form of EIIANtr(K75D) were plotted as the percent of total EIIANtr(K75D).
The GAF domain of EINtr senses nitrogen availability in vivo
The canonical signal of nitrogen limitation in enteric bacteria is the intracellular glutamine to α-ketoglutarate ratio, which increases under conditions of nitrogen sufficiency (high concentration of ammonium ion) and decreases under conditions of nitrogen starvation (the presence of other nitrogen sources or concentrations of ammonium ion lower than 2 mM) (Doucette et al., 2011; Ninfa and Atkinson, 2000). We assumed that this might explain why glutamate did not show any effect on the phosphorylation of EIIANtr(K75D) and tryptophan fluorescence quenching of EINtr while α-ketoglutarate and glutamine did (Figs. 3 and 6), although these three amino acids can readily exchange amino groups by glutamate dehydrogenase, glutamine synthetase and glutamate synthase in E. coli and other bacteria. We showed above that the phosphorylated form of EIIANtr significantly increased in E. coli cells supplemented with 20 mM alanine in W salts medium compared with cells supplemented with 20 mM ammonium salt (left panel of Fig. 2B). To further correlate the phosphorylation state of EIIANtr with the ratio of α-ketoglutarate to glutamine concentrations in the cell, we tested another condition known to increase this ratio for the effect on the in vivo phosphorylation state of the PTSNtr. As expected from the in vitro studies (Figs. 3–6), the phosphorylated form of EIIANtr significantly increased when the culture was subjected to a nitrogen downshift from 20 to 1 mM ammonium salt (right panel of Fig. 2B). The western blots suggest that the total amount of EIIANtr is comparable under both growth conditions.
To investigate the direct effect of α-ketoglutarate and glutamine on the phosphorylation state of the PTSNtr in vivo, we monitored changes of the phosphorylation state of EIIANtr(K75D) dependent on the addition of α-ketoglutarate or glutamine to growth medium. The inclusion of 10 mM glutamine in W salts medium containing 20 mM alanine significantly increased the dephosphorylated form of EIIANtr, while the inclusion of 10 mM α-ketoglutarate showed the opposite effect to a small degree (Fig. 8A). One possibility for this result could be that the intracellular level of α-ketoglutarate in cells grown in the medium containing 20 mM alanine is already high enough to produce full activation of the autophosphorylation of EINtr in the cell. To investigate this possibility, we tested the effect of α-ketoglutarate in cells grown under a condition where the ratio of α-ketoglutarate to glutamine is low. However, the addition of 20 mM α-ketoglutarate in the medium supplemented with 20 mM ammonium salt had little effect on the phosphorylation state of EIIANtr, ruling out this possibility (Fig. S5). Another possibility is that α-ketoglutarate is not permeable enough to support its accumulation in the cytoplasm. Dimethyl-α-ketoglutarate (dm-α-KG), a membrane-permeable ester known to be cleaved by cellular esterases to form α-ketoglutarate in the cell, is often used to increase the intracellular level of α-ketoglutarate (Doucette et al., 2011). The addition of 20 mM dm-α-KG to medium supplemented with 20 mM ammonium salt significantly increased the phosphorylated form of EIIANtr in cells (left panel of Fig. 8B), supporting the notion that a high ratio of α-ketoglutarate to glutamine stimulates the phosphorylation of the PTSNtr.
Fig. 8.
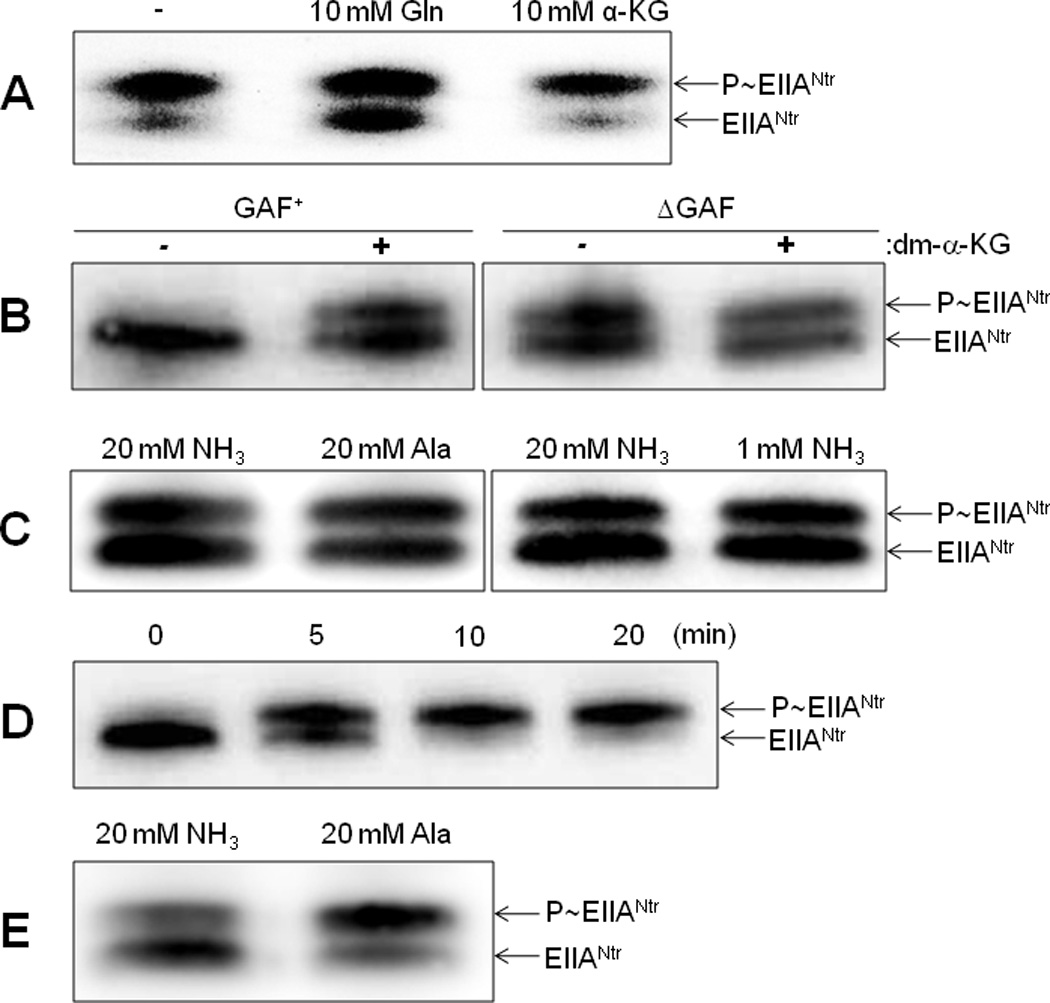
Metabolite effects on the in vivo phosphorylation state of EIIANtr. The intracellular phosphorylation state of EIIANtr(K75D) was determined in each strain as described in Experimental Procedures.
