Abstract
We examined how prenatally acquired vitamin A deficiency (VAD) modulates innate immune responses and human rotavirus (HRV) vaccine efficacy in a gnotobiotic (Gn) piglet model of HRV diarrhea. The VAD and vitamin A sufficient (VAS) Gn pigs were vaccinated with attenuated HRV (AttHRV) with or without concurrent oral vitamin A supplementation (100,000IU) and challenged with virulent HRV (VirHRV).
Regardless of vaccination status, the numbers of conventional and plasmacytoid dendritic cells (cDCs and pDCs) were higher in VAD piglets pre-challenge, but decreased substantially post-challenge as compared to VAS pigs. We observed significantly higher frequency of CD103 (integrin αEβ7) expressing DCs in VAS vs. VAD piglets post-challenge, indicating that VAD may interfere with homing (including intestinal) phenotype acquisition. Post VirHRV challenge, we observed longer and more pronounced diarrhea and higher VirHRV fecal titers in non-vaccinated VAD piglets. Consistent with higher VirHRV shedding titers, higher IFNα levels were induced in control VAD vs. VAS piglet sera at post-challenge day (PCD)2. Ex vivo HRV-stimulated mononuclear cells (MNCs) isolated from spleen and blood of VAD pigs pre-challenge also produced more IFNα. In contrast at PCD10, we observed reduced IFNα levels in VAD pigs that coincided with decreased TLR3+ MNC frequencies. Numbers of necrotic MNCs were higher in VAD pigs in spleen (coincident with splenomegaly in other VAD animals) pre-challenge and intestinal tissues (coincident with higher VirHRV induced intestinal damage) post-challenge. Thus, prenatal VAD caused an imbalance in innate immune responses and exacerbated VirHRV infection, whereas vitamin A supplementation failed to compensate for these VAD effects.
Introduction
Rotavirus (RV) is a leading cause of viral diarrhea in infants, children and young animals. Rotavirus infection is responsible for approximately 660,000 deaths worldwide, annually causing 5% of all deaths in children less than 5 years of age. More than 85% of RV-related fatalities occur in developing countries (1). Current licensed attenuated RV vaccines (RotaTeq®, Rotarix®) effective in developed countries show reduced efficacy in impoverished regions (2–5). Adequate levels of vitamin A are required to maintain intestinal homeostasis and increase resistance against many mucosal pathogens. Even marginal (subclinical) vitamin A deficiency (VAD) may compromise various aspects of innate and adaptive immune responses, resulting in enhanced susceptibility to infectious diseases. Additionally, micronutrient deficiencies, including low vitamin A, are suspected to reduce the efficacy of vaccines (6). The World Health Organization (WHO) has recommended vitamin A supplementation of 100,000–200,000 IU every 4 to 6 months to increase its levels in VAD infants and children. Supplementation of large dose vitamin A (with or w/o vaccination) in randomized placebo controlled clinical trials in VAD regions suggests an overall positive effect on infection-related morbidity and mortality, varying however, with pathogen, age and gender (7–11).
Serum retinol concentration is homeostatically controlled over the range of adequate liver stores; therefore an increase in serum retinol concentration after a single high dose supplementation is indicative of vitamin A deficiency (VAD) and a 30-day dose-response test is being used to reveal subclinical VAD (12–14). The transfer of retinol from mother (even vitamin A sufficient) to fetus is limited during gestation (15). Therefore neonates have low reserves of retinol and are dependent on vitamin A-rich breast milk (15, 16). However, gestational VAD may affect fetal development in-utero, including the immune system, and it is possible that some of these effects cannot be fully reversed by short term vitamin A supplementation after birth.
Antibody responses to tetanus toxoid and Newcastle disease virus were consistently reduced in VAD rats and poultry, respectively (17, 18). Vitamin A-deficient mice infected with RV produced significantly lower levels of serum RV antibody than mice pair-fed the control diet or fed ad libitum (19, 20). In all the experiments using adult animal models, repletion with vitamin A restored a normal level of antibody production. Morbidity and mortality rates after E. coli infection were greater in VAD chicks, but were also high in chicks that received an excess of vitamin A (21). The onset of herpetic keratitis was more rapid, and the clinical disease, inflammatory response, incidence of epithelial ulceration and necrosis were more severe in VAD rats than control rats (22). Vitamin A deficiency in rats has been associated with decreased IFN production by spleen cells and diminished NK cell function in spleen (17, 23, 24), both of which were restored after oral vitamin A supplementation (23). Vitamin A-deficient mice showed a moderate reduction in the T cell area of the spleen, a significant reduction in thymus mass and a reduced number of goblet cells per duodenal villus (20). There was a marked destruction of the villus tips in VAD mice infected with RV, but neither VAD nor RV infection alone produced such a marked effect. Recent studies of mice demonstrated that VAD alters splenic dendritic cell (DC) subpopulations, possibly contributing to skewed immune responses (25). However, in most of these studies that demonstrate vitamin A repletion effects, VAD was induced by dietary manipulations after birth, while the actual situation in humans may be more complex including both prenatal (maternal) and dietary VAD.
The adjuvant properties of retinol were first reported in 1968 by Dresser who showed that retinol-treated mice produced antibodies to soluble bovine gamma-globulin, which is not immunogenic in the mouse (26). Cytokine production, lymphocyte transformation and resistance to tumour cells have all been reported to be greater in normal animals supplemented with high doses of vitamin A, possibly by recruiting lymphocytes and monocytes to the circulation and altering membrane structure (27, 28). Phagocytosis by peritoneal macrophages in mice and S. typhimurium clearance in rats were greater in groups fed diets high in retinyl palmitate, probably through macrophage and T lymphocyte activation (29, 30). More recent studies on vitamin A adjuvant characteristics are somewhat contradictory and suggest that vitamin A adjuvancy can be pathogen/antigen and gender specific (31–35).
The immune function of retinoic acid (RA, a metabolite of vitamin A) includes enhancement of B and T cell mucosal homing (α4β7 and CCR9), generation of regulatory T cells, increase in IgA antibody secretion and restoration of NK cell activity, all of which are critical for RV clearance and protection against RV diarrhea (23, 36, 37). A number of recent findings suggests that the (38) immunomodulatory effects of RA are primarily mediated through gut DCs, which play a central role in generation of appropriate and balanced immune responses [T helper (Th) 1, Th2, Th3, Th17 and T reg] (25, 36).
Piglets resemble human infants in many biological and immunological aspects and are susceptible to HRV diarrhea for a prolonged time exhibiting gut pathology similar to infants (39–41). As an outbred model, piglets show heterogeneity in immune responses representative of humans, and gnotobiotic (Gn) pigs have no extraneous confounding microflora that may complicate interpretation of clinical trial results in humans. Additionally, dietary induced VAD in sows allows us to reproduce the actual situation in impoverished countries where maternal VAD may affect very early steps of fetal development including immune functions.
We induced dietary VAD in pregnant sows and fed the VAD Gn piglets after birth with a low vitamin A milk diet to maintain their VAD status. Control vitamin A sufficient (VAS) Gn pigs were derived dietary VAS sows. Both pregnant VAS sows and the derived Gn piglets (at birth) received supplemental vitamin A to maintain their VAS status. Using our Gn pig model, we investigated how gestational VAD affects innate immune responses to and protective efficacy of an AttHRV oral vaccine and the severity of VirHRV-induced disease. Additionally, we assessed whether it was possible to reverse VAD effects by large dose vitamin A supplementation with each of the 3-dose AttHRV vaccinations [following the WHO recommended regimen for children in developing countries with RV vaccinations and large-dose (100,000IU) oral vitamin A supplementation given concurrently] and whether vitamin A acts as an adjuvant in the AttHRV vaccinated piglets.
Materials and Methods
Virus
The virulent HRV (VirHRV) Wa strain was used for pig challenge at a dose of 1×105 fluorescent-forming units (FFU). The ID50 of VirHRV in pigs was determined as approximately 1 FFU (39, 42). Cell-culture adapted attenuated HRV (AttHRV) Wa strain was maintained in MA 104 monkey kidney cells and used as a vaccine [3 doses of 5 × 107 fluorescent-forming units (FFU)/dose] and positive control in the cell culture immunofluorescence assay (CCIF)(39, 42).
Experimental design
This study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Ohio State University and conducted in compliance with local and federal guidelines. Pregnant sows were assigned to VAD or VAS diet at approximately gestational day 34 (when pregnancy was confirmed) or about 80 days prior to derivation of Gn piglets. The sows fed VAS diet were given an additional 500,000IU of injectable vitamin A (Vedco, NDC50989-178-12) to compensate for the naturally declining vitamin A levels during gestation. The Gn pigs were derived by hysterectomy from pair-fed [vitamin A deficient (VAD) and conventional VAS diets] near-term sows (Landrace × Yorkshire White × Duroc crossbred) and maintained in sterile isolation units as described previously (38). Pigs were fed commercial ultra-high temperature-treated sterile milk (Parmalat). Vitamin A supplementation from the milk diet was estimated as 350IU daily (approximately 3 times less than recommended by ARC 1981 or NRC, 1998 for piglets), allowing us to maintain low vitamin A levels in VAD piglets, but not depleting vitamin A liver storage in the VAS piglets. Additionally, supplemental vitamin A (50,000IU dose) was given intramuscularly to VAS piglets at birth to stably maintain adequate vitamin A levels. Retinyl palmitate (Sigma-Aldrich, R3375) at 100,000IU dose was used for oral supplementation with each vaccine dose or mock inoculation. Pigs were randomly assigned to one of four treatment groups as follows: 1) vaccinated-non vitamin A supplemented (3xAttHRV)(VAD n=12; VAS n=11); 2) vaccinated-vitamin A supplemented with each vaccination (3xAttHRV, VitA 100,000IU) (VAD n=9; VAS n=10); 3) non-vaccinated – nonvitamin A supplemented (control) (VAD n=9; VAS n=9); and 4) non-vaccinated – vitamin A supplemented (control, VitA 100,000IU) (VAD n=9; VAS n=8). Non-vaccinated control pigs were given an equal volume of diluent in place of vaccine. All pigs were given 5 ml of 100 mM sodium bicarbonate to reduce gastric acidity 20 min before AttHRV, VirHRV or mock inoculation. After three sequential vaccinations with AttHRV [post-inoculation day (PID)0, PID10 and PID20], a subset of Gn piglets in each group was euthanized at PID26 to assess pre-challenge immune responses, and the others were challenged with VirHRV Wa strain and euthanized 10 days later (PID36) to assess post-challenge immune responses and vaccination efficacy. Rectal swabs (RS) were collected daily to assay VirHRV shedding (post-challenge day, PCD0-10). Pigs were examined daily for diarrhea post-challenge as described previously (38, 40, 41). Serum samples were collected at PID 0, 2, 4, and PID26/PCD0 and PID36/PCD10 to assess vitamin A and IFNα levels (1). Pigs were euthanized at PID26/PCD0 and PID36/PCD10 to isolate the mononuclear cells (MNC) from ileum, duodenum, spleen and peripheral blood for determining frequencies of DCs and apoptotic/necrotic MNCs. Liver samples were collected at euthanasia for assessment of vitamin A levels. Spleen and body weights were measured for VAS and VAD pigs and spleen/body weight ratios were calculated to estimate the relative spleen weight increases.
