Abstract
Background & Aims
Patients with diarrhea-predominant irritable bowel syndrome (IBS-D) could benefit from a gluten-free diet (GFD).
Methods
We performed a randomized controlled 4-week trial of a gluten-containing diet (GCD) or GFD in 45 patients with IBS-D; genotype analysis was performed for HLA-DQ2 and HLA-DQ8. Twenty-two patients were placed on the GCD (11 HLA-DQ2/8–negative and 11 HLA-DQ2/8–positive) and 23 on the GFD (12 HLA-DQ2/8−negative and 11 HLA-DQ2/8–positive. We measured bowel function daily, small bowel (SB) and colonic transit, mucosal permeability (by lactulose and mannitol excretion), and cytokine production by peripheral blood mononuclear cells (PBMCs) following exposure to gluten and rice. We collected rectosigmoid biopsies from 28 patients, analyzed levels of mRNAs encoding tight junction proteins, and performed hematoxylin and eosin staining and immunohistochemical analyses. Analysis of covariance models was used to compare data from the GCD and GFD groups.
Results
Subjects on the GCD had more bowel movements/day (P=.04); the GCD had a greater effect on bowel movements/day of HLA-DQ2/8–positive than −negative patients (P=.019). The GCD was associated with higher SB permeability (based on 0–2 hr levels of mannitol and lactulose:mannitol ratio); SB permeability was greater in HLA-DQ2/8–positive than −negative patients (P=.018). No significant differences in colonic permeability were observed. Patients on the GCD had a small decrease in expression of ZO-1 in SB mucosa and significant decreases in expression of ZO-1, claudin-1, and occludin in rectosigmoid mucosa; the effects of the GCD on expression were significantly greater in HLA-DQ2/8–positive patients. GCD vs GFD had no significant effects on transit or histology. PBMCs produced higher levels of interleukin-10, granulocyte colony-stimulating factor, and transforming growth factor-a in response to gluten than rice (unrelated to HLA genotype).
Conclusion
Gluten alters bowel barrier functions in patients with IBS-D, particularly in HLA-DQ2/8–positive patients. These findings reveal a reversible mechanism for the disorder.
Keywords: permeability, transit, immunity, cytokines
BACKGROUND
The relationship of gluten exposure and symptom generation in irritable bowel syndrome (IBS) is complex and not well understood though many patients are either counseled or decide to follow a gluten free diet. The prevalence of celiac disease in patients with functional chronic diarrhea (FD) or diarrhea-predominant IBS (IBS-D) is similar to that of healthy controls, approximately 0.4%.1. Non-celiac IBS-D patients who are HLA-DQ2/8 positive can show symptom improvement on a gluten-free diet (GFD). In view of the observation of celiac disease-associated serum IgG (not IgA) in 37% of patients with IBS-D, an adaptive immune mechanism in response to gluten exposure has been proposed to explain the improvement in symptoms with gluten withdrawal from the diet.2 Other factors such as alterations in gut motility or secretion,3 gut permeability, or inflammation may conceivably interact with immune mechanisms to result in symptoms in IBS-D patients exposed to gluten.
Alterations in intestinal permeability and jejunal mucosal tight junction (TJ) signaling have been described in IBS-D,4,5 including post-infectious IBS-D.6 However, the degree of increased small bowel (SB) permeability in patients considered gluten sensitive was low compared to that of celiac disease patients and healthy controls.7
The aim of this 4-week, randomized, clinical trial in HLA-DQ2/8 positive and negative patients with IBS-D was to assess the effects of a gluten-containing diet (GCD) compared to a GFD on bowel function, gut transit, SB and colonic barrier functions measured functionally by two-sugar excretion permeability test and mRNA expression of TJ proteins in mucosa of the SB and rectosigmoid (RS). We also assessed mucosal morphology and immune activation in response to the diets, and the proliferative and cytokine responses of peripheral blood mononuclear cells (PBMCs) to gluten and rice antigens.
METHODS
Study Design and Intervention
Between January 2010 and February 2012, we conducted a single-center, parallel-group, randomized, controlled, 4-week trial of GCD and GFD in 45 gluten-ingesting patients with IBS-D. They were recruited from a database of over 800 patients with irritable bowel syndrome who had been evaluated clinically by the investigators or clinical staff at Mayo Clinic; these patients reside within 150 miles of Mayo Clinic in Rochester, MN; the database is maintained in the laboratory of the principal investigator; 307 participants with known IBS-D were invited to participate by letter or electronic communication. The study was approved by the Mayo Clinic Institutional Review Board, and all participants signed informed consent.
The study was registered at Clinicaltrials.gov, # NCT01094041, and the authors had access to the study data and reviewed and approved the final manuscript. There were no adverse effects of the interventions or treatments in the entire study.
All studies were conducted on an outpatient basis at Mayo Clinic, and meals were ingested or prepared in the Mayo Clinical Research Unit. Participants were also provided snacks and advised to eat only the foods provided by the study dietitians during the entire study period. The macronutrient distribution of the meals was 20% protein, 30% fat and 50% carbohydrate. Three typical meals were prepared with the same macronutrient proportions in the gluten-free and gluten-containing diets. The typical food offerings in the diet are included in Appendix 1. Patients selected foods from these menus. We used the Harris Benedict equation8 plus an additional correction of calories required for different levels of physical activity to ensure weight maintenance. During the 4-week study, compliance to the diet was assessed by direct questioning by the dietitians when participants picked up the meal and snack supplies.
Randomization and Masking
The randomization sequence was generated by computer and communicated by a statistician to the dietitians in the Clinical Research Unit. Allocation to the different diets was concealed from all investigators except for the assigned lead dietitian (DJ, co-author).
Participants and Screening for Celiac Disease
The IBS-D patients (43 female, 2 male) had symptoms consistent with Rome II criteria9 and confirmed by a validated questionnaire.10 Psychological health was assessed at baseline using the Hospital Anxiety and Depression Inventory.11 All patients were ingesting gluten in their diet prior to starting the study. Dietary gluten intake (food servings containing gluten) prior to enrollment to the trial was assessed by a questionnaire.
