Abstract
Objectives
Prior studies of gender differences in AAA repair suggest there may be differences in presentation, suitability for EVAR, and outcomes between men and women.
Methods
We used the Vascular Study Group of New England database to identify all patients undergoing EVAR or open AAA repair (OAR). We analyzed demographics, comorbidities, and procedural, and perioperative data. Results were compared using Fisher’s exact test and student’s t-test. Multivariable logistic regression and Cox proportional hazards modeling was performed to identify predictors of mortality.
Results
We identified 4,026 patients who underwent AAA repair (78% male, 54% EVAR). Women were less likely than men to undergo EVAR for intact aneurysms (50% vs. 60% of intact AAA repairs of, P<.001) but not for ruptured aneurysms (26% vs. 20%, P=.23). Women were older (median age 75 vs. 72 years for intact, P<.001; 78 vs. 73 years for rupture, P<.001) with smaller aortic diameters (57 vs. 59mm for elective, P<.001; 71 vs. 79mm for rupture, P<.001). Arterial injury was more common in women (5.4% vs. 2.7%, P=0.013) among patients undergoing EVAR for intact aneurysms and women stayed in the hospital longer (4.3 vs. 2.7 days, P=.018) and had a lower odds of being discharged home, even after adjusting for age.. Among patients undergoing open repair for intact aneurysms, women more frequently experienced leg ischemia/emboli (4% vs. 1%, P=.001) and bowel ischemia (5% vs. 3%, P=.044). Women had higher 30-day mortality after OAR for both intact (4% vs. 2%, P=.03) and rupture (48% vs. 34%, P=.03) repairs. However, 30-day mortality after EVAR was similar for both intact (1% in men vs. 1% in women, P=.57) and rupture (29% in men vs. 27% in women, P=1.00) repairs. Late survival was worse in women than men only for patients undergoing open repair of ruptured aneurysms (HR 1.8, 95% CI 1.0–3.1, P=.04). After controlling for age, type of repair, urgency at presentation (i.e. elective/intact vs. ruptured), comorbidities, and other relevant risk factors, gender was not predictive of 30-day or 1-year mortality.
Conclusion
Women with AAA are being treated at older ages and smaller diameters, and undergoing rupture repair at smaller diameters than men. Women are more likely to experience perioperative complications as a result of less favorable vascular anatomy. Age >80 years, comorbidity, presentation, and type of repair are more important predictors of mortality than gender.
INTRODUCTION
Although abdominal aortic aneurysm (AAA) is a disease primarily of men with a 4:1 male to female predominance1, women who develop AAAs tend to fare worse than men. Women with AAAs are older, have faster growing aneurysms, a 3–4 fold higher rupture risk, and rupture at smaller diameters than men2–5). When undergoing repair, women are less likely to undergo the less morbid endovascular approach (EVAR)6. Even when EVAR is undertaken in women, it has been suggested that the survival gain over open repair by women is inferior to gains observed in men7. Regardless of the urgency of presentation or type of repair, studies frequently show that women have worse morbidity and both short- and long-term mortality, even after adjustment for age and comorbidities8–11.
Theories about the etiology of these disparities abound. Differences in hormonal milieu result in delayed presentation in women and different degrees of atherosclerosis and matrix metalloproteinase production12. The vascular anatomy of women may limit their eligibility for EVAR and leave them more prone to vessel injury and procedural complications13. Further, because women are generally smaller in body size, some have argued that an aneurysm of a certain diameter in a woman represents more advanced disease than the same sized aneurysm in a man14, 15. Lastly, it is documented that women have a higher rate of undiagnosed cardiovascular disease16 and are undertreated even when comorbidities are recognized, leaving them at higher risk of perioperative cardiovascular events17.
There is still no formal consensus as to whether men and women with AAAs should be managed differently. Our objective was therefore to describe differences in the presentation, choice of repair, and mortality among men and women undergoing AAA repair. Although the outcomes we studied are not unique, the strength of this study lies in the database, which is not only large but also comprehensive of both academic and community hospitals, contains detailed clinical information not found in administrative databases, and has a representative proportion of women.
METHODS
We performed a retrospective review of open and endovascular AAA repairs in the Vascular Study Group of New England (VSGNE) database. The VSGNE is a voluntary collaboration among vascular surgeons, cardiologists, and radiologists from 30 academic and community hospitals in all 6 New England states. Formed as a quality improvement initiative, it represents a pool of clinical data related to several frequently performed vascular procedures that has been collected since 2003. In addition to detailed perioperative and procedural information, longitudinal outcomes are available at one year. Further details about this registry are available at http://www.vsgne.org. At the time of this analysis, there were 1,867 open and 2,159 endovascular repairs between 2003 and 2011 recorded in the VSGNE.
Patient presentation was categorized as either intact (including patients who were symptomatic as well as those undergoing elective repair) or ruptured. Four subgroup analyses were performed: intact EVAR, ruptured EVAR, intact open repair, and ruptured open repair. For the purposes of multivariable modeling, intact EVAR served as the referent group.