Detection of rectal HRV shedding by cell culture immunofluorescent assay (CCIF)
Rectal swab samples were used to quantitate infectious HRV post-challenge by CCIF assay as previously described (42, 43). Briefly, rectal swab samples were initially diluted 1:25 in serum-free minimum essential medium, then diluted 1:4 and serially diluted 10-fold thereafter. Rectal swab fluids from mock-inoculated pigs were used as negative controls. The HRV-positive rectal swab fluids from reference HRV-inoculated pigs were used as positive controls. The final CCIF titers were calculated and expressed as the reciprocal of the highest dilution showing positive fluorescing cells.
Serum and liver vitamin A levels
Serum and liver samples were submitted to the Diagnostic Center for Population and Animal Health, Michigan State University, Lansing, MI, and vitamin A concentrations were measured by HPLC.
Isolation of mononuclear cells (MNCs)
Spleen, blood, duodenum and ileum were collected the day of euthanasia and processed for isolation of MNC as previously described (38, 41). After isolation, the cells were diluted in Roswell Park Memorial Institute (RPMI) 1640 medium (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (Atlas biological, Fort Collins, CO), 1% L-glutamine, sodium pyruvate, non-essential amino acids and ampicilin/gentamicin (20μg/ml/200μg/ml) (Invitrogen) (DC media) and kept at room temperature (RT) until testing. Flow cytometry stainings were performed the same day immediately after the isolation of all tissue-derived cells. MNCs (400,000) for qRT-PCR (RBP4/RARα gene expression) were frozen at −70°C in RNALater Buffer (Qiagen, Valencia, CA).
Flow cytometry to assess plasmacytoid and conventional dendritic cell (pDCs/cDCs) frequencies and distribution
Procedures for flow cytometry staining (including buffers used) were performed as described previously (44–47) with minor modifications. The 96 deep well plates (Eppendorf AG - Hamburg, Germany 0030 521.102) were used to perform all flow cytometry stainings. To assess DC subset frequencies and distribution, cells were stained with specific and cross-reactive monoclonal antibodies (mAb) to porcine and human cell surface markers, respectively, isotype control and secondary antibodies (Table I). Swine plasmacytoid DCs were defined as SWC3a+CD4+CD11R1− and conventional DCs were defined as SWC3a+CD4−CD11R1+ (48). Expression of CD103 marker (Table I) was assessed for both DC subsets and the total MNC population. After the cell surface marker stainings were completed, the cells were permeabilized using Cytofix/Cytoperm [Becton Dickinson (BD), 554714], washed in Perm/Wash Buffer (Becton Dickinson, 554723) and resuspended in Perm/Wash Buffer. Intracellular staining for TLR3+ (Table I) was performed as recommended by the manufacturer and acquisition of 50,000 events was done using AccuriC6 flow cytometer (BD, Accuri cytometers, Inc.). Analyses were performed using CFlow software (BD, Accuri cytometers, Inc.).
Table I.
Antibodies used for flow cytometry analyses.
| Marker | Fluorochrome* | Antibody/Vendor/Cat. # | Isotype | Note |
|---|---|---|---|---|
| SWC3a (porcine) | PE | Mouse Anti-porcine Monocyte/Granulocyte-PE/Southern Biotech/ 4525-09 | IgG1 | Human analog CD172 |
| CD4 (porcine) | SPRD | Mouse Anti-porcine CD4a/Southern Biotech/4515-13 | IgG2b | |
| CD11R1 (porcine) | No | Mouse Anti-pig CD11R1/Serotec/MCA1220 | IgG1 | Human analog CD11b |
| CD103 (human) | FITC | Mouse Anti-Pig CD103 FITC Conjugated, clone B-Ly7/eBiosciences/11-1038-73 | IgG1 | Cross reacts with porcine CD103 |
| TLR3 (human) | PE | PE-Anti-Human TLR3/eBiosciences/12-9039-82 | IgG1 | Cross reacts with porcine TLR3 |
| Isotype control, IgG1-PE | PE | Mouse IgG1-PE/Southern Biotech/0102-09 | IgG1 | Isotype control SWC3a PE |
| Isotype control, IgG2b-SPRD | SPRD | Mouse IgG2b-SPRD/Southern Biotech/0104-13 | IgG2b | Isotype control CD4 SPRD |
| Isotype control, IgGI-FITC | FITC | Mouse IgG1 K Isotype Control FITC/eBiosciences/11-4714-42 | IgG1 | Isotype control CD103 FITC |
| Secondary antibody, anti-mouse IgG1, APC | APC | APC Rat Anti-Mouse IgG1, clone X56/BD biosciences/550874 | IgG1 | Secondary antibody for CD11R1 |
PE - R-phycoerythrin; SPRD – SpectralRed; FITC - Fluorescein isothiocyanate; APC – Allophycocyanin.
Frequencies and tissue distribution of apoptotic and necrotic MNC by flow cytometry
Annexin V Apoptosis Detection Kit APC (eBiosciences, 88-8007-74) and Propidium Iodide Staining Solution (eBiosciences, 006990-50) were used according to the manufacturer protocols to detect and discriminate apoptotic and necrotic MNCs. Within 4 hours after the staining, acquisition of 50,000 events was performed using AccuriC6 flow cytometer and analyses were performed using CFlow software.
RNA isolation and real-time RT-PCR for the retinol binding protein 4 (RBP4) and retinoic acid receptor-alpha (RARα) mRNA quantification
Total RNA was extracted from the purified MNCs using an RNeasy mini kit (Qiagen) according to the manufacturers' protocols. RNA was digested with DNase I (RNase-Free DNase set; Qiagen). RT-PCR was conducted using an equal amount of total RNA (150 ng) with QuantiTect SYBR Green RT-PCR Kit (Qiagen, USA). The real-time quantitative RT-PCR was done in a final volume of 20 μl, which contained 10 μl of 2× SYBR Mix, 0.2 μl of RT Mix and 25 pmol of each primer for the detection of RBP4, RARα, and β-actin that was used as a housekeeping gene. All reactions were done in duplicate in PCR 8-tube strips using the EppendorfMastercycler EP Realplex (Eppendorf, Germany). The following primers were designed in this study and used for RBP4 and RARα mRNA quantification, respectively: pRBP4-NF: GCAAGATGGAATGGGTTTG (sense)/pRBP4-NR: GTTCTCTTTGACTCGGAAGCTG (antisense) and pRARa-F: AGCTGGGCAAATACACTACGAA (sense)/pRARa-R: GGCAGCYGCTTGGCRAACTC (antisense).
All-trans retinoic acid (ATRA) treatment of splenic MNCs and proliferation assay
A stock solution of 1mM retinoic acid (Sigma-Aldrich, R2625) was prepared in 95% ethanol. Splenic MNCs were resuspended in enriched RPMI-1640 media (Gibco-Invitrogen) supplemented with 10% fetal bovine serum, 2 mM glutamine, and 100 IU of Ampicillin and 100 mg of gentamicin/ml and stimulated ex vivo with different final concentrations (0.1μM, 1 μM, and 10μM) of ATRA for 24 hrs. To assess ex vivo proliferation we used Click-iT® EdU Alexa Fluor® 488 Flow Cytometry Assay Kit (Invitrogen-Life Technologies, Grand Island, NY) according to the manufacturer's instructions.
Interferon-α production by splenic MNCs after ex vivo re-stimulation with HRV
MNCs/DCs (400,000 cells/well, each sample in duplicate) were cultured for 24 hrs at 37°C, with 5% CO2, in 24-well plates in enriched RPMI-1640 in the presence of HRV antigen or phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) as a control added at final concentrations of 12μg/ml and 25μg/ml, respectively. After 24 hrs, cells were removed by centrifugation, and supernatants were stored at −80°C.
IFNα bioassay
The cytopathic effect inhibition bioassay for IFNα has been described elsewhere (49, 50). Briefly, monolayer cultures of 1 day old Madin Darby Bovine Kidney (MDBK) cells in 96-well microtiter plates were rinsed twice and incubated with 4-fold serial dilutions of samples (sera or MNC supernatants) or standard (recombinant porcine IFNα) for 16 hrs, and then rinsed twice and challenged with vesicular stomatitis virus (VSV, Indiana strain). Forty-eight hours later, when complete cytopathic effects (CPE) developed in VSV control wells (w/o IFNα), the medium was discarded from all wells, the plates were rinsed and fresh medium was added, then 10μl of AlamarBlue (Invitrogen) was added to each well. Five hrs later, the fluorescence was read at 530–560 and 590nm using Fluoroskan Ascent FL and Ascent software (Thermo Electron Corporation). One international unit per milliliter (IU/ml) is defined here as that concentration which in the bioassay protects 50% of the cells in a culture against VSV-induced CPE (reciprocal of the dilution producing 50% CPE × 20).
Statistical analyses
Fisher's exact test was used to compare proportions of pigs with diarrhea and virus shedding among groups. One-way analysis of variance (ANOVA-general linear model), followed by Duncan's multiple range test, were used to compare mean duration of virus shedding and diarrhea, vitamin A and IFNα levels. The frequencies of cell populations measured by flow cytometry were compared among or within groups using the Kruskal-Wallis rank sum (non parametric) test. Statistical significance was assessed at p ≤0.05 for all comparisons. All statistical analyses were performed using the Minitab 16 program (Minitab Inc, PA, USA).