The three main exclusion criteria were: dietary gluten exclusion prior to enrollment to the trial based on a dietary questionnaire; evidence on the history that the patient had previously responded to gluten restriction or exclusion; and evidence of celiac disease by positive serum tissue transglutaminase (TTG) IgA or IgG, or a recorded SB biopsy suggestive of celiac disease, or a positive serum anti-endomysial antibody test, if TTG was equivocal. TTG IgA and IgG were measured by commercial enzyme-linked immunoassay kits (Abnova, San Diego, CA). Other procedurally-related exclusion criteria were use of: tobacco products within the past 6 months, NSAIDs or aspirin within the past week (since they affect intestinal permeability); artificial sweeteners from 2 days before the study (to avoid contamination of the 2-sugar permeability test); medications which affect gastrointestinal motility for 2 days before the transit studies; and medications that increase risk of bleeding from mucosal biopsies.
Twenty-eight of the 45 participants agreed to undergo upper gastrointestinal endoscopy and flexible sigmoidoscopy; all these participants underwent both procedures under conscious sedation performed by the same three endoscopists (MV-R, JAM, MC).
Assessment of Stool Frequency, Consistency and Ease of Passage
During the 14-day baseline and the 28-day study periods, patients completed a daily bowel pattern diary [that included the 7-point Bristol Stool Form Scale,12 ranging from 1 or hard lumpy stool to 7 or watery diarrhea] to record their bowel habits. The bowel pattern diary was dispensed at the screening visit, and the completed bowel diary was collected at the end of the study. The diary also recorded the date and timing of each bowel movement, the ease of passage (to assess evacuation) and completeness of evacuation, and any medications received. The instrument has been used extensively over a decade since its incorporation into pharmacodynamic studies in our laboratory.13
HLA Genotyping
DNA was extracted from peripheral blood for HLA typing of DQ alleles. HLA-DQ2 and 8 were determined using six HLA-tagging single-nucleotide polymorphisms (SNPs).14
Measurement of Gastric Emptying, Small Bowel and Colonic Transit with Scintigraphy
Measurement of gastric emptying (GE), BS and colonic transit with scintigraphy was conducted as in prior studies that describe the performance characteristics of the methods15–17 and involved ingestion of a radiolabeled solid 296kcal meal and radiolabeled activated charcoal delivered by means of a pH-sensitive capsule to the distal small bowel to measure colonic transit.
Anterior and posterior gamma camera images were obtained immediately after radiolabeled meal ingestion, hourly through 8 hours, and then at 24 and 48 hours after radiolabeled meal ingestion. Two standardized meals were ingested at 4 hours (530 kcal chicken meal) and 8 hours (750 kcal, roast beef sandwich, which includes two slices of bread).
A variable region of interest program was used to measure isotope counts in each region and, thereby, derive a transit measurement (after correction for radioisotope decay and tissue attenuation) to estimate the proportion of 99mTc emptied from the stomach at each time point or filling the colon at 6 hours, and the proportion of 111In in each colonic region at specified times. Gastric emptying was measured over 4 hours after meal ingestion. Filling of the colon at 6 hours serves as a valid surrogate for small bowel (SB) transit.15
Overall colonic transit was summarized as the colonic geometric center [GC, weighted average of counts in the colonic regions (ascending, transverse, descending, rectosigmoid and stool, respectively, numbers 1 to 5)] at 24 and 48 hours.
Measurement of Small Bowel and Colonic Permeability
Measurement of SB and colonic permeability was conducted in the Clinical Research Unit, as in prior studies with standardized meals during the first 8 hours and water allowed ad libitum throughout the day.4,18 Lactulose, 1000 mg (L7877, Sigma-Aldrich, St. Louis, MO 63103), and mannitol, 200 mg (M8429, Sigma-Aldrich, St. Louis, MO 63103), were used to determine the urine sugar excretions at different times as markers of SB (0–2h) and colonic (8–24h) mucosal permeability after oral ingestion of the sugars in aqueous solution. Urine was collected every 30 minutes for the first 2 hours and cumulated for the entire 2 hours (validated measure of SB permeability), every 2 hours for the next 6 hours, and from 8 to 24 hours (validated measure of colonic permeability). The total volume of each collection was measured, and an aliquot was stored at −20° Celsius until it was thawed for analysis.
Participants ingested standardized meals during the first 8 hours, specifically, 500mL water was given 30 minutes after sugar administration to aid in the collection of urine, a breakfast of egg and toast was given after 2 hours, and a lunch of chicken and potato was offered after 6 hours. Water was allowed ad libitum throughout the day and during meals.
Urinary saccharide concentrations were measured by high performance liquid chromatography-tandem mass spectrometry. Details of the method were previously described.18,19
Cumulative excretion in each collection (0–2h and 8–24h) was calculated by:
[concentration of sugar (µg/mL)]* total urine volume (mL)
The lactulose: mannitol ratio (L:M ratio) was calculated by:
L:M ratio = 0.2 × (cumulative excretion lactulose) / (cumulative excretion mannitol)
Quantitation of Tight Junction Proteins Using Real-Time PCR
We used real-time PCR to quantitate mRNA expression of TJ proteins [zonula occludens 1 (ZO-1), occludin (OCLN), claudin-1 (CLDN-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH, control)] in SB and RS biopsy samples from 28 IBS-D patients who consented to undergo endoscopy solely for research.
Small bowel and rectosigmoid colon biopsy samples were submerged in RNAlater Solution (Ambion, Austin, TX) and stored at −80°C. RNA extraction was performed as in the manufacturer’s instructions (RNeasy Mini Kit, Qiagen, Valencia, CA). cDNA synthesis was performed using 0.2 µg of total RNA with the High Capacity Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Taqman gene expression assays for zonula occludens 1 (ZO-1), occludin (OCLN), claudin-1 (CLDN-1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were carried out in triplicate for each gene on an ABI Prism 7900HT Real-time PCR System (Applied Biosystems) according to manufacturer's instructions using the comparative ΔΔCT method for relative quantification.
The expression of each gene was normalized to the endogenous control, GAPDH. The post-diet fold change was calculated individually with respect to the pre-diet biopsy. Mean values of mRNA expressions were used for the statistical analysis.
Small Bowel and Colonic Mucosal Morphology
The SB and colonic biopsies (obtained in 28/45 IBS-D patients) were stained with H&E to quantitatively assess intra-epithelial lymphocytes (IEL) and villous to crypt ratio and CD3 and CD8 lymphocytes. Section orientation was controlled by counting cells in areas with three adjacent villi in small bowel biopsies and three adjacent crypts in colonic biopsies viewable for their entire length. The methods used for immunochemistry and quantitative and semi-quantitative morphological analyses are summarized in Appendix 2.
