Abstract
Purpose
Diet is a potentially modifiable risk factor for Barrett's esophagus (BE). We investigated the associations between intakes of fruits and vegetables and risk of BE.
Methods
We identified study subjects from 1,859 participants who underwent the endoscopy in a single VA Medical Center in the U.S between 2008 and 2011. Dietary intake in the previous year was elicited using a self-administered Block food frequency questionnaire (FFQ). Logistic regression model was used to estimate odds ratio (OR) and its 95% confidence interval (CI) for BE.
Results
A total of 151 cases with definite BE and 777 controls completed the FFQ. When highest tertile of intake was compared with the lowest, the OR (95% CI) was 0.46 (0.26-0.81) for dark green vegetables, 0.52 (0.30- 0.90) for legumes, 0.50 (0.28-0.90) for total fiber, 0.45 (0.25-0.81) for isoflavones, 0.52 (0.30- 0.67) for total folate, and 0.45 (0.26-0.79) for lutein, adjusting for multiple confounding factors including use of aspirin or proton pump inhibitor, gastro-esophageal reflux symptoms, and physical activity. The association for dark green vegetables was attenuated after adjustment for lutein, total fiber, and total folate (OR = 0.82; 95% CI, 0.30-2.22).
Conclusion
Higher intake of dark green vegetables was associated with a decreased risk of BE in a veteran population. Such an inverse association may be partially mediated by lutein, fiber and folate. The novel findings on the association between intake of lutein, total folate, or isoflavones and risk of BE need further confirmation.
Keywords: diet, Barrett's esophagus, risk, case-control
Introduction
Dietary factors are potentially modifiable risk factors for both Barrett's esophagus (BE) and esophageal adenocarcinoma (EAC). However, the studies on diet and risk of BE are relatively limited. Among four population-based case-control studies that have examined diet and risk of BE [1-4], two studies from the U.S. showed a significant inverse association between total fruit and vegetable intake and risk of BE [1,2], while two studies from Ireland (the FINBAR study) did not find such an association [3,4].
Fruits and vegetables are sources of many nutrient components including fiber, antioxidants, and folate, which may explain the possible protective effect of fruits or vegetables on risk of BE. Two population-based case-control studies reported an inverse association between total fiber intake and the risk of BE [5,6]. However, the study findings on antioxidants were inconsistent. The U.S. study reported an inverse association with vitamin C, vitamin E, and β-carotene [1] whereas the FINBAR study found that the overall antioxidant index, a measure of the combined intake of vitamin C, vitamin E, total carotenoids, and selenium, was associated with a reduced risk of EAC, but not of BE [4]. Likewise, high intake of folate-containing foods was associated with a reduced risk of EAC, but not with BE in one population-based case-control study in Australia [7].
Given the inconsistent findings of the few published studies, more studies are required to examined the association between fruits and vegetables and their nutrient components and risk of BE. Greater understanding of the dietary factors that modify the risk of BE may assist with developing preventive strategies for BE as well as for EAC. The primary purpose of this study was to evaluate fruits, vegetables, total fiber, folate, and antioxidants in association with risk of BE in an endoscopy clinic-based case-control study.
Material and methods
Study design and study population
To obtain the cases and controls, we first conducted two cross-sectional studies in subjects seeking health care at the Michael E. DeBakey Veterans Affairs Medical Center (MEDVAMC) in Houston, Texas, United States from February 1, 2008 to July 31, 2011. One of the cross-sectional studies was conducted among consecutive eligible patients undergoing an elective upper endoscopy, and the other was conducted among patients eligible for a screening colonoscopy who were randomly identified at primary care facilities in the MEDVAMC and agreed to undergo an upper endoscopy examination at the same time as their colonoscopy.
This study was approved by the Institutional Review Boards at both Baylor College of Medicine and the MEDVAMC in 2008.
Cases and controls
All study participants had to be 40-80 years of age (and 50-80 years for primary care patients), and undergo an upper endoscopy in which the findings of suspected BE cases were systematically recorded. We excluded participants with the following conditions prior to enrollment: 1) previous gastro-esophageal surgery; 2) previous/current neoplastic epithelial diseases; 3) currently taking anticoagulants; 4) platelet count <70,000, ascites, or known gastro-esophageal varices; or 5) history of major stroke or mental condition that could limit ability to obtain valid information from the interview.
Cases were subjects who were diagnosed with definite BE as confirmed by the presence of intestinalized columnar epithelia (confirmed by Alcian-PAS stain) in at least one biopsy samples obtained from tubular esophagus. Participants with only suspected BE, i.e., those not confirmed on histopathology, were excluded from this analysis. Subjects who were not diagnosed with a definitive or suspected BE made up our control group. The majority of the control subjects were enrolled from the upper endoscopy clinic (endoscopy controls), and the remaining control subjects were enrolled from the colonoscopy clinic (colonoscopy controls). We further excluded the participants who did not respond to the Block food frequency questionnaire (FFQ), and then those with the reported daily energy intake < 800 Kcal and > 5000 Kcal because their responses to FFQs were considered to be unreliable [8].
Study measurements
We interviewed all study participants before the endoscopy to collect data on age, sex, ethnicity, current and previous smoking status, alcohol consumption, physical activity, medical history, frequency of medication use including proton pump inhibitors (PPI) and aspirin, and onset, frequency and severity of gastro-esophageal reflux (GER) symptoms (heartburn and acid regurgitation). Height and weight were measured prior to endoscopy and were used to calculate body mass index (BMI) as well as waist and hip circumference to calculate waist-to-hip ratio (WHR).