We compared nine postoperative complications between men and women. Acute myocardial infarction (MI) was documented if any of the following were present: isolated troponin elevation, EKG change, or clinical evidence of MI. Postoperative dysrhythmias were defined as new rhythm disturbances that required treatment with medications or cardioversion. Postoperative congestive heart failure was defined as pulmonary edema with requirement for monitoring or treatment in an intensive care unit (ICU) or step-down unit. Respiratory complications consisted of pneumonia (lobar infiltrate on CXR and pure growth of recognized pathogen or 4+ growth of recognized pathogen in presence of mixed growth) or ventilator requirement after initial extubation. Change in renal function was defined as a creatinine rise > .5mg/dl or the institution of dialysis (peritoneal dialysis, hemodialysis, or hemo-filtration), whether temporary or permanent. Leg ischemia/emboli was defined as the loss of previously palpable pulses, loss of previously present Doppler signals, decrease of >0.15 in ABI, blue toe, or tissue loss. Criteria for diagnosing bowel ischemia included colonoscopic evidence of ischemia, bloody stools in a patient who died before colonoscopy or laparotomy could be performed, or a presumptive diagnosis with conservative treatment. Wound complications ranged from a superficial separation of the incision to problems requiring return to the operating room. Any complication that required a trip to the operating room was tabulated. Bleeding complications included minor hematomas, those that required fresh frozen plasma or hemostatic agents to be administered, and those that required reoperation or other invasive intervention to control.
Comparisons between genders were made using the Pearson χ2 and Fisher’s exact test for categorical variables and the student’s t-test or Wilcoxon rank sum test for continuous variables. Multivariable logistic regression was used to determine predictors of 30-day mortality. Individual survival curves for each presentation/treatment group were evaluated for differences in survival between men and women. Cox proportional hazards modeling was used to determine predictors of long-term mortality. Survival was measured by postoperative number of days until death or most recent follow-up. For regression modeling, presentation/treatment subgroup was categorized into a single variable using intact EVAR as the referent group. Statistical significance was defined as a p-value of <.05. Analyses were performed using Stata version 12.0 (Stata Corp, College Station, Tex). This study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center.
RESULTS
We identified 4,026 patients who underwent AAA repair, 78% of whom were male (Table I). Rupture was the presenting indication for repair in 11% of men and 9% of women. Women undergoing intact repair were 3 years older than men (median age 75 vs. 72 years, P<.001) while those undergoing ruptured repair were 5 years older (median age 78 vs. 73 years, P<.001). As a proportion of all repairs, use of EVAR increased from 40% in 2003 to 73% in 2011. Men were more likely to undergo EVAR than open repair for intact repairs (60% EVAR for men vs. 50% EVAR for women, P<0.001), but not ruptured repairs (20% EVAR for men vs. 26% EVAR for women, P=.234). Women had smaller aortic diameters than men for both intact (57mm vs. 59mm, P<.001) and ruptured (71mm vs. 78mm, P<.001) aneurysms.
TABLE I.
Presentation of men and women with abdominal aortic aneurysms and the types of repair they underwent
| Men (N=3122, 78%) | Women (N=904, 22%) | P | |
|---|---|---|---|
| Presentation, % | |||
| Intact | 89 | 91 | 0.142 |
| Ruptured | 11 | 9 | |
| Age (years), median [IQR] | |||
| Intact | 72 [66,78] | 75 [69, 79] | <.001 |
| Ruptured | 73 [67, 79] | 78 [71, 83] | <.001 |
| EVAR, % | |||
| Intact | 60 | 50 | <.001 |
| Ruptured | 20 | 26 | 0.234 |
| Max. AAA AP diameter (mm), mean ± SD | |||
| Intact | 59 ± 12 | 57 ±11 | <.001 |
| Ruptured | 79 ± 19 | 71 ± 20 | <.001 |
From 2003 to 2011, the mean diameter of intact aneurysms being repaired decreased from 60.5mm to 57.3mm for men (P<.010) and 58.0mm to 57.1mm for women (P=.118). The proportion of patients undergoing repair of intact small (<5.5cm) aneurysms increased from 33% in 2003 to 44% in 2011 for men and from 33% to 47% in women. These individuals were younger and had lower rates of CHF and COPD (Table II). Of all the intact <5.5cm aneurysms repaired in both men and women, only 5% were symptomatic. Perioperative and 1-year mortality were lower for men and women undergoing repair of smaller aneurysms. EVAR constituted a higher proportion of repairs for small aneurysms for both men and women (Online Supplemental Appendix, Figures 1 & 2).
Table II.
Demographics and mortality of patients undergoing repair of intact small (<5.5cm) vs. large (≥5.5cm) aneurysms
| Men | Women | |||||
|---|---|---|---|---|---|---|
| <5.5 cm N=986 (36%) |
≥5.5 cm N=1791 (64%) |
P | <5.5 cm N=350 (43%) |
≥5.5 cm N=470 (57%) |
P | |
| Age (median) ± SD | 70 ± 8 | 73 ± 8 | <.001 | 72 ± 8 | 75 ± 8 | <.001 |
| EVAR, % | 65 | 57 | <.001 | 56 | 45 | 0.003 |
| Symptoms, % | 4 | 8 | <.001 | 7 | 11 | 0.025 |
| Smoking history, % | 89 | 90 | 0.725 | 88 | 83 | 0.038 |
| HTN, % | 82 | 84 | 0.093 | 82 | 88 | 0.015 |
| DM, % | 18 | 18 | 0.856 | 15 | 16 | 0.902 |
| CAD, % | 34 | 37 | 0.155 | 25 | 27 | 0.657 |
| CHF, % | 6 | 11 | <.001 | 7 | 11 | 0.063 |
| CABG/PCI, % | 33 | 33 | 0.875 | 19 | 18 | 0.838 |
| COPD, % | 30 | 35 | 0.010 | 42 | 43 | 0.688 |
| Dialysis, % | 0.9 | 0.8 | 0.715 | 0.0 | 0.9 | 0.084 |
| 30-day survival, % | 0.7 | 1.5 | 0.067 | 1.1 | 3.4 | 0.038 |
| 1-year survival, % | 4 | 7 | 0.007 | 3 | 8 | 0.003 |
FIGURE 1.