Proliferative Responses and in vitro Cytokine Responses of PBMCs to Gluten and Rice
In celiac disease, DQ2+ and DQ8+ restricted gluten specific inflammatory T cells mediate inflammation through the production of IFNγ. There is also an inflammatory innate response to gluten that is mediated partly through the production of TNFα.20 To help determine whether the immune response to gluten in the IBS patients evaluated is an inflammatory adaptive T cell response to gluten or an inflammatory innate immune response to gluten, the PBMCs of patients were tested for the ability to proliferate in response to in vitro gluten stimulation, and the supernatants were evaluated for IFNγ, TNFα, and other cytokines.
The method used to assess the proliferative responses and in vitro cytokine responses of PBMCs to gluten and rice is described in Appendix 2.
Statistical Analysis
The primary endpoints were colonic GC at 24 hours, urine mannitol excretion at 0–2 hours and 8–24 hours. Secondary endpoints were GE t1/2 (min), colonic filling at 6 hours (CF6, %), GC at 48 hours, ascending colon (AC) t1/2, urine lactulose 8–24 hours, urine lactulose: mannitol ratio (LMR) at 0–2 hours and 8–24 hours, mucosal inflammation, and bowel functions (frequency, consistency, ease of passage). The α level was set at 0.05 for the primary endpoints, and we did not adjust the α level for diet comparisons of the secondary endpoints. The primary analyses used analysis of variance (AOV) or covariance (ANCOVA) models (using pre-diet values and, where relevant, BMI as a covariate) to assess the effects of diet. Thus, the effects of the diet on transit and bowel function were direct comparisons of post-diet A vs. postdiet B observations, adjusted for the pre-diet measurements in each individual patient. Differential diet effects depending on HLA status were assessed using ANCOVA models by also including an overall HLA term and an HLA by diet interaction term. An intention to treat (ITT) analysis was used, including all subjects randomized or those consenting to the biopsy portion. Student’s t test was used to compare cytokine production in vitro in response to rice and gluten. Other details of the statistical analysis and statistical power are included in Appendix 2.
All authors had access to the study data and reviewed and approved the final manuscript.
RESULTS
Demographics and Baseline Characteristics
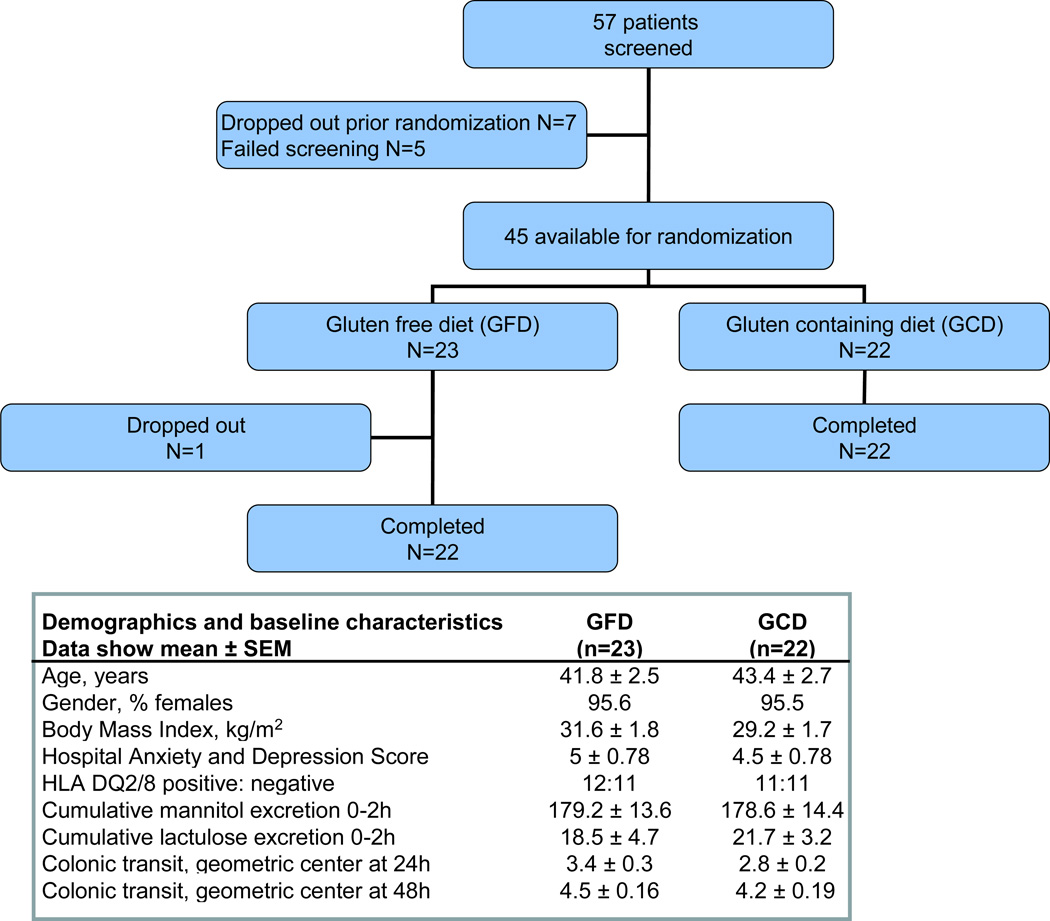
Figure 1 shows the trial flow and summarizes the baseline demographic data. There were no differences in age, gender distribution, Hospital Anxiety and Depression scores, BMI, baseline 0–2 hour excretion of mannitol and lactulose, and baseline colonic transit in the groups assigned to each diet. As expected in this patient population, there was a female predominance. The pre-study diet questionnaire demonstrated that the patients were not ingesting a gluten free diet prior to the study. The range of the number of gluten containing food servings per day was 1 to 15 (mean: 3.10±0.46). Only two patients were ingesting 15 servings of gluten containing food per day; 90% of participants ingested between 1 and 4.4 servings of gluten containing food per day. Participant compliance with the diets was uniformly excellent in the dietitians’ reports based on direct questions when patients picked up assigned diets and snacks.
Figure 1.
Trial flow according to the CONSORT guidelines and baseline demographics showing comparability of the two diet treatment groups.