We used the 110-item Block FFQ (version 2005) to ascertain dietary intake during the previous year. The Block FFQ is a self-administered instrument that takes approximately 40-50 minutes to complete and has been extensively used and validated in epidemiologic research [9]. If study subjects could not complete the FFQ in the endoscopy visit due to the time constraint, they were asked to mail the completed FFQ back after the visit. Participants were asked to report their usual frequency of consumption of various foods and typical portion sizes. Each food item had nine options for frequency (ranging from “never or less than once per month” to “2+ times per day”) and three options for portion size (small, medium, and large). Pictures were provided to enhance accuracy of quantification of portion size. The Block FFQ was also used to ascertain the frequency and dosage of use of vitamin supplements over the previous year. The raw data of FFQ were processed at the NutritionQuest service. The USDA MyPyramid Equivalents Database (MPED) version 1.0 [10] was merged with the FFQ data to calculate cup equivalents for fruits and vegetables (1 cup = 237 mL). In general, a cup equivalent is 1 cup of raw or cooked vegetable or fruit and 2 cups of raw leafy salad greens, and 4 ounce equivalents for dry peas and beans (legumes).
Statistical analysis
Tertiles of each food and nutrient intake were generated based on the distributions of the intake in the combined control group. Folate intake was analyzed according to the following four sources: 1) food folate (a combination of natural folate and fortified folic acid), 2) fortified folic acid (folic acid added to food), 3) supplemental folic acid (folic acid from vitamin supplements), and 4) total folate intake (a combination of food folate and supplemental folic acid). We calculated an antioxidant score by adding up the decile category of each nutrient for each subject. For example, a subject with an antioxidant intake in the first decile for each of the eight micronutrients (vitamin C, vitamin E, α-carotene, β-carotene, selenium, cryptoxanthin, lutein and lycopene) had an antioxidant score equal to 4, while a subject in the tenth decile for each micronutrient had an antioxidant score equal to 40, as previously described [2,3].
We compared the cases with the combined group of controls in terms of demographic and lifestyle factors using χ2 test or two sample Student's t test. The daily average intake of foods or nutrients (total fruits, total as well as components of vegetables, total fiber, folate, isoflavones, and antioxidants) was energy adjusted using the density method [11]. Correlations between intake of vegetables and their nutrient components were evaluated by Spearman's rank correlation coefficients.
We used unconditional multivariate logistic regression model to obtain odds ratios (ORs) and their 95% confidence intervals (CIs) for BE while controlling for potential confounders. The lowest tertile was used as the reference category. The base model contained the fixed variables of age and energy intake (all continuous). The final model further contained sex, ethnicity, smoking status (never, former and current), alcohol consumption (never, former, and current), regular aspirin use in the previous three months, PPI use any time in the past, frequency of GER symptoms, BMI (continuous), WHR (tertile), and physical activity (low, moderate, and high according to metabolic equivalent of task (MET) for recreational activity). These variables changed the β value for risk estimate by >10% or they are potential risk factors for BE [12]. For antioxidants, we examined the association between individual antioxidants as well as the antioxidant score and risk of BE. A linear trend test was performed to examine for differences across tertiles.
Several additional analyses were conducted. First, we examined whether the major nutrient components of vegetables explained the observed association between vegetables intake and risk of BE by including the components individually and jointly in the multivariate models. A mediation effect was evaluated. Second, saturated fat intake was evaluated as a potential confounding factor because saturated fat intake increased risk of BE in our study population. The OR was 1.78 (95% CI, 1.02-3.11) for the third tertile of intake compared with the lowest. Third, stratified analyses were performed according to the presence of GER symptoms (never and ever). Finally, we examined the association comparing cases with each control group separately (endoscopy versus colonoscopy controls). The cutoff points for tertile were derived separately according to the distribution of the foods/nutrients in each control group.
All analyses were conducted using STATA 12.0 (Stata Corporation, College Station, TX). We considered 2-sided probability of P ≤ 0.05 as statistically significant.
Results
We identified 1,859 participants comprising of 237 cases with definite BE, 122 cases with suspected (endoscopic finding only) BE, and 1,500 individuals with no BE (1,021 endoscopy controls and 479 colonoscopy controls). We excluded 122 cases with suspected BE, and then 697 participants who did not complete the FFQ. The response rate to the Block FFQ was 70% for the definite cases, 56% for the endoscopy controls and 62% for the colonoscopy controls. Those who did not complete the FFQ were two years older on average, and more likely to be African American or current smokers, had lower self-reported physical activity, than those who completed the FFQ. We further excluded 112 individuals with daily energy intake of < 800 or > 5000 Kcal. Therefore, our present analysis was based on 928 study participants: 151 cases with definite BE and 777 controls that consisted of 521 endoscopy controls and 256 colonoscopy controls.
Colonoscopy controls were older, more likely to be African American men, had lower physical activity and higher BMI, and reported less use of PPI or aspirin and less GER symptoms than endoscopy controls. However, the differences between two control groups did not affect the direction or the significance of the examined association between diet and risk of BE. We therefore present the results derived from comparing the BE cases with two control groups combined.
Most study participants (> 90%) were men as expected in this VA population. Compared with the combined control group, the cases were more likely to be older, Caucasian, or report more frequent PPI use and GER symptoms while controls reported more frequent use of aspirin (Table 1). Cases and controls did not differ significantly in BMI, but cases had a higher average WHR than controls. The distributions of education levels, smoking status, and alcohol consumption were not significantly different between cases and controls.
Table 1.