A. CR301 cells harboring the pCR3(K75D) plasmid expressing EIIANtr(K75D) were grown in W salts medium containing 20 mM alanine in the presence of 10 mM glutamine or 10 mM α-ketoglutarate.
B. CR301 (left panel, GAF+) and KM201 (right panel, ΔGAF) cells harboring the pCR3(K75D) plasmid were grown in W salts medium containing 20 mM (NH4)2SO4 and 0.2% glucose in the presence and absence of 10 mM dimethyl-α-ketoglutarate (dm-α-KG).
C. KM201/pCR3(K75D) cells were grown in W salts medium containing 0.2% glucose with 20 mM (NH4)2SO4, 20 mM alanine, or 1 mM (NH4)2SO4. The intracellular phosphorylation state of EIIANtr(K75D) was determined when A600 reached 0.8 in A–C.
D. Effect of nitrogen depeletion on the phosphorylation state of EIIANtr. CR301/pCR3(K75D) cells were grown in W salts medium containing 20 mM (NH4)2SO4 and 0.2% glucose to mid-logarithmic phase, then centrifuged, washed and resuspended in W salts medium containing 0.2% glucose with no nitrogen source. After incubation for the indicated time periods, intracellular phosphorylation states of EIIANtr were determined.
E. Effect of nitrogen availability on the PTSNtr activity in ilvG+ E. coli. CR303 cells harboring the plasmid pCR3(K75D) were grown in W salts medium containing 20 mM (NH4)2SO4 and 0.2% glucose to mid-logarithmic phase, then centrifuged, washed and resuspended in W salts medium containing 0.2% glucose with 20 mM (NH4)2SO4 or 20 mM alanine. When A600 reached 0.8, the intracellular phosphorylation state of EIIANtr(K75D) was determined as described in Experimental Procedures.
To test whether the GAF domain is necessary to sense nitrogen availability in vivo, we constructed a strain (KM201) deleted for both the ptsN gene (encoding EIIANtr) and that encoding the N-terminal 169 amino acids covering the GAF domain in the ptsP gene (encoding EINtr). Expression of the EINtr(ΔGAF) protein was confirmed by Western blotting with an antibody raised against EINtr (Fig. S6) and from the fact that EIIANtr(K75D) could be phosphorylated in the KM201 strain harboring the pCR3(K75D) plasmid whereas it was not phosphorylated in the ptsP deletion mutant (CR103) harboring pCR3(K75D) (compare Figs. 2A and 8C). The data in Fig. 8C show that the phosphorylation state of EIIANtr(K75D) is not influenced by the nitrogen sources in KM201/pCR3(K75D) cells. Furthermore, the effect of dm-α-KG was also dependent on the presence of the GAF domain (right panel of Fig. 8B), indicating the requirement of the GAF domain of EINtr for sensing nitrogen availability in vivo.
We further investigated how the phosphorylation state of EIIANtr changes when cells are moved from an ammonium-rich environment to nitrogen depletion. CR301 cells harboring pCR3(K75D) were grown in W salts medium with 0.2% glucose as carbon source and 20 mM ammonium ion as nitrogen source to mid-logarithmic phase, at which point the culture was subjected to nitrogen depletion by moving cells to the same medium lacking the nitrogen source. It is well-known that α-ketoglutarate concentration in E. coli is rapidly elevated within a few minutes of nitrogen depletion with a concomitant decrease by an order of magnitude in the glutamine level. We monitored the phosphorylation state of EIIANtr upon nitrogen depletion over the course of 20 minutes. While EIIANtr exists mainly in a dephosphorylated form in cells growing in ammonia-rich medium, EIIANtr was about 70% phosphorylated in 5 minutes and it was almost completely phosphorylated in 10 minutes after cells were shifted to a medium depleted of nitrogen source (Fig. 8D). These data support the model that glutamine and α-ketoglutarate together control the phosphorylation state of the PTSNtr in response to nitrogen availability in vivo.
In our previous study (Lee et al., 2007), we demonstrated that the dephosphorylated form of EIIANtr inhibited the Trk K+ transport system by forming a tight complex with TrkA resulting in a decrease of the intracellular concentrations of K+. Therefore, we determined whether the nitrogen source can influence intracellular K+ levels. As shown in Fig. 9, intracellular K+ levels in cells grown in the presence of 20 mM (NH4)2SO4 were about 30% lower than those grown in the presence of 20 mM alanine as the sole nitrogen source, while K+ levels were not affected by nitrogen sources in cells deleted for the GAF domain of EINtr. These data suggest that glutamine and α-ketoglutarate modulate cellular potassium levels by regulating the phosphorylation state of the PTSNtr and that the GAF domain of EINtr is essential for this regulation.
Fig. 9.