Results
Hepatic vitamin A levels were significantly higher in VAS and vitamin A supplemented piglets
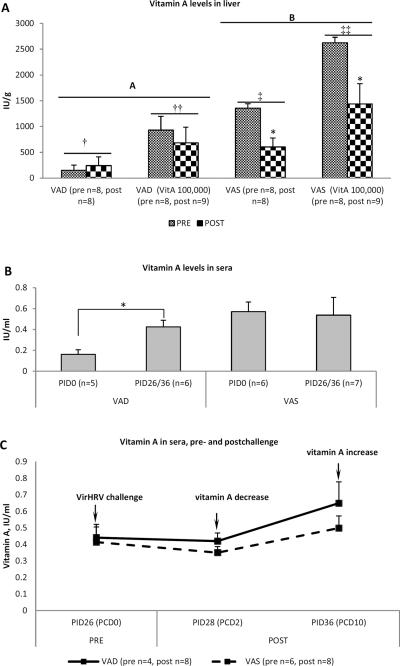
Hepatic vitamin A levels for VAD piglets were significantly lower than for VAS piglets in all treatment groups (Fig. 1a). Hepatic vitamin A levels of non-supplemented piglets were significantly lower than those of supplemented piglets, respectively on PID26/PCD0 and PID36/PCD10. Serum vitamin A levels of VAS piglets were within the normal range defined for nursing piglets (0.3–1IU/ml) and stayed at similar levels throughout the experiment reflecting that liver and serum vitamin A levels are under homeostatic regulation (Fig. 1b). In contrast, in VAD piglets, during the course of experiment, serum vitamin A levels increased ~2 fold (Fig. 1b) suggesting a positive “30 day dose response” that indicates the presence of subclinical VAD as observed in human clinical studies (12). The 3 dose vitamin A supplementation (100,000IU) did not affect serum vitamin A levels in VAD or VAS piglets (data not shown).
Fig. 1.
(a) Hepatic vitamin A in VAD and VAS piglets, pre- and post-challenge. Significantly (p≤0.05) higher vitamin A levels were observed in livers of vitamin A supplemented (†† and ‡‡) compared to non-supplemented († and ‡) piglets, in livers of VAS piglets pre-challenge compared to post-challenge (*) and in livers of VAS (B) compared to VAD (A) piglets. The results are grouped regardless of vaccination status as no effect of vaccination on hepatic vitamin A levels was evident. (b) Serum vitamin A levels in VAD and VAS pigs, pre- and post-challenge. Asterisk indicates that vitamin A levels differed significantly between PID0 and PID26/36 for VAD pigs. The results are grouped regardless of vaccination or vitamin A supplementation status as no effect of vaccination or vitamin A supplementation on serum vitamin A levels was evident. (c) Transient decrease in circulating vitamin A in most VAD and VAS piglets at post-challenge day 2. The results are grouped regardless of vaccination or vitamin A supplementation status as no effect of vaccination or vitamin A supplementation on serum vitamin A levels was evident. Pooled data are the mean ± SEM from two (c) or three (a and b) independent experiments.
Interestingly, VirHRV challenge of VAS piglets resulted in significantly reduced hepatic vitamin A post-challenge (PCD10) (Fig. 1a) and a transient decrease in serum vitamin A early after the challenge (PCD2) that was restored by PCD10, likely by means of hepatic vitamin A release into serum (Fig. 1c). Thus VirHRV or VirHRV induced diarrhea may affect vitamin A metabolism or absorption.
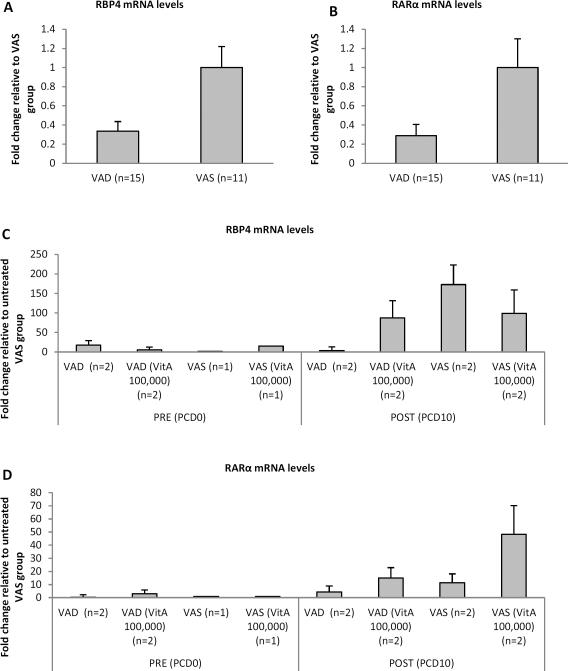
RBP4 and RARα mRNA levels were higher in VAS piglets
RBP4 and RARα mRNA levels were measured by quantitative RT-PCR in MNCs isolated from ileum, duodenum, spleen and blood at pre- (PID26/PCD0) and post- (PID36/PCD10) VirHRV challenge. However, detectable levels of RBP4 and/or RARα mRNA and differences between the treatment groups were only observed for splenic MNCs. Consistent with vitamin A levels, RBP4 and RARα mRNA levels were affected by vitamin A status, vitamin A supplementation and VirHRV challenge, but not by the vaccination status. Overall they were higher in splenic MNCs of VAS pigs as compared to VAD pigs (Fig. 2a,b). Interestingly, RBP4 and RARα mRNA expression was increased post-challenge in both VAS and VAD animals (Fig. 2c,d). RARα mRNA levels were increased in vitamin A supplemented VAS and VAD pigs post-challenge (Fig. 2d), whereas RBP4 mRNA levels were decreased in vitamin A supplemented VAS and increased in vitamin A supplemented VAD pigs post-challenge (Fig. 2c).
Fig. 2.
Relative RBP4 and RARα mRNA levels in splenic MNCs of VAD/VAS pigs measured by real-time RT-PCR and expressed as fold change relative to VAS group (a,b) or to untreated (no vitamin A supplementation, vaccination or challenge) VAS group (c,d). (a) and (b) fold change is shown regardless of treatment (vitamin A supplementation, vaccination or challenge) for RBP4 and RARα mRNA levels, respectively. (c) and (d) fold change is shown pre- and post-challenge for each group for RBP4 and RARα mRNA levels, respectively. Data are the mean ± SEM from one experiment.
HRV shedding and diarrhea were higher in VAD piglets
VAD control piglets had significantly more severe and longer duration of diarrhea compared to VAS piglets (Table II). Noteworthy, in control VAS piglets, fecal scores peaked by PCD 3 and declined thereafter, whereas in control VAD piglets, fecal scores were significantly higher at PCD3–5 and peaked at PCD4 (data not shown). VAD control piglets had longer fecal HRV shedding and higher HRV titers and diarrhea post-challenge as compared to VAS control piglets (Table II). All vaccinated VAS (with or w/o vitamin A supplementation), but only vaccinated and vitamin A supplemented VAD piglets were completely protected from RV shedding (Table II). Only 75% (3/4) of VAD vaccinated non-supplemented piglets were protected against VirHRV fecal shedding. These results indicate that the oral AttHRV vaccine-induced protective efficacy and adaptive immune responses (unpublished data) were not as severely compromised as innate immunity suggesting that even low level postnatal vitamin A supplementation (in the milk diet) may have had beneficial effects. Mean cumulative fecal score and average peak fecal HRV titers were significantly higher in control VAD vs. VAS pigs (Table II). Mean HRV shedding duration differed numerically, but did not differ significantly between these two groups. Thus, VAD increases HRV-induced diarrhea severity and fecal HRV shedding titers and duration, whereas the 3 dose vitamin A supplementation to VAD pigs did not provide consistent compensatory effects in the Gn pig model.
Table II.
Diarrhea and virus shedding in VAD/VAS Gn pigs vaccinated with AttHRV (with or without vitamin A supplementation) and challenged with VirHRV Wa.
| Virus shedding | Diarrhea | Protection rate (%) against shedding | Protection rate (%) against diarrhea | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
||||||||||
| N | % shed | Mean days to onset of shedding | Mean duration days | Avg. peak titer shed (FFU/ml)* | %with diarrhea | Mean duration days** | Mean cumulative fecal scoret† | |||
| Treatment | ||||||||||
| VAD | ||||||||||
| 3xAttHRV Wa | 4 | 25 | 3.5A‡ | 1.3A | 1587A | 0 | 0A | 5.1A | 75 | 100 |
| 3xAttHRV Wa (VitA 100,000) | 7 | 0 | - | 0A | ≤12.5A | 0 | 0A | 3.1A | 100 | 100 |
| Control | 5 | 100 | 1.5B | 5B | 47188B | 100 | 3.5B | 11.8B | 0 | 0 |
| Control (VitA 100,000) | 4 | 100 | 1.5B | 4.3B | 30875B | 100 | 4.5B | 12.1B | 0 | 0 |
| VAS | ||||||||||
| 3xAttHRV Wa | 5 | 0 | - | 0A | ≤12.5A | 0 | 0A | 1.7C | 100 | 100 |
| 3xAttHRVWa (VitA 100,000) | 5 | 0 | - | 0A | ≤12.5A | 0 | 0A | 2.0C | 100 | 100 |
| Control | 5 | 100 | 1.5B | 2.67AB | 3667A | 100 | 0.33C | 5.8A | 0 | 0 |
| Control (VitA 100,000) | 5 | 100 | 1.5B | 3.67AB | 375A | 100 | 0.33C | 4.3A | 0 | 0 |
N- Number of pigs/group; FFU - fluorescent focus forming units;
Determined by cell culture immunofluorescence infectivity assay; ≤12.5FFU/ml represents negative result, as lowest dilution tested was 1:25;
Duration of diarrhea determined by number of days with fecal scores > 1: feces were scored as follows: 0=normal; 1=pasty; 2=semiliquid; 3=liquid;
Mean cumulative score=[(sum of fecal consistency scores for 10 days post-challenge)/N];
Means in the same column, with different superscript letters differed significantly, p≤0.05 (One way ANOVA, Minitab).
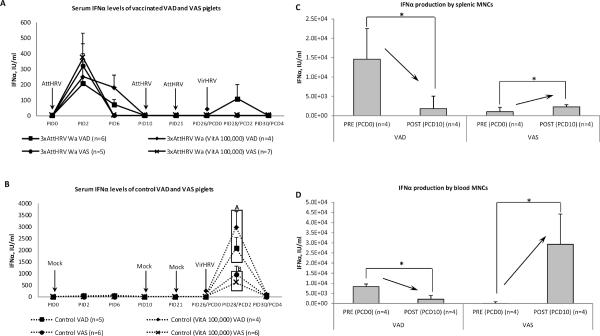
Circulating IFNα levels were higher in sera from VAD piglets post-challenge
At PID2, vaccinated and, surprisingly, some non-vaccinated control VAD piglets had very low but detectable IFNα levels in sera; while in VAS piglets, IFNα was detectable only in vaccinated animals (data not shown). This suggests that in VAD pigs, IFNα production may be induced via other than a TLR3 (viral dsRNA) dependent pathway.