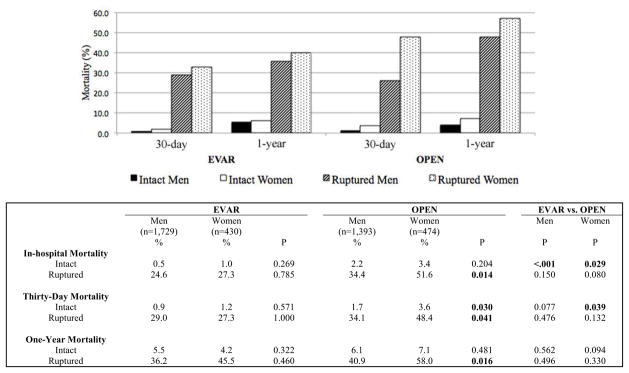
Gender differences in in-hospital, thirty-day, and one-year mortality following endovascular and open repair of intact and ruptured abdominal aortic aneurysms
FIGURE 2.
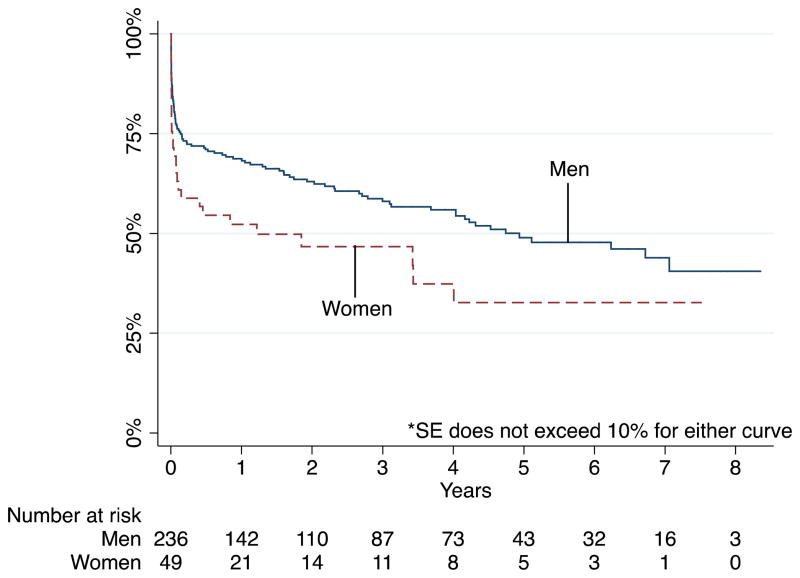
Survival of patients undergoing open repair of ruptured aneurysms
Demographics & Comorbidities
EVAR
Compared to men, women undergoing EVAR were less likely to have a smoking history, coronary artery disease (CAD), history of coronary artery bypass graft or percutaneous coronary intervention (CABG/PCI), but more likely to have chronic obstructive pulmonary disease (Table III). The proportion of patients with a history of hypertension was similar. Preoperatively, women were less likely to be on an aspirin or a statin.
TABLE III.
Baseline demographics, comorbidities, and preoperative medication use of men and women undergoing endovascular and open repair of abdominal aortic aneurysms
| EVAR
|
OPEN
|
|||||
|---|---|---|---|---|---|---|
| Male (n=1,729) % |
Female (n=430) % |
P | Male (n=1,393) % |
Female (n=474) % |
P | |
| Age, median [IQR] | 74 [67, 80] | 77 [71, 81] | <.001 | 71 [64, 77] | 73 [68, 78] | <.001 |
| White | 98 | 98 | 0.851 | 98 | 98 | 1.000 |
| Comorbidities | ||||||
| Smoking history | 87 | 80 | <.001 | 92 | 88 | 0.011 |
| HTN | 85 | 84 | 0.881 | 81 | 86 | 0.008 |
| DM | 20 | 19 | 0.634 | 15 | 13 | 0.361 |
| CAD | 37 | 27 | <.001 | 35 | 27 | 0.001 |
| CHF | 11 | 12 | 0.442 | 7.4 | 6.8 | 0.757 |
| CABG/PCI | 33 | 18 | <.001 | 31 | 19 | <.001 |
| COPD | 34 | 41 | 0.010 | 33 | 45 | <.001 |
| Dialysis | 1.0 | 0.5 | 0.398 | 0.6 | 0.4 | 1.000 |
| Preoperative medications | ||||||
| Aspirin | 71 | 65 | 0.011 | 69 | 63 | 0.040 |
| Plavix | 7 | 6 | 0.386 | 5.0 | 6.2 | 0.341 |
| Statin | 69 | 61 | 0.001 | 61 | 58 | 0.327 |
| Beta-blocker | 76 | 75 | 0.704 | 80 | 79 | 0.508 |
| Family history of AAA | 13 | 13 | 0.619 | 15 | 16 | 0.392 |
| Prior aortic surgery | 2.7 | 2.6 | 1.000 | 3.4 | 4.3 | 0.392 |
| Nursing home resident | 1.0 | 1.4 | 0.434 | 0.5 | 1.7 | 0.031 |
OPEN
Paralleling trends observed for patients undergoing EVAR, women undergoing open repair were also less likely to have a smoking history, CAD, and history of CABG/PCI than men. Like women undergoing EVAR, they also had higher rates of COPD and were less likely to be on aspirin preoperatively. However, rates of statin use were similar between men and women undergoing open repair.