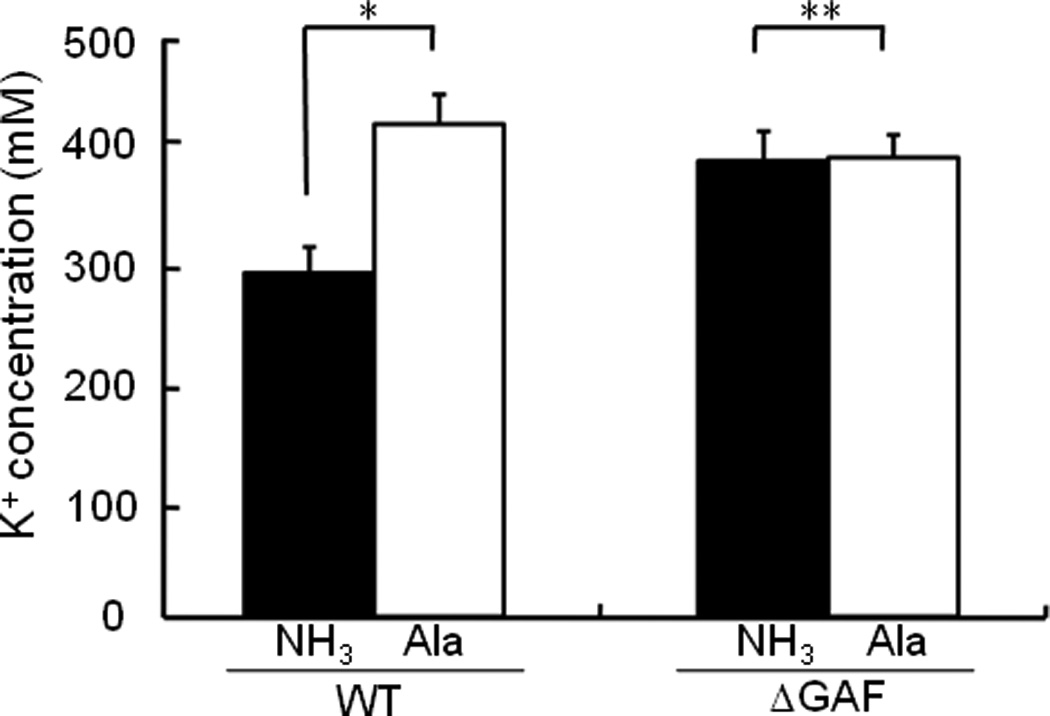
Effect of nitrogen source on the intracellular level of potassium. E. coli MG1655 (WT) and KM101 (ΔGAF) cells were grown in W salts medium supplemented with 20 mM (NH4)2SO4 or alanine as the sole nitrogen source to A600 = 0.5 and intracellular K+ concentrations were measured as described previously (Lee et al., 2007). Each column represents the mean ± S.D. of 5 measurements and statistical significance of differences between groups was analyzed by Student’s t test: *, p < 0.001; **, p > 0.1.
We previously indicated that some E. coli K-12 strains such as MG1655 are sensitive to leucine-containing peptides (LCP) due to a frameshift mutation in ilvG encoding acetohydroxy acid synthase (AHAS) II and have shown that a ptsN mutant is extremely sensitive whereas ptsP and ptsO mutants are more resistant than wild type MG1655 cells to LCPs (Lee et al., 2005). In E. coli, there are three isozymes of AHAS, which catalyze the first common step in the biosynthetic pathway of the three branched-chain amino acids. While AHAS I and III are sensitive to valine, AHAS II is valine-resistant. Because AHAS II is not expressed in some E. coli K-12 strains, these strains are extremely sensitive to valine (Lawther et al., 1981) and, albeit to a less extent, to LCPs (Gollop et al., 1982; Lee et al., 2005). Since LCPs in the medium induce the accumulation of abnormally high cellular concentrations of leucine for an unknown reason, biosynthesis of isoleucine and valine is feedback-inhibited in ilvG mutant cells; therefore, growth of E. coli MG1655 cells is retarded in the presence of LCPs. The extreme sensitivity of a ptsN mutant of E. coli MG1655 to LCPs was shown to be due to a further decrease in both the level and total activity of AHAS caused by the increase in cellular potassium; dephosphorylated EIIANtr was shown to be required to neutralize the sensitivity of E. coli K12 strains to LCPs (Lee et al., 2007). Therefore, we compared the KM101 strain, deleted for the GAF domain of EINtr, with wild-type MG1655 for sensitivity to the Ala-Leu dipeptide in the presence of different nitrogen sources, based on the length of the lag period for the growth of cultures. While the wild-type strain was more resistant to Ala-Leu in a nitrogen-rich medium than in a nitrogen-limited medium, the sensitivity of KM101 cells to Ala-Leu was not influenced by nitrogen sources; KM101 cells were equally sensitive to Ala-Leu in both nitrogen sources (Fig. 10). This result further supports the thesis that the GAF domain is necessary to sense nitrogen availability in vivo.
Fig. 10.
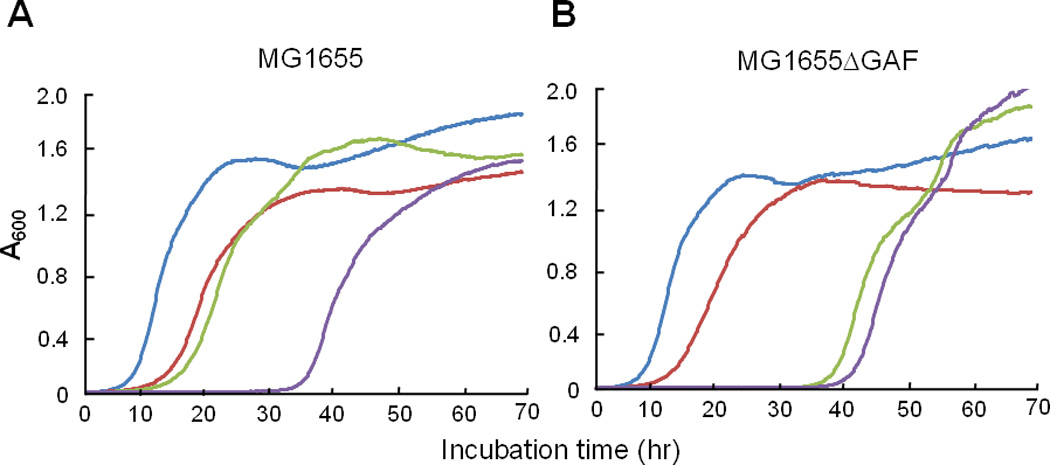
The GAF domain and nitrogen availability affect the sensitivity of E. coli MG1655 cells to Ala-Leu dipeptide. Wild-type (A) and KM101 (B) cells grown in LB medium overnight were washed with M9 medium, inoculated into W salts medium containing 0.5% glucose with 20 mM (NH4)2SO4 (blue) or 20 mM alanine (red), and growth was recorded by measuring the optical density at 600 nm. To test the effect of a leucine-containing peptide, 0.5 mM Ala-Leu was added into W salts medium containing 0.5% glucose with 20 mM (NH4)2SO4 (green) or 20 mM alanine (purple).