At PCD2 (peak of HRV-induced IFNα in serum), we observed 4 times higher levels of IFNα in serum samples of control VAD vs. VAS piglets (Fig. 3b), likely due to higher VirHRV shedding levels (Table II) and/or the imbalanced immune responses. At PCD4, IFNα was still detectable in challenged control VAD piglets, but not in VAS piglets (Fig. 3b). Notably, serum IFNα levels appeared to coincide with the extent of HRV replication, occurring in all vaccinated pigs after the primary AttHRV vaccine dose (Fig. 3a). Post-challenge, high IFNα levels in control pigs compared to vaccinated pigs (Fig. 3b) coincided with the control pigs having higher HRV shedding titers post-challenge, but also corresponding to the 25% shedding rate in VAD vaccinated pigs only (Table II).
Fig. 3.
Circulating interferon-α in VAD and VAS vaccinated (a) and control (b) piglets' sera pre- and post-challenge. Different capital letters indicate that IFNα level was significantly lower (p<0.05) in VAS piglet sera at PCD2. Pooled data are the mean ± SEM from three independent experiments Interferon-α production by splenic (c) and blood (d) MNCs (post HRV antigen stimulation) of control VAD and VAS piglets pre- and post-challenge. Pooled data are the mean ± SEM from two independent experiments. Asterisks indicate that IFNα production by splenic and blood MNCs was significantly decreased (arrow down) for VAD piglets and significantly increased (arrow up) for VAS piglets post-challenge.
Similarly to IFNα levels in vivo, after ex vivo stimulation (HRV antigen), MNCs from spleen and blood of VAD piglets pre-challenge also produced 10- and 100-fold, respectively, higher amounts of IFNα than MNCs from VAS piglets (Fig. 3c,d). In contrast after VirHRV challenge, the situation was the opposite - IFNα production by MNCs isolated from VAD piglets decreased 4- and 8-fold (for blood and spleen MNCs) and IFNα production by MNCs isolated from VAS piglets increased 2-fold for spleen and 430-fold for blood MNCs (Fig. 3c,d).
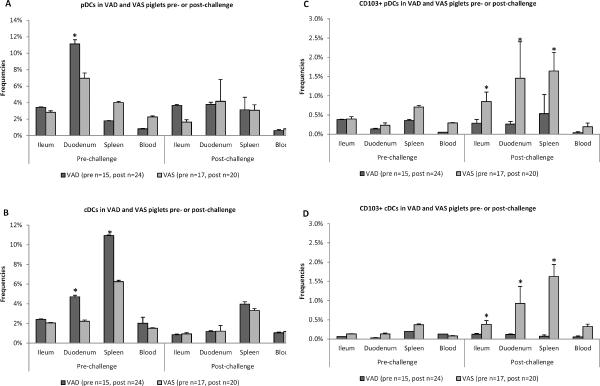
Total DC frequencies were increased in VAD piglets pre-challenge whereas frequencies of CD103 expressing DC were decreased in VAD piglets post-challenge
In the Gn pigs, in steady state or upon VirHRV challenge, regardless of treatments (vaccination or vitamin A status) the major residential tissue for pDCs was duodenum, while the highest frequencies of cDCs were observed in spleen (Fig. 4a,b). Total pDC and cDC frequencies were lower post VirHRV challenge. Total frequencies of pDCs were significantly higher in gut tissues of VAD piglets, but in systemic tissues of VAS piglets pre-challenge, with lower and similar (between VAD and VAS) levels post-challenge (Fig. 4a). Conventional DC frequencies were higher in all tissues of VAD piglets pre-challenge and were also decreased and similar to those of VAS piglets post-challenge (Fig. 4b). These enhanced frequencies of DCs in VAD piglets in the absence of pathogen (pre-challenge) may subsequently lead to immune system imbalance and debilitation, with inability to control VirHRV post-challenge. The observed trends were not affected by vaccination status and vitamin A supplementation, so the data were collapsed for analysis.
Fig. 4.
Total frequencies of pDCs (a) and cDCs (b) in local and systemic tissues of VAD and VAS piglets pre- and post-challenge regardless of treatments (vitamin A supplementation or vaccination). Asterisks indicate that pDC (a) and cDC (b) frequencies were significantly higher (p<0.05) in duodenum (pDCs and cDCs) and spleen (cDCs) of VAD piglets compared to VAS piglets, prechallenge. Total frequencies of CD103 expressing pDCs (c) and cDCs (d) in local and systemic tissues of VAD and VAS piglets pre- and post-challenge regardless of treatments (vitamin A supplementation or vaccination). Asterisks indicate that CD103+ pDC (c) and cDC (d) frequencies were significantly higher (p<0.05) in ileum, duodenum and spleen of VAS piglets post-challenge. Pooled data are the mean ± SEM from three independent experiments. The dot plots were gated on total MNCs, and the numbers on the graphs are the percentages of total pDCs (a), total cDCs (b), CD103+ pDCs (c) and CD103+ cDCs among total MNCs.
Frequencies of specialized CD103+ (αEβ7) DCs (pDCs and cDCs) were consistently (pre- and post-challenge) and significantly (post-challenge) higher in VAS vs. VAD piglets (Fig. 4c,d), suggesting a potential for subsequent less inflammatory and more regulatory immune profiles in VAS piglets. Notably, whereas in VAS piglets, frequencies of CD103+ DCs increased 2–4-fold post-challenge, in VAD piglets those frequencies were unchanged providing a possible explanation for the less efficient resolution of VirHRV post-challenge in VAD piglets (Fig. 4c,d). Additionally, no effect of vitamin A supplementation on DC subsets was observed pre- or post-challenge in VAD and VAS control and vaccinated piglets (data not shown).
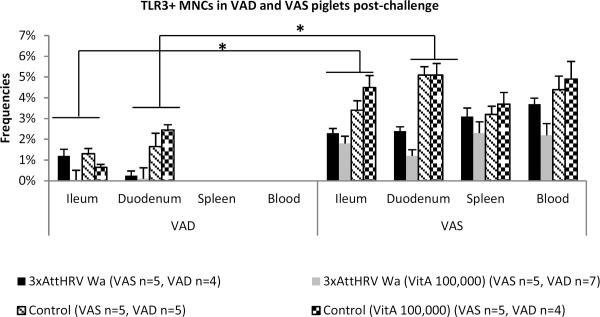
TLR3+ MNC frequencies were significantly higher in intestinal and systemic tissues of VAS piglets
TLR3+ MNCs were detectable only post-challenge and their frequencies were significantly higher in all tissues of VAS as compared to VAD piglets (Fig. 5). This is consistent with decreased IFNα production by splenic and blood MNCs of VAD piglets at PCD10, suggesting that post-challenge IFNα production is activated via a TLR3 dependent pathway.
Fig. 5.
Frequencies of TLR3+ MNCs in local and systemic tissues of VAD and VAS piglets post-challenge. Asterisks indicate that TLR3+ MNC frequencies were significantly higher in all tissues of VAS piglets. TLR3+ MNCs were not detectable in spleen and blood of VAD piglets. Pooled data are the mean ± SEM from two independent experiments. The dot plots were gated on total mononuclear cells, and the numbers on the graphs are the percentages of total TLR3+ MNCs among total MNCs.
Decreased TLR3 expression by MNCs from vaccinated piglets compared to control piglets likely reflects their partial protection against VirHRV replication. A similar situation is probable in the case of marginally lower TLR3+ MNC frequencies for vaccinated and vitamin A supplemented piglets (as compared to vaccinated, non-supplemented): the vitamin A supplemented piglets also had improved protection against VirHRV replication.
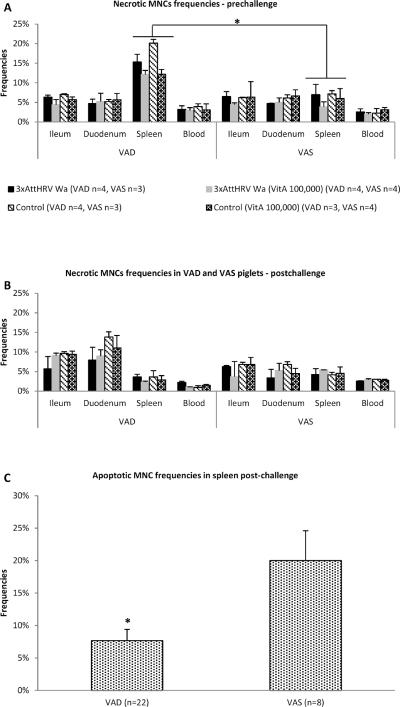
Necrotic MNC frequencies were increased and apoptotic MNC frequencies were decreased in local and systemic tissues of VAD piglets
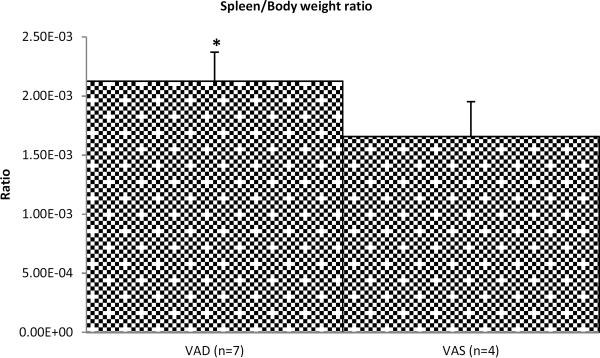
We observed significantly higher (~2.5-fold) frequencies of necrotic MNCs in spleens of VAD piglets pre-challenge (Fig. 6a). There was a slight moderation in the frequencies of necrotic MNCs due to vitamin A supplementation as evident pre-challenge (Fig. 6a). Higher cDC (Fig. 4b) and necrotic MNC (Fig. 6a) frequencies, potentially accompanied by inflammatory cell (including macrophages and neutrophils) infiltration and cell debris accumulation in spleens of VAD piglets pre-challenge, could contribute to the relative increase in spleen/body weight ratio observed in this study (Fig. 7).
Fig. 6.