Operative Details and Perioperative Outcomes
Intact EVAR (Online Supplemental Appendix, Table I)
There were 2,068 patients who underwent EVAR for intact aneurysms (80% men). Women underwent repair of intact aneurysms at smaller aortic diameters than men (56mm vs. 58mm, P=.004). For patients undergoing EVAR for intact aneurysms, endografts used more commonly in men were the AneuRx (16.6 vs. 12.6%), Talent (8.3 vs. 7.5%), Zenith (26.4 vs. 20.3%), Powerlink (7.4 vs. 6.8%), and Endurant (3.9 vs. 3.3%). Endografts used more frequently in women were the Excluder (42.4 vs. 32.4%), Cordis (0.2 vs. 0.1%), Zenith Low Profile (0.7 vs. 0.2%) and the Aptus (5.8 vs. 4.2%). The p-value for the t-test comparing endograft type was 0.008. Women were more likely to have unintentional coverage of the hypogastric arteries (one: 3.4% vs. 1.8%, both: 1.0% vs. 0.2%, P=.013) but rates of intentional coverage did not differ. Rates of unplanned graft extensions and presence of endoleak at the end of the procedure did not significantly differ between genders but arterial injury was more common in women (5.4% vs. 2.7%, P=.013). Procedural times were longer in women (175 min. vs. 163 min, P=.006) but there were no differences in the amount of contrast used, the estimated blood loss (EBL), or the intraoperative units of blood transfused. Postoperatively, vasopressors were administered more often in women (7.4% vs. 3.5%, P=.001), as was transfusion of packed red blood cells (RBCs) (0.4 units vs. 0.1 units, P<0.001). Women required return to the operating room more frequently (3.4% vs. 1.6%, P=.025), stayed in the hospital longer (4.3 days vs. 2.7 days, P=.018) and were discharged somewhere other than home more often (14.1% vs. 6.2%, P<.001). Women had a lower odds of being discharged home even after adjusting for age (HR 0.5, 95% CI 0.3–0.7, P<.001).
Ruptured EVAR (Online Supplemental Appendix, Table II)
Ninety-one patients underwent EVAR for rupture (76% men). Women with ruptured aneurysms undergoing EVAR had smaller aneurysms than men but this difference was not statistically significant (73mm vs. 76 mm, P=.510). There were no differences in type of endografts used, presence of endoleak, rates of arterial injury, amounts of contrast used, EBL, units of RBCs transfused, or total procedural time. Postoperatively, women stayed in the ICU longer than men (8.0 days vs. 3.4 days, P=.015) but did not differ in the rate of vasopressor administration or postoperative blood transfusion. Wound complications were more common in women (19.1% vs. 1.6%, P=.013). However, there were only 69 men and 22 women undergoing rupture EVAR such that the absolute difference was only 3 wound complications (1 among men and 4 among women), suggesting this comparison may have been significant by chance, particularly because there was no difference in wound complications among men and women undergoing EVAR for intact aneurysms. There were no other significant differences in the rate of other postoperative complications but women nonetheless remained in the hospital longer (15.3 days vs. 6.8 days, P=.009).
Intact Open Repair (Online Supplemental Appendix, Table III)
Open repair for an intact aneurysm was performed in 1,529 patients (73% men). Women underwent open repair of intact aneurysms at smaller diameters than men (58mm vs. 62mm, P<.001). The proportion of repairs representing conversion from EVAR was higher in women but this difference was not statistically significant (1.7% vs. 0.5%, P=.052). Rates of retroperitoneal exposure also were not different between genders. Women were more likely to get tube grafts than men (59% vs. 50%) and among bifurcated repairs, women were more likely to have femoral vs. iliac anastomoses (femoral: 18.5% vs. 14.3%, common iliac: 3.7% vs. 7.9%, external iliac: 18.7% vs. 27.6%, P<.001). Women were less likely to have an infra-renal proximal clamp position (65% vs. 74%, P=.010). Women had lower EBL (1261ml vs. 1563ml, P<.001), and less autotransfusion (568ml vs. 788ml, P<.001) but greater intraoperative transfusion of RBCs (0.9 units vs. 0.6 units, P=0.001). Women were in the ICU longer (5.2 days vs. 3.5 days, P=.001) and more frequently developed complications of dysrhythmia (17% vs. 12%, P=.024), CHF (7% vs. 4%, P=.056), leg ischemia/emboli (4% vs. 1%, P=.001), and bowel ischemia (5.0% vs. 3%, P=.044). Not surprisingly, on average they stayed in the hospital more than a day longer than men (10.8 days vs. 9.3 days, P=.050) and more frequently were not discharged home (28.5% vs. 17.4%, P<.001). Again, women had a lower odds of being discharged home even after adjusting for age (HR 0.6, 95% CI 0.5–0.8, P=.001).