Sensing of nitrogen availability by the PTSNtr is independent of the ilvG genotype
Besides the sensitivity to leucine or LCPs, a growth defect of the ptsN mutant on certain organic nitrogen sources was recently shown to be observed only in E. coli strains lacking a functional ilvG gene (Reaves and Rabinowitz, 2011). Therefore, involvement of the PTSNtr in nitrogen regulation has been challenged (Ninfa, 2011). To clarify whether the effect of the ratio of glutamine to α-ketoglutarate on the PTSNtr activity is dependent on the ilvG genotype in vivo, we constructed an ilvG+ revertant of the E. coli MG1655 strain and tested effects of nitrogen sources on the phosphorylation state of the PTSNtr in cells. As shown in Fig. 8E, the dephosphorylated form of EIIANtr prevails in E. coli MG1655 ilvG+ cells supplemented with 20 mM ammonium sulfate, whereas exposure of these cells to the same concentration of alanine favors the phosphorylated state. These data show that the effect of glutamine and α-ketoglutarate on the phosphorylation state of the PTSNtr is independent of the ilvG genotype.
In aggregate, the studies presented here make a strong case that glutamine and α–ketoglutarate levels, as the canonical signals of nitrogen limitation, determine the state of phosphorylation of EIIANtr and that the mediator of the regulation is the GAF domain of EINtr.
Discussion
The work described here required the development of a method to evaluate the state of phosphorylation of EIIANtr. While the wild-type form of EIIAGlc exhibits a PDMS, that of EIIANtr does not (Fig. 1A). To search for the appropriate modification of EIIANtr that would result in a PDMS, systematic site-directed mutagenesis was carried out. The creation (K75D mutant) of a form of EIIANtr exhibiting both physiological activity (Fig. 1B) and a PDMS (Fig. 1A) provided the foundation for defining the mechanism by which the in vivo phosphorylation state of EIIANtr is regulated.
The measurement of the in vivo state of phosphorylation (Figs. 2 and 8) of EIIANtr(K75D) was consistent with the in vitro results (Figs. 3 and 4). The presence of glutamine increased while α–ketoglutarate decreased the level of the dephosphorylated form of EIIANtr(K75D). The overall results from both in vitro and in vivo tests are consistent with the model that glutamine and α–ketoglutarate serve as regulatory signals of the nitrogen PTS.
Rabus et al. previously searched for specific regulators of EINtr (Rabus et al., 1999), but effects of glutamine and α-ketoglutarate were not observed. Major differences between their and our current studies are that we used EINtr that was purified as a soluble form without denaturation as well as a direct protein phosphorylation assay while they used a protein solubilized with urea, then renatured, and an indirect mannitol phosphorylation assay.
It is interesting to note that, while EINtr autophosphorylation is stimulated by α-ketoglutarate (this study), the opposite effect (inhibition by α-ketoglutarate) appears to operate with the enzyme I of the sugar PTS (EIsugar) (Doucette et al., 2011). The present data demonstrate that the interaction of α-ketoglutarate with EINtr requires the presence of the GAF domain present in EINtr but not in EIsugar. The explanation for the GAF domain-independent interaction with EIsugar remains to be elucidated.
Despite the finding that the “nitrogen” PTS encoding NPr and EIIANtr was located in the same operon with rpoN encoding the nitrogen-related sigma factor and the observation of a growth defect of ptsN mutants on certain poor nitrogen sources (Powell et al., 1995), there has been little evidence that any component of the so-called PTSNtr is directly involved in nitrogen metabolism or influences the genetic regulatory system for nitrogen assimilation, the bona fide Ntr system. In the present work, the phosphorylation state of the nitrogen PTS was shown to be regulated by glutamine and α-ketoglutarate, the canonical signals of nitrogen metabolism. Autophosphorylation of EINtr was inhibited by glutamine and activated by α-ketoglutarate.
This regulatory mechanism is somewhat similar to that of the bona fide Ntr system, the genetic regulatory system for the activity of glutamine synthetase and transcription of other nitrogen-related genes; glutamine inhibits the uridylyltransferase activity of GlnD and the adenylyl-removing activity of GlnE and activates the uridylyl-removing activity of GlnD and the adenylyltransferase activity of GlnE to decrease nitrogen-regulated (Ntr) gene expression, whereas α-ketoglutarate exerts the opposite effects. α-Ketoglutarate also directly binds to PII and regulates the ability of PII to interact with GlnE and NRII to increase Ntr gene expression (Jiang et al., 1998; Jiang et al., 2007; Kamberov et al., 1995; Ninfa and Jiang, 2005). Our demonstration of the role of glutamine and α-ketoglutarate in regulating PTSNtr provides an important link to the regulation of nitrogen metabolism.
The finding that the regulatory effects of glutamine and α-ketoglutarate are mediated by EINtr places the locus of the regulatory signals at the first step of the nitrogen PTS pathway. The PTSNtr is highly conserved in all proteobacterial branches except for the ε-subdivision. A major structural difference between EI and EINtr is the presence of the GAF domain in EINtr. Until now, the function of the GAF domain in EINtr has been elusive. The finding that the regulatory effects of glutamine and α-ketoglutarate require the presence of the GAF domain has now explained the rationale for its presence in EINtr. GAF domains were initially found in cGMP phosphodiesterases, Anabaena adenylate cyclases, and E. coli FhlA and have been shown to be important for signal perception. The GAF domain (N-terminal 127 amino acids) of EINtr exhibits a homology throughout its length to the N-terminal GAF domains of NifA proteins of the free-living diazotrophs with 21–26% identity (Reizer et al., 1996) and displays a slightly less homology to sodium-responsive GAF domains in Anabaena adenylate cyclases, the formate-responsive GAF domain of E. coli FhlA, and cGMP phosphodiesterases. Intriguingly, the GAF domain of the transcriptional activator NifA was previously shown to directly bind α-ketoglutarate to resist inhibition by NifL under nitrogen-limiting condition in Azotobacter vinelandii, although the effect of glutamine was not tested (Little and Dixon, 2003). Therefore, binding of glutamine and α-ketoglutarate to GAF domains of EINtr and thus regulation of the phosphorylation of the PTSNtr in response to nitrogen availability might be widely conserved among proteobacteria.