Selected characteristics of cases and the combined control group
| Characteristics % or mean (SD) | Casesa n =151 | Controlsa n = 777 | P valueb |
|---|---|---|---|
| Age (n (%)) | 0.03 | ||
| 40-50 | 4 (2.6) | 52 (6.7) | |
| 50-60 | 41 (27.2) | 256 (33.0) | |
| 60-70 | 87 (57.6) | 353 (45.4) | |
| 70-79 | 19 (12.6) | 116 (14.9) | |
| Sex (n (%)) | 0.01 | ||
| Men | 147 (97.4) | 713 (91.8) | |
| Women | 4 (2.6) | 64 (8.2) | |
| Racial group (n (%)) | < 0.0001 | ||
| Non-Hispanic White | 127 (84.1) | 435 (56.0) | |
| Non-Hispanic Black | 12 (8.0) | 252 (32.5) | |
| Hispanic White | 8 (5.3) | 54 (6.9) | |
| Others | 2 (1.3) | 25 (3.2) | |
| Unknown | 2 (1.3) | 11 (2.4) | |
| Education levels (n (%)) | 0.26 | ||
| High school or less | 18 (12.7) | 121 (16.5) | |
| Tech school/diploma | 95 (66.9) | 484 (66.7) | |
| University or beyond | 30 (21.4) | 119 (16.8) | |
| Physical activity in the past weekc (n (%)) | 0.05 | ||
| Low | 91 (63.2) | 519 (71.1) | |
| Moderate | 23 (16.0) | 115 (15.7) | |
| High | 30 (20.8) | 99 (13.2) | |
| Smoking status (n (%)) | 0.26 | ||
| Never smokers | 32 (22.5) | 201 (27.7) | |
| Former smokers | 72 (50.7) | 316 (43.5) | |
| Current smokers | 38 (26.8) | 209 (28.8) | |
| Alcohol consumption (n (%)) | 0.38 | ||
| Never drinker | 11 (7.6) | 69 (9.4) | |
| Former drinker | 53 (36.8) | 296 (40.5) | |
| Current drinker | 80 (55.6) | 367 (50.1) | |
| Aspirin use in the past 3 months (yes, %) | 42.2 | 50.3 | 0.07 |
| PPI use ever (yes, %) | 70.6 | 50.1 | < 0.001 |
| Frequency of GER symptom (yes, %) | 0.04 | ||
| Never | 21.4 | 34.5 | |
| ≤ 1 per month | 9.66 | 11.9 | |
| ≥ 1 per week | 35.2 | 31.4 | |
| Daily | 33.8 | 22.2 | |
| Body mass index, mean (SD) | 30.3 (5.6) | 30.0 (5.9) | 0.65 |
| Waist to hip ratio, mean (SD) | 0.98 (0.07) | 0.95 (0.06) | < 0.0001 |
GER, gastro-esophageal reflux; PPI, proton pump inhibitors; SD, standard deviation.
The number may not add up to a total because of the missing value.
P value for χ2 test for categorical variables or t test for continuous variables.
Table 2 shows the associations between risk of BE and daily MyPyramid servings (cup equivalent) of fruits and vegetables in multivariate analyses. Intake of total vegetables, in particular dark green vegetables and legumes, was associated with a strong risk reduction of BE. The risk of BE in the participants with highest intake of total fiber and isoflavones was lower than that for those with the lowest intake. Neither total fruit (including juice) nor other types of vegetables intake was associated with risk of BE.
Table 2.
Risk of Barrett's esophagus and daily intake of fruit, vegetable, fiber, and isoflavones (151 cases and 777 controls)
| Food groupa (cup/1000 Kcal) | Tertile 1 | Tertile 2 | Tertile 3 | P valued |
|---|---|---|---|---|
| Total fruit (including juice) | ||||
| Median (Range) | 0.16 (<0.26) | 0.36 (0.26-0.49) | 0.67(0.49-2.25) | |
| Case/control | 63/259 | 43/259 | 45/259 | |
| ORb (95% CI) | 1.00 | 0.64 (0.41-0.99) | 0.68 (0.44-1.05) | 0.07 |
| ORc (95% CI) | 1.00 | 0.66 (0.40-1.10) | 0.81 (0.47-1.38) | 0.35 |
| Total vegetables | ||||
| Median (Range) | 0.77 (<1) | 1.28 (1-1.58) | 2.12 (1.58-8.04) | |
| Case/control | 53/259 | 59/259 | 39/259 | |
| ORb (95% CI) | 1.00 | 1.12 (0.73-1.71) | 0.74 (0.47-1.18) | 0.22 |
| ORc (95% CI) | 1.00 | 0.94 (0.56-1.56) | 0.61 (0.35-1.06) | 0.08 |
| Dark green vegetables | ||||
| Median (Range) | 0.04 (<0.08) | 0.12 (0.08-0.18) | 0.28 (0.18-1.85) | |
| Case/control | 64/259 | 52/259 | 35/259 | |
| ORb (95% CI) | 1.00 | 0.80 (0.53-1.22) | 0.51 (0.32-0.81) | 0.005 |
| ORc (95% CI) | 1.00 | 0.84 (0.50-1.39) | 0.46 (0.26-0.81) | 0.008 |
| Orange vegetables | ||||
| Median (Range) | 0.02 (<0.03) | 0.04 (0.03-0.06) | 0.09 (0.06-0.58) | |
| Case/control | 53/259 | 54/259 | 44/259 | |
| ORb (95% CI) | 1.00 | 1.16 (0.73-1.84) | 0.76 (0.46-1.27) | 0.31 |
| ORc (95% CI) | 1.00 | 1.01 (0.61-1.69) | 0.76 (0.43-1.32) | 0.34 |
| White potatoes | ||||
| Median (Range) | 0.06 (<0.08) | 0.10 (0.08-0.14) | 0.20 (0.14-0.82) | |
| Case/control | 42/259 | 55/259 | 54/259 | |
| ORb (95% CI) | 1.00 | 1.24 (0.76-2.02) | 1.30 (0.80-2.14) | 0.30 |
| ORc (95% CI) | 1.00 | 1.09 (0.63-1.86) | 1.08 (0.62-1.88) | 0.78 |