Stool Frequency, Consistency and Ease of Passage
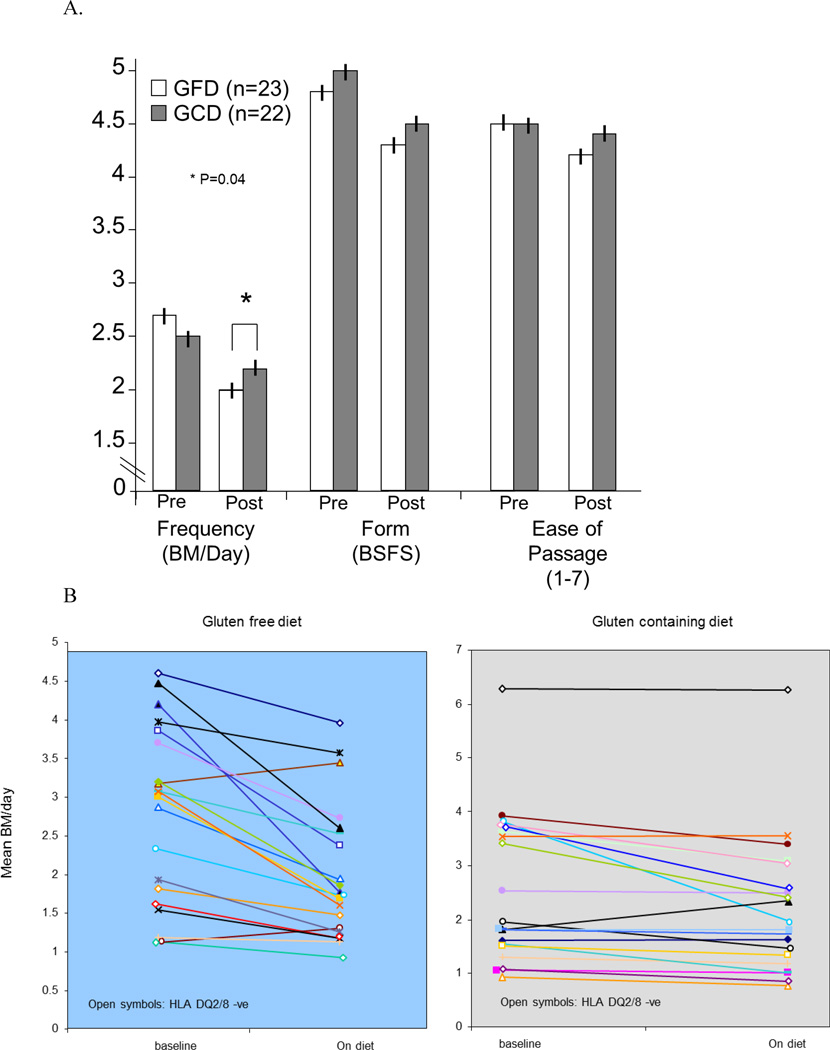
There was a diet effect on stool frequency in the overall groups, with a statistically significant decrease in subjects who were on a GFD compared to subjects on a GCD (p=0.04, Figure 2A, 2B). The 95% confidence interval (CI) of the difference in mean daily stool frequency for the entire study period between GFD and GCD was −0.652 to −0.015.
Figure 2.
A. Diet effect on stool frequency, (* p= 0.04) form and ease of passage; the effect on stool frequency was greater in HLA-DQ2 or 8 positive patients (p=0.019).
B. Mean bowel movements per day during 14-day baseline and 28-day diet treatment periods in each patient randomized to gluten free and gluten containing diets. HLA-DQ2/8 negative patients are indicated by the open symbols.
The diet effects on stool frequency were more pronounced in subjects who were HLA-DQ2 or 8 positive (p=0.019). In HLA-DQ2 or 8 negative patients, the 95% CI of the difference in mean daily stool frequency for the entire study period between GFD and GCD was −0.5712 to 0.344; in the HLA-DQ2 or 8 positive patients, the 95% CI was −1.005 to −0.092.
There was no significant diet effect (GFD vs. GCD) on mean daily stool form for the entire study cohort (overall 95% CI −0.475 to 0.251) with similar non-significant differences in the HLA-DQ 2 or 8 positive or negative groups.
There was no significant diet effect (GFD vs. GCD) on mean ease of passage score for the entire study cohort (overall 95% CI −0.337 to 0.010 [p=0.064]) with similar non-significant differences in the HLA-DQ 2 or 8 positive or negative groups.
Gastrointestinal and Colonic Transit
In the overall study cohort or HLA-DQ groups, there were no diet effects on GE and CF6; similarly, the GFD did not delay colonic transit at 24 or 48 hours (Table 1).
Table 1.
Effects of GCD and GFD on Barrier Functions, Tight Junction Proteins andColonic Transit
| GFD | GCD | P: effect of diet |
P: effect of diet in HLA + HLA− |
||
|---|---|---|---|---|---|
| Δ cum. urine mannitol 0–2h, mg | −48.6 ± 12.3 | 83.0 ± 70.9 | 0.028 | 0.586 | 0.01 |
| Δ cum. urine lactulose 0–2h, mg | 3.7 ± 4.0 | −3.5 ± 2.5 | 0.207 | 0.150 | 0.708 |
| Δ lactulose:mannitol ratio 0–2h | 0.008 ± 0.004 | −0.005 ± 0.004 | 0.0012 | 0.006 | 0.043 |
| Δ cum. urine mannitol 8–24h, mg | −21.6 ± 13.4 | −35.2 ± 12.7 | 0.358 | 0.999 | 0.203 |
| Δ cum. urine lactulose 8–24h, mg | −1.71 ± 3.22 | −5.25 ± 4.98 | 0.858 | 0.540 | 0.396 |
| Δ lactulose:mannitol ratio 8–24h | 0.027 ± 0.022 | 0.059 ± 0.024 | 0.531 | 0.445 | 0.919 |
| ZO-1 fold change, SB | 1.57 ± 0.24 | 1.11 ± 0.24 | 0.065 | 0.119 | 0.218 |
| Occludin fold change, SB | 1.14 ± 0.07 | 1.03 ± 0.08 | 0.28 | 0.017 | 0.490 |
| Claudin fold change, SB | 1.64 ± 0.31 | 1.13 ± 0.12 | 0.24 | 0.32 | 0.41 |
| ZO-1 fold change, colon | 1.97 ± 0.56 | 1.04 ± 0.26 | 0.025 | 0.038 | 0.161 |
| Occludin fold change, colon | 1.47 ± 0.16 | 0.96 ± 0.13 | 0.004 | 0.006 | 0.178 |
| Claudin fold change, colon | 1.63 ± 0.23 | 1.01 ± 0.15 | 0.036 | 0.015 | 0.203 |
| Colonic transit, GC24h | 3.2 ± 0.19 | 2.6 ± 0.2 | 0.182 | 0.364 | 0.251 |
| Colonic transit, GC48h | 4.4 ± 0.16 | 4.0 ± 0.2 | 0.304 | 0.352 | 0.548 |
Data show mean ± SEM. Δ = difference post- / pre-diet or fold change post- / pre-diet.