Experimental Procedures
Bacterial strains, plasmids, and culture conditions
The bacterial strains and plasmids used in this study are listed in Table 1. All plasmids were constructed using standard PCR-based cloning procedures and verified by sequencing. Bacterial cells were grown as described previously (Lee et al., 2010). Escherichia coli MG1655 derivatives with deletions of the GAF domain (residues 1–169) of EINtr were constructed using the lambda red recombination method (Datsenko and Wanner, 2000). The kanamycin resistance (KmR) cassette from plasmid pKD13 was amplified using the following primers: forward primer, 5'- ACA CCA GGT GCT GCC GGT AAT GCG CGG ATT CGC ATC GCT TGG CGA TAT TGG TGT AGG CTG GCG CTG CTT-3' and reverse primer, 5'- CAC AAA ACG CAT CTG CTT ATC GAC GTA AAA GAG GTT AAG TCA CGC CAA TTA TTC CGG GGA TCC GTC GAC C-3'. A new ATG start codon was inserted in the forward primer (in boldface type) to express a truncated EINtr protein.
Table 1.
Escherichia coli strains and plasmids used in this study.
| Strain or plasmid | Genotype or phenotype | Source or Reference |
|---|---|---|
| Strains | ||
| MG1655 | F− λ− ilvG− rfb-50 rph-1. Wild type E. coli K-12 | (Blattner et al., 1997) |
| MG1655 ilvG+ | F− λ− rfb-50 rph-1 | This study |
| GI698 | F− λ − lacIq lacPL8 ampC::Ptrp cI | (LaVallie et al., 1993) |
| CR103 | MG1655 ptsP::cat ptsN::TetR | This study |
| CR301 | MG1655 ptsN::TetR | (Lee et al., 2005) |
| CR303 | MG1655 ilvG+ ptsN::TetR | This study |
| KM101 | MG1655 ΔGAF | This study |
| KM201 | MG1655 ptsN::TetR ΔGAF | This study |
| Plasmids | ||
| pRE1 | Expression vector under control of λPL promoter, Ampr | (Reddy et al., 1989) |
| pCR3 | pRE1-based expression vector for EIIANtr | (Lee et al., 2005) |
| pCR3(H73A) | pRE1-based expression vector for EIIANtr(H73A) | (Lee et al., 2005) |
| pCR3(K75D) | pRE1-based expression vector for EIIANtr(K75D) | This study |
| pCR1H | pRE1-based expression vector for EINtr with C-terminal 6 histidines | This study |
| pCR1H(ΔGAF) | pRE1-based expression vector for EINtr(ΔGAF) with N-terminal 6 histidines | This study |
| pCR3H | pRE1-based expression vector for EIIANtr with N-terminal 6 histidines | This study |
| pCR3H(K75D) | pRE1-based expression vector for EIIANtr(K75D) with N-terminal 6 histidines | This study |
| pCR3H(I69D) | pRE1-based expression vector for EIIANtr(I69D) with N-terminal 6 histidines | This study |
| pCR3H(A70D) | pRE1-based expression vector for EIIANtr(A70D) with N-terminal 6 histidines | This study |
| pCR3H(I71D) | pRE1-based expression vector for EIIANtr(I71D) with N-terminal 6 histidines | This study |
| pCR3H(P72D) | pRE1-based expression vector for EIIANtr(P72D) with N-terminal 6 histidines | This study |
| pCR3H(G74D) | pRE1-based expression vector for EIIANtr(G74D) with N-terminal 6 histidines | This study |
| pCR3H(L76D) | pRE1-based expression vector for EIIANtr(L76D) with N-terminal 6 histidines | This study |
| pPR3H(E86A) | pRE1-based expression vector for EIIAGlc(E86A) with N-terminal 6 histidines | This study |
| pKD13 | Template plasmid, Kmr Ampr | (Datsenko and Wanner, 2000) |
| pKD46 | Vector encoding arabinose-inducible λ-Red recombinase, Ampr | (Datsenko and Wanner, 2000) |
ilvG+ revertants of E. coli MG1655 were generated using the lambda red recombination method (Datsenko and Wanner, 2000). The functional ilvG gene was amplified from an E. coli BL21 strain by PCR and introduced into E. coli MG1655 harboring pKD46. E. coli MG1655 ilvG+ revertants were selected based on valine resistance by growing on M9 medium supplemented with 0.5% glucose and 0.2 mM valine. After the plasmid pKD46 was cured, the ilvG+ genotype was confirmed by DNA sequencing.
Purification of overexpressed proteins
Purification of soluble His-tagged proteins (His-EI, His-HPr, His-EIIAGlc, EINtr-His, His-EINtr(ΔGAF), His-EIIANtr and His-EIIANtr(K75D) was accomplished as described previously (Lee et al., 2005), with slight modifications. E. coli GI698 harboring specific plasmids were grown and protein expression was induced as described previously for overproduction (LaVallie et al., 1993). His-tagged proteins were purified using BD TALON™ metal affinity resin (BD Biosciences Clontech) according to the manufacturer’s instructions and bound proteins were eluted with binding buffer containing 200 mM imidazole. The fractions containing His-tagged proteins were pooled and concentrated in a 3K Macrosep centrifugal concentrator (Pall Gelman Laboratory). To obtain homogeneous proteins (>98% pure) and to remove imidazole, the concentrated pool was chromatographed on a HiLoad 16/60 Superdex 75 prepgrade column (GE Healthcare Life Sciences) equilibrated with buffer A (20 mM HEPES-KOH, pH 8.0 containing 200 mM NaCl). Note that purified EINtr containing the GAF domain has a tendency to aggregate (Piszczek et al., 2011). Therefore, meaningful studies of the properties of this protein are limited to relatively dilute solutions.