Frequencies of necrotic MNCs in local and systemic tissues of VAD and VAS piglets pre-(a) and post-challenge (b) regardless of treatments (vitamin A supplementation or vaccination). Asterisk (a) indicates that necrotic frequencies of splenic MNCs were significantly higher in VAD piglets. Boxed areas (b) show higher necrotic MNCs frequencies in duodenum and ileum of VAD compared to VAS piglets. (c) Frequencies of apoptotic MNCs in spleens of VAD and VAS piglets post-challenge; asterisk indicates that apoptotic frequencies of splenic MNCs were significantly decreased in VAD piglets post-challenge. Pooled data are the mean ± SEM from two independent experiments. The dot plots were gated on total mononuclear cells, and the numbers on the graphs are the percentages of necrotic (a and b) and apoptotic (c) MNCs among total MNCs.
Fig. 7.
Spleen to body weight ratio of VAD/VAS piglets pre-challenge. Asterisk indicates that this ratio was significantly higher for VAD piglets, which reflects an increase in relative spleen weight. Pooled data are the mean ± SEM from two independent experiments.
Additionally, necrotic MNC numbers were increased (1.5–2 times) in ileum and duodenum of VAD piglets compared to VAS piglets post-challenge, likely due to a combined effect of VAD and HRV infection (Fig. 6b). Apoptotic MNC frequencies post-challenge were significantly higher in spleen of VAS pigs as compared to VAD pigs, suggesting insufficiency of immunoregulatory function of the latter pigs (Fig. 6c) with no effect of vitamin A supplementation evident (data not shown).
Splenic MNCs from VAS piglets were more responsive to ATRA treatment ex vivo
In our study we did not observe a consistent effect (or minor if observed) of 3 dose vitamin A supplementation on innate immune responses in either VAD or VAS neonatal piglets. In VAS piglets with adequate circulating and hepatic vitamin A levels, all supplemented vitamin A is likely being stored in the liver; whereas in VAD piglets (with positive 30 day dose response indicating subclinical VAD), we expected that some supplemental vitamin A would be converted into ATRA and compensate for negative VAD effects. To address the observed lack of such consistent compensatory effects of vitamin A supplementation in vivo, we analyzed the effects of ATRA (at concentrations of 0.1 μM, 1 μM and 10 μM) ex vivo on splenic MNCs derived from VAD and VAS control pigs. The ATRA effect on proliferation, apoptosis and CD103 expression was dose-dependent (data not shown). Interestingly, ex vivo stimulation with ATRA (1 μM) resulted in decreased proliferation and increased apoptosis of MNCs from VAS, but not from VAD pigs (Table III). ATRA stimulation improved viability of VAD MNCs as reflected by decreased frequency of necrosis, whereas in VAS MNCs it was lower initially and did not change (Table III). Additionally, ATRA treatment significantly increased CD103 expression by VAS MNCs while no effect on VAD MNCs was apparent (Table III). These findings demonstrate that VAD MNCs were less responsive to ATRA stimulation (no effect of ATRA except for necrosis frequencies) than VAS MNCs and suggest that vitamin A absorption efficiency and/or metabolism may be compromised in VAD neonatal piglets. Alternatively it may require a longer time period to improve the immune function and alter the life cycle of immune cells in vivo by means of vitamin A supplementation.
Table III.
The effect of all-trans retinoic acid (ATRA) treatment on the frequencies of proliferating, necrotic, apoptotic and CD103+ MNCs in the spleen of control VAD/VAS Gn pigs.
| VAD | VAS | |||
|---|---|---|---|---|
|
|
||||
| ATRA (1μM)(n=4/9)* | No ATRA(n=4/10)* | ATRA (1μM) (n=2/16)* | No ATRA(n=2/17)* | |
| Frequency of: | ||||
| Proliferation | 1.99% | 2.02% | 1.08% | 2.25% |
| Necrosis | 4.12% | 3.10% | 2.10% | 2.02% |
| Apoptosis | 7.85% | 8.00% | 11.85% | 9.75% |
| CD103 expression | 3.58% | 3.43% | 9.52%A | 6.38%B |
Proliferation: VAD ATRA n=4, VAD No ATRA n=4, VAS ATRA n=2, VAS No ATRA n=2; Necrosis, apoptosis and CD103 expression: VAD ATRA n=9, VAD No ATRA n=10, VAS ATRA n=16, VAS No ATRA n=17. Numbers in bold indicate that an ATRA effect was observed. Means in the same row, with different superscript letters differed significantly, p≤0.05 (One way ANOVA, Minitab)
Discussion
Reduced efficacy of oral vaccines and increased mortality rates from infectious diseases are prevalent in children in impoverished countries and often are associated with their poor micronutrient status. In particular, VAD is strongly associated with an increased risk of respiratory and alimentary tract diseases. Rotavirus causes life-threatening gastroenteritis with high fatality rates in developing countries, where VAD is prevalent in preschool children (1, 51). We have successfully established a VAD and VAS Gn pig model and studied the impact of VAD and vitamin A supplementation (following the WHO recommendations) among AttHRV vaccinated and VirHRV challenged groups. In our Gn pig model we recreated subclinical VAD, with significantly decreased liver vitamin A levels and positive serum 30-day dose-response test (12). VirHRV challenge depressed vitamin A levels in serum early post-challenge (PCD2) as it was previously shown for measles virus (52). This finding suggests that VirHRV, which causes intestinal villous atrophy and malabsorption (41), may affect vitamin A metabolism or its absorption from the milk diet. Non-vaccinated VAD piglets had more severe diarrhea and higher HRV shedding compared to VAS piglets suggesting that vitamin A mediates its effect by maintaining the epithelial barrier and/or by regulating immune functions. Consistent with this, higher frequencies of necrotic MNCs in ileum and duodenum are reflective of more prominent intestinal damage in VAD piglets. Vitamin A supplementation had no consistent effect on diarrhea or HRV shedding. Our neonatal Gn pig model with VAD induced in mothers may represent severe effects of VAD development in the prenatal period. We speculate that earlier (shortly prior to vaccination or virulent HRV exposure) or longer vitamin A supplementation or vitamin A supplementation of both mothers and their offspring may be needed to improve intestinal health and normalize immune responses. Also the effect of vitamin A supplementation may vary for different pathogens or even strains as was shown previously (53, 54).
According to the previously published results, RBP4 and RARα mRNA expression is affected by VAD and they are reflective of retinol-binding protein 4 (RBP4) and retinoic acid receptor (RAR) protein levels, respectively (55–59). RBP4 is secreted by adipocytes and carries retinol through the plasma to extrahepatic tissues (60). Although liver is considered to be the major source of RBP4, other RBP4 expression sites are suggested including human macrophages that are closely related genetically to adipocytes and are capable of RBP4 expression (61). We showed for the first time that porcine RBP4 mRNA is expressed by splenic MNCs and its levels are affected by VAD, vitamin A supplementation and VirHRV challenge, similar to the latter effects on vitamin A levels. Notably, vitamin A supplementation increased RBP4 mRNA levels in VAD piglets post-challenge and decreased RBP4 mRNA levels in VAS piglets, possibly indicating vitamin A saturation in the latter pigs.
In our study, RARα mRNA expression by splenic MNCs was positively correlated with vitamin A status, supplementation and VirHRV challenge. We observed decreased RARα mRNA levels and lower relative responses to supplemental vitamin A in VAD vs. VAS pigs post-challenge. This suggests that in VAD pigs, supplemental vitamin A may not be metabolized to ATRA efficiently or insufficient RARα expression results in aberrant cell signaling and the observed lack of consistent compensatory effects of such supplementation. Whether post-challenge increases of RBP4 and RARα mRNA expression was a compensatory response to the transient drop in serum vitamin A at PCD2 and decreased hepatic vitamin A storage post-challenge remains to be determined.
Numerous studies have demonstrated that VAD decreases resistance to and aggravates respiratory and enteric infections by depressing immune function; thus, we expected to see reduced innate immune responses (IFNα, pDCs and cDCs) in our study. In our experiments, however, we observed not just a decrease, but VAD-induced dysregulation of IFNα production in response to AttHRV or VirHRV. Significantly higher amounts of circulating IFNα in VAD piglets at PCD2 were consistent with higher VirHRV replication titers and more prominent intestinal inflammation (as reflected by higher necrotic MNC frequencies). However, at PCD 10 the capacity of IFNα production by MNCs from VAD piglets ex vivo was significantly decreased as compared to pre-challenge and to MNCs from VAS piglets post-challenge. This, together with higher amounts of IFNα in VAD piglets' sera, suggests that imbalanced IFNα release by immune cells may contribute to increased inflammation, and does not efficiently control HRV infection. Additionally, although RVs are known to be potent IFNα inducers, the role of IFNα in RV clearance is uncertain and varies between homologous and heterologous infections (62–64). Moreover, type I and II interferons were not demonstrated to be major inhibitors of RV replication in mice (63, 64). In this study we did not observe a negative correlation between IFNα levels and HRV fecal shedding in either VAS or VAD pigs, which could be attributable to HRV replication in the porcine (heterologous) host or reflect the failure of IFNs to inhibit RV replication (63, 64).
Consistent with IFNα response dysregulation, we observed higher total frequencies of pDCs (gut tissues) and cDCs (all tissues) in VAD piglets pre-challenge compared to VAS piglets regardless of vaccination or vitamin A supplementation status. This result is in agreement with previous findings by others that VAD causes a systemic expansion of myeloid cells in SENCAR mice and an increase in polymorphonuclear neutrophils, lymphoid DCs, and memory CD8+ T cells in C57BL/6J mice (25, 65). Vitamin D deficiency was previously shown to be prevalent in patients with Systemic Lupus Erythematosus and associated with overactive DCs and overexpression of IFN regulated genes (66). This, together with our findings, suggests that some universal immunoregulatory mechanisms involve DC overactivation and are affected by various vitamin deficiencies.
Declines in pDC and cDC frequencies in both VAD and VAS piglets post-challenge may have been due to VirHRV infection or VirHRV-induced apoptosis. A marked reduction in splenic pDCs in mice was reported following herpes simplex virus 1, VSV, and murine cytomegalovirus infections, with IFN-I contributing to pDC death through induction of the expression of proapoptotic molecules and caspase activation (67). Decreased numbers of circulating pDCs have also been observed in patients infected with human immunodeficiency virus, Hepatitis B or C and measles viruses (68–72). Our data provide the first in vivo evidence that an acute gastrointestinal infection may cause at least a transient decrease in cDC and pDC frequencies in local and systemic tissues.