Small Bowel and Colonic Permeability
There were two participants who had >5 times the ingested mass of mannitol excreted in urine at baseline. This was deemed to be evidence of unrecognized ingestion of mannitol from a dietary contaminant. Therefore, the permeability was analyzed using intention to treat (Table 1) and per protocol principles.
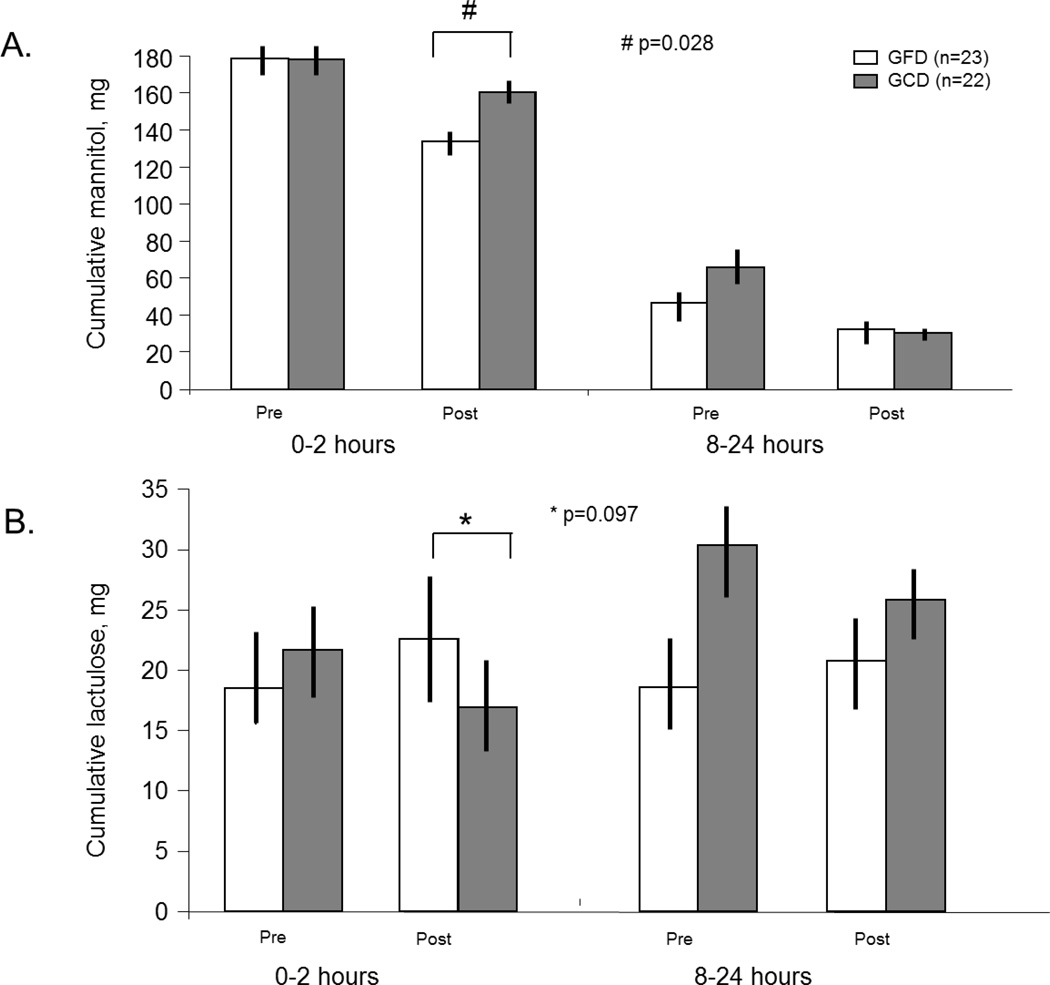
There was no diet effect on SB permeability for cumulative lactulose excretion in the ITT analysis (p=0.21); however, the per protocol analysis showed p=0.097 for the l diet effect in the overall cohort (Figure 3), and p=0.06 in the HLA-DQ2 or 8 positive patients (detailed below).
Figure 3.
Small intestinal and colonic permeability by the cumulative mannitol (A) and lactulose (B) excretion at 0–2h and 8–24h, respectively. There was increased small intestinal permeability with GCD, as shown by both cumulative mannitol excretion and lactulose to mannitol ratio (ITT analysis, # p=0.028 and p=0.0012). The effect on lactulose excretion was borderline significant (* p=0.097).
In the overall study cohort, there was an increased SB permeability with GCD relative to GFD, as shown by both cumulative mannitol excretion (Figure 3) and L:M ratio in the ITT analysis (p=0.028 and p=0.0012); these effects were also significant in the per protocol analysis (p=0.05; p=0.0035, respectively).
In the overall study cohort and the HLA-DQ subgroups, there were no diet effects on colonic permeability for the cumulative mannitol and lactulose excretion (Figure 3) or the L:M ratio (Table 1).
Impact of HLA-DQ Status on Small Bowel Mucosal Permeability
The effect of GCD on 0–2 hour cumulative mannitol excretion was stronger in HLA-DQ2 or 8 positive patients in both ITT and per protocol analysis (p=0.0097 and p=0.0086, respectively).
Similarly, the effects on L:M ratio at 0–2 hours were more pronounced in HLA-DQ2 or 8 positive patients in the ITT and per protocol analysis (p=0.006 and p=0.014, respectively).
Tight Junction mRNA Expression in Small Bowel and Colonic Mucosa
There was a p value of 0.065 for the comparison of the diet effect on ZO-1 mRNA expression in the SB, but no significant effect on occludin and claudin-1 mRNA in all patients in each diet group (Table 1). However, diet-associated changes in occludin expression in SB mucosa were significant (p=0.017) in the HLA-DQ2 or 8 positive group (Table 1).
Expressions of ZO-1, occludin, and claudin-1 mRNA in colonic mucosa were significantly lower in GCD relative to GFD in the overall groups, particularly in subjects with HLA-DQ2 or 8 positive status.
Small Bowel and Colonic Mucosal Morphology
There were no diet effects on SB IEL, CD3, CD8 CD4, CD68 and CD79 cells and tryptase positive (mast) cells. There were no diet effects on SB ZO-1 staining as determined by percent of cells and intensity of the staining (Table 2). Normal villus-to-crypt ratios confirmed the absence of celiac disease in all participants at baseline; there were no diet effects on villus-to-crypt ratios.