Purification of insoluble His-tagged proteins (His-EIIANtr(I69D), His-EIIANtr(A70D), His-EIIANtr(I71D), His-EIIANtr(P72D), His-EIIANtr(G74D) and His-EIIANtr(L76D)) was accomplished using 6 M urea as described previously (Kim et al., 2011) with some modifications. After disruption of cells, overexpressed proteins in inclusion bodies were solubilized with 6 M urea and centrifuged. The supernatant solution was mixed with 500 µl TALON metal affinity resin (BD Biosciences Clontech) and agitated for 20 min at 4 °C. The resin was then centrifuged and washed sequentially with 5 volumes of Buffer A containing 3, 1.5, 0.75, and 0 M urea. Proteins were then eluted with 1 volume of Elution Buffer (20 mM HEPES-KOH containing 300 mM NaCl and 200 mM imidazole, pH 8.0). The concentrated pool was chromatographed on a HiLoad 16/60 Superdex 75 prepgrade column to remove imidazole and any remaining urea.
In vitro phosphorylation assays
All reactions were performed with purified proteins in the presence of 1 mM PEP, 0.1 M Tris00B7HCl, pH 7.5, 2 mM MgCl2, 1 mM EDTA,10 mM KCl, 0.5 mM dithiothreitol in a total volume of 20 µl. To measure a PDMS of EIIAGlc, EIIAGlc (2 µg) was incubated with EI (1 µg) and HPr (1 µg) and to measure a PDMS of EIIANtr, EIIANtr (2 µg) was incubated with EINtr (1 µg) and NPr (1 µg). After incubation at 37 °C for 10 min, reactions were stopped by the addition of 5 µl of SDS-PAGE sample buffer (250 mM Tris·Cl, pH 6.8, 10% glycerol, 1% SDS, 150 mM 2-mercaptoethanol) and then analyzed by SDS PAGE. The proteins were stained with Coomassie Brilliant Blue.
To examine the effect of various metabolites on the phosphotransferase activity of the PTSNtr, EIIANtr(K75D) (3 µg) was incubated with trace amounts of EINtr or EINtr(ΔGAF) and NPr in the presence of each metabolite tested (5 mM). After incubation for the indicated times at room temperature, reactions were stopped by the addition of 5 µl of SDS-PAGE sample buffer and analyzed by 14% SDS-PAGE. The proteins were stained with Coomassie Brilliant Blue.
To test the effect of metabolites on the autophosphorylation of EINtr and EI Ntr(ΔGAF), 0.5 µg of purified proteins were incubated with [32P]PEP (20 µM) and various concentrations of metabolites. After incubation for 1 min at room temperature, reactions were stopped by the addition of 5 µl of SDS-PAGE sample buffer, and then analyzed by SDS-PAGE (4–20% gradient gel). The labeled proteins were visualized by autoradiography.
Measurement of the in vivo phosphorylation state of EIIANtr
To determine the in vivo phosphorylation state of EIIANtr, we made polyclonal antibodies against EIIANtr using female ICR mice. The CR301 and KM201 cells harboring the expression vector pCR3(K75D) (See Table 1) were grown in W salts medium containing 20 mM (NH4)2SO4 and 0.2% glucose to mid-logarithmic phase. Cells were harvested, washed and resuspended in W salts medium containing 0.2% glucose with different nitrogen sources as indicated in each figure and legend. After growth at 37 °C to A600 = 0.8 or mid-logarithmic phase, an aliquot (0.2 ml) of the cell culture was quenched to stabilize the phosphorylation state of the PTSNtr components by adding 20 µl of 10 M NaOH followed by vortexing for 10 s, and then 180 µl of 3 M sodium acetate (pH 5.2) and 1 ml of ethanol were added. Samples were chilled at −70 °C for at least 15 min, thawed and centrifuged at 4 °C. The pellet was rinsed with 70% ethanol and resuspended in 100 µl of SDS sample buffer, and 20 µl of this solution was run on SDS–PAGE (15% gel). Proteins were then electrotransferred onto immobilin-P (Millipore, MA) following the manufacturer's protocol and were detected by immunoblotting using antiserum against EIIANtr.
Intrinsic tryptophan fluorescence measurements
Fluorescence measurements were carried out with a CARY Eclipse fluorescence spectrophotometer with excitation at 280 nm at room temperature. The spectral bandwidths were 5 and 10 nm, respectively for excitation and emission. The emission spectrum was monitored between 300 and 400 nm. EINtr and EINtr(ΔGAF) were present at 95 µg/ml in 50 mM Tris (pH 7.5) containing 20 mM NaCl. For determination of the respective Kd values, various concentrations of metabolites (0.5–32 mM) were examined for the degree of fluorescence quenching.
Supplementary Material
Acknowledgements
This work was supported by the Korea Research Foundation Grant (NRF 2010-0017384) and the WCU program (R31-2009-000-10032-0) from Ministry of Education, Science, and Technology, and by the Marine and Extreme Genome Research Center Program of the Ministry of Land, Transportation and Maritime Affairs, Republic of Korea. C.R.L. was supported by BK21 Research Fellowship. A.P. was supported by the Intramural Research Program of NHLBI, National Institutes of Health.
Footnotes
Conflict of interest statements: The authors declare that they have no conflicts of financial interests.