Ruptured Open Repair (Online Supplemental Appendix, Table IV)
TABLE IV.
Multivariable predictors of 30-day and long-term mortality
| 30-day Survival | OR | 95% C.I. | P |
|---|---|---|---|
| Age | |||
| <60 years | - | - | - |
| 60–69 years | 1.9 | 1.0–3.5 | 0.042 |
| 70–79 years | 3.1 | 1.6–6.0 | 0.001 |
| ≥80 years | 10.9 | 3.3–36.5 | <.001 |
| Female | 1.7 | 1.0–2.8 | 0.063 |
| CAD | 1.7 | 1.0–2.8 | 0.043 |
| Dialysis | 9.5 | 1.1–85.7 | 0.044 |
| Treatment subgroup | |||
| Intact EVAR | - | - | - |
| Intact OAR | 3.3 | 1.2–9.0 | 0.024 |
| Ruptured EVAR | 48.4 | 14.8–157.9 | <.001 |
| Ruptured OAR | 83.8 | 30.8–227.7 | <.001 |
| Long-term Survival | |||
| Age | |||
| <60 years | |||
| 60–69 years | 1.6 | 1.2–2.0 | 0.001 |
| 70–79 years | 2.7 | 2.0–3.5 | <.001 |
| ≥80 years | 4.8 | 2.6–8.7 | <.001 |
| Gender | 1.1 | 0.9–1.4 | 0.445 |
| CAD | 1.4 | 1.1–1.7 | 0.004 |
| CHF | 1.4 | 1.0–1.8 | 0.025 |
| COPD | 1.5 | 1.2–1.8 | <.001 |
| Treatment subgroup | |||
| Intact EVAR | - | - | - |
| Intact OAR | 1.3 | 0.9–1.6 | 0.112 |
| Ruptured EVAR | 4.3 | 2.6–7.3 | <.001 |
| Ruptured OAR | 2.7 | 1.9–3.8 | <.001 |
There were 338 patients who underwent open repair for a ruptured aneurysm (82% men). Aneurysm diameter was smaller in women (71.0mm vs. 79.3mm, P<.001). As with intact open repairs, the distal anastomosis was more frequently to the aorta and common femoral artery in women and to the common and external iliac arteries in men. However, this difference was not significant for open repair of ruptured AAAs.. Other than a lower rate of auto-transfusion (3336ml vs. 3997ml, P=.013), women did not differ from men in terms of renal visceral ischemia time, EBL, intraoperative or postoperative blood transfusion, or procedural time. Postoperatively, vasopressor requirement and ICU stay also did not differ significantly between genders. Women had higher rates of dysrhythmia (33% vs. 20%, P=.045) and tended to have more leg ischemia/emboli complications (15% vs. 7%, P=.055). However, rates of other complications were comparable. A similar proportion of men and women were not discharged home (52 vs. 67%, P=.169).
Mortality
In-hospital deaths were the majority of deaths that occurred within the first 30 days (Figure 1). Crude in-hospital, 30-day, and 1-year mortality were similar between men and women undergoing EVAR for both intact and ruptured aneurysms. In contrast, 30-day mortality was higher in women undergoing open repair for intact aneurysms than men (3.6% vs. 1.7%, P=.030). In-hospital, 30-day, and 1-year mortality were also higher in women after open repair for ruptured aneurysms (in-hospital: 51.6% vs. 34.4%, P=.014; 30-day: 48.4% vs. 34.1%, P=.041; 1-year: 58.0% vs. 40.9%, P=.016). For men, crude in-hospital mortality was lower after EVAR than after open repair for intact (0.5% for EVAR vs. 2.2% for open, P<.001) but not ruptured (34.4% for EVAR vs. 24.6% for open, P=.150) aneurysms. There was no survival benefit seen in men undergoing EVAR over men undergoing open repair at 30-days and 1-year. For women, crude in-hospital and 30-day mortality were lower for EVAR than open repair for intact (in-hospital: 1.0% for EVAR vs. 3.4% for open, P=.029; 30-day: 1.2% for EVAR vs. 3.6% for open, P=.039) but these differences did not attain statistical significance for ruptured (inhospital: 27.3% for EVAR vs. 51.6% for open, P=.080; 30-day: 27.3% for EVAR vs. 48.4% for open, P=.132) aneurysms. A sample size calculation assuming an α of .05 and 90% power indicated a minimum of 87 women would be needed in each group to detect a 25% mortality difference (among the women in our cohort, 22 underwent EVAR and 62 underwent open repair for rupture). Additionally, there were no differences in one-year mortality between women undergoing EVAR vs. women undergoing open repair for both intact and ruptured repairs.
Multivariable predictors of 30-day mortality were age, CAD, preoperative dialysis dependence, and presentation/treatment subgroup (intact vs. ruptured and EVAR vs. open repair) (Table IV). Age greater than 80 years increased the odds of 30-day mortality by 10.9 and rupture increased the odds by 48.4 for EVAR and 83.8 for open repair. Female gender did not reach statistical significance as a predictor of 30-day mortality.