Table 2.
Small Bowel and Colon Histology and Immunohistochemistry (available in 28 patients)
| Data show mean ± SEM | GFD (n=14) | GCD (n=14) | P: effect of diet | |
|---|---|---|---|---|
| Duodenum | ||||
| IEL/100 epithelial cells | 28.5±5.0 | 23.5±6.1 | Ns | |
| CD3 lymphocytes/100 IEL | 30.5±5.4 | 23.9±5.5 | Ns | |
| CD4 lymphocytes# | ||||
| 1 | 1 | 4 | ||
| 2 | 11 | 10 | ||
| 3 | 1 | 0 | ||
| CD8 lymphocytes/100 IEL | 29.8±5.8 | 24.8±4.5 | Ns | |
| CD68 lymphocytes# | ||||
| 1 | 10 | 9 | ||
| 2 | 3 | 5 | ||
| CD79 lymphocytes# | ||||
| 1 | 1 | 3 | ||
| 2 | 11 | 10 | ||
| 3 | 1 | 1 | ||
| Mast cells #/hpf | 21.3±1.8 | 20.2±1.6 | Ns | |
| ZO-1 IHC | 152.3±22.3 | 165±21.5 | ||
| Villus:crypt ratio, % patients | ||||
| 3 | 38.5 | 35.7 | Ns | |
| 4 | 61.5 | 64.3 | Ns | |
| Colon | ||||
| IEL/100 epithelial cells | 3.1±0.4 | 2.6±0.5 | Ns | |
| CD4 lymphocytes# | ||||
| 1 | 13 | 9 | ||
| 2 | 0 | 5 | ||
| CD68 lymphocytes# | ||||
| 1 | 8 | 10 | ||
| 2 | 5 | 4 | ||
| CD79 lymphocytes# | ||||
| 1 | 9 | 10 | ||
| 2 | 3 | 3 | ||
| 3 | 1 | 1 | ||
| ZO-1 IHC | 115.4±19.8 | 135.7±18.6 | ||
CD4, CD68 and CD79a are scored semi-quantitatively (grade 1 to 4) and ZO-1immunohistochemistry was scored semi-quantitatively as described in methods section based on positive cells and intensity of staining. No statistical comparisons were conducted for the descriptive observations of CD4, CD68, CD79 lymphocytes and ZO-1 IHC, in contrast to the quantitative data on IELs, CD3, CD8 and mast cells.
Ns=non-significant
Relationships between Stool Frequency, Transit and Barrier Function
After diet intervention, we found a significant correlation in the overall study cohort between stool frequency and consistency and GC24, r=0.42 (p=0.03) and r=0.41 (p=0.03), respectively. There was no significant correlation between SB and colonic mRNA expression of TJ protein and bowel functions.
In vitro Proliferative Responses and Cytokine Responses of PBMCs to Gluten and Rice
The overall proliferation of PBMCs to gluten or rice in IBS patients was not different, and there was not an increased proliferative response to gluten in the DQ2 or 8 positive patients, suggesting that gluten-induced proliferation is not enhanced by DQ status and could be arising from non-T cells in both groups of patients.
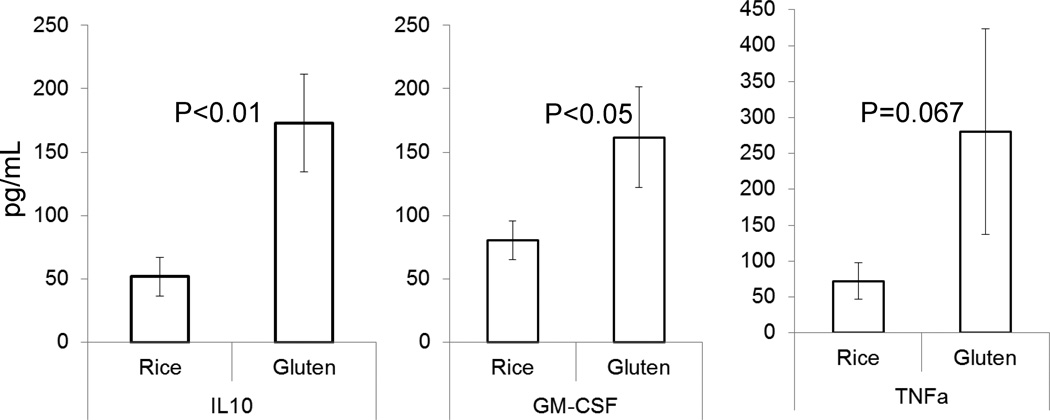
Gluten induced greater IL-10 and G-CSF by PBMCs compared to rice stimulation (p<0.01 and p<0.05, respectively; Figure 4). Interferon-gamma was not consistently changed; however, TNF-α production appeared to be increased in the PBMCs of IBS subjects with some gluten specificity (p=0.076). In general, cytokine responses were not predicted by DQ status.
Figure 4.
In vitro cytokine production by peripheral blood mononuclear cells in response to stimulation with either rice (control) or gluten. Note the increased production of IL-10 and GM-CSF, and the borderline increased TNF-α in response to gluten, compared to rice.
DISCUSSION
In this 4-week, randomized, controlled, diet intervention study, the intervention was associated with several significant effects. Subjects on a GCD had increased stool frequency compared to GFD; this effect was greater in HLA-DQ 2 or 8 positive patients. While the absolute difference in stool frequency is small, it is important to appreciate that this increased frequency is on a background of the typical increase in stool frequency (average 2.6 bowel movements / day at baseline) and consistency in patients with IBS-D. In addition, the patients studied were not selected on the basis of prior clinical demonstration of sensitivity to gluten; therefore, our observations are generalizable to the broader spectrum of IBS-D, rather than those with prior response to GFD. Patients receiving GCD also had increased SB permeability demonstrated by increased mannitol excretion and increased lactulose to mannitol ratio and, overall, this effect was greater in the HLA-DQ2 or 8 positive patients. GCD resulted in reduced mRNA expression of mucosal TJ proteins [most clearly demonstrated in colonic mucosa, and borderline significant for ZO-1 (p=0.065) in SB mucosa] in the absence of mucosal inflammatory markers. Effects on mRNA expression of TJ proteins were also associated with HLA-DQ2 or 8 positive status. Compared to GFD intervention, the GCD was not associated with significant effects on colonic transit, immunocyte activation or intra-epithelial lymphocytes in SB or colonic mucosa, or SB villus : crypt ratios. A limitation in the quantification of TJ protein expression is that it was solely based on PCR (mRNA expression), which may not necessarily match protein expression. Future studies will incorporate Western blot and other methods to directly identify these proteins and their distribution.