Small intestinal CD103+ DCs are imprinted with an ability to metabolize vitamin A (retinol) and generate gut-tropic T cells (expressing CC chemokine receptor-9 and α4β7). It was recently shown that RA regulates generation of gut-tropic migratory DC precursors that give rise to CD103+ intestinal pDCs and cDCs (73). Lamina propria DCs expressing CD103 were shown to convert naϊve CD4+ T cells into Foxp3+ Treg cells (74). Interestingly, we observed slightly lower CD103+ pDC and cDC frequencies in ileum, duodenum and spleen of VAD piglets pre-challenge, and they were decreased significantly post-challenge as compared to VAS piglets. The loss of CD103 integrin by colonic DCs during experimentally induced colitis was described in mice (75), suggesting that VAD-induced MNC necrosis, possibly reflecting intestinal inflammation in our Gn pig model, could contribute to, or be the result of the significantly decreased CD103+ DC frequencies. CD103+ DCs express the tight junction proteins (zonula, occluding and claudin I) preserving the integrity of the epithelial barrier and preventing the overt inflammatory reactions to intestinal pathogens (76, 77). Additionally, αEβ7 (CD103) integrin was demonstrated to influence cellular intraepithelial morphogenesis and motility (78) which are critical for the proper communication between pathogen, DCs and T and B lymphocytes. Therefore, the VAD-induced loss of CD103 expression by DCs, that we observed in our experiments, could have resulted in aberrant innate immune responses against VirHRV and compromised resolution of the infection. Ultimately, disruption of signaling between DCs and T/B-lymphocytes may be due to lack of proper antigen presentation to the latter cells resulting in lowered HRV specific IgA antibody titers that we observed (Chattha et al., unpublished) and diminished NK cell function (Saif and Gourapura, unpublished) in VAD piglets.
Interestingly, in this study, frequencies of apoptotic MNCs were slightly higher in all treatment groups of VAS piglets pre-challenge (data not shown) confirming that adequate vitamin A levels improve immunoregulatory function and programmed cell death – a mechanism involved in regulation of autoimmunity and T cell tolerance (79). Additionally, we observed an increase in apoptotic MNC frequencies post-challenge in VAD and VAS pigs (data not shown), supporting previous findings regarding RV induced apoptosis in various cell lines of human, simian and rat origin (80–84).
Mice with VAD (65) and mice treated with an RA receptor antagonist showed accumulation of immature myeloid cells similar to the immature myeloid-suppressive cells found in cancer patients (85). Additionally, a recent study demonstrated that RA suppresses immune cell proliferation and induces tolerogenic DC development which indicates its therapeutic potential for the treatment of numerous inflammatory or autoimmune conditions (86). In our present study despite the higher total frequencies of cDCs, we observed significantly lower numbers of specialized CD103+ cDCs in VAD piglets regardless of vaccination/challenge status. This confirms previous findings on VAD effects on the immune system including development of autoimmune conditions and tumorogenesis. Interestingly, we also observed significantly higher frequencies of apoptotic MNCs in VAS piglets in all groups, indicating that a mechanism of programmed cell death is affected by VAD and therefore untreated VAD may result in an overactive immune system and subsequently contribute to the development of autoimmune conditions.
Overexpansion of myeloid cells due to VAD in mice was reported previously and also coincided with increased spleen size (65). Also it was suggested that inflammatory responses and accumulation of cell debris may have contributed to increased spleen size (splenomegaly) observed in VAD mice and lambs (65, 87, 88). Intriguingly, our observations of RBP4/RARα mRNA expression in spleen, increased relative spleen weight and necrotic MNC frequencies in VAD pigs indicate that spleen – a systemic lymphoid organ - may play an important role in vitamin A metabolism and retinoid signaling and is affected by VAD thereby altering immune responses at the systemic level. Additionally, we observed a decreased ability of splenic MNCs from VAD pigs to respond to vitamin A supplementation in vivo (as reflected by RBP4/RARα mRNA levels) or to ATRA stimulation ex vivo indicating that prenatal VAD has a profound effect on intrinsic properties and life cycle of the immune cells.
In conclusion, our results indicate that under steady state, VAD in Gn pigs results in innate immune system overactivity and possibly disrupted cell signaling. This suggests that one of the major vitamin A functions is to maintain the immunoregulatory profile in the gut (mucosal) and at systemic immune sites. The VAD induced loss of this immunoregulatory function may lead to imbalanced innate immune responses and subsequent development of autoimmune conditions as reported by others. CD103+ DCs appeared to be the only subset examined that was significantly increased in VAS piglets, suggesting their critical role in efficient immune responses to HRV. Finally, a 3× high dose vitamin A supplementation concurrent with the AttHRV vaccine did not compensate for the VAD effects or act as an adjuvant for AttHRV vaccine possibly indicating a need for maternal, longer, or higher levels of postnatal vitamin A supplementation.
Acknowledgements
We gratefully acknowledge the technical assistance of Dr. Juliette Hanson, Rich McCormick, Lindsey Good, Celina G. Vega, Marina Bok, Ozkan Timurkan, Joshua Amimo, Kyle T. Scheuer, Ning Chen and Clarissa Smith. We would also like to thank the Diagnostic Center for Population and Animal Health, Michigan State University, Lansing, MI, for hepatic and serum vitamin A analysis.
Footnotes
This work was supported by a grant from the National Institutes of Health (R21AI083546 to LJS) and federal funds appropriated to the Ohio Agricultural Research and Development Center (OARDC) of The Ohio State University, Wooster, Ohio.
Disclosures The authors have no financial conflicts of interest.
References
- 1.Parashar UD, Gibson CJ, Bresse JS, Glass RI. Rotavirus and severe childhood diarrhea. Emerg Infect Dis. 2006;12:304–306. doi: 10.3201/eid1202.050006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widdowson MA, Steele D, Vojdani J, Wecker J, Parashar U. Global rotavirus surveillance: determining the need and measuring the impact of rotavirus vaccines. J Infect Dis. 2009;200(Suppl 1):S1–8. doi: 10.1086/605061. [DOI] [PubMed] [Google Scholar]
- 3.Zaman K, Dang DA, Victor JC, Shin S, Yunus M, Dallas MJ, Podder G, Vu DT, Le TP, Luby SP, Le HT, Coia ML, Lewis K, Rivers SB, Sack DA, Schodel F, Steele AD, Neuzil KM, Ciarlet M. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:615–623. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 4.Armah GE, Sow SO, Breiman RF, Dallas MJ, Tapia MD, Feikin DR, Binka FN, Steele AD, Laserson KF, Ansah NA, Levine MM, Lewis K, Coia ML, Attah-Poku M, Ojwando J, Rivers SB, Victor JC, Nyambane G, Hodgson A, Schodel F, Ciarlet M, Neuzil KM. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. Lancet. 2010;376:606–614. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 5.Bresee JS, Glass RI, Ivanoff B, Gentsch JR. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine. 1999;17:2207–2222. doi: 10.1016/s0264-410x(98)00376-4. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman DR, De Calisto J, Simmons NL, Cruz AN, Villablanca EJ, Mora JR, Barouch DH. Vitamin A deficiency impairs vaccine-elicited gastrointestinal immunity. J Immunol. 2011;187:1877–1883. doi: 10.4049/jimmunol.1101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diness BR, Christoffersen D, Pedersen UB, Rodrigues A, Fischer TK, Andersen A, Whittle H, Yazdanbakhsh M, Aaby P, Benn CS. The effect of high-dose vitamin A supplementation given with bacille Calmette-Guerin vaccine at birth on infant rotavirus infection and diarrhea: a randomized prospective study from Guinea-Bissau. J Infect Dis. 2010;202(Suppl):S243–251. doi: 10.1086/653569. [DOI] [PubMed] [Google Scholar]
- 8.Diness BR, Martins CL, Bale C, Garly ML, Ravn H, Rodrigues A, Whittle H, Aaby P, Benn CS. The effect of high-dose vitamin A supplementation at birth on measles incidence during the first 12 months of life in boys and girls: an unplanned study within a randomised trial. Br J Nutr. 2011:1–4. doi: 10.1017/S0007114510005532. [DOI] [PubMed] [Google Scholar]
- 9.Benn CS, Fisker AB, Napirna BM, Roth A, Diness BR, Lausch KR, Ravn H, Yazdanbakhsh M, Rodrigues A, Whittle H, Aaby P. Vitamin A supplementation and BCG vaccination at birth in low birthweight neonates: two by two factorial randomised controlled trial. BMJ. 2010;340:c1101. doi: 10.1136/bmj.c1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahmathullah L, Tielsch JM, Thulasiraj RD, Katz J, Coles C, Devi S, John R, Prakash K, Sadanand AV, Edwin N, Kamaraj C. Impact of supplementing newborn infants with vitamin A on early infant mortality: community based randomised trial in southern India. BMJ. 2003;327:254. doi: 10.1136/bmj.327.7409.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rotondi MA, Khobzi N. Vitamin A supplementation and neonatal mortality in the developing world: a meta-regression of cluster-randomized trials. Bull World Health Organ. 2010;88:697–702. doi: 10.2471/BLT.09.068080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferraz IS, Daneluzzi JC, Vannucchi H, Jordao AA, Jr., Ricco RG, Del Ciampo LA, Martinelli CE, Jr., Engelberg AA, Bonilha LR, Flores H. Detection of vitamin A deficiency in Brazilian preschool children using the serum 30-day dose-response test. Eur J Clin Nutr. 2004;58:1372–1377. doi: 10.1038/sj.ejcn.1601978. [DOI] [PubMed] [Google Scholar]