References
- Bahr T, Lüttmann D, März W, Rak B, Görke B. Insight into bacterial phosphotransferase system-mediated signaling by interspecies transplantation of a transcriptional regulator. J Bacteriol. 2011;193:2013–2026. doi: 10.1128/JB.01459-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner FR, Plunkett G, 3rd, Bloch CA, Perna NT, Burland V, Riley M, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- Cases I, Velázquez F, de Lorenzo V. Role of ptsO in carbon-mediated inhibition of the Pu promoter belonging to the pWW0 Pseudomonas putida plasmid. J Bacteriol. 2001;183:5128–5133. doi: 10.1128/JB.183.17.5128-5133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Shin D, Yoon H, Kim J, Lee CR, Kim M, et al. Salmonella pathogenicity island 2 expression negatively controlled by EIIANtr-SsrB interaction is required for Salmonella virulence. Proc Natl Acad Sci USA. 2010;107:20506–20511. doi: 10.1073/pnas.1000759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci USA. 2000;97:6640–6645. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70:939–1031. doi: 10.1128/MMBR.00024-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette CD, Schwab DJ, Wingreen NS, Rabinowitz JD. α-Ketoglutarate coordinates carbon and nitrogen utilization via enzyme I inhibition. Nat Chem Biol. 2011;7:894–901. doi: 10.1038/nchembio.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollop N, Tavori H, Barak Z. Acetohydroxy acid synthase is a target for leucine containing peptide toxicity in Escherichia coli. J Bacteriol. 1982;149:387–390. doi: 10.1128/jb.149.1.387-390.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa F, Edelstein PH. Potential virulence role of the Legionella pneumophila ptsP ortholog. Infect Immun. 2001;69:4782–4789. doi: 10.1128/IAI.69.8.4782-4789.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogema BM, Arents JC, Bader R, Eijkemans K, Inada T, Aiba H, Postma PW. Inducer exclusion by glucose 6-phosphate in Escherichia coli. Mol Microbiol. 1998;28:755–765. doi: 10.1046/j.1365-2958.1998.00833.x. [DOI] [PubMed] [Google Scholar]
- Jiang P, Mayo AE, Ninfa AJ. Escherichia coli glutamine synthetase adenylyltransferase (ATase, EC 2.7.7.49): kinetic characterization of regulation by PII, PII-UMP, glutamine, and α-ketoglutarate. Biochemistry. 2007;46:4133–4146. doi: 10.1021/bi0620510. [DOI] [PubMed] [Google Scholar]
- Jiang P, Peliska JA, Ninfa AJ. Enzymological characterization of the signal-transducing uridylyltransferase/uridylyl-removing enzyme (EC 2.7.7.59) of Escherichia coli and its interaction with the PII protein. Biochemistry. 1998;37:12782–12794. doi: 10.1021/bi980667m. [DOI] [PubMed] [Google Scholar]
- Kamberov ES, Atkinson MR, Ninfa AJ. The Escherichia coli PII signal transduction protein is activated upon binding 2-ketoglutarate and ATP. J Biol Chem. 1995;270:17797–17807. doi: 10.1074/jbc.270.30.17797. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee CR, Kim M, Peterkofsky A, Seok YJ. Dephosphorylated NPr of the nitrogen PTS regulates lipid A biosynthesis by direct interaction with LpxD. Biochem Biophys Res Commun. 2011;409:556–561. doi: 10.1016/j.bbrc.2011.05.044. [DOI] [PubMed] [Google Scholar]
- Koo BM, Yoon MJ, Lee CR, Nam TW, Choe YJ, Jaffe H, et al. A novel fermentation/respiration switch protein regulated by enzyme IIAGlc in Escherichia coli. J Biol Chem. 2004;279:31613–31621. doi: 10.1074/jbc.M405048200. [DOI] [PubMed] [Google Scholar]
- Lüttmann D, Göpel Y, Görke B. The phosphotransferase protein EIIANtr modulates the phosphate starvation response through interaction with histidine kinase PhoR in Escherichia coli. Mol Microbiol. 2012;86:96–110. doi: 10.1111/j.1365-2958.2012.08176.x. [DOI] [PubMed] [Google Scholar]
- Lüttmann D, Heermann R, Zimmer B, Hillmann A, Rampp IS, Jung K, Görke B. Stimulation of the potassium sensor KdpD kinase activity by interaction with the phosphotransferase protein IIANtr in Escherichia coli. Mol Microbiol. 2009;72:978–994. doi: 10.1111/j.1365-2958.2009.06704.x. [DOI] [PubMed] [Google Scholar]
- LaVallie ER, DiBlasio EA, Kovacic S, Grant KL, Schendel PF, McCoy JM. A thioredoxin gene fusion expression system that circumvents inclusion body formation in the E. coli cytoplasm. Biotechnology (NY) 1993;11:187–193. doi: 10.1038/nbt0293-187. [DOI] [PubMed] [Google Scholar]
- Lawther RP, Calhoun DH, Adams CW, Hauser CA, Gray J, Hatfield GW. Molecular basis of valine resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1981;78:922–925. doi: 10.1073/pnas.78.2.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Cho SH, Kim HJ, Kim M, Peterkofsky A, Seok YJ. Potassium mediates Escherichia coli enzyme IIANtr-dependent regulation of sigma factor selectivity. Mol Microbiol. 2010;78:1468–1483. doi: 10.1111/j.1365-2958.2010.07419.x. [DOI] [PubMed] [Google Scholar]
- Lee CR, Cho SH, Yoon MJ, Peterkofsky A, Seok YJ. Escherichia coli enzyme IIANtr regulates the K+ transporter TrkA. Proc Natl Acad Sci USA. 2007;104:4124–4129. doi: 10.1073/pnas.0609897104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CR, Koo BM, Cho SH, Kim YJ, Yoon MJ, Peterkofsky A, Seok YJ. Requirement of the dephospho-form of enzyme IIANtr for derepression of Escherichia coli K-12 ilvBN expression. Mol Microbiol. 2005;58:334–344. doi: 10.1111/j.1365-2958.2005.04834.x. [DOI] [PubMed] [Google Scholar]
- Lee JW, Helmann JD. Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. J Biol Chem. 2006;281:23567–23578. doi: 10.1074/jbc.M603968200. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jeong CS, An YJ, Lee HJ, Park SJ, Seok YJ, et al. FrsA functions as a cofactor-independent decarboxylase to control metabolic flux. Nat Chem Biol. 2011;7:434–436. doi: 10.1038/nchembio.589. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Boos W, Bouche JP, Plumbridge J. Signal transduction between a membrane-bound transporter, PtsG, and a soluble transcription factor, Mlc, of Escherichia coli. EMBO J. 2000;19:5353–5361. doi: 10.1093/emboj/19.20.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R, Dixon R. The amino-terminal GAF domain of Azotobacter vinelandii NifA binds 2-oxoglutarate to resist inhibition by NifL under nitrogen-limiting conditions. J Biol Chem. 2003;278:28711–28718. doi: 10.1074/jbc.M301992200. [DOI] [PubMed] [Google Scholar]