| Legumes | ||||
| Median (Range) | 0.02 (<0.03) | 0.04 (0.03-0.07) | 0.12 (0.07-1.82) | |
| Case/control | 57/259 | 61/259 | 42/259 | |
| ORb (95% CI) | 1.00 | 1.06 (0.70-1.62) | 0.75 (0.48-1.19) | 0.24 |
| ORc (95% CI) | 1.00 | 0.89 (0.54-1.48) | 0.52 (0.30-0.90) | 0.02 |
| Other starchy vegetables | ||||
| Median (Range) | 0.31 (< 0.40) | 0.53 (0.41-0.68) | 0.93 (0.68-3.87) | |
| Case/control | 51/259 | 57/259 | 43/259 | |
| ORb (95% CI) | 1.00 | 1.09 (0.71-1.67) | 0.84 (0.53-1.32) | 0.46 |
| ORc (95% CI) | 1.00 | 0.90 (0.54-1.52) | 0.77 (0.45-1.33) | 0.36 |
| Other vegetables | ||||
| Median (Range) | 0.21 (< 0.28) | 0.34 (0.28-0.42) | 0.58 (0.42-2.50) | |
| Case/control | 57/259 | 61/259 | 42/259 | |
| ORb (95% CI) | 1.00 | 0.97 (0.63-1.49) | 0.85 (0.54-1.32) | 0.48 |
| ORc (95% CI) | 1.00 | 0.88 (0.52-1.49) | 0.68 (0.39-1.17) | 0.16 |
| Total fiber (gram) | ||||
| Median (Range) | 5.84 (< 6.8) | 7.76 (6.83-8.96) | 11.0 (8.9-32.1) | |
| case/control | 59/259 | 60/259 | 41/259 | |
| ORb (95% CI) | 1.00 | 1.02 (0.69-1.53) | 0.64 (0.41-1.01) | 0.06 |
| ORc (95% CI) | 1.00 | 1.12 (0.67-1.85) | 0.50 (0.28-0.90) | 0.03 |
| Isoflavones (mg) | ||||
| Median (Range) | 0.36 (< 0.49) | 0.60 (0.49-0.75) | 1.01(≥ 0.75) | |
| case/control | 71/259 | 46/259 | 43/259 | |
| ORb (95% CI) | 1.00 | 1.22 (0.80-1.84) | 0.63 (0.39-1.01) | 0.07 |
| ORc (95% CI) | 1.00 | 1.12 (0.68-1.86) | 0.45 (0.25-0.81) | 0.008 |
Please refer to reference 10 for food composition. For example, dark green vegetables include broccoli, kale, mustard greens, collard greens, romaine lettuce, spinach, turnip greens, mustard cabbage, watercress, arugula, balsam-pear tips, beet greens, bitter melon leaves, , chard, chicory, cilantro, cress, dandelion greens, endive, escarole, grape leaves, lambsquarters, parsley, poke greens, pumpkin leaves, sweet potato leaves, and taro leaves.
OR was adjusted for age and energy intake.
OR was adjusted for age, energy intake, sex, ethnicity, smoking status, alcohol consumption, waist to hip ratio (tertile), recent use of aspirin, ever or never use of proton pump inhibitor, frequency of GER symptoms, and physical activity (low, moderate, and high according to metabolic equivalent of task).
P value for trend across tertiles.
Table 3 presents the findings on the association between folate intake and BE. A significant inverse association was seen for total folate intake, and possibly folate from food. We did not observe a significant association for folic acid supplement nor fortified folate from foods.
Table 3.
Risk of Barrett's esophagus and daily intake of folate (151 cases and 777 controls)
| Food groups | Tertile 1 | Tertile 2 | Tertile 3 | P valuec |
|---|---|---|---|---|
| Folate from foods (μg) | ||||
| Median (Range) | 92.2 (<109) | 125 (109-141) | 168 (141-542) | |
| case/control | 59/259 | 49/259 | 43/259 | |
| ORa (95% CI) | 1.00 | 0.81(0.53-1.24) | 0.67 (0.43-1.05) | 0.08 |
| ORb (95% CI) | 1.00 | 0.85(0.51-1.41) | 0.58 (0.33-1.00) | 0.05 |
| Fortified folic acid (μg) | ||||
| Median (Range) | 37.3 (<48.6) | 58.9 (48.6-70.7) | 94.3 (70.7-588) | |
| case/control | 62/259 | 50/259 | 39/259 | |
| ORa (95% CI) | 1.00 | 0.81 (0.53-1.24) | 0.66 (0.42-1.03) | 0.07 |
| ORb (95% CI) | 1.00 | 0.75 (0.45-1.24) | 0.74 (0.43-1.28) | 0.25 |
| Folate acid supplement (μg) | ||||
| Dose | 0 | < 400 | ≥ 400 | |
| case/control | 69/383 | 12/77 | 70/317 | |
| ORa (95% CI) | 1.00 | 0.97 (0.50-1.90) | 1.24 (0.85-1.80) | 0.27 |
| ORb (95% CI) | 1.00 | 1.21 (0.56-2.59) | 1.19 (0.76-1.88) | 0.45 |
| Total folate (μg) | ||||
| Median (Range) | 179 (< 209) | 230 (209-260) | 316 (260-1123) | |
| case/control | 67/259 | 45/259 | 39/259 | |
| ORa (95% CI) | 1.00 | 0.68 (0.44-1.04) | 0.57 (0.36-0.88) | 0.01 |
| ORb (95% CI) | 1.00 | 0.59 (0.36-1.01) | 0.52 (0.30-0.67) | 0.02 |
OR was adjusted for age and energy intake.
OR was adjusted for age, energy intake, sex, ethnicity, smoking status, alcohol consumption, waist to hip ratio (tertile), ever or never use of aspirin, ever or never use of proton pump inhibitor, frequency of the GER symptoms, and physical activity (low, moderate, and high according to metabolic equivalent of task).