Using Kaplan-Meier survival analysis, we observed similar survival between men and women undergoing intact repairs out to three years for both EVAR and open repair. For patients with ruptured aneurysms undergoing EVAR, survival at 1 year was 54% in women and 67% in men (HR 1.1, 95% CI 0.5–2.4, P=.741). One-year survival among patients with ruptured aneurysms undergoing open repair was 50% in women vs. 68% in men (HR 1.6, 95% CI 1.0–2.4, P=.033) (Figure 2). The highest mortality for all patients with rupture occurred within the first year and it was also during this time that the greatest gender disparity in mortality became apparent. Long-term mortality was predicted by age, comorbidities (CAD, CHF, and COPD) and presentation/treatment subgroup. Of note, patients undergoing EVAR for rupture had higher odds of long-term mortality compared to those undergoing open repair for rupture. Gender was not predictive of long-term mortality.
DISCUSSION
In this study, we sought to characterize gender disparities in presentation, treatment, and outcomes after AAA repair using a large multicenter database inclusive of both community and academic hospitals that contained a representative sample of women. Women presented at an older age and ruptured at smaller aortic diameters than men but the proportion of ruptured repairs was not higher in women. When undergoing elective repair, women were repaired at smaller diameters.
From 2003 to 2011, the size of aneurysms at elective repair has decreased for both genders but to a greater degree for men than women. This could in part be due to an increase in aneurysm detection through screening, but because we do not have data about ruptures treated non-operatively, we cannot speculate whether this trend has lowered the proportion of patients who present with rupture. Despite evidence from randomized controlled trials suggesting no survival benefit to repairing small (4.0–5.5cm) aneurysms regardless of repair type18, 35% of men and 43% of women with intact aneurysms in our study underwent repair at diameters <5.5cm and ultimately experienced lower perioperative and long-term mortality. The decision to pursue elective AAA repair is dependent on factors besides just aneurysm size, such as patient surgical risk, life expectancy, and personal preference. These variables were not precisely captured in the VSGNE database. However, we did note that patients undergoing repair of small aneurysms were younger, and generally had fewer comorbidities, and were less likely to undergo open repair. The lower perioperative and 1-year mortality observed in the group with small aneurysms suggests that surgeons may be selecting patients according to Society of Vascular Surgery guidelines which state that repair at smaller sizes (4.5–5.5cm) may be indicated in younger low-risk patients who prefer early repair (19.).
Women were less likely to undergo EVAR for intact aneurysms. The most likely reason for this is that women less frequently meet the anatomic criteria for EVAR. This has been observed in several studies (20, 24), including one by Sweet et al.13, who showed that even after adjustment for age and aneurysm size, women have decreased neck length, increased neck angulation, and smaller iliac access. Indeed, women undergoing EVAR in the VSGNE had significantly smaller graft limb diameters (right limb: 14.3 vs. 15.7mm, P<.001, left limb: 14.2 vs. 15.9mm, P<.001). In the study by Sweet et al, these features resulted in a lower likelihood of women meeting device Instructions for Use criteria. Though we did not have iliac artery size measurements, we did observe that lower-profile endografts, such as the Excluder, Cordis, and Zenith Low Profile, were more frequently used in women.
When women did undergo EVAR, they experienced higher rates of intraoperative complications, including arterial injury and unplanned graft extension. We suspect that this is again related to less favorable iliac anatomy, making graft placement more difficult or complicated by injury necessitating extension and coverage., Postoperatively, women undergoing EVAR also experienced greater morbidity, as evidenced by longer ICU stays, higher rates of bowel ischemia and complications requiring return to the operating room. Abedi et al.9 described gender disparities among patients undergoing EVAR using the ACS-NSQIP dataset. Although preoperative, intra-operative, and complication data captured in the NSQIP at the time were not specific to AAA patients, they also observed more postoperative complications in women, reporting women had 1.7 higher odds of operative morbidity than men.
Gender disparities in perioperative outcomes were also evident in patients who underwent open repair of intact aneurysms. Our study is one of very few to offer a comparison of intraoperative and procedural details and vascular complications specific to AAA repair between men and women. The primary outcome reported in the vast majority of prior studies has been mortality. Of the few that did report specific postoperative complications, most did not find significant differences between men and women, possibly due to smaller cohort sizes. In their study of 675 patients from 1983 to 2003, Bonamigo et al. found no differences in duration of surgery or EBL25. Postoperatively, men and women in their study did not differ significantly in the rates of coronary, pulmonary, renal, gastrointestinal complications, infectious, or vascular complications, although there was a trend toward higher rates in women for the latter two. Johnston similarly found no gender differences in early or late vascular complications in his study of 679 patients undergoing intact open AAA repair26. Our analysis identified longer ICU stays and higher rates of dysrhythmia, CHF, and embolic and bowel complications in women.
Results of our analyses of mortality were surprising. Despite higher intra- and postoperative complications, there were no differences in 30-day and 1-year mortality between men and women undergoing EVAR, regardless of urgency of presentation. Only in a small subgroup of patients undergoing open repair of ruptured aneurysms did we see a significant mortality difference between men and women. Thus, our findings are consistent with an earlier VSGNE report on mortality after elective AAA repair, in which gender was not found to be a significant predictor of mortality27. One consistent multivariable predictor of both 30-day and long-term mortality was advanced age, particularly 80 years of age or older, suggesting greater weight should be given to projected life expectancy when balancing the risks and benefits of AAA repair.