- 13.Underwood BA. Methods for assessment of vitamin A status. J Nutr. 1990;120(Suppl 11):1459–1463. doi: 10.1093/jn/120.suppl_11.1459. [DOI] [PubMed] [Google Scholar]
- 14.Underwood BA. Biochemical and histological methodologies for assessing vitamin A status in human populations. Methods Enzymol. 1990;190:242–251. doi: 10.1016/0076-6879(90)90029-z. [DOI] [PubMed] [Google Scholar]
- 15.Moore T. Vitamin A transfer from mother to offspring in mice and rats. Int J Vitam Nutr Res. 1971;41:301–306. [PubMed] [Google Scholar]
- 16.Wallingford JC, Underwood BA. Rapid preparation of anhydroretinol and its use as an internal standard in determination of liver total vitamin A by high-performance liquid chromatography. J Chromatogr. 1986;381:158–163. doi: 10.1016/s0378-4347(00)83575-6. [DOI] [PubMed] [Google Scholar]
- 17.Sijtsma SR, Rombout JH, West CE, van der Zijpp AJ. Vitamin A deficiency impairs cytotoxic T lymphocyte activity in Newcastle disease virus-infected chickens. Vet Immunol Immunopathol. 1990;26:191–201. doi: 10.1016/0165-2427(90)90067-3. [DOI] [PubMed] [Google Scholar]
- 18.Lavasa S, Kumar L, Chakravarti RN, Kumar M. Early humoral immune response in vitamin A deficiency--an experimental study. Indian J Exp Biol. 1988;26:431–435. [PubMed] [Google Scholar]
- 19.Ahmed F, Jones DB, Jackson AA. The interaction of vitamin A deficiency and rotavirus infection in the mouse. Br J Nutr. 1990;63:363–373. doi: 10.1079/bjn19900122. [DOI] [PubMed] [Google Scholar]
- 20.Ahmed F, Jones DB, Jackson AA. Effect of undernutrition on the immune response to rotavirus infection in mice. Ann Nutr Metab. 1990;34:21–31. doi: 10.1159/000177566. [DOI] [PubMed] [Google Scholar]
- 21.Friedman A, Meidovsky A, Leitner G, Sklan D. Decreased resistance and immune response to Escherichia coli infection in chicks with low or high intakes of vitamin A. J Nutr. 1991;121:395–400. doi: 10.1093/jn/121.3.395. [DOI] [PubMed] [Google Scholar]
- 22.Nauss KM, Anderson CA, Conner MW, Newberne PM. Ocular infection with herpes simplex virus (HSV-1) in vitamin A-deficient and control rats. J Nutr. 1985;115:1300–1315. doi: 10.1093/jn/115.10.1300. [DOI] [PubMed] [Google Scholar]
- 23.Bowman TA, Goonewardene IM, Pasatiempo AM, Ross AC, Taylor CE. Vitamin A deficiency decreases natural killer cell activity and interferon production in rats. J Nutr. 1990;120:1264–1273. doi: 10.1093/jn/120.10.1264. [DOI] [PubMed] [Google Scholar]
- 24.Nauss KM, Newberne PM. Local and regional immune function of vitamin A-deficient rats with ocular herpes simplex virus (HSV) infections. J Nutr. 1985;115:1316–1324. doi: 10.1093/jn/115.10.1316. [DOI] [PubMed] [Google Scholar]
- 25.Duriancik DM, Hoag KA. Vitamin A deficiency alters splenic dendritic cell subsets and increases CD8(+)Gr-1(+) memory T lymphocytes in C57BL/6J mice. Cell Immunol. 2010;265:156–163. doi: 10.1016/j.cellimm.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dresser DW. Adjuvanticity of vitamin A. Nature. 1968;217:527–529. doi: 10.1038/217527a0. [DOI] [PubMed] [Google Scholar]
- 27.Forni G, Cerruti Sola S, Giovarelli M, Santoni A, Martinetto P, Vietti D. Effect of prolonged administration of low doses of dietary retinoids on cell-mediated immunity and the growth of transplantable tumors in mice. Journal of the National Cancer Institute. 1986;76:527–533. [PubMed] [Google Scholar]
- 28.Nuwayri-Salti N, Murad T. Immunologic and anti-immunosuppressive effects of vitamin A. Pharmacology. 1985;30:181–187. doi: 10.1159/000138067. [DOI] [PubMed] [Google Scholar]
- 29.Hatchigian EA, Santos JI, Broitman SA, Vitale JJ. Vitamin A supplementation improves macrophage function and bacterial clearance during experimental salmonella infection. Proc Soc Exp Biol Med. 1989;191:47–54. doi: 10.3181/00379727-191-42888. [DOI] [PubMed] [Google Scholar]
- 30.Moriguchi S, Werner L, Watson RR. High dietary vitamin A (retinyl palmitate) and cellular immune functions in mice. Immunology. 1985;56:169–177. [PMC free article] [PubMed] [Google Scholar]
- 31.Kjolhede CL, Chew FJ, Gadomski AM, Marroquin DP. Clinical trial of vitamin A as adjuvant treatment for lower respiratory tract infections. J Pediatr. 1995;126:807–812. doi: 10.1016/s0022-3476(95)70416-7. [DOI] [PubMed] [Google Scholar]
- 32.Dias WO, Horton DSPQ, Cainelli Gebara VCB, Furuyama N, Risoleo L, Ferreira VRF, Raw I. Vitamin A as adjuvant to the mouse immune response to pertussis, tetanus and diphtheria vaccines. Biotech Lett. 2002;24:1515–1518. [Google Scholar]
- 33.Livingston KA, Klasing KC. Retinyl palmitate does not have an adjuvant effect on the antibody response of chicks to keyhole limpet hemocyanin regardless of vitamin A status. Poult Sci. 2011;90:965–970. doi: 10.3382/ps.2010-01085. [DOI] [PubMed] [Google Scholar]
- 34.DePaolo RW, Abadie V, Tang F, Fehlner-Peach H, Hall JA, Wang W, Marietta EV, Kasarda DD, Waldmann TA, Murray JA, Semrad C, Kupfer SS, Belkaid Y, Guandalini S, Jabri B. Co-adjuvant effects of retinoic acid and IL-15 induce inflammatory immunity to dietary antigens. Nature. 2011;471:220–224. doi: 10.1038/nature09849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu M, Vajdy M. A novel retinoic acid, catechin hydrate and mustard oil-based emulsion for enhanced cytokine and antibody responses against multiple strains of HIV-1 following mucosal and systemic vaccinations. Vaccine. 2011;29:2429–2436. doi: 10.1016/j.vaccine.2011.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science. 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 38.Yuan L, Ward LA, Rosen BI, To TL, Saif LJ. Systematic and intestinal antibody-secreting cell responses and correlates of protective immunity to human rotavirus in a gnotobiotic pig model of disease. J Virol. 1996;70:3075–3083. doi: 10.1128/jvi.70.5.3075-3083.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saif LJ, Ward LA, Yuan L, Rosen BI, To TL. The gnotobiotic piglet as a model for studies of disease pathogenesis and immunity to human rotaviruses. Arch Virol Suppl. 1996;12:153–161. doi: 10.1007/978-3-7091-6553-9_17. [DOI] [PubMed] [Google Scholar]
- 40.Yuan L, Saif LJ. Induction of mucosal immune responses and protection against enteric viruses: rotavirus infection of gnotobiotic pigs as a model. Vet Immunol Immunopathol. 2002;87:147–160. doi: 10.1016/S0165-2427(02)00046-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ward LA, Rosen BI, Yuan L, Saif LJ. Pathogenesis of an attenuated and a virulent strain of group A human rotavirus in neonatal gnotobiotic pigs. J Gen Virol. 1996;77(Pt 7):1431–1441. doi: 10.1099/0022-1317-77-7-1431. [DOI] [PubMed] [Google Scholar]
- 42.Saif L, Yuan L, Ward L, To T. Comparative studies of the pathogenesis, antibody immune responses, and homologous protection to porcine and human rotaviruses in gnotobiotic piglets. Adv Exp Med Biol. 1997;412:397–403. doi: 10.1007/978-1-4899-1828-4_62. [DOI] [PubMed] [Google Scholar]
- 43.Saif LJ, Redman DR, Smith KL, Theil KW. Passive immunity to bovine rotavirus in newborn calves fed colostrum supplements from immunized or nonimmunized cows. Infect Immun. 1983;41:1118–1131. doi: 10.1128/iai.41.3.1118-1131.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W, Azevedo MS, Gonzalez AM, Saif LJ, Van Nguyen T, Wen K, Yousef AE, Yuan L. Influence of probiotic Lactobacilli colonization on neonatal B cell responses in a gnotobiotic pig model of human rotavirus infection and disease. Vet Immunol Immunopathol. 2008;122:175–181. doi: 10.1016/j.vetimm.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Azevedo MS, Wen K, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Probiotic Lactobacillus acidophilus enhances the immunogenicity of an oral rotavirus vaccine in gnotobiotic pigs. Vaccine. 2008;26:3655–3661. doi: 10.1016/j.vaccine.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang W, Wen K, Azevedo MS, Gonzalez A, Saif LJ, Li G, Yousef AE, Yuan L. Lactic acid bacterial colonization and human rotavirus infection influence distribution and frequencies of monocytes/macrophages and dendritic cells in neonatal gnotobiotic pigs. Vet Immunol Immunopathol. 2008;121:222–231. doi: 10.1016/j.vetimm.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez AM, Azevedo MS, Jung K, Vlasova A, Zhang W, Saif LJ. Innate immune responses to human rotavirus in the neonatal gnotobiotic piglet disease model. Immunology. 2010;131:242–256. doi: 10.1111/j.1365-2567.2010.03298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Summerfield A, McCullough KC. The porcine dendritic cell family. Developmental and comparative immunology. 2009;33:299–309. doi: 10.1016/j.dci.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Artursson K, Wallgren P, Alm GV. Appearance of interferon-alpha in serum and signs of reduced immune function in pigs after transport and installation in a fattening farm. Vet Immunol Immunopathol. 1989;23:345–353. doi: 10.1016/0165-2427(89)90146-3. [DOI] [PubMed] [Google Scholar]
- 50.Diaz de Arce H, Artursson K, L'Haridon R, Perers A, La Bonnardiere C, Alm GV. A sensitive immunoassay for porcine interferon-alpha. Vet Immunol Immunopathol. 1992;30:319–327. doi: 10.1016/0165-2427(92)90102-v. [DOI] [PubMed] [Google Scholar]
- 51.West KP., Jr. Extent of vitamin A deficiency among preschool children and women of reproductive age. J Nutr. 2002;132:2857S–2866S. doi: 10.1093/jn/132.9.2857S. [DOI] [PubMed] [Google Scholar]
- 52.West CE. Vitamin A and measles. Nutr Rev. 2000;58:S46–54. doi: 10.1111/j.1753-4887.2000.tb07803.x. [DOI] [PubMed] [Google Scholar]