P value for trend across tertiles.
Table 4 presents the association between antioxidants and risk of BE. Among the eight antioxidants examined, only highest intake of vitamin E or lutein was associated with reduced risk of BE. The second, but not the highest tertile of vitamin C intake, was associated with significantly lower risk of BE. A higher antioxidant score derived from the intake of these eight antioxidants was also associated with statistically significant lower risk of BE. The OR was 0.95 (95% CI, 0.90-0.99) for each unit increment of the score. The overall score ranges from 8 to maximum 79 in our study.
Table 4.
Risk of Barrett's esophagus and daily intake of antioxidants (151 cases and 777 controls)
| Antioxidants | Tertile 1 | Tertile 2 | Tertile 3 | P valuec |
|---|---|---|---|---|
| Vitamin C (mg) | ||||
| Median (Range) | 25.1 (<34.7) | 42.5 (34.7-54.2) | 73.3 (54.2-298) | |
| Case/control | 70/259 | 36/259 | 45/259 | |
| ORa (95% CI) | 1.00 | 0.57 (0.37-0.87) | 0.64 (0.42-0.98) | 0.03 |
| ORb (95% CI) | 1.00 | 0.52 (0.30-0.89) | 0.79 (0.47-1.34) | 0.28 |
| Vitamin E (mg) | ||||
| Median (Range) | 2.95 (<3.4) | 3.77 (3.4-4.2) | 4.93 (4.2-16.4) | |
| Case/control | 60/259 | 54/259 | 37/259 | |
| ORa (95% CI) | 1.00 | 0.80 (0.53-1.20) | 0.61 (0.39-0.94) | 0.03 |
| ORb (95% CI) | 1.00 | 0.90 (0.54-1.48) | 0.46 (0.26-0.83) | 0.01 |
| α-carotene (μg) | ||||
| Median (Range) | 79.2 (<117) | 157 (117-217) | 347 (218-2190) | |
| Case/control | 52/259 | 44/259 | 55/259 | |
| ORa (95% CI) | 1.00 | 1.00 (0.65-1.54) | 1.00 (0.65-1.53) | 0.99 |
| ORb (95% CI) | 1.00 | 0.82 (0.49-1.40) | 0.88 (0.52-1.49) | 0.64 |
| β-carotene (μg) | ||||
| Median (Range) | 863 (<1245) | 1665 (1245-2164) | 2976 (1264-12316) | |
| Case/control | 60/259 | 50/259 | 41/259 | |
| ORa (95% CI) | 1.00 | 0.81 (0.53-1.22) | 0.64 (0.41-0.98) | 0.04 |
| ORb (95% CI) | 1.00 | 0.89 (0.54-1.49) | 0.64 (0.37-1.10) | 0.12 |
| Cryptoxanthin (μg) | ||||
| Median (Range) | 27.4 (<41.2) | 55.9 (41.2-74.5) | 115 (74.5-414.9) | |
| Case/control | 67/259 | 37/259 | 47/259 | |
| ORa (95% CI) | 1.00 | 0.58 (0.36-0.89) | 0.69 (0.46-1.04) | 0.06 |
| ORb (95% CI) | 1.00 | 0.58 (0.34-0.99) | 0.93 (0.56-1.56) | 0.54 |
| Lutein (μg) | ||||
| Median (Range) | 628 (<941) | 1267 (941-1691) | 2565 (1691-11771) | |
| Case/control | 66/259 | 47/259 | 38/259 | |
| ORa (95% CI) | 1.00 | 0.71 (0.47-1.08) | 0.55 (0.35-0.85) | 0.006 |
| ORb (95% CI) | 1.00 | 0.81 (0.49-1.34) | 0.45 (0.26-0.79) | 0.007 |
| Selenium (μg) | ||||
| Median (Range) | 40.1 (<46.4) | 50.4 (46.4-54.4) | 60.9 (54.4-119) | |
| Case/control | 51/259 | 47/259 | 53/259 | |
| ORa (95% CI) | 1.00 | 0.85 (0.55-1.33) | 0.95 (0.62-1.46) | 0.81 |
| ORb (95% CI) | 1.00 | 0.87 (0.51-1.48) | 0.99 (0.58-1.68) | 0.87 |
| Lycopene (μg) | ||||
| Median (Range) | 1064 (<1556) | 1987 (1556-2558) | 3505 (2558-27772) | |
| Case/control | 52/272 | 49/272 | 57/273 | |
| ORa (95% CI) | 1.00 | 0.95 (0.61-1.46) | 1.06 (0.69-1.61) | 0.79 |
| ORb (95% CI) | 1.00 | 0.82 (0.48-1.40) | 0.75 (0.45-1.26) | 0.28 |
| Antioxidant score | ||||
| Median (Range) | 28 (8-38) | 45 (39-51) | 60 (52-79) | |
| case/control | 69/257 | 42/260 | 40/2260 | |
| ORa (95% CI) | 1.00 | 0.58 (0.37-0.89) | 0.52 (0.33-0.80) | 0.003 |
| ORb (95% CI) | 1.00 | 0.44 (0.25-0.78) | 0.60 (0.36-1.00) | 0.05 |
ORs were adjusted for age and energy intake.
ORs were adjusted for age, energy intake, sex, ethnicity, smoking status, alcohol consumption, waist to hip ratio (tertile), ever or never use of aspirin, ever or never use of proton pump inhibitor, frequency of GER symptoms (i.e., heartburn and acid regurgitation), and physical activity (low, moderate, and high according to metabolic equivalent of task).
P value for trend across tertiles.