Patients on a GCD had increased SB permeability relative to GFD. While the clinical significance of these changes in permeability are not demonstrated in the current study, the abundant experimental evidence from the published literature that increased mucosal permeability enhances inflammation and leads to increased sensitivity is summarized elsewhere.21,22 Increased intestinal permeability has been described in previous studies in post-infectious IBS patients,6,23 but the effect of gluten in intestinal permeability in IBS-D patients has not been previously reported. Increased mucosal permeability may conceivably result in greater fluid flux towards the lumen (thereby altering stool consistency) or activation of sensory pathways that result in hypersensitivity.24 The mechanism underlying the host interaction with gluten that causes increased SB permeability in IBS-D is not well understood. However, it appears that HLA-DQ2 or 8 genotype is a susceptibility factor, as the overall effects of gluten on barrier function (lactulose : mannitol ratio and on mRNA expression of TJ proteins) were more pronounced in HLA-DQ2 or 8 positive IBS-D patients A prior study in gluten-sensitive patients with IBS exposed to a randomized dietary intervention did not document the effect of HLA genotype on permeability, but there was no significant effect of diet on permeability overall.25 Decreased ZO-1 expression in jejunal mucosa of IBS-D patients has been recently reported,5 and our study demonstrates one dietary factor (gluten) that may alter mRNA expression of TJ proteins in IBS-D, particularly, occludin, with greater susceptibility in HLA-DQ2 or 8 positive patients.
The association with HLA-DQ genotype suggests that an adaptive immune response may explain the effects of gluten on barrier function. It is also conceivable that gluten may mediate an immune response through the innate pathway, since recent studies have shown increased levels of toll-like receptors (TLR) in mucosa of gluten-sensitive patients.7 One hypothesis, based on the latter observation,7 is that the TLR interact with gluten and activate immune responses that may lead to changes in mucosal barrier function. Alternatively, the PBMC proliferative and cytokine responses to gluten and rice antigens suggest an increased response to gluten and rice, and, in support of this hypothesis, we have observed increased gut mucosal permeability in patients with IBS-D,4 which was also confirmed in these same patients at baseline compared to controls.26 The PBMC cytokine responses (particularly TNF-α and GM-CSF), which are increased in response to gluten, suggest monocytic stimulation or innate immune responses that are not DQ2/8 restricted. While increased PBMC cytokine responses to gluten have been well documented in celiac disease,27 this is the first documentation of PBMC cytokine responses in non-celiac patients with IBS-D. The lack of proliferation and IFNγ expression in response to gluten stimulation of the PBMCs strongly suggests that the gluten induced effects that are generated in the gluten sensitive IBS patients are not mediated by INFγ producing gluten specific CD4+ T cells like those seen in celiac disease. The increased production of the combination of cytokines, IL-10, GM-CSF, and TNFα in response to gluten over rice (a control grain) would suggest that other cells (potentially monocytes, dendritic cells, eosinophils, or NK T cells) are being stimulated by gluten. Overall, the increased production of TNFα in the absence of IFNγ production after in vitro treatment of PBMCs with gluten fragments is consistent with the stimulation of monocytes and, possibly, dendritic cells directly by gluten in an innate fashion.28 Further experiments are needed to determine which cell groups could be contributing to this particular cytokine pattern in the periphery and whether the same cytokine profile is observed in the gut.
Our data convincingly demonstrated effects of gluten on the increased mRNA expression of all the measured TJ proteins in colonic tissue relative to GFD. One limitation of the study was the inability to document alterations in colonic permeability using the 2-sugar excretion profile from 8 to 24 hours. We perceive that this may represent lack of sensitivity of the lactulose and mannitol excretion test, for example, due to the metabolism of both sugars by colonic bacteria.29 There are advantages to measuring both tissue and in vivo markers of barrier function. Another limitation is that the mechanism for improvement in stool frequency on a GFD in the absence of changes in colonic transit was not elucidated by our studies. Since it is unclear whether gluten or its metabolic products induce specific secretory mechanisms, the current hypothesis is that change in stool frequency may reflect change in fluid secretion from increased mucosal permeability. Our current studies did not evaluate effects of gluten on the microbiome, afferent functions, or cytokine expression in the mucosal biopsies from patients before and after the interventions. These would be interesting parameters to include in future studies. Finally, our study does not specifically address the effects of gluten protein per se, and it is possible that other proteins in wheat flour may be responsible for the changes observed.
Overall, our data provide mechanistic explanations for the observation that gluten withdrawal may improve patient symptoms in IBS, and the data also explain, in part, the observation of Wahnschaffe et al. the beneficial effects of gluten withdrawal in view of our observation that biological effects of gluten observed in our study were associated with HLA-DQ2 or 8 genotype.2 The data suggest that the relationship of dietary factors, innate and adaptive immune responses and mucosal interactions in IBS-D deserve further study, and they support the need for further clinical intervention studies to evaluate the clinical effects of gluten withdrawal in patients with IBS-D.
Acknowledgments
Grant Support: This study was supported in part by NIH 1RC1-DK086182 and NIH R01-DK092179 (Dr. Camilleri) and NIH CTSA grant UL1 TR000135. We thank Michael Ryks and Debbie Rhoten for technical support and Cindy Stanislav for secretarial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: JAM has received grants from Alba Therapeutics for clinical trials with the drug, larazotide. The other authors have no conflicts of interest.