Mehta et al.7, who recently published the results of their single-institution 7-year experience with 2,631 open and endovascular AAA repairs, found no differences in 30-day mortality among men and women undergoing elective open, ruptured open, or ruptured endovascular repair. However, among their patients undergoing elective EVAR, the odds of 30-day mortality was 3.4 times higher in women after adjustment for age, aneurysm size, and other risk factors. It is unclear why disparities were only seen among their patients undergoing elective EVAR (a finding not previously reported in other gender-based studies) and why these were not seen in our data. The cohort undergoing elective EVAR in their study was comparable to ours in sample size, gender-specific mean aneurysm sizes, and comorbidities. However, as the authors themselves acknowledged, their study was based on the experience of a small group of surgeons from a single institution and given that they serve as a referral center, was likely affected by selection bias.
Population-based studies have reported worse mortality in women following AAA repair2, 8, 9–11. Certainly, these studies had considerably larger study populations than our current study or that of Mehta et al. Perhaps we would have seen significant gender-associated differences in mortality if our study population had been larger. We did observe a trend toward higher 30-day mortality rates among women. Although not quite statistically significant, the magnitude and direction of the hazard ratio mirrors those reported in population-based studies, suggesting a possible type II error due to limitations in population size.
Although our study is retrospective, all data in the VSGNE are collected prospectively and this current study represents the largest study utilizing detailed clinical information derived from a broad population, enhancing its generalizability. Another potential limitation was selection bias. The decision to pursue repair and the method of repair to perform can depend on a myriad of factors and differential selection for repair based on gender may have led to confounding by unmeasured covariates.
CONCLUSION
Compared to men, women are older and have smaller aneurysms when undergoing AAA repair for both intact and ruptured aneurysms. When undergoing aneurysm repair, women are less likely than men to undergo EVAR and have higher rates of procedural complications. Differences in operative and late mortality are driven primarily by rupture, open repair versus EVAR, age, and comorbidity rather than gender. In fact, gender is not predictive of 30-day and 1-year mortality for both intact and ruptured aneurysms.
Acknowledgments
This work was supported by the NIH T32 Harvard-Longwood Research Training in Vascular Surgery grant HL007734.
Footnotes
Author Disclosures: R.C. Lo, none; R.P. Bensley, none; J Darling, none; S Dahlberg, none; A Hamdan, Endologix Consultant; M Wyers, Endologix Consultant, Boston Scientific Consultant; J Adams, none; M.L. Schermerhorn, Endologix Consultant, Medtronic Consultant.
Presented at the Spring Meeting of the Peripheral Vascular Surgery Society in Washington, D.C. on 6/6/12.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Harthun NL. Current issues in the treatment of women with abdominal aortic aneurysm. Gend Med. 2008;5(1):36–43. doi: 10.1016/s1550-8579(08)80006-x. [DOI] [PubMed] [Google Scholar]
- 2.McPhee JT, Hill JS, Eslami MH. The impact of gender on presentation, therapy, and mortality of abdominal aortic aneurysm in the United States, 2001–2004. J Vasc Surg. 2007;45(5):891–899. doi: 10.1016/j.jvs.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 3.Long-term outcomes of immediate repair compared with surveillance of small abdominal aortic aneurysms. N Engl J Med. 2002;346(19):1445–1452. doi: 10.1056/NEJMoa013527. [DOI] [PubMed] [Google Scholar]
- 4.Mofidi R, V, Goldie J, Kelman J, Dawson AR, Murie JA, Chalmers RT. Influence of sex on expansion rate of abdominal aortic aneurysms. Br J Surg. 2007;94(3):310–314. doi: 10.1002/bjs.5573. [DOI] [PubMed] [Google Scholar]
- 5.Brown LC, Powell JT. Risk factors for aneurysm rupture in patients kept under ultrasound surveillance. UK Small Aneurysm Trial Participants. Ann Surg. 1999;230(3):289–296. doi: 10.1097/00000658-199909000-00002. discussion 296-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dillavou ED, Muluk SC, Makaroun MS. A decade of change in abdominal aortic aneurysm repair in the United States: Have we improved outcomes equally between men and women? J Vasc Surg. 2006;43(2):230–238. doi: 10.1016/j.jvs.2005.09.043. discussion 238. [DOI] [PubMed] [Google Scholar]
- 7.Mehta M, Byrne WJ, Robinson H, Roddy SP, Paty PS, Kreienberg PB, et al. Women derive less benefit from elective endovascular aneurysm repair than men. J Vasc Surg. 2012;55(4):906–913. doi: 10.1016/j.jvs.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 8.Egorova NN, Vouyouka AG, McKinsey JF, Faries PL, Kent KC, Moskowitz AJ, et al. Effect of gender on long-term survival after abdominal aortic aneurysm repair based on results from the Medicare national database. J Vasc Surg. 2011;54(1):1–12. e16. doi: 10.1016/j.jvs.2010.12.049. discussion 11-12. [DOI] [PubMed] [Google Scholar]