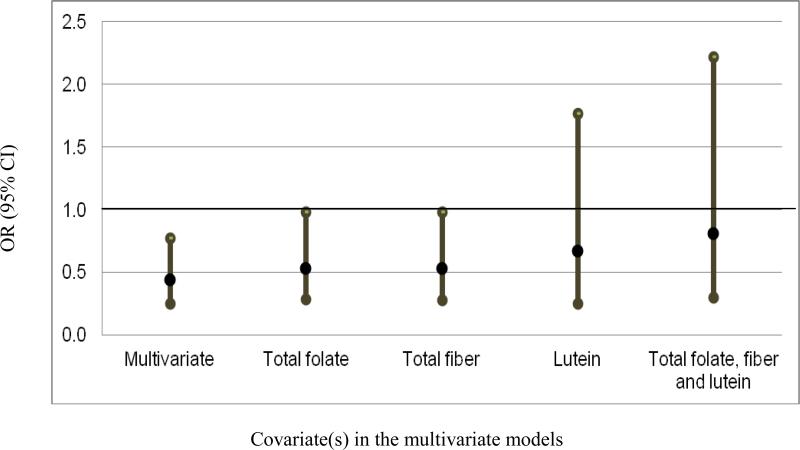
We further explored the potential explanatory effect of total fiber, total folate, vitamin E, and lutein on the observed association between dark green vegetables and risk of BE. Intake of dark green vegetable had a significant positive correlation with intakes of lutein (r = 0.93), vitamin E (r = 0.52), total fiber (r = 0.51) or total folate (r = 0.44). Based on the multivariate OR (OR = 0.46; 95% CI, 0.26-0.81) presented in Table 2, the further adjustment for total fiber, total folate or lutein individually attenuated the association between dark green vegetables and BE by 20%, 20%, and 50%, respectively. The OR for dark green vegetables was 0.69 (95% CI, 0.26-1.84) when lutein was included in the model and was 0.82 (95% CI, 0.30-2.22) when total folate and total fiber were included in the model additionally. The mediation analysis showed that the proportion of total effect mediated by these three factors was 48%. Figure 1 presents the ORs and their 95% CIs of BE for the highest tertile of intake of dark green vegetables compared with the lowest in the multivariate model, and in models subsequently adjusted for total folate, total fiber and/or lutein. Adjustment for vitamin E did not change the risk estimate.
Fig 1.
The OR and its 95% CI of BE with the highest tertile of dark green vegetable intake compared with the lowest in a multivariate model adjusted for age, energy intake, sex, ethnicity, smoking status, alcohol consumption, waist to hip ratio, aspirin use, PPI use, frequency of GER symptoms, and physical activity; and in models that additionally adjusted for dietary intake of total folate, total fiber, and lutein individually and in combination (all variables were on a tertile scale).
The main findings were robust in several additional analyses. The adjustment for saturated fat did not change the risk estimates presented in Tables 2 to 4 by more than 10%. The inverse associations with the consumption of dark green vegetables, total fiber, total folate, isoflavones, vitamin E, and lutein were observed among patients who reported having (31 cases and 255 controls) or not having (114 cases and 484 controls) GER symptoms. Lastly, we examined the association by comparing BE cases with each control group separately. We found that the inverse association was stronger when the comparisons were made with the colonoscopy control group. Specifically, when the endoscopy or colonoscopy controls were used, the ORs (95% CIs) of BE were 0.55 (0.30-1.01) versus 0.38 (0.16-0.90) for total fiber intake; 0.52 (0.29-0.92) versus 0.35 (0.15-0.80) for total folate intake, and 0.44 (0.24-0.79) versus 0.38 (0.17-0.85) for lutein intake, respectively.
Discussion
In this endoscopy clinic-based case-control study evaluating diet and risk of BE, we confirmed the previous finding on an inverse association between total vegetable intake and risk of BE. Specifically, we found an inverse association between intake of dark green vegetables or legumes and risk of BE. Total fiber, total folate, antioxidants lutein may partially mediate the association between dark green vegetables and risk of BE.
We found that daily consumption of greater than 1.58 cup/per 1000 Kcal of total vegetable and 0.18 cup/per 1000 Kcal of dark green vegetables was associated with a reduced risk of BE. Total vegetables intake has been associated with a reduced risk of BE in two previous population-based case-control studies in the U. S [1,2]. In one study of 170 hospital-based cases and 182 population controls in Washington State, total vegetable intake above the lower tertile was associated with 60-70% risk reduction for BE. The inverse association between total fruit intake alone and risk of BE was not statistically significant [2]. In the Kaiser Permanente study of 296 cases, total fruit and vegetable intake was inversely associated with the risk of BE compared with 309 population controls; but not compared with 308 GER disease controls [1]. In the population-based FINBAR study of 224 cases and 260 controls, the inverse association between fruit and vegetable intake and risk of BE was attenuated after adjustment for GER disease [3]. Dark green vegetable intake and risk of BE was not evaluated in these three studies. However, intake of dark green vegetables or leafy vegetables has been consistently associated with risk reduction of EAC [13]. No study has reported the analysis on legume intake in association with BE. We found that legume intake greater than 0.07 cup/1000 Kcal per day was associated with a reduced risk of BE. Nevertheless, a randomized clinical trial of 87 men and women with BE did not find any effect of dietary intervention (reduction of energy and fat and increase of vegetable and fruit intake) on biomarkers of cell proliferation in BE [14]. However, the direct relevance of the intermediate markers to the intervention was unclear.
In our study, total fiber intake in the highest tertile was associated with an approximate 50% reduction in BE risk. This observation was consistent with two previous studies [5,6]. The Kaiser Permanente study reported that only fiber from fruits and vegetables (but not grains or beans) was associated with lower risk of BE [6]. We could not further examine the source of fiber because of missing information for 70% of study subjects.