- Lux R, Jahreis K, Bettenbrock K, Parkinson JS, Lengeler JW. Coupling the phosphotransferase system and the methyl-accepting chemotaxis protein-dependent chemotaxis signaling pathways of Escherichia coli. Proc Natl Acad Sci USA. 1995;92:11583–11587. doi: 10.1073/pnas.92.25.11583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michiels J, Van Soom T, D'Hooghe I, Dombrecht B, Benhassine T, de Wilde P, Vanderleyden J. The Rhizobium etli rpoN locus: DNA sequence analysis and phenotypical characterization of rpoN, ptsN, and ptsA mutants. J Bacteriol. 1998;180:1729–1740. doi: 10.1128/jb.180.7.1729-1740.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam TW, Cho SH, Shin D, Kim JH, Jeong JY, Lee JH, et al. The Escherichia coli glucose transporter enzyme IICBGlc recruits the global repressor Mlc. EMBO J. 2001;20:491–498. doi: 10.1093/emboj/20.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam TW, Park YH, Jeong HJ, Ryu S, Seok YJ. Glucose repression of the Escherichia coli sdhCDAB operon, revisited: regulation by the CRP*cAMP complex. Nucleic Acids Res. 2005;33:6712–6722. doi: 10.1093/nar/gki978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa AJ. Unnecessary signaling: poorly named? J Bacteriol. 2011;193:4571–4573. doi: 10.1128/JB.05682-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninfa AJ, Atkinson MR. PII signal transduction proteins. Trends Microbiol. 2000;8:172–179. doi: 10.1016/s0966-842x(00)01709-1. [DOI] [PubMed] [Google Scholar]
- Ninfa AJ, Jiang P. PII signal transduction proteins: sensors of α-ketoglutarate that regulate nitrogen metabolism. Curr Opin Microbiol. 2005;8:168–173. doi: 10.1016/j.mib.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Park YH, Lee BR, Seok YJ, Peterkofsky A. In vitro reconstitution of catabolite repression in Escherichia coli. J Biol Chem. 2006;281:6448–6454. doi: 10.1074/jbc.M512672200. [DOI] [PubMed] [Google Scholar]
- Peterkofsky A, Wang G, Seok YJ. Parallel PTS systems. Arch Biochem Biophys. 2006;453:101–107. doi: 10.1016/j.abb.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Pflüger-Grau K, Görke B. Regulatory roles of the bacterial nitrogen-related phosphotransferase system. Trends Microbiol. 2010;18:205–214. doi: 10.1016/j.tim.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Pflüger K, de Lorenzo V. Growth-dependent phosphorylation of the PtsN (EIINtr) protein of Pseudomonas putida. J Biol Chem. 2007;282:18206–18211. doi: 10.1074/jbc.M611110200. [DOI] [PubMed] [Google Scholar]
- Piszczek G, Lee JC, Tjandra N, Lee CR, Seok YJ, Levine RL, Peterkofsky A. Deuteration of Escherichia coli enzyme INtr alters its stability. Arch Biochem Biophys. 2011;507:332–342. doi: 10.1016/j.abb.2010.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell BS, Court DL, Inada T, Nakamura Y, Michotey V, Cui X, et al. Novel proteins of the phosphotransferase system encoded within the rpoN operon of Escherichia coli. Enzyme IIANtr affects growth on organic nitrogen and the conditional lethality of an erats mutant. J Biol Chem. 1995;270:4822–4839. doi: 10.1074/jbc.270.9.4822. [DOI] [PubMed] [Google Scholar]
- Prell J, Mulley G, Haufe F, White JP, Williams A, Karunakaran R, et al. The PTSNtr system globally regulates ATP-dependent transporters in Rhizobium leguminosarum. Mol Microbiol. 2012;84:117–129. doi: 10.1111/j.1365-2958.2012.08014.x. [DOI] [PubMed] [Google Scholar]
- Rabus R, Reizer J, Paulsen I, Saier MH., Jr Enzyme INtr from Escherichia coli. A novel enzyme of the phosphoenolpyruvate-dependent phosphotransferase system exhibiting strict specificity for its phosphoryl acceptor, NPr. J Biol Chem. 1999;274:26185–26191. doi: 10.1074/jbc.274.37.26185. [DOI] [PubMed] [Google Scholar]
- Reaves ML, Rabinowitz JD. Characteristic phenotypes associated with ptsN-null mutants in Escherichia coli K-12 are absent in strains with functional ilvG. J Bacteriol. 2011;193:4576–4581. doi: 10.1128/JB.00325-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Peterkofsky A, McKenney K. Hyperexpression and purification of Escherichia coli adenylate cyclase using a vector designed for expression of lethal gene products. Nucleic Acids Res. 1989;17:10473–10488. doi: 10.1093/nar/17.24.10473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J, Reizer A, Merrick MJ, Plunkett G, 3rd, Rose DJ, Saier MH., Jr Novel phosphotransferase-encoding genes revealed by analysis of the Escherichia coli genome: a chimeric gene encoding an Enzyme I homologue that possesses a putative sensory transduction domain. Gene. 1996;181:103–108. doi: 10.1016/s0378-1119(96)00481-7. [DOI] [PubMed] [Google Scholar]
- Segura D, Espin G. Mutational inactivation of a gene homologous to Escherichia coli ptsP affects poly-β-hydroxybutyrate accumulation and nitrogen fixation in Azotobacter vinelandii. J Bacteriol. 1998;180:4790–4798. doi: 10.1128/jb.180.18.4790-4798.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senior PJ. Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: studies with the continuous-culture technique. J Bacteriol. 1975;123:407–418. doi: 10.1128/jb.123.2.407-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seok YJ, Sondej M, Badawi P, Lewis MS, Briggs MC, Jaffe H, Peterkofsky A. High affinity binding and allosteric regulation of Escherichia coli glycogen phosphorylase by the histidine phosphocarrier protein, HPr. J Biol Chem. 1997;272:26511–26521. doi: 10.1074/jbc.272.42.26511. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Kimata K, Aiba H. A novel regulatory role of glucose transporter of Escherichia coli: membrane sequestration of a global repressor Mlc. EMBO J. 2000;19:5344–5352. doi: 10.1093/emboj/19.20.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chen Y, Potvin E, Sanschagrin F, Levesque RC, McCormack FX, Lau GW. Comparative signature-tagged mutagenesis identifies Pseudomonas factors conferring resistance to the pulmonary collectin SP-A. PLoS Pathog. 2005;1:259–268. doi: 10.1371/journal.ppat.0010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem. 2000;275:25046–25051. doi: 10.1074/jbc.M002526200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