- 53.Sudfeld CR, Navar AM, Halsey NA. Effectiveness of measles vaccination and vitamin A treatment. Int J Epidemiol. 2010;39(Suppl 1):i48–55. doi: 10.1093/ije/dyq021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Long KZ, Garcia C, Ko G, Santos JI, Al Mamun A, Rosado JL, DuPont HL, Nathakumar N. Vitamin A modifies the intestinal chemokine and cytokine responses to norovirus infection in Mexican children. J Nutr. 2011;141:957–963. doi: 10.3945/jn.110.132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bobbert P, Weithauser A, Andres J, Bobbert T, Kuhl U, Schultheiss HP, Rauch U, Skurk C. Increased plasma retinol binding protein 4 levels in patients with inflammatory cardiomyopathy. Eur J Heart Fail. 2009;11:1163–1168. doi: 10.1093/eurjhf/hfp153. [DOI] [PubMed] [Google Scholar]
- 56.Broch M, Auguet MT, Ramirez R, Olona M, Aguilar C, Megia A, Alcaide MJ, Pastor R, Martinez S, Caubet E, Garcia-Espana A, Richart C. Parallel downregulation of retinol-binding protein-4 and adiponectin expression in subcutaneous adipose tissue of non-morbidly obese subjects. European journal of endocrinology / European Federation of Endocrine Societies. 2009;161:87–94. doi: 10.1530/EJE-08-0866. [DOI] [PubMed] [Google Scholar]
- 57.Dvorak Z, Vrzal R, Ulrichova J, Macejova D, Ondkova S, Brtko J. Expression, protein stability and transcriptional activity of retinoic acid receptors are affected by microtubules interfering agents and all-trans-retinoic acid in primary rat hepatocytes. Molecular and cellular endocrinology. 2007;267:89–96. doi: 10.1016/j.mce.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 58.Kato S, Mano H, Kumazawa T, Yoshizawa Y, Kojima R, Masushige S. Effect of retinoid status on alpha, beta and gamma retinoic acid receptor mRNA levels in various rat tissues. The Biochemical journal. 1992;286(Pt 3):755–760. doi: 10.1042/bj2860755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vitkova M, Klimcakova E, Kovacikova M, Valle C, Moro C, Polak J, Hanacek J, Capel F, Viguerie N, Richterova B, Bajzova M, Hejnova J, Stich V, Langin D. Plasma levels and adipose tissue messenger ribonucleic acid expression of retinol-binding protein 4 are reduced during calorie restriction in obese subjects but are not related to diet-induced changes in insulin sensitivity. The Journal of clinical endocrinology and metabolism. 2007;92:2330–2335. doi: 10.1210/jc.2006-2668. [DOI] [PubMed] [Google Scholar]
- 60.Frey SK, Vogel S. Vitamin A metabolism and adipose tissue biology. Nutrients. 2011;3:27–39. doi: 10.3390/nu3010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broch M, Ramirez R, Auguet MT, Alcaide MJ, Aguilar C, Garcia-Espana A, Richart C. Macrophages are novel sites of expression and regulation of retinol binding protein-4 (RBP4) Physiological research / Academia Scientiarum Bohemoslovaca. 2010;59:299–303. doi: 10.33549/physiolres.931714. [DOI] [PubMed] [Google Scholar]
- 62.Feng N, Kim B, Fenaux M, Nguyen H, Vo P, Omary MB, Greenberg HB. Role of interferon in homologous and heterologous rotavirus infection in the intestines and extraintestinal organs of suckling mice. J Virol. 2008;82:7578–7590. doi: 10.1128/JVI.00391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Angel J, Franco MA, Greenberg HB, Bass D. Lack of a role for type I and type II interferons in the resolution of rotavirus-induced diarrhea and infection in mice. J Interferon Cytokine Res. 1999;19:655–659. doi: 10.1089/107999099313802. [DOI] [PubMed] [Google Scholar]
- 64.Vancott JL, McNeal MM, Choi AH, Ward RL. The role of interferons in rotavirus infections and protection. J Interferon Cytokine Res. 2003;23:163–170. doi: 10.1089/107999003321532501. [DOI] [PubMed] [Google Scholar]
- 65.Kuwata T, Wang IM, Tamura T, Ponnamperuma RM, Levine R, Holmes KL, Morse HC, De Luca LM, Ozato K. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood. 2000;95:3349–3356. [PubMed] [Google Scholar]
- 66.Ben-Zvi I, Aranow C, Mackay M, Stanevsky A, Kamen DL, Marinescu LM, Collins CE, Gilkeson GS, Diamond B, Hardin JA. The impact of vitamin D on dendritic cell function in patients with systemic lupus erythematosus. PloS one. 2010;5:e9193. doi: 10.1371/journal.pone.0009193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Swiecki M, Wang Y, Vermi W, Gilfillan S, Schreiber RD, Colonna M. Type I interferon negatively controls plasmacytoid dendritic cell numbers in vivo. J Exp Med. 2011 doi: 10.1084/jem.20110654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feldman S, Stein D, Amrute S, Denny T, Garcia Z, Kloser P, Sun Y, Megjugorac N, Fitzgerald-Bocarsly P. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 69.Duan XZ, Wang M, Li HW, Zhuang H, Xu D, Wang FS. Decreased frequency and function of circulating plasmocytoid dendritic cells (pDC) in hepatitis B virus infected humans. Journal of clinical immunology. 2004;24:637–646. doi: 10.1007/s10875-004-6249-y. [DOI] [PubMed] [Google Scholar]
- 70.Kanto T, Inoue M, Miyazaki M, Itose I, Miyatake H, Sakakibara M, Yakushijin T, Kaimori A, Oki C, Hiramatsu N, Kasahara A, Hayashi N. Impaired function of dendritic cells circulating in patients infected with hepatitis C virus who have persistently normal alanine aminotransferase levels. Intervirology. 2006;49:58–63. doi: 10.1159/000087264. [DOI] [PubMed] [Google Scholar]
- 71.Della Bella S, Crosignani A, Riva A, Presicce P, Benetti A, Longhi R, Podda M, Villa ML. Decrease and dysfunction of dendritic cells correlate with impaired hepatitis C virus-specific CD4+ T-cell proliferation in patients with hepatitis C virus infection. Immunology. 2007;121:283–292. doi: 10.1111/j.1365-2567.2007.02577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fugier-Vivier I, Servet-Delprat C, Rivailler P, Rissoan MC, Liu YJ, Rabourdin-Combe C. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J Exp Med. 1997;186:813–823. doi: 10.1084/jem.186.6.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zeng R, Oderup C, Yuan R, Lee M, Habtezion A, Hadeiba H, Butcher EC. Retinoic acid regulates the development of a gut-homing precursor for intestinal dendritic cells. Mucosal immunology. 2012 doi: 10.1038/mi.2012.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Belkaid Y, Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Strauch UG, Grunwald N, Obermeier F, Gurster S, Rath HC. Loss of CD103+ intestinal dendritic cells during colonic inflammation. World J Gastroenterol. 2010;16:21–29. doi: 10.3748/wjg.v16.i1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rescigno M, Rotta G, Valzasina B, Ricciardi-Castagnoli P. Dendritic cells shuttle microbes across gut epithelial monolayers. Immunobiology. 2001;204:572–581. doi: 10.1078/0171-2985-00094. [DOI] [PubMed] [Google Scholar]
- 77.Rescigno M, Urbano M, Valzasina B, Francolini M, Rotta G, Bonasio R, Granucci F, Kraehenbuhl JP, Ricciardi-Castagnoli P. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 78.Schlickum S, Sennefelder H, Friedrich M, Harms G, Lohse MJ, Kilshaw P, Schon MP. Integrin alpha E(CD103)beta 7 influences cellular shape and motility in a ligand-dependent fashion. Blood. 2008;112:619–625. doi: 10.1182/blood-2008-01-134833. [DOI] [PubMed] [Google Scholar]
- 79.Jin CJ, Hong CY, Takei M, Chung SY, Park JS, Pham TN, Choi SJ, Nam JH, Chung IJ, Kim HJ, Lee JJ. All-trans retinoic acid inhibits the differentiation, maturation, and function of human monocyte-derived dendritic cells. Leuk Res. 2010;34:513–520. doi: 10.1016/j.leukres.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 80.Martin-Latil S, Mousson L, Autret A, Colbere-Garapin F, Blondel B. Bax is activated during rotavirus-induced apoptosis through the mitochondrial pathway. J Virol. 2007;81:4457–4464. doi: 10.1128/JVI.02344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bagchi P, Dutta D, Chattopadhyay S, Mukherjee A, Halder UC, Sarkar S, Kobayashi N, Komoto S, Taniguchi K, Chawla-Sarkar M. Rotavirus nonstructural protein 1 suppresses virus-induced cellular apoptosis to facilitate viral growth by activating the cell survival pathways during early stages of infection. J Virol. 2010;84:6834–6845. doi: 10.1128/JVI.00225-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaibi C, Cotte-Laffitte J, Sandre C, Esclatine A, Servin AL, Quero AM, Geniteau-Legendre M. Rotavirus induces apoptosis in fully differentiated human intestinal Caco-2 cells. Virology. 2005;332:480–490. doi: 10.1016/j.virol.2004.11.039. [DOI] [PubMed] [Google Scholar]
- 83.Sato A, Iizuka M, Nakagomi O, Suzuki M, Horie Y, Konno S, Hirasawa F, Sasaki K, Shindo K, Watanabe S. Rotavirus double-stranded RNA induces apoptosis and diminishes wound repair in rat intestinal epithelial cells. J Gastroenterol Hepatol. 2006;21:521–530. doi: 10.1111/j.1440-1746.2005.03977.x. [DOI] [PubMed] [Google Scholar]
- 84.Superti F, Ammendolia MG, Tinari A, Bucci B, Giammarioli AM, Rainaldi G, Rivabene R, Donelli G. Induction of apoptosis in HT-29 cells infected with SA-11 rotavirus. J Med Virol. 1996;50:325–334. doi: 10.1002/(SICI)1096-9071(199612)50:4<325::AID-JMV8>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 85.Walkley CR, Yuan YD, Chandraratna RA, McArthur GA. Retinoic acid receptor antagonism in vivo expands the numbers of precursor cells during granulopoiesis. Leukemia. 2002;16:1763–1772. doi: 10.1038/sj.leu.2402625. [DOI] [PubMed] [Google Scholar]
- 86.VanGundy Z, Taylor C, White A, Baker J, Muth D, Papenfuss TL. Tolerogenic DC differentiation and development of immunosupressive DC precursors. 99th Annual Meeting of The American Association of Immunologists; Boston, Massachusetts. 2012. p. 38. [Google Scholar]
- 87.Smith SM, Levy NS, Hayes CE. Impaired immunity in vitamin A-deficient mice. J Nutr. 1987;117:857–865. doi: 10.1093/jn/117.5.857. [DOI] [PubMed] [Google Scholar]
- 88.Bruns NJ, Webb KE., Jr. Vitamin A deficiency: serum cortisol and humoral immunity in lambs. J Anim Sci. 1990;68:454–459. doi: 10.2527/1990.682454x. [DOI] [PubMed] [Google Scholar]