Folate is a water-soluble B vitamin that occurs naturally in dark green leafy vegetables, legumes and citrus fruits. A meta-analysis showed that high folate intake is significantly associated with a reduced risk of EAC [15]. The association between folate intake and risk of BE has not been well studied. We found that total folate intake and possibly folate from food were inversely associated with risk of BE. There was no significant association with the use of folic acid supplement. One population-based case-control study in Australia found that high intake of folate-containing foods did not reduce risk of BE, whereas the use of folic acid supplement increased risk of BE [7]. Further studies on genetic polymorphisms of folate and one-carbon metabolizing genes may help understand the role of folate in the development of BE.
Oxidative stress plays a role in the pathogenesis and neoplastic progression of BE [16]. Consistent with the Kaiser Permanente study [1], we showed that dietary vitamin E and the comprehensive antioxidant score were inversely associated with risk of BE. On the other hand, the FINBAR study did not find a significant association between antioxidants and risk of BE [4]. In a molecular epidemiologic study of 36 patients with BE, 32 patients with erosive esophagitis, and 35 patient controls, patients with BE had significantly lower plasma concentrations of selenium, vitamin C, β cryptoxanthin, and lutein/xanthophyll compared with the other groups [17]. Therefore, the data are conflicting on the role of antioxidants in BE development, with more evidence suggesting a protective role of vitamin C, E, and lutein. However, in our study, the second, but not the third tertile, of vitamin C intake was associated with lower risk of BE.
We further found that the possible beneficial effect of intake of dark green vegetables was explained mostly by intake of lutein, and to a less extent, by total fiber and total folate. Lutein is one of the xanthophyll carotenoids found in large quantities in dark green leafy vegetables such as spinach, kale, turnip greens, and collard greens. It is more hydrophilic and hence more readily absorbed than other carotenoids in blood and tissues [18]. One Italian study of 304 cases and 743 controls found that dietary intake of lutein assessed by FFQ was associated with 60% decreased risk of esophageal squamous cell carcinoma [19]. However, one case-cohort study nested in a prospective study in China did not find an association between serum levels lutein/xanthophylls and risk of esophageal squamous cell carcinoma [20]. More studies of lutein in BE and esophageal cancer are needed.
The strengths of our studies included the adjustment of multiple potential confounders such as PPI and aspirin use, GER symptoms and physical activity. Our study had low possibility of recall bias given that the self-administrated FFQ was completed without knowing the report for biopsy. We included only histopathologically diagnosed BE to avoid outcome misclassification.
The limitations of this case-control study included: 1) the inability to make causal inferences from the observed associations or offer possible explanations for all the associations;.2) the possible non-differential misclassifications of dietary intake due to inaccurate self-reported assessment that may drive the estimates towards the null; 3) selection bias in the endoscopy controls. It is possible that the patients recommended for upper endoscopy would already have experienced the symptoms of GER and therefore changed their diet. Such reverse causation bias could mean that the inverse association observed between dark green vegetables intake and risk of BE was diluted by using the endoscopy controls. Selection bias was a less concern in the colonoscopy controls because the study subjects were randomly selected from the primary care clinic and were recommend mostly for screening colonoscopy. Although the characteristics of the two controls groups used in this study were not entirely the same in terms of age, BMI, GER symptom, and PPI use, the risk estimates derived from two control groups were similar thus did not compromise the internal validity of the study; 4) although our findings on the association between intake of vegetable, fiber, or antioxidant and risk of BE were consistent with previous studies [1,5], we did not detect the association with intake of fruit as observed in previous studies [1,2]. The range of the intake of certain foods in our study population, such as total fruit, was below the recommended levels (2 cups daily) [10]. Therefore, the beneficial effect of intake of certain nutrients/foods on risk of BE may not be detectable if there is a threshold for such an effect. In addition, the design was different across existing studies of dietary risk factors for BE in terms of the study settings, control groups, tools for diet ascertainment, and scale of measurement of food consumption. These differences may have contributed to the discrepant findings among these (including ours) observational studies. Likewise, in this study comprised of mostly men of lower socioeconomic status, the findings may not be generalized to women or wealthier individuals; 5) non-response bias may have occurred. We had to exclude 37% of the study participants who did not respond to the self-administered FFQ. In our study, the control groups had slightly lower response rate than cases. If the non-response was related to the unfavorable lifestyle such as an unhealthy diet, the risk estimates may be biased towards the null. Lastly, we examined multiple nutrients in this study, and the positive findings may have been due to type 1 error.
In summary, our study suggested that an increased consumption of dark green vegetables and legumes may be beneficial for preventing BE. Our novel findings of the inverse association between intake of total folate, lutein and isoflavones and risk of BE need confirmation in large and prospective studies.
Acknowledgments
Thanks to Liang Chen for her assistance in preparing the manuscript.
This work was supported by grant R01-116845 (to El-Serag, HB) from the National Cancer Institute, National Institutes of Health. Hashem B. El-Serag is supported by grant K24-04-107 from National Institute of Diabetes and Digestive and Kidney Diseases. This work is supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, and the Houston VA Health Services Research and Development Center of Excellence (HFP90-020), and by the Texas Digestive Disease Center NIH DK58338. The funding agencies did not influence the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviation
- BE
Barrett's esophagus
- BMI
Body mass index
- CI
Confidence interval
- EAC
Esophageal adenocarcinoma
- FFQ
Food frequency questionnaire
- GER
Gastro-esophageal reflux
- MEDVAMC
Michael E. DeBakey Veterans Affairs Medical Center
- MET
Metabolic equivalent of task
- MPED
MyPyramid Equivalents Database
- OR
Odds ratio
- PPI
Proton pump inhibitors
- SD
Standard deviation
- WHR
Waist-to-hip ratio
Footnotes
Conflict of interest: The authors declare that they have no conflict of interest.
Contributor Information
Li Jiao, Houston VA Health Services Research and Development Center of Excellence, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA; Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
Jennifer R. Kramer, Houston VA Health Services Research and Development Center of Excellence, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
Massimo Rugge, Surgical Pathology & Cytopathology Unit, Department of Medicine-DIMED, University of Padova, Padova, Italy.