- 9.Abedi NN, Davenport DL, Xenos E, Sorial E, Minion DJ, Endean ED. Gender and 30-day outcome in patients undergoing endovascular aneurysm repair (EVAR): an analysis using the ACS NSQIP dataset. J Vasc Surg. 2009;50(3):486–491. 491 e481–484. doi: 10.1016/j.jvs.2009.04.047. [DOI] [PubMed] [Google Scholar]
- 10.Giles KA, Hamdan AD, Pomposelli FB, Wyers MC, Dahlberg SE, Schermerhorn ML. Population-based outcomes following endovascular and open repair of ruptured abdominal aortic aneurysms. J Endovasc Ther. 2009;16(5):554–564. doi: 10.1583/09-2743.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giles KA, Schermerhorn ML, O’Malley AJ, Cotterill P, Jhaveri A, Pomposelli FB, et al. Risk prediction for perioperative mortality of endovascular vs open repair of abdominal aortic aneurysms using the Medicare population. J Vasc Surg. 2009;50(2):256–262. doi: 10.1016/j.jvs.2009.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ailawadi G, Eliason JL, Roelofs KJ, Sinha I, Hannawa KK, Kaldjian EP, et al. Gender differences in experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2004;24(11):2116–2122. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 13.Sweet MP, Fillinger MF, Morrison TM, Abel D. The influence of gender and aortic aneurysm size on eligibility for endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011;54(4):931–937. doi: 10.1016/j.jvs.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 14.Forbes TL, Lawlor DK, DeRose G, Harris KA. Gender differences in relative dilatation of abdominal aortic aneurysms. Ann Vasc Surg. 2006;20(5):564–568. doi: 10.1007/s10016-006-9079-y. [DOI] [PubMed] [Google Scholar]
- 15.Heikkinen M, Salenius JP, Auvinen O. Ruptured abdominal aortic aneurysm in a well-defined geographic area. J Vasc Surg. 2002;36(2):291–296. doi: 10.1067/mva.2002.125479. [DOI] [PubMed] [Google Scholar]
- 16.Mikhail GW. Coronary heart disease in women. Bmj. 2005;331(7515):467–468. doi: 10.1136/bmj.331.7515.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller M, Byington R, Hunninghake D, Pitt B, Furberg CD. Sex bias and underutilization of lipid-lowering therapy in patients with coronary artery disease at academic medical centers in the United States and Canada. Prospective Randomized Evaluation of the Vascular Effects of Norvasc Trial (PREVENT) Investigators. Arch Intern Med. 2000;160(3):343–347. doi: 10.1001/archinte.160.3.343. [DOI] [PubMed] [Google Scholar]
- 18.Filardo G, Powell JT, Martinez MA, Ballard DJ. Surgery for small asymptomatic abdominal aortic aneurysms. Cochrane Database Syst Rev. 2012;3:CD001835. doi: 10.1002/14651858.CD001835.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brewster DC, Cronenwett JL, Hallett JW, Jr, Johnston KW, Krupski WC, Matsumura JS. Guidelines for the treatment of abdominal aortic aneurysms. Report of a subcommittee of the Joint Council of the American Association for Vascular Surgery and Society for Vascular Surgery. J Vasc Surg. 2003;37(5):1106–1117. doi: 10.1067/mva.2003.363. [DOI] [PubMed] [Google Scholar]
- 20.Sampaio SM, Panneton JM, Mozes GI, Andrews JC, Noel AA, Karla M, et al. Endovascular abdominal aortic aneurysm repair: does gender matter? Ann Vasc Surg. 2004;18(6):653–660. doi: 10.1007/s10016-004-0106-6. [DOI] [PubMed] [Google Scholar]
- 21.Langhammer A, Johnsen R, Gulsvik A, Holmen TL, Bjermer L. Sex differences in lung vulnerability to tobacco smoking. Eur Respir J. 2003;21(6):1017–1023. doi: 10.1183/09031936.03.00053202. [DOI] [PubMed] [Google Scholar]
- 22.Sorheim IC, Johannessen A, Gulsvik A, Bakke PS, Silverman EK, DeMeo DL. Gender differences in COPD: are women more susceptible to smoking effects than men? Thorax. 2010;65(6):480–485. doi: 10.1136/thx.2009.122002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gan WQ, Man SF, Postma DS, Camp P, Sin DD. Female smokers beyond the perimenopausal period are at increased risk of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Respir Res. 2006;7:52. doi: 10.1186/1465-9921-7-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biebl M, Hakaim AG, Hugl B, Oldenburg WA, Paz-Fumagalli R, McKinney JM, et al. Endovascular aortic aneurysm repair with the Zenith AAA Endovascular Graft: does gender affect procedural success, postoperative morbidity, or early survival? Am Surg. 2005;71(12):1001–1008. [PubMed] [Google Scholar]
- 25.Bonamigo TPLM, Erling N., Jr Surgical treatment of abdominal aortic aneurysms: is there a difference in the results between men and women? J Vasc Brasil. 2006;5:101–108. [Google Scholar]
- 26.Johnston KW. Influence of sex on the results of abdominal aortic aneurysm repair. Canadian Society for Vascular Surgery Aneurysm Study Group. J Vasc Surg. 1994;20(6):914–923. doi: 10.1016/0741-5214(94)90228-3. discussion 923-916. [DOI] [PubMed] [Google Scholar]
- 27.Beck AW, Goodney PP, Nolan BW, Likosky DS, Eldrup-Jorgensen J, Cronenwett JL. Predicting 1-year mortality after elective abdominal aortic aneurysm repair. J Vasc Surg. 2009;49(4):838–843. doi: 10.1016/j.jvs.2008.10.067. discussion 843-834. [DOI] [PubMed] [Google Scholar]