Authors’ Contributions:
M.I. Vazquez-Roque: fellow investigator, recruitment of participants, authorship of paper
REFERENCES
- 1.Cash BD, Rubenstein JH, Young PE, et al. The prevalence of celiac disease among patients with nonconstipated irritable bowel syndrome is similar to controls. Gastroenterology. 2011;141:1187–1193. doi: 10.1053/j.gastro.2011.06.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wahnschaffe U, Schulzke JD, Zeitz M, Ullrich R. Predictors of clinical response to gluten-free diet in patients diagnosed with diarrhea-predominant irritable bowel syndrome. Clin Gastroenterol Hepatol. 2007;5:844–850. doi: 10.1016/j.cgh.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 3.Verdu EF, Huang X, Natividad J, et al. Gliadin-dependent neuromuscular and epithelial secretory responses in gluten-sensitive HLA-DQ8 transgenic mice. Am J Physiol. 2008;294:G217–G225. doi: 10.1152/ajpgi.00225.2007. [DOI] [PubMed] [Google Scholar]
- 4.Rao AS, Camilleri M, Eckert DJ, et al. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol. 2011;301:G919–G928. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez C, Vicario M, Ramos L, et al. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107:736–746. doi: 10.1038/ajg.2011.472. [DOI] [PubMed] [Google Scholar]
- 6.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol. 2006;101:1288–1294. doi: 10.1111/j.1572-0241.2006.00672.x. [DOI] [PubMed] [Google Scholar]
- 7.Sapone A, Lammers KM, Casolaro V, et al. Divergence of gut permeability and mucosal immune gene expression in two gluten-associated conditions: celiac disease and gluten sensitivity. BMC Med. 2011;9:23–34. doi: 10.1186/1741-7015-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pellett PL. Food energy requirements in humans. Am J Clin Nutr. 1990;51:711–722. doi: 10.1093/ajcn/51.5.711. [DOI] [PubMed] [Google Scholar]
- 9.Drossman DA, Corazziari E, Talley N, et al., editors. The Functional Gastrointestinal Disorders. 2nd ed. McLean, VA: Degnon Associates; 2000. Functional Bowel Disorders and Functional Abdominal Pain. [Google Scholar]
- 10.Talley NJ, Phillips SF, Wiltgen CM, Zinsmeister AR, Melton LJ., 3rd Assessment of functional gastrointestinal disease: the bowel disease questionnaire. Mayo Clin Proc. 1990;65:1456–1479. doi: 10.1016/s0025-6196(12)62169-7. [DOI] [PubMed] [Google Scholar]
- 11.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 12.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 13.Coulie B, Szarka LA, Camilleri M, Burton DD, McKinzie S, Stambler N, Cedarbaum JM. Recombinant human neurotrophic factors accelerate colonic transit and relieve constipation in humans. Gastroenterology. 2000;119:41–50. doi: 10.1053/gast.2000.8553. [DOI] [PubMed] [Google Scholar]
- 14.Monsuur AJ, de Bakker PI, Zhernakova A, et al. Effective detection of human leukocyte antigen risk alleles in celiac disease using tag single nucleotide polymorphisms. PLoS One. 2008;3:e2270. doi: 10.1371/journal.pone.0002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cremonini F, Mullan BP, Camilleri M, Burton DD, Rank MR. Performance characteristics of scintigraphic transit measurements for studies of experimental therapies. Aliment Pharmacol Ther. 2002;16:1781–1790. doi: 10.1046/j.1365-2036.2002.01344.x. [DOI] [PubMed] [Google Scholar]
- 16.Burton DD, Camilleri M, Mullan BP, Forstrom LA, Hung JC. Colonic transit scintigraphy labeled activated charcoal compared with ion exchange pellets. J Nucl Med. 1997;38:1807–1810. [PubMed] [Google Scholar]
- 17.Deiteren A, Camilleri M, Bharucha AE, et al. Performance characteristics of scintigraphic colon transit measurement in health and irritable bowel syndrome and relationship to bowel functions. Neurogastroenterol Motil. 2010;22:415–423. doi: 10.1111/j.1365-2982.2009.01441.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camilleri M, Nadeau A, Lamsam J, et al. Understanding measurements of intestinal permeability in healthy humans with urine lactulose and mannitol excretion. Neurogastroenterol Motil. 2010;22:e15–e26. doi: 10.1111/j.1365-2982.2009.01361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lostia AM, Lionetto L, Principessa L, et al. A liquid chromatography/mass spectrometry method for the evaluation of intestinal permeability. Clin Biochem. 2008;41:887–892. doi: 10.1016/j.clinbiochem.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Jelínková L, Tucková L, Cinová J, Flegelová Z, Tlaskalová-Hogenová H. Gliadin stimulates human monocytes to production of IL-8 and TNF-alpha through a mechanism involving NF-kappaB. FEBS Lett. 2004;571:81–85. doi: 10.1016/j.febslet.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 21.Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camilleri M, Lasch K, Zhou W. The confluence of increased permeability, inflammation, and pain in irritable bowel syndrome. Am J Physiol. 2012;303:G775–G785. doi: 10.1152/ajpgi.00155.2012. [DOI] [PubMed] [Google Scholar]
- 23.Marshall JK, Thabane M, Garg AX, et al. Intestinal permeability in patients with irritable bowel syndrome after a waterborne outbreak of acute gastroenteritis in Walkerton, Ontario. Aliment Pharmacol Ther. 2004;20:1317–1322. doi: 10.1111/j.1365-2036.2004.02284.x. [DOI] [PubMed] [Google Scholar]
- 24.Bielefeldt K, Levinthal D. Pieces of a puzzle: Permeability, proinflammatory pathways and pain? Pain. 2009;146 doi: 10.1016/j.pain.2009.06.008. 7–8.23. [DOI] [PubMed] [Google Scholar]
- 25.Biesiekierski JR, Newnham ED, Irving PM, et al. Gluten causes gastrointestinal symptoms in subjects without celiac disease: a double-blind randomized placebo-controlled trial. Am J Gastroenterol. 2011;106:508–514. doi: 10.1038/ajg.2010.487. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, O'Neill J, Carlson P, Lamsam J, Eckert DJ, Janzow D, Burton DD, Ryks M, Rhoten D, Zinsmeister AR. Association of HLA-DQ Gene with Bowel Transit, Barrier Function and Inflammation in Irritable Bowel Syndrome with Diarrhea. Am J Physiol Gastrointest Liver Physiol. 2012 Oct 4; doi: 10.1152/ajpgi.00294.2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stepniak D, Koning F. Celiac disease--sandwiched between innate and adaptive immunity. Hum Immunol. 2006;67:460–468. doi: 10.1016/j.humimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Cinova J, Palová-Jelínková L, Smythies LE, Cerná M, Pecharová B, Dvorák M, Fruhauf P, Tlaskalová-Hogenová H, Smith PD, Tucková L. Gliadin peptides activate blood monocytes from patients with celiac disease. J Clin Immunol. 2007;27:201–209. doi: 10.1007/s10875-006-9061-z. [DOI] [PubMed] [Google Scholar]
- 29.Meddings JB, Gibbons I. Discrimination of site-specific alterations in gastrointestinal permeability in the rat. Gastroenterology. 1998;114:83–92. doi: 10.1016/s0016-5085(98)70636-5. [DOI] [PubMed] [Google Scholar]