Paola Parente, Casa Sollievo della Sofferenza, Department of Pathology, San Giovanni Rotondo, Italy.
Gordana Verstovsek, Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
Abeer Alsarraj, Houston VA Health Services Research and Development Center of Excellence, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA; Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
Hashem B. El-Serag, Houston VA Health Services Research and Development Center of Excellence, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas, USA Department of Medicine, Baylor College of Medicine, Houston, Texas, USA.
References
- 1.Kubo A, Levin TR, Block G, et al. Dietary antioxidants, fruits, and vegetables and the risk of Barrett's esophagus. Am J Gastroenterol. 2008;103:1614–1623. doi: 10.1111/j.1572-0241.2008.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson OM, Beresford SA, Kirk EA, Vaughan TL. Vegetable and fruit intakes and risk of Barrett's esophagus in men and women. Am J Clin Nutr. 2009;89:890–896. doi: 10.3945/ajcn.2008.26497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson LA, Watson RG, Murphy SJ, et al. Risk factors for Barrett's esophagus and esophageal adenocarcinoma: results from the FINBAR study. World J Gastroenterol. 2007;13:1585–1594. doi: 10.3748/wjg.v13.i10.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Murphy SJ, Anderson LA, Ferguson HR, et al. Dietary antioxidant and mineral intake in humans is associated with reduced risk of esophageal adenocarcinoma but not reflux esophagitis or Barrett's esophagus. J Nutr. 2010;140:1757–1763. doi: 10.3945/jn.110.124362. [DOI] [PubMed] [Google Scholar]
- 5.Mulholland HG, Cantwell MM, Anderson LA, et al. Glycemic index, carbohydrate and fiber intakes and risk of reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma. Cancer Causes Control. 2009;20:279–288. doi: 10.1007/s10552-008-9242-6. [DOI] [PubMed] [Google Scholar]
- 6.Kubo A, Block G, Quesenberry CP, Buffler P, Corley DA. Effects of dietary fiber, fats, and meat intakes on the risk of Barrett's esophagus. Nutr Cancer. 2009;61:607–616. doi: 10.1080/01635580902846585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibiebele TI, Hughes MC, Pandeya N, et al. High intake of folate from food sources is associated with reduced risk of esophageal cancer in an Australian population. J Nutr. 2011;141:274–283. doi: 10.3945/jn.110.131235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willett WC. Nutritional Epidemiology. 2nd ed. Oxford University Press; New York: 1998. [Google Scholar]
- 9.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America's Table Study. Am J Epidemiol. 2001;154:1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 10.Friday JE, Bowman SA. MyPyramid Equivalents Database for USDA Survey Food Codes, 1994-2002 Version 1.0. USDA; Agricultural Research Service; Beltsville Human Nutrition Research Center; Community Nutrition Research Group; Beltsville, MD: 2006. [Google Scholar]
- 11.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado G, Greenland S. Simulation study of confounder-selection strategies. Am J Epidemiol. 1993;138:923–936. doi: 10.1093/oxfordjournals.aje.a116813. [DOI] [PubMed] [Google Scholar]
- 13.Kubo A, Corley DA, Jensen CD, Kaur R. Dietary factors and the risks of oesophageal adenocarcinoma and Barrett's oesophagus. Nutr Res Rev. 2010;23:230–246. doi: 10.1017/S0954422410000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kristal AR, Blount PL, Schenk JM, et al. Low-fat, high fruit and vegetable diets and weight loss do not affect biomarkers of cellular proliferation in Barrett esophagus. Cancer Epidemiol Biomarkers Prev. 2005;14:2377–2383. doi: 10.1158/1055-9965.EPI-05-0158. [DOI] [PubMed] [Google Scholar]
- 15.Larsson SC, Giovannucci E, Wolk A. Folate intake, MTHFR polymorphisms, and risk of esophageal, gastric, and pancreatic cancer: a meta-analysis. Gastroenterology. 2006;131:1271–1283. doi: 10.1053/j.gastro.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Sihvo EI, Salminen JT, Rantanen TK, et al. Oxidative stress has a role in malignant transformation in Barrett's oesophagus. Int J Cancer. 2002;102:551–555. doi: 10.1002/ijc.10755. [DOI] [PubMed] [Google Scholar]
- 17.Clements DM, Oleesky DA, Smith SC, et al. A study to determine plasma antioxidant concentrations in patients with Barrett's oesophagus. J Clin Pathol. 2005;58:490–492. doi: 10.1136/jcp.2004.023721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van het Hof KH, Brouwer IA, West CE, et al. Bioavailability of lutein from vegetables is 5 times higher than that of beta-carotene. Am J Clin Nutr. 1999;70:261–268. doi: 10.1093/ajcn.70.2.261. [DOI] [PubMed] [Google Scholar]
- 19.Franceschi S, Bidoli E, Negri E, et al. Role of macronutrients, vitamins and minerals in the aetiology of squamous-cell carcinoma of the oesophagus. Int J Cancer. 2000;86:626–631. doi: 10.1002/(sici)1097-0215(20000601)86:5<626::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 20.Abnet CC, Qiao YL, Dawsey SM, et al. Prospective study of serum retinol, beta-carotene, beta-cryptoxanthin, and lutein/zeaxanthin and esophageal and gastric cancers in China. Cancer Causes Control. 2003;14:645–655. doi: 10.1023/a:1025619608851. [DOI] [PubMed] [Google Scholar]
- 21.Craig CL, Marshall AL, Sjostrom M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 22.IPAQ Research Committee . Guidelines for data processing and analysis of the international physical activity questionnaire (IPAQ) Huddinge; Sweden: 2005. [Google Scholar]